Abstract
A new low molecular weight fluorescent probe, Col-F, that exhibits affinity to collagen and elastin, was used successfully in imaging of extracellular matrix in freshly excised animal tissues. Col-F readily penetrates between live cells into tissues and binds to fibers of collagen and elastin by a noncovalent mechanism. Fibers of collagen and elastin have been stained in a variety of tissues, including tendon, skeletal muscle, connective tissue, and arteries. Cells migrating in a Col-F-stained collagenous biomaterial were also imaged. No phototoxic effects were detected when live keratocytes were imaged in the in vitro culture in the presence of Col-F. In conclusion, Col-F provides a simple and convenient tool for fluorescence three-dimensional imaging of intricate collagenous and elastic structures in live and fixed animal tissues, as well as in collagen-containing biomaterials.
Keywords: extracellular matrix, collagen, elastin, fluorescence microscopy, live cell imaging, confocal microscopy
INTRODUCTION
Collagens and elastin are synthesized primarily by fibroblasts and constitute principal components of extracellular matrix (ECM). Collagen molecules form a triple helix and, once outside cells, assemble into fibrils and fibers that provide mechanical strength to almost all animal tissues (1,2,3,4). Elastin secreted by cells assembles into fibers and cross-links to form a mechanically flexible network of fibers and sheets (5). Fibers of collagen and elastin form scaffolds that mechanically support various animal tissues. Interaction between cells and ECM fibers play a key role in embryogenesis, tissue remodeling and regeneration. Imaging dynamic changes of ECM in live tissue can provide new insights into the structure and role of ECM in biological processes.
Several microscopy techniques of visualizing fibers of ECM in fixed tissues are known, but only a few methods of visualizing ECM in live tissues in situ and in freshly excised tissues are available. Collagen fibers polymerized under laboratory conditions can be imaged in an optical microscope, using dark field (6), differential interference (Nomarski), or polarization contrast (7). Immunofluorescence, autofluorescence, and scattered light imaging, supplemented with Fourier transform in 3D space, have been used in confocal imaging of collagenous fibers ex vivo (8). In fixed tissues, collagen fibers are typically visualized by picro-syrius red (9), aniline blue, silver staining (for optical microscopy) (10), or negative staining (for electron microscopy). Several histochemical methods of staining elastin were reported over the years, including resorcin-fuchsin by Weigert (11), orcein by Schmorl (12), or iron hematoxylins by Weigert and Verhoeff. In live tissues, collagen and elastin fibers can be imaged by detecting their autofluorescence, although the specificity of fluorescent signals and their strength varies between tissues. Collagen can also be imaged using fluorescently labeled collagen binding proteins (13). Staining of elastin in live tissues by sulforhodamine B has been reported (14). Two sophisticated imaging techniques can image collagen - multiphoton confocal microscopy in an instrument equipped for detection of second harmonic generation signals, and a coherent anti-Stokes Raman scattering (CARS) imaging (15,16). To our knowledge, there are no simple, inexpensive, or widely available techniques for 3-dimensional imaging of collagen and elastin fibers in live animals, or excised metabolically active tissues.
We describe a new, simple method of fluorescent labeling of elastic and collagenous structures in excised metabolically active tissues for standard wide field fluorescence and confocal microscopy. A low molecular weight fluorescent probe, Col-F (fluorescein conjugated to physostigmine), exhibits affinity to fibrillar proteins of extracellular matrix. Col-F readily penetrates into tissue via a mechanism that does not involve its penetration into the cell interior, and subsequently binds noncovalently to ECM fibers. Confocal imaging of Col-F-stained freshly excised tissues can reveal a stunning variety of intricate 3D collagenous and elastic structures.
