ABSTRACT
While health inequities are well documented, and there are helpful frameworks to understand health disparities, implementation frameworks are also needed to focus the design, evaluation, and reporting on interventions targeting populations at increased risk. This study aims to describe how the reach, effectiveness, adoption, implementation, and maintenance (RE-AIM) framework can be used for these purposes and illustrate its application in the context of a randomized, pragmatic weight loss and hypertension self-management intervention. RE-AIM was used to both plan and evaluate the Be Fit Be Well program for urban community health center patients. The RE-AIM framework helped to focus attention on and produce high rates of adoption and reach. Implementation rates varied across components. Weight losses were statistically significant, but not clinically significant. They were robust across a variety of patient characteristics, and the program was relatively of low cost. Individual weight losses and blood pressure reductions were maintained throughout the 24-month period, but the program was not sustained at any of the three settings. Implementation frameworks such as RE-AIM can help design pragmatic interventions that focus on both the context for disparities reduction and the ultimate goal of public health impact.
KEYWORDS: Weight loss, Health disparities, Low income, RE-AIM, Pragmatic trial, Implementation science
Despite widespread attention from the public health community and increasingly from policymakers, there has been uneven progress in improving health disparities. Indeed, during the past several decades, already pressing racial/ethnic and socioeconomic gaps have increased for a number of conditions, including type 2 diabetes and obesity [1]. As recognition about the severity and potential intractability of health disparities has grown, so, too, have intervention efforts designed to improve the health of affected groups. Nevertheless, there has been limited translation of these intervention efforts into real-world clinical and public health practice settings [2].
To address the Healthy People 2020 goal of reducing or eliminating health disparities [3], it is prudent to integrate dissemination and implementation considerations at the outset of intervention planning, especially when working in a community and low-resource settings [4]. We hypothesized that the reach, effectiveness, adoption, implementation, and maintenance (RE-AIM) framework would be useful for this task. RE-AIM was designed to guide the consideration of criteria that relate to translation, dissemination, and public health impact [5, 6].
Additionally, more pragmatic trials are needed to adequately address complex and pervasive issues that arise in chronic disease prevention and management interventions [7]. Features of pragmatic studies, as discussed by the CONSORT authors and others, are the following: (1) that they are conducted from the perspective of and with ongoing engagement of stakeholders; (2) the outcomes measured are those of importance to the end users [patients, clinicians, and decision makers (e.g., clinic administrators)]; (3) that comparison conditions are real-world alternatives; and (4) the settings and samples studied are chosen to be generalizable to real-world settings [8, 9].
The purpose of this paper is to apply the RE-AIM framework to highlight issues infrequently addressed but important for the dissemination and implementation of interventions designed for populations with disproportionate health risks. Although some limited primary outcome data are presented to support this purpose, this paper does not focus on the primary outcomes of the original study, which are described elsewhere [10]. Instead, we describe the health disparities implications for each of the five key RE-AIM dimensions and assess how Be Fit, Be Well (BFBW) addressed these issues. This builds on earlier work [5, 11, 12] and provides a concrete example of how the RE-AIM model can be used to design and evaluate pragmatic trials intended for disproportionately affected populations. A recent publication describes how RE-AIM was used to facilitate cultural adaptation for a Latina population [13], but this paper is the first, to our knowledge, to apply RE-AIM specifically to the reduction of health inequities.
METHODS
Approach
The RE-AIM framework [4, 6, 14] has been utilized to plan, evaluate, and review a variety of health promotion and disease management interventions [6, 15]. It was developed, in part, to create a balanced emphasis on internal and external validity issues [5]. It is intended to help bridge the gap between efficacy and effectiveness trials to obtain greater community acceptance, impact, and sustainability by increasing the focus on intervention reach, effectiveness, adoption, implementation, and maintenance [5]. Implicit in the RE-AIM framework is the assumption that, by focusing on these issues in the design phase, there will be a reduction in the health disparities that often occur as a result of unequal participation and accrual of benefit across populations [11].
In recent years, there have been a number of dissemination models and frameworks that have been developed and explored [16, 17]. RE-AIM was chosen because it is one of the frameworks that address both implementation and dissemination, as well as intervention design and evaluation, and it identifies key frequent translation challenges that need to be anticipated. For the purposes of this paper, we demonstrate how the RE-AIM framework was used to design BFBW and to evaluate its public health impact and dissemination potential. We also address some key translational challenges including reach, adoption, and consistent implementation.
RE-AIM components considered in intervention design
BFBW, a 24-month randomized weight loss and hypertension self-management intervention trial, has been described in detail elsewhere [10, 18, 19]. Briefly, the 365 participants in BFBW were primary care patients at one of three community health centers (CHC) in Boston, MA. Eligibility criteria included measured body mass index (BMI) 30–50 kg/m2, pharmacologic treatment for hypertension, age 21 years or older, receipt of primary care of at one of the three participating CHCs, and ability to speak and read either English or Spanish. Participants were randomized to either usual care or the BFBW intervention. Participants received a $50 gift card at their baseline, 6-, 12-, and 18-month visits and a $75 gift card at their 24-month visit. Intervention participants received a scale at their 6-month visit and a blood pressure monitor at their 12-month visit to aid their behavioral self-monitoring. Participants with difficulty getting to clinic sites for assessment visits were also offered taxi vouchers.
BFBW’s design [19] addressed these RE-AIM issues in several ways. First, to decrease commonly found burdens of transportation, time, and access to services, intervention content was largely delivered by phone and Internet (reach). Participants without regular access to the internet were still able to fully participate in the program using exclusively the phone and print materials. Second, it purposefully engaged health center staff in the design phase of the intervention to increase buy-in while at the same time ensuring low demand on clinic resources and staff during implementation, to be context-appropriate and widely applicable (adoption). In particular, they advised on ways to make the introduction of the intervention fit into existing clinic flow.
