Abstract
We report measurements of the absolute threshold of cone vision, which has been previously underestimated due to sub-optimal conditions or overly strict subjective response criteria. We avoided these limitations by using optimized stimuli and experimental conditions while having subjects respond within a rating scale framework. Small (1′ fwhm), brief (34 msec), monochromatic (550 nm) stimuli were foveally presented at multiple intensities in dark-adapted retina for 5 subjects. For comparison, 4 subjects underwent similar testing with rod-optimized stimuli. Cone absolute threshold, that is, the minimum light energy for which subjects were just able to detect a visual stimulus with any response criterion, was 203 ± 38 photons at the cornea, ∼0.47 log units lower than previously reported. Two-alternative forced-choice measurements in a subset of subjects yielded consistent results. Cone thresholds were less responsive to criterion changes than rod thresholds, suggesting a limit to the stimulus information recoverable from the cone mosaic in addition to the limit imposed by Poisson noise. Results were consistent with expectations for detection in the face of stimulus uncertainty. We discuss implications of these findings for modeling the first stages of human cone vision and interpreting psychophysical data acquired with adaptive optics at the spatial scale of the receptor mosaic.
Keywords: cones, absolute threshold, sensitivity, signal detection theory, uncertainty, detection models, psychophysics
Introduction
The absolute threshold of cone vision, or the minimum light energy required to just detect a visual stimulus with cone photoreceptors, is fundamentally important for understanding and modeling the earliest stages of photopic vision. However, prior measurements have likely yielded overestimates due to suboptimal testing conditions or the use of overly strict subjective response criteria. Adaptive optics now allows psychophysical and physiological experiments with the potential to probe detection and perception at the scale of individual retinal cones (see for example Arathorn, Yang, Vogel, Zhang, Tiruveedhula, & Roorda, 2007; Hofer, Singer, & Williams, 2005; Makous, Carroll, Wolfing, Lin, Christie, & Williams, 2006; Poonja, Patel, Henry, & Roorda, 2005; Putnam, Hofer, Doble, Chen, Carroll, & Williams, 2005; Rossi & Roorda, 2010; Sinchich, Zhang, Tiruveedhula, Horton, & Roorda, 2009). Enormous potential exists to elucidate the impact of the photoreceptor mosaic, as well as subsequent neural limits, on spatial and color vision, but full realization of this potential requires an accurate model of the cone detection process. This is because these experiments yield information about the retinal light distribution, but to specify the link between the retinal mosaic and perception, or to create any model of the visual process that can explain these data (see Brainard, Williams, & Hofer, 2008 for one example), one requires the ensemble of photoreceptor responses.
To obtain cone responses from a retinal light distribution requires detailed knowledge of the detection process governing cone vision. For example it is not possible to determine whether a specific cone will absorb enough light to significantly contribute to detection or perception of a visual stimulus without knowledge of a host of factors, including (but not limited to) quantum efficiency, sensitivity, spatial pooling, and noise. None of these factors are well specified for foveal cone detection. In this paper we take the first step and measure the absolute threshold of foveal cone vision.
While the limits of rod vision have been extensively studied, both psychophysically (Barlow, 1956; Hecht, Shlaer & Pirenne, 1942; Sakitt, 1972; van der Velden, 1946) and electrophysiologically (Baylor, Lamb & Yau, 1979; Baylor, Nunn & Schnapf, 1984; Field & Reike, 2002; Schneeweis, & Schnapf, 2000), much less is known about the ultimate limits of cone detection. Of the numerous previous investigators (including, but not limited to: Dillon & Zegers, 1958; Marriott, 1963; Vimal, Pokorny, Smith & Shevell, 1989; Wesner, Pokorny, Shevell & Smith, 1991) who investigated cone thresholds, Marriott (1963) arguably used the most favorable experimental conditions (reviewed in Donner, 1992) but unfortunately instructed his subjects to use very strict response criteria. This is problematic since it is well known that sensitivity can generally be increased, and detection thresholds reduced, by adopting more lenient response criteria (see Fig 1). For example, Sakitt (1972) has shown in her classic rating scale experiments, that rod thresholds are reduced well below those obtained with the typical ‘yes-no’ report method (used, for example, in the famous experiments of Hecht et al., 1942), simply by allowing subjects to use more lenient response criteria. Her results imply continually increasing access to stimulus information with more lenient response criteria consistent with an effective Poisson noise limit to detection (Sakitt, 1972; Donner, 1992), with this noise source often referred to as ‘dark light’ (Barlow, 1957- note however, that the data are equivocal as to relevant contributions of receptoral, retinal, and central noise to this dark light- Barlow, 1977; also reviewed in Field, Sampath & Reike 2005). This raises the possibility that cone thresholds, like rod thresholds, may be modifiable by changing the detection criterion. If so we would also expect to observe cone thresholds much lower than those previously reported (Marriot, 1963) by having subjects intentionally adopt more lenient subjective response criteria. Here we determine the absolute threshold of cone vision, and the trade-off between sensitivity and detection criterion in this regime, by essentially repeating Sakitt's rating scale experiment with stimuli optimized for cone detection.
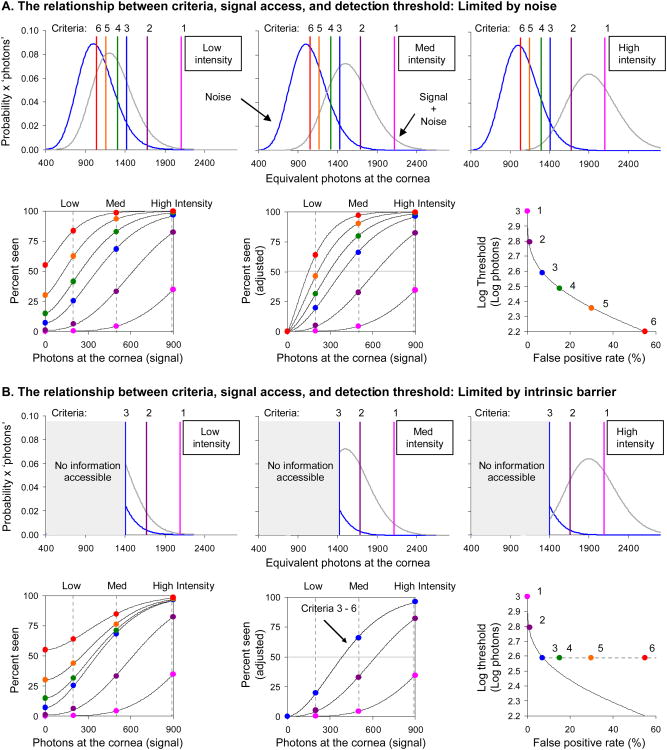
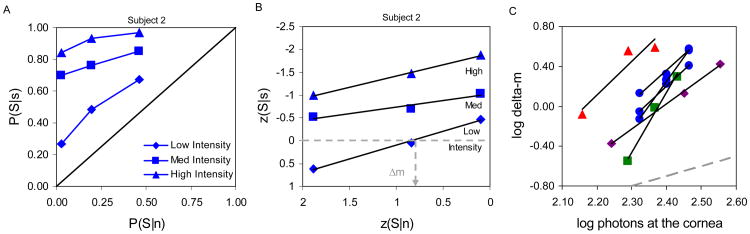
Figure 1.
Signal detection theory (SDT; Green & Swets, 1966) predicts that in distinguishing a signal (grey distribution) from noise (blue distribution) observers can increase sensitivity and lower detection thresholds by using more lenient response criteria (represented by colored vertical lines and points, ranging from pink to red, and numbered 1 (strictest) – 6 (most lenient)). A) Low, medium, and high intensity stimuli (left, center and right; row 1, also indicated by the dashed vertical grey lines in row 2) are detected against irreducible noise. Detection thresholds are interpolated at 50% seen (at the intersection of the solid grey line and the frequency of seeing curves, row 2, center) after adjusting for the non-zero false positive rate as in Equation 1. Detection thresholds are still seen to decrease after this adjustment (row 2, right) indicating a net gain in performance with more lenient criteria. Row 2 right shows the expected Poisson noise-limited reduction in detection threshold (solid curve). B) An intrinsic barrier limits access to signal information (grey boxes, row 1). Subjects cannot reduce the detection criterion below this point. They can continue to increase the false positive rate by guessing, but the rate of seeing and of false positives increase in step and there is no further improvement in threshold (row 2). Row 2, right shows limited threshold reduction (plateau, dashed line) in the face of an intrinsic barrier to information access. All distributions shown are Poisson probability density functions.
The relationship between criteria, signal access, and detection threshold
Before describing the experimental methods and results it is worthwhile to discuss the expected trade-off between sensitivity and detection criterion if the only impediment to cone detection is an effective dark light, as suggested for rods. We will apply the insights of Signal Detection Theory (see Green & Swets, 1966; the familiar reader may wish to skip ahead), which assumes that detection of a visual stimulus takes place against an irreducible background of noise- an appropriate assumption in our case since photoreceptors are known to spontaneously isomerize in the absence of light (Baylor, Matthews & Yau, 1980- rods; Fu, Kefalov, Luo, Xue & Yau, 2008; Rieke & Baylor, 2000- cones). The subject's task is to distinguish between trials where a signal (stimulus) is absent and trials where a signal (stimulus) is present. The average visual response in these two cases is N and S+N respectively, but there is a distribution of responses (shown in Figure 1A, row 1) so that the actual visual response varies from trial to trial. When the average signal greatly exceeds the noise the subject can correctly identify the presence or absence of the stimulus by adopting some suitable response criterion between the two distributions, responding ‘seen’ if the visual response exceeds this criterion, and ‘not seen’ if it does not. However as stimulus intensity decreases, as shown in Figure 1A (top row), the noise and signal plus noise distributions will overlap and the presence or absence of the stimulus on each trial cannot be reliably distinguished. If the subject adopts a high criterion there will be very few false positives, but many missed stimuli. Conversely, a low response criterion leads to fewer missed stimuli, but to more false positives, resulting in increases in both sensitivity (percent seen) and false positive rate (Figure 1A, second row, left). However, sensitivity increases faster than the false positive rate, indicating a net gain in detection performance via increasing access to stimulus information. This can be easily seen by adjusting the percent seen to account for the non-zero false positive rate as follows (Figure 1A, second row, center panel):
| (1) |
Where p(S|s)* is the percent seen adjusted for the non-zero false positive rate, and p(S|s) and p(S|n) are the measured seen and false positive rates, respectively. After this adjustment, often called a guessing correction because it removes the effects of chance guessing (as shown further below), sensitivity is still seen to increase (Figure 1A, second row, middle), demonstrating a true increase in performance. If the sensitivity increase were due to chance guessing alone the adjusted curves shown in the middle panel of the second row of Figure 1A would collapse onto a single curve. The improvement in detection performance can further be seen if we calculate a detection threshold, which we will define throughout this paper as the stimulus intensity required for 50% seeing after adjusting for the false positive rate as describe in Equation 1. This is akin to taking the stimulus intensity required for half of the stimuli to be ‘correctly seen’. Figure 1A (second row, right) shows that the detection thresholds obtained in this manner depend on the response criterion such that ever higher false positive rates are associated with ever lower detection thresholds. In this sense, provided nothing impedes the subject from continually lowering the response criterion, an absolute threshold in the strict sense will not exist, since even one quantum (or the neural equivalent), given a great many trials, will still be detected at a rate higher than chance. We may still refer to an ‘absolute threshold’ in a looser sense though, that is, the lowest threshold subjects can be practically induced to achieve.
The situation just described is an ideal case with detection limited only by noise. We now consider a non-ideal case where full information may not be available, for example, the presence of an intrinsic barrier below which the subject is not able to access any information when deciding how to respond on each trial (Figure 1B, first row). Figure 1B, second row shows that when the visual response (due to either noise alone or the signal plus noise) exceeds this intrinsic barrier, the subject's response frequencies and false positive rates vary with changes in the subjective criterion just as in the ideal detection scenario described above. But, below this barrier the frequency of seeing can only be increased by effectively guessing. In this case sensitivity and the false positive rate increase in step and there is no net gain in terms of the detection threshold (Figure 1B, second row, center and right). The intrinsic barrier prevents the visual system from accessing all information about the stimulus that it could if detection were limited by the noise alone.
We can derive more specific predictions for the trade-off of sensitivity with increasing false positive rate by noting that both the noise and the signal plus noise distributions are appropriately described as Poisson (at least at the level of the cone quantum catch) when detecting a stimulus encoded in a noisy cone mosaic. If detection is optimal, in the sense of being impeded by nothing other than the irreducible Poisson noise, the probability of detection as a function of criteria is given by:
| (2) |
Where P gives the probability of reporting ‘seen’ with a response criterion C for a stimulus causing S average isomerizations given N average noise events, and where the actual values of s and n vary from flash to flash as a result of quantum fluctuations. The relationship between threshold and false positive rate for this ideal case (given by Equation 2) is shown in Figure 1 (A&B, row 2, right, solid black curve). It is worthwhile to note that this relationship is invariant to the strength of both the signal and the noise, so if detection in cone vision is effectively dark light-limited, thresholds and false positive rates should vary similarly with changes in the detection criterion. On the other hand, failure of subjects' data to conform to this expectation would imply some impediment to detection that cannot be expressed in terms of an equivalent Poisson dark light. This would necessarily indicate an inability to make optimal use of the stimulus information encoded in the cone mosaic.
Methods
Subjects
We measured the absolute threshold of cone vision and its dependence on subjective response criterion with a rating scale method in five subjects. Subjects 1 and 2 were the authors, subjects 4 and 5 were naïve subjects and subject 3 was a practiced psychophysical observer but naïve to the purposes of the study. Subjects 1, 2, and 4 as well as an additional naïve subject (subject 6) participated in a similar rod threshold experiment. Subjects 1, 2, and 4 also participated in two-alternative forced-choice (2AFC) experiments for comparison. Subjects' ages were 36, 32, 31, 35, 20, and 22 years, respectively. All subjects had normal vision correctable to at least 20/20. The research followed the tenets of the Declaration of Helsinki and all subjects gave informed consent after an explanation of the study procedure and any possible risks. All study procedures were approved by the Institutional Review Board of the University of Houston.