MATERIALS AND METHODS
Fluorescent probes and staining
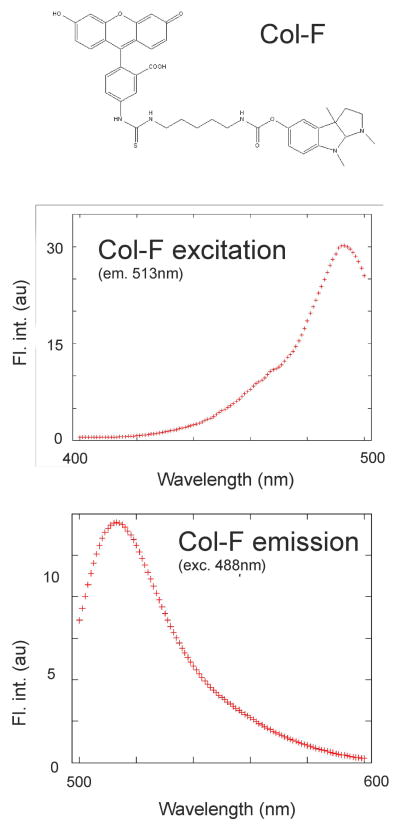
Col-F is a conjugate of physostigmine and fluorescein (Fig. 1a; patent pending). Spectral characteristics are similar to fluorescein (Fig. 1b,c); the fluorescence quantum efficiency of Col-F dissolved in PBS is 0.30. Excitation and emission curves were collected using a Hitachi fluorescence spectrophotometer F-450. Col-F was originally synthesized (under the name Ph-F, US provisional patent application 1579.004PRV) with an intention to probe cholinesterases (17). Although the compound does show limited affinity to cholinesterases in biochemical assays, our attempts to image cholinergic nerves by fluorescence confocal microscopy were unsuccessful, presumably due to weak fluorescence signals. Col-F (Immunochemistry Technologies, Bloomington, MN, USA) was dissolved in DMSO and stored frozen. Tissue fragments were stained by adding 1 μl of Col-F stock solution (20mM) to culture medium, in which the specimen was submerged. Depending on tissue type, the final concentration used was 10, 15 or 20 μM, and incubation times varied from 5 minutes to several hours. DRAQ5 (Biostatus, Cardiff, UK), TMRE (Molecular Probes, Eugene, OR) and sulforhodamine B (SRB) (Sigma-Aldrich) stock solutions were stored at 2°C and added to culture medium to a final concentration of 5 μM (DRAQ5 and TMRE), and 1.73 μM (SRB).
Fig. 1.
Chemical structure and excitation and emission spectra of Col-F.
Polymerized collagen in vitro
A sterile solution of monomeric collagen type I from bovine dermis (Vitrogen, USA) was maintained in 0.012 N HCl, at 2°C. 0.8 ml of the solution was placed in a custom-made steel holder with a glass bottom made of a 0.17 mm thick, 22 mm diameter coverslip (Menzel, Germany). 0.1 ml of 10x times concentrated culture medium and 0.1 ml 0.1 M NaOH was added and gently mixed. The sample was fixed in a microscope stage microincubator (Life Science Resources, Cambridge, UK). Polymerization of collagen was initiated by increasing temperature to 37°C (18). This procedure leads to formation of fibrils of a diameter of 20–70 nm (19).
Animal tissues
Mice were sacrificed by cervical dislocation. Tissues were removed and placed in culture medium (DMEM without phenol red and bicarbonate, pH 7.4 (Sigma)) supplemented with 1% fetal bovine serum, Col-F, DRAQ5 and TMRE. For imaging tissue fragments were placed on glass bottom Petri dishes (MatTek, USA). All laboratory procedures conformed with the animal license issued by Local Ethics Commission for Animal Experiments at Jagiellonian University.
Frozen sections preparation
Tissues were frozen in liquid nitrogen and then stored at −80°C. These tissues were subsequently cut into 8 μm sections and incubated in PBS dyes solution.
Collagen wound dressing
A 4×4 mm fragment of a sterile sheet of collagen-alginate dressing (Fibracol, Johnson-Johnson, Inc.) was cut and placed onto a glass bottom Petri dish containing culture medium and imaged. Another fragment was incubated with a logarithmically growing culture of HeLa cells. After 24 hours of incubation the fragment of a dressing was populated with migrating cells. It was than transferred to a new sterile Petri dish, submerged in culture medium supplemented with Col-F, TMRE and DRAQ5 and imaged at room temperature.
