Abstract
Significance: Appropriately controlled epigenetic regulation is critical for the normal development and health of an organism. Misregulation of epigenetic control via deoxyribonucleic acid (DNA) methylation or histone methylation has been associated with cancer and chromosomal instability syndromes. Recent Advances: The main function of the proteins in the base excision repair (BER) pathway is to repair DNA single-strand breaks and deamination, oxidation, and alkylation-induced DNA base damage that may result from chemotherapy, environmental exposure, or byproducts of cellular metabolism. Recent studies have suggested that one or more BER proteins may also participate in epigenetic regulation to facilitate gene expression modulation via alteration of the state of DNA methylation or via a reaction coupled to histone modification. BER proteins have also been reported to play an essential role in pluripotent stem cell reprogramming. Critical Issues: One emerging function for BER in epigenetic regulation is the repair of base lesions induced by hydrogen peroxide as a byproduct of lysine-specific demethylase 1 (LSD1) enzymatic activity (LSD1/LSD2-coupled BER) for transcriptional regulation. Future Directions: To shed light on this novel role of BER, this review focuses on the repair of oxidative lesions in nuclear DNA that are induced during LSD1-mediated histone demethylation. Further, we highlight current studies suggesting a role for BER proteins in transcriptional regulation of gene expression via BER-coupled active DNA demethylation in mammalian cells. Such efforts to address the role of BER proteins in epigenetic regulation could broaden cancer therapeutic strategies to include epigenetic modifiers combined with BER inhibitors. Antioxid. Redox Signal. 18, 2429–2443.
Introduction
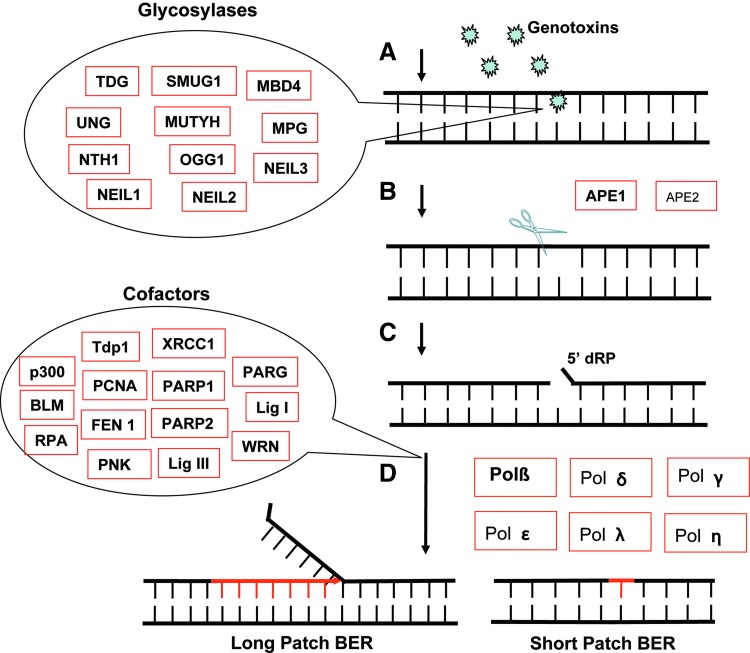
The proteins of the base excision repair (BER) pathway participate in the repair of dozens of base modifications that result from oxidative deoxyribonucleic acid (DNA) damage (88). Such damage can arise from numerous exogenous and endogenous sources, resulting in a multitude of detrimental cellular effects, including mutations, genome rearrangements, altered gene expression, and the onset of cell death or senescence (9,49,50). As depicted in Figure 1 and extensively reviewed elsewhere (88), many proteins are involved in the BER pathway to remove the base lesions and complete repair. Briefly, there are 11 human DNA glycosylases that remove the induced base lesions and thereby function to initiate BER. Once the base lesions are removed, the product, an apurinic/apyrimidinic (abasic or AP) site, is a substrate for an endonuclease specific for AP sites, the AP endonucleases APE1 or APE2 (although the majority activity results from APE1) (3). There is general consensus that the resulting DNA single-strand break after APE1 (or APE2) cleavage forms a nucleation site for scaffold proteins such as poly(adenosine diphosphate [ADP]-ribose) polymerase-1 (PARP1) and XRCC1, followed by recruitment of the proteins needed to complete repair. DNA polymerase β (Polβ) is considered the major end-trimming and DNA polymerase in BER (bolded, Fig. 1D). Alternate DNA polymerases have also been suggested to participate in BER, either in a short-patch repair subpathway or an alternate long-patch repair subpathway, as depicted in Figure 1. As mentioned above, BER has been considered to have a biological role limited to nuclear (and mitochondrial) genome repair in response to endogenous and exogenous genotoxins. However, recently, it has been suggested that these proteins may also have been co-opted to facilitate and enhance gene regulation, as will be discussed herein.
FIG. 1.
General model for base excision repair (BER). The BER pathway includes three essential steps: lesion recognition/strand scission, gap tailoring, and deoxyribonucleic acid (DNA) synthesis/ligation. (A) When DNA is damaged by genotoxins, BER is initiated with one of 11 human DNA glycosylases to remove base lesions and form the apurinic/apyrimidinic (abasic or AP) site or hydrolyze the DNA strand (depending on the glycosylase). (B) The AP endonucleases APE1 or APE2 are recruited to the AP site to cleave the DNA backbone and form a single-strand break. (C,D) The BER cofactor proteins are recruited to the lesion site working with DNA polymerase β (Polβ) (or other DNA polymerases) to complete the repair. Alternate DNA polymerases have also been suggested to participate in BER, either in a short-patch repair subpathway or an alternate long-patch repair subpathway. The enzymes depicted with smaller fonts suggest that they play a minor role in BER.
Innovation.
Proteins of the base excision repair (BER) pathway help maintain the stability of the genome by repairing deoxyribonucleic acid (DNA) base damage and DNA single-strand breaks that may arise from oxidative stress and cellular metabolism. Recent studies have suggested that one or more BER proteins may also play a role in epigenetic regulation of gene expression. To shed light on this novel role of BER, this review focuses on the repair of oxidative lesions in nuclear DNA that are induced during histone demethylation. Further, we highlight current studies suggesting a role for BER proteins in DNA demethylation.
The genome of more than 350 species has been sequenced (34), yet the gap between our understanding of the genotype and phenotype is still considerable. Through a series of spatiotemporal developmental steps, stem and progenitor cells of a multicellular organism are differentiated into different cell types with unique gene expression profiles to perform their specific functions (11,16,46,68). It is now quite clear that among the many biological and genetic alterations that define cellular function, the pattern of DNA and histone modification plays a main role to define the cellular phenotypes (54). The balance between the DNA methylation/demethylation and histone modification status can impact the structure of chromatin, define the on/off switch for many genes, and eventually change the physiologic outcome. For example, the fate of a honeybee (Apis mellifera) as either a worker or a queen is determined by its DNA methylation pattern (82). Approximately 3%–4% of genomic cytosine in a typical mammalian cell is methylated to 5-methylcytosine (5mC) as an epigenetic mark (24). These marks generally occur at the dinucleotide CpG of the promoter region of a gene. Methylation of cytosine to 5mC at the CpG sites is an important step in epigenetic transcriptional regulation related to transcriptional repression, X-chromosome inactivation, imprinting, and suppression of parasitic sequences (13,60,83). Abnormal DNA methylation patterns at the CpG sites are often observed in disease states such as global genome hypomethylation and tumor suppressor gene hypermethylation in cancer (28,31).
In addition, because the DNA is wrapped around histone proteins packed into the chromatin structure, post-translational modification of histone proteins is an important method for controlling DNA access and transcriptional regulation (14,41). The tails of H3 and H4 histones can be covalently modified on several residues by methylation, acetylation, phosphorylation, ubiquitylation, SUMOylation, citrullination, and ADP-ribosylation (19,41,64). Based on the status of these histone modifications, the expression of the modified gene might be upregulated or silenced.
Recent studies have demonstrated that BER may be involved or coupled with both active DNA demethylation (BCADD) and lysine-specific demethylase 1 (LSD1)-mediated histone demethylation (LC-BER) (40,76). In BCADD, three families of enzymes are involved: the Ten–eleven translocation (Tet) protein family, activation-induced deaminase (AID)/apolipoprotein B mRNA-editing enzyme complex (APOBEC), and proteins from the BER pathway. The TET proteins are responsible for the hydroxylation of 5mC to yield 5-hydroxy-methyl-cytosine (5hmC), which can be further oxidized to 5-formylcytosine (5fC) and 5-carboxylcytosine (5caC). AID/APOBEC proteins can deaminate 5mC (or 5hmC) to form thymine or 5-hydroxymethyluracil (5hmU). In the latter case, the resulting modified base would be mispaired with guanine. Once these modified bases are formed, the BER pathway is initiated by TDG to remove base modifications such as 5fC, 5caC, or 5hmU (22).
With regard to histone modification, BER is suggested to be coupled to LSD1-mediated histone demethylation to repair base lesions induced by hydrogen peroxide (H2O2) as a byproduct of the LSD1 (or LSD2) enzymatic activity (LC-BER). LSD1 is the first protein demethylase discovered that converts histone H3K4me2 to H3K4me1 or H3K4me0 through a flavin adenine dinucleotide (FAD)-dependent oxidative reaction. In the amine oxidase-mediated demethylation reaction, the cofactor FAD is reduced to FADH2 and then reoxidized to FAD by oxygen with the generation of H2O2 (Fig. 2). The DNA damaged by H2O2 from LSD1-mediated demethylation triggers the recruitment of the BER machinery to the promoter and regulatory response sites. It is suggested that it is the repair of the LSD1-induced DNA damage that facilitates and enhances transcription initiation (78). Therefore, it is likely that the BER machinery is not only critical for preventing reactive oxygen species (ROS)-induced genome instability, but it is also possible that BER is an essential component of ROS-mediated transcriptional regulation. In this review, the two main epigenetic modifications, DNA demethylation and histone demethylation, are discussed in detail with an emphasis on the roles of BER in transcriptional regulation.
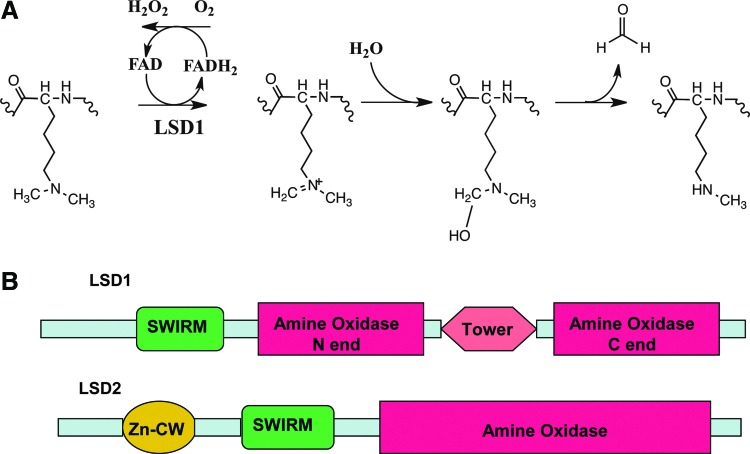
FIG. 2.
Comparative analysis of the mammalian flavin-dependent histone demethylases lysine-specific demethylase 1 (LSD1) and LSD2. (A) The reaction of LSD1/LSD2 demethylation: LSD1/LSD2 demethylates H3K4me2 via an amine oxidation reaction. Flavin adenine dinucleotide (FAD) is needed as a cofactor. The reaction generates hydrogen peroxide (H2O2), which results in local DNA base oxidation. The formation of an imine intermediate is necessary. The imine intermediate further hydrolyzes to an unstable carbinolamine, which spontaneously degrades to H3K4Me1 with the release of formaldehyde. (B) The domain differences between LSD1 and LSD2: The first 150 amino acids of LSD1 are predicted to be disordered. On the contrary, LSD2 has a CW-type zinc-finger domain in its N-terminus. Additionally, LSD2 does not have a Tower domain. The Tower domain of LSD1 is responsible for the binding of LSD1 to CoRest.