Stimuli
Cone detection
Stimuli for cone detection were small, brief (34 msec; 6 msec for subject 6), monochromatic (550 nm, 40 nm bandwidth) spot stimuli displayed through a 2 mm artificial pupil. This wavelength is approximately at the peak of the eye's photopic spectral sensitivity function while the duration is within Bloch's interval for complete temporal summation in cone vision (50-100 msec; Karn, 1936; Marriott, 1963; Sperling & Jolliffe, 1965), hence maximizing cone detection. The extent of complete spatial summation in cone vision is not precisely known, but believed to be no larger than the smallest stimulus that can be projected to the retina with normal optics (Davila & Geisler, 1991). For this reason, we used point stimuli created by backlighting a 25 μm pinhole subtending ∼0.2′ with a broadband halogen light source in conjunction with interference and neutral density filters. Retinal extent for these stimuli when well refracted is limited by diffraction and ∼1′ full-width at half maximum (verified by convolving the stimulus aperture with point-spread functions derived from measured ocular aberrations, see Appendix A.2). This stimulus size is appropriate for maximizing cone detection since it is approximately as small as possible with natural optical conditions.
To favor cone-mediated detection, stimuli were monocularly presented to the central fovea after at least 10 minutes of dark adaptation in an otherwise completely dark visual field, save for two small dim white fixation dots presented in Maxwellian view. Subjects fixated midway between these two dots, which were separated by 2.25°. This fixation target was chosen to minimize interference at the fovea without introducing large fixation errors. Reported accuracy of fixation for similar targets is 3-6′ (Rattle, 1969) which is not significantly different from that reported by Ditchburn (1973) for fixation of small central fixation targets. The fixation target was kept just bright enough for accurate fixation so as to minimize any impact on detection. An auditory cue preceded each stimulus (or interval for 2AFC) by 250 msec. A preliminary experiment on subject 1 indicated this was an appropriate cue-stimulus interval for facilitating detection.
Subjects wore their habitual spectacle correction (subject 2), or were corrected with either trial lenses (subjects 3 and 5) and/or by translating a movable stage in a badal optometer. Thresholds for subjects 2, 3, and 5 were corrected for light loss at lens surfaces by assuming 1% overall loss in antireflection coated spectacle lenses, or 4% light loss at each trial lens surface. No dilating or cycloplegic agents were used, since subjects' natural pupils were always larger than the 2 mm artificial pupil. This small pupil size also provided a large depth of focus, minimizing the potential impact of accommodative drifts and fluctuations on the experimental results (see Appendix A.2).
Rod detection
Experimental conditions for rod detection were similar to those for cone detection, except that rod-optimized stimuli were used. After at least 30 minutes of dark adaptation subjects viewed a 490 nm, 27′ diameter, 34 msec spot presented 11° temporal to a single dim red fixation spot. This stimulus size and duration are within the window of complete spatial (Hallett, 1963; Sakitt, 1971) and temporal summation (Baumgardt & Hillmann, 1961) for rod detection while the wavelength is approximately that of peak sensitivity for rods. The red fixation spot was chosen to allow accurate fixation by foveal cones while minimizing any potential interference in rod detection.
Psychophysical procedure
General
We employed two experimental paradigms to measure rod and cone detection thresholds, rating scale and 2AFC, described in more detail below. In both cases trials were self-paced. Although trials were self-paced, the rate of repetition was effectively constrained by software to be less than ∼1.4 Hz. A preliminary experiment on one subject (subject 1) indicated that thresholds obtained with this rate were not significantly different from those measured when imposing a longer four second minimum inter-stimulus interval (indicating no significant adaptation effects). After practice trials and a preliminary experiment to determine the appropriate range of stimulus energies to sample the psychometric curve, the experiment was performed in blocks of 80-160 trials each consisting of stimuli at 4 randomly interleaved intensities, one of which was blank. Timing and presentation were controlled by a custom Matlab program (The Mathworks, Natick, MA) incorporating Psychophysics Toolbox extensions (Brainard, 1997; Pelli, 1997). Subjects typically performed four to five trial blocks per session with frequent breaks when needed.
Stimulus energy at the eye's pupil was measured without intervening neutral density and interference filters with the integrating function of a Newport 1930C power meter. Attenuating factors for each filter were determined in separate measurements. After adjusting for interference and neutral density filters, stimulus energy at each intensity level was converted to quantal values (i.e. photons at the cornea) with the following relationship: photons = energy in Joules × stimulus peak wavelength × 5.0341 × 1015 J−1 m−1. For some sessions only relative intensity information was collected.
Rating scale response method
The rating scale method has been used and described before in acoustics (Egan, Schulman, & Greenberg, 1959) and vision science (Nachmias, & Steinman, 1963; Sakitt, 1972; Swets, Tanner, & Birdsall, 1961; Weintraub, & Hake, 1962). In this method the subject uses multiple response categories rather than just a single all or none (yes-no) response. Subjects were instructed to rate each stimulus on a scale from ‘1’ to ‘6’ indicating the subjective certainty of seeing a test flash as follows: 1 ≈ definitely not seen, 2 ≈ not seen but uncertain, 3 ≈ seen but uncertain, 4 ≈ dim flash seen, 5 ≈ moderate flash seen, and 6 ≈ bright flash seen. No feedback was provided. After practice the final rating scales varied slightly, e.g. some subjects felt more comfortable using only five ratings instead of six, or attached slightly different meanings to the particular ratings. As long as subjects could use the ratings consistently, and used ratings that resulted in a variety of false positive rates, the particular number of ratings and the internal meaning a subject associated with each rating were not relevant to the experimental results.
Subjects practiced until they were comfortable with the rating task and could perform it consistently; practice trials were excluded from data analysis. Rating use was evaluated for consistency by inspection of histograms of the response distributions. Subjects were typically able to consistently respond within the rating framework after two to three trial blocks. Some subjects required additional practice trials to reach their best performance on the detection task; these trials were also excluded from data analysis. The raw cone rating data accepted for analysis for all subjects is provided in Table A2 in Appendix A.4.
Frequency of seeing curves were created for each response criterion, C2 to C6 (C2, rating of 2 or more – the lowest criterion; C3, rating of 3 or more, etc.) after adjusting for the false positive rate using Equation 1. We then calculated the threshold stimulus intensity for each response criterion by fitting the adjusted frequency of seeing data to Weibull functions and interpolating at 50% seen. As already mentioned, the thresholds determined in this manner are invariant to the rate of chance guessing, so that if a difference in threshold persists even after this adjustment it reflects a true increase in the extent to which the subject was able to make use of the information encoded in the retinal mosaic. Since we also make the same adjustment to the various model outputs (described later), there is no impediment to proper interpretation of the results.
Subjects 1 through 5 participated in the cone rating scale experiment. The total number of trials included in analysis for each subject was approximately 5700, 600, 800, 800, and 250, respectively. Subjects 1, 2, 4, and 6 participated in the rod rating scale experiment. The total number of trials included in analysis for each subject was approximately 5300, 1600, 2900, and 1300, respectively. Variation in the number of trials reflects differences in subject stamina and availability, all data for all subjects after the point at which performance stabilized was considered in the analysis.
2Afc
2AFC is a criterion-free method of determining thresholds where, in our case, the stimulus is presented in one of two temporal intervals. Observers record the interval thought to most likely have contained the stimulus. 2AFC testing was performed with both rod and cone stimuli, with stimulus parameters as described above. As with the rating scale method, stimuli were presented at four intensity levels, one of which was blank. Auditory feedback was provided. This feedback was randomized for the blank trials. 2AFC thresholds were taken as the stimulus intensity required for 75% correct. Subjects 1 and 4 participated in the cone 2AFC experiment (∼800 and 500 trials), and subjects 1, 2, and 4 participated in the rod 2AFC experiment (∼500, 120, and 200 trials).
Results
Absolute cone threshold
The absolute threshold of cone vision, or the lowest threshold possible with any response criterion, was 203 ± 38 photons at the cornea averaged across four subjects (see Table 1; absolute values were not known for subject 4, but the relative change in threshold is shown in Figure 4). This is ∼0.47 log units lower than the 606 photons at the cornea reported by Marriott (1963) for nine subjects. This discrepancy most likely reflects the use of extremely strict (zero false positives) response criteria by his subjects. Because the most lenient criterion is not the same for all subjects, Table 1 also shows the criterion-free 2AFC thresholds predicted from the rating scale data using the method described by Sakitt (1972; as per her equation 15). The 2AFC thresholds were predicted at 75% correct, roughly the stimulus intensity with discriminability (d′) of 1 (Gescheider, 1997).
Table 1.
Cone absolute threshold, i.e. the threshold number of photons at the cornea when using the most lenient response criterion (middle column). Thresholds were interpolated at 50% probability of detection after adjusting for the non-zero false positive rate with Equation 1. The average threshold for Marriott's (1963) nine subjects, who used strict response criteria, is also shown for comparison. Values for subject 1 reflect measurements on three different days. Data for subject 4 are not included because only relative stimulus intensity information was available. To facilitate comparison across different subjects, who may have employed different internal criteria, we also report predicted 2AFC thresholds derived from empirical rating scale data using the method described by Sakitt (1972). The probability of correctly detecting stimuli in the 2AFC task is equivalent to the area under the receiver operating characteristic curve (Green & Swets, 1966), here we predict 2AFC threshold at 75% correct, roughly equivalent to the stimulus intensity with d' of 1 (Gescheider, 1997).
| Cone Thresholds (photons at the cornea) | Predicted 2AFC Thresholds (photons at the cornea) | |
|---|---|---|
| Subject 1 | 212 ± 21 | 220 ± 15 |
| Subject 2 | 169 | 168 |
| Subject 3 | 252 | 250 |
| Subject 5 | 178 | 213 |
| Average | 203 ± 38 | 213 ± 34 |
| Marriott | 606 ± 126 | n/a |
Figure 4.
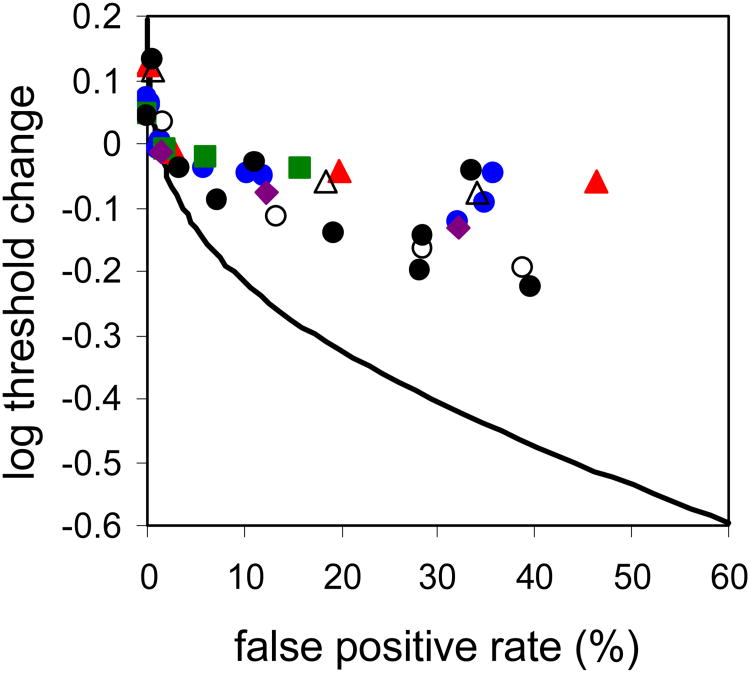
Rod thresholds for our four subjects (Subjects 1 (blue circles), 2 (red triangles), 4 (purple diamonds) and 6 (orange squares)) and Sakitt's three subjects BS, LF and KD (grey squares, diamonds and triangles; Sakitt 1972). As in Figure 3, to better compare across subjects, curves were shifted vertically with the aid of a detection model (described in detail in the Discussion) so that the 1% false positive point, which loosely reflects a typical ‘yes-no’ threshold, coincides with a log threshold change of zero. Thresholds are significantly more responsive to reductions in response criteria for rods than for cones; range for cone data shown as light gray lines).
Cone thresholds cannot be arbitrarily lowered by reducing the response criterion
In addition to specifying the minimum energy required to just detect a visual stimulus in cone vision, i.e. the cone absolute threshold, we measured the trade-off between sensitivity and false positive rate to asses whether one can obtain increasingly more stimulus information by reducing the response criteria, as has been described in rods (Sakitt, 1972; Teich, Prucnal, Vannucci, Breton & McGill, 1982). Figure 2 shows, for one subject (subject 2), that performance in cone detection is only modestly improved with more lenient response criteria after a certain point. Figure 3 shows, for all subjects, how cone thresholds change when criterion is reduced as a function of the associated increase in false positive rate. For each subject the thresholds associated with the two most lenient criteria, which were determined after adjusting for the non-zero false positive rate with Equation 1 as described in the Methods, were not significantly different (based on the 95% confidence limits returned from Weibull fits to the frequency of seeing data). This stands in contrast to behavior previously reported for rod detection (Sakitt, 1972, Teich, et al, 1982) and suggests an absolute threshold to cone vision in the strictest sense.
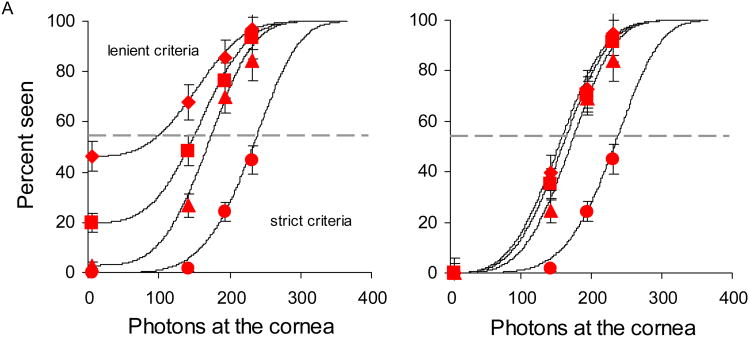
Figure 2.
