Abstract
Background
Elderly people suffer from skeletal muscle disorders that undermine their daily activity and quality of life; some of these problems can be listed as but not limited to: sarcopenia, changes in central and peripheral nervous system, blood hypoperfusion, regenerative changes contributing to atrophy, and muscle weakness. Determination, proliferation and differentiation of satellite cells in the regenerative process are regulated by specific transcription factors, known as myogenic regulatory factors (MRFs). In the elderly, the activation of MRFs is inefficient which hampers the regenerative process. Recent studies found that low intensity laser therapy (LILT) has a stimulatory effect in the muscle regeneration process. However, the effects of this therapy when associated with aging are still unknown.
Objective
This study aimed to evaluate the effects of LILT (λ=830 nm) on the tibialis anterior (TA) muscle of aged rats.
Subjects and methods
The total of 56 male Wistar rats formed two population sets: old and young, with 28 animals in each set. Each of these sets were randomly divided into four groups of young rats (3 months of age) with n=7 per group and four groups of aged rats (10 months of age) with n=7 per group. These groups were submitted to cryoinjury + laser irradiation, cryoinjury only, laser irradiation only and the control group (no cryoinjury/no laser irradiation). The laser treatment was performed for 5 consecutive days. The first laser application was done 24 h after the injury (on day 2) and on the seventh day, the TA muscle was dissected and removed under anesthesia. After this the animals were euthanized. Histological analyses with toluidine blue as well as hematoxylin-eosin staining (for counting the blood capillaries) were performed for the lesion areas. In addition, MyoD and VEGF mRNA was assessed by quantitative polymerase chain reaction.
Results
The results showed significant elevation (p<0.05) in MyoD and VEGF genes expression levels. Moreover, capillary blood count was more prominent in elderly rats in laser irradiated groups when compared to young animals.
Conclusion
In conclusion, LILT increased the maturation of satellite cells into myoblasts and myotubes, enhancing the regenerative process of aged rats irradiated with laser.
Keywords: aged rats, phototherapy, low intensity laser therapy, muscle regeneration, MyoD, VEGF
1 Introduction
Aging is associated to loss of muscle mass and consequently muscle function impairment [1]. These characteristics are worse and increase the risk falls and other injuries in the elderly [2]. In addition, the elderly show regenerative changes [1] influenced by modification in the neuromuscular system, blood hypoperfusion and sarcopenia [3]. Furthermore, studies show that there is a change in the activation of myogenic regulatory factors (MRFs) during aging [4, 5]. Although the causes of this change are not well defined it is possible that this influences the regenerative process.
The MRFs are part of the family of transcription factors for alpha-helix-helix genes that regulate muscle-specific, being composed of four members: MyoD, Myf-5, MRF4 and myogenin. Two groups of MRFs were identified: the primary MRFs are the MyoD and Myf-5, which has a role in determining myogenesis, doing the transformation of precursor cells into myoblasts, and the secondary MRFs that are myogenin and MRF4 responsible to the cellular differentiation of myoblasts in myocyte differentiation and to the mature muscle fiber [6-8].
It has been shown that MyoD and myogenin are expressed in high levels during the first months of life. In adult muscle of animals they are detectable in the processes of plasticity and regeneration. However in the elderly, muscle MRFs are often increased and may be related to blood hypoperfusion, insufficient innervation and subsequent muscle atrophy [1, 5, 9].
Unfortunately, studies that investigated laser as a treatment for muscle regeneration in aged rats are difficult to access. However, other studies show positive effects of low intensity laser (LILT) in regenerating muscle of young rats, the increase of myotubes, myofibrils and angiogenesis [4, 10-16]. These previous results in young murine animals are interesting for the development of therapeutic strategies that may enhance muscle regenerative capacity in the elderly. If these results are confirmed in the elderly model, LILT could be considered as a therapeutic strategy. Based on these statements, we hypothesized that LILT could improve the muscle regeneration process in elderly rats. Therefore, the aim of this study was to analyze the effect of LILT during the muscle regenerative process of young compared to old rats.
2 Subjects and methods
2.1 Animal care and experimental groups
In this study we used 56 male Wistar rats, 28 of them were 3 months old (266±30 g), thus in the young category, and 28 of them were 10 months old (674±85 g) and comprised the old category. All the animals were housed in plastic cages and kept in a room with controlled environmental conditions, and with free access to water and standard food. The experimental procedures were approved by the Ethics Committee of the Federal University of São Carlos, SP, Brazil and conducted in accordance with the ‘Guide for Care and Use of Laboratory Animals’.
The animals were divided into four experimental groups for both young (Y) and old (O) animals, with seven animals per each group (n=7). A group of young animals (Y) was paired with a group of old animals (O) and the paired groups were subjected to the same experimental conditions; thus, we had four pairing groups: one comprised of young animals and one of old animals, and the experiments that we performed ran parallel in the designated young and old paired groups.
The muscle subjected to cryolesioning was the right tibialis anterior (TA). Laser treatment, when done, was applied for 29 s at wavelength of λ=830nm, and energy of 0.87 J.
-
–
Groups 1 (IL-Y) and 2 (IL-O): muscles of young and old rats, respectively, were subjected to cryolesioning and were treated with laser irradiation.
-
–
Groups 3 (I-Y) and 4 (I-O): muscles of young and old rats, respectively, were subjected to cryolesioning but were not irradiated.
-
–
Groups 5 (L-Y) and 6 (L-O): muscles of young and old rats, respectively, were not submitted to cryolesioning, but were treated with laser irradiation in the right TA.
-
–
Groups 7 (C-Y) and 8 (C-O): muscles of young and old rats, respectively, were submitted neither to cryolesioning nor to laser irradiation, thus were the absolute control groups.