Confocal imaging
Fluorescence and backscattered light images were collected using a Bio-Rad MRC1024 confocal system (Bio-Rad, Microscience, Hemel Hemsptead, UK), interfaced with an inverted microscope Nikon Diaphot (Nikon, Amsterdam, The Netherlands), and equipped with three fluorescence detection channels, 100 mW Ar+ ion (ILT, Salt lake City, UT, USA), 15 mW KrAr (ALC, Salt Lake City, UT, USA) lasers, a 60x NA 1.4 oil immersion lens (Nikon) and a 40x NA 1.2 WI lens (Nikon). Col-F, TMRE, SRB and DRAQ5 were excited simultaneously by 488 and 568 laser-lines. Conditions of measurements were: primary triple dichroic mirror 488/568/647 (T1 block), secondary dichroic 560LP (T2A), a 620LP fixed dichroic facing PMT3, emission filters 540/30, 580 LP, 630 LP. 8 bit 512×512 or 1024×1024 images were collected at a rate of 1 or 0.3/s scan. Conditions for simultaneous detection of backscattered light and green fluorescence: excitation 488nm, primary beam splitter 527DRLP (A1 block), secondary dichroic 565DRLP (A2 block), fluorescence detection in PMT2 (emission filter 540/30), backscattered light detection in PMT3 (no emission filter). In some experiments a Leica SP5 SMD confocal microscope (Leica Microsystems) was used. Col-F, TMRE, SRB and DRAQ5 were excited simultaneously by 488 and 561 nm laser lines. Fluorescence was collected in the range of 500–550nm (Col-F), 600–700 nm (TMRE and SRB) and 700–800 nm (DRAQ5). In the images displayed below Col-F, TMRE and DRAQ5 are shown as green, red and blue, respectively. Images were collected with a scanning frequency of 200 Hz, resolution 512 × 512 or 1024 × 1024 pixels, using a 63x HCX PL APO CS NA 1.4 lens.
FRAP studies
A freshly excised mouse tendon and mesentery were stained with Col-F and a 123×123 μm area was imaged. Subsequently, the laser beam (360 μW) was scanned over a 10 × 10 μm2 area (30 scans, frequency of scanning 200 lines/s; 512×512 pixels). Recovery of fluorescence (in the spectral range 500–560nm) in the bleached area was recorded by subsequent time lapse imaging of the original 123 × 123 μm region (5 scans every 2,5 sec; subsequently 140 scans every 7.5 sec.). The fluorescence recovery curves were obtained by calculating the average values of fluorescence intenisties in the bleached region and plotting against time.
Image analysis
Images of Col-F, TMRE and DRAQ5 fluorescence were deconvolved (AutoDeblur, AutoQuant, USA) prior to reconstructing maximum projection images.
RESULTS
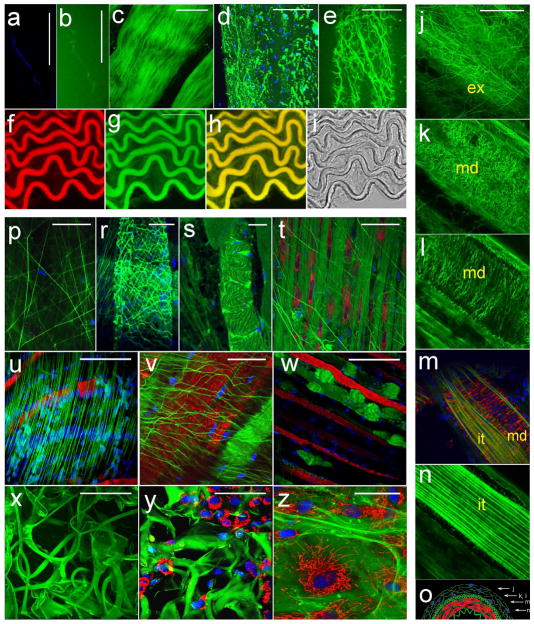
When freshly isolated mouse tissues are exposed to culture medium supplemented with Col-F, a network of fibers is readily stained and becomes visible in fluorescence microscopy. In order to identify the types of fibers to which Col-F exhibits affinity, we imaged various samples containing known proteins or tissues with a known content and architecture of various ECM fibers. We demonstrated the affinity of Col-F to polymerized collagen type I in vitro by exposing a meshwork of fibrils of this polymer to the dye and imaging the selected fibril simultaneously by an established method, i.e. backscattered light microscopy (20) (Fig. 2a), and by detecting fluorescence of Col-F bound to the same fiber (Fig. 2b). We further confirmed the affinity of Col-F for polymerized collagens by staining a freshly isolated mouse tendon (Fig. 2c). Col-F stained collagen in de-mineralized bone (Fig. 2d), indicating that the probe has affinity not only to native, but to a denatured collagen as well.
Fig. 2.
Binding of Col-F to ECM fibers.
