Abstract
Background
A challenge in prostate cancer (PCa) management is identifying potentially lethal disease at diagnosis. Inflammation, focal prostatic atrophy and prostatic intraepithelial neoplasia (PIN) are common in prostate tumor specimens, but it is not clear whether these lesions have prognostic significance.
Methods
We conducted a case-control study nested in a cohort of men diagnosed with stage T1a-b PCa through transurethral resection of the prostate in Sweden. Cases are men who died of PCa (n=228). Controls are men who survived more than 10 years after PCa diagnosis without metastases (n=387). Slides were assessed for Gleason grade, inflammation, PIN, and four subtypes of focal prostatic atrophy: simple atrophy (SA), post-atrophic hyperplasia (PAH), simple atrophy with cyst formation, and partial atrophy. We estimated odds ratios (ORs) and 95% confidence intervals (CIs) for odds of lethal PCa with multivariable logistic regression.
Results
Chronic inflammation and PIN were more frequently observed in tumors with PAH, but not SA. No specific type of atrophy or inflammation was significantly associated with lethal PCa overall, but there was a suggestion of a positive association for chronic inflammation. Independent of age, Gleason score, year of diagnosis, inflammation, and atrophy type, men with PIN were 89% more likely to die of PCa (95% CI:1.04-3.42).
Conclusions
Our data demonstrate that PIN, and perhaps presence of moderate or severe chronic inflammation, may have prognostic significance for PCa.
Impact
Lesions in tumor adjacent tissue, and not just the tumor itself, may aid in identification of clinically relevant disease.
Keywords: Prostate adenocarcinoma, Chronic inflammation, Post-atrophic hyperplasia, Lethal prostate cancer
Introduction
Accumulating evidence highlights the role of chronic or recurrent inflammation in human carcinogenesis [1-3]. Repeated tissue damage and regeneration in a microenvironment containing highly reactive oxygen and nitrogen species is thought to contribute to cancer development and progression. Chronic inflammation has also been hypothesized to influence prostate carcinogenesis, specifically. Both chronic and acute inflammation are commonly observed in prostate tumor specimens from prostatectomies, transurethral resections of the prostate (TURP), and biopsy samples [4-5]. Reactive molecules released by inflammatory cells, capable of interacting with DNA in the proliferating epithelium, may cause permanent genomic alterations such as rearrangements, deletions, and point mutations. In the prostate gland, chronic inflammation is associated with different types of focal glandular atrophy, especially post-atrophic hyperplasia (PAH) and simple atrophy (SA) [6-7]. These lesions, characterized by a highly proliferative epithelium are found most often in the peripheral zone of the gland, where prostate cancer most commonly develops [8-9]. The term proliferative inflammatory atrophy (PIA) has been proposed to designate proliferative glandular epithelium with morphologic appearance of PAH or SA in the presence of inflammation [6]. These lesions have been suggested as precursors of prostatic adenocarcinoma, either directly or indirectly via progression to intraepithelial neoplasia (PIN) [6, 10-12].
In support of the hypothesis, morphological studies have reported the transition from PIA to PIN and from PIA to cancer [12-14]. Proliferation in PAH lesions appears significantly greater than in benign prostatic epithelium and SA, but less than in PIN and prostate cancer [15]. Moreover, the association between chronic inflammation and PAH appears to be stronger than that with SA. Chromosome 8 gain has further been observed more frequently in PAH than in SA and PIN [8, 15-16]. Chromosome 8 gain is considered to be a marker for poor prognosis in prostate adenocarcinoma [17].
No study to date has to our knowledge examined the role of proliferative atrophy, inflammation, or PIN as predictors of lethal prostate cancer. In this population-based nested case-control study of men diagnosed with localized prostate cancer and followed up to 30 years after diagnosis, we evaluate chronic inflammation, type of focal atrophy, and PIN in adjacent tumor tissue as predictors of lethal prostate cancer.
Material and methods
The study is nested within a cohort of men with localized prostate cancer diagnosed in the Örebro and South East Health Care Regions of Sweden between 1977 and 1999 (earlier described in ref [18-20]). We initially identified a cohort of 1,367 men during the study period. Eligible patients were identified through population-based prostate cancer quality data-bases maintained in these regions. We included men who were diagnosed with incidental prostate cancer through TURP or adenoma enucleation, i.e. category T1a-b tumors. In accordance with standard treatment protocols, patients with early stage/localized prostate cancer were followed expectantly (“watchful waiting”).
The study cohort was followed for cancer-specific and all-cause mortality until March 1, 2006, through record linkages to the Swedish Death Register and Migration Register. Information on cause of death for each individual was obtained through a complete review of medical records by a study end-point committee. Deaths were classified as cancer specific when prostate cancer was the primary cause of death.
Because the tissue evaluation requires considerable time and effort, we used a novel nested study design that included men who either died from prostate cancer during follow up (lethal prostate cancer “cases”, n =228) or who survived at least 10 years following their diagnosis (indolent prostate cancer “controls”, n =387). The study design excluded 752 men with non-informative outcomes, namely those who died from other causes within 10 years after their cancer diagnosis (N=595). The remaining 157 men were excluded because the cases did not die of prostate cancer and did not have the opportunity to survive 10 years before the end of the study follow-up in 2006. The study was approved by the Ethical Review Boards in Örebro and Linköping, Sweden.