2.2 Surgical technique and cryoinjury protocol
The experiment was conducted in 7 days:
-
–
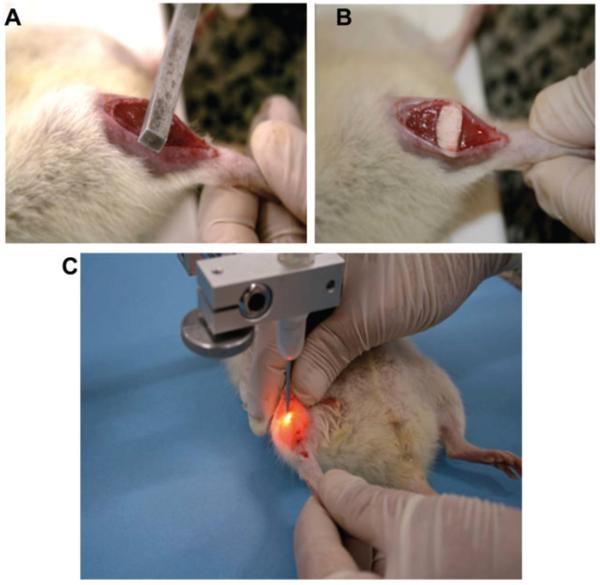
Day 1 – The animals were weighed and then anesthetized with ketamine hydrochloride (HCl) (0.05 mg/kg) and xylazine HCl (0.03 mg/kg) injections to the muscle tissue in accordance with the body weight. The muscle subjected to cryolesioning was the right TA. The skin covering the right TA was cleaned, and then a transverse incision (approx. 1 cm in length) was performed in the region corresponding to the belly of the muscle. For correct exposure of the TA it was still necessary to remove the fascia cover. Tissue injury was induced by freezing (cryolesioning) in the center of the muscle belly of the TA. For this, a stick of iron with 0.5 cm2 was previously immersed in liquid nitrogen (N2) and pressed transversely against on the belly of the muscle for 10 s (Figure 1 A, B). After further cooling of the bat, this procedure was repeated and then the skin was sutured (3-0 Nylon Yarn; Shalon Suturas Ltda., Goiania, Brazil) and cleaned with iodine alcohol. Injury by freezing (cryoinjury) is a common procedure used to induce damage [17].
-
–
Day 2 through 6 – The animals were subjected to the procedure of laser irradiation (Figure 1 C), for 5 consecutive days on the injured site.
-
–
Day 7 – The right TA muscle was carefully dissected and removed with the animals anesthetized. After removal, the muscle was weighed and then it was divided into two parts (with similar horizontal cuts through the belly). The distal portion was immediately frozen in liquid N2 for extraction of RNA. The proximal portion was pre-frozen in isopentane and used for histological analysis. The muscles were stored in a freezer at −86°C. All the animals of treated and control groups at the end were weighed, anesthetized and subjected to euthanasia with intracardiac injection of potassium chloride (3M KCl).
Figure 1.
Cryolesion generation (A, B) and laser irradiation (C).
2.3 RNA isolation and analysis
RNA was isolated from a frozen fragment from the distal ends of each muscle using 1 ml of Trizol reagent (Invitrogen, Carlsbad, CA, USA), according to the manufacturer’s instructions. The extracted RNA was dissolved in Tris-HCl and ethylene-diamine tetraacetic acid (pH 7.6) and quantified spectrophotometrically. The purity was assessed by determining the ratio of the absorbance at 260 nm and 280 nm. All samples had 260–280 nm ratios above 2.0. The integrity of the RNA was confirmed by inspection of ethidium bromide-stained 18S and 28S ribosomal RNA under ultraviolet light (Invitrogen, Carlsbad, CA, USA).
2.4 Reverse transcription
Total RNA was reverse transcribed into cDNA in two steps. In the first step, 1 μl of oligo (dT) primer (Invitrogen, Carlsbad, CA, USA) and 9.5 μl of water was added to 1 μg of isolated total RNA, heated to 70°C for 10 min and quickly cooled on ice. In the second step, 4 μl of 5× reverse transcription buffer, 1 μl of a dNTP (Promega, Madison, WI, USA) mixture containing 0.2 mm each of dATP, dCTP, dGTP, and 0.1 m dTTP, 2 μl of 0.1 m dithiothreitol, and 0.5 μl of M-MLV RT enzyme (Promega, Madison, WI, USA) in a total volume of 20 μl; the total mixture was incubated at 42°C for 60 min for each sample. To minimize any possible variation in the reverse transcription reaction, all RNA sample groups were reverse transcribed simultaneously.
2.5 Primers
The primers used for polymerase chain reactions in MyoD were (sense: GGAGACATCCTCAAGCGATGC; antisense: AGCACCTGGTAAATCGGATTG) as described in [18]; in vascular endothelial growth factor (VEGF) (sense: GGAGATCCTTCGAGGAGCACTT; antisense: GGCGATTTAGCAGCAGATATAAGAA) as described in [19]; in GAPDH (sense: CCACCAACTGCTTAGCCC; antisense: GCCAAATTCGTTGTCATACC), as described in our previous paper [4].
2.6 Analysis by real-time polymerase chain reactions
The RNA transcript levels for the different experimental and control muscles were analyzed simultaneously and the reactions were carried out in duplicate using SYBR green fluorescent dye (Applied Biosystems, Foster City, CA, USA) in a sequence detection system (GeneAmp 5700; Applied Biosystems).
2.7 Histological analysis
To evaluate the presence of signs of muscle injury induced via cryoinjury and the possible presence of capillary vessels, histological cross-sections of the right TA muscles were analyzed. For this, the medial parts of the middle belly muscle were frozen in isopentane pre-cooled in liquid N2, and stored in a freezer (−80°C). Serial cross-sections of 10 μm thickness (to see muscle injury) and 7 μm thickness (to see capillary blood) were then obtained from the frozen soleus muscle using a microtome cryostat (HE 505; Microm International GmbH, Jena, Germany) maintained at −20°C and alternate serial cross-sections of the muscles stained with 1% toluidine blue (TB)/1% borax. TB staining was used to evaluate the morphological pattern of the muscle fibers and the presence of muscle-fiber injury, because it permits the identification of the myonuclei, areas of myonecrosis, and the basophilic regions of the muscle fibers. For the capillary blood analysis the alternative serial cross-sections were stained with hematoxylin/eosin (H&E), were processed to obtain a mosaic showing the total extent of the histological section made by cutting five areas, and five sections per animal and the images were captured with the 10× objective and analyzed by optical microscope (Axioplan 2; Carl Zeiss, Jena, Germany). The capillary blood images were analyzed in Imagetool 3.0 software (University of Texas Health Science Center at San Antonio, TX, USA), which were recorded in the amount of blood capillaries in the photomicrography.
