Abstract
Background
Small conductance calcium activated potassium (SK) channels are responsible for afterhyperpolarization that suppresses nerve discharges.
Objectives
To test the hypotheses that low-level vagus nerve stimulation (LL-VNS) leads to the upregulation of SK2 proteins in the LSG.
Methods
Six dogs (Group 1) underwent 1-wk LL-VNS of the left cervical vagus nerve. Five normal dogs (Group 2) were used as control. SK2 protein levels were examined by western blotting. The ratio between SK2 and glyceraldehydes-3-phosphate-dehydrogenase (GAPDH) levels was used as an arbitrary unit (AU).
Results
We found higher SK2 expression in Group 1 (0.124 ± 0.049 AU) than Group 2 (0.085 ± 0.031 AU, P < 0.05). Immunostaining showed that the density of nerve structures stained with SK2 antibody was also higher in Group 1 (11,546 ± 7,271 μm2/mm2) than in Group 2 (5,321 ± 3,164 μm2/mm2, P < 0.05). There were significantly more ganglion cells without immunoreactivity to TH in Group 1 (11.4 ± 2.3%) than Group 2 (4.9 ± 0.7%; P < 0.05). The TH-negative ganglion cells mostly stained positive for choline acetyltransferase (ChAT) (95.9 ± 2.8% in Group 1 and 86.1 ± 4.4% in Group 2, P = 0.10). Immunofluorescence confocal microscopy revealed a significant decrease in the SK2 staining in the cytosol but an increase in the SK2 staining on the membrane of the ganglion cells in Group 1 compared to Group 2.
Conclusion
Left LL-VNS results in the upregulation of SK2 proteins, increased SK2 protein expression in the cell membrane and the increased TH-negative (mostly ChAT-positive) ganglion cells in the LSG. These changes may underlie the antiarrhythmic efficacy of LL-VNS in ambulatory dogs.
Keywords: Autonomic nervous system, Vagus nerve stimulation, Stellate ganglion, Small conductance calcium activated potassium channel, Western blot
Introduction
Small conductance calcium activated K+ (SK) channels are widely expressed in nerve structures.1 The SK channels are known to be sensitive to intracellular calcium (Cai) and are primarily responsible for the slow afterhyperpolarization (sAHP) that hyperpolarizes neurons and reduces the frequency of neuronal discharges.1,2 Apamin, a Western honeybee toxin which specifically inhibits SK channels, reduces the sAHP, increases neuronal discharges and breaks down the burst-termination patterns.2,3 Because similar burst-termination patterns are also observed in the left stellate ganglion (LSG) of ambulatory dogs,4 it is possible that SK channels also play a role in regulating sympathetic outflow. Manipulating SK channel expression may be a novel approach to regulating sympathetic outflow and controlling cardiac arrhythmias. We5 recently showed that continuous left low-level vagus nerve stimulation (LL-VNS) suppressed LSG nerve activity and increased tyrosine hydroxylase (TH)-negative nerve structures in the LSG. These changes are associated with a reduced incidence of paroxysmal atrial tachyarrhythmias, including atrial fibrillation (AF). The increase of TH-negative cells suggests that LL-VNS can cause adrenergic neurons to lose their ability to produce catecholamines. However, whether or not these TH-negative cells undergo phenotypic switching to cholinergic ones, i.e. choline acetyltransferase (ChAT)-positivity, remains unknown. The purpose of the present study was to test the hypothesis that LL-VNS induces significant LSG neural remodeling, including upregulation of SK proteins, increased SK protein trafficking to the cell membrane and increased percentage of TH-negative cells. Immunostaining was used to test the hypothesis that most of the TH-negative ganglion cells stain positive for ChAT.
Methods
Chronic LL-VNS in Ambulatory Dogs
The study protocol was approved by the Institutional Animal Use and Care Committee and conforms to the Guide for the Care and Use of Laboratory Animals. Six male adult mongrel dogs (Group 1) were used for LL-VNS. Under isoflurane inhalation general anesthesia, an incision was made on the left anterior side of the neck. A bipolar pacing lead and an anchor lead were placed around the left cervical vagus nerve (Figure 1) and connected to a subcutaneously positioned Cyberonics Demipulse neurostimulator (Cyberonics Inc, Houston, TX). The wound was closed and the dog was allowed to recover for one week. We then defined the cardiac threshold by stimulating the left cervical vagus nerve at 15 Hz and 500-μs pulse duration. The stimulus amplitude (mA) that elicited an abrupt decrease of heart rate by > 20% from baseline was defined as the stimulation threshold. We then programmed the neurostimulator output to 1 mA below the stimulation threshold 5,6 and confirmed that this stimulus strength (1.1 ± 0.4 mA, range 0.5–3 mA) did not cause any heart rate changes. The stimulator was programmed to 60-s ON and 12-s OFF for one week while the dogs were ambulatory. The dogs were then euthanized and the LSG harvested for protein analyses and histological examinations. An additional five normal dogs (Group 2) were used as control. We did not perform any nerve recordings in these dogs because surgical implantation of recording wires may cause irritation and potentially change the underlying histology and protein expression.
