Abstract
'Cancer' is a disease that can spread to the other organs over time. The prognosis of cancer patients with metastasis is generally poor. Accordingly, there is an urgent need to establish a greater understanding of metastatic processes. It is highly likely that cancer stem cells (CSCs) are the key cells that mediate metastases, even while the cellular origin of CSCs remains unknown. Growing evidence has also revealed that the microenvironment has profound effects on the regulation of CSCs. Recently, it has been shown that bone metastatic cancer cells target the microenvironment or 'niche', which houses hematopoietic stem cells (HSCs). The major function of the HSC niche is to maintain 'stemness' of HSCs. These findings suggest that by targeting the HSC niche, metastatic cells parasitize the very foundation of hematopoiesis to maintain their stemness. These observations suggest that there will be a need to target the HSC niche to provide effective therapies to eradicate metastatic CSCs.
Introduction
'Cancer' is defined as a group of diseases in which cells grow uncontrollably and invade other tissues. Cancer can also spread to distant organs through the blood stream and the lymphatic system. Early detection and treatment can lead to better disease prognosis. Conventional treatment approaches, including surgery, radiotherapy, chemotherapy or hormonal therapy, are effective for localized cancer. However, cancer with distant metastasis remains incurable. Therefore, one of the most important issues in cancer biology is to identify cells with high metastatic potential.
Accumulating evidence suggests that primary tumors consist of heterogeneous populations of the cancer cells. A subpopulation of these, also known as 'tumor-initiating cells' or 'cancer stem cells (CSCs)', have the ability to self-renew and eventually form diverse tumor cell population.1 In addition, clinical observations strongly suggest that CSCs are responsible for metastasis.2 Tumors derived from an early stage of CSCs (or progenitor cells) have high metastatic potential, whereas tumors rarely metastasize as 'mature' or differentiated tumor cells.2 Moreover, tumors that do not maintain a sizable stem cell population do not metastasize at all.2 Other evidence that supports this notion is that CSCs express high levels of the chemokine receptor CXCR4, which are critical for the homing process.3 The bone marrow strongly expresses CXCL12, the ligand of CXCR4, and the circulating hematopoietic stem cells (HSCs) migrate toward the bone marrow microenvironment, or the niche, through the CXCL12/CXCR4 axis.4 Similar to the HSC homing to the marrow, the CXCL12/CXCR4 axis also mediates the metastasis of solid tumors to the bone marrow.5,6 Recent work by our group has demonstrated that metastatic prostate cancer (PCa) cells target the bone marrow HSC niche during dissemination and compete for occupancy of the niche with HSCs.7 The main function of the niche is to promote HSC self-renewal, keep them quiescent and protect them from exhaustion or apoptosis.
These findings therefore suggest that the disseminated tumor cells (DTCs) take up residence in the bone marrow HSC niche to become dormant and facilitate resistance to current therapeutic approaches. Whether 'non-stem DTCs' preferentially home to the bone marrow and utilize the niche to become CSCs remains unclear and highly controversial. Regardless of whether the niche is able to transform non-stem DTCs and/or maintain CSCs in a stem-like state represents a major advance in our understanding of bone marrow metastasis. Clearly, a better understanding of the CSCs biology could lead to the development of novel preventive and therapeutic strategies for cancer recurrence. In this review, we will discuss the CSC hypothesis including recent debates and strive to provide insights into bone marrow microenvironments as potential therapeutic targets for eradicating CSCs.