We attempted to increase effectiveness of this intervention both by using evidence-based principles such as self-monitoring, individually tailored behavior change recommendations, and also by using cultural- and literacy-appropriate intervention materials [10, 19]. Skills training materials, offered in print and on the web, were adapted to be appropriate for the population. Though ethnically diverse, this population overall had low levels of educational attainment and were of low income. To meet the needs of this population, all materials were written at a fourth grade reading level. Existing materials from National Institutes of Health (NIH) and National Heart, Lung, and Blood Institute were incorporated into print and web materials, including “Delicious Heart Healthy Latino Recipes” and “Heart Healthy Home Cooking, African-American Style.” Research staff took care not to recommend uncommon cooking tools or high-cost ingredients in print and web materials and offered tips on how to shop on a budget. Materials also included neighborhood guides, lists of safe places to walk, or to do other free or low-cost physical activity opportunities.
Implementation was addressed by offering user-friendly interfaces, participant choice of Internet or IVR modalities, and by training lay community health educators to provide health coaching over the phone. Health coach training occurred over 2 days. This training included a review of research ethics and IRB review, lessons on facilitating adult learning, cultural competence, and the LEARN model of cross-cultural guidelines for health practitioners, motivational interviewing principles, and strategies to encourage participants to self-monitor. In the days following the training, health coaches were asked to complete a series of practice coaching calls and group sessions with their supervisors and were given performance feedback.
We planned for maintenance from the outset by continuing intervention for 24 months by planning to make the website and resources available after the study ended, and by addressing social–environmental determinants of obesity. Identified health disparities issues and the intervention considerations for each RE-AIM dimension are listed in more detail in Table 1.
Table 1.
Health disparities issues, by RE-AIM dimension, and how Be Fit Be Well (BFBW) addressed these issues
| RE-AIM element | Key disparities issues | How the BFBW design addressed issues |
|---|---|---|
| Reach | • Accessing disparate populations through targeted outreach methods and program options (i.e., transportation, hours, etc.) | Intervention largely mediated by phone and internet with no added visits; participants selected times for calls based on their schedule; use of community health workers (CHW) instead of physicians; Collected basic demographic information on people who declined participation |
| • Understanding characteristics of those who participated vs. those who declined | ||
| Effectiveness | • Assessing broader, patient-centered outcomes, quality of life, and unanticipated consequences | Allowed intervention tailoring—choice (goals and modalities); use of multiple channels; follow-up contacts; culturally appropriate—English and Spanish materials and CHWs; use of evidence-based treatment; data collection for broad array of demographics and subgroups; designed for low literacy |
| • Understanding the impact of the context on results | ||
| • Considering Minimal Intervention Needed for Change | ||
| • Analyzing results by disparity-related subgroups—consider disparities broadly (e.g., demographics, risk, experience, residence, literacy) | ||
| Adoption | • Documenting and enhancing participation of low-resource settings and a variety of staff | Intervention designed with staff for buy-in and used CHWs; incorporated medical adherence for MDs; used pragmatic design; made feasible in context and placed low demands on staff and resources |
| • Understanding and addressing reasons for non-participation by setting/staff | ||
| Implementation | • Monitoring delivery to different subgroups and by different staff | Provided staff training in motivational interviewing and offered certification; offered feedback on delivery and implemented self-monitoring; planned to minimize (and track) resources and costs; kept web and IVR novel and fresh |
| • Understanding and tracking costs of delivery | ||
| • Transparently documenting adaptations to original program | ||
| Maintenance | ||
| Individual | • Assessing long-term results across subgroups and identifying inequities and reason | Ongoing assessment for 2 years; addressed social environment barriers and facilitators; website remains for study participants to use |
| • Providing infrastructure and links to community resources for individuals to sustain program results | ||
| Setting | • Planning for and supporting sustainability of program after initial evaluation | |
| • Preparing delivery settings with tools to guide monitoring and adaption of the program long term | ||
As seen in Table 1, there are recurring themes across all five RE-AIM dimensions of the need to plan for dissemination and feasibility from the beginning; to focus on broad and multi-level contextual factors as well as individual behavior change; to be transparent in reporting, including lessons learned and adaptations made; and to use pragmatic approaches that begin and end with stakeholders’ input and address their concerns. To enhance implementation and effectiveness, monthly (for the first 12 months) and bimonthly (for the second 12 months) calls from the interventionist provided social support and technical assistance.
BFBW intervention
The intervention was designed to promote hypertension self-management and weight loss through four main components: (1) tailored behavioral goals; (2) skills training; (3) behavior self-monitoring; and (4) social support via BFBW interventionists, community/environmental resources, and health-care team. Components were delivered through daily tracking of behavioral goals via either a website or interactive voice response system (IVR), provision of print materials for skills training and self-monitoring, monthly interventionist-delivered coaching calls, and monthly group sessions. The original study’s primary outcome was weight change at 24 months with a secondary outcome of change in blood pressure at 24 months.
Analyses
Univariate statistics were generated to describe the sociodemographics of participants. Means and frequencies of the gender, age, BMI, and diabetes comorbidity status were computed from the electronic medical record data of patients who were invited but declined participation in the study. Bivariate comparisons using two-way ANOVA and chi-square tests were conducted to evaluate reach and compare participants to those who declined.
Building on previous analyses [10], select BFBW baseline demographic variables were introduced as potential interaction terms into the mixed-effect models developed to evaluate effectiveness. Although the subgroup analyses are not traditional measures of effectiveness, the evaluation of the results across key population subgroups provides important data in regard to the health equity and translation perspective of effectiveness. Each demographic variable was tested for an interactive effect on the profile of weight change and systolic blood pressure change by participant characteristic, treatment group, and study visit, after adjusting for baseline measurements, gender, and community health center main effects (Table 2).
Table 2.