Figure 1.
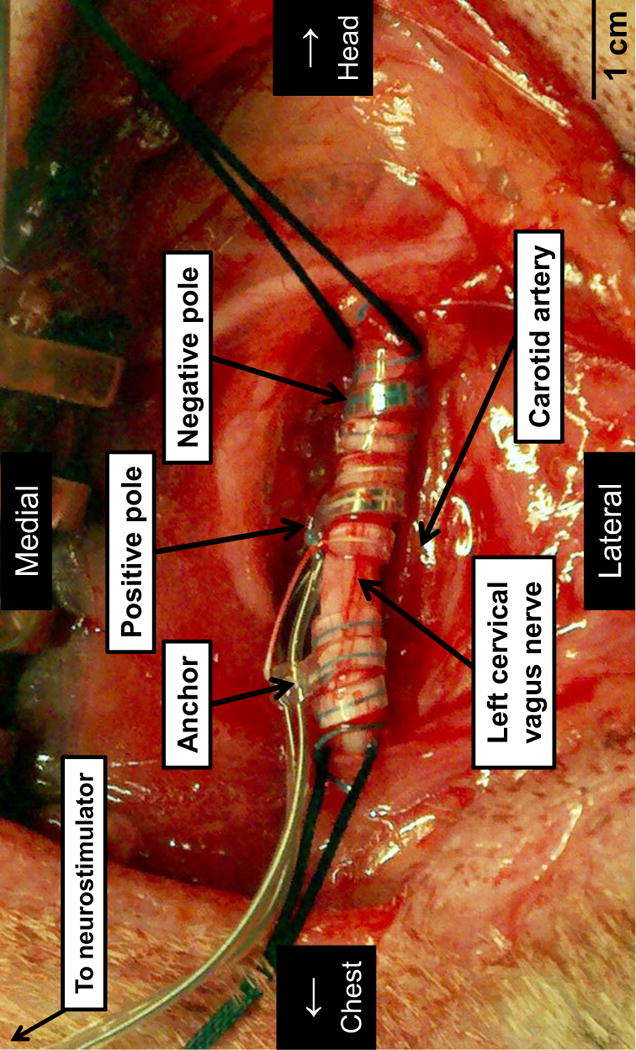
Implantation of electrodes on left cervical vagus nerve. A bipolar pacing lead and an anchor lead were placed around the left cervical vagus nerve and connected to a subcutaneously positioned Cyberonics Demipulse neurostimulator. The electrode orientation ensures that the negative pole is cranial to the positive pole, which is the configuration used clinically for the implanted Cyberonics stimulators.
Western blotting
The rostral half of the LSG was used for the western blot to quantify the amount of protein. We loaded 10 μg of homogenates of LSG on a SDS-PAGE and transferred to a nitrocellulose membrane. The blot was probed with an anti-subtype 2 SK (SK2) polyclonal antibody (1:600, Abcam, Cambridge, MA). The SK channels have 3 subtypes.1,7 Among them, SK1 is insensitive to apamin.8 SK3 is intermediately sensitive to apamin and is expressed in very low levels in atria and ventricles.9 In the sensory ganglia, SK3 is expressed only in satellite glial cells and not in ganglion neurons.10 On the other hand, SK2 is known to play an important role in regulating nerve activities of sympathetic ganglions.11 Therefore, we chose to rocus our errorts in studying SK2 expression in stellate ganglia. Antibody-binding protein bands were visualized by 125I protein A and quantified with a Bio-Rad Personal Fx phosphorimager, not by densitometry of the film. We adjusted the exposure to ensure that the protein bands were not saturated. Expression of SK2 for each sample was normalized to glyceraldehydes-3-phosphate-dehydrogenase (GAPDH) levels and expressed as arbitrary units (AU). The mean of intensity values from 5 cycles of western blots was used as the value for that LSG.
Immunohistochemistry Studies
We used the caudal half of the LSG for immunohistochemical analyses. The LSG samples were fixed in 4% formalin for 45 to 60 minutes, followed by storage in 70% alcohol.12 The tissues were processed routinely, paraffin embedded, and cut into 5 μm–thick sections.
Immunohistochemical staining was performed with antibodies against SK2 using rabbit polyclonal anti-KCNN2 (Abcam, Cambridge, MA), antibodies against TH using mouse monoclonal anti-TH (Accurate Chemical, Westbury, NY) and antibodies against choline acetyltransferase (ChAT) using goat polyclonal anti-ChAT (Chemicon, Billerica, MA).
