Dear Editor,
There is increasing awareness of an on-going epidemic of sexually-transmitted acute hepatitis C virus (HCV) infection among HIV-infected men who have sex with men (MSM). The percentage of all HIV infected people who also have HCV ranges from 15% to 30%, and one study reported that 37% of people with HIV were co-infected with HCV.1,2 Unrecognized HIV/HCV co-infection has serious implications on liver health, as it may accelerate the progression of HCV liver disease.1,2 Early detection of people co-infected with HIV and HCV is essential in managing treatment of these infections. Thus in 2009, the European Aids Clinical Society (EACS) guidelines3 introduced the notion of systematic annual HCV screening among HIV-infected patients. All HIV-infected patients should be screened for HCV infection using sensitive immunoassays licensed for detection of antibody to HCV in blood.2 HCV-seronegative patients at risk for acquiring HCV infection should undergo repeat testing annually.
We evaluated, retrospectively, staff knowledge, HCV screening rates, and seroconversion rates for HCV of individuals enrolled in our AIDS Reference Centre in order to determine physicians' adherence to HCV-screening recommendations. This study was designed on a criterion-based medical audit framework, with annual HCV serology in non-immune as the selected standard, and all HIV patients as a target. Eight physicians (HIV specialists) were interviewed on recommendations and perceived adherence to EACS clinical guidelines regarding HCV screening. Self-reported knowledge and adherence were assessed through face-to-face interviews with each of the physicians.
Patients on regular follow-up each year from 2008 to 2011 in the Centre were included. We considered a patient to be on regular follow-up when records showed at least two clinical reviews and one HIV viral load testing during the year. Demographic features and HCV serology tests were collected from the operating software of our institution.
Adherence to guidelines was measured by dividing the percentage of the patient population receiving the service (HCV screening) by the percentage of the patient population who should have received the service according to the guidelines, in this case, the whole HIV-positive population on follow-up. Diagnosis of HCV was retained when serology became positive and HCV RNA was detected. Percentage of patients screened were compared using the X2 test, and a two-tailed p<0.05 was considered statistically significant.
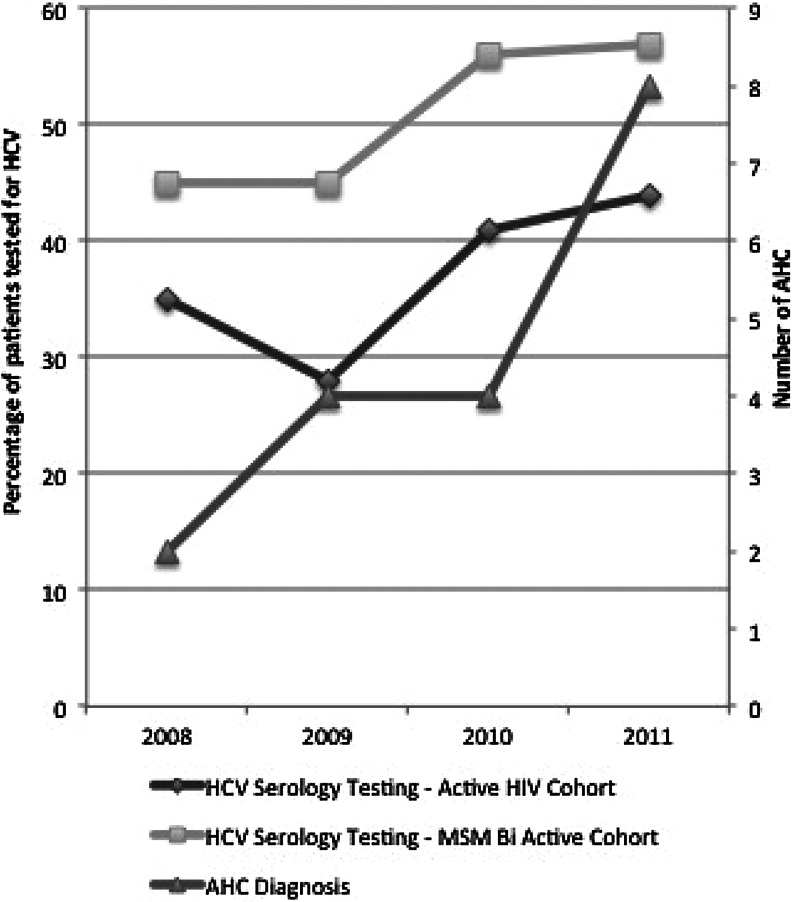
Though knowledge of current guidelines was excellent (100%), physicians claimed an 87.5% (7/8) adherence rate to these recommendations. The objective rate of screening rose gradually between 2008 and 2011, especially after introduction of EACS guidelines in 2009 (Table 1 and Fig. 1). The maximal screening rate was obtained in 2011, with 44% of patients tested among the general HIV population and 57% among MSM–bisexual patients. This trend was statistically significant in both populations (p<0.01). Additional analyses on screening rates were done according to risk factors. No active intravenous drug user was observed among our patients from 2007 to 2011. In our cohort, MSM were significantly more tested than heterosexual patients (X2, p<0.0001). The year 2011 displayed a marked increase in diagnosis of HCV infection, with 8 new patients diagnosed among the 963 patients who met inclusion criteria; all of them were MSM.