2.8 Sources of light and irradiation protocol
A diode laser (Model: Theralase; DMC Equipamentos Ltda., São Carlos, Brazil), operating with parameters given in Table 1 was used.
Table 1.
Parameters used in the laser irradiation of the rat muscle.
| Parameters of laser (GaAlAs) | Values |
|---|---|
| Wavelength | 830 nm |
| Optical power output | 30 mW |
| Beam diameter | 0.6 mm |
| Beam area | 0.0028 cm2 |
| Divergence angle | 1.5 degrees |
| Fluence | 30 J/cm2 |
| Irradiation time | 29 s |
| Total energy | 0.87 J |
The animals were irradiated directly on the site of muscle damage, every 24 h for 5 consecutive days, with the first application 24 h after induction of injury, directly on the skin covering the belly of the TA muscle. During the irradiation process animals were kept under sedation with low doses of ketamine HCl and xylazine to allow smooth laser application time.
2.9 Statistical analysis
Levene’s test was applied first to evaluate the homogeneity of the results. ANOVA was used to identify possibly differences amongst groups. When differences were observed, the Tukey test was performed. For all tests, the significance level was set at 5%.
3 Results
3.1 Morphological analysis
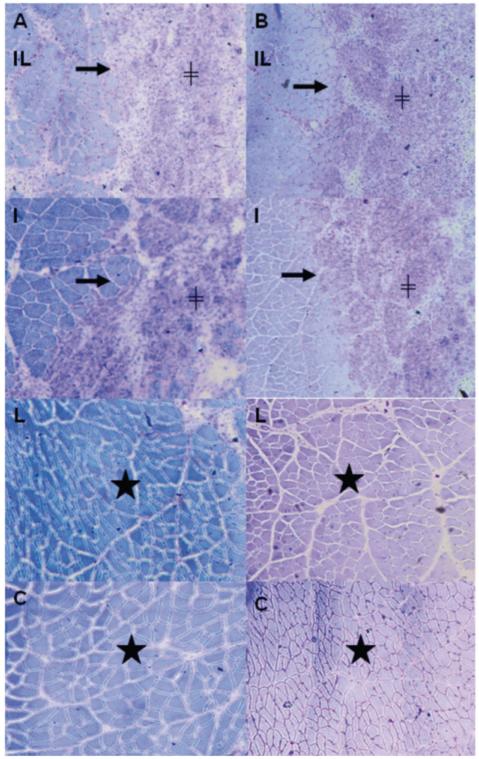
The qualitative histological procedures were analyzed in muscles under normal conditions and with influence of laser irradiation. Images stained with TB (Figure 2 A, B) in groups only cryoinjured and in groups cryoinjured and irradiated showed a well-defined injured region that remains restricted to the surface of the TA muscle and that could also be seen at the transition regions among the intact and injured tissue. In the site of injury in young rats (Figure 2B, image I young and IL young), near the region of transition, fibers with centralized nuclei and smaller cross-sectional area can be identified. At the surface of the lesion, it is possible to identify a high concentration of mononuclear cells (inflammatory and/or myogenic), abundant extracellular matrix, high concentration of lysosomes, and absence of young muscle fibers. In the irradiated only (L) and absolute control group (C), the intact region of the injured groups (Figure 2) shows the same characteristics of a healthy skeletal muscle tissue as a polygonal shape, peripheral nucleus, organization issues and no change in the morphology of the fibers.
Figure 2.
Transverse serial sections of the middle region of the right TA muscle, stained with toluidine blue; row (A) is the elderly group and row (B) the young group; IL, injured and laser irradiated groups; I, injured only groups; L, irradiated only groups; C, total control groups. View → is the interface between normal and injured regions; ≠ region corresponding to injury, ★ denotes normal muscle fibers.
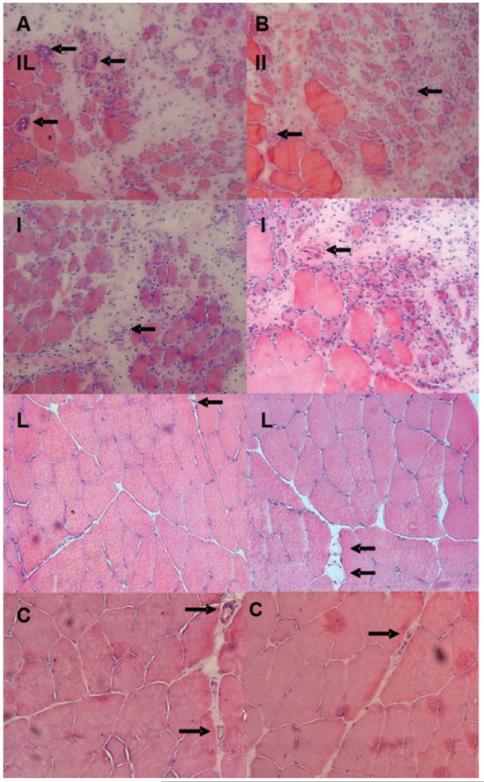
Images stained with H&E (Figure 3) enabled to observe the presence of blood capillaries in slides of control C (both C-Y and C-O), irradiated only L (L-Y and L-O), injured only I (I-Y and I-O), and injured and irradiated IL (IL-Y and IL-O) groups of animals.
Figure 3.
Transverse serial sections of the middle region of the right TA muscle stained with H&E. The row (A) is the elderly group, and row (B) the young group; IL, injured and irradiated groups; I, injured groups only; L, irradiated groups only; C, absolute control groups. → points to blood vessels.