Immunofluorescence Confocal Microscopy
Immunofluorescence confocal microscopy was performed and imaged using a Zeiss LSM 700 confocal laser scanning microscope. The study of the LSG tissue sections from Groups 1 and 2 was performed in a blinded fashion. Tissue sections (4 μm in thickness) were de-paraffinized and hydrated using the following series of washes: two Xylene washes (5 min each), followed by two 100% ethanol rinses (5 min each), followed by 95% ethanol, 70% ethanol, 50% ethanol, 30% ethanol, followed by water and Tris buffered saline with Tween 20 (TBST) wash for 5 min on a shaker. The slides were blocked using the blocking solution (Dulbecco’s phosphate buffered saline, catalog #21-031-CV from Mediatech, Inc. with 10% serum from the host of the secondary antibody) for one hour. Primary antibody (anti-SK2 antibody from Sigma-Aldrich, catalog #P0483, a polyclonal antibody raised in rabbit against a purified peptide corresponding to amino acid residues 542–559 of rat SK2 located in COOH terminus, 1:20 dilution) was then added in the tissue sections and incubated overnight in the humidified chamber (4 ºC). The slides were washed three times with TBST (3 min each on a shaker) followed by the addition of a secondary antibody (Cy3 goat anti-rabbit antibody, Invitrogen) diluted in the blocking solution and incubated for one hour at room temperature. The slides were washed three times with TBST (3 min each on a shaker) and rinsed with water. Finally, the tissue sections were mounted using mounting medium containing DAPI (4′, 6-diamidino-2-phenylindole, VectaMount, catalog# H-5000 Vector Laboratories, Inc.).
Data Analyses
We first analyzed the density (μm2/mm2) of nerve structures stained with SK2 antibody. We then manually determined the number of TH-negative ganglion cells among all ganglion cells in a stained slide.5 A blinded observer was asked to randomly select 5 high-power (20X) fields with highest ganglion cell density in the LSG from each dog. A second blinded investigator used these digital pictures to manually determine the percentage of TH-negative ganglion cells among all ganglion cells in the stained slides. We counted all cells with any part of the cells visible in the picture. The mean of those 5 selected fields was used as the value for that LSG. A third analysis was to compare the TH and ChAT staining of two serial slides and see if the TH-negative ganglion cells were ChAT-positive. The first blinded observer was asked to randomly select 3 high-power (20X) fields with highest density of TH-negative cells and then find the corresponding fields in the ChAT staining slides. He then obtained a digital picture. The second blinded investigator used these digital pictures to manually determine the percentage of the TH-negative ganglion cells that were ChAT-positive. For data obtained by confocal microscopy, the SK2 staining in LSG were quantified using Image J software from the National Institutes of Health. Regions of interest (ROIs) were drawn to measure the SK2 signals on the plasma membrane and in the cytosol of LSG neurons. The ratio of membrane/cytosol was obtained for each cell analyzed.
Statistical Analyses
The data were represented as mean followed by standard deviation. One-tailed t tests were used to compare the densitometric values of the western blot and immunostaining of the LSG of Group 1 and Group 2 dogs. To compare the membrane/cytosol SK2 fluorescence ratio in confocal microscopy, we first calculated the mean of the ratio of a number of cells for each animal. We then performed the Wilcoxon rank-sum test on those means between Group 1 and Group 2 using exact null distribution. The statistics were computed using the PASW Statistics (version 18; SPSS Inc, Chicago, IL) and SAS 9.2 (SAS Inc, Cary, NC). P ≤ 0.05 was considered significant.
Results
SK2 Protein in the LSG
Western blotting studies demonstrated the signal ratio of SK2 protein to glyceraldehydes-3-phosphate-dehydrogenase (GAPDH) of Group 1 (0.124 ± 0.049 arbitrary unit, AU) was significantly higher than that of Group 2 (0.085 ± 0.031 AU, P < 0.05, Figures 2A and 2B), indicating an upregulation of SK2 protein in the LSG among dogs undergoing LL-VNS. The SK2 protein levels before normalization were 3516 ± 795 for Group 1 and 3447 ± 1099 for Group 2. Immunostaining showed that the density of nerve structures stained with SK2 antibody was also higher in Group 1 (11,546 ± 7,271 μm2/mm2) than in Group 2 (5,321 ± 3,164 μm2/mm2, P < 0.05, high-power view in Figures 2C–2D and low-power view in Figures 2E–2F).
Figure 2.