Baseline characteristics of participants
| Characteristic | Usual care (N = 185) | Intervention (N = 180) |
|---|---|---|
| Female | 122 (66) | 128 (71.1) |
| Male | 63 (34.1) | 52 (28.9) |
| Race/ethnicity, N (%) | ||
| Non-Hispanic Black/African-American | 131 (70.8) | 129 (71.7) |
| Hispanic | 23 (12.4) | 25 (13.9) |
| Other | 31 (16.72) | 26 (14.6) |
| Language N (%) | ||
| English | 163 (88.1) | 157 (87.2) |
| Spanish | 22 (11.9) | 23 (12.8) |
| Education N (%) | ||
| <High school diploma | 73 (39.5) | 47 (26.1) |
| ≥High school diploma | 112 (60.5) | 133 (74) |
| Income N (%) | ||
| <$25,000 | 105 (56.8) | 94 (52.2) |
| ≥$25,000 | 80 (43.2) | 86 (47.7) |
| Employment N (%) | ||
| Employed | 98 (53) | 94 (52.2) |
| Unemployed | 87 (47) | 86 (47.4) |
| Health Insurance N (%) | ||
| Medicaid or Medicare | 99 (53.5) | 99 (55) |
| Private and other | 86 (46.5) | 81 (45) |
| Medication, N (%) | ||
| Diabetes | 64 (34.6) | 44 (24.4) |
| Lipids | 66 (35.7) | 66 (36.7) |
| Health center, N (%) | ||
| Center 1 | 51 (27.6) | 52 (28.9) |
| Center 2 | 82 (44.3) | 76 (42.2) |
| Center 3 | 52 (28.1) | 52 (28.9) |
| Miscellaneous | ||
| Age (year) | 54.6 ± 11.1 | 54.4 ± 10.8 |
| Weight (kg) | 100.2 ± 17.5 | 100.6 ± 18.6 |
| BMI (kg/m2) | 37.0 ± 5.2 | 37.0 ± 5.0 |
| SBP (mm Hg) | 128.5 ± 19.7 | 130.2 ± 18.9 |
| DBP (mmHg) | 77.4 ± 13.8 | 79.3 ± 12.7 |
SBP systolic blood pressure, DBP diastolic blood pressure, BMI body mass index
Adoption rates were assessed by calculating the number of clinics that were approached and those that agreed to participate, as well as the assessing the percentage of individual physicians within those clinics who participated. Although there are a multitude of definitions for implementation, for the purposes of the analyses, we are using those from RE-AIM and focusing on treatment delivery, receipt, and adherence [4, 6, 14]. As such, implementation rates of counseling calls were calculated by summing successful contacts over the study for each participant and dividing by their expected sums. Calculations were conducted for the proportion of participants completing 70 % of the calls. The BFBW interventionists kept a record indicating whether goals, barriers, and strategies were evaluated each (per call scripts). Chi-square tests were conducted to explore the association of these counseling call variables with study site, preferred language, and income to evaluate factors potentially influencing implementation.
The percentage of study weeks that self-monitoring data were reported was calculated by summing the weeks with either a successful IVR call or submission of a tracking form on the intervention website, divided by the number of expected weeks with self-monitoring. The proportion of participants who completed their self-monitoring for more than half the weeks in the study was calculated. The self-monitoring rates of participants grouped by study site and preferred language were compared with chi-square tests.
RESULTS
Reach
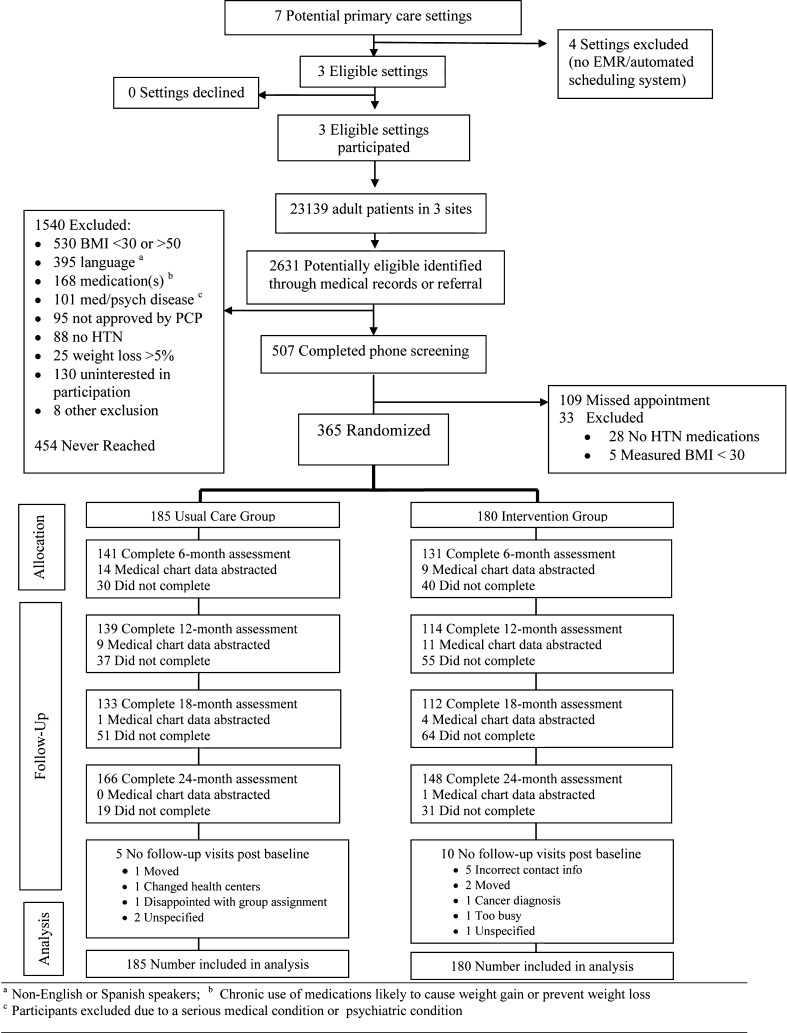
As can be seen in Fig. 1 and Table 3, BFBW enrolled approximately 60 % of potentially eligible patients invited to participate. As shown, due to standardized selection criteria of the parent POWER program, a significant percentage of patients were excluded from participation, despite this being the most pragmatic of the POWER studies [18, 20]. The most common reasons for exclusion were as follows: not speaking or reading either English or Spanish (n = 395) and being on medications likely to interfere with weight loss (n = 168). Relatively few patients meeting the medical eligibility criteria were excluded for other reasons. Of the 604 able to be reached and have phone eligibility criteria confirmed, 507 (84 %) agreed to participate, and 365 of these (60.4 % or 365/604 participation rate [21]) completed baseline, final eligibility criteria, and then were randomized.
Fig 1.
CONSORT diagram for recruitment and adoption [10]
Table 3.