A) Frequency of seeing curves for subject 2 for criteria C2 (diamonds), C3 (squares), C4 (triangles) and C5 (circles), ranging from lenient to strict. B) After adjusting for the non-zero false positive rate using Equation 1 the curves for the two most lenient criteria, C2 and C3, collapse onto each other, indicating no net benefit to detection. Solid curves are nonlinear least-squares Weibull fits to the frequency of seeing data.
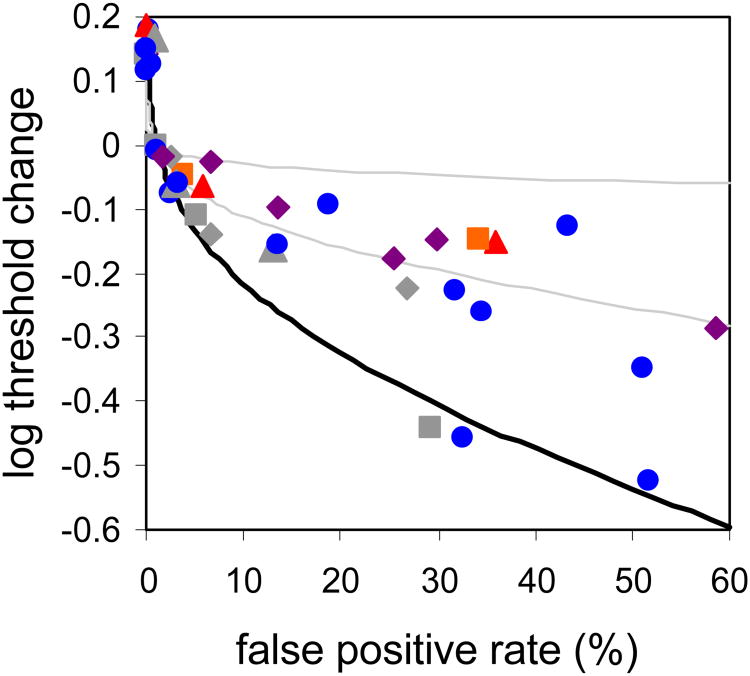
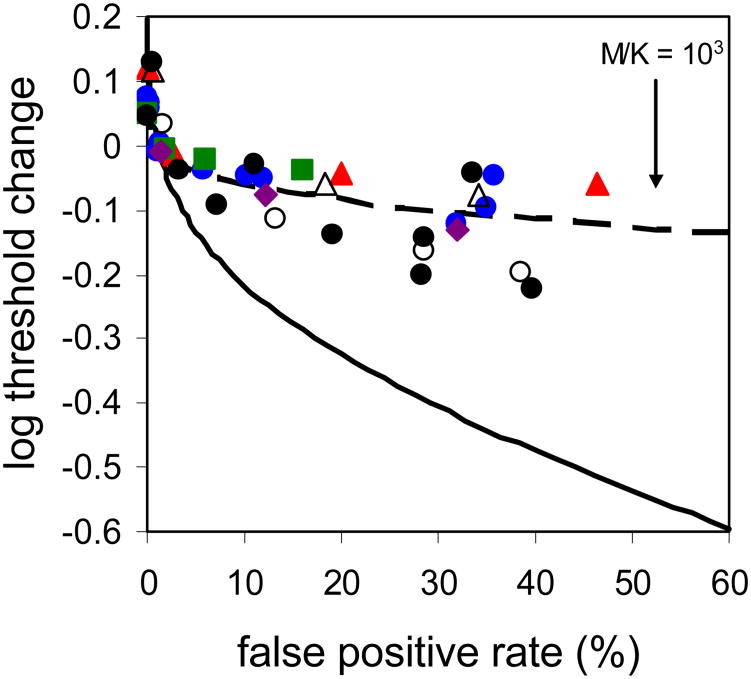
Figure 3.
Change in cone threshold as a function of false positive rate for subjects 1 (blue circles), 2 (red triangles), 3 (green squares) & 4 (purple diamonds).To better compare across subjects curves were shifted vertically with the aid of a detection model (described in detail in the Discussion section) so that the 1% false positive point, which loosely reflects a typical ‘yes-no’ threshold, coincides with a log threshold change of zero. Subjects' cone thresholds are significantly less responsive to reductions in the response criteria than expected for a linear Poisson noise-limited process (solid black line). Filled black circles are three additional trial runs for subject 1 where only relative and not absolute stimulus intensity information was available. Open symbols are the results for subjects 1 and 2 with an adapting background that are described later in the Discussion section.
In Figure 3 we chose to display the threshold reduction as a function of false positive rate, instead of subjective response criterion, because the false positive rate reflects the internal criterion yet is also easily and directly observable. For comparison, we also show the expected reduction in threshold (determined with the same adjustment for non-zero false positive rate as described for the subject data) with more lenient criteria if cone detection follows the ideal strategy given a noisy cone mosaic limited only by an effective dark light (which is a linear Poisson noise-limited process, described by Equation 2; solid black line). The discrepancy between this prediction and subjects' actual behavior is striking. Cone thresholds are much less responsive to changes in the detection criterion than expected for a dark light-limited process. Thresholds generally depart from the Poisson noise-limited expectation once observers adopt a criterion resulting in a false positive rate of more than ∼5%, and there is little substantial benefit with increases in the false positive rate beyond 10-20%.
To assist in quantitatively comparing data across subjects and conditions we estimated threshold reduction between the 1% and 30% false positive rates. This choice of endpoints is admittedly arbitrary, but since these points loosely correspond to subjects' performance on ‘yes-no’ and 2AFC tasks, respectively, it seems a reasonable choice. The estimated threshold reduction for different subjects over this range varied from 0.05 to 0.20 log units, substantially less than the ∼0.4 log units predicted by the Poisson model of detection for a linear, noise-limited system (solid curve in Figure 3). These results indicate that absolute threshold in cone vision is not ultimately limited by Poisson noise, and is therefore not compatible with optimal extraction of stimulus information from a noisy ensemble of photoreceptor responses.
Before further discussion of the potential factors underlying the observed behavior and their implications, we first address several potentially confounding methodological factors to evaluate whether they may have inadvertently contributed to the results.
Stimulus size and variability coupled with inhomogeneity in the retinal mosaic
The inhomogeneity in spatial sensitivity across the cone mosaic increases the variability in the number of photons absorbed by the retinal mosaic beyond that expected by the quantum nature of light alone. Although negligible for large stimuli, this inhomogeneity becomes increasingly more important when stimuli approach the scale of the underlying retinal receptors. For example, with very small stimuli (assuming they could be delivered to the retina) whether they fall directly on a cone or in between cones will have a large impact on whether or not they are seen. Monte Carlo simulations using a spatial model of retinal sensitivity and light capture similar to that described by Hofer et al., (2005) indicated that the stimuli used in the cone detection task (550 nm, ∼1′ full width at half maximum) were sufficiently large that the spatial variation in retinal sensitivity due to the grain of the photoreceptor mosaic could be safely ignored, and hence this cannot account for the failure of subjects' data to conform to the Poisson noise-limited predictions generated with Equation 2. Similar simulations incorporating variability in the retinal profile of the stimulus due to fluctuations in accommodation during the experiment indicated that fluctuations in optical quality during the experiment were also unlikely to significantly impact the experimental results (more details are provided in Appendices A.1 and A.2). Note, however, that for smaller stimuli, such as can be produced with adaptive optics, these factors would no longer be negligible.
Difficulty of the rating scale task is not a limiting factor
Another possibility is that our subjects were, for one reason or another, incapable of effectively employing the rating scale method for evaluating thresholds in cone vision. We addressed this concern with two additional experiments. First, we performed the rating scale experiment on a subset of subjects with rod stimuli and compared results with prior work (Sakitt, 1972). We then compared detection threshold measurements made with the simpler 2AFC method to predictions based on the rating scale data for a subset of subjects.
Rod thresholds
Subjects 1, 2, 4, and 6 participated in rod threshold testing using a rating scale method and a stimulus optimized for rod detection (see Methods). Sakitt (1972) has shown with the same stimuli and methods that rod thresholds can be reduced by ∼0.4 log units below typical ‘yes-no’ thresholds simply by adopting more lenient detection criteria, supporting a dark light limit to rod detection (as suggested in Barlow, 1956 and 1957). Table 2 lists rod thresholds with the most lenient detection criterion for our four subjects and for Sakitt's three subjects. The two data sets are remarkably consistent. Since rod thresholds, unlike cone thresholds, are highly dependent on subjective response criteria, comparison across subjects is aided by calculating the expected 2AFC threshold from the rating data (per Sakitt 1972). Note that there was no significant difference between our subjects and her subjects on this measure, with a predicted 2AFC threshold of ∼50 photons at the cornea in both cases.
Table 2.
Rod ‘absolute’ threshold, i.e. the threshold number of photons at the cornea when using the most lenient response criterion (middle column). Sakitt's (1972) average rod threshold for three subjects is shown for comparison. Values reported for subjects 1 and 4 are the mean and range for two data sets. To facilitate comparison across subjects the criterion-free 2AFC thresholds predicted from each subject's rating scale data using the method described in Sakitt (1972) are also shown (right column). Note the agreement in both data sets.
| Rod Threshold at lowest criterion (photons at the cornea) | Predicted 2AFC Thresholds (photons at the cornea) | |
|---|---|---|
| Subject 1 | 44 (27 - 62) | 47 (23 - 71) |
| Subject 2 | 70 | 74 |
| Subject 4 | 54 (46 - 62) | 55 (51 - 59) |
| Subject 6 | 32 | 32 |
| Average | 50 ± 16 | 52 ± 17 |
| Sakitt | 42 ± 15 | 50 ± 8 |
Figure 4 shows the relative change in rod threshold with increasing false positive rate for our four subjects and includes the dark light expectation (the linear Poisson noise-limited model described by Equation 2; solid curve) and data from Sakitt's three subjects (gray symbols, calculated from data reported in Sakitt, 1972). The range for the cone threshold data from Figure 3 is also included (light gray lines) to allow easier comparison of rod and cone threshold behavior. While rod thresholds for our subjects, on average, did not entirely meet the dark light expectation (see Table 3) they were significantly more responsive to changes in response criterion than cone thresholds (0.08 ± 0.04 vs 0.17± 0.05 average threshold reduction for cones and rods respectively going from 1% to 30% false positive rate, p = 0.04).
Table 3.
The slope of the cone detection, z-transformed ROC curve (σN/σSN) for each stimulus strength, and the exponent values (ρ) describing the rate of change of discriminability (Δm) with the stimulus strength (Δm ∝ Sρ) for cone and rod detection. Cone and rod values reported for subject 1 are the mean and standard deviation across 6 and 4 data sets, respectively. Rod values reported for subject 4 are the mean and range for 2 data sets. Cone ROC slopes for subject 1 only and averaged across all subjects were significantly less than 1 (p < 0.05*, p < 0.005**; unpaired, 2-tailed t-test). ρ was greater for cone than rod detection, but in both cases significantly higher than 1 (p = 0.03, cones; p = 0.02, rods). High Intensity ROC slope for subject 5 could not be calculated due to 100% seeing frequency. Also shown is the average ρ value calculated for Sakitt's three subjects from the detection data reported in Sakitt (1972).
| ROC slope = σN/σSN (cone detection) | Exponent (ρ) (where Δm ∝Sρ) | ||||
|---|---|---|---|---|---|
| Condition | Low intensity | Medium intensity | High intensity | Cones | Rods |
| Subject 1 | 0.77 ± 0.19* | 0.62 ± 0.17** | 0.56 ± 0.12** | 2.99 ± 0.99* | 1.60 ± 0.37 |
| Subject 2 | 0.60 | 0.28 | 0.50 | 3.36 | 1.64 |
| Subject 3 | 0.52 | 0.31 | 0.41 | 5.91 | n/a |
| Subject 4 | 0.69 | 0.55 | 0.65 | 2.51 | 1.99 (1.52 - 2.47) |
| Subject 5 | 0.73 | 0.28 | n/a | 2.03 | n/a |
| Subject 6 | n/a | n/a | n/a | n/a | 1.33 |
| Average | 0.66 ± 0.10** | 0.41 ± 0.16** | 0.53 ± 0.10** | 3.36 ± 1.51* | 1.64 ± 0.27* |
| Sakitt, 1972 | n/a | n/a | n/a | n/a | 1.18 ± 0.22 |
Practice
After following up cone threshold testing with rod threshold testing, where behavior was closer to that expected for a dark light-limited process, we wondered if the improved threshold responses in rods compared with cones could be due simply to prolonged experience with ratings (a practice effect). To investigate this, subject 1 repeated cone testing (solid black circles in Figure 3, sessions 4-6 in Table A2) and subject 4, formerly having only done rod testing, underwent threshold testing in cones (purple diamonds in Figure 3). We found that subject 1, despite additional practice, could not significantly improve the cone threshold response to more lenient criteria (threshold reduction for the first data set 0.078±0.032, threshold reduction for the second set taken after extended practice: 0.131±0.079, p-value = 0.3372). Subject 4′s threshold reduction in cones (0.13, one session) was similar to the other subjects, despite having undergone the rod testing first, and hence having more overall practice with the rating scale task prior to first acquiring cone data. Our general observation is that extended practice is beneficial in rod detection, but has only a limited effect, if any, on cone detection. We conclude that practice effects, specifically a lack of adequate practice, cannot explain the limited responsiveness of cone thresholds observed when subjects intentionally adopt more lenient response criteria.