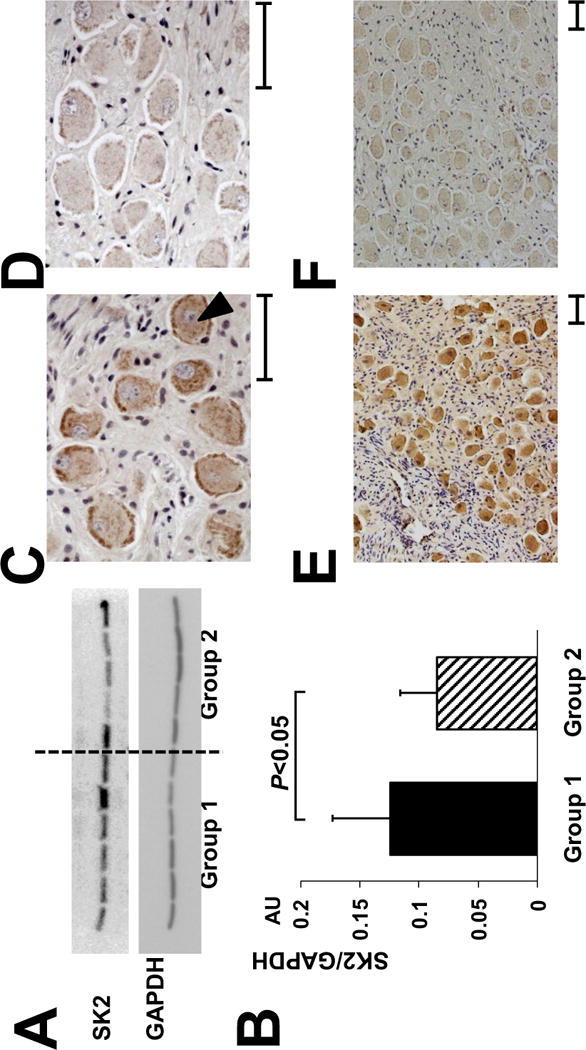
Upregulation of SK2 protein in the LSG with LL-VNS. A, Representative western blots show that the signal ratio of SK2 protein to GAPDH of Group 1 was significantly higher than that of Group 2. B, There is an upregulation of SK2 protein level in the LSG in Group 1 dogs after being normalized to GAPDH. C-D, Representative immunostaining of SK2 protein in the LSG The density of SK2-positive nerve structures (as pointed by a black arrowhead) is significantly higher in Group 1 dogs (Panel C), compared to Group 2 dogs (Panel D). E-F, Representative low-power view of immunostaining of SK2 protein in the LSG, that clearly demonstrates higher SK2 density in Group 1 dogs (Panel E), compared to Group 2 dogs (Panel F). SK2, Small conductance calcium-activated potassium channels subtype 2; GAPDH, glyceraldehydes-3-phosphate-dehydrogenase; AU, arbitrary units. Calibration bar = 50 μm.
Neurotransmitter Phenotypes in the LSG
We compared the results of TH immunostaining of the LSG between Group 1 and Group 2 dogs. There were significantly more ganglion cells without immunoreactivity to TH in dogs with LL-VNS (11.4 ± 2.3%) than in control (4.9 ± 0.7%; P < 0.05). These results were consistent with a previously-published study5 but the absolute percentages for both groups were higher because the cells on the edges of the picture were included in the present study but not in that previous study.5 We also compared the TH and ChAT serial staining slides in Group 1 and Group 2 dogs. There were high percentages of TH-negative cells that were also ChAT-positive in both Group 1 dogs (95.9 ± 2.8%) and Group 2 dogs (86.1 ± 4.4%, P = 0.10). Figure 3A shows a representative TH-staining of the LSG from a Group 1 dog. Dashed arrows point to three TH-negative ganglion cells. Figure 3B shows a serial slide from the same LSG used for ChAT immunostaining. The same three ganglion cells that are TH-negative are positive for ChAT (arrows).
Figure 3.
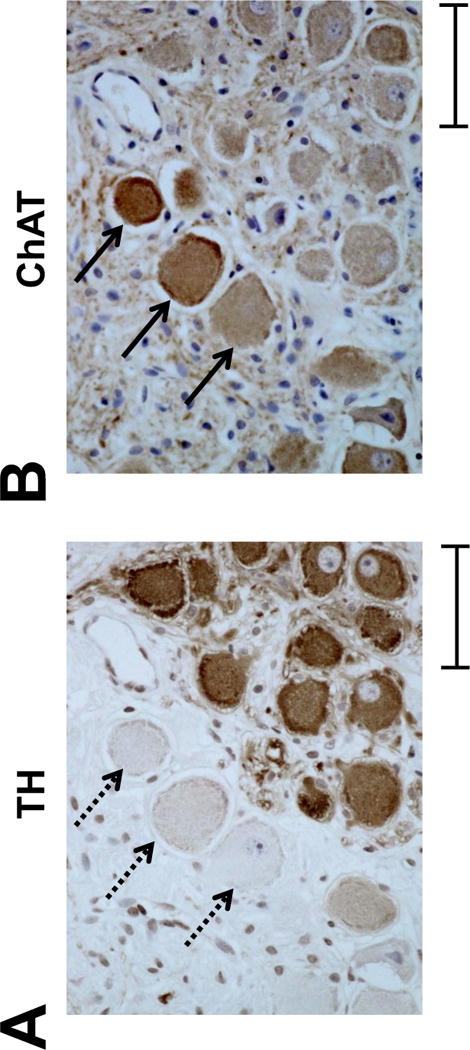
TH-negative cells are ChAT-positive in the LSG A, Representative TH-staining of the LSG from a Group 1 dog. Dashed arrows point to three TH-negative ganglion cells. B, A serial slide from the same LSG is used for ChAT immunostaining. The same three ganglion cells that are TH-negative are positive for ChAT (arrows). This development of cholinergic phenotype is observed in a vast majority of TH-negative cells, whose number is increased by LL-VNS. TH, tyrosine hydroxylase; ChAT, choline acetyltransferase. Calibration bar = 50 μm.