Tissue collection and evaluation
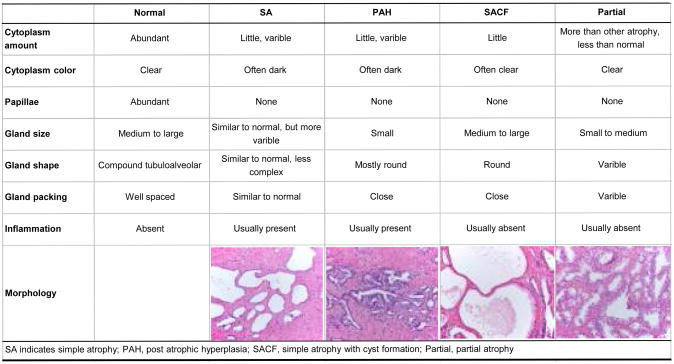
Tumor tissue specimens from TURP were available from 92% (1,256) of the men with localized prostate cancer in the original cohort. All TURP specimens were assessed to identify tumor areas and confirm cancer diagnosis. For the 615 patients selected for the present case-control study, H&E slides for the corresponding paraffin-embedded formalin-fixed blocks were re-reviewed to confirm cancer status, Gleason score and other notable histopathological features by a single pathologist (M.F.) blinded to disease outcome and other clinical data. All slides were assessed for the presence and type of inflammation, either acute or chronic, according to cells of the inflammatory infiltrate. Chronic inflammation was semi-quantitatively graded as mild, moderate or severe when the area of non-neoplastic prostate tissue covered by inflammatory cells was ≤10%, ≥10-20%, and ≥20%, respectively. Focal prostate atrophy was characterized according to the atrophy classification, proposed in 2006 by the Working Group for Histologic Classification of Prostate Atrophy Lesions [21] with the following subtypes: SA, simple atrophy with cyst formation (SACF), PAH, and partial atrophy. Figure 1 describes the major characteristics of the four types of lesions.
Figure 1. Key Histological Criteria of Focal Prostate Atrophy set up by Working Group Classification of Focal Prostate Atrophy Lesions.
Statistical analyses
Chi-square tests were used to evaluate associations between the types of focal atrophy, presence of PIN, chronic inflammation, and tumor characteristics. T-tests were used to compare age of diagnosis according to inflammation, atrophy, and PIN status. We used unconditional logistic regression to estimate odds ratios (ORs) and 95% confidence intervals (CIs) for lethal prostate cancer according to type of focal atrophy, degree of chronic inflammation, and PIN. Statistical significance was determined by a Wald test for the dichotomous focal atrophy variables. For chronic inflammation analyses, we included a three-level ordinal variable for the categories of none, mild, and moderate/severe. We also ran multivariate models adjusted for age at diagnosis (continuous), calendar year of diagnosis (1977-1982, 1983-1988, 1989-1993, and 1994-1998), Gleason score at diagnosis in four categories (4-6, 7:3+4, 7:4+3, and 8-10), presence of SA, presence of PAH, presence of acute inflammation, and chronic inflammation (none vs. mild vs. moderate/severe). A final model additionally controlled for tumor stage (T1a vs. T1b) and percent of tumor in specimen (continuous). We hypothesized that chronic inflammation and focal atrophy or PIN may act synergistically to influence prostate cancer progression. Thus, we specifically assessed the potential interactions between chronic inflammation and SA, PAH, and PIN on lethal prostate cancer. To assess statistical significance of the interaction, we used a 2 d.f. likelihood ratio test to compare unconditional logistic regression models that included age at diagnosis, calendar year of diagnosis, Gleason score category, acute inflammation, SA, PAH, PIN and the product term of degree of chronic inflammation (none/mild vs. moderate/severe) with type of focal atrophy or PIN, to models without the product term. All statistical analyses were carried out using SAS Statistical Software version 9.2 (Cary, NC).
Results
We evaluated prostate specimens from 615 men who were diagnosed with incidental prostate cancer through TURP or adenoma enucleation. In Table 1 we present the overall frequency of acute and chronic inflammation, the four atrophy types, and PIN, as well as the associations between these features. We found some evidence of chronic inflammation in three-quarters of the TURP specimens, with moderate or severe chronic inflammation apparent in 26%. Acute inflammation was apparent somewhat less frequently (13%), but acute and chronic inflammation were positively associated (p<0.0001). The morphologic classification showed that SA was the most common type of focal atrophy in this material, identified in 365 of 615 men (59%), followed by PAH (20%), SACF (6.2%), and partial atrophy (1.8%). PIN lesions were identified in 13% of the cases overall. Men with moderate or severe chronic inflammation were more likely to have PAH lesions (30.4% vs 16.9%; p=0.003). Frequencies of other types of atrophy and PIN were similar regardless of severity of chronic inflammation. PAH was less commonly observed among men with SA lesions (15.9% vs. 26.8%; p=0.001). The frequency of PIN did not vary according to the presence of SA (13.2% in SA positive vs. 12.8% in SA negative; p=0.90), but PIN was more frequently observed in tumor specimens with evidence of PAH (22.4% vs. 10.6%; p<0.001), PIN was not related to chronic or acute inflammation.