Comparison of rating scale and 2AFC methods
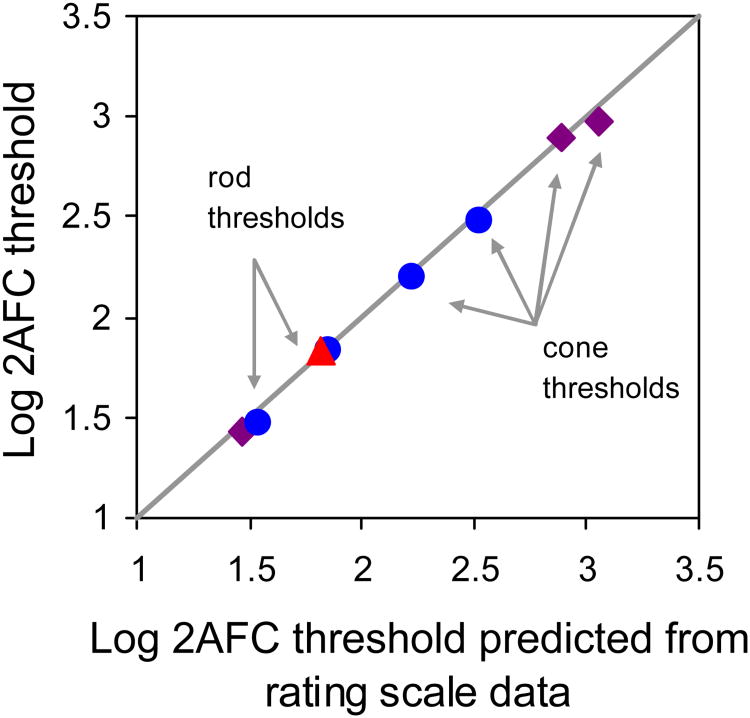
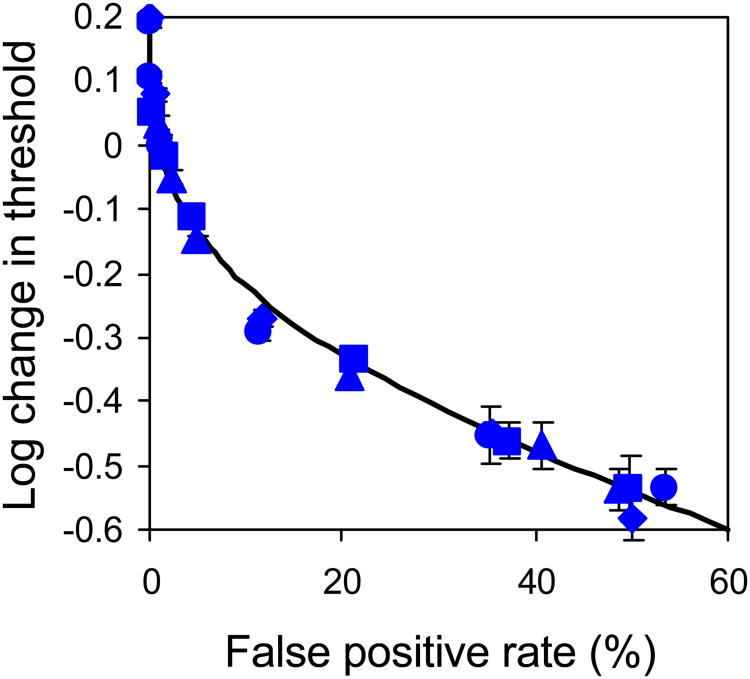
To further ensure that the cognitive burden of responding within the rating scale framework was not somehow limiting the increase in sensitivity achievable by relaxing the response criterion, we compared predictions based on the rating scale data to thresholds measured with the simpler 2AFC method for a subset of subjects (subjects 1, 2, and 4, see Figure 5). The results for both rod and cone detection fall along the unit line, indicating that the results of the more cognitively complex, criterion-dependent rating scale method are consistent with the results of the simpler, criterion-free 2AFC method. We conclude that subjects could effectively perform the rating scale task and that neither its complexity, nor factors such as the potential for criterion variability that are intrinsic to the rating task (see Appendix A3), can offer an alternative methodological explanation for the limited cone threshold reduction we observe.
Figure 5.
Measured 2AFC thresholds compared with predictions from rating scale data for subjects 1 (blue circles), 2 (red triangle) and 4 (purple diamonds). Thresholds were interpolated at 75% seen. Both rod and cone data lie along the line of unit slope, indicating high agreement between measured and predicted thresholds. This demonstrates that the complexity of the rating scale task did not adversely impact thresholds. Data shown for subjects 2 and 4 reflect relative and not absolute thresholds, since absolute stimulus intensity information was not available for these subjects. The prediction of 2AFC thresholds from rating data is described in detail in Sakitt (1972).
Receiver operating characteristic analysis: cone thresholds are inconsistent with a linear Poisson process
Receiver operating characteristic (ROC) analysis provides additional insight into the factors limiting detection at cone absolute threshold. The probability of correctly detecting a stimulus, P(S|s), is plotted against the false positive probability, P(S|n) to form a receiver operating characteristic curve (ROC curve) from which various criterion-free indices of signal discriminability can be derived (McNicol, 2005; see also Figure 6). We chose to calculate one such index of discriminability, Δm, for each subject and condition. Δm, like the more common d′, is useful because it is a measure of the separation between the noise and the signal plus noise distributions that is independent of the subject's response criterion (see Figure 1). Unlike d′, though, it is applicable to those instances in which the variances of the signal plus noise and noise distributions are not equal, i.e. the ratio σn/σsn ≠ 1, as is generally the case with our data (see Figure 6). Δm is defined by the following equation:
Figure 6.
Receiver operating characteristic (ROC) analysis and the relationship between signal discriminability, Δm, and stimulus intensity for cone detection at absolute threshold. A) Typical ROC curves for subject 2. B) ROC curves for the same subject now on a double probability plot and with a sample estimation of discriminability from linear fits to the low intensity curve - Δm equals z(S|n) on the ROC curve where z(S|s) equals zero (dashed gray lines). On double probability plots slopes (σn/σsn) < 1 imply unequal variance in the noise and signal plus noise distributions; slopes for our subjects are uniformly < 1 (see also Table 3). C) Log Δm vs log stimulus energy for subjects 1 (blue circles), 2 (red triangles), 3 (green squares) and 4 (purple diamonds). Photons at the cornea for subject 4 are relative, all others are absolute. Datasets 4-6 for subject 1, where only relative thresholds were obtained, are not included but are included in Table 3 below. Unit slope, as expected for Poisson noise-limited detection, is shown for comparison (gray dashed line). All subjects demonstrate an acceleration of discriminability with signal strength in cone detection that is inconsistent with a linear Poisson noise-limited process.
| (3) |
Where SN, S and N are the means of the signal plus noise, signal, and noise distributions, respectively, and σn is the standard deviation of the noise distribution, which for Poisson noise is equal to √N. When detection is limited by a linear Poisson process the noise and signal strengths are independent and Δm is directly proportional to signal strength.
For each subject we calculated the dependence of discriminability, Δm, on signal strength from the cone rating data. Discriminability was not directly proportional to, but rather an accelerating function of, signal strength (Δm ∝ Sρ, with ρ > 1), as seen in Figure 6. This behavior has been noted before for increment and contrast thresholds under different experimental conditions (for example: Cohn, Thibos & Kleinstein, 1974; Nachmias & Kocher, 1970; Pelli, 1985; Stromeyer & Klein, 1974); although it has not been described for detection at cone absolute threshold. This lack of proportionality is another indicator that cone detection cannot be properly described by a linear Poisson process, as would be expected if cone detection were limited by an effective dark light. While ρ was significantly greater than 1 for rods as well as cones (one sample t-test, hypothesized mean = 1, p = 0.02 and 0.03, respectively), it was on average much closer to one for rods than for cones. This indicates that rod detection more nearly meets the assumption of proportionality for Poisson noise-limited systems, as can be seen in Table 3.
Discussion
What factors limit detection at cone absolute threshold?
The limited improvement in cone thresholds with more lenient detection criteria and the acceleration of discriminability (Δm) with increasing stimulus strength, indicate that cone detection at absolute threshold is not consistent with linear processing of cone signals limited by Poisson noise, i.e. it is not appropriately described as being limited by an effective dark light. This implies a detection strategy that does not make optimal use of the information present in the cone quantum catch. Two limiting factors consistent with the observed behavior have been identified: nonlinear transduction and uncertainty. In the first case detection is governed by a ‘nonlinear transducer’ function (Foley & Legge, 1981; Nachmias & Kocher, 1970; Nachmias & Sansbury, 1974), a suboptimal processing strategy if the goal is to detect a weak stimulus in Poisson noise. A fixed internal threshold [incidentally, the assumption made by Hecht et al., (1942)], and an accelerating nonlinearity are two examples. In the second, the subject is assumed to have imperfect knowledge about the stimulus and detection is impacted by this uncertainty (Cohn, Thibos & Kleinstein, 1974; Nachmias & Kocher, 1970; Pelli, 1985; Tanner, 1961). For example, a subject either does not know, or cannot remember, exactly where or when the stimulus will occur and so monitors a number of sensory channels, M, only K of which will actually contain stimulus-relevant information (hence the uncertainty).
Although nonlinear transducers and uncertainty have been discussed as if they are mutually exclusive (see attempts to discount one or the other factor in Cohn & Lasley, 1985; Foley and Legge, 1981; Lasley & Cohn, 1981a; Legge & Kersten, 1987), neither explanation has been satisfactorily disproven and a general detection model incorporating both factors can be written. For example, in the model that follows a subject detects a stimulus when the decision variable, DV is equal to or greater than the response criterion, C, with DV given by:
| (4) |
Where rsi and rnj are the responses within the ith and jth stimulus-relevant and stimulus-irrelevant channels respectively, nl is late additive Poisson noise, and β is the Minkowski exponent for summation across channel responses (Quick, 1974 & 1978), β of 1 and infinity refer to linear summation and independent detection (incidentally the optimal strategy when uncertainty exists) across the relevant and irrelevant channels, respectively. Allowing an intermediate, finite, β is a generalization of the classical model of uncertainty with linear transduction, in which detection across relevant and irrelevant channels is independent (described for example in Pelli, 1985). K and M refer to the number of stimulus-relevant and total channels, and together they define the amount of uncertainty (M/K). K also indicates the extent of summation, for example when K =1, the signal is assumed to occupy only a single channel, as would occur when spatio-temporal summation over a stimulus-relevant extent is complete. Nonlinearity in the channel responses is accommodated if the functional form for rs and rn is chosen to depend nonlinearly on each channel's input.
For this model to display an acceleration of discriminability (limited threshold improvement with more lenient response criteria) consistent with our data, one or both of the following must occur: nonlinear transduction with a suitable amount of late additive noise (n1), or uncertainty combined with nonlinear summation (β > 1). Neither nonlinear transduction without late additive noise, nor uncertainty without nonlinear summation, nor nonlinear summation without uncertainty, is sufficient. Multiplicative noise, although not further considered here since its effects are difficult to disentangle from those of additive noise, is also unable to produce an acceleration of discriminability in the absence of at least one of the these factors (see Appendix A.3).
Further insight requires simplifying assumptions, since this general model (as with simpler models: see for example Donner, 1992; and an extensive review of rod threshold data by Field et al., 2005) is not well-constrained by the data, a situation not aided by the broad range in physiological estimates of receptor quantum efficiency and dark noise (which in the case of cone ‘dark noise’, is already outside the range consistent with psychophysical behavior; see Schnapf, Nunn, Meister & Baylor, 1990 and Schneeweis & Schnapf, 1999). We will retain uncertainty, as it is likely present to some extent in the cone detection task given the small simulus size, and its negative impact on detection has been demonstrated (Cohn & Wardlaw, 1985; Davis, Kramer & Graham, 1983- spatial uncertainty; Lasley & Cohn, 1981b-temporal uncertainty; Greenhouse & Cohn, 1978-chromatic uncertainty). The potential role of nonlinear transduction is less clear. Cohn (2003) describes that linear responses have been found at the level of the retinal ganglion cells (Cohn, Green & Tanner, 1975; Levick, Thibos, Cohn, Cantanzarro & Barlow, 1983) and the complex cells of the visual cortex (Tolhurst, Movshon & Dean, 1983), and points out that overall response nonlinearity need not be an inevitable consequence of an early transduction nonlinearity. For example, a response nonlinearity is required in a neuron with limited dynamic range, however if the location of this nonlinearity is adaptable, resulting in locally linear behavior around the mean signal level (in our case corresponding to the non-zero receptor noise), no limit on threshold improvement is expected.
For further insight into the potential role of nonlinear transduction, we performed the same cone detection task on a bright background in subjects 1 and 2. In this case the background may elevate the combined signal, background, and noise above the effective barrier established by the nonlinearity (thereby ‘linearizing’ the response as predicted by Birdsall's theorem, see Klein & Levi, 2009 for a more extensive discussion), resulting in Poisson noise-limited behavior. As shown in Figure 3 (open symbols), adding the bright background (1.8 log photopic trolands; bright enough to elevate detection thresholds into the Weber's regime) did not result in a significant change in the amount of cone threshold reduction achievable by utilizing more lenient response criteria for these two subjects (0.05 versus 0.07 log unit threshold reduction with and without background for subject 2; 0.17 versus a range of 0.05 - 0.20 for subject 1). While this does not necessarily imply purely linear processing, it does imply that any nonlinear transduction must act after the site of light adaptation, with the bulk of the noise associated with cone detection occurring after this site as well [see the following references for detailed discussions of these issues: Cohn & Lasley (1985), Foley & Legge (1981), and Lasley & Cohn (1981a)]. This makes incorporating nonlinear transduction, in our opinion, less attractive since it is known that the predominant noise associated with cone detection is retinal (Borghuis, Sterling & Smith, 2008) and that psychophysical cone thresholds are already lower than expected based on physiological estimates of cone dark noise (Schnapf et al., 1990; Schneeweis & Schnapf, 1999).