Images a – i. Col-F binding to collagen and elastin fibers. Scale bars 50 μm
a and b - image of a collagen type I fibril, obtained using the backscattered light approach (20,22) and image of the same fibril stained with Col-F
c - collagen fibers in a tendon, stained with Col-F
d - collagen in demineralized bone (green) and cell nuclei (blue)
e - elastin in mesentery
f, g, h, i - a frozen section of aorta. (f – Col-F staining, g – SRB staining, h - the Col-F and SRB images overlaid, i – transmitted light image
j – o - images and schematics of a wall of an intact, live muscular artery (in the images lamina externa is marked with ‘ex’, media with ‘md’, and interna with ‘it’): j - adventitia (ex), k - the crosslinked fibers of elastin in elastic lamina externa (md), l - tunica media, fibers running perpendicular to a long axis of the blood vessel, between smooth muscle cells (mitochondria and nuclei in smooth muscle are stained red and green, respectively), m - an optical section through the layer of smooth muscle showing a fragment adjacent to internal elastic membrane, m, n - internal elastic membrane - a part of intima, n - elastic lamina interna, o - a schematic representation of the layers of the wall of an artery; the positions where the images j, k, l, m, n were taken are marked by arrows.
Images p–w. ECM in live tissues, a biomaterial and in a culture of primary fibroblasts. Various architectures of ECM networks in tissues in vivo, stained with Col-F, TMRE and DRAQ5 (in panels d-s: green - collagen; red - active mitochondria, blue - cell nuclei; scale bars 50 μm
p - elastin in epimysium of a skeletal muscle
r - elastin wrapping surrounding a muscle in a diaphragm,
s - collagen in dense connective tissue of a diaphragm and
t - collagen bundles and collagen-producing fibroblasts in connective tissue of a diaphragm
u - elastin in basal lamina on the surface of a skeletal muscle
w - collagen bundles arranged parallel to muscle fibers in a mouse diaphragm; images u and w demonstrate two architectures of parallel fibers supporting the basal lamina in a diaphragm
x, y - structure of alginate-collagen dressing (x) and fibroblasts migrating into the dressing (y)
z - primary fibroblasts 2D culture with ECM proteins produced by those fibroblasts.
Affinity of Col-F to elastin was demonstrated by imaging elastin fibers, decorated with Col-F, in a freshly isolated mouse mesentery, a tissue that is known to contain a characteristic array of elastin fibers. (Fig. 2e). We also stained a frozen section of mouse aorta with Col-F (Fig. 2f–i). Aorta has a characteristic architecture of elastic fibers in the vessel wall, in tunica media. Medial lamellar unit of the wall of aorta is very regular, with elastin arranged in concentric lamellae and smooth muscle cells surrounded by collagen fibers (21,22), which lies between those lamellae. A small amount of elastin is also present in the form of interlamellar rafts (23). Elastin in aorta was first stained with SRB, as described in (14) (Fig. 2f); subsequently Col-F was added and the sample was imaged. There was a clear match between the Col-F-stained fibers (Fig. 2g), and SRB-stained elastin fibers (Fig. 2f) in the image that represented an overlap of Col-F and SRB signals (Fig. 2h). Structures that stained with SRB and Col-F matched the structures seen in a transmitted light image (Fig. 2i) as well. These observations reinforce a notion that Col-F exhibits affinity to elastin.
Binding of Col-F to elastin and collagen was further confirmed by staining tunica adventitia (tunica externa) (Fig. 2j), media (Fig. 2k,l,m) and elastic lamina interna (Fig. 2m, n), in the wall of a freshly excised muscular artery. Adventitia is known to be composed primarily of collagen, media is built of smooth muscle surrounded by collagen and elastic fibers as well as a dense network while lamina interna contains numerous elastin fibers. All these structures were contrasted efficiently following exposure of tissue to medium supplemented with Col-F. The collagen fibers that attach the blood vessel were easily discerned in Fig. 2j and 2l (Fig. 2o shows a schematics of the cross-section of a blood vessel and indicates the positions of the confocal sections in relation to the vessel wall layers). The elastic fibers arranged in a characteristic transverse manner, between the muscle cells, were clearly seen. The smooth muscle cells were visualized by staining active mitochondria with TMRE and nuclei with DRAQ5 (Fig. 2m). The innermost layer contains elastin fibers that are readily stained by Col-F. This region has an appearance of longitudal wrinkles. The inner surface of blood vessels, which is generally smooth, adopts this corrugated appearance following excision of the tissue fragment.