Table 1. Association between inflammation and histological features in TURP specimens from the Swedish Watchful Waiting Cohort (N=615).
| Overall Frequency, N (%) | Frequency of Patients Positive for Feature According to Types of Inflammation, Atrophy, and PIN, N (%) | ||||||
|---|---|---|---|---|---|---|---|
| Moderate/Severe Chronic Inflammation | Simple Atrophy | PAH | SACF | Partial Atrophy* | PIN | ||
| Acute Inflammation | |||||||
| Yes | 84 (13.7) | 54 (64.3) | 319 (60.1) | 20 (23.8) | 6 (7.1) | 2 (2.4) | 13 (15.5) |
| No | 531 (86.3) | 104 (19.59) | 46 (54.8) | 105 (19.8) | 32 (6.0) | 9 (1.7) | 67 (12.6) |
| p<0.0001 | p=0.36 | p=0.39 | p=0.69 | p=0.65 | p=0.47 | ||
| Chronic Inflammation | |||||||
| Moderate/Severe | 158 (25.7) | 92 (58.2) | 48 (30.4) | 13 (8.2) | 2 (1.3) | 21 (3.4) | |
| None/Mild | 457 (74.3) | 273 (59.7) | 77 (16.9) | 25 (5.5) | 9 (2.0) | 59 (12.9) | |
| p=0.74 | p=0.003 | p=0.21 | p=0.74 | p=0.90 | |||
| Simple Atrophy | |||||||
| Yes | 365 (59.3) | 58 (15.9) | 18 (4.9) | 7 (1.9) | 48 (13.2) | ||
| No | 250 (40.7) | 67 (26.8) | 20 (8.0) | 4 (1.6) | 32 (12.8) | ||
| p=0.001 | p=0.12 | p=0.99 | p=0.90 | ||||
| PAH | |||||||
| Yes | 125 (20.3) | 8 (6.4) | 2 (1.6) | 28 (22.4) | |||
| No | 490 (79.7) | 30 (6.2) | 9 (1.8) | 52 (10.6) | |||
| p=0.91 | p=0.69 | p=0.001 | |||||
| SACF | |||||||
| Yes | 38 (6.2) | 3 (7.9) | 5 (13.2) | ||||
| No | 577 (93.8) | 8 (1.4) | 75 (13.0) | ||||
| p=0.03 | p=0.98 | ||||||
| Partial Atrophy | |||||||
| Yes | 11 (1.8) | 0 (0.0) | |||||
| No | 604 (98.2) | 80 (13.3) | |||||
| p=0.37 | |||||||
| PIN | |||||||
| Yes | 80 (13.0) | ||||||
| No | 535 (87.0) | ||||||
Due to small cell counts, p-values were estimated from a Fisher Exact Test. All other p-values from a Chi-square test.
Clinical characteristics overall, according to indolent or lethal status, and with respect to the various morphologic features are shown in Table 2. On average men were 73 years at diagnosis. Approximately half of the tumors evaluated were Gleason score 2-6 (47%). Men diagnosed with T1a tumors comprised 41% of the study population, with the remainder being T1b. Compared to indolent controls, lethal cases were significantly older at diagnosis (74.9 years vs. 71.9 years; p<0.0001); less likely to be diagnosed between 1992-1999 (40.3% vs. 52.7%; p=0.01); and more likely to have T1b tumors (75.2% vs. 49.2%; p<0.0001), tumors with a Gleaon score of 4+3, 8, 9, or 10 at diagnosis (63.1% vs. 15.9%; ptrend<0.0001), and a larger tumor volume (ptrend<0.0001). Acute inflammation was inversely associated with Gleason score (p=0.009). We found no association between Gleason score and mild/severe chronic inflammation (ptrend=0.80), presence of SA (ptrend=0.15) or PAH (ptrend=0.85). SA was inversely associated with the percent of tumor in the TURP specimen (p=0.01). PIN lesions were more commonly observed in specimens with T1b compared to T1a tumors (17% vs. 7%; p=0.0004), tumors with higher Gleason score (20% among Gleason 8-10 tumors vs 6% among Gleason 2-6 tumors; ptrend<0.0001), and in specimens with a higher percentage of tumor (29% in the upper quartile of tumor percent vs. 10% in the lowest quartile; ptrend=0.0004).