Characteristics of BFBW participants compared to those who declined participation
| Characteristics/category | BFBW participants [mean (SD) or %] (N = 365) | Declined for whom data available [mean (SD) or %] (N = 130) | Sign. of diff |
|---|---|---|---|
| Health Center | 0.05 | ||
| Center 1 | 103 (28.2) | 34 (26.2) | |
| Center 2 | 158 (43.3) | 71 (54.6) | |
| Center 3 | 104 (28.5) | 25 (19.2) | |
| Gender | 0.87 | ||
| Female | 250 (68.5) | 88 (67.7) | |
| Male | 115 (31.5) | 42 (32.3) | |
| Diagnosed with diabetesa | 0.26 | ||
| Yes | 240 (66.1) | 93 (71.5) | |
| No/unknown | 123 (33.9) | 37 (28.5) | |
| Ageb | 54.6 ± 10.9 | 58.3 ± 13.1 | 0.005 |
| BMIc | 37.0 ± 5.1 | 35.8 ± 4.9 | 0.03 |
aBFBW participants who were ever told by a doctor that they had diabetes outside of pregnancy. Refusers who had a diabetes diagnosis in their medical records
bAge at enrollment for BFBW participants. Age at study start for refusers
cOnly 115 refusers had weight and height available in their records to calculate BMI
Recruitment analyses indicated that participants, compared to nonparticipants, were younger (mean ± SD: 54.6 vs. 58.3 years, P = 0.005) and had a higher mean BMI (mean ± SD: 37.0 vs. 35.8, P < .05) but did not significantly differ by gender or comorbid diabetes status, as seen in Table 3.
Effectiveness
Outcomes are reported in more detail elsewhere [10, 22]. In this article, we report primarily on implementation outcomes and only secondarily on outcomes of weight loss and SBP reduction, and on behaviors directly related to these outcomes as they pertain to health disparities. As previously reported [10], patients randomized to the intervention experienced a modest but significant increased weight loss compared to those in the control condition and maintained it over the course of the study (24-month difference: −1.0 kg; 95 % confidence interval (CI), −2.0 to −0.03 kg). Twenty percent of intervention participants and 19.5 % of usual care lost at least 5 % of their initial body weight over 24 months. On average, intervention participants lost a greater percentage of body weight by 24 months compared to those receiving usual care (difference: −1.02 %; 95 % CI −2.02, −0.005) after adjusting for gender and clinic. Intervention patients also had lower systolic blood pressure throughout the course of the study (24-month difference: −3.7 mm/Hg; 95 % CI, −7.9 to 0.5). As can be seen in Table 4, outcomes were relatively uniform across a variety of patient characteristics.
Table 4.
Primary outcomes by condition and characteristic for weight loss and by condition and subgroup for systolic blood pressure
| Adjusted mean (S.E.)a | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Visit | Baseline | 6 months | 12 months | 18 months | 24 months | P value | |||||
| Condition | Int | UC | Int | UC | Int | UC | Int | UC | Int | UC | |
| I. Weight loss | |||||||||||
| Overall | 102.2 (1.5) | 102.4 (1.4) | 100.9 (1.5) | 102.2 (1.4) | 100.8 (1.5) | 102.0 (1.4) | 100.9 (1.5) | 102.0 (1.4) | 100.6 (1.5) | 101.9 (1.4) | 0.15 |
| Race/ethnicity | 0.97 | ||||||||||
| African-American | 102.8 (1.7) | 103.6 (1.6) | 101.6 (1.7) | 103.4 (1.6) | 101.7 (1.7) | 103.3 (1.6) | 101.6 (1.7) | 103.2 (1.6) | 101.2 (1.7) | 103.1 (1.6) | |
| Latino | 97.4 (3.6) | 95.8 (3.8) | 96.6 (3.6) | 95.9 (3.8) | 95.7 (3.6) | 95.3 (3.8) | 96.4 (3.6) | 96.1 (3.9) | 95.3 (3.6) | 94.8 (3.8) | |
| Other | 102.1 (3.4) | 100.7 (3.2) | 100.9 (3.4) | 100.7 (3.2) | 99.8 (3.4) | 100.4 (3.2) | 100.4 (3.4) | 100.5 (3.2) | 101.8 (3.4) | 100.5 (3.2) | |
| Education | 0.81 | ||||||||||
| <12th Grade | 101.9 (2.7) | 102.2 (2.1) | 100.7 (2.7) | 101.9 (2.1) | 100.1 (2.7) | 101.9 (2.1) | 100.5 (2.7) | 102.3 (2.1) | 100.4 (2.7) | 101.5 (2.1) | |
| High school or more | 102.2 (1.6) | 102.5 (1.8) | 101.0 (1.7) | 102.4 (1.8) | 101.0 (1.7) | 102.2 (1.8) | 101.0 (1.7) | 101.9 (1.8) | 100.7 (1.7) | 102.1 (1.8) | |
| Sex | 0.76 | ||||||||||
| Female | 96.8 (1.7) | 95.4 (1.7) | 95.4 (1.7) | 95.4 (1.7) | 95.4 (1.7) | 95.1 (1.7) | 95.5 (1.7) | 94.9 (1.7) | 95.4 (1.7) | 94.9 (1.7) | |
| Male | 106.6 (2.4) | 110.2 (2.3) | 105.5 (2.4) | 109.8 (2.3) | 105.3 (2.5) | 109.8 (2.3) | 105.4 (2.5) | 110.2 (2.3) | 104.5 (2.5) | 109.8 (2.3) | |
| Income | 0.49 | ||||||||||