Immunofluorescence Confocal Microscopic Examination
To directly assess the subcellular distribution of SK2 protein after left LL-VNS in ambulatory dogs, immunofluorescence confocal microscopy was performed using anti-SK2 antibody and LSG tissue sections from the two groups of animals. Figure 4 shows typical immunofluorescence confocal images from the two groups of animals. LL-VNS resulted in the alteration in the distribution of SK2 protein. Specifically, control ganglion cells from the LSG of all Group 2 dogs showed homogenous SK2 staining in the cytosol. In contrast, LL-VNS (Group 1) resulted in a significant decrease in cytosolic SK2 staining but a significant increase in the staining in the periphery of the ganglion cells. These findings were observed in four Group 1 dogs. In the remaining 2 dogs, the stain quality was not good enough to clearly demonstrate this phenomenon. The ratio of membrane/cytosol SK2 fluorescence was obtained from a total of 33 cells from 4 Group 1 dogs and 3 Group 2 dogs (3–7 cells per dog). The fluorescence ratio of Group 1 and Group 2 dogs averaged 2.3 ± 1.6 and 0.85 ± 0.06, respectively (P = 0.057).
Figure 4.
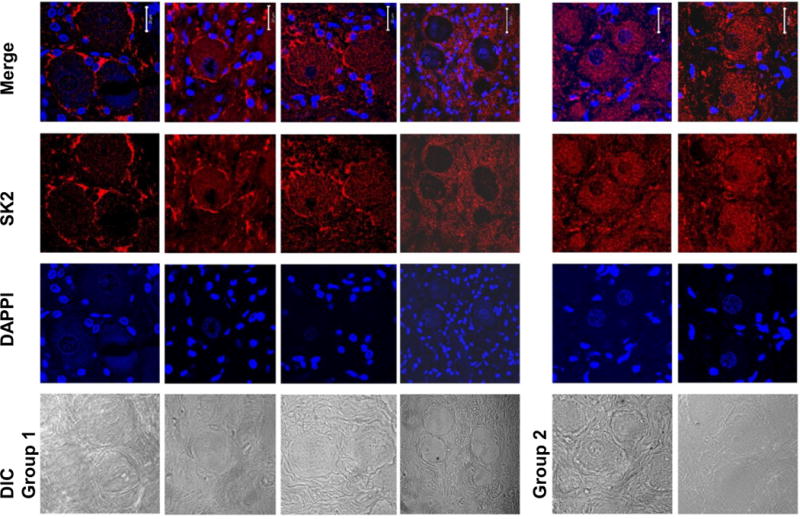
LL-VNS changes the subcellular distribution of SK2 protein. Immunofluorescence confocal microscope images of four Group 1 (LL-VNS) LSG and two Group 2 (control) LSG using anti-SK2 antibody and DAPI (4′,6-diamidino-2-phenylindole to identify nuclei). Corresponding differential interference contrast (DIC) images are shown on the left. The merged picture shows that in Group 1 LSG, there was a significant increase in SK2 staining in the periphery of ganglion cells but decrease in the cytosol. In contrast, in Group 2 LSG, the SK2 staining was homogeneous. DAPI, 4′, 6-diamidino-2-phenylindole. The white calibration bars are 20 μM in length.
Discussion
Mechanisms of SK2 Upregulation
The SK channels are constitutively recycled from the postsynaptic membrane. Activation of β receptors removes SK channels from the excitatory synapses.13 Because vagal stimulation may exert significant effects on cardiac sympathetic fiber discharges,5,14,15 it may indirectly impede the removal and recycling of the SK channels, leading to increased protein expression. The same mechanisms might also contribute to the upregulation of SK current densities in the failing ventricles16 because heart failure is known to significantly downregulate β-receptors.17,18
SK protein and Effects of VNS
Shen et al5 observed two phases of LSG nerve activity reduction during continuous LL-VNS in ambulatory dogs. The first phase occurred immediately and persisted for 3 days, consistent with a direct suppression of LSG nerve activity by VNS. A second phase occurred 4 days after the onset of LL-VNS, when LSG nerve activity demonstrated further reduction. The delayed onset of VNS effects is consistent with the time needed to result in induction of SK protein upregulation and phenotypic switching within the LSG. Both of these remodeling changes may lead to further reduction of the sympathetic tone. Furthermore, we have shown that there is increased expression of SK proteins on the membrane of ganglion cells. The increased protein trafficking to the cell membrane induced by VNS could also contribute to the reduced sympathetic tone. In the central nervous system, SK channels are known to be involved in long term potentiation and postsynaptic regulation.13,20 We propose that LL-VNS directly affects the activation patterns of the LSG. These physiological changes then induce secondary LSG remodeling and reduce the overall sympathetic tone. The upregulation of the SK2 proteins, the trafficking of the SK2 protein to the cell membrane and the phenotypic switching within the LSG also explains the carry-over effects after the termination of the VNS5.