Table 2. Association of inflammation and histological features with clinical characteristics in the Swedish Watchful Waiting Cohort (N=615).
| Overall, N (%) | Frequency of Clinical Characteristics According to Case/Control Status, N (%) | Frequency of Patients Positive for Feature According to Clinical Tumor Characteristics, N (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Indolent PCa Controls N=228 | Lethal PCa Cases N=387 | Acute Inflamm. | Moderate/Severe Chronic Inflamm. | Simple Atrophy | PAH | SACF | Partial Atrophy | PIN | ||
| Age at Dx, Mean (Std)* | 73.0 (6.6) | 71.9 (6.3) | 74.9 (6.7) | 73.1 (6.4) | 72.8 (6.6) | 73.3 (6.5) | 71.9 (6.9) | 728 (6.8) | 72.1 (6.2) | 72.6 (6.7) |
| p<0.0001 | p=0.96 | p=0.66 | p=0.27 | p=0.03 | p=0.87 | p=0.63 | p=0.55 | |||
| Calendar Year of Dx** | ||||||||||
| 1977-1982 | 22 (3.6) | 13 (3.4) | 9 (3.9) | 4 (18.2) | 8 (36.4) | 12 (54.6) | 3 (13.6) | 6 (27.3) | 2 (9.1) | 1 (4.6) |
| 1982-1986 | 39 (6.3) | 27 (7.0) | 12 (5.2) | 8 (20.5) | 13 (33.3) | 28 (71.8) | 6 (15.4) | 4 (10.3) | 3 (7.7) | 2 (5.1) |
| 1987-1991 | 258 (42.0) | 142 (36.9) | 116 (50.4) | 32 (12.4) | 65 (25.2) | 150 (58.1) | 63 (24.4) | 14 (5.4) | 2 (0.8) | 34 (13.2) |
| 1992-1999 | 296 (48.1) | 203 (52.7) | 93 (40.3) | 40 (13.5) | 72 (24.3) | 175 (59.1) | 53 (17.9) | 14 (4.7) | 4 (1.4) | 43 (14.5) |
| p=0.01 | p=0.52 | p=0.42 | p=0.41 | p=0.18 | p=0.0002 | p=0.001 | p=0.25 | |||
| Tumor Stage* | ||||||||||
| T1a | 252 (41.0) | 195 (50.8) | 57 (24.8) | 32 (12.7) | 57 (22.6) | 156 (61.9) | 49 (19.4) | 14 (5.6) | 6 (2.4) | 18 (7.4) |
| T1b | 362 (59.0) | 189 (49.2) | 173 (75.2) | 51 (14.1) | 101 (27.9) | 209 (57.7) | 75 (20.7) | 24 (6.6) | 5 (1.4) | 61 (16.9) |
| p<0.0001 | p=0.62 | p=0.14 | p=0.30 | p=0.70 | p=0.59 | p=0.37 § | p=0.0004 | |||
| Gleason Score§§ | ||||||||||
| 2-6 | 288 (46.8) | 240 (62.3) | 48 (20.8) | 48 (16.7) | 71 (24.7) | 179 (62.2) | 53 (18.4) | 22 (7.6) | 8 (2.8) | 18 (6.3) |
| 3+4 | 121 (19.7) | 84 (21.8) | 37 (16.1) | 17 (14.1) | 31 (25.6) | 71 (58.7) | 31 (25.6) | 2 (1.7) | 3 (2.5) | 20 (16.5) |
| 4+3 | 86 (14.0) | 41 (10.7) | 45 (19.6) | 11 (12.8) | 28 (32.6) | 49 (57.0) | 19 (22.1) | 8 (9.3) | 0 (0.0) | 19 (22.1) |
| 8-10 | 120 (19.5) | 20 (5.2) | 100 (43.5) | 8 (6.7) | 28 (23.2) | 66 (55.0) | 22 (18.3) | 6 (5.0) | 0 (0.0) | 23 (19.7) |
| p<0.0001 | p=0.009 | p=0.80 | p=0.15 | p=0.85 | p=0.54 | NS | p<0.0001 | |||
| Tumor percent§§ | ||||||||||
| Q1: <2 | 201 (35.7) | 164 (45.9) | 37 (18.0) | 29 (14.4) | 48 (23.9) | 132 (65.7) | 34 (16.9) | 12 (6.0) | 4 (2.0) | 19 (9.5) |
| Q2: >2 - <10 | 152 (27.0) | 98 (27.5) | 54 (26.2) | 21 (13.8) | 42 (27.6) | 93 (61.2) | 37 (24.3) | 14 (9.2) | 4 (2.6) | 16 (10.5) |
| Q3: >10 - <60 | 158 (28.1) | 79 (22.1) | 79 (38.4) | 17 (10.8) | 42 (26.6) | 87 (55.1) | 34 (21.5) | 9 (5.7) | 3 (1.9) | 26 (16.5) |
| Q4: >60 | 52 (9.2) | 16 (4.5) | 36 (17.5) | 6 (11.5) | 13 (25.0) | 26 (50.0) | 8 (15.4) | 2 (3.9) | 0 (0.0) | 15 (28.9) |
| -- | p<0.0001 | p=0.32 | p=0.68 | p=0.01 | p=0.69 | p=0.61 | NS | p=0.0004 | ||
P-value from a T-test
P-value from a Chi-square test
Due to small cell counts, p-values were estimated from a Fisher Exact Test.