Once the affinity of Col-F to collagen and elastin was demonstrated by the experiments described above, we imaged Col-F-stained ECM structures in various mouse tissues, including a skeletal muscle and a diaphragm (Fig. 2p–w), in order to examine the usefulness of Col-F for rapid staining and imaging of ECM fibers ex vivo, in freshly excised tissue samples. Image 2p shows elastin in epimysium of a skeletal muscle, 2r - elastin wrapping surrounding a muscle in a diaphragm, 2s – collagen in dense connective tissue of a diaphragm and 2t - collagen bundles and fibroblasts in connective tissue of a diaphragm. Here again live cells (fibroblasts) were visualized by staining with TMRE (Fig. 2t–w) and DRAQ5 (Fig. 2p–w). Images in Fig. 2u and 2v demonstrate elastin in basal lamina on the surface of a skeletal muscle (and live cells stained as above), and Fig. 2w shows an image of collagen bundles (green) arranged parallel to muscle fibers in a mouse diaphragm. Mitochondria that are known to be aligned parallel to actin and myosin fibers (red) and cell nuclei (blue) located at the periphery of muscle fibers are clearly seen.
In addition to imaging ECM in excised tissues we also used Col-F to visualize the 3D structure of an extracellular matrix substitute, collagen-alginate dressing. This biomaterial is used in treatment of wounds and burns. The image shown in Fig. 2x is a maximum projection of a stack of confocal cross-sections and demonstrates a complicated 3D structure of the biomaterial. In Fig. 2z the attached, migrating cells are imaged as well. Further, we used Col-F to visualize ECM fibers that are externalized by primary human fibroblasts in a 6 days old monolayer culture (Fig. 2z).
In order to shed more light on affinity of Col-F to various components of ECM, we also performed preliminary surface plasmon resonance studies (SPR) and molecular modeling (data not shown). SPR experiments confirmed that Col-F has affinity to collagen type I, but not to fibronectin. The results of molecular modeling are consistent with a hypothesis that Col-F binds to collagen and elastin via hydrogen bonds and hydrophobic interactions.
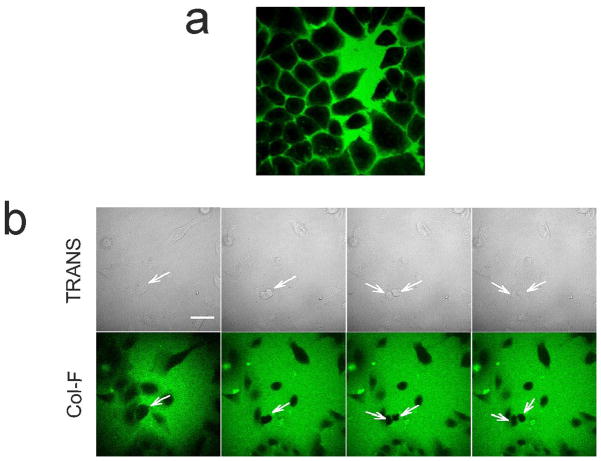
The usefulness of fluorescent probes in studies of live cells and tissues is heavily dependent on their toxicity and phototoxicity. As mentioned before, in order to examine viability and metabolic activity of cells in Col-F-stained tissues we imaged active mitochondria using a potential-sensitive dye, TMRE. Cells were also counter-stained with a plasma membrane permeant anthracycline fluorochrome, DRAQ5, which binds to nuclear DNA. Thus, mitochondrial staining shown in Fig. 2m,t–w demonstrates that cells in these Col-F-stained tissues were metabolically active. We have also demonstrated that phototoxic effects exerted by Col-F are negligible during time-lapse imaging of primary human keratocytes submerged in culture medium supplemented with Col-F. Cells migrated and divided normally in the presence of Col-F during a 2h long experiment (Fig. 3b). However, as the chromophore in Col-F is fluorescein, which is known to produce singlet oxygen upon exposure to exciting light, detectable phototoxic effects are to be expected when higher light doses are used.
Fig. 3.
Interaction of Col-F with live cells
a. Image of Col-F and live cells in an in vitro culture. Fluorescence of Col-F (green) is detected exclusively in culture medium surrounding the cells, the dye does not penetrate through intact plasma membranes
b. keratocytes migrate and divide in the presence of Col-F. Cell division is marked with arrows. A quickly migrating cell can be seen in the upper part of the image (12 frames, 256×256 pixels, duration 2h).
In order to investigate whether Col-F can cross plasma membranes of live cells, we incubated HeLa cells with the dye in an in vitro culture, in the absence of collagen. Col-F remained extracellular during a 60 min incubation, indicating a lack of penetration through intact plasma membranes (Fig. 3a). Col-F did not enter interiors of live cells even during a 60h incubation (data not shown). Thus, we postulate that Col-F, when applied to a excised tissue, penetrates by diffusing through extracellular space, between live cells.