Development of Cholinergic Phenotypes in the LSG
In the present study, we show that the vast majority of the TH-negative ganglion cells stained positively for ChAT. LL-VNS increases the percentage of these cholinergic cells, suggesting that some cells have undergone phenotypic switching and converted from using norepinephrine as their neurotransmitter to acetylcholine. Previous studies showed that a switch from a fully functional noradrenergic phenotype to a cholinergic phenotype in the stellate ganglion can occur due to the presence of target-derived cytokines such as cholinergic differentiation factor (CDF) (also known as leukemia inhibiting factor, LIF), interleukin-6 and ciliary neutrophic factor.21–23 CDF/LIF expression is dynamically adjusted and may increase dramatically in the sympathetic ganglia in response to injury24 and myocardial infarction.25 It is possible that LL-VNS can similarly increase the expression of cytokines that induce the phenotypic switching from noradrenergic to cholinergic phenotypes in the LSG through its effects on the myocardium. Because the percentage of TH-positive ganglion cells in the LSG is reduced by VNS, smaller amount of catecholamines are produced and released during LSG activation. Therefore, the phenotypical switching may be one of the mechanisms by which VNS reduces sympathetic tone.
LL-VNS induces SK2 redistribution to cell membrane
LL-VNS induces both an increased total SK2 protein levels and the redistribution of the SK2 proteins to the cell membrane. In the COS7 cell expression system, direct Phosphorylation by cAMP-dependent protein kinase causes a dramatic decrease in surface localization of the SK2 channels,26 suggesting that sympathetic nerve activity plays a role in SK2 protein distribution. Intermittent burst pacing in rabbit atria is known to cause SK2 channel trafficking to the cell membrane, leading to increased apamin-sensitive K current (Ikas) and accelerates the repolarization of the pulmonary veins.19 In addition, Lu et al27 showed that the SK2 channel localization on the cell-surface membrane is dynamically regulated. One of the critical steps includes the process of cytoskeletal anchoring of SK2 channel by its interacting protein, alpha-actinin2, as well as endocytic recycling via early endosome back to the cell membrane. The mechanisms by which LL-VNS induces trafficking of the SK2 to cell membrane remain unclear. However, it is possible that the LL-VNS has activated the sensory component within the vagus nerve, leading to altered central inputs to the left stellate ganglion. These neural signals then increase the protein expression and cause SK2 protein to traffic to the cell membrane. The increased cell membrane localization of the SK2 may play an important role of increased Ikas and reduced nerve discharges in the LSG.
Conclusion
Left LL-VNS results in the upregulation of SK2 proteins, increased SK2 protein expression in the cell membrane and the increased TH-negative (mostly ChAT-positive) ganglion cells in the LSG. These changes may underlie the antiarrhythmic efficacy of LL-VNS in ambulatory dogs.
Limitations of the Study
Because this study focuses on the neural remodeling of the LSG,5,28,29 whether or not the same remodeling occurs in the right stellate ganglion was not investigated. We did not isolate the ganglion cells from the stellate ganglion to test for afterhyperpolarization. Therefore, it is unclear if the changes of expression and intracellular distribution of SK2 protein causes increased afterhyperpolarization in the ganglion cells. We have used blinded observers in this study. This approach minimizes but may not completely eliminate the observer bias. We did not have data on heart rhythm in the present study. However, in a previous study we have documented that similar methods of VNS may suppress atrial arrhythmias in ambulatory dogs.5
Acknowledgments
We thank Lei Lin, Jian Tan and Nicole Courtney for their assistance; and Jason Begnaud of the Cyberonics Inc for technical assistance in programming the vagal stimulator. We also thank Amy Chang for her assistance in the preparation of this manuscript.
Funding Sources
This study was supported in part by NIH Grants P01HL78931, R01HL78932, R01HL71140, R21HL106554, a Heart Rhythm Society Fellowship in Cardiac Pacing and Electrophysiology (M.J.S.), R01HL85844, R01HL85727, VA Merit Review Grant I01BX000576 (N.C.), a Medtronic-Zipes Endowment (P.-S.C.) and an Indiana University Health-Indiana University School of Medicine Strategic Research Initiative.