| ≤$10,000 | 101.1 (2.9) | 103.8 (2.5) | 100.1 (2.9) | 103.9 (2.5) | 99.7 (2.9) | 103.9 (2.5) | 99.2 (2.9) | 103.7 (2.5) | 98.5 (2.9) | 103.7 (2.5) | |
| >$10,000 | 102.5 (1.6) | 101.8 (1.6) | 101.2 (1.6) | 101.6 (1.6) | 101.1 (1.6) | 101.3 (1.6) | 101.4 (1.6) | 101.4 (1.6) | 101.3 (1.6) | 101.1 (1.6) | |
| Language | 0.98 | ||||||||||
| English | 102.9 (1.5) | 103.1 (1.5) | 101.6 (1.5) | 102.8 (1.5) | 101.6 (1.5) | 102.8 (1.5) | 101.6 (1.5) | 102.7 (1.5) | 101.4 (1.5) | 102.6 (1.5) | |
| Spanish | 95.2 (3.7) | 94.1 (3.9) | 94.1 (3.7) | 95.0 (3.9) | 93.1 (3.7) | 93.7 (3.9) | 94.0 (3.7) | 94.4 (3.9) | 93.4 (3.7) | 93.8 (3.9) | |
| Health center | 0.93 | ||||||||||
| Center 1 | 102.7 (2.4) | 97.9 (2.4) | 100.8 (2.4) | 97.2 (2.4) | 101.2 (2.4) | 97.4 (2.4) | 102.1 (2.4) | 97.4 (2.4) | 101.7 (2.4) | 97.5 (2.4) | |
| Center 2 | 102.1 (2.0) | 106.1 (1.9) | 101.4 (2.0) | 106.6 (1.9) | 100.9 (2.0) | 106.0 (1.9) | 100.3 (2.0) | 106.0 (1.9) | 100.1 (2.0) | 105.9 (1.9) | |
| Center 3 | 102.3 (2.4) | 101.6 (2.4) | 101.0 (2.4) | 101.3 (2.4) | 101.0 (2.4) | 101.2 (2.4) | 101.2 (2.5) | 101.2 (2.4) | 101.0 (2.4) | 100.7 (2.4) | |
| II. Systolic blood pressure | |||||||||||
| Overall | 132.5 (1.5) | 130.7 (1.5) | 133.0 (1.7) | 132.5 (1.6) | 131.1 (1.8) | 134.1 (1.6) | 132.3 (1.8) | 136.3 (1.7) | 134.1 (1.7) | 136.0 (1.6) | 0.07 |
| Race/ethnicity | 0.51 | ||||||||||
| African American | 131.4 (1.7) | 130.9 (1.7) | 131.5 (1.9) | 131.2 (1.9) | 130.5 (2.0) | 132.5 (1.9) | 130.1 (2.0) | 134.6 (1.9) | 132.4 (1.8) | 134.4 (1.8) | |
| Latino | 136.3 (3.9) | 121.9 (4.1) | 133.0 (4.4) | 131.1 (4.4) | 131.7 (4.5) | 131.5 (4.5) | 133.1 (4.8) | 131.0 (5.4) | 139.5 (4.1) | 132.5 (4.4) | |
| Other | 126.7 (3.7) | 129.9 (3.4) | 132.6 (4.4) | 132.5 (3.9) | 125.8 (4.6) | 136.7 (4.1) | 135.5 (4.6) | 140.6 (3.7) | 129.6 (4.2) | 138.8 (3.5) | |
| Education | 0.83 | ||||||||||
| <12th Grade | 132.3 (2.8) | 127.8 (2.3) | 135.1 (3.4) | 129.9 (2.5) | 132.7 (3.3) | 132.7 (2.5) | 131.4 (3.4) | 131.8 (2.6) | 139.5 (3.1) | 134.7 (2.4) | |
| High school or more | 132.7 (1.7) | 132.8 (1.9) | 132.4 (1.9) | 134.3 (2.0) | 130.7 (2.0) | 135.1 (2.1) | 132.7 (2.0) | 139.1 (2.1) | 132.3 (1.9) | 137.0 (1.9) | |
| Gender | 0.19 | ||||||||||
| Female | 130.5 (1.8) | 128.7 (1.8) | 130.5 (1.9) | 129.5 (2.0) | 128.9 (2.0) | 130.8 (1.9) | 128.8 (2.0) | 133.0 (1.9) | 132.8 (1.8) | 131.9 (1.8) | |
| Male | 133.8 (2.6) | 132.3 (2.4) | 135.2 (3.0) | 136.0 (2.6) | 132.6 (3.3) | 138.0 (2.7) | 138.2 (3.4) | 140.5 (2.8) | 132.6 (3.1) | 141.5 (2.5) | |
| Income | 0.75 | ||||||||||
| ≤$10,000 | 128.3 (3.0) | 129.5 (2.7) | 130.8 (3.7) | 128.9 (3.0) | 130.5 (3.7) | 133.1 (2.9) | 130.1 (3.6) | 132.3 (3.0) | 133.8 (3.3) | 135.0 (2.8) | |
| >$10,000 | 133.6 (1.7) | 131.1 (1.7) | 133.4 (1.8) | 133.7 (1.9) | 131.1 (2.0) | 134.3 (1.9) | 132.7 (2.0) | 137.8 (1.9) | 134.0 (1.8) | 136.3 (1.8) | |
| Language | 0.32 | ||||||||||
| English | 132.3 (1.6) | 131.1 (1.6) | 133.2 (1.8) | 132.0 (1.7) | 132.1 (1.9) | 133.8 (1.7) | 132.4 (1.9) | 136.1 (1.7) | 133.9 (1.8) | 135.7 (1.6) | |
| Spanish | 134.3 (4.0) | 128.3 (4.2) | 131.8 (4.5) | 136.5 (4.7) | 125.1 (4.6) | 136.0 (4.7) | 131.6 (4.6) | 138.6 (5.1) | 135.2 (4.2) | 138.9 (4.4) | |
| Health center | 0.24 | ||||||||||
| Center 1 | 134.0 (2.6) | 139.2 (2.6) | 141.0 (2.9) | 139.1 (2.9) | 137.8 (3.1) | 138.1 (3.2) | 135.6 (3.1) | 142.3 (3.1) | 138.2 (2.8) | 141.2 (2.8) | |
| Center 2 | 130.2 (2.2) | 123.9 (2.1) | 128.0 (2.4) | 126.1 (2.3) | 126.9 (2.6) | 129.6 (2.2) | 127.0 (2.5) | 129.6 (2.3) | 127.8 (2.4) | 129.8 (2.2) | |
| Center 3 | 130.4 (2.6) | 128.5 (2.6) | 128.2 (3.0) | 131.4 (3.0) | 126.6 (3.1) | 131.6 (2.9) | 133.0 (3.3) | 136.7 (3.0) | 135.3 (2.9) | 135.9 (2.7) | |
Int intervention, UC usual care
aModels are adjusted for clinic and gender (where they are not a part of the interaction of interest). Visits after bariatric surgery are censored
We also reported elsewhere [10] that intervention participants showed significantly greater change in medication adherence at 6 months (difference: −1.22; 95 % CI −0.15, −0.012; P = 0.001) and 12 months (difference: −0.85; 95 % CI −1.40, −0.30; P = 0.002). However, no significant between-group difference was observed at 18 months (difference: −0.31; 95 % CI −0.86, 0.25; P = 0.28) and 24 months (difference: −0.36; 95 % CI −0.88, 0.15; P = 0.17).