3.2 Morphometric analysis
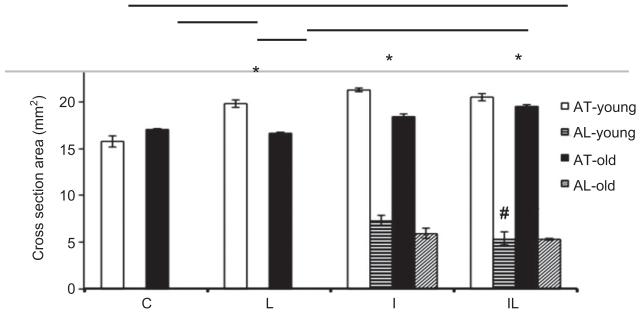
Figure 4 shows an expansion of the total area of cross section of young and elderly groups, but a significant increase in this area occurred only in the groups IL-Y, I-Y, and L-Y. The figure also shows that there was a decrease in affected areas with statistical difference only for the IL-Y group compared with the I-Y one.
Figure 4.
Morphometric analyses. Total area (AT) and injured area (AL), where *: p<0.05 corresponds intragroup comparisons, i.e., the significant differences between groups IL, L, I, and C on the young and old paired groups; #: p<0.05 is significant difference between the IL and I young subjects groups. The horizontal bars in the upper portion of the figure indicate the intergroup comparisons showing statistical differences (p<0.05), between young and elderly subjects groups.
3.3 Quantification of capillary blood vessels
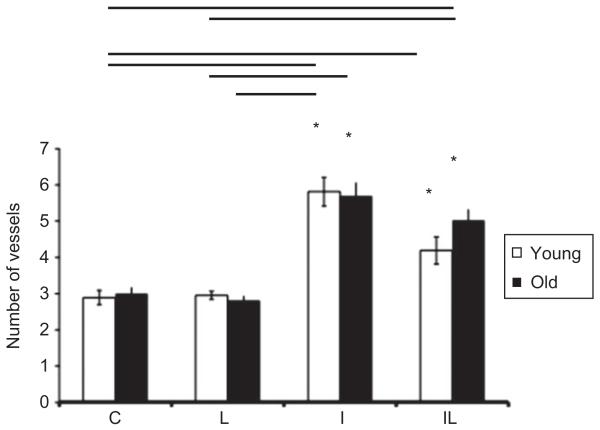
The result of counting of the blood capillary vessels, as seen in Figures 3 and 5, shows a significant increase in the amount of blood capillaries in groups IL-Y, IL-O, I-Y and I-O. The young and elderly irradiated group showed no changes in the total number of blood capillaries.
Figure 5.
Count of blood vessels by light microscopy photomicrographs obtained in groups of young and old specimens, where *: p<0.05 corresponds intragroup comparisons, i.e., the significant differences between groups IL young, I young, and compared to absolute control (C) young; and between groups IL, I elderly compared to absolute control (C) group of elderly. The horizontal bars in the upper portion of the figure indicate the intergroup comparisons showing statistical differences (p<0.05), between young and elderly groups.
3.4 mRNA gene expression of MyoD
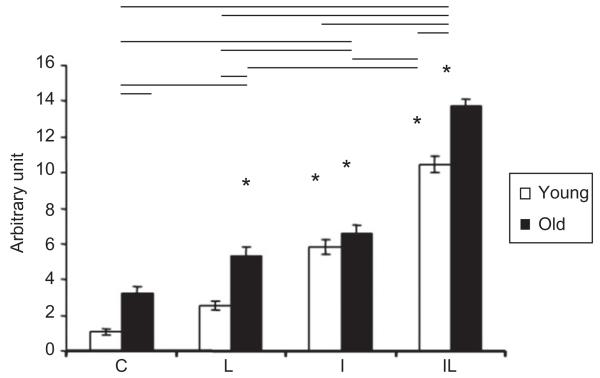
MyoD mRNA gene expression proved to be at elevated levels in both young and elderly of L, I, and IL groups in comparison with the control group C (for both young and elderly). The level of gene expression differences reached an even more significant state, if we compared group C with the IL-O, I-O, and L-O groups. In the young animals, we already see statistical difference of MyoD mRNA expression levels in comparison with the control C-Y, and IL-Y, and I-Y groups (Figure 6). If we compare the young with the elderly groups, we can see a more pronounced response in the levels of mRNA of MyoD in the elderly groups.
Figure 6.
Levels of MyoD mRNA in the TA muscle of rats, where *: p<0.05 corresponds intragroup comparisons, i.e., the significant differences between groups IL, I, and absolute control (C) of young specimens; and between groups IL, I, and L in the elderly group with absolute control (C) of the elderly specimens. The horizontal bars in the upper part of the figure indicate the intergroup comparisons showing statistical differences, between young and elderly groups.
3.5 Gene expression of VEGF mRNA
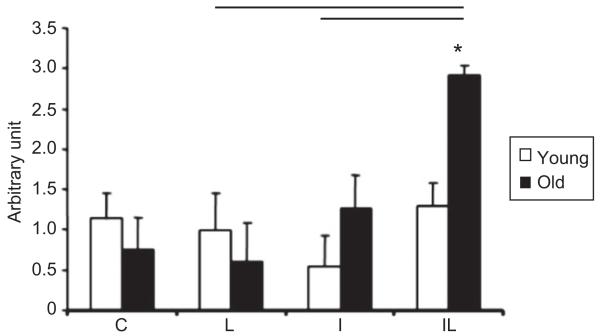
The gene expression of VEGF mRNA in elderly IL and I groups appears to be with increased expression levels, whereas in the elderly IL and C groups, it reaches significant-increase levels. In contrast, while the young IL and C groups showed an increase in the expression of VEGF mRNA, the young IL and I groups showed a decrease in the expression levels (Figure 7).
Figure 7.