Gene Expression Regulation by Histone Acetylation and Methylation
The basic unit of chromatin consists of 146 base pairs (bp) of DNA wrapped around a histone octamer, which is composed of two copies of each of the four core histones: H2A, H2B, H3, and H4. Thus, post-translational modifications of histones alter the interactions between DNA and histones and modulate DNA access by transcription factors or other regulatory proteins. These alterations result in chromatin structure condensation or relaxation, which leads to the regulation of the expression of a targeted gene (6). The two most common histone modifications are acetylation and methylation. Histone acetylation and deacetylation are highly regulated dynamic processes typically catalyzed by enzymes with a histone acetyltransferase (HAT) or histone deacetylase (HDAC) activity. Acetylation of the (normally) positively charged lysine residue results in an uncharged lysine residue, causing a decreased interaction between the histone and DNA that is generally associated with active transcription. Histone acetylation and deacetylation are usually coregulated with histone methylation and demethylation to facilitate regulation of gene expression. Unlike histone acetylation, methylation of histones does not influence the net charge of the newly modified lysine residues, and hence has no effect on the DNA–histone interaction. However, the histone methylation status is important for the interaction between chromatin and regulatory proteins and therefore can impact transcription. The presence of epsilon-N-methyl-lysine in calf thymus histones was first demonstrated in 1964, and it was suggested that the methyl group might come from methionine (73). In the same year, Allfrey and colleagues reported the possible role of histone methylation and acetylation in regulating RNA synthesis (1). Decades of work on histone modification and regulation of gene expression have developed into a histone-code hypothesis that links the function of histone modifications in chromatin with transcriptional activation or suppression states of related genes (55,87,95). Histone methylation is usually thought to occur at the N-terminal domains of H3 and H4. Histone lysine methyl transferases (HKMTs) can mainly be classified into two families: Su(var)3–9/Enhancer of zeste/Trithorax (SET) domain-containing proteins and the DOT1-like proteins without a SET domain (41). Proteins from both families can transfer a methyl group from S-adenosylmethionine to the epsilon-amino-group of lysine, resulting in the formation of S-adenosylhomocysteine and methyl-lysine. Most HKMTs belong to the SET family and use the SET domain as their catalytic core and almost exclusively act near the N-termini of the histone proteins. On the other hand, DOT1/DOT1L proteins target the lysine tail region of the histone, and DOT1 is the only enzyme known to methylate a lysine residue in the globular core of the histone (103).
Histone Demethylation and Oxidization of DNA
For a long time, histone methylation was considered a permanent and irreversible histone modification and therefore used as an epigenetic mark because of the high thermodynamic stability of the N–CH3 bond (93). The first histone demethylase, LSD1, discovered in 2004 by Shi and coworkers, brought a completely new field to light that suggested a dynamically controlled balance between HKMTs and demethylases in response to the extracellular or intracellular signals needed for transcriptional regulation (81). LSD1 is often upregulated in various tumors and hence is considered a promising oncogenic target. In line with this thought, the inhibition of LSD1 activity has been reported to reduce cancer cell proliferation or block tumor metastasis (52). LSD1 also plays an important role in embryo development, demonstrated by the observation that a null mutation of the mouse isoform of LSD1 (Kdm1a) causes embryonic lethality (100). LSD1 demethylates its histone substrate via an FAD-dependent amine oxidase reaction (Fig. 2A). Because demethylation of trimethylated lysine residues requires a protonated methyl ammonium group for LSD1-catalyzed oxidation, LSD1 is unable to demethylate trimethylated lysines (81). Subsequently, the other histone demethylase family that contains the Jumonji (JmjC) catalytic domain was identified and characterized and shown to demethylate trimethylated lysines (20,62,94,101,104). The JmjC-driven demethylase reaction is compatible with demethylation of mono-, di-, and trimethylated lysines and has a substrate preference for trimethylated lysine demethylation (23,75). Unlike the LSD1 family proteins, JmjC proteins catalyze lysine demethylation of histones through an oxidative reaction that requires Fe(II) and α-ketoglutarate (α-KG) as cofactors. This demethylation mechanism will not result in DNA damage and therefore does not invoke the BER system. As such, these demethylases are not the focus of this review. The details on the JmjC family of demethylases have been reviewed elsewhere (80).
Based on the proposal that amine oxidases might remove the methyl group from histones via an oxidation reaction (7) and the highly homologous sequences between LSD1 and amine oxidases, Shi et al.81 demonstrated that LSD1 catalyzes the removal of the methyl group from both H3K4me2 and H3K4me1, but not H3K4me3 in vitro via an FAD-dependent oxidization reaction (Fig. 2A). Forneris et al. then demonstrated that molecular oxygen was utilized as the electron acceptor. After exposure of the reduced LSD1, which was generated from the demethylation of H3K4Me2 under anaerobic conditions, to air for a few minutes, the flavin cofactor of LSD1 was reoxidized by oxygen. The reoxidized LSD1 has the same absorption spectrum as the native enzyme (35). In LSD1-catalyzed histone demethylation, the amino-group of the methylated lysine is oxidized, presumably to generate an imine intermediate that will spontaneously hydrolyze to produce formaldehyde and the corresponding amine residue. Substrate oxidation leads to the two-electron reduction of the cofactor FAD, which is reoxidized by molecular oxygen to produce H2O2, which was initially detected with a peroxidase-coupled assay. Because of the formation of the imine intermediate, LSD1-mediated demethylation is critically dependent on the protonation of the nitrogen. Thus, this enzyme can only catalyze demethylation of mono- and dimethylated lysines. In 2009, Karytinos and colleagues discovered a second FAD-dependent H3 lysine demethylase, LSD2. LSD2 was demonstrated to be specific for demethylation of H3K4me1 and H3K4me2 via a mechanism similar to LSD1 (58). Both proteins have a C-terminal amine oxidase domain hosting the FAD cofactor for catalytic activity that is preceded by a SWIRM domain, a six-α-helical structural module frequently found in chromatin-associated proteins and important to LSD1 protein stability (Fig. 2B). The main difference between LSD1 and LSD2 is in the N-terminus. Unlike the N-terminal sequence of LSD2 that forms a CW-type zinc-finger domain (residues 130–200), the first 150 amino acids of LSD1 are predicted to be disordered (58). Another difference is that LSD2 does not have a Tower domain that in LSD1 is important for CoRest binding, which therefore precludes the possibility of LSD2/CoRest complex formation (58). In addition, the N-terminal CW-type zinc-finger domain found only in LSD2 suggests the possibility of an interaction between LSD2 and nucleosomal DNA (58). The sequence and structural differences between LSD1 and LSD2 may foreshadow functional specificities of the two demethylases. Recently, Huang and colleagues showed that inhibition of LSD1 activity by the monoamine oxidase inhibitor pargyline or reduction of LSD1 expression by small interfering RNA (siRNA) resulted in increased acetylation of H3K9 (AcH3K9), an epigenetic mark for transcriptionally active chromatin. Further, the reactivation of many tumor suppressor genes resulted in growth inhibition of breast cancer cells. However, the reduction of LSD2 expression by siRNA-mediated knockdown did not change the level of AcH3K9. This result suggests that LSD2 activity may not functionally complement the HDAC activity of LSD1 (52). In 2010, van Essen and colleagues demonstrated that LSD2 was required for the removal of the methyl group from H3K9me2 at the Mdc and Il12b promoters when stimulus-induced NF-κB was recruited to the promoter region for activating those genes (97). In addition, Fang et al. reported that LSD2 mainly binds to the gene bodies (but not promoters) to demethylate H3K4me2 and may be associated with elongation factors to regulate gene transcription after initiation (29). In both the LSD1- and LSD2-mediated demethylation reaction, the H2O2 product generated oxidative damage on the nearby DNA (21). It has been reported that when LSD1-mediated H3K4 demethylation occurred, the percentage of cells labeled with a fluorescein-tagged 8-oxo-7,8-dihydro-2′-deoxyguanosine (8-oxodG)-binding protein increased from 5% at a basal level to 74%, close to the percentage (84%) that resulted from the treatment of H2O2 as a positive control (21). Although only the oxidatively modified form of deoxyguanosine (8-oxodG) was reported after LSD1-mediated histone demethylation (4,78), all four deoxynucleosides (deoxyadenosine, deoxyguanosine, thymidine, and deoxycytidine) and the methylated form of deoxycytidine (5-methyl-deoxycytidine) can be oxidatively damaged (88). The most common and intensively studied oxidatively damaged base is 8-oxodG (59). Other major oxidative lesions include 2,6-diamino-4-hydroxy-5-formamidopyrimidine. With the exception of DNA polymerase iota (ι), DNA replication in mammalian cells is not significantly blocked by 8-oxodG damage, and thus the 8-oxodG DNA lesion is minimally cytotoxic and in fact is mostly mutagenic (106). Since 8-oxodG potentially mispairs with A, either DNA replication or DNA repair-mediated DNA synthesis opposite the 8-oxodG lesion yields deoxyadenosine monophosphate (dAMP) insertion (47,65,67,85,107). Eventually, this results in a G⇒T substitution mutation (85). In general, the 8-oxodG lesion is mainly repaired by the BER pathway, and it has been suggested that the repair (BER) synthesis mediated by Pol-lambda (λ) preferentially inserts deoxycytidine triphosphate (dCTP) opposite 8-oxodG (69,70,98,99). Similarly, BER synthesis mediated by Polβ prefers insertion of dCTP (by a factor of 2:1) over insertion of the mutagenic dAMP base (71). If not repaired, the 8-oxodG lesion can be further oxidized to yield several mutagenic base lesions, including guanidinohydantoin and spiroiminodihydantoin (44,74).
Deoxyadenosine can be oxidized into two major products: 8-oxo-7,8-dihydro-2′-deoxyadenosine (8-oxodA) and 4,6-diamino-5-formamidopyrimidine (FapydA) (12). The FapydA lesion was first detected in both normal and cancerous tissues (77) and is the most abundant of the adenine lesions induced by γ-radiation (18). Both lesions are weakly mutagenic (57,92), and tandem 8-oxodA lesions can be induced by hydroxyl radicals. BER is the major pathway to repair such DNA lesions. The major 2′-deoxycytidine-oxidized product is 5-hydroxy-2′-deoxycytidine (OH5dC). Inaccurate replication across the OH5dC lesion incorporated into DNA tends to generate the C⇒T transition mutations (30). Thymine can be oxidized to thymine glycol (Tg). The Tg lesion is formed by a hydroxyl radical attack on the double bond of thymine at C5 or C6 or by hydrogen abstraction from the methyl group (56). Tg was originally identified in purified DNA after oxidation with ionizing radiation (36). Although the Tg lesion blocks human replicative DNA polymerases, the lesion only results in a minimal increase in mutations, since several translesion DNA polymerases such as DNA polymerase η, κ, ν, β, and λ readily bypass the lesion (10,33,66,91). Additional oxidized thymine analogs include 5,6-dihydro-thymine and 5-hydroxy-5,6-dihydro-thymine. Further details on the repair of oxidative DNA base lesions have been reviewed previously (88).