P-value from a Chi-square test of trend
We investigated whether acute or chronic inflammation, SA, PAH, or PIN were associated with the odds of dying of prostate cancer (Table 3). In unadjusted analyses, we found no association between acute inflammation, chronic inflammation, SA, or PAH and odds of death from prostate cancer. However, PIN was associated with a more than 2-fold increase in the odds of lethal prostate cancer in crude analyses (OR: 2.16; 95% CI: 1.35-3.37). In a model including age at diagnosis, calendar year of diagnosis, Gleason score, acute inflammation, SA, and PAH, the association between PIN and lethal prostate cancer was attenuated slightly but remained statistically significant (OR: 1.89; 95% CI: 1.04-3.42). In the multivariate model there was a suggestion of a positive association for lethal prostate cancer comparing moderate/severe chronic inflammation to no chronic inflammation (OR: 1.61; 95% CI: 0.89-2.93).
Table 3. Odds ratios (ORs) and 95% confidence intervals (CIs) of lethal prostate cancer, Swedish Watchful Waiting Cohort, 1977-2006.
| Indolent, N (%) | Lethal, N (%) | OR1(95%CI) | OR2(95%CI) | OR3 (95% CI) | |
|---|---|---|---|---|---|
| Acute Inflammation | |||||
| No | 329 (85.5) | 202 (87.8) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Yes | 56 (14.5) | 28 (12.2) | 0.81 (0.50-1.32) | 0.96 (0.52-1.77) | 0.80 (0.41-1.57) |
| Chronic inflammation | |||||
| No | 97 (25.2) | 67 (29.1) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Mild | 198 (51.4) | 95 (41.3) | 0.70 (0.47-1.03) | 0.96 (0.58-1.60) | 0.94 (0.55-1.62) |
| Moderate/Severe | 90 (23.4) | 68 (29.6) | 1.10 (0.70-1.70) | 1.61 (0.89-2.93) | 1.73 (0.92-3.27) |
| SA | |||||
| No | 155 (40.3) | 95 (41.3) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Yes | 230 (59.7) | 135 (58.7) | 0.96 (0.69-1.34) | 1.07 (0.71-1.63) | 1.20 (0.76-1.87) |
| PAH | |||||
| No | 304 (79.0) | 186 (80.9) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Yes | 81 (21.0) | 44 (19.1) | 0.89 (0.59-1.34) | 0.82 (0.48-1.39) | 0.92 (0.53-1.61) |
| PIN | |||||
| No | 348 (90.4) | 187 (81.3) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Yes | 37 (9.6) | 43 (18.7) | 2.16 (1.35-3.47) | 1.89 (1.04-3.42) | 1.75 (0.94-3.28) |
OR unadjusted;
OR adjusted for age at diagnosis, calendar period of diagnosis (1977-1982, 1983-1988, 1989-1993, and 1994-1998),Gleason categories (4-6, 7:3+4, 7:4+3, and 8-10), acute inflammation, chronic inflammation (none vs. mild vs. moderate/severe), SA, PAH, and PIN;
OR additionally adjusted for tumor stage (T1a vs. T1b) and tumor percent (missing data for 52 patients)
When we explored whether the association between chronic inflammation and lethal prostate cancer was modified by presence of PAH, we found evidence of an interaction (p for interaction = 0.02; data not shown). Among men without PAH, we found no association comparing moderate/severe chronic inflammation to no/mild chronic inflammation with respect to lethal prostate cancer (OR: 1.27; 95% CI: 0.73-2.23) controlling for age at diagnosis, calendar year of diagnosis, Gleason score, acute inflammation, SA, and PIN. However, among men with PAH, presence of moderate/severe chronic inflammation was associated with a statistically significant nearly 5-fold increase in odds of dying of prostate cancer (OR: 4.88; 95% CI: 1.55-15.30). We found no other statistically significant interactions between the inflammation status and atrophy or PIN.
Discussion
In line with earlier studies, our investigation revealed that chronic inflammation and focal atrophy lesions are commonly found in tissue adjacent to prostate adenocarcinomas [22-23]. Our data further suggests PIN, and perhaps moderate/severe chronic inflammation especially in the presence of PAH, may be associated with an increased risk of prostate cancer-specific death. There is relatively little existing data on the associations between these features and cancer outcomes. One previous study of 65 patients did not find any difference in the incidence of newly identified prostate cancer at the time of re-biopsy according to the presence of focal atrophy [24]. Other studies have positively associated chronic inflammation and focal atrophy lesions with clinical covariates and biochemical recurrence [25-26]. This is to our knowledge the first large evaluation of chronic inflammation and proposed precancerous lesions (PAH and PIN) as determinants of prostate cancer-specific death.
Given the consistency of data on the common occurrence of prostatic inflammation, and the suggestion that chronic inflammation is associated with prostate cancer death, it seems important to consider the potential sources of such inflammation. Infections are well known to trigger inflammation and consequently inflammatory response. Two large case-control studies revealed that presence of antibodies against Trichomonas vaginalis was positively associated with the incidence of prostate cancer [27][28]. Furthermore, recent reports show that the bacterium Propionibacterium acnes was detected with high frequency in prostate tissue from men with prostate cancer but absent in other cancerous tissue biopsies [29][30]. Our study provides additional support for the continued investigation of infectious agents in the etiology of prostate cancer.