CSC hypothesis
Although the CSC hypothesis has provided new insights into our understanding of tumorigenesis, there are still vigorous debates surrounding the existence of CSCs. The first controversy is the identification or isolation of CSCs based on cell surface marker profiles. It has been believed that a subpopulation of tumor cells has tumor-initiating ability in both hematologic malignancies and solid tumors. Identification of these rare cells, within the bulk of tumor cells, by fluorescence-activated cell sorting using a combination of cell surface markers has been a major area of study. In 1994, the existence of CSCs in hematologic malignancy was first reported.8 In this study, 1 in 250 000 cells expressing the CD34+CD38− phenotype in human acute myeloid leukemia are able to engraft into immunodeficeint mice, whereas cells derived from either CD34+CD38+ or CD34− fraction are not capable of engrafting.8 In solid tumors, CD44+CD24−/lowLin− breast cancer cells were identified as being capable of generating tumors when the cells were implanted into immunodeficeint mice orthotopically.9 Subsequently, CSCs of other cell types including brain tumor,10 PCa11 and pancreatic cancer12 have been identified. Although many markers, such as CD24, CD34, CD44, CD133, aldehyde dehydrogenase and Hoechst 33342, have been combined to segregate CSC, no marker or a combination of markers successfully isolate a single CSC subset as of yet. In addition, it was recently demonstrated that many tumorigenic markers reversibly switch between tumorigenic melanoma cells and non-tumorigenic melanoma cells.13 These findings suggest that some cancers may not follow a stem cell model. For instance, all tumorigenic melanoma cells obtained from patients were able to form tumors in immunodeficient mice.13 Moreover, none of the tested 22 markers were capable of enriching tumorigenic cells or sorting out the large population of tumor cells that fail to form tumors.13 While clearly variations on the stem cell model are possible, the finding that many cancers do follow the model represents a major change in our understanding of tumor progression.
The second controversial issue is the origin of CSCs. Two hypothetical models exist. The 'traditional' CSC hypothesis suggests that cancer is heterogeneous because a single cell, or CSC, is capable of both self-renewal and sustaining tumor formation while the vast bulk of tumor cells have restricted reproductive ability.14 This hierarchical CSC model suggests that cancers are organized in a hierarchy similar to the hematopoietic system. With this model, CSCs are thought to be a source of disease recurrence. The basis of this concept is that CSCs are likely to be resistant to both radiotherapy and chemotherapy as radiotherapy and the majority of existing chemotherapies are dependent on targeting proliferating cells. If tumors are initiated by CSCs, therapies that target CSCs specifically would be desirable. Although many of the tumors, including leukemia8 and breast cancer,9 appear to follow the hierarchal CSC model; others are likely to follow a stochastic model.13 As mentioned previously, nearly 30% of immunodeficient mice develop tumors when single melanoma cells obtained from various stages of patients were implanted.13 This model implies that some cancers are comprised of heterogeneous cells many of which maintain the capacity to proliferate perpetually and have ability to form tumors. In fact, melanomas may not be the only tumor type, which deviates from a hierarchal model. A recent investigation suggests even variations in breast cancer may occur as CD44low non-CSCs spontaneously convert into CD44hi CSCs both in vitro and in vivo.15
Another important and controversial issue in stem cell research is whether or not all stem cells cycle at the same rate. Recent evidence in several systems including the bone marrow, intestinal epithelium and the hair follicle argues for the existence of two types of stem cells: those that are involved in day-to-day tissue turnover and regeneration that regularly enter into proliferative cycles, and those that are deeply quiescent.16,17 These deeply quiescent cells are thought to rarely contribute to normal tissue maintenance but are kept as reserve cells—largely immune from tissue or environmental impact—which when necessary can be called upon to participate in regeneration. These two subpopulations of stem cells are thought to coexist in multiple tissues; however, whether they exist in separate or adjoining locations remains unclear. Importantly, whether separate or integrated signaling pathways exist for each subpopulation has yet to be evaluated. It is not yet clear if CSCs also have subpopulations that participate in the bulk of tumor maintenance and growth, and those that participate in establishing dormancy. Conceptually attractive is the concept that CSCs also have subpopulations that mimic those findings in the stem cell field. Unfortunately, there are little data to guide us in whether subtypes of CSCs exist, but the concept makes considerable sense given how effective chemotherapeutics may be in eliminating many stem and progenitor populations in the bulk of neoplasms. Moreover, whether there are different niches that regulate each of the CSCs within metastatic sites remains to be determined.