BER Pathway and the Repair of Oxidatively Damaged DNA
Both environmental and endogenous factors can induce DNA base damage, requiring repair by multiple DNA repair pathways to maintain genome stability and to prevent cell malignant transformation. The BER pathway is the predominant DNA repair pathway for repairing oxidatively damaged DNA. The BER pathway consists of three functional steps: (i) lesion recognition/strand scission, (ii) gap tailoring, and (iii) DNA synthesis/ligation (3,88). The initial step of recognition and strand scission involves the removal of the oxidatively damaged DNA lesion by one of several DNA glycosylases (Fig. 1) (88). This is followed by hydrolysis of the DNA backbone primarily by APE1, an AP endonuclease. Gap tailoring can be conducted by several enzymes depending on the initial lesion, and finally DNA synthesis allows the insertion of the correct DNA base followed by ligation to seal the DNA backbone (3). There are several classes of DNA glycosylases, monofunctional and bifunctional glycosylases, which differ based on function. Monofunctional glycosylases hydrolyze the N-glycosidic bond to remove the damaged base. This class includes UNG, SMUG1 TDG, MBD4, MPG, and MUTYH. Monofunctional DNA glycosylases such as SMUG1 and MYH use an activated water molecule to cleave the N-glycosidic bond. After monofunctional glycosylases remove the damaged base, an AP endonuclease (APE1 or APE2) is required to hydrolyze the phosphodiester bond of DNA, creating a single-strand break with a 5′deoxyribose-phosphate (5′dRP) group that is ultimately gap-tailored by Polβ (86). In contrast, bifunctional glycosylases possess an AP lyase activity in addition to the glycosylase activity. OGG1, NTHL1, and NEIL3 are bifunctional DNA glycosylases that have an associated β-elimination activity, whereas NEIL1 and NEIL2 are bifunctional DNA glycosylases that have an associated β,δ-elimination activity. In human cells, NTHL1 (NTH1), NEIL1, or NEIL2 is usually involved in removing oxidative pyrimidine lesions while OGG1 is primarily responsible for the removal of oxidatively modified purine bases. SMUG1 excises a subset of oxidative base damage, including 5-hydroxyuracil, 5hmU, and 5-formyluracil. MUTYH (MYH) can remove the normal A-base when misincorporated opposite the template 8-oxoguanine (8-oxodG) during DNA replication or repair synthesis. Interestingly, it has been reported that MYH mutations are related to a colorectal adenoma syndrome (MYH-associated polyposis) and high colorectal cancer risk (17). Bifunctional DNA glycosylases (NTH1, NEIL1, NEIL2, and OGG1) use Lys or Pro for direct attack on sugar C1’ to hydrolyze the N-glycosidic bond. Based on the initiating lesion and the mechanism of base removal, the BER pathway can be classified into either a long-patch BER subpathway (Fig. 1) or three short-patch subpathways, including a monofunctional DNA glycosylase-initiated subpathway, bifunctional DNA glycosylases with associated β-elimination-initiated subpathway, and bifunctional DNA glycosylases with associated β,δ-elimination-initiated subpathway (88). The monofunctional DNA glycosylase-initiated subpathway uses the classic short-patch BER mechanism starting with hydrolysis of the N-glycosidic bond to form an abasic site (3). The gap margins with a 3′-OH and a 5′-dRP group are tailored by the 5′-dRP lyase activity of Polβ, followed by Polβ-mediated gap filling (DNA synthesis). Either DNA ligase I (LigI) or a complex of DNA ligase III (LigIII) and XRCC1 seals the DNA chain (3). Recent evidence indicating that LigIII is not required for nuclear BER may suggest that LigI is the preferred ligase in BER (37,84). Strand-break repair and signaling are mediated by XRCC1 together with PARP1, PARP2, and PARG. XRCC1 acts as a scaffold for protein complex formation (2,3).
The BER subpathway initiated by bifunctional DNA glycosylases associated with β-elimination is the predominant BER mechanism for the removal of oxidatively damaged DNA. A bifunctional DNA glycosylase such as OGG1, NTHL1, or NEIL3 hydrolyzes the DNA backbone, 3′ to the incised base, leaving a 3′-unsaturated aldehyde after β-elimination and a 5′-phosphate at the termini of the repair gap. NEIL3 mainly is used for nuclear DNA repair, whereas OGG1 and NTHL1 can repair oxidatively damaged DNA in both the nucleus and the mitochondria. The gap tailoring is performed by the 3′-phosphodiesterase activity of APE1 (3). Next, gap filling (new DNA synthesis) is mediated by Polβ, and the DNA backbone is sealed by the XRCC1/LigIII heterodimer or LigI (3). In some cases, replicative DNA polymerases (δ and ɛ) or low-fidelity DNA polymerases may insert a wrong base opposite many oxidative lesions. To prevent the accumulation and the eventual onset of G⇒T substitution mutations, failure to repair the 8-oxodG lesion also triggers repair of the A-base opposite the 8-oxodG lesion by the MYH glycosylase (26,85).
Another BER subpathway is initiated by bifunctional DNA glycosylases with an associated β,δ-elimination (NEIL1 and NEIL2). These DNA glycosylases are followed by an APE1-independent repair mechanism. Binding of NEIL2 to the lesion recruits XRCC1 to the DNA (15). It has not yet been established if the same process of XRCC1 recruitment is mediated by NEIL1. NEIL1 or NEIL2 generates a 5′- and 3′-phosphate at the ends of the DNA in the single-base gap, resulting from hydrolysis of the glycosidic bond to release the base, cleavage of the DNA-3′ to the abasic site via β-elimination, and then cleavage of the DNA-5′ to the abasic site via δ-elimination, releasing the trans-4-hydroxy-2,4-pentadienal (25,102). The phosphatase PNKP is subsequently recruited to the site to remove the 3′-phosphate in the gap and complete the gap-tailoring step. The repair is finalized by DNA synthesis with Polβ and ligation with XRCC1/LigIII (25,102).
BER and Epigenetic Regulation via Induction and Repair of 8-oxodG
In almost all types of cells, a detectable low basal level of oxidative DNA modifications (e.g., 8-oxodG) is observed, likely due to a steady state between continuous generation of these and related DNA modifications by ROS and simultaneous repair mainly by BER mechanisms (51). The endogenous oxidative nuclear DNA damage is traditionally thought to arise from ROS generated via the mitochondrial electron transport chain. This concept was challenged by Hoffmann and colleagues in 2004 (51). The authors reported that a reduction in mitochondrial ROS production by depletion of mitochondrial DNA or an increase in mitochondrial ROS production via interfering with the mitochondrial electron transport chain did not change the density of nuclear DNA damage (51). The observation implied there may be additional sources of ROS that contribute to the steady-state level of endogenously induced nuclear DNA base modifications. Moreover, Ziel et al. proposed that the oxidative DNA modifications might be utilized as signals for transcriptional regulation (109). Here, the authors observed DNA oxidative modifications enriched at hypoxic-response elements located in the promoter region of the VEGF gene. Moreover, compared to the reporter gene with wild-type hypoxic elements, luciferase activity increased with AP-site-modified hypoxic elements. As described above, the AP sites are repair intermediates formed after glycosylase-mediated base lesion removal (Fig. 1). Because AP sites are repaired by the BER pathway, these results suggested that the BER pathway may play a role in transcriptional regulation (109). More recent studies directly demonstrated that the BER pathway may participate in estrogen-induced target gene expression and Myc-related transcriptional regulation (see below).
BER and Estrogen-Induced Epigenetic Regulation
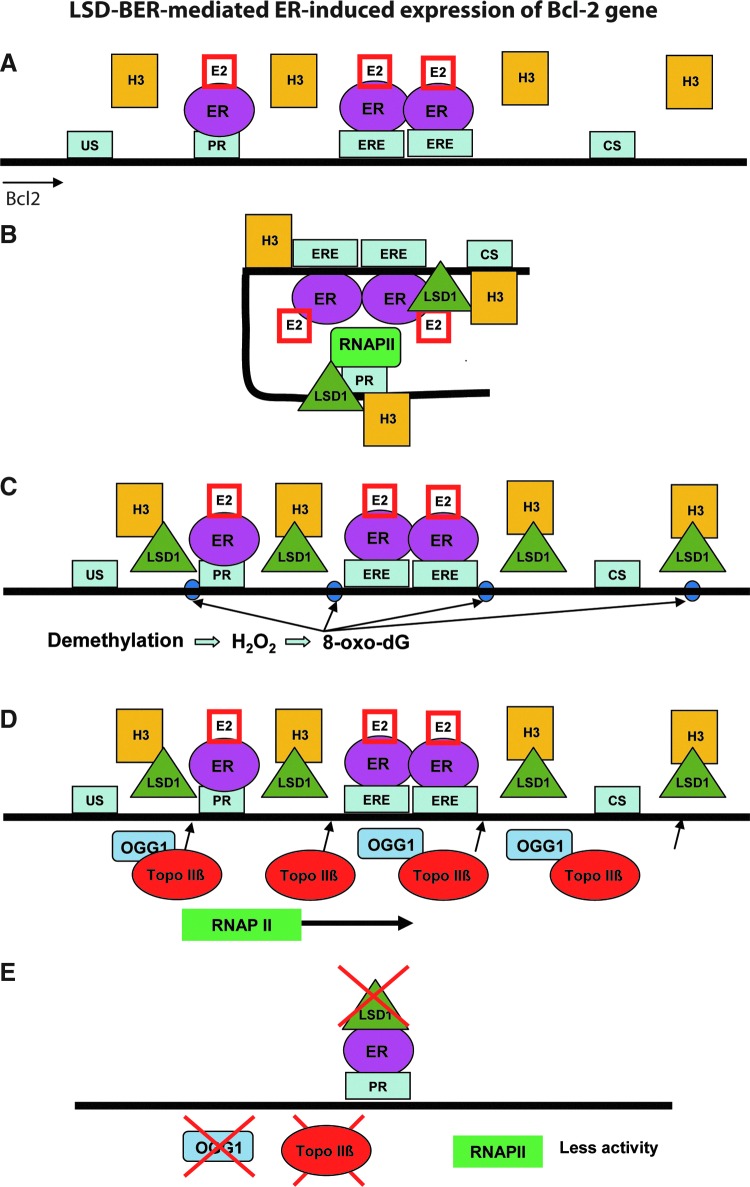
The first direct evidence of LC-BER and the involvement of BER in transcriptional regulation was reported by Perillo et al. in a study of estrogen-induced target gene expression mediated by LSD1 (78). As shown in Figure 3, the authors used a model system (estrogen receptor [ER]-positive MCF7 cells treated with 17β-estradiol [E2]), demonstrating that the ER was recruited to the promoter and estrogen-response elements (EREs) and enhancers (Fig. 3A). Binding of activated ER by E2 to both the B-cell CLL/lymphoma 2 (bcl-2) promoter and the ERE enhancer regions, which are 1.5-kb apart, formed a chromatin loop between the promoter and the ERE enhancer (Fig. 3B). The chromatin looping lasted for 60 minutes after E2 addition and then disappeared. Assembly of the transcription complex occurred between 30 and 45 minutes after E2 addition. Meanwhile, H3K9me2 demethylation occurred at the promoter and enhancer sites of the bcl-2 or pS2 genes. Both chromatin looping and estrogen-induced transcription were prevented when the LSD1 activity was inhibited by the free radical-scavenging drug N-acetyl-l-cysteine (NAC) or the LSD1 inhibitor pargyline. The dimethyl lysine at H3K9me2 in both the promoter and enhancer regions is demethylated by LSD1 via an FAD-dependent oxidative reaction, yielding the generation of local H2O2 that can oxidize the nearby guanine bases to 8-oxo-G (Fig. 3C). Five minutes after E2 treatment, 8-oxodG could be detected by immunofluorescence, and 45 minutes after E2 treatment, the immunofluorescence intensity specific for 8-oxodG was similar to the control experiment after H2O2 treatment (78). As expected, deactivation of LSD1 with NAC or pargyline, as well as siRNA knockdown of LSD1, abolished the accumulation of the 8-oxodG lesion. Next, it was demonstrated that removal of the 8-oxodG lesions from the promoter and enhancer regions of the DNA occurs primarily by OGG1, which specifically recognizes and removes 8-oxodG as a part of the BER pathway (78). Another repair enzyme, topoisomerase IIβ (TopoIIb), which is responsible for recognizing and repairing single-stranded DNA breaks, was present in both sites as well (Fig. 3D). Inhibition of TopoIIb expression with siRNA strongly reduced loading of the activated RNA Pol II, of which Ser5 was phosphorylated on the promoter. A decrease of loading or assembly of the phospho-RNA polymerase by 30%–40% was achieved by the siRNA-mediated knockdown of OGG1 (Fig. 3E). These results indicated that targeting both TopoIIb and OGG1 to these sites drove transcription initiation.