Levels of VEGF mRNA in the TA muscle of rats, where *: p<0.05 corresponds to intragroup comparisons, i.e., the significant differences between IL elderly groups and elderly groups I, L and C. The horizontal bars in the upper part of the figure indicate the intergroup comparisons showing statistical differences (p<0.05), between young and elderly groups.
4 Discussion
LILT has been used extensively in the clinic rehabilitation [4] and several studies are showing the positive action of LILT in muscle regeneration [15, 20, 21]. However, there are few studies that examine the mechanisms by which this therapy interacts with the muscle tissue during the regeneration process, especially in the elderly. In our current study we demonstrate the influence of LILT in regenerating skeletal muscles in elderly rats. Our results support the idea that LILT treatment enhanced the regenerative process of skeletal muscle in irradiated aged rats where cryolesions increased the maturation of satellite cells into myoblasts and myotubes. These results are very interesting for the development of novel therapeutic strategies and modalities, such as including the LILT as a treatment option (in addition to those that are already in place), that may enhance muscle regenerative capacity in the elderly.
This study was designed to observe and evaluate the response to injury during the acute phase of the inflammation induced by cryoinjury, as well as to evaluate how the inflammation may be affected by laser irradiation. As such, our experimental set-up is reflecting the inflammation development; thus, in vivo studies followed the 5 days benchmark.
We induced the cryoinjuries with a liquid N2 cooled rod and marked the spot twice with a cooling the rod in between times. The injured spot is clearly seen in Figure 1. The lower image is of laser irradiation performed on the TA muscle.
Morphological analyses of the images (generated with TB staining) of the right TA muscles cross-sections, are rendering only qualitative information. Looking at these images one does not see pronounced difference in the muscle regeneration patterns of young and old rats that have undergone both cryolesioning and irradiation treatments, when compared with the young and old rat groups that have only been through a cryolesioning treatment (Figure 2). However, the borderlines of the cryoinjury and cell structure damage are visible and pronounced.
To assess the regenerative effect of irradiation morphometric analyses of the stained tissue had to be done (over the total area of imaging), and by that it was possible to verify the regenerative pattern differences in the elderly and young rat groups. Our results show that only the young rats’ group were cryolesioned and irradiated (Figure 4), gain a positive outlook for repairing of the injured area, with unequivocal narrowing of the damaged area. In this regard, the LILT was effective for muscle regeneration in the 5-day treatment process, enabling the proliferation and/or differentiation of satellite cells recruited during the initial phase of the regeneration [22, 23]. In the elderly rats’ cryolesioned and irradiated group, it was not possible to see such decrease of the damaged area. However, neither did this area increase, which shows that the regenerative process in the elderly rats is poor [24], and that the LILT nevertheless stimulated the regeneration such that it did not stall nor did it take a turn for the worse. Thus, it is important to evaluate treatments with different times and different doses using laser irradiation.
The reduced regenerative capacity is related to aging in association with changes in the function of satellite cells [25]. However, activation of satellite cells requires a controlled increase in the MRFs expression and the muscle-specific genes [6]. In the muscles of older rats the MRFs expression are high enough; however, they are ineffective in action [1, 5]. According to Alway et al. [1], gene expression of MRFs can increase in aged muscles; however, it cannot increase as much and with the same rates after injury, which is an impairing factor in the regenerative process.
Our recent studies [4], as well as Nakano et al. [26], have shown that there is an increase in the number of molecular markers linked to muscle regeneration in young rats and mice treated with LILT. In our previous study [4], using lasers with wavelengths of 685 and 830 nm, and irradiation doses of 2.6 and 8.4 J/cm2, respectively, we showed that enzymes lactate dehydrogenase and citrate synthase levels increased upon treatment; pointing towards the fact that this type of therapy influences the proliferation and metabolic functions of satellite cells during the muscle regeneration process. Nakano et al. [26] demonstrated that laser diode with λ=830 nm has a stimu latory effect over cellular activity and that it increases the number of capillaries as well as the fibroblast growth factor levels.
In our current study where aged rats are used for the first time in this type of experimental model we show that treatment with LILT had a positive influence on elevating the gene expression levels of MyoD in the young groups; thus we hypothesized that the laser irradiation activates quiescent satellite cells and leads to activation of the initial phase of the cell cycle and as such has mediating effects over cell proliferation. This is in line with arguments of other groups as well [11, 22]. We see a similar response in aged groups rats where MRFs expressions are upregulated.
In examining the effects of LILT on the gene expression levels of MyoD, it was observed that the gene initiates the activation of proliferation and differentiation of the satellite cells during the muscle regenerative process [6]. Thus, it is clear that satellite cells are essential for regeneration. However, during aging even though satellite cells ability to spread is maintained, their quantity and capacity to differentiate is reduced. These changes are caused by the lack of induction of MRFs in the cells, an action crucial to the regenerative process and to the transcription of muscle-specific genes [26, 27]. In our previous study [28], with different study conditions and only a young mice population, we showed that an 808 nm laser irradiation treatment applied population had elevated mRNA levels of the transcription factors MyoD and myogenin. Our current results, as seen in Figure 6, show that LILT exerted great influence on the mRNA expression of MyoD in older groups, but viewing the injured area of the TA muscle, we can say that the IL group showed positive responses in young muscle regeneration which is more pronounced than in group IL elderly. For this, Degens [27] emphasizes that the high levels of mRNA of MRFs do not necessarily reflect high levels of muscle-specific proteins.
The relationship between muscle regeneration and adequate blood supply is very important [19] in helping the migration of satellite cells to the injury site [29]. With that in mind we analyzed the gene expression of VEGF (as an angiogenic factor playing an important role in vascular development) [29], an active participant in the muscle regenerative process [19].