The ability of cancers to maintain plasticity in its stem cell compartment provides new challenges and opportunities for cancer treatments. If cancers follow the stochastic model, a potential treatment strategy could be 'Kill Them All' approach or 'differentiation therapy'. If CSCs and non-CSCs can reversibly convert into one another, then it may be possible to eradicate cancers by inducing permanent differentiation of the CSCs. Consequently, differentiation therapy could convert aggressive cancer into benign neoplasms. The most thoroughly examined and clinically successful differentiation treatment is retinoic acid in treatment of acute promyelocytic leukemia.18 However, differentiation therapy in solid tumors is yet not well defined. An understanding of the cellular signals that control CSC fate could lead to the development of a new therapeutic approach to ultimately eradicate cancers. As the findings will have great relevance to treatment, further research on the clinical, genetic, molecular and cellular aspects of CSCs will be clearly needed.
Bone marrow microenvironment and CSCs
Among the most well-defined microenvironments in the stem cell field is the bone marrow. In hematopoiesis, HSCs are known to reside in specific microenvironments to escape from the cytotoxic insults. This microenvironment that supports stem cell function is commonly referred to as the stem cell niche.19 Despite continuing controversy,20,21 the stem cell niche is believed to be dominated by two major types of cells: cells of the osteoblastic19,22,23,24 and endothelial lineages,25 while the participation of other cell types is clearly known.26,27,28 The stem cell niche is thought to have a crucial role in the homing, self-renewal, proliferation and differentiation of HSCs. It is suggested that the endosteal or osteoblastic niche maintains the long-term survival of HSCs, whereas the endothelial or vascular niche regulates HSCs that are immediately able to respond to physiological stresses, including infection and bleeding.29 The osteoblastic niche is also involved in the homing process of HSCs; circulating HSCs or hematopoietic progenitor cells use CXCR4 to home to the osteoblastic niche, which is a major source of CXCL12.19
Like HSCs, recent studies have demonstrated that solid tumor cells also utilize the CXCL12/CXCR4 axis when they metastasize to distant organs.5,6 In addition, both HSCs and PCa use the same adhesion molecule, annexin II (Anxa2), to adhere to the osteoblastic niche.30,31 We recently observed that Anxa2 signaling has an important role in maintaining HSCs as the number of HSCs are fewer in Anxa2 knockout mice compared with wild-type mice.30 By blocking Anxa2 signaling, the engraftment of HSCs is significantly inhibited.30 Similarly, by blocking Anxa2 or its receptor, short-term and long-term dissemination of PCa were prevented in the xenograft animal model.31 Moreover, Anxa2 interacts synergistically with CXCL12 to stimulate the homing of HSCs.32 While Anxa2 is clearly important in regulating niche function, other molecules are also important. For example, it is also well established that α4β1 integrin (VLA-4) have an important role in the HSC homing to bone marrow through adhesion to vascular cell adhesion molecule-1 or fibronectin expressed by bone marrow niche.33 Likewise, upregulation of α4β1 integrin in Chinese hamster ovary cells and K562 human leukemia cell line enhanced bone metastasis in vivo, and these activities are countered by antibody against α4 or vascular cell adhesion molecule-1.34 Like the data on Anxa2, these findings strongly suggest that cancer cells use similar mechanisms as HSCs do to gain access to the marrow during metastasis.