No significant between group differences were observed when we examined changes in health-related quality of life based on the population preference-weighted health index score from the EQ-5D at 12 months (difference: −0.01; 95 % CI −0.05, 0.03; P = 0.48) or at 24 months (difference: −0.004; 95 % CI −0.04, 0.03; P = 0.84).
Adoption
The program was successful in recruiting all three of the CHC primary care clinics invited to participate and in getting 19 of 20 (95 %) primary care providers to refer their patients to the program. Four potential CHCs were excluded because they did not have any electronic records or scheduling systems to use for population-based recruitment purposes.
Implementation
Details on implementation and costs of this complex multifaceted intervention are reported in greater detail elsewhere [10, 22]. Here, we focus on overall implementation rates and patient characteristics related to implementation. Overall program delivery rates by the interventionists were relatively high with a 70.6 % completion rate of counseling calls and 63.3 % of participants completing more than 70 % of their calls. The completion rate of counseling calls did not differ by study site or participant-preferred language, but calls were significantly more likely to be completed with participants making over $10,000 a year (73.1 vs. 62.2 %, P < 0.0001).
Significant differences in the evaluation of goals, barriers, and strategies were seen by study site, preferred language, and income. Each of these evaluation points were more consistently completed at center 1 and less consistently at center 3 (P < 0.0001). English speakers were more likely to have goals, barriers, and strategies evaluated (P < 0.0001), as were participants making more than $10,000 (P < 0.001). Weekly self-monitoring rates were variable, with participants completing 42.2 % of the expected tracking events across the 2-year intervention and 40.0 % of participants tracking on more than half of study weeks. Self-monitoring rates were better when IVR tracking was utilized compared to web tracking (44.7 vs. 34.0 %, P < 0.0001). English speakers had a higher self-monitoring rate than participants who preferred to speak Spanish (42.6 vs. 39.5 %, P = 0.004).
Maintenance
In RE-AIM, maintenance has indicants at both the individual and setting levels. As can be seen in Table 4, at the individual level, maintenance out to the 24-month follow-up was good. Patient characteristics of race, education, gender, or income did not moderate maintenance results. Likewise, the profiles of systolic blood pressure change by treatment group did not significantly differ between these demographic subgroups.
In regards to setting-level maintenance, interviews with clinic staff indicated that sites were interested in adapting the elements of BFBW regarding hypertension medication adherence and blood pressure control. However, none of the clinics had formally adopted any of the components of BFBW. Some have added walking fitness programs, but it is unclear if these can be attributed to their participation in BFBW.
DISCUSSION
The BFBW program was successful at reaching and helping patients with low income, high risk, and from predominantly African-American obese community health center to stay engaged with a program to improve health-promoting behaviors, make modest reductions in blood pressure and less so in weight, and to maintain these gains for at least 24 months. However, lower income and Spanish-speaking patients were less engaged in the intervention and that the program was not sustained at any of the participating clinics. As described in more detail in the main outcome study, this is one of the few weight loss programs designed specifically for low-income African-Americans and Latinos facing such challenging socioeconomic conditions to produce encouraging, albeit modest, long-term results [10, 23, 29].
The purpose of this article was to describe the use of the RE-AIM model to help design and evaluate this intervention. There are likely multiple reasons for the recruitment, adoption, implementation, and success of BFBW (and the mixed maintenance success—good at the patient level, poor at the setting level), but we conclude that factors related to RE-AIM issues such as designing a program with the input of key stakeholders and placing minimal burden on the primary care providers (both addressing adoption); that was attractive and accessible to patients and did not require much travel or extra visits (reach); and that had user-friendly interfaces, patient choice of tracking modalities, and community health workers to provide ongoing support and prompts (implementation and maintenance) were at least partially responsible. To address setting-level maintenance challenges, from a RE-AIM perspective, we might recommend actions including ensuring that the program is aligned with clinic mission, exploring the fit with new health policy changes such as the enhanced community health workforce to have ongoing responsibility, and advocacy to make the reimbursement policies worth the time of clinics to invest in obesity prevention and treatment.
We further speculate that other keys to success were likely “designing for dissemination” [4] at the outset and the focus on keeping both time and resource demands on an already overworked and underfunded community health center staff low [22]. Compatible with pragmatic and adaptive trials [7], we also made adjustments during the study to enhance recruitment, keep the website and IVR components novel, respond to participants’ concerns, and maintain a high level of intervention engagement.
RE-AIM analyses of the percent and representativeness of results at multiple levels and across different outcomes, including reach, adoption, effectiveness, implementation and maintenance generally revealed robust results, with modest differences across patient characteristics. These results are encouraging and tentatively suggest that BFBW may have broad appeal to a relatively wide range of community health centers and patients, and are of at least modest benefit even to high-risk patients. A separate publication documented that the BFBW study was significantly more pragmatic than other POWER weight loss studies funded under the same grant mechanism [18].
The BFBW program was not uniformly successful and could likely be improved and further adapted to fit different settings and populations to make it more effective and generalizable. For example, the group meetings were poorly attended, eventually discontinued, and likely did not add much to the intervention effectiveness. Magnitude of weight loss that was not clinically significant might be increased by increasing program intensity or contacts. Despite use of local community health workers, there were implementation differences by income and preferred language. Lower-income and Spanish-speaking participants were less engaged than others despite considerable efforts to make the intervention culturally and contextually appropriate. One possible reason for the discrepancies in call completion among the lowest-income participants could be that they had less consistent access to telephones and could not afford the costs of the calls. At 24-month follow-up, questions of economic hardship were assessed, including phone disconnections. Approximately 20 % of the participants at follow-up indicated that their phone line had been disconnected at least once within the 2-year study period. It is possible that either use of text messaging or expanding the intervention to more directly address fundamental determinants of health (e.g., housing, food insecurity, racism) would help to reduce these implementation subgroup differences.