It is known that VEGF gene has an important regulatory role in basal capillarization of muscle tissue and in the mitigation of motoneurons loss in the elderly [30] by accelerating the fusion of myogenic satellite cells to form myotubes during regeneration [19, 31]. Studies show that the endothelial dysfunction related to age and the reduced expression of VEGF are possible mechanisms of reducing age-dependent angiogenesis [30, 32], leading to a delay in the formation of blood capillaries in the elderly [29]. Bryan et al. [33] showed that VEGF is coordinately regulated and increasing its expression during myogenesis, and also they found that increase of VEGF levels depends on the myogenic transcription factor, the MyoD. Thus, changes in gene expression of the mRNA of MyoD that occurs during aging may clearly influence the reduced expression of the VEGF gene. Results of our study (Figures 6 and 7) show that LILT irradiation upregulated the gene expression of the mRNA of MyoD, and of VEGF, as well as increased the number of blood vessels in the muscle groups of the right TA. Our findings corroborate the effects of LILT on angiogenesis through increasing expression levels of VEGF [34, 35] (Figure 7); increasing the count of blood capillaries [16, 36], (Figures 3 and 5); and increasing the expression of satellite cells [22] through the MyoD effect (Figure 6).
In our study we observed that there was a decrease in the number of blood vessels in the IL group compared with the I group (Figures 3 and 5). This response may be explained by the effect of LILT in the modulation of local inflammatory response through its systemic effect, such as increased superoxide dismutase activity [25]; increased lymphocytes and the regulation of reactive oxygen species [37].
According to the theory of photochemical LILT, the interaction of electromagnetic radiation with the chromophores of the mitochondria and the subsequent biochemical cascade, photostimulation occurs mainly during the unstable redox state [38] and our study shows that the effect of LILT generates a better response in the elderly IL group compared to the young groups having analyzed the mRNA levels of MyoD and VEGF, as seen in the Figures 6 and 7. We found that responses were important in the LILT treated elderly group and we believe that the elevated levels of gene expression of MyoD and VEGF also influenced the increase in capillary blood vessels formation, which in turn prevented the expansion of the injury (injured area) and increased the total area of the muscle cross section in the elderly group IL. This indicates that lesion formation is activating the muscle gene program to start proliferation of the satellite cells, specifically forming muscle fibers and replacing the injured fibers (arising from the cryolesioning), as well as reversing muscle atrophy and intrinsic aging.
The L group, irradiated with the laser and serving as a control group for injury irradiation, was used to verify the action of photostimulation in biological tissues without metabolic changes in the young group and in the old group (with metabolic changes arising from the advancement of age) [39]. The results show that there is an elevation in the gene expression levels of MyoD and the total area of muscle cross section in the elderly; possibly due to a positive reply from upregulation of MRFs that are present in muscle fibers but are lost in the attempt to reverse the muscle atrophy because of intrinsic aging. Despite the positive responses to 5 days of irradiation, we believe that more days of treatment with LILT are needed for the aged muscle to have more pronounced and effective responses during the regeneration process and natural muscle atrophy.
In summary, the study indicates that LILT accelerated the process of muscle regeneration after cryolesioning in young and elderly rats because it activated the expression of genes MyoD and VEGF, probably by increasing the maturation of satellite cells into myoblasts and myotubes, and reducing muscle atrophy in irradiated elderly rats, as well as by the improvement of blood supply during this regene ration process.
5 Conclusions
Our results clearly demonstrate that there are differences between the regenerative responses in young and old animals. These responses during the acute phase of inflammation (set in after induction of cryoinjury) show signs of body’s physiological response necessary to repair the injured tissue. The LILT apparently acts as a response modulating factor over the myogenic factors (MyoD) and the vasculature (VEGF); however, we suspect that there are many other implications that are signaled from these initial mechanisms.
Our research showed that in elderly individuals, because there is a greater oxidative stress and a lengthy natural regeneration process, it appears that the laser treatment has a greater and more pronounced overall effect. The LILT shows a great response in the young population as well, which is expected; but even there, the irradiation treatment improves the muscle regeneration when compared with untreated young subjects.
Acknowledgements
We appreciate the support offered by the National Council for Scientific and Technological Development (CNPq) [200824/20111-2] and Fundacao de Amparo a Pesquisa do Estado de Sao Paulo (FAPESP) [2010/07194-7, 2011/06240-8 and 2012/05919-0], both from Brazil. MRH was supported by US NIH (grant RO1AI050875).
Footnotes
FV and NCR contributed to the paper equally.
Contributor Information
Fatma Vatansever, Wellman Center for Photomedicine, Massachusetts General Hospital, Boston, MA, USA; and Department of Dermatology, Harvard Medical School, Boston, MA, USA.
Natalia C. Rodrigues, Physiotherapy Department, Federal University of São Carlos, SP, Brazil; and Biotechnology Post-Graduate Program, Federal University of São Carlos, SP, Brazil
Livia L. Assis, Physiotherapy Department, Federal University of São Carlos, SP, Brazil
Sabrina S. Peviani, Physiotherapy Department, Federal University of São Carlos, SP, Brazil
Joao L. Durigan, Physiotherapy Department, Federal University of São Carlos, SP, Brazil
Fernando M.A. Moreira, Physics Department, Federal University of São Carlos, SP, Brazil; and Biotechnology Post-Graduate Program, Federal University of São Carlos, SP, Brazil
Michael R. Hamblin, Wellman Center for Photomedicine, Massachusetts General Hospital, Boston, MA, USA; Department of Dermatology, Harvard Medical School, Boston, MA, USA; and Harvard-MIT Division of Health Science and Technology, Cambridge, MA, USA
Nivaldo A. Parizotto, Biotechnology Post-Graduate Program, Federal University of São Carlos, SP, Brazil.