From both a simplicity and efficiency standpoint the nonlinear transducer is also less attractive, since it is a decidedly suboptimal strategy for detecting weak stimuli in noise and requires more parameters to account for subject data than a model based on uncertainty alone. While future experiments may allow disentanglement of the roles of uncertainty and non-linear transduction, either by incorporating external noise (common in contrast sensitivity meaurements, as described for example in Goris, Wagemans & Wichmann, 2008 and Goris, Zaenen & Wagemans, 2008), or by analyzing second responses in a multi-alternative forced-choice scheme (as described by Solomon, 2007), for the present we will simplify our detection model by assuming linear transduction without late additive noise. If nonlinear transduction is also present, but not accounted for, then, since it can impact the data in a manner similar to uncertainty, the extent of uncertainty may be overestimated.
Cone absolute threshold data is consistent with detection in the face of uncertainty
Having restricted our attention to linear detection models in the context of uncertainty without later additive noise, we'll simplify further and assume independent channels (β → ∞). This limiting form is both the most efficient, in the sense of representing the optimal detection strategy given some irreducible amount of uncertainty, and requires the fewest free parameters (only two- uncertainty and receptor dark noise) to describe subjects' data. Constraining β to infinity and nl to zero allows both a lower bound to be placed on the amount of uncertainty, and an upper bound on the dark noise associated with cones, consistent with subjects' data.
In this simplified model, which has been applied to various increment threshold and contrast sensitivity scenarios (Cohn, Thibos & Kleinstein, 1974; Nachmias & Kocher, 1970; Pelli, 1985; Tanner, 1961) but not, to our knowledge, to describe cone absolute sensitivity, the overall probability of detecting is given by one minus the probability of not detecting with any of the relevant or irrelevant channels and can be expressed analytically as follows:
| (5) |
Where Ps is the probability that the combination of signal and noise exceeds the subjective criterion in any one of the signal-relevant channels:
| (6) |
And Pn is the probability that noise in any one of the irrelevant channels exceeds subjective criterion:
| (7) |
Note the predictions of this model in terms of the change in detection threshold for a given increase in false positive rate depends only on uncertainty (M/K) and are indifferent to the value of K. Hence, an estimate of the uncertainty governing detection can be derived from the shape of the threshold reduction curve predicted by the uncertainty model without knowing the extent of spatial pooling of stimulus-relevant information at detection threshold. (This is also true for finite β).
Because the predicted impact on threshold behavior varies slowly with changes in uncertainty, it is often easier to describe uncertainty (M/K) by orders of magnitude. Figure 7 shows that cone threshold data are well fit by a detection model with a degree of uncertainty of ∼1,000, or 103 (best fit M/K = 10 for rods, not shown). This value was determined by fitting the uncertainty model described by Equations 5, 6 and 7 to subjects' aggregate detection data by minimizing the root-mean-square difference between data and model curves, with uncertainty (M/K) varying from 1 to 109 in steps of 0.25 log units. Aggregate fits were preferred to an arithmetic mean of uncertainty values obtained from individual fits due to large individual fit uncertainty and to the fact that greater uncertainty leads to smaller changes in the steepness of the model curves, thus potentially biasing towards large values of uncertainty (M/K). (Uncertainty can also be estimated from the ρ values in Table 3, however Monte Carlo simulations indicated the estimates with this method are less accurate). Since these uncertainty estimates were obtained assuming independent detection across stimulus relevant and irrelevant channels without late additive noise, they represent the minimum uncertainty consistent with subjects' detection data given linear transduction.
Figure 7.
Cone threshold behavior is well described by a model of detection incorporating uncertainty, described by Equations 5-7. An uncertainty (M/K) of ∼1000 best fits subjects' aggregate cone detection data: subject 1 (circles), 2 (green squares), 3 (red triangles) and 4 (purple diamonds). Subject 5′s data were not included due to false positive rates too low to allow confident fits. As in Figures 3 and 4, individual subjects' data were shifted so that a false positive rate of 1% (loosely indicating a typical ‘yes-no’ threshold) corresponds to a log threshold change of zero.
Figure 8 shows that the introduction of a given amount of uncertainty elevates thresholds in addition to reducing the responsiveness of thresholds to criterion reductions. In this case, if detection was not impacted by uncertainty but was instead limited only by Poisson noise, we would have observed even lower thresholds. For example at 1% and 30% false positive rate we could have expected thresholds ∼0.26 and 0.57 log units lower, or ∼112 and 55 photons at the cornea, respectively. Interestingly, this would result in nearly equal rod and cone absolute thresholds.
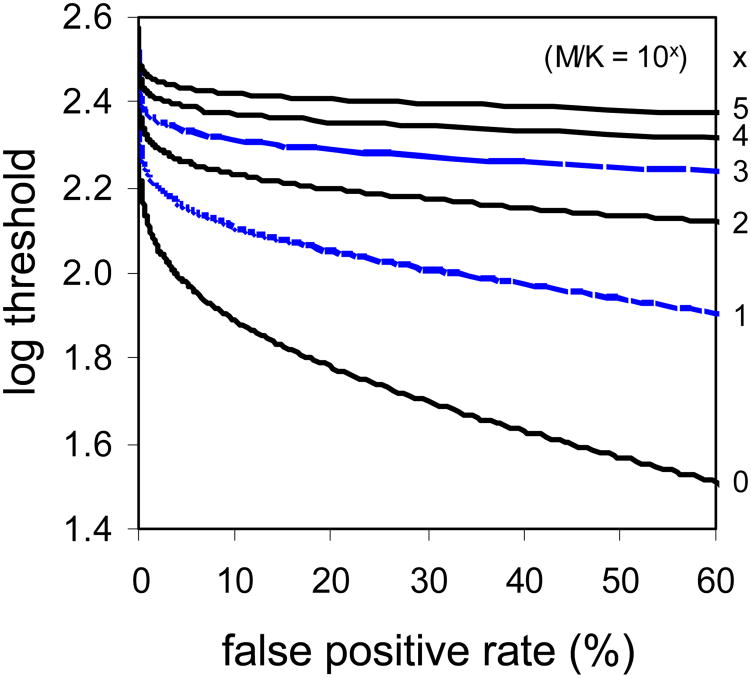
Figure 8.
Uncertainty, M/K, elevates thresholds and decreases the responsiveness of threshold to changes in response criteria (false positive rate). Shown are predictions of how uncertainty impacts thresholds, and the responsiveness of threshold to changes in criteria, for different values of M/K. These curves were calculated from Equations 5-7. Log(M/K) of 3 and 1 best accounted for subjects' aggregate cone and rod data.
We've shown that cone detection is consistent with a variety of scenarios, one of which is optimal detection given both noise and uncertainty (see also Kontsevich & Tyler, 1999 on a distraction model with predictions similar to uncertainty). In this scenario an uncertainty of approximately 3 orders of magnitude is required to account for subjects' data. Spatial uncertainty is expected in the cone detection task if fixation instability impairs the subject's ability to predict retinal stimulus location. In this case, assuming minimal spatial summation, the degree of uncertainty would be approximately equal to the ratio of the area of the fixational instability to the stimulus area. For fixation instability with a reasonable standard deviation∼ 5′ of arc, this would correspond to an uncertainty in cone detection of roughly 25, a value much lower than the uncertainty consistent with cone detection for our subjects (Table 4). However, spatial uncertainty may be larger. Stevenson, Kumar & Roorda (2007) and Kumar (2008) reported imprecision in location judgments (i.e. subjects' ability to identify ‘where they were looking’) about twice as large as fixation instability. If this effect causes spatial uncertainty in cone detection, it would be associated with an uncertainty of ∼100 (again assuming fixation instability with standard deviation of 5′). This is at the lower end of the range for our subjects' cone data, suggesting the intriguing possibility that this ‘inability to know where one is looking’ may represent an irreducible barrier to cone detection. Note that since the circular area of such error would be less than the area of summation in rod vision at our stimulus location, this localization uncertainty would not be expected to impact rod detection. Lastly, it may be that nonlinear transduction is present as a contributing factor and the amount of uncertainty required to explain our subjects' data may be lower. But, due to the required pairing of significant late additive noise (nl) with nonlinear transduction, this would require a decrease in cone dark noise, potentially broadening the discrepancy between psychophysically and physiologically derived estimates.
Table 4.
Log uncertainty (M/K) for both cones and rods. Uncertainty was estimated by fitting threshold data with a detection model incorporating uncertainty, described by Equations 5-7. Subject 5's uncertainty could not be reliably estimated due to a low false positive rate. Cone and rod values reported for subject 1 are the mean and standard deviation across 6 and 4 data sets, respectively. Rod values reported for subject 4 are the mean and range for 2 data sets. Uncertainty (M/K) is significantly higher for cone detection than for rod detection on average (p = 0.03). Since these estimates were obtained assuming independent detection across stimulus relevant and irrelevant channels without late additive noise, they represent the minimum uncertainty consistent with subject's detection data given linear transduction.
| Log M/K | ||
|---|---|---|
| Cones | Rods | |
| Subject 1 | 4.13 ± 2.78 | 1.13 ± 1.16 |
| Subject 2 | 6.50 | 2.25 |
| Subject 3 | 7.00 | n/a |
| Subject 4 | 2.25 | 2.13 (1.26 – 3.00) |
| Subject 6 | n/a | 2.00 |
Relevance for psychophysical modeling
Ultimately we have shown that detection thresholds in cone vision cannot be described as dark light-limited, implying some additional limiting factor beyond that imposed by the cone mosaic. We've shown that stimulus uncertainty, possibly in conjunction with nonlinear transduction, can provide this limit. This has important implications for understanding psychophysics at threshold. For example, it is common practice to estimate an upper bound on the noise associated with detection from the slope of the psychometric function (Barlow, 1956; Donner, 1992; Marriott, 1963), under the assumption that any type of noise results in shallower curves. Under the additional assumption that the main noise source impacting detection is the quantal nature of light, the slope is used to estimate the minimum required stimulus events for detection (Cicerone & Nerger, 1989; Hecht et al., 1942; Marriot, 1963; Pirenne & Marriott, 1955; Sakitt, 1972; Vimal et al., 1989; Williams, MacLeod, & Hayhoe, 1981). However, there are at least two, often overlooked, reasons why it is not appropriate to rely on the slope of the psychometric function for such estimates. First, if the quantum efficiency of photon absorption is not known precisely, then the detection noise, N, can trade-off with the threshold minimum number of signal induced events, S, when fitting empirical data (Barlow, 1956; discussed extensively in Donner 1992). Second, whereas what is traditionally thought of as noise flattens the psychometric function, uncertainty and nonlinear transduction actually steepen the psychometric function by disproportionately affecting detection of weak stimuli (Cohn, 1981; Cohn, Thibos, & Kleinstein, 1974; Nachmias & Sansbury, 1974; Nolte & Jaarsma, 1967; Peterson, Birdsall, & Fox, 1954). As pointed out by Cohn (1981), this steepening will cause an underestimation of the detection noise (or underestimation of quantum efficiency if detection noise is presumed to be known) if estimated solely from the psychometric curve slope. Hence, in the face of uncertainty and/or nonlinear transduction the slope of the detection curve does not provide any reliable estimate of the noise associated with detection (or the minimum number of stimulus events required for detection).
Since uncertainty depends on the psychophysical procedure, e.g. spatial and temporal characteristics, psychophysical models of visual performance may need to be tailored with estimates of uncertainty derived from the specific task. For example, the choice of fixation target, the degree of spatial and temporal predictability (for example Hofer et al., 2005 and Makous et al., 2006 presented stimuli at multiple locations, which may have increased uncertainty), and how a visual stimulus is cued (auditory tone, pedestal) may all impact detection uncertainty. Since uncertainty elevates threshold regardless of subjective criterion, failure to account for it will result in inaccurate estimates of both noise and signal to noise ratios at detection threshold. With high uncertainty, stimuli will have to be made brighter to be seen, which for tiny stimuli (e.g. stimuli created with adaptive optics) will mean that more cones will meaningfully participate in detection than otherwise would have had uncertainty been minimized.
Comparison of rod and cone detection
Isomerizations per receptor at absolute threshold
Cone absolute threshold, in terms of photons at the wavelength of peak sensitivity, is approximately 4× higher than for rods (203 vs 51 photons at the cornea on average, see Table 5). Using reasonable ranges for the quantum efficiency of cones and rods at the eccentricities of our stimuli of 0.11-0.35 and 0.13-0.30, respectively (reviewed by Donner, 1992), this corresponds to 22-71 total cone isomerizations and 7-15 total rod isomerizations at detection threshold (Table 5). Further, using average cone density estimates of 199,000 cones mm−2 at the fovea and 130,000 rods mm−2 at 10 degrees (Curcio, 1990) and retinal stimulus dimensions of 2′ and 27′, assumed to encompass 14 and 1718 cones and rods respectively, we estimate the average number of photons per photoreceptor at threshold to be 2-5 for cones and 0.004-0.009 for rods. (Note that if we had considered individual variation in receptor density these ranges would be somewhat more expanded). These rod threshold values are consistent with estimates derived from Sakitt's (1972) prior work (the only comparable data set due to strict criterion usage by other investigators) after compensating for the unrealistic estimate of rod quantum efficiency of 0.05 that she used in her original calculations (see both Donner's, 1992; and Makous', 1990 re-analysis of Sakitt's original data). However, the cone thresholds are significantly lower than previously reported (Marriot, 1963), most likely due overly strict criterion usage on the part of his subjects. Note that the numerical estimates provided above do not require any assumption regarding either spatial summation, the noise contributing to detection, or any other specific details of the detection model.
Table 5.