FIG. 3.
LSD1/BER-mediated estrogen receptor (ER)-induced expression of the bcl-2 gene. (A) Thirty minutes after E2 stimulation, the E2-ER complex is recruited to the promoter (Pr) and estrogen-response elements (EREs) and enhancer (Enh) sites. (B) After E2-ER complex binding to Pr and ERE, a chromatin loop is formed between Pr and Enh. At the same time, LSD1 is recruited to these sites to demethylate H3K9me2. (C) H2O2, a byproduct generated from the reaction of LSD1-mediated H3K9me2 demethylation, oxidizes the nearby guanine bases to 8-oxo-7,8-dihydro-2′-deoxyguanosine (8-oxodG). (D) To initiate DNA repair-coupled transcription, OGG1 and TOPO IIβ are recruited to the lesion sites (purple dots) to initiate the repair of 8-oxodG. RNA polymerase II assembly starts the transcription of the bcl-2 gene. (E) The results from inhibition experiments indicate that without E2, LSD1 is not recruited to the Pr and Enh sites. The loss of function of OGG1 or TOPO IIβ by using targeted siRNA reduces the loading of activated RNAP II on promoters. US, upstream sequence; CS, coding sequence.
The proposed mechanism of histone demethylation coupling with BER (LC-BER) for transcriptional regulation is as follows: LSD1 demethylation results in the accumulation of 8-oxodG lesions via the generation of H2O2 at the sites of demethylation. The 8-oxodG lesions are repaired by OGG1, ultimately generating DNA single-strand breaks during BER that are recognized by TopoIIb to facilitate chromatin relaxation and formation of the complex process of transcription initiation. As such, H3K9me2 demethylation produced temporally limited and localized H2O2 at the promoter and enhancer regions, providing a local signal for the assembly of transcription initiation complex proteins mediated in part by BER proteins such as OGG1 and APE1. Overall, this study demonstrated that LSD1-mediated histone demethylation is coupled with BER to initiate hormone-dependent gene expression (78).
BER and Myc-Induced Epigenetic Regulation
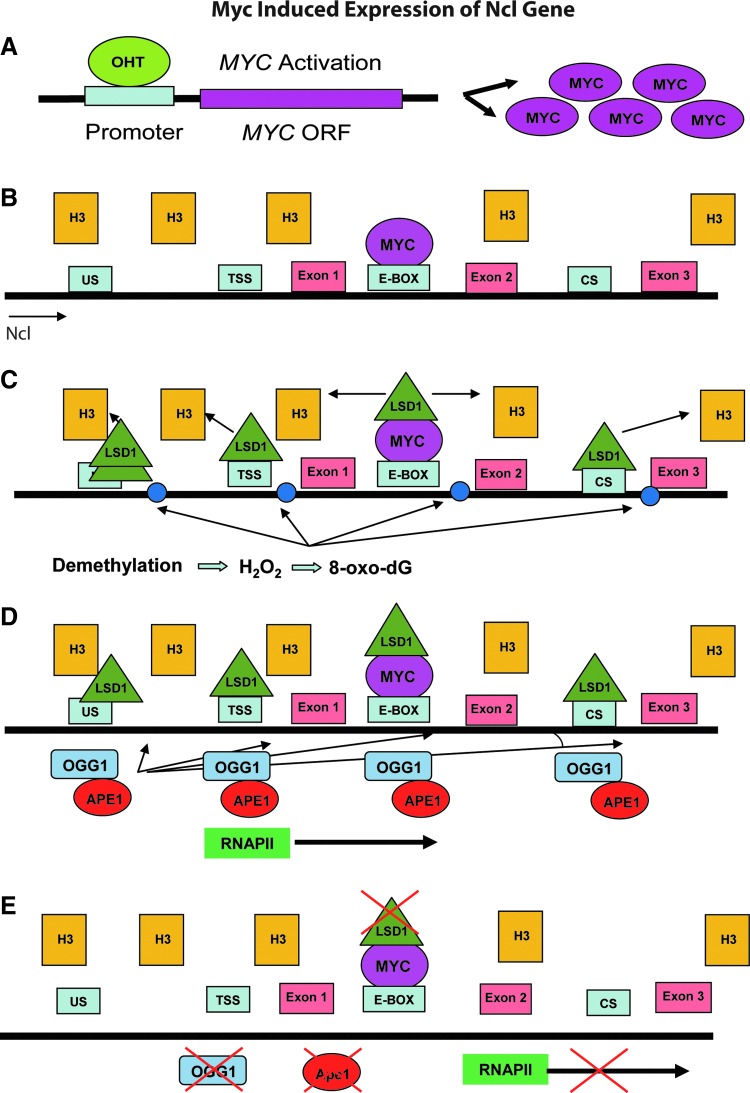
More recently, the same group observed LSD1-BER-mediated transcriptional regulation in Myc-activated transcription of the Myc-target genes Ncl and CAD (4,5). Myc is one of the most common activators of cell proliferation used by cancer cells to drive disease progression. Because the E-box sequence (CACGTG) for Myc binding was estimated to exist in 15% of the all promoter regions in the human genome (32,42,105), the involvement of BER in the mechanism of Myc-induced transcriptional regulation will have broad significance for cancer therapy research. Amente and colleagues demonstrated that LSD1-BER-coupled epigenetic regulation via demethylation of H3K4Me2 by LSD1 at the promoter and E-box sites drives Myc-mediated gene expression (Fig. 4) (4). In this study, the activation of Myc with tamoxifen (OHT) was set as the start point (Fig. 4A). About 30–60 minutes after Myc activation, Myc was recruited mainly on the E-boxes of both the Ncl and CAD genes and stably accumulated at the E-box until the end point (240 minutes) (Fig. 4B). LSD1 was recruited to the transcription start site (TSS) and the coding region (CR) of both genes shortly after Myc activation (Fig. 4C). The authors suggested that the recruitment of LSD1 to the promoters is an early and transient event, because the existence of LSD1 on both the TSS and the E-box overlapped with H3K4me2 demethylation on those sites only in the period from 30 to 60 minutes. Ncl and Cad mRNA accumulation was observed 1 hour after Myc induction. Four hours later, not only the methylation status of both sites was back to normal but also the BER proteins were no longer present at the sites. Stable acetylated histone H4 accumulation was detected on the TSS and CR of both the genes with a peak level at 240 minutes after Myc activation. During the demethylation of H3K4me2, the fluorescent signal for detection of 8-oxodG was significantly increased in Myc-activated cells (Fig. 4C), similar to the level of cells exposed to H2O2, but not in the Myc-null cells. In separate experiments, addition of siRNA specific for Myc or addition of pargyline (an LSD1 inhibitor) abolished the signal for 8-oxodG detection. It was also demonstrated that two BER enzymes (OGG1 and APE1) were engaged at the TSS and E-boxes of the Ncl and CAD genes after Myc activation (4). OGG1 appears to be required for the removal of the 8-oxodG lesion, and APE1 was required to complete repair (Fig. 4D). However, no additional BER proteins were evaluated in this initial report. The functional role of BER for transcriptional control was confirmed by the loss of expression of either OGG1 or APE1 via targeted siRNA treatment, dramatically reducing the expression of both the Ncl and CAD genes after Myc activation (Fig. 4E). The proposed mechanism for Myc-induced transcription is that Myc recruits LSD1 to the target E-box site and initiates histone H3 demethylation, which is then linked to oxidation and DNA repair (LC-BER) to promote the assembly of the transcript initiation complex.
FIG. 4.
Myc-induced expression of the Ncl gene. (A) The expression of Myc was induced by adding tamoxifen (OHT) to cultured cells. (B) Thirty minutes after Myc activation, Myc is recruited to the E-BOX. (C) After Myc binds to the E-BOX, LSD1 is recruited to US, transcription start site (TSS), E-BOX, and CR sites, and H3K4me2 is then demethylated. LSD1-mediated demethylation of H3K4me2 generates H2O2, which results in DNA damage (8-oxodG). (D) OGG1 and APE1 are recruited to the lesion sites to repair DNA damage. This couples with RNAP II to start the transcription of the Ncl genes. (E) Knockdown of LSD1, OGG1, or APE1 abolishes the expression of Ncl even with the expression of Myc.
Epigenetic Regulation via BCADD
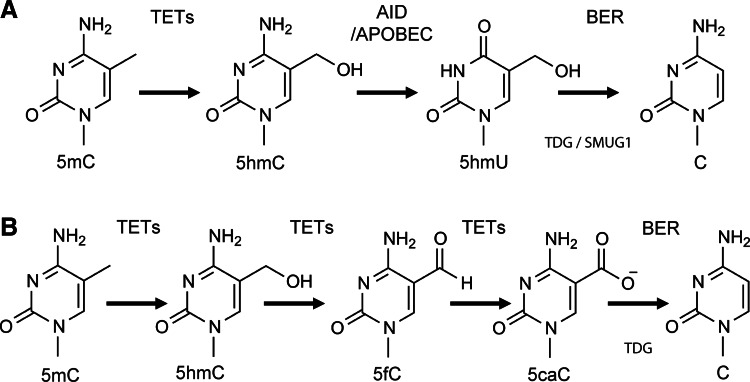
In 1998, Um and colleagues reported that TDG directly interacted with the retinoic acid receptor and the retinoid X receptor (RXR) in a ligand-independent manner (96). Overexpression of TDG induced a fourfold increase of the β-galactosidase reporter gene expression controlled by a retinoic acid-responsive promoter (96). Subsequently, using chicken 5-methylcytosine DNA glycosylase (5-MCDG), a homolog of human TDG, Zhu and colleagues discovered the upregulation of reporter gene expression induced by a similar 5-MCDG-RXR complex that was the result of DNA demethylation of the downstream ecdysone–retinoic acid-responsive enhancer (108). In a nonmammalian model, participation of BER in DNA demethylation is clearly documented. In Arabidopsis, the BER proteins Demeter and ROS1 directly remove 5mC via their glycosylase activities (39,72). In Xenopus, growth arrest and DNA damage-inducible protein 45-alpha (Gadd45a) is the major protein to initiate DNA demethylation, and the interaction of Gadd45a with the nucleotide excision repair protein XPG is suggested (8). In zebrafish embryos, 5mC is converted to thymine by AID, and then the G:T mismatch is removed by the zebrafish thymine glycosylase MBD4, associating Gadd45 with the BER pathway (79). In a mammalian model, the participation of BER in active demethylation has recently been documented using mouse embryos during genome-wide epigenetic reprogramming. In 2010, Hajkova reported the initiation of epigenetic reprogramming at embryonic day 7.25 (E7.25), followed by genome-wide DNA demethylation. Involvement of BER was proposed after an increase in expression of APE1, XRCC1, and the formation of PAR, suggesting the involvement of BER in mouse primordial germ cells, but not the neighboring somatic cells (45). The mechanism of participation of BER proteins in mammalian demethylation was not clear until the discovery of the TET protein family (see Fig. 5) (90). TET protein family members are α-KG and Fe(II)-dependent dioxygenases that include the isoforms TET1, TET2, and TET3. TET1 is mainly expressed in embryonic stem cells (ESCs), whereas TET2 and TET3 are more ubiquitously expressed (90). All TETs can oxidize 5mC to 5hmC (22,48,89,90) (Fig. 5A, B). TET2 mutations were linked to many types of myeloid malignancies and resulted in a significant decrease in the level of 5hmC in bone marrow cells isolated from patients with acute myeloid leukemia as compared to healthy individuals (63). BER was proposed to participate in TET-mediated DNA demethylation by repairing the 5hmU that is formed from 5hmC by the AID/APOBEC family of cytidine deaminases (43) (Fig. 5A). In addition, recent studies in mammalian cells demonstrated two new cytosine modifications, formylcytosine and carboxylcytosine, which are generated by two successive oxidation reactions of 5hmC catalyzed by the TET proteins (22,53) (Fig. 5B). It has recently been reported that the existence of 5fC or 5caC in a DNA template dramatically reduced the RNA Pol II elongation efficiency compared to the DNA templates that had unmodified C, 5mC, or 5hmC bases. It was demonstrated that the DNA templates with either 5fC or 5caC resulted in much lower GTP incorporation efficiencies than the others. In addition, 5fC- and 5caC-modified bases also caused a significant level of RNA Pol II complex backtracking, further suggesting that RNA Pol II shifted from an active state to a paused state. In addition, 5fC greatly reduced the fidelity of nucleotide incorporation (61). TDG was shown to be the glycosylase responsible for removal of the carboxylcytosine base that was then followed by complete BER processing to restore the normal cytosine (Fig. 5B). A depletion of TDG leads to accumulation of carboxylcytosine in mouse ESCs (48). Moreover, a loss of TDG function is lethal to mouse embryos in an early developmental stage associated with epigenetic aberrations affecting the expression of developmental genes (22).