Abbreviations and Acronyms
- AF
atrial fibrillation
- AU
arbitrary unit
- CDF
cholinergic differentiation factor
- ChAT
choline acetyltransferase
- GAPDH
glyceraldehydes-3-phosphate-dehydrogenase
- LIF
leukemia inhibiting factor
- LL-VNS
low-level vagus nerve stimulation
- LSG
left stellate ganglion
- sAHP
slow afterhyperpolarization
- SK
small conductance calcium activated K+
- TH
tyrosine hydroxylase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
Cyberonics Inc donated the neurostimulator used in the present study. Dr Peng-Sheng Chen is a consultant to Cyberonics Inc.
THIS PAPER WAS PROCESSED BY A GUEST EDITOR
References
- 1.Adelman JP, Maylie J, Sah P. Small-Conductance Ca(2+)-Activated K(+) Channels: Form and Function. Annu Rev Physiol. 2011 doi: 10.1146/annurev-physiol-020911-153336. [DOI] [PubMed] [Google Scholar]
- 2.Nagahara D, Nakata T, Hashimoto A, et al. Predicting the need for an implantable cardioverter defibrillator using cardiac metaiodobenzylguanidine activity together with plasma natriuretic peptide concentration or left ventricular function. J Nucl Med. 2008;49(2):225–233. doi: 10.2967/jnumed.107.042564. [DOI] [PubMed] [Google Scholar]
- 3.del Negro CA, Hsiao CF, Chandler SH. Outward currents influencing bursting dynamics in guinea pig trigeminal motoneurons. J Neurophysiol. 1999;81(4):1478–1485. doi: 10.1152/jn.1999.81.4.1478. [DOI] [PubMed] [Google Scholar]
- 4.Ogawa M, Zhou S, Tan AY, et al. Left stellate ganglion and vagal nerve activity and cardiac arrhythmias in ambulatory dogs with pacing-induced congestive heart failure. Journal of the American College of Cardiology. 2007;50:335–343. doi: 10.1016/j.jacc.2007.03.045. [DOI] [PubMed] [Google Scholar]
- 5.Shen MJ, Shinohara T, Park HW, et al. Continuous low-level vagus nerve stimulation reduces stellate ganglion nerve activity and paroxysmal atrial tachyarrhythmias in ambulatory canines. Circulation. 2011;123(20):2204–2212. doi: 10.1161/CIRCULATIONAHA.111.018028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li S, Scherlag BJ, Yu L, et al. Low-level vagosympathetic stimulation: a paradox and potential new modality for the treatment of focal atrial fibrillation. Circ Arrhythm Electrophysiol. 2009;2(6):645–651. doi: 10.1161/CIRCEP.109.868331. [DOI] [PubMed] [Google Scholar]
- 7.Tuteja D, Rafizadeh S, Timofeyev V, et al. Cardiac small conductance Ca2+-activated K+ channel subunits form heteromultimers via the coiled-coil domains in the C termini of the channels. Circ Res. 2010;107(7):851–859. doi: 10.1161/CIRCRESAHA.109.215269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ishii TM, Maylie J, Adelman JP. Determinants of apamin and d-tubocurarine block in SK potassium channels. J Biol Chem. 1997;272(37):23195–23200. doi: 10.1074/jbc.272.37.23195. [DOI] [PubMed] [Google Scholar]
- 9.Tuteja D, Xu D, Timofeyev V, et al. Differential expression of small-conductance Ca2+-activated K+ channels SK1, SK2, and SK3 in mouse atrial and ventricular myocytes. Am J Physiol Heart Circ Physiol. 2005;289(6):H2714–2723. doi: 10.1152/ajpheart.00534.2005. [DOI] [PubMed] [Google Scholar]
- 10.Vit JP, Jasmin L, Bhargava A, Ohara PT. Satellite glial cells in the trigeminal ganglion as a determinant of orofacial neuropathic pain. Neuron Glia Biol. 2006;2(4):247–257. doi: 10.1017/s1740925x07000427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maingret F, Coste B, Hao J, et al. Neurotransmitter modulation of small-conductance Ca2+-activated K+ channels by regulation of Ca2+ gating. Neuron. 2008;59(3):439–449. doi: 10.1016/j.neuron.2008.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cao JM, Chen LS, KenKnight BH, et al. Nerve sprouting and sudden cardiac death. Circulation Research. 2000;86:816–821. doi: 10.1161/01.res.86.7.816. [DOI] [PubMed] [Google Scholar]
- 13.Faber ES, Delaney AJ, Power JM, Sedlak PL, Crane JW, Sah P. Modulation of SK channel trafficking by beta adrenoceptors enhances excitatory synaptic transmission and plasticity in the amygdala. J Neurosci. 2008;28(43):10803–10813. doi: 10.1523/JNEUROSCI.1796-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schwartz PJ, Pagani M, Lombardi F, Malliani A, Brown AM. A cardiocardiac sympathovagal reflex in the cat. Circ Res. 1973;32(2):215–220. doi: 10.1161/01.res.32.2.215. [DOI] [PubMed] [Google Scholar]