Estimates of cone and rod detection parameters. P and I refer to estimates for either complete spatial summation (cone pooling) across the dimensions of the stimulus or independent cone detection. Uncertainty values were obtained from fits to aggregate subject data as described in the Discussion. Stimulus size, cone density, and quantum efficiency used in these calculations are provided in the text. For the noise calculations we assumed that noise is pooled over 100 msec for both rods and cones, and over a spatial extent of 1° (assumed to encompass 8482 rods) for rods. For the cone pooling scenario we assumed that noise is pooled over the extent of the stimulus (2′ estimated to encompass 14 cones).
| Parameter | Cones | Rods |
|---|---|---|
| Threshold at most lenient criterion (photons at the cornea) | 203 ± 38 | 50 ± 16 |
| Total photons absorbed (S) | 22-71 | 7-15 |
| Photons absorbed per receptor | 2-5 | 0.004-0.009 |
| Total noise events summed with the stimulus (N) | 23-253 (P) 7-73 (I) | 4-21 |
| Noise events per receptor per second (dark noise) | 17-180 (P) 5-52 (I) | 0.004-0.025 |
| S/N at absolute threshold | 3.3 – 3.9 (P) | 2.0-2.5 |
| S/N per receptor | 0.9 – 1.1 (P) 1.1 – 1.6 (I) | 0.06 – 0.08 |
| Previous psychophysical estimates of dark noise, (noise events per receptor per second) | 112* 10-100** | 0.011* 0.004-0.11† |
| Previous physiological estimates of dark noise (noise events per receptor per second) | 3800†† | 0.0063‡ 0.008 - 0.225‡‡ |
| Log Uncertainty (Log(M/K)) | 3.0 | 1.0 |
Parameters are arranged from least (top) to most (bottom) required assumptions. For example, total photons absorbed and S/N per receptor both require an assumed quantum efficiency range, but the latter also requires assumptions about summation time and area, and uncertainty- all assumptions are described in the text.
Noise events per receptor
As previously mentioned, in the context of linear transduction, we can provide an upper bound on the receptor dark noise consistent with subjects' behavior by fitting the data with the simplified uncertainty model described by Equations 5-7, since any elaborations to this model (for example allowing later additive noise) result in higher thresholds for given values of quantum efficiency and receptor dark noise. These psychophysically derived dark noise estimates are highly sensitive to the degree of stimulus-relevant spatial pooling assumed at threshold (the parameter K in the model). We assumed rod pooling of 1 degree at the eccentricity of our stimulus (11 deg). Since cone pooling at detection threshold is unknown, we calculated the noise required to account for the measured absolute thresholds for the limiting cases of complete spatial summation over the extent of the stimulus (the optimal strategy) and complete cone independence. The rod dark noise estimates ranged from 0.004 to 0.025 events per rod per second. Cone dark noise estimates ranged from 17 to 180 events per cone per second, if spatial summation over the stimulus is assumed complete, to 5 to 52 events per cone per second if cones detect the stimulus independently.
Our estimates of rod noise are consistent with prior psychophysically- and electrophysiologically-derived estimates (see Table 5). Our estimates of cone noise are also consistent with previous psychophysical estimates (Donner, 1992; Barlow, 1958); especially if we take into account the effects of uncertainty and differing assumptions about the number of cones illuminated by the stimulus). However, cone noise estimates are at least 1-2 orders of magnitude lower than electrophysiological estimates, with even greater discrepancy under the independent cone detection scenario (which may argue somewhat against this scenario's plausibility).
The discrepancy between psychophysically- and electrophysiologically-derived estimates of cone noise is already known (Donner, 1992; Schneeweis & Schnapf, 1999), so that we too observe a discrepancy is not surprising. It is possible that there is a degree of calculation inaccuracy associated with the physiological estimates, given the difficulty in estimating the psychophysically relevant cone dark noise from electrophysiological data. Another possibility is that we have either underestimated the quantum efficiency of cone detection or overestimated the temporal window over which noise is pooled, resulting in an underestimation of the dark noise required to account for our psychophysical data. Even if cone noise is not summed over the 100 msec we assumed, but instead limited to the 34 msec duration of the stimulus, the quantum efficiency of cone detection would have to be as high as 0.86 for our psychophysical estimates to be consistent with the 3800 noise events per cone per second estimated by Schneeweis & Schnapf (1999). Since this is well outside the physically reasonable range for the quantum efficiency of cone detection, we conclude that this does not offer a plausible explanation for the discrepancy between the psychophysical and physiological estimates of cone noise. This discrepancy remains perplexing, and suggests that unless the cone noise in vivo is substantially different from physiologically derived estimates to date, the visual system's strategy for cone detection must be highly efficient, attaining close to optimal performance given uncertainty, with little significant impact of nonlinearity in response or additional late stage noise.
Spatial summation
One difficulty in estimating the noise associated with detection (cone dark noise) at absolute threshold is that the area of complete spatial summation in the fovea is not known. This is because the eye's optics limit the minimum stimulus size that can be displayed to the fovea, so previous estimates provide only an upper bound on the true extent of foveal cone pooling at threshold (Davila & Geisler, 1991; Glezer, 1965; Lie, 1981). Thus, the number of cones participating in the detection process and the amount of noise they contribute cannot be accurately determined. Consequently, in Table 5 we present calculations for two limiting scenarios, complete spatial summation over the extent of the stimulus and cone independence. Further constraint awaits improved estimates of the extent of cone pooling in the dark adapted fovea at detection threshold, such as may be obtainable in the future by investigating areal summation with adaptive optics, a technique that allows stimuli to be delivered to the retina roughly twice as small as with conventional means. Dalimier and Dainty (2010) recently took the first step in this direction by investigating spatial summation in light adapted foveal cones with adaptive optics, with their data suggesting a minimum pooling extent larger than a single cone, although the potential influence of uncontrolled optical factors could not be excluded. Note that if cones are found to be able to detect independently with sufficiently small retinal stimuli, provided that detection uncertainty increases no more than expected based on the change in stimulus size, our data suggest that the sensitivity of cone vision, in terms of total photons absorbed by the retina, may even surpass that of rods.
Conclusions
Absolute cone sensitivity has been previously underestimated due to the requirement that subjects use strict response criteria to minimize errors in detection. With a rating scale method and carefully optimized experimental conditions we found the absolute foveal cone threshold to be 203 ± 38 photons at the cornea, on average. These results were consistent with the results of 2AFC measurements on the same subjects. Our results suggest that 2-5 photons per photoreceptor are required for detection by foveal cones and that cone thresholds are less responsive than rod thresholds to reductions in the response criteria. The limited threshold reduction and the observed lack of proportionality between discriminability and signal strength indicate that cone detection cannot be properly described as a dark light-limited process, and implies a limit to detection beyond that imposed by the cone mosaic. Stimulus uncertainty, either alone or in conjunction with nonlinear transduction, can describe the observed behavior. It may be necessary to account for uncertainty when developing task-specific psychophysical models as different experimental paradigms include different amounts of uncertainty.
Acknowledgments
We thank the anonymous reviewers for helpful comments, and we thank Hope Queener for software assistance. These results were partially reported in poster form at the Association for Research in Vision and Ophthalmology, 2009. This work has been supported by National Institute of Health Grants, RO1 EY019069, T32 EY007024 and P30 EY07551, and the Minnie F. Turner Memorial Fund for Impaired Vision Research Award.
A.1 Discrete sampling and spatial sensitivity variation in the retinal mosaic
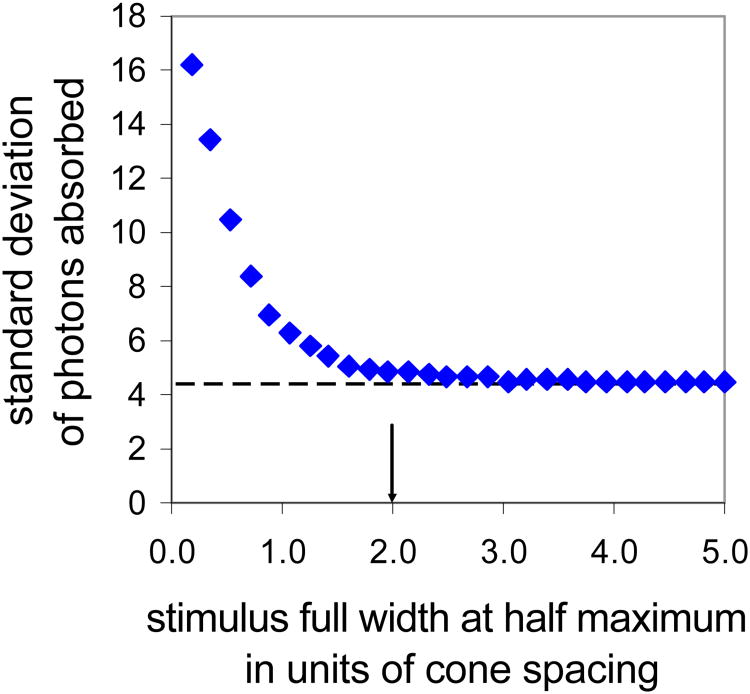
The variation in spatial sensitivity of the cone mosaic (i.e. the loss of light between cones) increases the variability in the number of photons absorbed by the retinal mosaic beyond that expected by the quantum nature of light alone. This variability is negligible for large stimuli but becomes increasingly more important when stimuli approach the scale of the underlying retinal receptors. We performed Monte Carlo simulations of the detection process using a model based on that described by Hofer et al., (2005) that included realistic photoreceptor locations, Gaussian cone apertures with full width at half maximum of 0.6 average cone spacing (Qi, 1996), stimuli with Gaussian retinal profiles, and photon absorption governed by Poisson statistics. The results of these simulations, shown in Figure A1, indicate that as long as the retinal stimulus has a full width at half height greater than ∼2 cone diameters the variance in the number of photons absorbed will be essentially the same as expected from a purely Poisson process. Additional Monte Carlo simulations indicated that the cone stimulus size used did not impact either the threshold, or the slope of the psychometric function. We used a foveal cone stimulus with a full width at half height of ∼1′ on the retina (∼2′ full width), this is 2-3 foveal cone diameters and large enough that the effects of discrete sampling and spatial sensitivity variation in the cone mosaic can be safely ignored. These simulations demonstrated that the use of Equation 2 remains valid at the spatial scale of our stimuli.
Figure A1.
Monte Carlo simulations indicate that the 1′ full width at half maximum cone detection stimulus is large enough to ignore the impact of spatial variation in the sensitivity of the cone mosaic on detection. Stimulus size is reported as the full width at half maximum in units of cone spacing, with the cone stimuli ranging from ∼2-3 units of cone spacing depending on subjects' foveal cone density. For these stimulus sizes actual variability in absorbed photons (blue diamonds) approaches that due to Poisson quantum variability (dashed line) alone. Error bars (± 1 SD) are the size of the symbols and are not included; each symbol represents the average of 5 simulated runs with 1500 trials per run.
A.2 The impact of accommodative fluctuations
Fluctuations in accommodation could impact detection behavior if they cause either a decrease in the number of photons absorbed or increase in the variability of the number of photons absorbed. We assessed the potential impact of accommodative fluctuations by performing Monte Carlo simulations, similar to those described in Appendix A.1, with realistically varying estimates of the stimulus profile obtained from wavefront sensor measurements. Wavefront sensing over a 6 mm pupil was used to measure short (within trial) and long term variability in accommodation and higher order aberrations for two subjects (subjects 1 and 2), under conditions similar to those employed during the cone threshold experiment. The range of accommodative change was found to be similar in both subjects. We calculated time-resolved point spread functions (PSFs) for a 2 mm pupil from the measured wave aberrations, and then convolved these PSFs with the known stimulus profile to obtain estimates of the retinal stimulus profile and its variation from trial to trial.
The mean and variance of the number of photons absorbed for 1500 trials that included random presentation of the individual retinal stimulus profiles was compared with that using the average profile. The mean number of photons absorbed varied by no more than ∼0.4%, and the variance was on average only ∼2% higher for the individual retinal stimulus profiles than with the average profile. These results suggest that fluctuations in accommodation and optical quality during the experiment had a negligible impact on the number and variability of photons absorbed across trials.
A.3 Cascaded noise, Signal quantization, and Criterion variability
We also explored whether any of several other alternative explanations could account for the limited cone threshold reduction observed when our subjects used increasingly more lenient response criteria without requiring the additional assumption of either nonlinearly responding channels or uncertainty. These included: multiple sources of noise acting in series, signal quantization, and uncertainty in the use of the subjective response criterion.
Cascaded noise
Monte Carlo simulations indicated that the relationship between threshold and the false positive rate in a linear system limited by Poisson noise (as described Equation 2), remains unchanged even with the addition of multiple, cascaded sources of Poisson noise (see Figure A2), as might be the case if there are multiple sources of quantum noise in the visual system relevant to the detection task. This is true regardless of the nature of summation across channels.
Signal quantization
We also used Monte Carlo simulation to investigate the potential effect of signal compression and quantization on threshold behavior, in an otherwise linear system limited by Poisson noise, both alone and in combination with multiple cascaded noise sources. This, too, was found not to affect the shape of the theoretical noise-limited relationship between detection threshold and the false positive rate, as shown in Figure A2.
Criterion uncertainty
In signal detection theory the signal strength and the strength of the perceptual response are assumed to vary randomly, usually in a Poisson fashion. Generally though, the value of the subjective response criterion is assumed to be constant. In reality it is unlikely that the subjective criterion is constant even within trials (Nachmias & Kocher, 1970; Wickelgren, 1968). We used Monte Carlo simulation to investigate the impact of criterion uncertainty on threshold behavior by allowing the value of C in Equation 2 to vary randomly from trial to trial in a Poisson manner. We found that criterion uncertainty has no impact on the expected relationship between detection threshold and the false positive rate in a Poisson noise-limited system (see Figure A2).
Figure A2.