FIG. 5.
BER participates in active demethylation. (A) DNA demethylation via 5-hydroxymethylcytosine (5hmC)/5-hydroxymethyluracil (5hmU): TET proteins (TET1–3) convert 5mC to 5hmC. Next, an AID/APOBEC enzyme deaminates 5hmC to 5hmU, which is a substrate for TDG or SMUG1. The removal of 5hmU by TDG or SMUG1 results in an abasic (AP) site, inducing recruitment of other BER proteins to complete repair and the conversation of 5hmU to C. (B) DNA demethylation via 5-formylcytosine (5fC)/5-carboxylcytosine (5caC): TET enzymes iteratively oxidize 5mC to 5hmC and then to 5fC and finally to 5caC. TDG excises the 5caC and forms an AP site. This initiates the BER pathway to convert 5caC to C.
Further, a recent report demonstrated that another BER protein, PARP1, is involved in the regulation of the process required for reprogramming somatic cells into pluripotent stem cells (iPSCs) when using the pluripotency factors Oct4, Sox2, Klf4, and c-Myc (OSKM) to mouse embryonic fibroblasts (MEFs) prepared from WT and Parp1−/− 13.5-day embryos and tail-tip fibroblasts prepared from WT and Tet2−/− mice (27). In this mechanism, PARP1 and Tet2 are recruited to the Nanog and Esrrb loci to erase the somatic epigenetic signatures of DNA methylation or histone modification and establish alternative epigenetic marks characteristic of ESCs. In a functional screen for epigenetic modification factors that promoted OSKM-mediated somatic cell reprogramming in MEFs, overexpression of PARP1 potently induced OSKM-MEF reprogramming to generate more iPSCs. Moreover, PARP1 deficiency suppressed the reprogramming of iPSCs. The quantification of total cytosine methylation (5mC plus 5hmC) at the regulatory regions of the pluripotency-related genes Nanog or Esrrb indicated that PARP1 appeared as a regulator of 5mC. The loss of PARP1 function induced accumulation of 5mC, but not 5hmC, at both the Nanog and Esrrb loci. However, overexpression of PARP1 did not significantly change the 5mC or 5hmC levels. PARP1 also affected accessibility of the reprogramming factor Oct4 to the regulatory sites of both the Nanog and Esrrb genes. PARP1 deficiency significantly diminished Oct4 occupancy at both pluripotency loci, as measured by the chromatin immunoprecipitation (ChIP) analysis. Further, PARP1 overexpression robustly promoted exogenous Oct4 binding to the pluripotency loci. PARP1 affected the chromatin state of the Nanog and Esrrb loci as well. Deficiency of PARP1 diminished the H3K4me2 chromatin mark, which is enriched at the pluripotency loci of iPSCs (both the Nanog and Esrrb loci) (27). It has also been shown that Tet2 played an important role in iPSC reprogramming. Expression of Tet2 gradually increased along with iPSC reprogramming and kept elevating in iPSCs. In contrast, expression of Tet1 or Tet3 remained at basal levels. iPSC colony formation was abolished when Tet2 expression was knocked down using targeted shRNA. The ChIP analysis indicated that Tet2 was present at the Nanog and Esrrb pluripotency loci with or without functional PARP1. Tet2 knockdown suppressed the induction of 5hmC and abolished the H3K4Me2 chromatin mark at the pluripotency loci of both the Nanog and Esrrb genes (27). Overall, these data clearly support a role for PARP1 in the induction of PSCs and suggest that additional BER proteins may have a supportive role. Given the potential for PARP inhibitors to induce the death of cancer cells defective in homologous recombination DNA repair, the participation of PARP1 in epigenetic regulation may provide support for the use of PARP inhibitors combined with epigenetic inhibitors in cancer. For example, it has been reported that the HDAC inhibitor MS275 potentiated the cytotoxic effect of the PARP inhibitors KU-0058948 and PJ34 in PARP inhibitor-sensitive leukemic cells. However, the DNA methyltransferase inhibitor 5′-aza-2′-deoxycytidine failed to increase the cytotoxicity of PARP inhibitors against primary myeloid leukemic cells and myeloid leukemic cell lines (38). More investigations are clearly needed to fully understand the mechanism of HDAC inhibitor-mediated potentiation of PARP inhibitors with the ultimate goal of improving therapy options using combinations of epigenetic regulators and BER inhibitors.
Summary
Gene expression in eukaryotic cells is regulated at multiple levels of transcriptional control to allow response to growth factors, cellular stress, DNA damage, or development. The primary control comes from genetic information encoded in the DNA sequence, which defines protein sequences, signal sequences, and noncoding regulatory elements for transcription factors, enhancers, or silencers. A secondary, but critical, level of control of gene expression is at the epigenetic level via DNA methylation and histone modification. The BER pathway has recently been demonstrated to be necessary for both DNA methylation- and histone modification-mediated epigenetic regulation separate from its main function in maintaining genome stability. Impaired BER can also have significant effects on the cellular DNA methylation status. For example, knockout of TDG resulted in mass DNA methylation changes in many gene promoter regions in mouse embryos and resulted in early embryonic lethality (22). The discovery of the function of the TET family proteins suggests that the dynamic regulation of DNA methylation by active demethylation requires the BER pathway as the final effector. Two possible TET-BER-regulated active demethylations were proposed. Cortellino et al. proposed that 5hmC may first be deaminated by the AID/APOBEC family of cytidine deaminases to generate 5hmU. BER is then engaged to repair the 5hmU:G mismatch, because both TDG and SMUG1 were demonstrated to effectively repair 5hmU in the 5hmU:G mispair in dsDNA, yielding the correct C:G base pair (22). He and colleagues proposed another active DNA demethylation pathway in which the 5mC lesion is iteratively oxidized by TET proteins into 5hmC, 5fC, and 5caC, followed by a conversion back to an unmethylated cytosine via TDG-initiated BER (48). Further studies are needed to determine the condition for activating these two pathways. BER also plays an important role in transcriptional regulation at sites of histone demethylation. As we discussed above, defects in BER directly reduced estrogen- or Myc-induced target gene expression. The 8-oxodG lesion, produced from H2O2, generated by LSD1 or LSD2 during the process of histone demethylation, serves as the signal to recruit BER enzymes to hydrolyze and relax high GC-content promoter regions, facilitating and enhancing transcription initiation and elongation (5).
Appropriately controlled epigenetic regulation is critical for the normal development and health of an organism. Misregulation of epigenetic control, regarding either DNA methylation or histone methylation, has been associated with cancer, chromosomal instability syndromes, and mental retardation. Recent reports show that BER is indispensable for epigenetic events such as hormone-modulated gene expression and iPSC reprogramming. Such efforts to address the role of BER proteins in epigenetic regulation could broaden cancer therapeutic strategies to include epigenetic modifiers combined with BER inhibitors.
Abbreviations Used
- α-KG
α-ketoglutarate
- 5caC
5-carboxylcytosine
- 5′dRP
5′deoxyribose-phosphate
- 5fC
5-formylcytosine
- 5hmC
5-hydroxy-methyl-cytosine
- 5hmU
5-hydroxymethyluracil
- 5mC
5-methylcytosine
- 5-MCDG
5-methylcytosine DNA glycosylase
- 8-oxodA
8-oxo-7,8-dihydro-2′-deoxyadenosine
- 8-oxodG
8-oxo-7,8-dihydro-2′- deoxyguanosine
- A
adenine
- AcH3K9
acetylation of histone H3 lysine9
- ADP
adenosine diphosphate
- AID
activation-induced deaminase
- AP
apurinic/apyrimidinic
- APE1
apurinic/apyrimidinic endonucleases 1
- APE2
apurinic/apyrimidinic endonucleases 2
- APOBEC
apolipoprotein B mRNA-editing enzyme complex
- BCADD
BER-coupled active DNA demethylation
- bcl-2
B-cell CLL/lymphoma 2
- BER
base-excision repair
- bp
base pairs
- C
cytosine
- CAD
carbamoyl-phosphate synthetase 2, aspartate transcarbamylase, and dihydroorotase
- ChIP
chromatin immunoprecipitation
- CoRest
chromatin-modifying corepressor
- CpG
deoxy-cytidylate-phosphate-deoxy-guanylate
- CR
coding region
- CW-type
cysteine and tryptophan-type zinc-finger motif
- dAMP
deoxyadenosine monophosphate
- dCTP
deoxycytidine triphosphate
- Demeter
transcriptional activator DEMETER
- DNA
deoxyribonucleic acid
- DOT1
disruptor of telomeric silencing 1
- DOT1L
disrupter of telomere silencing 1-like
- E-box
CACGTG
- E2
17β-estradiol
- Enh
enhancers
- ER
estrogen receptor
- EREs
estrogen-response elements
- ESCs
embryonic stem cells
- FAD
flavin adenine dinucleotide
- FADH
flavin adenine dinucleotide, reduced
- FapydA
4,6-diamino-5-formamidopyrimidine
- G
Guanine
- Gadd45a
DNA damage-inducible protein 45-alpha
- H2A
histone H2A
- H2B
histone H2B
- H2O2
hydrogen peroxide
- H3
histone H3
- H3K4me0
unmethylated histone H3 lysine 4
- H3K4me1
monomethylated histone H3 lysine 4
- H3K4me2
dimethylated histone H3 lysine 4
- H3K4me3
trimethylated histone H3 lysine 4
- H3K9me2
dimethylated histone H3 lysine 9
- H4
histone H4
- HAT
histone acetyltransferase
- HDAC
histone deacetylase
- HKMTs
histone lysine methyl transferases
- Il12b
interleukin 12B (natural killer cell stimulatory factor 2
- iPSCs
inducible pluripotent stem cells
- JmjC
Jumonji
- Kdm1a
lysine-specific demethylase 1
- Klf4
Kruppel-like factor 4
- LC-BER
LSD1/LSD2-coupled BER
- LigI
DNA ligase I
- LigIII
DNA ligase III
- LSD1
lysine-specific demethylase 1
- LSD2
lysine-specific demethylase 2
- Lys
lysine
- MBD4
methyl-CpG-binding domain protein 4
- Mdc
ADAM metallopeptidase domain 11
- MEFs
mouse embryonic fibroblasts
- MPG
methyl purine-DNA glycosylase
- MUTYH
mutY homolog
- Myc
V-myc myelocytomatosis viral oncogene homolog (avian)
- NAC
N-acetyl-l-cysteine
- Ncl
nucleolin
- NEIL1
Nei endonuclease VIII-like 1
- NEIL2
Nei endonuclease VIII-like 2
- NEIL3
Nei endonuclease VIII-like 3
- NF-κB
nuclear factor kappa light-chain enhancer of activated B cells
- NTHL1
endonuclease III-like protein 1
- OGG1
8-oxoguanine glycosylase
- OH5dC
5-hydroxy-2′-deoxycytidine
- OHT
tamoxifen
- OSKM
Oct4, Sox2, Klf4, and c-Myc
- PAR
poly(ADP)ribose
- PARG
poly (ADP-ribose) glycohydrolase
- PARP1
poly (ADP-ribose) polymerase 1
- PARP2
poly (ADP-ribose) polymerase 2
- PNKP
bifunctional polynucleotide phosphatase/kinase
- Pol
polymerase
- Polβ
DNA polymerase β
- Pr
promoter
- Pro
proline
- RNA Pol II
RNA polymerase II
- ROS
reactive oxygen species
- ROS1
repressor of silencing1
- RXR
retinoid X receptor
- Ser
serine
- SET
Su(var)3–9/Enhancer of zeste/Trithorax
- siRNA
small interfering RNA
- SMUG1
single-strand selective monofunctional uracil DNA glycosylase
- Sox2
sex determining region Y-box 2
- SUMO
small ubiquitin-related modifier
- SWIRM
Swi3p, Rsc8p, and Moira
- T
thymine
- TDG
thymine-DNA glycosylase
- Tet
Ten–eleven translocation
- Tet1
Ten–eleven translocation 1
- Tet2
Ten–eleven translocation 2
- Tet3
Ten–eleven translocation 3
- Tg
thymine glycol
- TopoIIb
topoisomerase IIβ
- TSS
transcription start site
- UNG
uracil–DNA glycosylase
- US
upstream sequence
- VEGF
vascular endothelial growth factor A
- XPG
xeroderma pigmentosum, complementation group G
- XRCC1
X-ray repair cross-complementing protein 1
Acknowledgments
This work was supported by grants from National Institutes of Health (GM087798, CA148629, ES019498, ES021116, and GM099213) to R.W.S.