References
- [1].Alway SE, Lowe DA, Chen KD. The effects of age and hindlimb supension on the levels of expression of the myogenic regulatory factors MyoD and myogenin in rat fast and slow skeletal muscles. Exp Physiol. 2001;86(4):509–17. doi: 10.1113/eph8602235. [DOI] [PubMed] [Google Scholar]
- [2].Oliveira DM, Narciso FM, Santos ML, Pereira DS, Coelho FM, Dias JM, Pereira LS. Muscle strength but not functional capacity is associated with plasma interleukin-6 levels of community-dwelling elderly women. Braz J Med Biol Res. 2008;41(12):1148–53. doi: 10.1590/s0100-879x2008001200017. [DOI] [PubMed] [Google Scholar]
- [3].Mozdziak PE, Greaser ML, Schultz E. Myogenin, MyoD, and myosin expression after pharmacologically and surgically induced hypertrophy. J Appl Physiol. 1998;84(4):1359–64. doi: 10.1152/jappl.1998.84.4.1359. [DOI] [PubMed] [Google Scholar]
- [4].Amaral AC, Parizotto NA, Salvini TF. Dose-dependency of low-energy HeNe laser effect in regeneration of skeletal muscle in mice. Lasers Med Sci. 2001;16(1):44–51. doi: 10.1007/pl00011336. [DOI] [PubMed] [Google Scholar]
- [5].Dedkov EI, Kostrominova TY, Borisov AB, Carlson BM. MyoD and myogenin protein expression in skeletal muscles of senile rats. Cell Tissue Res. 2003;311(3):401–16. doi: 10.1007/s00441-002-0686-9. [DOI] [PubMed] [Google Scholar]
- [6].Marsh DR, Criswell DS, Carson JA, Booth FW. Myogenic regulatory factors during regeneration of skeletal muscle in young, adult, and old rats. J Appl Physiol. 1997;83(4):1270–5. doi: 10.1152/jappl.1997.83.4.1270. [DOI] [PubMed] [Google Scholar]
- [7].Ishibashi J, Perry RL, Asakura A, Rudnicki MA. MyoD induces myogenic differentiation through cooperation of its NH2- and COOH-terminal regions. J Cell Biol. 2005;171(3):471–82. doi: 10.1083/jcb.200502101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Buchberger A, Nomokonova N, Arnold HH. Myf5 expression in somites and limb buds of mouse embryos is controlled by two distinct distal enhancer activities. Development. 2003;130(14):3297–307. doi: 10.1242/dev.00557. [DOI] [PubMed] [Google Scholar]
- [9].Musarò A, Cusella De Angelis MG, Germani A, Ciccarelli C, Molinaro M, Zani BM. Enhanced expression of myogenic regulatory genes in aging skeletal muscle. Exp Cell Res. 1995;221(1):241–8. doi: 10.1006/excr.1995.1372. [DOI] [PubMed] [Google Scholar]
- [10].Weiss N, Oron U. Enhancement of muscle regeneration in the rat gastrocnemius muscle by low energy laser irradiation. Anat Embryol (Berl) 1992;186(5):497–503. doi: 10.1007/BF00185463. [DOI] [PubMed] [Google Scholar]
- [11].Shefer G, Partridge TA, Heslop L, Gross JG, Oron U, Halevy O. Low-energy laser irradiation promotes the survival and cell cycle entry of skeletal muscle satellite cells. J Cell Sci. 2002;115(Pt 7):1461–9. doi: 10.1242/jcs.115.7.1461. [DOI] [PubMed] [Google Scholar]
- [12].Oliveira NM, Parizzotto NA, Salvini TF. GaAs (904 nm) laser radiation does not affect muscle regeneration in mouse skeletal muscle. Lasers Surg Med. 1999;25(1):13–21. doi: 10.1002/(sici)1096-9101(1999)25:1<13::aid-lsm3>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- [13].Morrone G, Guzzardela GA, Orienti L, Giavaresi G, Fini M, Rocca M, Torricelli P, Martini L, Giardino R. Muscular trauma treated with a Ga-Al-As diode laser: in vivo experimental study. Lasers Med Sci. 1998;13(4):293–8. doi: 10.1007/s101030050011. [DOI] [PubMed] [Google Scholar]
- [14].Bibikova A, Oron U. Promotion of muscle regeneration in the toad (Bufo viridis) gastrocnemius muscle by low-energy laser irradiation. Anat Rec. 1993;235(3):374–80. doi: 10.1002/ar.1092350306. [DOI] [PubMed] [Google Scholar]
- [15].Bibikova A, Oron U. Attenuation of the process of muscle regeneration in the toad gastrocnemius muscle by low energy laser irradiation. Lasers Surg Med. 1994;14(4):355–61. doi: 10.1002/lsm.1900140408. [DOI] [PubMed] [Google Scholar]
- [16].Bibikova A, Belkin V, Oron U. Enhancement of angiogenesis in regenerating gastrocnemius muscle of the toad (Bufo viridis) by low-energy laser irradiation. Anat Embryol (Berl) 1994;190(6):597–602. doi: 10.1007/BF00190110. [DOI] [PubMed] [Google Scholar]
- [17].Durigan JL, Peviani SM, Russo TL, Delfino GB, Ribeiro JU, Cominetti MR, Selistre-de-Araujo HS, Salvini TF. Effects of alternagin-C from Bothrops alternatus on gene expression and activity of metalloproteinases in regenerating skeletal muscle. Toxicon. 2008;52(6):687–94. doi: 10.1016/j.toxicon.2008.07.018. [DOI] [PubMed] [Google Scholar]
- [18].Hill M, Wernig A, Goldspink G. Muscle satellite (stem) cell activation during local tissue injury and repair. J Anat. 2003;203(1):89–99. doi: 10.1046/j.1469-7580.2003.00195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Wagatsuma A, Tamaki H, Ogita F. Sequential expression of vascular endothelial growth factor, Flt-1, and KDR/Flk-1 in regenerating mouse skeletal muscle. Physiol Res. 2006;55(6):633–40. doi: 10.33549/physiolres.930811. [DOI] [PubMed] [Google Scholar]