The mode of binding of Col-F to ECM fibers was studied using the fluorescence recovery after photobleaching (FRAP) technique (24,25). Fluorescence intensity swiftly recovered in the photobleached areas, while fluorescence intensity in other regions of the labeled fibers decreased (Fig. 4a–d) indicating that the bound probe was readily exchanging with the unbound molecules in the surrounding solution. Rapid fluorescence recovery implies that interaction between Col-F and the ECM fiber involves only weak chemical bonding mechanisms rather than a covalent linkage. It also explains a surprisingly high (and very convenient) stability of fluorescent signals, which we observed during data collection.
Fig. 4.
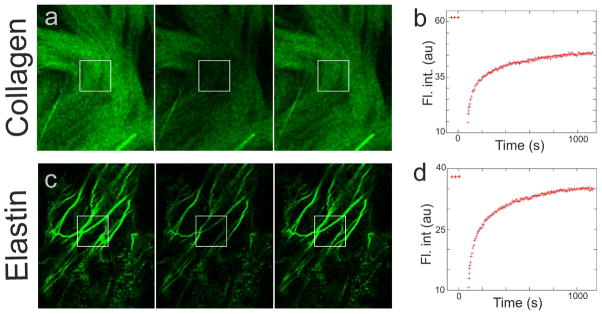
FRAP studies of binding of Col-F to ECM fibers
a, c - images of Col-F bound to collagen in an excised mouse tendon (a) and elastin in mesentery (c)
Left panel - image prior to photobleaching
Center panel - image immediately after photobleaching a 10×10 μm area (marked with a square)
Right panel - image 17 minutes after photobleaching, showing the recovery of fluorescence in the bleached region, and an equilibrium between the staining intensity in the bleached and unbleached region of the tendon and mesentery.
b, d - FRAP curves of Col-F bound to collagen in a mouse tendon (b) and elastin in mesentery (d).
DISCUSSION
Imaging of collagen and elastin in live tissues, as well as other components of extracellular matrix, is required in many research fields (26), including studies of interactions between tumor cells and extracellular matrix, rearrangements of extracellular matrix, cell migration, mechanical properties of blood vessels, wound healing, etc. In order to facilitate such studies, a method of imaging the scaffold of extracellular matrix must provide good contrast, introduce minimum disturbance to tissue, and be as simple and rapid as possible.
Currently, noninvasive imaging of elastic and collagenous structures in live tissues can be best achieved by detecting their autofluorescence or second harmonic generation (SHG) signals (15,16,27,28). The advantage of this method is the ability to contrast ECM fibers without introducing any exogenous probes. This technique of imaging ECM is not entirely specific for ECM, since several tissue components, including actin, yield SHG signals as well (29). Moreover, the signal depends on the angle between the polarization of exciting radiation and the long axis of the imaged fiber, leading to image interpretation artifacts (30). SHG is expensive and technically demanding since it requires a multiphoton confocal microscope and a dedicated detector. High quality images are difficult to obtain.
The advantages of the method of labeling and imaging of ECM fibers, which employs Col-F and fluorescence microscopy, are its simplicity (the dye is directly applied to tissue in a single step procedure), rapidity (staining is instantaneous, and penetration into the depth detectable by confocal microscopy occurs within minutes), high stability of fluorescent signals (due to equilibrium between the bound and the dissolved dye), versatility (elastin and collagens of several types in all tissues examined so far are stained readily), low cost (can be used in any fluorescence microscope). Potential limitations include phototoxicity during long-term imaging, non-specific staining of damaged cells and an inability to distinguish collagen and elastin. Since Col-F molecule contains physostigmine, an inhibitor of acetylcholinesterase, staining of cholinergic nerves or motor plates might be anticipated. The latter could arise from binding of Col-F to an active center of the enzyme as well as with the collagen-Q moiety of the membrane-bound enzyme. However, we have not detected such structures on the images collected in a large variety of tissues that we examined. The inability to detect binding of Col-F to cholinesterases may be due to the fact that either conjugation of fluorescein to physostigmine reduces binding of the inhibitor to the enzyme or that content/local density of cholinesterases in the studied structures was inadequate to be detectable. We conclude that Col-F staining of ECM offers a potentially useful approach to general imaging of the architecture of collagen and elastin fibers in excised live tissues.