Modeling of cascaded noise sources (squares), signal compression and quantization (diamonds), and subjective criterion uncertainty (triangles) reveals how they do not change the expected behavior for Poisson noise-limited detection processes (control (circles) and solid curve). Error bars are SD of log threshold for 5 simulated runs at 300 trials each for each scenario.
We conclude that none of these factors – multiple sources of cascaded noise, signal quantization and compression, or subjective criterion uncertainty - can explain the observed limit on threshold reduction.
A.4 Cone rating data
Table A2. The total trial counts and number of stimuli placed in each rating category are shown for each stimulus type (blank, weak, medium and strong) for each subject. Also shown are either the absolute or relative (blue italics) stimulus energy measurements reported as photons at the cornea. An asterisk (*) in the photons column indicates values after a correction for estimated light loss due to reflections from antireflection coated spectacle lenses (1%, subjects 2 and 4) or trial lenses (8%, subjects 3 and 5). Trials with reported ratings of ‘1’ were judged as definitely not seen and trials with reported ratings of 2 to 6 were judged to be seen with increasing confidence. In general the ratings ‘2’ and ‘3’ were used when the subject was uncertain as to whether or not the stimulus was seen and ratings of ‘4’, ‘5’, or ‘6’ reflected subjectively increasing brightness. Non-zero blank intensities are reported because the method used to control the illumination through the pinhole directed some residual light even on the darkest setting. The values reported, generally at least 2.5 log units lower that the lowest stimulus values used, are upper estimates on the maximum light at the cornea during blank presentations, the real values possibly being less due to uncertainties in accurately measuring the stimulus intensities at the lowest light levels. These non-zero blank values were used in fitting subjects' frequency of seeing curves and interpolating thresholds, but false positive rates were left uncorrected since the required correction was insignificantly small compared with the inherent uncertainty in the data.
| Subject 1 - Ratings - Absolute Sensitivity, 1 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Intensity | Photons | Total | 1 | 2 | 3 | 4 | 5 | 6 |
| Blank | 5 | 240 | 75 | 81 | 59 | 24 | 1 | 0 |
| Weak | 210 | 241 | 38 | 47 | 63 | 76 | 17 | 0 |
| Medium | 251 | 239 | 20 | 31 | 43 | 93 | 40 | 12 |
| Strong | 292 | 240 | 10 | 21 | 23 | 90 | 66 | 30 |
| Subject 1 - Ratings - Absolute Sensitivity , 2 | ||||||||
| Intensity | Photons | Total | 1 | 2 | 3 | 4 | 5 | 6 |
| Blank | 5 | 241 | 156 | 71 | 10 | 3 | 1 | 0 |
| Weak | 210 | 239 | 101 | 48 | 36 | 34 | 19 | 1 |
| Medium | 251 | 240 | 61 | 34 | 38 | 73 | 26 | 8 |
| Strong | 292 | 240 | 28 | 14 | 40 | 61 | 74 | 23 |
| Subject 1 - Ratings - Absolute Sensitivity , 3 | ||||||||
| Intensity | Photons | Total | 1 | 2 | 3 | 4 | 5 | 6 |
| Blank | 5 | 239 | 167 | 49 | 21 | 2 | 0 | 0 |
| Weak | 210 | 235 | 62 | 59 | 46 | 54 | 13 | 1 |
| Medium | 251 | 251 | 44 | 36 | 46 | 73 | 45 | 7 |
| Strong | 292 | 235 | 9 | 11 | 28 | 85 | 80 | 22 |
| Subject 1 - Ratings - Absolute Sensitivity , 4 | ||||||||
| Intensity | Photons | Total | 1 | 2 | 3 | 4 | 5 | 6 |
| Blank | 5 | 365 | 220 | 42 | 77 | 26 | 0 | 0 |
| Weak | 229 | 362 | 112 | 29 | 114 | 104 | 3 | 0 |
| Medium | 339 | 364 | 81 | 24 | 84 | 149 | 26 | 0 |
| Strong | 431 | 369 | 16 | 5 | 32 | 182 | 134 | 0 |
| Subject 1 - Ratings - Absolute Sensitivity , 5 | ||||||||
| Intensity | Photons | Total | 1 | 2 | 3 | 4 | 5 | 6 |
| Blank | 5 | 151 | 108 | 14 | 24 | 5 | 0 | 0 |
| Weak | 229 | 150 | 84 | 8 | 43 | 14 | 1 | 0 |
| Medium | 364 | 128 | 36 | 7 | 27 | 47 | 11 | 0 |
| Strong | 477 | 171 | 23 | 10 | 37 | 74 | 27 | 0 |
| Subject 1 - Ratings - Absolute Sensitivity , 6 | ||||||||
| Intensity | Photons | Total | 1 | 2 | 3 | 4 | 5 | 6 |
| Blank | 4 | 179 | 119 | 40 | 19 | 1 | 0 | 0 |
| Weak | 157 | 197 | 72 | 33 | 83 | 8 | 1 | 0 |
| Medium | 202 | 159 | 20 | 12 | 90 | 34 | 3 | 0 |
| Strong | 263 | 185 | 6 | 4 | 53 | 109 | 13 | 0 |
| Subject 2 - Ratings - Absolute Sensitivity | ||||||||
| Intensity | Photons* | Total | 1 | 2 | 3 | 4 | 5 | 6 |
| Blank | 5 | 136 | 73 | 36 | 23 | 4 | 0 | 0 |
| Weak | 153 | 139 | 45 | 27 | 30 | 35 | 2 | 0 |
| Medium | 208 | 157 | 23 | 14 | 10 | 72 | 33 | 5 |
| Strong | 249 | 134 | 4 | 5 | 12 | 53 | 45 | 15 |
| Subject 3 - Ratings - Absolute Sensitivity | ||||||||
| Intensity | Photons* | Total | 1 | 2 | 3 | 4 | 5 | 6 |
| Blank | 5 | 207 | 173 | 17 | 13 | 4 | 0 | 0 |
| Weak | 193 | 193 | 122 | 26 | 9 | 26 | 8 | 2 |
| Medium | 231 | 208 | 106 | 15 | 11 | 36 | 33 | 7 |
| Strong | 269 | 192 | 66 | 17 | 14 | 38 | 41 | 16 |
| Subject 4 - Ratings - Absolute Sensitivity | ||||||||
| Intensity | Photons* | Total | 1 | 2 | 3 | 4 | 5 | 6 |
| Blank | 7 | 203 | 138 | 40 | 22 | 3 | 0 | 0 |
| Weak | 654 | 203 | 98 | 51 | 29 | 20 | 5 | 0 |
| Medium | 1055 | 192 | 60 | 28 | 42 | 51 | 11 | 0 |
| Strong | 1344 | 202 | 15 | 22 | 38 | 101 | 26 | 0 |
| Subject 5 - Ratings - Absolute Sensitivity | ||||||||
| Intensity | Photons* | Total | 1 | 2 | 3 | 4 | 5 | 6 |
| Blank | 10 | 62 | 58 | 3 | 0 | 1 | 0 | 0 |
| Weak | 276 | 62 | 15 | 7 | 6 | 32 | 2 | 0 |
| Medium | 402 | 64 | 9 | 0 | 6 | 37 | 12 | 0 |
| Strong | 503 | 60 | 0 | 0 | 2 | 32 | 26 | 0 |
| Subject 1 - Ratings - Threshold Elevating Background | ||||||||
| Intensity | Photons | Total | 1 | 2 | 3 | 4 | 5 | 6 |
| Blank | 37 | 119 | 73 | 12 | 18 | 14 | 2 | 0 |
| Weak | 2393 | 135 | 34 | 11 | 24 | 52 | 14 | 0 |
| Medium | 3275 | 119 | 11 | 3 | 8 | 47 | 50 | 0 |
| Strong | 4362 | 127 | 3 | 1 | 2 | 27 | 94 | 0 |
| Subject 2 - Ratings - Threshold Elevating Background | ||||||||
| Intensity | Photons* | Total | 1 | 2 | 3 | 4 | 5 | 6 |
| Blank | 37 | 179 | 118 | 28 | 32 | 1 | 0 | 0 |
| Weak | 1141 | 174 | 89 | 24 | 53 | 8 | 0 | 0 |
| Medium | 1727 | 167 | 33 | 15 | 90 | 29 | 0 | 0 |
| Strong | 2393 | 176 | 9 | 6 | 66 | 83 | 12 | 0 |
Footnotes
Commercial relationships: none.
References
- Arathorn DW, Yang Q, Vogel CR, Zhang Y, Tiruveedhula P, Roorda A. Retinally stabilized cone-targeted stimulus delivery. Optics Express. 2007;15(21):13731–13744. doi: 10.1364/oe.15.013731. [DOI] [PubMed] [Google Scholar]
- Barlow HB. Retinal noise and absolute threshold. Journal of the Optical Society of America. 1956;46(8):634–639. doi: 10.1364/josa.46.000634. [DOI] [PubMed] [Google Scholar]
- Barlow HB. Increment thresholds at low intensities considered as signal/noise discriminations. Journal of Physiology. 1957;136:469–488. doi: 10.1113/jphysiol.1957.sp005774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow HB. Visual Problems of Colour. II. London: Her Majesty's Stationery Office; 1958. Intrinsic noise of cones; pp. 617–630. [Google Scholar]
- Barlow HB. Retinal and central factors in human vision limited by noise. In: Barlow HB, Fatt P, editors. Vertebrate Photoreception. San Fransisco, California: Academic Press; 1977. [Google Scholar]
- Baumgardt E, Hillmann B. Duration and size as determinants of peripheral retinal response. Journal of the Optical Society of America. 1961;51(3):340–344. doi: 10.1364/josa.51.000340. [DOI] [PubMed] [Google Scholar]
- Baylor DA, Lamb TD, Yau KW. Responses of retinal rods to single photons. Journal of Physiology. 1979;288:613–634. [PMC free article] [PubMed] [Google Scholar]
- Baylor DA, Matthews G, Yau KW. Two components of electrical dark noise in toad retinal rod outer segments. Journal of Physiology. 1980;309:591–621. doi: 10.1113/jphysiol.1980.sp013529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor DA, Nunn BJ, Schnapf JL. The photocurrent, noise and spectral sensitivity of rods in the monkey Macaca fascicularis. Journal of Physiology. 1984;357:575–607. doi: 10.1113/jphysiol.1984.sp015518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghuis BG, Sterling P, Smith RG. Loss of sensitivity in an analog neural circuit. Journal of Neuroscience. 2009;29(10):3045–3058. doi: 10.1523/JNEUROSCI.5071-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard DH. The Psychophysics Toolbox. Spatial Vision. 1997;10:433–436. [PubMed] [Google Scholar]
- Brainard DH, Williams DR, Hofer H. Trichromatic reconstruction from the interleaved cone mosaic: Bayesian model and the color appearance of small spots. Journal of Vision. 2008;8(5):1–23. doi: 10.1167/8.5.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicerone CM, Nerger JL. The relative numbers of long-wavelength-sensitive to middle-wavelength-sensitive cones in the fovea centralis. Vision Research. 1989;29(1):115–128. doi: 10.1016/0042-6989(89)90178-8. [DOI] [PubMed] [Google Scholar]
- Cohn TE. Absolute threshold: analysis in terms of uncertainty. Journal of the Optical Society of America. 1981;71(6):783–785. doi: 10.1364/josa.71.000783. [DOI] [PubMed] [Google Scholar]
- Cohn TE. Thresholds and noise. In: Chalupa LM, Werner JS, editors. The Visual Neurosciences. Vol. 1. Cambridge, Massachusetts: MIT Press; 2003. pp. 811–824. [Google Scholar]
- Cohn TE, Green DG, Tanner WP. Receiver operating characteristic analysis. Application to the study of quantum fluctuation effects in optic nerve of Rana pipiens. Journal of General Physiology. 1975;66:583–616. doi: 10.1085/jgp.66.5.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn TE, Lasley DJ. Site of the accelerating nonlinearity underlying luminance-change detection. Journal of the Optical Society of America A. 1985;2(2):202–205. doi: 10.1364/josaa.2.000202. [DOI] [PubMed] [Google Scholar]
- Cohn TE, Wardlaw JC. Effect of large spatial uncertainty on foveal luminance increment detectability. Journal of the Optical Society of America A. 1985;2(6):820–825. doi: 10.1364/josaa.2.000820. [DOI] [PubMed] [Google Scholar]
- Cohn TE, Thibos LN, Kleinstein RN. Detectability of a luminance increment. Journal of the Optical Society of America. 1974;64(10):1321–1327. doi: 10.1364/josa.64.001321. [DOI] [PubMed] [Google Scholar]
- Curcio CA, Sloan KR, Kalina RE, Hendrickson AE. Human photoreceptor topography. Journal of Comparative Neurology. 1990;292:497–523. doi: 10.1002/cne.902920402. [DOI] [PubMed] [Google Scholar]
- Dalimier E, Dainty C. Role of ocular aberrations in photopic spatial summation in the fovea. Optics Letters. 2010;35(4):589–591. doi: 10.1364/OL.35.000589. [DOI] [PubMed] [Google Scholar]
- Davila KD, Geisler WS. The relative contributions of pre-neural and neural factors to areal summation in the fovea. Vision Research. 1991;31(7/8):1369–1380. doi: 10.1016/0042-6989(91)90058-d. [DOI] [PubMed] [Google Scholar]
- Davis ET, Kramer P, Graham N. Uncertainty about spatial frequency, spatial position, or contrast of visual patterns. Perception & Psychophysics. 1983;33(1):20–28. doi: 10.3758/bf03205862. [DOI] [PubMed] [Google Scholar]
- Dillon DJ, Zegers RT. Quantal determination and statistical evaluation of absolute foveal luminosity thresholds and of threshold variability. Journal of the Optical Society of America. 1958;48(12):877–883. doi: 10.1364/josa.48.000877. [DOI] [PubMed] [Google Scholar]
- Ditchburn RW. Eye-movements and visual perception. Oxford: Clarendon Press; 1973. [Google Scholar]
- Donner K. Noise and the absolute threshold of cone and rod vision. Vision Research. 1992;32(5):853–866. doi: 10.1016/0042-6989(92)90028-h. [DOI] [PubMed] [Google Scholar]
- Egan J, Schulman AI, Greenberg GZ. Operating characteristics determined by binary decisions and ratings. Journal of the Acoustical Society of America. 1959;31(6):768–773. [Google Scholar]
- Field GD, Rieke F. Mechanisms regulating variability of the single photon responses of mammalian rod photoreceptors. Neuron. 2002;35:733–747. doi: 10.1016/s0896-6273(02)00822-x. [DOI] [PubMed] [Google Scholar]