In addition to the observed or suggested associations between chronic inflammation and PIN and disease aggressiveness, some observations in our study are consistent with the hypothesis that the onset of chronic inflammation, initiated by infections or some other source, might influence normal prostate epithelia to transform into PAH, which in turn may give rise to prostate cancer both through or independently of PIN, as proposed by De Marzo et al [6]. First, as in previous studies [8, 15-16, 31], chronic inflammation was present in almost all specimens with PAH lesions and was more common and severe among men with PAH lesions than in men without evidence of PAH. Second, we found a correlation between the presence of PAH and PIN, potentially suggesting that PAH could be an early transformation in the prostate epithelium and that a subgroup of cells within the lesion has down-regulated tumor suppressor genes resulting in a more vulnerable state for genomic alterations. Nonetheless, our study is cross-sectional with respect to evaluation of the lesions and thus cannot determine temporality of lesion development,
We found that PAH lesions, but not SA lesions, were associated with PIN. This observation provide evidence that SA and PAH differ from each other not only morphologically, but also potentially as they relate to prostate cancer development and progression. Previous studies have also identified similarities between PAH and PIN and between PAH and prostate cancer. Besides the fact that some atrophic cell lesions express low levels of GSTP1, a common finding in both PIN and prostate cancer, lower expression of the cyclin-dependent kinase inhibitor p27Kip1 in the same lesions has also been reported [6]. p27Kip1 has been observed to be down-regulated at high frequencies in both PIN [32] and PCa [33]. Moreover, Shah et al. used FISH to evaluate chromosome 8 gain [15], which has been suggested as a common alteration in PIN and prostate cancer [34-35]. They found a higher amplification frequency of chromosome 8 in PAH lesions compared with SA lesions. Observations in prostatectomy specimens have also revealed a significant increase in nuclear proliferation gradually ranging from benign prostate (1.20%), SA (2.67%), PAH (3.62%), PIN (6.14%), to PCa (12%) [15].
All 615 prostate cancer patients evaluated in the present study were diagnosed by TURP. While utilizing TURP specimens could be viewed as a limitation given that prostate cancer originates most frequently in the peripheral zone, approximately 25% of prostate adenocarcinomas are thought to develop within the transition zone [36]. In this study, we found a high proportion of large tumors encompassing both zones, and approximately 20% of the men in the original cohort of T1a-b tumors died from their prostate cancer. Interestingly, the frequency of focal atrophy lesions and PIN in the present study is also very similar to that of a large U.S.-based radical prostatectomy cohort1.
Because the evaluation of atrophy and inflammation status requires a tremendous time commitment from the pathologist, we opted for a case-control design to increase efficiency. The sampling approach we utilized is less statistically powerful than evaluating the entire cohort. Nonetheless, there was likely a relatively modest loss in statistical power compared to the cohort analysis approach because the “true” lethal or indolent status of the excluded men could not be determined, most often due to competing causes of death. Given that a subset of the excluded men would have become lethal cases in the absence of competing causes, the excluded men had more poorly differentiated tumors and were more likely to be stage T1b than the men selected as indolent controls. As PIN was positively associated with both Gleason score and tumor stage, two relatively strong predictors of prostate cancer death, it is possible that the crude odds ratio for the association between PIN and lethal prostate cancer was somewhat overestimated. In light of the fact that adjusting for Gleason score and tumor stage only slightly attenuated the odds ratio estimates, it seems unlikely that this potential bias could account for the entire observed association. Strengths of the study include the long follow-up allowing for a sufficient number of prostate cancer deaths to occur and the fact that all men were managed without initial treatment, enabling a study of the natural disease course.
In summary, our data provide evidence that men with PIN adjacent to their tumor are nearly two times as likely to die of their prostate cancer. Moreover, we found data to support that chronic inflammation in the presence of PAH is associated with greater likelihood of prostate cancer death. Only studies that evaluate focal atrophy and inflammation in prostate tissue prior to cancer diagnosis can help to clarify whether these lesions predispose men to the development of prostate cancer. Nonetheless, if confirmed, our findings suggest that strategies to reduce prostatic inflammation may improve survival for men with prostate cancer.
Acknowledgments
Financial support: This study was supported by Department of Defense Prostate Cancer Idea Development Award PC060389, DF/HCC Prostate SPORE Career Development Award NIH/NCIP50 CA90381 (JRR), Prostate Cancer Foundation (LAM), and by Swedish Cancer Society, CAN 2006/1341 (KF)
Footnotes
Rider JR (Stark), Mucci LA, Fall K, Andrén O, Andersson S-O, Stampfer MJ, Loda M, Fiorentino M. The Epidemiological and Pathological Implications of Focal Atrophy Lesions: Data from cohorts from the U.S. and Sweden. Italian Society of Uro-Oncology Annual Meeting, June 25, 2010, Rome, Italy.