Acknowledgments
Grant sponsors: MNSW; Grant numbers: 2067/PO1/2007/32 (EB, JG, JWD) and EU structural funds grant BMZ no. POIG.02.01.00–12–064/08;. NCI; CA 28704 and Robert A. Welke Foundation for Cancer Research (ZD);
We thank Prof. Z. Stok osowa, Prof. Z. D browski, Prof. J. Styrna, Dr J. Druka a for stimulating discussions and help, and Dr A. Polit for help with measurements of quantum efficiency of Col-F. This research was supported by a grant 2067/P01/2007/32 from Ministry of Science and Higher Education in Warsaw (JD) as well as NIH NCI RO1 28–704 and Robert A. Welke Cancer Research Foundation (ZD).
Footnotes
Conflict of interests. The authors declare a conflict of interests; BJ and GLJ represent the manufacturer of Col-F.
References
- 1.Kadler K. Matrix loading: assembly of extracellular matrix collagen fibrils during embryogenesis. Birth Defects Res C Embryo Today. 2004;72(1):1–11. doi: 10.1002/bdrc.20002. [DOI] [PubMed] [Google Scholar]
- 2.Burrage PS, Mix KS, Brinckerhoff CE. Matrix metalloproteinases: role in arthritis. Front Biosci. 2006;11:529–43. doi: 10.2741/1817. [DOI] [PubMed] [Google Scholar]
- 3.Baxter BT. Heritable diseases of the blood vessels. Cardiovasc Pathol. 2005;14(4):185–8. doi: 10.1016/j.carpath.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 4.Wess TJ. Collagen fibril form and function. Adv Protein Chem. 2005;70:341–74. doi: 10.1016/S0065-3233(05)70010-3. [DOI] [PubMed] [Google Scholar]
- 5.Patel A, Fine B, Sandig M, Mequanint K. Elastin biosynthesis: The missing link in tissue-engineered blood vessels. Cardiovascular Res. 2006;71(1):40–9. doi: 10.1016/j.cardiores.2006.02.021. [DOI] [PubMed] [Google Scholar]
- 6.Liu MY, Yeh ML, Luo ZP. In vitro regulation of single collagen fibril length by buffer compositions and temperature. Biomed Mater Eng. 2005;15(6):413–20. [PubMed] [Google Scholar]
- 7.Petroll WM. Differential interference contrast and confocal reflectance imaging of collagen organization in three-dimensional matrices. Scanning. 2006;28(6):305–10. doi: 10.1002/sca.4950280602. [DOI] [PubMed] [Google Scholar]
- 8.Rajwa B. PhD Thesis. Jagiellonian University; Kraków, Poland: 2002. Three-dimensional visualization of extracellular matrix-based tissue models. [Google Scholar]
- 9.Sweat F, Puchtler H, Rosenthal SI. Sirius Red F3BA as a stain for connective tissue. Arch Pathol. 1964;78:69–72. [PubMed] [Google Scholar]
- 10.Hwang WS, Ngo K, Saito K. Silver staining of collagen fibrils in cartilage. Histochem J. 1990;22(9):487–90. doi: 10.1007/BF01007233. [DOI] [PubMed] [Google Scholar]
- 11.Weigert C. Uber eine methode zur farbung elastischer faser. Zentbl Allg Pathol Anat. 1898;9:289–302. [Google Scholar]
- 12.Puchtler H, Meloan SN. Orcein, collastin and pseudo-elastica: a re-investigation of Unna’s concepts. Histochemistry. 1979;64(2):119–30. doi: 10.1007/BF00490093. [DOI] [PubMed] [Google Scholar]
- 13.Krahn KN, Bouten CVC, van Tuijl S, van Zandvoort MaMJ, Merkx M. Fluorescently labeled collagen binding proteins allow specific visualization of collagen in tissues and live cell culture. Anal Biochem. 2006;350(2):177–85. doi: 10.1016/j.ab.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 14.Ricard C, Vial JC, Douady J, van der Sanden B. In vivo imaging of elastic fibers using sulforhodamine B. J Biomed Opt. 2007;12(6):064017. doi: 10.1117/1.2821421. [DOI] [PubMed] [Google Scholar]
- 15.Mansfield J, Yu J, Attenburrow D, Moger J, Tirlapur U, Urban J, Cui Z, Winlove P. The elastin network: its relationship with collagen and cells in articular cartilage as visualized by multiphoton microscopy. J Anat. 2009;215(6):682–91. doi: 10.1111/j.1469-7580.2009.01149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fritze O, Schleicher M, Konig K, Schenke-Layland K, Stock U, Harasztosi C. Facilitated Noninvasive Visualization of Collagen and Elastin in Blood Vessels. Tissue Eng Part C Methods. 2010;16(4):705–10. doi: 10.1089/ten.TEC.2009.0309. [DOI] [PubMed] [Google Scholar]