Author Disclosure Statement
R.W.S. is a scientific consultant for Trevigen, Inc. The remaining authors state that there is no conflict of interest.
References
- 1.Allfrey VG. Faulkner R. Mirsky AE. Acetylation and methylation of histones and their possible role in the regulation of RNA synthesis. Proc Natl Acad Sci U S A. 1964;51:786–794. doi: 10.1073/pnas.51.5.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Almeida KH. Sobol RW. Increased specificity and efficiency of base excision repair through complex formation. In: Siede W, editor; Doetsch PW, editor; Kow YW, editor. DNA Damage Recognition. New York: Marcel Dekker Inc.; 2005. pp. 33–64. [Google Scholar]
- 3.Almeida KH. Sobol RW. A unified view of base excision repair: lesion-dependent protein complexes regulated by post-translational modification. DNA Repair. 2007;6:695–711. doi: 10.1016/j.dnarep.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amente S. Bertoni A. Morano A. Lania L. Avvedimento EV. Majello B. LSD1-mediated demethylation of histone H3 lysine 4 triggers Myc-induced transcription. Oncogene. 2010;29:3691–3702. doi: 10.1038/onc.2010.120. [DOI] [PubMed] [Google Scholar]
- 5.Amente S. Lania L. Avvedimento EV. Majello B. DNA oxidation drives Myc mediated transcription. Cell Cycle. 2010;9:3002–3004. doi: 10.4161/cc.9.15.12499. [DOI] [PubMed] [Google Scholar]
- 6.Bannister AJ. Kouzarides T. Reversing histone methylation. Nature. 2005;436:1103–1106. doi: 10.1038/nature04048. [DOI] [PubMed] [Google Scholar]
- 7.Bannister AJ. Schneider R. Kouzarides T. Histone methylation: dynamic or static? Cell. 2002;109:801–806. doi: 10.1016/s0092-8674(02)00798-5. [DOI] [PubMed] [Google Scholar]
- 8.Barreto G. Schafer A. Marhold J. Stach D. Swaminathan SK. Handa V. Doderlein G. Maltry N. Wu W. Lyko F. Niehrs C. Gadd45a promotes epigenetic gene activation by repair-mediated DNA demethylation. Nature. 2007;445:671–675. doi: 10.1038/nature05515. [DOI] [PubMed] [Google Scholar]
- 9.Baute J. Depicker A. Base excision repair and its role in maintaining genome stability. Crit Rev Biochem Mol Biol. 2008;43:239–276. doi: 10.1080/10409230802309905. [DOI] [PubMed] [Google Scholar]
- 10.Belousova EA. Maga G. Fan Y. Kubareva EA. Romanova EA. Lebedeva NA. Oretskaya TS. Lavrik OI. DNA polymerases beta and lambda bypass thymine glycol in gapped DNA structures. Biochemistry. 2010;49:4695–4704. doi: 10.1021/bi901792c. [DOI] [PubMed] [Google Scholar]
- 11.Bhattacharya B. Puri S. Puri RK. A review of gene expression profiling of human embryonic stem cell lines and their differentiated progeny. Curr Stem Cell Res Ther. 2009;4:98–106. doi: 10.2174/157488809788167409. [DOI] [PubMed] [Google Scholar]
- 12.Bjelland S. Seeberg E. Mutagenicity, toxicity and repair of DNA base damage induced by oxidation. Mutat Res. 2003;531:37–80. doi: 10.1016/j.mrfmmm.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 13.Boyes J. Bird A. Repression of genes by DNA methylation depends on CpG density and promoter strength: evidence for involvement of a methyl-CpG binding protein. EMBO J. 1992;11:327–333. doi: 10.1002/j.1460-2075.1992.tb05055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Butler JS. Koutelou E. Schibler AC. Dent SY. Histone-modifying enzymes: regulators of developmental decisions and drivers of human disease. Epigenomics. 2012;4:163–177. doi: 10.2217/epi.12.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Campalans A. Marsin S. Nakabeppu Y. O'Connor TR. Boiteux S. Radicella JP. XRCC1 interactions with multiple DNA glycosylases: a model for its recruitment to base excision repair. DNA Repair (Amst) 2005;4:826–835. doi: 10.1016/j.dnarep.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 16.Cedar H. Bergman Y. Epigenetics of haematopoietic cell development. Nat Rev Immunol. 2011;11:478–488. doi: 10.1038/nri2991. [DOI] [PubMed] [Google Scholar]
- 17.Chen H. Xu L. Qi Q. Yao Y. Zhu M. Wang Y. A haplotype variation affecting the mitochondrial transportation of hMYH protein could be a risk factor for colorectal cancer in Chinese. BMC Cancer. 2008;8:269. doi: 10.1186/1471-2407-8-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chetsanga CJ. Grigorian C. A dose-response study on opening of imidazole ring of adenine in DNA by ionizing radiation. Int J Radiat Biol Relat Stud Phys Chem Med. 1983;44:321–331. doi: 10.1080/09553008314551261. [DOI] [PubMed] [Google Scholar]
- 19.Chi P. Allis CD. Wang GG. Covalent histone modifications—miswritten, misinterpreted and mis-erased in human cancers. Nat Rev Cancer. 2010;10:457–469. doi: 10.1038/nrc2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cloos PA. Christensen J. Agger K. Maiolica A. Rappsilber J. Antal T. Hansen KH. Helin K. The putative oncogene GASC1 demethylates tri- and dimethylated lysine 9 on histone H3. Nature. 2006;442:307–311. doi: 10.1038/nature04837. [DOI] [PubMed] [Google Scholar]
- 21.Cooke MS. Loft S. Olinski R. Evans MD. Bialkowski K. Wagner JR. Dedon PC. Moller P. Greenberg MM. Cadet J. Recommendations for standardized description of and nomenclature concerning oxidatively damaged nucleobases in DNA. Chem Res Toxicol. 2010;23:705–707. doi: 10.1021/tx1000706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cortellino S. Xu J. Sannai M. Moore R. Caretti E. Cigliano A. Le Coz M. Devarajan K. Wessels A. Soprano D. Abramowitz LK. Bartolomei MS. Rambow F. Bassi MR. Bruno T. Fanciulli M. Renner C. Klein-Szanto AJ. Matsumoto Y. Kobi D. Davidson I. Alberti C. Larue L. Bellacosa A. Thymine DNA glycosylase is essential for active DNA demethylation by linked deamination-base excision repair. Cell. 2011;146:67–79. doi: 10.1016/j.cell.2011.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Couture JF. Collazo E. Ortiz-Tello PA. Brunzelle JS. Trievel RC. Specificity and mechanism of JMJD2A, a trimethyllysine-specific histone demethylase. Nat Struct Mol Biol. 2007;14:689–695. doi: 10.1038/nsmb1273. [DOI] [PubMed] [Google Scholar]
- 24.Dahl C. Gronbaek K. Guldberg P. Advances in DNA methylation: 5-hydroxymethylcytosine revisited. Clin Chim Acta; International Journal Of Clinical Chemistry. 2011;412:831–836. doi: 10.1016/j.cca.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 25.Das A. Wiederhold L. Leppard JB. Kedar P. Prasad R. Wang H. Boldogh I. Karimi-Busheri F. Weinfeld M. Tomkinson AE. Wilson SH. Mitra S. Hazra TK. NEIL2-initiated, APE-independent repair of oxidized bases in DNA: evidence for a repair complex in human cells. DNA Repair (Amst) 2006;5:1439–1448. doi: 10.1016/j.dnarep.2006.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.David SS. O'Shea VL. Kundu S. Base-excision repair of oxidative DNA damage. Nature. 2007;447:941–950. doi: 10.1038/nature05978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Doege CA. Inoue K. Yamashita T. Rhee DB. Travis S. Fujita R. Guarnieri P. Bhagat G. Vanti WB. Shih A. Levine RL. Nik S. Chen EI. Abeliovich A. Early-stage epigenetic modification during somatic cell reprogramming by Parp1 and Tet2. Nature. 2012;488:652–655. doi: 10.1038/nature11333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eads CA. Lord RV. Wickramasinghe K. Long TI. Kurumboor SK. Bernstein L. Peters JH. DeMeester SR. DeMeester TR. Skinner KA. Laird PW. Epigenetic patterns in the progression of esophageal adenocarcinoma. Cancer Res. 2001;61:3410–3418. [PubMed] [Google Scholar]
- 29.Fang R. Barbera AJ. Xu Y. Rutenberg M. Leonor T. Bi Q. Lan F. Mei P. Yuan GC. Lian C. Peng J. Cheng D. Sui G. Kaiser UB. Shi Y. Shi YG. Human LSD2/KDM1b/AOF1 regulates gene transcription by modulating intragenic H3K4me2 methylation. Mol Cells. 2010;39:222–233. doi: 10.1016/j.molcel.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feig DI. Sowers LC. Loeb LA. Reverse chemical mutagenesis: identification of the mutagenic lesions resulting from reactive oxygen species-mediated damage to DNA. Proc Natl Acad Sci U S A. 1994;91:6609–6613. doi: 10.1073/pnas.91.14.6609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feinberg AP. Ohlsson R. Henikoff S. The epigenetic progenitor origin of human cancer. Nat Rev Genet. 2006;7:21–33. doi: 10.1038/nrg1748. [DOI] [PubMed] [Google Scholar]
- 32.Fernandez PC. Frank SR. Wang L. Schroeder M. Liu S. Greene J. Cocito A. Amati B. Genomic targets of the human c-Myc protein. Genes Dev. 2003;17:1115–1129. doi: 10.1101/gad.1067003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fischhaber PL. Gerlach VL. Feaver WJ. Hatahet Z. Wallace SS. Friedberg EC. Human DNA polymerase kappa bypasses and extends beyond thymine glycols during translesion synthesis in vitro, preferentially incorporating correct nucleotides. J Biol Chem. 2002;277:37604–37611. doi: 10.1074/jbc.M206027200. [DOI] [PubMed] [Google Scholar]
- 34.Flicek P. Amode MR. Barrell D. Beal K. Brent S. Carvalho-Silva D. Clapham P. Coates G. Fairley S. Fitzgerald S. Gil L. Gordon L. Hendrix M. Hourlier T. Johnson N. Kahari AK. Keefe D. Keenan S. Kinsella R. Komorowska M. Koscielny G. Kulesha E. Larsson P. Longden I. McLaren W. Muffato M. Overduin B. Pignatelli M. Pritchard B. Riat HS. Ritchie GR. Ruffier M. Schuster M. Sobral D. Tang YA. Taylor K. Trevanion S. Vandrovcova J. White S. Wilson M. Wilder SP. Aken BL. Birney E. Cunningham F. Dunham I. Durbin R. Fernandez-Suarez XM. Harrow J. Herrero J. Hubbard TJ. Parker A. Proctor G. Spudich G. Vogel J. Yates A. Zadissa A. Searle SM. Ensembl 2012. Nucleic Acids Res. 2012;40:D84–90. doi: 10.1093/nar/gkr991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Forneris F. Binda C. Vanoni MA. Mattevi A. Battaglioli E. Histone demethylation catalysed by LSD1 is a flavin-dependent oxidative process. FEBS Lett. 2005;579:2203–2207. doi: 10.1016/j.febslet.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 36.Frenkel K. Goldstein MS. Duker NJ. Teebor GW. Identification of the cis-thymine glycol moiety in oxidized deoxyribonucleic acid. Biochemistry. 1981;20:750–754. doi: 10.1021/bi00507a014. [DOI] [PubMed] [Google Scholar]