Along with these findings, more recent work by our group has revealed that metastatic PCa cells target the same area where HSCs reside.7 First, engraftment of transplanted HSCs to bone marrow was dramatically prevented by disseminated PCa cells from subcutaneously implanted primary tumor.7 Bone marrow rescue in lethally irradiated mice was also inhibited by simultaneously injected PCa cells.7 Furthermore, human PCa cells prevent the engraftment of human HSCs into the bone marrow of recipient mice.7 Immunohistochemical analyses confirmed that HSCs and disseminated PCa cells colocalize with each other at bone-lining osteoblastic niche.7 Manipulating the size of niche also altered the frequency of bone metastases.7 When the osteoblastic niche was expanded with parathyroid hormone treatments,22 significantly more metastatic PCa cells were found in the marrow.7 Conversely, when the osteoblastic niche was ablated using thymidine kinase-transformed bone tissues,35 the number of metastatic cells was significantly reduced.7 Premobilization of HSCs from the niche using HSC-mobilizing agents help recruit disseminated PCa cells into the niche.7 Interestingly, the same HSC-mobilizing agents could mobilize disseminated PCa cells from the marrow.7 Surprisingly, PCa with CSC properties preferably metastasized to the marrow and bound to osteoblast niche, compared with non-CSC populations.7 Finally, disseminated PCa cells displaced HSCs from the HSC niche, drove them into hematopoietic progenitor cells and affect their gene profiles.7 Critically, these observations were further confirmed with patients' samples.7
Collectively, work in PCa models of dissemination demonstrate that PCa cells target the bone marrow HSC niche during metastases and compete with HSCs for the occupancy of the osteoblastic niche. In a similar fashion, it has been reported that leukemia stem cells home to the osteoblastic region in the bone marrow and share the niche with normal stem cells to become resistant to chemotherapy.36,37 A very recent study also revealed the vascular niche also may be a target of metastasis. Here the interaction of vascular niche and skin cancer cells initiates the semness of skin cancers through VEGF–NRP1 pathway.38 Thus, accumulating evidence including our findings implies that the HSC niche is pivotal in controlling the stemness of cancer in the marrow. As the predominant role of the HSC niche is to maintain HSCs as quiescent, it is natural to assume that cancer cells take advantage of this function to maintain stemness and perhaps even to become CSC as in the case of metastatic non-CSCs to survive for an extended period in the marrow.
This last point is pivotal. If indeed CSCs target and engage the HSC niche, there is a high probability that these cells would engage the niche machinery to become dormant and therefore acquire the ability to escape from the existing radiotherapy and chemotherapy that target rapidly dividing cells. Although identifying the self-renewal pathways of CSCs is obviously necessary, efficient blockage of crosstalk between CSCs and the niche could also be a potential target to eradicate this small population of cancer cells.39 Based upon our recent findings,7 niche-engaging DTCs can be mobilized from the niche using HSC-mobilizing agents. If CSCs maintain their stemness by anchoring to the niche, CSCs could also be transformed into progenitor-like state in which cancer cells are actively dividing by releasing from the niche. Accordingly, the released CSCs are likely to be responsive to the current therapy that attacks the cells in the cycle. In fact, it has been demonstrated that pre-treatment of AMD3100, a CXCR4 antagonist and also known as a HSC-mobilizing agent,40 reverses the chemosensitivity of acute leukemia41 and myeloma cells,42 suggesting that the combination of mobilization and succeeding chemotherapy is a potential therapy for bone marrow malignancy. By interfering with the interactions between acute myeloid leukemia and bone marrow cells by blocking VLA-4 pathway also restored the chemosensitivity of acute myeloid leukemia.43 Furthermore, it was suggested that HSC mobilization initiated by granulocyte colony-stimulating factor and/or AMD3100 are enhanced in combination with VLA-4 blockage.44,45 Therefore, a combination of mobilizing agents and the blockage of adhesion molecules could prove to be a promising strategy for driving dormant CSCs into the cycle. Although few therapies that target CSCs are available, interference with CSC–niche interactions are attractive targets for eradicating CSCs.