- 15.Kyoung-suk Rhee, Chia-Hsiang Hsueh, Hyung Wook Park, et al. Effects of suprathreshold vagal stimulation on stellate ganglion nerve activity in ambulatory dogs. Heart Rhythm. 2012;9(suppl):S406. [Google Scholar]
- 16.Chua SK, Chang PC, Maruyama M, et al. Small-Conductance Calcium-Activated Potassium Channel and Recurrent Ventricular Fibrillation in Failing Rabbit Ventricles. Circ Res. 2011;108:971–979. doi: 10.1161/CIRCRESAHA.110.238386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bristow MR, Ginsburg R, Minobe W, et al. Decreased catecholamine sensitivity and beta-adrenergic-receptor density in failing human hearts. N Engl J Med. 1982;307(4):205–211. doi: 10.1056/NEJM198207223070401. [DOI] [PubMed] [Google Scholar]
- 18.Liang CS, Fan TH, Sullebarger JT, Sakamoto S. Decreased adrenergic neuronal uptake activity in experimental right heart failure. A chamber-specific contributor to beta-adrenoceptor downregulation. J Clin Invest. 1989;84(4):1267–1275. doi: 10.1172/JCI114294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ozgen N, Dun W, Sosunov EA, et al. Early electrical remodeling in rabbit pulmonary vein results from trafficking of intracellular SK2 channels to membrane sites. Cardiovasc Res. 2007;75(4):758–769. doi: 10.1016/j.cardiores.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Behnisch T, Reymann KG. Inhibition of apamin-sensitive calcium dependent potassium channels facilitate the induction of long-term potentiation in the CA1 region of rat hippocampus in vitro. Neurosci Lett. 1998;253(2):91–94. doi: 10.1016/s0304-3940(98)00612-0. [DOI] [PubMed] [Google Scholar]
- 21.Rao MS, Sun Y, Escary JL, et al. Leukemia inhibitory factor mediates an injury response but not a target-directed developmental transmitter switch in sympathetic neurons. Neuron. 1993;11(6):1175–1185. doi: 10.1016/0896-6273(93)90229-k. [DOI] [PubMed] [Google Scholar]
- 22.Habecker BA, Landis SC. Noradrenergic regulation of cholinergic differentiation. Science. 1994;264(5165):1602–1604. doi: 10.1126/science.8202714. [DOI] [PubMed] [Google Scholar]
- 23.Oh YJ, O’Malley KL. IL-6 increases choline acetyltransferase but not neuropeptide transcripts in sympathetic neurons. Neuroreport. 1994;5(8):937–940. doi: 10.1097/00001756-199404000-00021. [DOI] [PubMed] [Google Scholar]
- 24.Banner LR, Patterson PH. Major changes in the expression of the mRNAs for cholinergic differentiation factor/leukemia inhibitory factor and its receptor after injury to adult peripheral nerves and ganglia. Proc Natl Acad Sci USA. 1994;91(15):7109–7113. doi: 10.1073/pnas.91.15.7109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oh YS, Jong A, Kim D, et al. Relationship between nerve sprouting and neurotrophic gene expression in a mouse model of myocardial infarction. Heart Rhythm. 2004;1:S191. [Google Scholar]
- 26.Ren Y, Barnwell LF, Alexander JC, et al. Regulation of surface localization of the small conductance Ca2+-activated potassium channel, Sk2, through direct phosphorylation by cAMP-dependent protein kinase. J Biol Chem. 2006;281(17):11769–11779. doi: 10.1074/jbc.M513125200. [DOI] [PubMed] [Google Scholar]
- 27.Lu L, Timofeyev V, Li N, et al. Alpha-actinin2 cytoskeletal protein is required for the functional membrane localization of a Ca2+-activated K+ channel (SK2 channel) Proc Natl Acad Sci USA. 2009;106(43):18402–18407. doi: 10.1073/pnas.0908207106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tan AY, Zhou S, Ogawa M, et al. Neural mechanisms of paroxysmal atrial fibrillation and paroxysmal atrial tachycardia in ambulatory canines. Circulation. 2008;118:916–925. doi: 10.1161/CIRCULATIONAHA.108.776203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou S, Jung BC, Tan AY, et al. Spontaneous stellate ganglion nerve activity and ventricular arrhythmia in a canine model of sudden death. Heart Rhythm. 2008;5(1):131–139. doi: 10.1016/j.hrthm.2007.09.007. [DOI] [PubMed] [Google Scholar]