- 37.Gao Y. Katyal S. Lee Y. Zhao J. Rehg JE. Russell HR. McKinnon PJ. DNA ligase III is critical for mtDNA integrity but not Xrcc1-mediated nuclear DNA repair. Nature. 2011;471:240–244. doi: 10.1038/nature09773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gaymes TJ. Shall S. MacPherson LJ. Twine NA. Lea NC. Farzaneh F. Mufti GJ. Inhibitors of poly ADP-ribose polymerase (PARP) induce apoptosis of myeloid leukemic cells: potential for therapy of myeloid leukemia and myelodysplastic syndromes. Haematologica. 2009;94:638–646. doi: 10.3324/haematol.2008.001933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gehring M. Huh JH. Hsieh TF. Penterman J. Choi Y. Harada JJ. Goldberg RB. Fischer RL. DEMETER DNA glycosylase establishes MEDEA polycomb gene self-imprinting by allele-specific demethylation. Cell. 2006;124:495–506. doi: 10.1016/j.cell.2005.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gehring M. Reik W. Henikoff S. DNA demethylation by DNA repair. Trends Genet. 2009;25:82–90. doi: 10.1016/j.tig.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 41.Greer EL. Shi Y. Histone methylation: a dynamic mark in health, disease and inheritance. Nat Rev Genet. 2012;13:343–357. doi: 10.1038/nrg3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guccione E. Martinato F. Finocchiaro G. Luzi L. Tizzoni L. Dall’ Olio V. Zardo G. Nervi C. Bernard L. Amati B. Myc-binding-site recognition in the human genome is determined by chromatin context. Nat Cell Biol. 2006;8:764–770. doi: 10.1038/ncb1434. [DOI] [PubMed] [Google Scholar]
- 43.Guo JU. Su Y. Zhong C. Ming GL. Song H. Emerging roles of TET proteins and 5-hydroxymethylcytosines in active DNA demethylation and beyond. Cell Cycle. 2011;10:2662–2668. doi: 10.4161/cc.10.16.17093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hailer MK. Slade PG. Martin BD. Rosenquist TA. Sugden KD. Recognition of the oxidized lesions spiroiminodihydantoin and guanidinohydantoin in DNA by the mammalian base excision repair glycosylases NEIL1 and NEIL2. DNA Repair (Amst) 2005;4:41–50. doi: 10.1016/j.dnarep.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 45.Hajkova P. Jeffries SJ. Lee C. Miller N. Jackson SP. Surani MA. Genome-wide reprogramming in the mouse germ line entails the base excision repair pathway. Science. 2010;329:78–82. doi: 10.1126/science.1187945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hammoud SS. Cairns BR. Carrell DT. Analysis of gene-specific and genome-wide sperm DNA methylation. Methods Mol Biol. 2013;927:451–458. doi: 10.1007/978-1-62703-038-0_39. [DOI] [PubMed] [Google Scholar]
- 47.Hanes JW. Thal DM. Johnson KA. Incorporation and replication of 8-oxo-deoxyguanosine by the human mitochondrial DNA polymerase. J Biol Chem. 2006;281:36241–36248. doi: 10.1074/jbc.M607965200. [DOI] [PubMed] [Google Scholar]
- 48.He YF. Li BZ. Li Z. Liu P. Wang Y. Tang Q. Ding J. Jia Y. Chen Z. Li L. Sun Y. Li X. Dai Q. Song CX. Zhang K. He C. Xu GL. Tet-mediated formation of 5-carboxylcytosine and its excision by TDG in mammalian DNA. Science. 2011;333:1303–1307. doi: 10.1126/science.1210944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hegde ML. Hegde PM. Rao KS. Mitra S. Oxidative genome damage and its repair in neurodegenerative diseases: function of transition metals as a double-edged sword. J Alzheimer's Dis: JAD. 2011;24(Suppl 2):183–198. doi: 10.3233/JAD-2011-110281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hoeijmakers JH. Genome maintenance mechanisms for preventing cancer. Nature. 2001;411:366–374. doi: 10.1038/35077232. [DOI] [PubMed] [Google Scholar]
- 51.Hoffmann S. Spitkovsky D. Radicella JP. Epe B. Wiesner RJ. Reactive oxygen species derived from the mitochondrial respiratory chain are not responsible for the basal levels of oxidative base modifications observed in nuclear DNA of mammalian cells. Free Radic Biol Med. 2004;36:765–773. doi: 10.1016/j.freeradbiomed.2003.12.019. [DOI] [PubMed] [Google Scholar]
- 52.Huang Y. Vasilatos SN. Boric L. Shaw PG. Davidson NE. Inhibitors of histone demethylation and histone deacetylation cooperate in regulating gene expression and inhibiting growth in human breast cancer cells. Breast Cancer Res Treat. 2012;131:777–789. doi: 10.1007/s10549-011-1480-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ito S. Shen L. Dai Q. Wu SC. Collins LB. Swenberg JA. He C. Zhang Y. Tet proteins can convert 5-methylcytosine to 5-formylcytosine and 5-carboxylcytosine. Science. 2011;333:1300–1303. doi: 10.1126/science.1210597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jaenisch R. Bird A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat Genet. 2003;33(Suppl):245–254. doi: 10.1038/ng1089. [DOI] [PubMed] [Google Scholar]
- 55.Jenuwein T. Allis CD. Translating the histone code. Science. 2001;293:1074–1080. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- 56.Jovanovic SV. Simic MG. Mechanism of OH radical reaction with thymine and uracil derivatives. J Am Chem Soc. 1986;108:5968–5972. doi: 10.1021/ja00279a050. [DOI] [PubMed] [Google Scholar]
- 57.Kalam MA. Haraguchi K. Chandani S. Loechler EL. Moriya M. Greenberg MM. Basu AK. Genetic effects of oxidative DNA damages: comparative mutagenesis of the imidazole ring-opened formamidopyrimidines (Fapy lesions) and 8-oxo-purines in simian kidney cells. Nucleic Acids Res. 2006;34:2305–2315. doi: 10.1093/nar/gkl099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Karytinos A. Forneris F. Profumo A. Ciossani G. Battaglioli E. Binda C. Mattevi A. A novel mammalian flavin-dependent histone demethylase. J Biol Chem. 2009;284:17775–17782. doi: 10.1074/jbc.M109.003087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kasai H. Nishimura S. Hydroxylation of deoxyguanosine at the C-8 position by ascorbic acid and other reducing agents. Nucleic Acids Res. 1984;12:2137–2145. doi: 10.1093/nar/12.4.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kass SU. Landsberger N. Wolffe AP. DNA methylation directs a time-dependent repression of transcription initiation. Curr Biol. 1997;7:157–165. doi: 10.1016/s0960-9822(97)70086-1. [DOI] [PubMed] [Google Scholar]
- 61.Kellinger MW. Song CX. Chong J. Lu XY. He C. Wang D. 5-Formylcytosine and 5-carboxylcytosine reduce the rate and substrate specificity of RNA polymerase II transcription. Nat Struct Mol Biol. 2012;19:831–833. doi: 10.1038/nsmb.2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Klose RJ. Kallin EM. Zhang Y. JmjC-domain-containing proteins and histone demethylation. Nat Rev Genet. 2006;7:715–727. doi: 10.1038/nrg1945. [DOI] [PubMed] [Google Scholar]
- 63.Ko M. Huang Y. Jankowska AM. Pape UJ. Tahiliani M. Bandukwala HS. An J. Lamperti ED. Koh KP. Ganetzky R. Liu XS. Aravind L. Agarwal S. Maciejewski JP. Rao A. Impaired hydroxylation of 5-methylcytosine in myeloid cancers with mutant TET2. Nature. 2010;468:839–843. doi: 10.1038/nature09586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kooistra SM. Helin K. Molecular mechanisms and potential functions of histone demethylases. Nat Rev Mol Cell Biol. 2012;13:297–311. doi: 10.1038/nrm3327. [DOI] [PubMed] [Google Scholar]
- 65.Krahn JM. Beard WA. Miller H. Grollman AP. Wilson SH. Structure of DNA polymerase beta with the mutagenic DNA lesion 8-oxodeoxyguanine reveals structural insights into its coding potential. Structure. 2003;11:121–127. doi: 10.1016/s0969-2126(02)00930-9. [DOI] [PubMed] [Google Scholar]
- 66.Kusumoto R. Masutani C. Iwai S. Hanaoka F. Translesion synthesis by human DNA polymerase eta across thymine glycol lesions. Biochemistry. 2002;41:6090–6099. doi: 10.1021/bi025549k. [DOI] [PubMed] [Google Scholar]
- 67.Lee DH. Pfeifer GP. Translesion synthesis of 7,8-dihydro-8-oxo-2′-deoxyguanosine by DNA polymerase eta in vivo. Mutat Res. 2008;641:19–26. doi: 10.1016/j.mrfmmm.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liang Y. Russell I. Walworth C. Chen C. Gene expression in stem cells. Crit Rev Eukaryot Gene Expr. 2009;19:289–300. doi: 10.1615/critreveukargeneexpr.v19.i4.30. [DOI] [PubMed] [Google Scholar]
- 69.Markkanen E. Hubscher U. van Loon B. Regulation of oxidative DNA damage repair: the adenine:8-oxo-guanine problem. Cell Cycle. 2012;11:1070–1075. doi: 10.4161/cc.11.6.19448. [DOI] [PubMed] [Google Scholar]
- 70.Markkanen E. van Loon B. Ferrari E. Parsons JL. Dianov GL. Hubscher U. Regulation of oxidative DNA damage repair by DNA polymerase lambda and MutYH by cross-talk of phosphorylation and ubiquitination. Proc Natl Acad Sci U S A. 2012;109:437–442. doi: 10.1073/pnas.1110449109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Miller H. Prasad R. Wilson SH. Johnson F. Grollman AP. 8-oxodGTP incorporation by DNA polymerase beta is modified by active-site residue Asn279. Biochemistry. 2000;39:1029–1033. doi: 10.1021/bi991789x. [DOI] [PubMed] [Google Scholar]