Concluding remarks
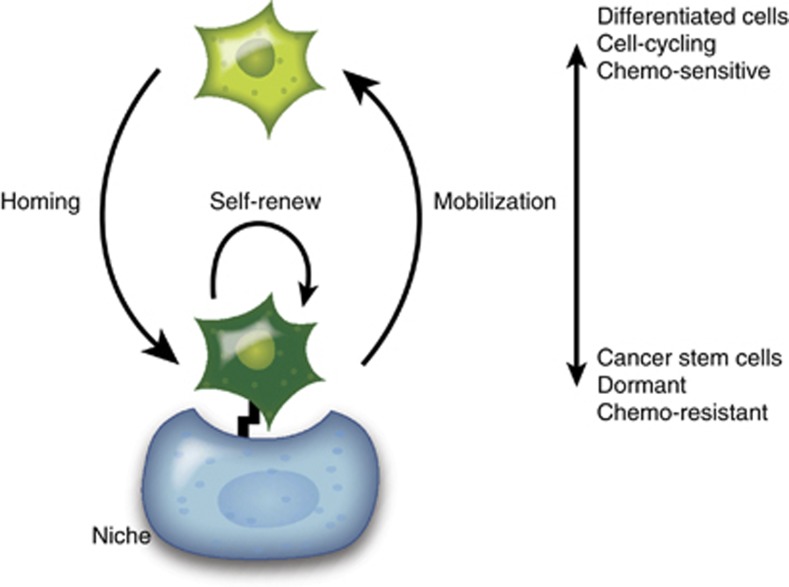
Paget46 proposed that each cancer type has a preferential microenvironment for its metastatic spread in his seed and soil hypothesis. It is plausible that bone metastatic cancer cells target the microenvironments that maintain the HSC function,7 and may in fact parasitize these microenvironments, or the niches, to establish future metastatic growth in the bone marrow47 (Figure 1). As the microenvironment of metastatic cancer cells is studied further, more light undoubtedly will be shed on the complicated mechanisms of dormancy and metastatic growth in the bone marrow. Although which cancer cells are capable of self-renew remains a question, the bone marrow niche may have a crucial role, perhaps even a dominant role, in maintaining stemness and selecting which cells are capable of activating specific self-renewal pathways. If true, CSCs are likely to acquire cell adhesion-mediated drug resistance through the receptor–ligand interactions regulated by the niche. Therefore to eradicate metastatic cancer cells in the marrow, a better understanding of the self-renewal pathways in CSCs activated by the microenvironment is warranted.
Figure 1.
The roles of the microenvironment in bone metastatic disease. Metastatic cancer cells home to the bone marrow microenvironment, or the niche, using similar mechanisms as the homing of HSCs. When the metastatic cells engage to the niche, they acquire the self-renewal potential through the signals released from the niche most likely involving cooperative interactions with non-niche cells and by paracrine interactions. Consequently, cancer cells obtain the stem-like phenotype, undergo dormancy and are therefore protected from the cytotoxic insults. Conversely, cancer cells differentiate into progenitors that are believed to be in active cycle, and therefore become sensitive to the radiotherapy and chemotherapy, when they detach from the protective functions of the niche.
Acknowledgments
This work is directly supported by the National Cancer Institute (CA093900, RST; CA163124, YS and RST), the Department of Defense (YS and RST) and the Prostate Cancer Foundation (YS and RST).
Footnotes
The authors declare no conflict of interest.
References
- Al-Hajj M, Clarke MF. Self-renewal and solid tumor stem cells. Oncogene 2004;23:7274–7282. [DOI] [PubMed] [Google Scholar]
- Tu SM, Lin SH, Logothetis CJ. Stem-cell origin of metastasis and heterogeneity in solid tumours. Lancet Oncol 2002;3:508–513. [DOI] [PubMed] [Google Scholar]
- Moriyama T, Ohuchida K, Mizumoto K, Cui L, Ikenaga N, Sato N et al. Enhanced cell migration and invasion of CD133+ pancreatic cancer cells cocultured with pancreatic stromal cells. Cancer 2010;116:3357–3368. [DOI] [PubMed] [Google Scholar]
- Wright DE, Bowman EP, Wagers AJ, Butcher EC, Weissman IL. Hematopoietic stem cells are uniquely selective in their migratory response to chemokines. J Exp Med 2002;195:1145–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taichman RS, Cooper C, Keller ET, Pienta KJ, Taichman NS, McCauley LK. Use of the stromal cell-derived factor-1/CXCR4 pathway in prostate cancer metastasis to bone. Cancer Res 2002;62:1832–1837. [PubMed] [Google Scholar]
- Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME et al. Involvement of chemokine receptors in breast cancer metastasis. Nature 2001;410:50–56. [DOI] [PubMed] [Google Scholar]
- Shiozawa Y, Pedersen EA, Havens AM, Jung Y, Mishra A, Joseph J et al. Human prostate cancer metastases target the hematopoietic stem cell niche to establish footholds in mouse bone marrow. J Clin Invest 2011;121:1298–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature 1994;367:645–648. [DOI] [PubMed] [Google Scholar]
- Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA 2003;100:3983–3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T et al. Identification of human brain tumour initiating cells. Nature 2004;432:396–401. [DOI] [PubMed] [Google Scholar]
- Collins AT, Berry PA, Hyde C, Stower MJ, Maitland NJ. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res 2005;65:10946–10951. [DOI] [PubMed] [Google Scholar]
- Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V et al. Identification of pancreatic cancer stem cells. Cancer Res 2007;67:1030–1037. [DOI] [PubMed] [Google Scholar]
- Quintana E, Shackleton M, Foster HR, Fullen DR, Sabel MS, Johnson TM et al. Phenotypic heterogeneity among tumorigenic melanoma cells from patients that is reversible and not hierarchically organized. Cancer Cell 2010;18:510–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature 2001;414:105–111. [DOI] [PubMed] [Google Scholar]
- Chaffer CL, Brueckmann I, Scheel C, Kaestli AJ, Wiggins PA, Rodrigues LO et al. Normal and neoplastic nonstem cells can spontaneously convert to a stem-like state. Proc Natl Acad Sci USA 2011;108:7950–7955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Clevers H. Coexistence of quiescent and active adult stem cells in mammals. Science 2010;327:542–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs E. The tortoise and the hair: slow-cycling cells in the stem cell race. Cell 2009;137:811–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sell S. Stem cell origin of cancer and differentiation therapy. Crit Rev Oncol/Hematol 2004;51:1–28. [DOI] [PubMed] [Google Scholar]
- Taichman RS. Blood and bone: two tissues whose fates are intertwined to create the hematopoietic stem-cell niche. Blood 2005;105:2631–2639. [DOI] [PubMed] [Google Scholar]
- Li P, Zon LI. Resolving the controversy about N-cadherin and hematopoietic stem cells. Cell Stem Cell 2010;6:199–202. [DOI] [PubMed] [Google Scholar]
- Askmyr M, Sims NA, Martin TJ, Purton LE. What is the true nature of the osteoblastic hematopoietic stem cell niche? Trends Endocrinol Metab 2009;20:303–309. [DOI] [PubMed] [Google Scholar]
- Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature 2003;425:841–846. [DOI] [PubMed] [Google Scholar]
- Arai F, Hirao A, Ohmura M, Sato H, Matsuoka S, Takubo K et al. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell 2004;118:149–161. [DOI] [PubMed] [Google Scholar]
- Zhang J, Niu C, Ye L, Huang H, He X, Tong WG et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature 2003;425:836–841. [DOI] [PubMed] [Google Scholar]
- Kiel MJ, Yilmaz OH, Iwashita T, Yilmaz OH, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell 2005;121:1109–1121. [DOI] [PubMed] [Google Scholar]
- Naveiras O, Nardi V, Wenzel PL, Hauschka PV, Fahey F, Daley GQ. Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment. Nature 2009;460:259–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 2010;466:829–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama T, Kohara H, Noda M, Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity 2006;25:977–988. [DOI] [PubMed] [Google Scholar]
- Yin T, Li L. The stem cell niches in bone. J Clin Invest 2006;116:1195–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung Y, Wang J, Song J, Shiozawa Y, Havens A, Wang Z et al. Annexin II expressed by osteoblasts and endothelial cells regulates stem cell adhesion, homing, and engraftment following transplantation. Blood 2007;110:82–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiozawa Y, Havens AM, Jung Y, Ziegler AM, Pedersen EA, Wang J et al. Annexin II/annexin II receptor axis regulates adhesion, migration, homing, and growth of prostate cancer. J Cell Biochem 2008;105:370–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung Y, Shiozawa Y, Wang J, Patel LR, Havens AM, Song J et al. Annexin-2 is a regulator of stromal cell-derived factor-1/CXCL12 function in the hematopoietic stem cell endosteal niche. Exp Hematol 2011;39:151–166.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papayannopoulou T, Priestley GV, Nakamoto B, Zafiropoulos V, Scott LM. Molecular pathways in bone marrow homing: dominant role of alpha(4)beta(1) over beta(2)-integrins and selectins. Blood 2001;98:2403–2411. [DOI] [PubMed] [Google Scholar]