- [20].Cressoni MD, Dib Giusti HH, Casarotto RA, Anaruma CA. The effects of a 785-nm AlGaInP laser on the regeneration of rat anterior tibialis muscle after surgically-induced injury. Photomed Laser Surg. 2008;26(5):461–6. doi: 10.1089/pho.2007.2150. [DOI] [PubMed] [Google Scholar]
- [21].Liu XG, Zhou YJ, Liu TC, Yuan JQ. Effects of low-level laser irradiation on rat skeletal muscle injury after eccentric exercise. Photomed Laser Surg. 2009;27(6):863–9. doi: 10.1089/pho.2008.2443. [DOI] [PubMed] [Google Scholar]
- [22].Ben-Dov N, Shefer G, Irintchev A, Wernig A, Oron U, Halevy O. Low-energy laser irradiation affects satellite cell proliferation and differentiation in vitro. Biochim Biophys Acta. 1999;1448(3):372–80. doi: 10.1016/s0167-4889(98)00147-5. [DOI] [PubMed] [Google Scholar]
- [23].Salvini TF, Belluzzo SS, Selistre de Araujo HS, Souza DH. Regeneration and change of muscle fiber types after injury induced by a hemorrhagic fraction isolated from Agkistrodon contortrix laticinctus venom. Toxicon. 2001;39(5):641–9. doi: 10.1016/s0041-0101(00)00188-4. [DOI] [PubMed] [Google Scholar]
- [24].Ehrhardt J, Morgan J. Regenerative capacity of skeletal muscle. Curr Opin Neurol. 2005;18(5):548–53. doi: 10.1097/01.wco.0000177382.62156.82. [DOI] [PubMed] [Google Scholar]
- [25].Kim JS, Kosek DJ, Petrella JK, Cross JM, Bamman MM. Resting and load-induced levels of myogenic gene transcripts differ between older adults with demonstrable sarcopenia and young men and women. J Appl Physiol. 2005;99(6):2149–58. doi: 10.1152/japplphysiol.00513.2005. [DOI] [PubMed] [Google Scholar]
- [26].Nakano J, Kataoka H, Sakamoto J, Origuchi T, Okita M, Yoshimura T. Low-level laser irradiation promotes the recovery of atrophied gastrocnemius skeletal muscle in rats. Exp Physiol. 2009;94(9):1005–15. doi: 10.1113/expphysiol.2009.047738. [DOI] [PubMed] [Google Scholar]
- [27].Degens H. Age-related skeletal muscle dysfunction: causes and mechanisms. J Musculoskelet Neuronal Interact. 2007;7(3):246–52. [PubMed] [Google Scholar]
- [28].Assis L, Moretti AI, Abrahão TB, de Souza HP, Hamblin MR, Parizotto NA. Low-level laser therapy (808 nm) contributes to muscle regeneration and prevents fibrosis in rat tibialis anterior muscle after cryolesion. Lasers Med Sci. 2012 doi: 10.1007/s10103-012-1183-3. doi: 10.1007/s10103-012-1183-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Germani A, Di Carlo A, Mangoni A, Straino S, Giacinti C, Turrini P, Biglioli P, Capogrossi MC. Vascular endothelial growth factor modulates skeletal myoblast function. Am J Pathol. 2003;163(4):1417–28. doi: 10.1016/S0002-9440(10)63499-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ryan NA, Zwetsloot KA, Westerkamp LM, Hickner RC, Pofahl WE, Gavin TP. Lower skeletal muscle capillarization and VEGF expression in aged vs. young men. J Appl Physiol. 2006;100(1):178–85. doi: 10.1152/japplphysiol.00827.2005. [DOI] [PubMed] [Google Scholar]
- [31].Arsic N, Zacchigna S, Zentilin L, Ramirez-Correa G, Pattarini L, Salvi A, Sinagra G, Giacca M. Vascular endothelial growth factor stimulates skeletal muscle regeneration in vivo. Mol Ther. 2004;10(5) doi: 10.1016/j.ymthe.2004.08.007. [DOI] [PubMed] [Google Scholar]
- [32].Gennaro G, Menard C, Michaud SE, Rivard A. Age-dependent impairment of reendothelialization after arterial injury: role of vascular endothelial growth factor. Circulation. 2003;107(2):230–3. doi: 10.1161/01.cir.0000050652.47145.4c. [DOI] [PubMed] [Google Scholar]
- [33].Bryan BA, Walshe TE, Mitchell DC, Havumaki JS, Saint-Geniez M, Maharaj AS, Maldonado AE, D’Amore PA. Coordinated vascular endothelial growth factor expression and signaling during skeletal myogenic differentiation. Mol Biol Cell. 2008;19(3):994–1006. doi: 10.1091/mbc.E07-09-0856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Braverman B, McCarthy RJ, Ivankovich AD, Forde DE, Overfield M, Bapna MS. Effect of helium-neon and infrared laser irradiation on wound healing in rabbits. Lasers Surg Med. 1989;9(1):50–8. doi: 10.1002/lsm.1900090111. [DOI] [PubMed] [Google Scholar]
- [35].Tuby H, Maltz L, Oron U. Modulations of VEGF and iNOS in the rat heart by low level laser therapy are associated with cardioprotection and enhanced angiogenesis. Lasers Surg Med. 2006;38(7):682–8. doi: 10.1002/lsm.20377. [DOI] [PubMed] [Google Scholar]
- [36].Jarvinen M, Aho AJ, Lehto M, Toivonen H. Age dependent repair of muscle rupture. A histological and microangiographical study in rats. Acta Orthop Scand. 1983;54(1):64–74. doi: 10.3109/17453678308992871. [DOI] [PubMed] [Google Scholar]
- [37].Vladimirov YA, Osipov AN, Klebanov GI. Photobiological principles of therapeutic applications of laser radiation. Biochemistry (Mosc) 2004;69(1):81–90. doi: 10.1023/b:biry.0000016356.93968.7e. [DOI] [PubMed] [Google Scholar]
- [38].Karu TI, Pyatibrat LV, Afanasyeva NI. Cellular effects of low power laser therapy can be mediated by nitric oxide. Lasers Surg Med. 2005;36(4):307–14. doi: 10.1002/lsm.20148. [DOI] [PubMed] [Google Scholar]
- [39].Elmadfa I, Meyer AL. Body composition, changing physiological functions and nutrient requirements of the elderly. Ann Nutr Metab. 2008;52(Suppl 1):2–5. doi: 10.1159/000115339. [DOI] [PubMed] [Google Scholar]