- 72.Morales-Ruiz T. Ortega-Galisteo AP. Ponferrada-Marin MI. Martinez-Macias MI. Ariza RR. Roldan-Arjona T. DEMETER and REPRESSOR OF SILENCING 1 encode 5-methylcytosine DNA glycosylases. Proc Natl Acad Sci U S A. 2006;103:6853–6858. doi: 10.1073/pnas.0601109103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Murray K. The occurrence of epsilon-N-methyl lysine in histones. Biochemistry. 1964;3:10–15. doi: 10.1021/bi00889a003. [DOI] [PubMed] [Google Scholar]
- 74.Neeley WL. Essigmann JM. Mechanisms of formation, genotoxicity, and mutation of guanine oxidation products. Chem Res Toxicol. 2006;19:491–505. doi: 10.1021/tx0600043. [DOI] [PubMed] [Google Scholar]
- 75.Ng SS. Kavanagh KL. McDonough MA. Butler D. Pilka ES. Lienard BM. Bray JE. Savitsky P. Gileadi O. von Delft F. Rose NR. Offer J. Scheinost JC. Borowski T. Sundstrom M. Schofield CJ. Oppermann U. Crystal structures of histone demethylase JMJD2A reveal basis for substrate specificity. Nature. 2007;448:87–91. doi: 10.1038/nature05971. [DOI] [PubMed] [Google Scholar]
- 76.Niehrs C. Active DNA demethylation and DNA repair. Differentiation; Research In Biological Diversity. 2009;77:1–11. doi: 10.1016/j.diff.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 77.Olinski R. Zastawny T. Budzbon J. Skokowski J. Zegarski W. Dizdaroglu M. DNA base modifications in chromatin of human cancerous tissues. FEBS Lett. 1992;309:193–198. doi: 10.1016/0014-5793(92)81093-2. [DOI] [PubMed] [Google Scholar]
- 78.Perillo B. Ombra MN. Bertoni A. Cuozzo C. Sacchetti S. Sasso A. Chiariotti L. Malorni A. Abbondanza C. Avvedimento EV. DNA oxidation as triggered by H3K9me2 demethylation drives estrogen-induced gene expression. Science. 2008;319:202–206. doi: 10.1126/science.1147674. [DOI] [PubMed] [Google Scholar]
- 79.Rai K. Huggins IJ. James SR. Karpf AR. Jones DA. Cairns BR. DNA demethylation in zebrafish involves the coupling of a deaminase, a glycosylase, and gadd45. Cell. 2008;135:1201–1212. doi: 10.1016/j.cell.2008.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Seward DJ. Cubberley G. Kim S. Schonewald M. Zhang L. Tripet B. Bentley DL. Demethylation of trimethylated histone H3 Lys4 in vivo by JARID1 JmjC proteins. Nat Struct Mol Biol. 2007;14:240–242. doi: 10.1038/nsmb1200. [DOI] [PubMed] [Google Scholar]
- 81.Shi Y. Lan F. Matson C. Mulligan P. Whetstine JR. Cole PA. Casero RA. Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell. 2004;119:941–953. doi: 10.1016/j.cell.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 82.Shi YY. Huang ZY. Zeng ZJ. Wang ZL. Wu XB. Yan WY. Diet and cell size both affect queen-worker differentiation through DNA methylation in honey bees (Apis mellifera, Apidae) PLoS ONE. 2011;6:e18808. doi: 10.1371/journal.pone.0018808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Siegfried Z. Cedar H. DNA methylation: a molecular lock. Curr Biol. 1997;7:R305–307. doi: 10.1016/s0960-9822(06)00144-8. [DOI] [PubMed] [Google Scholar]
- 84.Simsek D. Furda A. Gao Y. Artus J. Brunet E. Hadjantonakis AK. Van Houten B. Shuman S. McKinnon PJ. Jasin M. Crucial role for DNA ligase III in mitochondria but not in Xrcc1-dependent repair. Nature. 2011;471:245–248. doi: 10.1038/nature09794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sobol RW. For MutY, it's all about the OG. Chem Biol. 2012;19:313–314. doi: 10.1016/j.chembiol.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sobol RW. Prasad R. Evenski A. Baker A. Yang XP. Horton JK. Wilson SH. The lyase activity of the DNA repair protein β-polymerase protects from DNA-damage-induced cytotoxicity. Nature. 2000;405:807–810. doi: 10.1038/35015598. [DOI] [PubMed] [Google Scholar]
- 87.Strahl BD. Allis CD. The language of covalent histone modifications. Nature. 2000;403:41–45. doi: 10.1038/47412. [DOI] [PubMed] [Google Scholar]
- 88.Svilar D. Goellner EM. Almeida KH. Sobol RW. Base excision repair and lesion-dependent sub-pathways for repair of oxidative DNA damage. Antioxid Redox Signal. 2011;14:2491–2507. doi: 10.1089/ars.2010.3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Szwagierczak A. Bultmann S. Schmidt CS. Spada F. Leonhardt H. Sensitive enzymatic quantification of 5-hydroxymethylcytosine in genomic DNA. Nucleic Acids Res. 2010;38:e181. doi: 10.1093/nar/gkq684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tahiliani M. Koh KP. Shen Y. Pastor WA. Bandukwala H. Brudno Y. Agarwal S. Iyer LM. Liu DR. Aravind L. Rao A. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science. 2009;324:930–935. doi: 10.1126/science.1170116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Takata K. Shimizu T. Iwai S. Wood RD. Human DNA polymerase N (POLN) is a low fidelity enzyme capable of error-free bypass of 5S-thymine glycol. J Biol Chem. 2006;281:23445–23455. doi: 10.1074/jbc.M604317200. [DOI] [PubMed] [Google Scholar]
- 92.Tan X. Grollman AP. Shibutani S. Comparison of the mutagenic properties of 8-oxo-7,8-dihydro-2′-deoxyadenosine and 8-oxo-7,8-dihydro-2′-deoxyguanosine DNA lesions in mammalian cells. Carcinogenesis. 1999;20:2287–2292. doi: 10.1093/carcin/20.12.2287. [DOI] [PubMed] [Google Scholar]
- 93.Tsukada Y. Hydroxylation mediates chromatin demethylation. J Biochem. 2012;151:229–246. doi: 10.1093/jb/mvs003. [DOI] [PubMed] [Google Scholar]
- 94.Tsukada Y. Zhang Y. Purification of histone demethylases from HeLa cells. Methods. 2006;40:318–326. doi: 10.1016/j.ymeth.2006.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Turner BM. Histone acetylation and an epigenetic code. Bioessays. 2000;22:836–845. doi: 10.1002/1521-1878(200009)22:9<836::AID-BIES9>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 96.Um S. Harbers M. Benecke A. Pierrat B. Losson R. Chambon P. Retinoic acid receptors interact physically and functionally with the T:G mismatch-specific thymine-DNA glycosylase. J Biol Chem. 1998;273:20728–20736. doi: 10.1074/jbc.273.33.20728. [DOI] [PubMed] [Google Scholar]
- 97.van Essen D. Zhu Y. Saccani S. A feed-forward circuit controlling inducible NF-kappaB target gene activation by promoter histone demethylation. Mol Cells. 2010;39:750–760. doi: 10.1016/j.molcel.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 98.van Loon B. Hubscher U. An 8-oxo-guanine repair pathway coordinated by MUTYH glycosylase and DNA polymerase lambda. Proc Natl Acad Sci U S A. 2009;106:18201–18206. doi: 10.1073/pnas.0907280106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.van Loon B. Markkanen E. Hubscher U. Oxygen as a friend and enemy: how to combat the mutational potential of 8-oxo-guanine. DNA Repair (Amst) 2010 doi: 10.1016/j.dnarep.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 100.Wang J. Scully K. Zhu X. Cai L. Zhang J. Prefontaine GG. Krones A. Ohgi KA. Zhu P. Garcia-Bassets I. Liu F. Taylor H. Lozach J. Jayes FL. Korach KS. Glass CK. Fu XD. Rosenfeld MG. Opposing LSD1 complexes function in developmental gene activation and repression programmes. Nature. 2007;446:882–887. doi: 10.1038/nature05671. [DOI] [PubMed] [Google Scholar]
- 101.Whetstine JR. Nottke A. Lan F. Huarte M. Smolikov S. Chen Z. Spooner E. Li E. Zhang G. Colaiacovo M. Shi Y. Reversal of histone lysine trimethylation by the JMJD2 family of histone demethylases. Cell. 2006;125:467–481. doi: 10.1016/j.cell.2006.03.028. [DOI] [PubMed] [Google Scholar]
- 102.Wiederhold L. Leppard JB. Kedar P. Karimi-Busheri F. Rasouli-Nia A. Weinfeld M. Tomkinson AE. Izumi T. Prasad R. Wilson SH. Mitra S. Hazra TK. AP endonuclease-independent DNA base excision repair in human cells. Mol Cells. 2004;15:209–220. doi: 10.1016/j.molcel.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 103.Wood A. Shilatifard A. Posttranslational modifications of histones by methylation. Adv Protein Chem. 2004;67:201–222. doi: 10.1016/S0065-3233(04)67008-2. [DOI] [PubMed] [Google Scholar]
- 104.Yamane K. Toumazou C. Tsukada Y. Erdjument-Bromage H. Tempst P. Wong J. Zhang Y. JHDM2A, a JmjC-containing H3K9 demethylase, facilitates transcription activation by androgen receptor. Cell. 2006;125:483–495. doi: 10.1016/j.cell.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 105.Zeller KI. Zhao X. Lee CW. Chiu KP. Yao F. Yustein JT. Ooi HS. Orlov YL. Shahab A. Yong HC. Fu Y. Weng Z. Kuznetsov VA. Sung WK. Ruan Y. Dang CV. Wei CL. Global mapping of c-Myc binding sites and target gene networks in human B cells. Proc Natl Acad Sci U S A. 2006;103:17834–17839. doi: 10.1073/pnas.0604129103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhang Y. Yuan F. Wu X. Taylor JS. Wang Z. Response of human DNA polymerase iota to DNA lesions. Nucleic Acids Res. 2001;29:928–935. doi: 10.1093/nar/29.4.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhang Y. Yuan F. Wu X. Wang M. Rechkoblit O. Taylor JS. Geacintov NE. Wang Z. Error-free and error-prone lesion bypass by human DNA polymerase kappa in vitro. Nucleic Acids Res. 2000;28:4138–4146. doi: 10.1093/nar/28.21.4138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhu B. Benjamin D. Zheng Y. Angliker H. Thiry S. Siegmann M. Jost JP. Overexpression of 5-methylcytosine DNA glycosylase in human embryonic kidney cells EcR293 demethylates the promoter of a hormone-regulated reporter gene. Proc Natl Acad Sci U S A. 2001;98:5031–5036. doi: 10.1073/pnas.091097298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ziel KA. Grishko V. Campbell CC. Breit JF. Wilson GL. Gillespie MN. Oxidants in signal transduction: impact on DNA integrity and gene expression. FASEB J. 2005;19:387–394. doi: 10.1096/fj.04-2805com. [DOI] [PubMed] [Google Scholar]