- Matsuura N, Puzon-McLaughlin W, Irie A, Morikawa Y, Kakudo K, Takada Y. Induction of experimental bone metastasis in mice by transfection of integrin alpha 4 beta 1 into tumor cells. Am J Pathol 1996;148:55–61. [PMC free article] [PubMed] [Google Scholar]
- Visnjic D, Kalajzic Z, Rowe DW, Katavic V, Lorenzo J, Aguila HL. Hematopoiesis is severely altered in mice with an induced osteoblast deficiency. Blood 2004;103:3258–3264. [DOI] [PubMed] [Google Scholar]
- Ishikawa F, Yoshida S, Saito Y, Hijikata A, Kitamura H, Tanaka S et al. Chemotherapy-resistant human AML stem cells home to and engraft within the bone-marrow endosteal region. Nat Biotechnol 2007;25:1315–1321. [DOI] [PubMed] [Google Scholar]
- Ninomiya M, Abe A, Katsumi A, Xu J, Ito M, Arai F et al. Homing, proliferation and survival sites of human leukemia cells in vivo in immunodeficient mice. Leukemia 2007;21:136–142. [DOI] [PubMed] [Google Scholar]
- Beck B, Driessens G, Goossens S, Youssef KK, Kuchnio A, Caauwe A et al. A vascular niche and a VEGF-Nrp1 loop regulate the initiation and stemness of skin tumours. Nature 2011;478:399–403. [DOI] [PubMed] [Google Scholar]
- Shiozawa Y, Pienta KJ, Taichman RS. Hematopoietic stem cell niche is a potential therapeutic target for bone metastatic tumors. Clin Cancer Res 2011;17:5553–5558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broxmeyer HE, Orschell CM, Clapp DW, Hangoc G, Cooper S, Plett PA et al. Rapid mobilization of murine and human hematopoietic stem and progenitor cells with AMD3100, a CXCR4 antagonist. J Exp Med 2005;201:1307–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nervi B, Ramirez P, Rettig MP, Uy GL, Holt MS, Ritchey JK et al. Chemosensitization of acute myeloid leukemia (AML) following mobilization by the CXCR4 antagonist AMD3100. Blood 2009;113:6206–6214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azab AK, Runnels JM, Pitsillides C, Moreau AS, Azab F, Leleu X et al. CXCR4 inhibitor AMD3100 disrupts the interaction of multiple myeloma cells with the bone marrow microenvironment and enhances their sensitivity to therapy. Blood 2009;113:4341–4351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsunaga T, Takemoto N, Sato T, Takimoto R, Tanaka I, Fujimi A et al. Interaction between leukemic-cell VLA-4 and stromal fibronectin is a decisive factor for minimal residual disease of acute myelogenous leukemia. Nat Med 2003;9:1158–1165. [DOI] [PubMed] [Google Scholar]
- Bonig H, Watts KL, Chang KH, Kiem HP, Papayannopoulou T. Concurrent blockade of alpha4-integrin and CXCR4 in hematopoietic stem/progenitor cell mobilization. Stem Cells 2009;27:836–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez P, Rettig MP, Uy GL, Deych E, Holt MS, Ritchey JK et al. BIO5192, a small molecule inhibitor of VLA-4, mobilizes hematopoietic stem and progenitor cells. Blood 2009;114:1340–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paget S. The distribution of secondary growths in cancer of the breast. Lancet 1889;1:571–573. [PubMed] [Google Scholar]
- Shiozawa Y, Havens AM, Pienta KJ, Taichman RS. The bone marrow niche: habitat to hematopoietic and mesenchymal stem cells, and unwitting host to molecular parasites. Leukemia 2008;22:941–950. [DOI] [PMC free article] [PubMed] [Google Scholar]