Similarly, lessons learned during recruitment could be applied, and alternative strategies, such as recruiting from a weight loss registry or directly from primary care visits, would likely reduce recruitment costs substantially. The site differences in results in multi-site studies are not unusual; however, many studies do not report on-site differences. This is a feature that we would recommend and think would enhance the transparency of reporting. For this study, we speculate that differences across sites were attributable to multiple factors. Administrative issues may have impacted the variance as recruitment at site 2 began 1–2 months before sites 1 and 3. Therefore, more individuals were assessed for eligibility at site 2, so while there were a larger number of refusals, there was not necessarily a higher rate of refusal. Additionally, language barriers may have resulted in some of the discrepancies. Site 3 had the highest percentage of Spanish speakers, and the materials were not translated into Spanish until about 4–5 months after recruitment started at site 2, therefore limiting the number of Spanish-speaking participants from that site. Additionally, site 1 had a number of ineligible participants due to language as that center has a large Cape Verdean clientele.
This study must be interpreted in context. Over the past 30 years, economic inequality has grown, and the divide between the rich and poor has deepened [24]. Growing economic inequality leads to worse health outcomes [25]. These problems are exacerbated by a poor health environment and an overstretched primary care system that is unable to recover from decades of underinvestment in fundamental health care and prevention. The Affordable Care Act [26], as its provisions take effect, promises to increase access to health care for many, but it faces a host of challenges in the coming years.
Within this challenging context, BFBW [10] attempted to engage the poor and underserved in healthy eating, physical activity, and blood pressure control mediated through support of healthy behaviors and enhanced interactions with their primary care clinic. We used RE-AIM as well as experience working in low-income settings to design and evaluate the BFBW intervention for community health centers. This focus on those most in need stands in contrast to many studies that (often unintentionally) exclude the most vulnerable because they cannot be reached at the outset, devote considerable time and resources, and/or face substantial barriers to access of needed resources. The studies closest in scope to ours focused on community health center or low-income-setting-based studies that explicitly recruited low income, largely African-American or Latino samples for weight loss interventions that we could locate, were by Clark et al. [27], Samuel-Hodge et al. [28], and Ockene et al. [29]. All three studies used in person rather than electronic interventions. The study of Clark et al. reported challenges with reach and implementation with 16 % of those eligible having intervention contact but only 2 % having ten or more contacts, which they found necessary for significant weight loss. The study of Samuel-Hodge et al. [28] reported larger weight losses but did not report on reach. Samuel-Hodge et al. [23] have, however, recently reported initial recruitment results from a follow-up study conducted in county health departments that demonstrated high reach and adoption by health department, but weight loss data have not yet been reported. Finally, Ockene et al. [29] recently reported results from a low-cost version of the Diabetes Prevention Program tailored for low-income Latino populations that was moderately successful at producing weight loss (2.5 lbs over 12 months) but also reported challenges with group meeting attendance.
This study has both limitations and strengths. Limitations include the fact that BFBW results are undoubtedly due to many factors, and we did not experimentally evaluate the use of RE-AIM vs. other implementation models to guide intervention and evaluation. Other limitations are that our conclusions must be limited to the types of community health centers and patient populations studied, the relatively small number of health centers, the moderate patient sample size, the limited magnitude of weight loss, and that the intervention was not continued after the study. Finally, the most important outcomes, namely factors such as long-term cost-effectiveness and levels of adoption and successful implementation by other settings, will not be available for several years, although initial cost-effectiveness results are shared elsewhere [22]. Strengths include the high-risk, diverse, and largely African-American sample studied, the multiple, low-resource urban community health center settings, the pragmatic intervention and study design, the 24-month evaluation period, the general robustness of results across multiple RE-AIM dimensions, and the systematic use of an implementation science framework such as RE-AIM for intervention planning and evaluation.
In conclusion, RE-AIM can be applied as a framework to help plan and analyze interventions to address health inequities. Further research using and comparing different implementation science models, with greater use of cost and economic analyses, and pragmatic research on interventions for low-income African-American and other underserved populations in low-resource service settings is clearly needed to address pressing health equity issues.
Acknowledgments
The findings and conclusions in this publication are those of the authors and do not necessarily represent the views of the National Cancer Institute, National Institutes of Health. This research was supported by the National Institutes of Health (NIH) Grant 5 U01 HL087071. Erica T. Warner was supported by the National Cancer Institute grant number 5T32CA009001-36 and by the National Institute of General Medical Sciences grant number 5R25GM055353-14.
Footnotes
Implications
Policy: Public health impact can be enhanced by use of planning and evaluation frameworks to address issues such as inequitable participation, engagement, outcomes, and sustainability of well-intended programs and policies.
Research: Use of implementation science models such as RE-AIM can be useful for both planning and reporting on programs intended to address health inequities.
Practice: Planning ahead to address frequent challenges to implementation can help enhance program reach, delivery, and reduce health disparities. Additional features are likely needed to increase magnitude of weight losses produced.
References
- 1.Bleich SN, Jarlenski MP, Bell CN, Laveist TA. Health inequalities: Trends, progress, and policy. Annual Review of Public Health. 2012;33:7–40. doi: 10.1146/annurev-publhealth-031811-124658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smedley BD, Stith AY, Nelson AR. Board on health sciences policy, eds. Unequal treatment: Confronting racial and ethnic disparities in health care. Washington, DC: The National Academies, Institute of Medicine; 2003. [PubMed] [Google Scholar]
- 3.Department of Health and Human Services. HHS action plan to reduce racial and ethnic health disparities: a nation free of disparities in health and health care. http://minorityhealth.hhs.gov/npa/files/Plans/HHS/HHS_Plan_complete pdf. 2012. Accessed 3/17, 2012.