- 17.Huang X, Lee B, Johnson G, Naleway J, Guzikowski A, Dai W, Darzynkiewicz Z. Novel assay utilizing fluorochrome-tagged physostigmine (Ph-F) to in situ detect active acetylcholinesterase (AChE) induced during apoptosis. Cell Cycle. 2005;4(1):140–7. doi: 10.4161/cc.4.1.1322. [DOI] [PubMed] [Google Scholar]
- 18.Brightman AO, Rajwa BP, Sturgis JE, McCallister ME, Robinson JP, Voytik-Harbin SL. Time-lapse confocal reflection microscopy of collagen fibrillogenesis and extracellular matrix assembly in vitro. Biopolymers. 2000;54(3):222–34. doi: 10.1002/1097-0282(200009)54:3<222::AID-BIP80>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 19.Kadler KE, Holmes DF, Trotter JA, Chapman JA. Collagen fibril formation. Biochem J. 1996;316(Pt 1):1–11. doi: 10.1042/bj3160001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Voytik-Harbin SL, Rajwa B, Robinson JP. Three-dimensional imaging of extracellular matrix and extracellular matrix-cell interactions. Methods Cell Biol. 2001;6:583–97. doi: 10.1016/s0091-679x(01)63031-0. [DOI] [PubMed] [Google Scholar]
- 21.Wolinsky H, Glagov S. Structural basis for the static mechanical properties of the aortic media. Circ Res. 1964;14:400–13. doi: 10.1161/01.res.14.5.400. [DOI] [PubMed] [Google Scholar]
- 22.Wolinsky H, Glagov S. A lamellar unit of aortic medial structure and function in mammals. Circ Res. 1967;20:99–111. doi: 10.1161/01.res.20.1.99. [DOI] [PubMed] [Google Scholar]
- 23.O’Connell MK, Murthy S, Phan S, Xu C, Buchanan J, Spilker R, Dalman RL, Zarins CK, Denk W, Taylor CA. The three-dimensional micro- and nanostructure of the aortic medial lamellar unit measured using 3D confocal and electron microscopy imaging. Matrix Biol. 2008;27(3):171–81. doi: 10.1016/j.matbio.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Axelrod D, Koppel DE, Schlessinger J, Elson E, Webb WW. Mobility measurement by analysis of fluorescence photobleaching recovery kinetics. Biophys J. 1976;16(9):1055–69. doi: 10.1016/S0006-3495(76)85755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trembecka DO, Kuzak M, Dobrucki JW. Conditions for using FRAP as a quantitative technique-influence of the bleaching protocol. Cytometry A. 2010;77A(4):366–370. doi: 10.1002/cyto.a.20866. [DOI] [PubMed] [Google Scholar]
- 26.Wolf K, Friedl P. Functional imaging of pericellular proteolysis in cancer cell invasion. Biochimie. 2005;87(3–4):315–20. doi: 10.1016/j.biochi.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 27.Teng SW, Tan HY, Peng JL, Lin HH, Kim KH, Lo W, Sun Y, Lin WC, Lin SJ, Jee SH, So PT, Dong CY. Multiphoton autofluorescence and second-harmonic generation imaging of the ex vivo porcine eye. Invest Ophthalmol Vis Sci. 2006;47(3):1216–24. doi: 10.1167/iovs.04-1520. [DOI] [PubMed] [Google Scholar]
- 28.Fine S, Hansen WP. Optical second harmonic generation in biological systems. Appl Opt. 1971;10:2350–2353. doi: 10.1364/AO.10.002350. [DOI] [PubMed] [Google Scholar]
- 29.Campagnola PJ, Loew LM. Second-harmonic imaging microscopy for visualizing biomolecular arrays in cells, tissues and organisms. Nat Biotechnol. 2003;21(11):1356–60. doi: 10.1038/nbt894. [DOI] [PubMed] [Google Scholar]
- 30.Williams RM, Zipfel WR, Webb WW. Interpreting second-harmonic generation images of collagen I fibrils. Biophys J. 2005;88(2):1377–86. doi: 10.1529/biophysj.104.047308. [DOI] [PMC free article] [PubMed] [Google Scholar]