Table 1.
Data Summary Comparing Patients on Regular Follow-Up (f/u) and Number of Patients Screened
| Year | Patients on regular f/u, n | MSM-bisexual patients on regular f/u, n (%) | HCV serology tests total active cohort, n (%) | HCV serology tests among MSM-bisexual patients, n (%) |
|---|---|---|---|---|
| 2008 | 818 | 258 (31) | 292 (35) | 118 (45) |
| 2009 | 869 | 290 (33) | 314 (28) | 132 (45) |
| 2010 | 938 | 308 (33) | 389 (41) | 198 (56) |
| 2011 | 963 | 335 (35) | 425 (44) | 193 (57) |
FIG. 1.
Evolution of HCV screening and acute HCV hepatitis diagnosis (2008–2011).
Clinical guidelines are increasingly being used to improve the quality of medical care. An important task in guideline-based quality improvement, the assessment of medical care quality, can be accomplished by retrospectively comparing clinician actions to the guideline recommendations. Performance measures are defined as ratios that determine the extent to which a clinician's action conform to the clinical practice guideline. However, it is still mostly limited to evaluating simple one-step elements of medical care.4 Literature on adherence to guidelines relies partly on self-reporting. Validity of such literature is questionable, as there is a possible response bias in self-report. When compared to more objective measures, Adams et al. confirmed the existence of a substantial overestimation of adherence (median absolute difference of 27%).5 Our team was no exception to this, with an absolute difference of 29.5% at best.
Though not many studies address the issue of adherence to preventive measures recommendations among HIV specialists, results show very variable rates of compliance. On the specific issues of hepatitis B or C screening, mostly studies addressing initial screening could be found. Our figures are consistent with those of Hoover et al., who evaluated screening rates for hepatitis B and C in a random sample of HIV-infected MSM in HIV clinics in the US to 52% and 54%, respectively.6 However, looking at the British national audit on co-infections, annual screening rates for HBV and HCV were 71% and 66%, respectively, though these figures rely on self-reported estimates.7 Among US veterans tested for HIV and found to be positive for HIV, Wright et al. observed that approximately 79.1% were co-tested for HCV.8 On the other hand, HIV screening in HCV-positive patients among the Veterans Health Administration, Fuller et al. demonstrated that only approximately one-third of veterans with HCV were tested for HIV (32%).9 The ECDC review on hepatitis B and C collected available data in several specific populations. Among IDUs for example, the proportion of patients screened for HCV varied between 5% in the United Kingdom and 88% in Ireland.10 These results, though quite variable, tend to demonstrate a trend towards low adherence to hepatitis screening guidelines. They underscore the need for providers and clinics to evaluate their data and implement effective and sustainable interventions to increase hepatitis screening.
In recent years, there have been reports of HCV infection acquisition in HIV-infected MSM linked to sexual transmission and associated with the use of nonparenteral recreational drugs.2,11 This was translated in previous guidelines as recommended annual HCV screening in HIV patients with selected risk factors. It probably explains the higher rates of screening among the MSM–bisexual population in our cohort. Garvey et al. suggested that reasons for suboptimal screening rates may include a clinical decision that a subject is at ‘low risk’, for example to HCV, thus making the test a low priority and other general time constraints in the outpatient setting.7 These seemed relevant in our centre, where only a third of the patients are MSM, and no active IDU is currently on follow-up. Qualitative research would be useful in identifying barriers to better adherence and in selecting appropriate interventions to improve quality of care.