- Field GD, Sampath AP, Rieke F. Retinal processing near absolute threshold: From behavior to mechanism. Annual Review of Physiology. 2005;67:491–514. doi: 10.1146/annurev.physiol.67.031103.151256. [DOI] [PubMed] [Google Scholar]
- Foley JM, Legge GE. Contrast detection and near-threshold discrimination in human vision. Vision Research. 1981;21:1041–1053. doi: 10.1016/0042-6989(81)90009-2. [DOI] [PubMed] [Google Scholar]
- Fu Y, Kefalov V, Luo DG, Xue T, Yau KW. Quantal noise from human red cone pigment. Nature Neuroscience. 2008;11(5):565–571. doi: 10.1038/nn.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gescheider GA. Psychophysics: The Fundamentals. New Jersey: Lawrence Erlbaum Associates; 1997. [Google Scholar]
- Glezer VD. The receptive field of the retina. Vision Research. 1965;5:497–525. doi: 10.1016/0042-6989(65)90084-2. [DOI] [PubMed] [Google Scholar]
- Goris RLT, Wagemans J, Wichmann FA. Modelling contrast discrimination data suggest both the pedestal effect and stochastic resonance to be caused by the same mechanism. Journal of Vision. 2008;8(15):1–21. doi: 10.1167/8.15.17. [DOI] [PubMed] [Google Scholar]
- Goris RLT, Zaenen P, Wagemans J. Some observations on contrast detection in noise. Journal of Vision. 2008;8(9):1–15. doi: 10.1167/8.9.4. [DOI] [PubMed] [Google Scholar]
- Green Dm, Swets JA. Signal detection theory and psychophysics. New York: Wiley; 1966. [Google Scholar]
- Greenhouse DS, Cohn TE. Effect of chromatic uncertainty on detectability of a visual stimulus. Journal of the Optical Society of America. 1978;68(2):266–267. doi: 10.1364/josa.68.000266. [DOI] [PubMed] [Google Scholar]
- Hallett PE. Spatial summation. Vision Research. 1963;3:9–24. [Google Scholar]
- Hecht S, Shlaer S, Pirenne Mh. Energy, quanta, and vision. Journal of General Physiology. 1942;25:819–840. doi: 10.1085/jgp.25.6.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer H, Singer B, Williams DR. Different sensations from cones with the same photopigment. Journal of Vision. 2005;5:444–454. doi: 10.1167/5.5.5. [DOI] [PubMed] [Google Scholar]
- Karn HW. Area and the intensity-time relation in the fovea. Journal of General Physiology. 1936;14:360–369. [Google Scholar]
- Klein SA, Levi DM. Stochastic model for detection of signals in noise. Journal of the Optical Society of America A. 2009;26(11):B110–B126. doi: 10.1364/JOSAA.26.00B110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontsevich Ll, Tyler Cw. Distraction of attention and the slope of the psychometric function. Journal of the Optical Society of America A. 1999;16(2):217–222. doi: 10.1364/josaa.16.000217. [DOI] [PubMed] [Google Scholar]
- Kumar G. The retinal origin: On the indepence of retinal loci targeted by saccades, pursuits and fixation and their relationship to the retinal point of perceived fixation Ph D dissertation. University of Houston - College of Optometry; Houston, TX: 2008. [Google Scholar]
- Lasley DJ, Cohn T. Why luminance discrimination may be better than detection. Vision Research. 1981a;21:273–278. doi: 10.1016/0042-6989(81)90121-8. [DOI] [PubMed] [Google Scholar]
- Lasley DJ, Cohn T. Detection of a luminance increment: effect of temporal uncertainty. Journal of the Optical Society of America. 1981b;71(7):845–850. doi: 10.1364/josa.71.000845. [DOI] [PubMed] [Google Scholar]
- Legge GE, Kersten D. Contrast discrimination in noise. Journal of the Optical Society of America A. 1987;4(2):391–404. doi: 10.1364/josaa.4.000391. [DOI] [PubMed] [Google Scholar]
- Levick WR, Thibos LN, Cohn TE, Catanzaro D, Barlow HB. Performance of cat retinal ganglion cells at low light levels. Journal of General Physiology. 1983;82:405–426. doi: 10.1085/jgp.82.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lie I. Visual detection and resolution as a function of adaptation and glare. Vision Research. 1981;21:1793–1797. doi: 10.1016/0042-6989(81)90213-3. [DOI] [PubMed] [Google Scholar]
- Makous W. Absolute sensitivity. In: Hess RF, Sharpe LT, Nordby K, editors. Night vision. Cambridge: Cambridge University Press; 1990. pp. 146–176. [Google Scholar]
- Makous W, Carroll J, Wolfing JI, Lin J, Christie N, Williams DR. Retinal microscotomas revealed with adaptive-optics microflashes. Investigative Ophthalmology and Visual Science. 2006;47:4160–4167. doi: 10.1167/iovs.05-1195. [DOI] [PubMed] [Google Scholar]
- Marriott FHC. The foveal absolute visual threshold for short flashes and small fields. Journal of Physiology. 1963;169:416–423. doi: 10.1113/jphysiol.1963.sp007267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNicol D. A primer of signal detection theory. New Jersey: Lawrence Erlbaum Associates, Inc., Publishers; 2005. [Google Scholar]
- Nachmias J, Kocher Ec. Visual detection and discrimination of luminance increments. Journal of the Optical Society of America. 1970;60(3):382–389. doi: 10.1364/josa.60.000382. [DOI] [PubMed] [Google Scholar]
- Nachmias J, Sansbury RV. Grating contrast: Discrimination may be better than detection. Vision Research. 1974;14:1039–1042. doi: 10.1016/0042-6989(74)90175-8. [DOI] [PubMed] [Google Scholar]
- Nachmias J, Steinman RM. Study of absolute visual detection by the rating-scale method. Journal of the Optical Society of America. 1963;53(10):1206–1213. doi: 10.1364/josa.53.001206. [DOI] [PubMed] [Google Scholar]
- Nolte LW, Jaarsma D. More on the detection of one of M orthogonal signals. Journal of the Acoustical Society of America. 1967;41(2):497–505. [Google Scholar]
- Pelli DG. Uncertainty explains many aspects of visual contrast detection and discrimination. Journal of the Optical Society of America A. 1985;2(9):1508–1532. doi: 10.1364/josaa.2.001508. [DOI] [PubMed] [Google Scholar]
- Pelli DG. The VideoToolbox software for visual psychophysics: Transforming numbers into movies. Spatial Vision. 1997;10:437–442. [PubMed] [Google Scholar]
- Peterson WW, Birdsall TG, Fox WC. The theory of signal detectability. Transactions of the IRE Professional Group on Information Theory. 1954;4:171–212. [Google Scholar]
- Pirenne MH, Marriott FHC. Absolute threshold and frequency-of-seeing curves. Journal of the Optical Society of America. 1955;45:909–912. doi: 10.1364/josa.45.000909. [DOI] [PubMed] [Google Scholar]
- Poonja S, Patel S, Henry L, Roorda A. Dynamic visual stimulus presentation in an adaptive optics scanning laser ophthalmoscope. Journal of Refractive Surgery. 2005;21(5):575–580. doi: 10.3928/1081-597X-20050901-30. [DOI] [PubMed] [Google Scholar]
- Putnam NM, Hofer Hj, Doble N, Chen L, Carroll J, Williams Dr. The locus of fixation and the foveal cone mosaic. Journal of Vision. 2005;5:632–639. doi: 10.1167/5.7.3. [DOI] [PubMed] [Google Scholar]
- Qi X. Spatial summation and antagonism of foveal cone signals at different illuminances in the human retina Ph D dissertation. University of Rochester; Rochester NY: 1996. [Google Scholar]
- Quick RF. A vector magnitude model of contrast detection. Kybernetik. 1974;16:65–67. doi: 10.1007/BF00271628. [DOI] [PubMed] [Google Scholar]
- Quick RF, Mullins WW, Reichert TA. Spatial summation effects on two-component grating thresholds. Journal of the Optical Society of America. 1978;68(1):116–121. doi: 10.1364/josa.68.000116. [DOI] [PubMed] [Google Scholar]
- Rattle JD. Effect of target size on monocular fixation. Optica Acta. 1969;16(2):183–192. doi: 10.1080/713818162. [DOI] [PubMed] [Google Scholar]
- Reike F, Baylor DA. Origin and functional impact of dark noise in retinal cones. Neuron. 2000;26:181–186. doi: 10.1016/s0896-6273(00)81148-4. [DOI] [PubMed] [Google Scholar]
- Rossi EA, Roorda A. The relationship between visual resolution and cone spacing in the human fovea. Nature Neuroscience. 2010;13(2):156–157. doi: 10.1038/nn.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakitt BS. Configuration dependence of scotopic spatial summation. Journal of Physiology. 1971;216:513–529. doi: 10.1113/jphysiol.1971.sp009538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakitt BS. Counting every quantum. Journal of Physiology. 1972;223:131–150. doi: 10.1113/jphysiol.1972.sp009838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnapf JL, Nunn BJ, Meister M, Baylor DA. Visual transduction in cones of the monkey Macaca fascicularis. Journal of Physiology. 1990;427:681–713. doi: 10.1113/jphysiol.1990.sp018193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneeweis DM, Schnapf JL. The photovoltage of macaque cone photoreceptors: adaptation, noise, and kinetics. Journal of Neuroscience. 1999;19(4):1203–1203. doi: 10.1523/JNEUROSCI.19-04-01203.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneeweis DM, Schnapf JL. Noise and light adaptation in rods of the macaque monkey. Visual Neuroscience. 2000;17:659–666. doi: 10.1017/s0952523800175017. [DOI] [PubMed] [Google Scholar]
- Sincich LC, Zhang Y, Tiruveedhula P, Horton JC, Roorda A. Resolving single cone inputs to visual receptive fields. Nature Neuroscience. 2009;12(8):967–967. doi: 10.1038/nn.2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon JA. Intrinsic uncertainty explains second responses. Spatial Vision. 2007;20:45–60. doi: 10.1163/156856807779369715. [DOI] [PubMed] [Google Scholar]
- Sperling HG, Jolliffe LL. Intensity-time relationship at threshold for spectral stimuli in human vision. Journal of the Optical Society of America. 1965;55:191–199. [Google Scholar]
- Stevenson SB, Kumar G, Roorda A. Psychophysical and oculomotor reference points for visual direction measured with the adaptive optics scanning laser ophthalmoscope. 2007;7(Number 9):137a. doi: 10.1167/7.9.137. [Abstract] 137. http://journalofvision.org/7/9/137/ [DOI] [Google Scholar]
- Stromeyer CF, Klein S. Spatial frequency channels in human vision as asymmetric (edge) mechanisms. Vision Research. 1974;14:1409–1420. doi: 10.1016/0042-6989(74)90016-9. [DOI] [PubMed] [Google Scholar]
- Swets JA, Tanner WP, Birdsall TG. Decision Processes in Perception. Psychological Review. 1961;68(5):301–340. [PubMed] [Google Scholar]
- Tanner WP. Physiological implications of psychophysical data. Annals of the New York Academy of Sciences. 1961;69:752–765. doi: 10.1111/j.1749-6632.1961.tb20176.x. [DOI] [PubMed] [Google Scholar]
- Teich MC, Prucnal PR, Vannucci G, Breton ME, McGill WJ. Multiplication noise in the human visual system at threshold: 1. Quantum fluctuations and minimum detectable energy. Journal of the Optical Society of America. 1982;72(4):419–431. doi: 10.1364/josa.72.000419. [DOI] [PubMed] [Google Scholar]
- Tolhurst DJ, Movshon JA, Dean AF. The statistical reliability of signals in single neurons in cat and monkey visual cortex. Vision Research. 1983;23(8):775–785. doi: 10.1016/0042-6989(83)90200-6. [DOI] [PubMed] [Google Scholar]
- van der Velden HA. The number of quanta necessary for the perception of light in the human eye. Ophthalmologica. 1946;111:321–331. doi: 10.1159/000300352. [DOI] [PubMed] [Google Scholar]
- Vimal RLP, Pokorny J, Smith VC, Shevell SK. Foveal cone thresholds. Vision Research. 1989;29(1):61–78. doi: 10.1016/0042-6989(89)90174-0. [DOI] [PubMed] [Google Scholar]
- Weintraub DJ, Hake HW. Visual discrimination: An interpretation in terms of detectability theory. Journal of the Optical Society of America. 1962;52:1179–1184. doi: 10.1364/josa.52.001179. [DOI] [PubMed] [Google Scholar]
- Wesner MF, Pokorny J, Shevell SK, Smith VC. Foveal cone detection statistics in color-normals and dichromats. Vision Research. 1991;31(6):1021–1037. doi: 10.1016/0042-6989(91)90207-l. [DOI] [PubMed] [Google Scholar]
- Wickelgren WA. Unidimensional strength theory and component analysis of noise in absolute and comparative judgments. Journal of Mathematical Psychology. 1968;5:102. [Google Scholar]
- Williams DR, MacLeod DIA, Hayhoe MM. Punctate sensitivity of the blue-sensitive mechanism. Vision Research. 1981;21:1357–1375. doi: 10.1016/0042-6989(81)90242-x. [DOI] [PubMed] [Google Scholar]