Disclosure of interest: No duality of interest to declare.
References
- 1.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–7. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–45. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 3.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–44. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 4.Nelson WG, De Marzo AM, Isaacs WB. Prostate cancer. N Engl J Med. 2003;349:366–81. doi: 10.1056/NEJMra021562. [DOI] [PubMed] [Google Scholar]
- 5.Blumenfeld W, Tucci S, Narayan P. Incidental lymphocytic prostatitis. Selective involvement with nonmalignant glands. Am J Surg Pathol. 1992;16:975–81. [PubMed] [Google Scholar]
- 6.De Marzo AM, Marchi VL, Epstein JI, Nelson WG. Proliferative inflammatory atrophy of the prostate: implications for prostatic carcinogenesis. Am J Pathol. 1999;155:1985–92. doi: 10.1016/S0002-9440(10)65517-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aus G, Abbou CC, Bolla M, Heidenreich A, Schmid HP, van Poppel H, et al. EAU guidelines on prostate cancer. Eur Urol. 2005;48:546–51. doi: 10.1016/j.eururo.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 8.Ruska KM, Sauvageot J, Epstein JI. Histology and cellular kinetics of prostatic atrophy. Am J Surg Pathol. 1998;22:1073–7. doi: 10.1097/00000478-199809000-00005. [DOI] [PubMed] [Google Scholar]
- 9.McNeal JE. Prostate cancer volume. Am J Surg Pathol. 1997;21:1392–3. doi: 10.1097/00000478-199711000-00018. [DOI] [PubMed] [Google Scholar]
- 10.Palapattu GS, Sutcliffe S, Bastian PJ, Platz EA, De Marzo AM, Isaacs WB, et al. Prostate carcinogenesis and inflammation: emerging insights. Carcinogenesis. 2005;26:1170–81. doi: 10.1093/carcin/bgh317. [DOI] [PubMed] [Google Scholar]
- 11.Platz EA, De Marzo AM. Epidemiology of inflammation and prostate cancer. J Urol. 2004;171:S36–40. doi: 10.1097/01.ju.0000108131.43160.77. [DOI] [PubMed] [Google Scholar]
- 12.Putzi MJ, De Marzo AM. Morphologic transitions between proliferative inflammatory atrophy and high-grade prostatic intraepithelial neoplasia. Urology. 2000;56:828–32. doi: 10.1016/s0090-4295(00)00776-7. [DOI] [PubMed] [Google Scholar]
- 13.Wang W, Bergh A, Damber JE. Morphological transition of proliferative inflammatory atrophy to high-grade intraepithelial neoplasia and cancer in human prostate. Prostate. 2009;69:1378–86. doi: 10.1002/pros.20992. [DOI] [PubMed] [Google Scholar]
- 14.De Marzo AM, Putzi MJ, Nelson WG. New concepts in the pathology of prostatic epithelial carcinogenesis. Urology. 2001;57:103–14. doi: 10.1016/s0090-4295(00)00952-3. [DOI] [PubMed] [Google Scholar]
- 15.Shah R, Mucci NR, Amin A, Macoska JA, Rubin MA. Postatrophic hyperplasia of the prostate gland: neoplastic precursor or innocent bystander? Am J Pathol. 2001;158:1767–73. doi: 10.1016/S0002-9440(10)64132-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anton RC, Kattan MW, Chakraborty S, Wheeler TM. Postatrophic hyperplasia of the prostate: lack of association with prostate cancer. Am J Surg Pathol. 1999;23:932–6. doi: 10.1097/00000478-199908000-00011. [DOI] [PubMed] [Google Scholar]
- 17.Macoska JA, Trybus TM, Wojno KJ. 8p22 loss concurrent with 8c gain is associated with poor outcome in prostate cancer. Urology. 2000;55:776–82. doi: 10.1016/s0090-4295(00)00468-4. [DOI] [PubMed] [Google Scholar]
- 18.Andren O, Fall K, Franzen L, Andersson SO, Johansson JE, Rubin MA. How well does the Gleason score predict prostate cancer death? A 20-year followup of a population based cohort in Sweden. J Urol. 2006;175:1337–40. doi: 10.1016/S0022-5347(05)00734-2. [DOI] [PubMed] [Google Scholar]
- 19.Aus G, Robinson D, Rosell J, Sandblom G, Varenhorst E. Survival in prostate carcinoma--outcomes from a prospective, population-based cohort of 8887 men with up to 15 years of follow-up: results from three countries in the population-based National Prostate Cancer Registry of Sweden. Cancer. 2005;103:943–51. doi: 10.1002/cncr.20855. [DOI] [PubMed] [Google Scholar]
- 20.Johansson JE, Andren O, Andersson SO, Dickman PW, Holmberg L, Magnuson A, et al. Natural history of early, localized prostate cancer. JAMA. 2004;291:2713–9. doi: 10.1001/jama.291.22.2713. [DOI] [PubMed] [Google Scholar]