Bonnard et al. already implemented a tool (ORCHESTRA Programme: automatic remind to test HCV) on an active cohort of 3000 HIV-infected patients. One year after implementing this system, the proportion of HIV-infected patients whose last negative HCV screening test dated back more than 3 years fell from 46% to 24%, however, these measures failed to increase HBV and HAV vaccine uptake.12
In our Centre, knowledge of EACS guidelines on screening for HCV was good but adherence to these recommendations is poor, though it improves over time. Education of clinicians is warranted to increase awareness and further improve adherence to guidelines. Qualitative research might help in identifying barriers and in selecting appropriate interventions to improve quality of care.
Acknowledgments
Institutional ethical committee approval was granted for this study (N°2012/13SEP/430) by the IRB (CEBH of the Université catholique de Louvain, Brussels, Belgium).
Author Disclosure Statement
All co-authors have participated in and agree with the content and conclusions This work is original and does not infringe any copyright.
References
- 1.Rockstroch JK. Spengler U. HIV and hepatitis C virus co-infection. Lancet. 2004;4:437–444. doi: 10.1016/S1473-3099(04)01059-X. [DOI] [PubMed] [Google Scholar]
- 2.Kim AY. Onofrey S. Church DR. An epidemiologic update on hepatitis C infection in persons living with or at risk of HIV infection. J Infect Dis. 2012;207:S1–S6. doi: 10.1093/infdis/jis927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.EACS guidelines Version 5, Clinical Management and Treatment of Chronic Hepatitis B & C
- 4.Advani A. Shahar Y. Musen M. Medical quality assessment by scoring adherence. J Am Med Inform Assoc. 2002;9:S92–S97. [Google Scholar]
- 5.Adams A. Soumerai S. Lomas J. Ross-Degnan D. Evidence of self-report bias in assessing adherence to guidelines. Int J Qual Health Care. 1999;11:187–192. doi: 10.1093/intqhc/11.3.187. [DOI] [PubMed] [Google Scholar]
- 6.Hoover KW. Butler M. Workowski KA, et al. Evaluation group for adherence to STD and hepatitis screening, low rates of hepatitis screening and vaccination of HIV-infected MSM in HIV clinics. Sex Transm Dis. 2012;39:349–353. doi: 10.1097/OLQ.0b013e318244a923. [DOI] [PubMed] [Google Scholar]
- 7.Garvey L. Curtis H. Brook G. The British HIV Association national audit on the management of subjects co-infected with HIV and hepatitis B/C. Int J STD AIDS. 2011;22:173–176. doi: 10.1258/ijsa.2010.010380. [DOI] [PubMed] [Google Scholar]
- 8.Wright TL. Yee H. Federal Practitioner. Vol. 20. Veterans Health Administration; 2003. VA treatment recommendations (version 5.0) pp. S1–S33. [Google Scholar]
- 9.Fuller B. Rodriguez V. Linke A. Hauser P. HIV co-testing among veterans with chronic hepatitis C. Open Infect Dis J. 2011;5:91–96. [Google Scholar]
- 10.European Centre for Disease Prevention and Control. Stockholm: ECDC; 2010. Hepatitis B and C in the EU neighbourhood: Prevalence, burden of disease and screening policies. [Google Scholar]
- 11.Taylor LE. DeLong AK. Maynard MA, et al. Acute hepatitis C virus in an HIV clinic: A screening strategy, risk factors, and perception of fisk. AIDS Patient Care STDs. 2011;26:571–577. doi: 10.1089/apc.2011.0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bonnard P. Pialoux G. Evaluation of a computer algorithm for promoting both annual hepatitis C screening and hepatitis B vaccination among HIV-infected individuals (ORCHESTRA) programme. Intl J STD AIDS. 2011;22:613–616. doi: 10.1258/ijsa.2011.011192. [DOI] [PubMed] [Google Scholar]