- 4.Klesges LM, Estabrooks PA, Dzewaltowski DA, Bull SS, Glasgow RE. Beginning with the application in mind: Designing and planning health behavior change interventions to enhance dissemination. Annals of Behavioral Medicine. 2005;29(Suppl):66–75. doi: 10.1207/s15324796abm2902s_10. [DOI] [PubMed] [Google Scholar]
- 5.Glasgow RE, Lichtenstein E, Marcus AC. Why don't we see more translation of health promotion research to practice? Rethinking the efficacy-to-effectiveness transition. American Journal of Public Health. 2003;93(8):1261–1267. doi: 10.2105/AJPH.93.8.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gaglio B, Glasgow RE. Evaluation approaches for dissemination and implementation research. In: Brownson RC, Colditz GA, Proctor EK, editors. Dissemination and implementation research in health: Translating science to practice. New York: Oxford University Press; 2012. pp. 327–356. [Google Scholar]
- 7.Zwarenstein M, Treweek S. What kind of randomised trials do patients and clinicians need? Evidence-Based Medicine. 2009;14(4):101–103. doi: 10.1136/ebm.14.4.101. [DOI] [PubMed] [Google Scholar]
- 8.Tunis SR, Stryer DB, Clancy CM. Practical clinical trials: Increasing the value of clinical research for decision making in clinical and health policy. Journal of the American Medical Association. 2003;290(12):1624–1632. doi: 10.1001/jama.290.12.1624. [DOI] [PubMed] [Google Scholar]
- 9.Thorpe KE, Zwarenstein M, Oxman AD, et al. A pragmatic-explanatory continuum indicator summary (PRECIS): a tool to help trial designers. Journal of Clinical Epidemiology. 2009;62(5):464–475. doi: 10.1016/j.jclinepi.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 10.Bennett GG, Warner ET, Glasgow RE, et al. Obesity treatment for socioeconomically disadvantaged patients in primary care practice. Archives of Internal Medicine. 2012;172(7):565–574. doi: 10.1001/archinternmed.2012.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koh HK, Oppenheimer SC, Massin-Short SB, Emmons KM, Geller AC, Viswanath K. Translating research evidence into practice to reduce health disparities: a social determinants approach. American Journal of Public Health. 2010;100(Suppl 1):S72–80. doi: 10.2105/AJPH.2009.167353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bennett GG, Glasgow RE. The delivery of public health interventions via the internet: Actualizing their potential. Annual Review of Public Health. 2009;30:273–292. doi: 10.1146/annurev.publhealth.031308.100235. [DOI] [PubMed] [Google Scholar]
- 13.Toobert DJ, Strycker LA, King DK, Barrera M, Jr, Osuna D, Glasgow RE. Long-term outcomes from a multiple-risk-factor diabetes trial for latinas: inverted exclamation markViva bien! Transl Behav Med. 2011;1(3):416–426. doi: 10.1007/s13142-010-0011-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glasgow RE, Vogt TM, Boles SM. Evaluating the public health impact of health promotion interventions: The RE-AIM framework. American Journal of Public Health. 1999;89(9):1322–1327. doi: 10.2105/AJPH.89.9.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glasgow RE, Klesges LM, Dzewaltowski DA, Bull SS, Estabrooks P. The future of health behavior change research: what is needed to improve translation of research into health promotion practice? Annals of Behavioral Medicine. 2004;27(1):3–12. doi: 10.1207/s15324796abm2701_2. [DOI] [PubMed] [Google Scholar]
- 16.Brownson RC, Colditz GA, Proctor EK, editors. Dissemination and implementation research in health: Translating science to practice. 1. New York: Oxford University Press, Inc.; 2012. [Google Scholar]
- 17.Tabak RG, Khoong EC, Chambers DA, Brownson RC. Bridging research and practice: Models for dissemination and implementation research. American Journal of Preventive Medicine. 2012;43(3):337–350. doi: 10.1016/j.amepre.2012.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Glasgow RE, Gaglio B, Bennett G, et al. Applying the PRECIS criteria to describe three effectiveness trials of weight loss in obese patients with comorbid conditions. Health Services Research. 2011;47(3):1051–-1067. doi: 10.1111/j.1475-6773.2011.01347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greaney ML, Quintiliani LM, Warner ET, King DK, Emmons KM, Colditz GA, Glasgow RE, Bennett GG. Weight management among patients at community health centers: the “be fit, be well” study. Obes Weight Manag. 2009;5(5):218–224. [Google Scholar]
- 20.Yeh HC, Clark JM, Emmons KE, et al. Independent but coordinated trials: Insights from the practice-based opportunities for weight reduction trials collaborative research group. Clinical Trials. 2010;7(4):322–332. doi: 10.1177/1740774510374213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Glasgow RE, Strycker LA, Kurz D, et al. Recruitment for an internet-based diabetes self-management program: Scientific and ethical implications. Annals of Behavioral Medicine. 2010;40(1):40–48. doi: 10.1007/s12160-010-9189-1. [DOI] [PubMed] [Google Scholar]
- 22.Ritzwoller D, Glasgow R, Sukhanova A, et al. Economic analyses of the be fit be well program: a weight loss program for community health centers. Journal of General Internal Medicine. Under Review. [DOI] [PMC free article] [PubMed]
- 23.Samuel-Hodge CD, Garcia BA, Johnston LF, et al. Rationale, design, and sample characteristics of a practical randomized trial to assess a weight loss intervention for low-income women: The weight-wise II program. Contemporary Clinical Trials. 2012;33(1):93–103. doi: 10.1016/j.cct.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 24.Congressional Budget Office. Trends in the distribution of household income between 1979 and 2007. 2011;CBO Publication No. 4031.
- 25.Wilkinson RG. The impact of inequality. Social Research. 2006;73(2):711–732. [Google Scholar]
- 26.Landmark: The inside story of America’s new health care law and what it means to all of us. 1. New York: Public Affairs; 2010. [Google Scholar]
- 27.Clark D, Chrysler L, Perkins A, et al. Screening, referral, and participation in a weight management program implemented in five CHCs. Journal of Health Care for the Poor and Underserved. 2010;21(2):617–628. doi: 10.1353/hpu.0.0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Samuel-Hodge CD, Johnston LF, Gizlice Z, et al. Randomized trial of a behavioral weight loss intervention for low-income women: the weight wise program. Obesity. 2009;17(10):1891–1899. doi: 10.1038/oby.2009.128. [DOI] [PubMed] [Google Scholar]
- 29.Ockene IS, Tellez TL, Rosal MC, et al. Outcomes of a Latino community-based intervention for the prevention of diabetes: The Lawrence Latino diabetes prevention project. American Journal of Public Health. 2012;102(2):336–342. doi: 10.2105/AJPH.2011.300357. [DOI] [PMC free article] [PubMed] [Google Scholar]