- 21.De Marzo AM, Platz EA, Epstein JI, Ali T, Billis A, Chan TY, et al. A working group classification of focal prostate atrophy lesions. Am J Surg Pathol. 2006;30:1281–91. doi: 10.1097/01.pas.0000213289.50660.be. [DOI] [PubMed] [Google Scholar]
- 22.Gerstenbluth RE, Seftel AD, MacLennan GT, Rao RN, Corty EW, Ferguson K, et al. Distribution of chronic prostatitis in radical prostatectomy specimens with up-regulation of bcl-2 in areas of inflammation. J Urol. 2002;167:2267–70. [PubMed] [Google Scholar]
- 23.Kaplan SA. Analysis of the inflammatory network in benign prostate hyperplasia and prostate cancer. J Urol. 2005;173:161. doi: 10.1016/s0022-5347(05)60807-5. [DOI] [PubMed] [Google Scholar]
- 24.Asimakopoulos AD, Miano R, Mauriello A, Costantini S, Pasqualetti P, Liberati E, et al. Significance of focal proliferative atrophy lesions in prostate biopsy cores that test negative for prostate carcinoma. Urol Oncol. 2010 doi: 10.1016/j.urolonc.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 25.Alcover J, Filella X, Luque P, Molina R, Izquierdo L, Auge JM, et al. Prognostic value of IL-6 in localized prostatic cancer. Anticancer Res. 2010;30:4369–72. [PubMed] [Google Scholar]
- 26.Lin HC, Liu CC, Kang WY, Yu CC, Wu TT, Wang JS, et al. Influence of cytokine gene polymorphisms on prostate-specific antigen recurrence in prostate cancer after radical prostatectomy. Urol Int. 2009;83:463–70. doi: 10.1159/000251189. [DOI] [PubMed] [Google Scholar]
- 27.Stark JR, Judson G, Alderete JF, Mundodi V, Kucknoor AS, Giovannucci EL, et al. Prospective study of Trichomonas vaginalis infection and prostate cancer incidence and mortality: Physicians' Health Study. J Natl Cancer Inst. 2009;101:1406–11. doi: 10.1093/jnci/djp306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sutcliffe S, Giovannucci E, Gaydos CA, Viscidi RP, Jenkins FJ, Zenilman JM, et al. Plasma antibodies against Chlamydia trachomatis, human papillomavirus, and human herpesvirus type 8 in relation to prostate cancer: a prospective study. Cancer Epidemiol Biomarkers Prev. 2007;16:1573–80. doi: 10.1158/1055-9965.EPI-07-0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cohen RJ, Shannon BA, McNeal JE, Shannon T, Garrett KL. Propionibacterium acnes associated with inflammation in radical prostatectomy specimens: a possible link to cancer evolution? J Urol. 2005;173:1969–74. doi: 10.1097/01.ju.0000158161.15277.78. [DOI] [PubMed] [Google Scholar]
- 30.Fassi Fehri L, Mak TN, Laube B, Brinkmann V, Ogilvie LA, Mollenkopf H, et al. Prevalence of Propionibacterium acnes in diseased prostates and its inflammatory and transforming activity on prostate epithelial cells. Int J Med Microbiol. 2011;301:69–78. doi: 10.1016/j.ijmm.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 31.Billis A, Magna LA. Inflammatory atrophy of the prostate. Prevalence and significance. Arch Pathol Lab Med. 2003;127:840–4. doi: 10.5858/2003-127-840-IAOTP. [DOI] [PubMed] [Google Scholar]
- 32.De Marzo AM, Nelson WG, Meeker AK, Coffey DS. Stem cell features of benign and malignant prostate epithelial cells. J Urol. 1998;160:2381–92. doi: 10.1097/00005392-199812020-00004. [DOI] [PubMed] [Google Scholar]
- 33.Yang RM, Naitoh J, Murphy M, Wang HJ, Phillipson J, deKernion JB, et al. Low p27 expression predicts poor disease-free survival in patients with prostate cancer. J Urol. 1998;159:941–5. [PubMed] [Google Scholar]
- 34.Qian J, Bostwick DG, Takahashi S, Borell TJ, Herath JF, Lieber MM, et al. Chromosomal anomalies in prostatic intraepithelial neoplasia and carcinoma detected by fluorescence in situ hybridization. Cancer Res. 1995;55:5408–14. [PubMed] [Google Scholar]
- 35.Sato K, Qian J, Slezak JM, Lieber MM, Bostwick DG, Bergstralh EJ, et al. Clinical significance of alterations of chromosome 8 in high-grade, advanced, nonmetastatic prostate carcinoma. J Natl Cancer Inst. 1999;91:1574–80. doi: 10.1093/jnci/91.18.1574. [DOI] [PubMed] [Google Scholar]
- 36.De Marzo AM, DeWeese TL, Platz EA, Meeker AK, Nakayama M, Epstein JI, et al. Pathological and molecular mechanisms of prostate carcinogenesis: implications for diagnosis, detection, prevention, and treatment. J Cell Biochem. 2004;91:459–77. doi: 10.1002/jcb.10747. [DOI] [PubMed] [Google Scholar]