Abstract
Aims: Urinary 8-oxo-7,8-dihydro-2′-deoxyguanosine (8-oxodG) is a widely used biomarker of oxidative stress. However, variability between chromatographic and ELISA methods hampers interpretation of data, and this variability may increase should urine composition differ between individuals, leading to assay interference. Furthermore, optimal urine sampling conditions are not well defined. We performed inter-laboratory comparisons of 8-oxodG measurement between mass spectrometric-, electrochemical- and ELISA-based methods, using common within-technique calibrants to analyze 8-oxodG-spiked phosphate-buffered saline and urine samples. We also investigated human subject- and sample collection-related variables, as potential sources of variability. Results: Chromatographic assays showed high agreement across urines from different subjects, whereas ELISAs showed far more inter-laboratory variation and generally overestimated levels, compared to the chromatographic assays. Excretion rates in timed ‘spot’ samples showed strong correlations with 24 h excretion (the ‘gold’ standard) of urinary 8-oxodG (rp 0.67–0.90), although the associations were weaker for 8-oxodG adjusted for creatinine or specific gravity (SG). The within-individual excretion of 8-oxodG varied only moderately between days (CV 17% for 24 h excretion and 20% for first void, creatinine-corrected samples). Innovation: This is the first comprehensive study of both human and methodological factors influencing 8-oxodG measurement, providing key information for future studies with this important biomarker. Conclusion: ELISA variability is greater than chromatographic assay variability, and cannot determine absolute levels of 8-oxodG. Use of standardized calibrants greatly improves intra-technique agreement and, for the chromatographic assays, importantly allows integration of results for pooled analyses. If 24 h samples are not feasible, creatinine- or SG-adjusted first morning samples are recommended. Antioxid. Redox Signal. 18, 2377–2391.
Introduction
The measurement of products of oxidatively damaged DNA in urine continues to receive widespread attention as a biomarker of in vivo oxidative stress (6). Analysis of urine minimizes consent issues, allowing studies to incorporate vulnerable subjects. The most frequently measured urinary biomarker of oxidatively-generated damage to 2′-deoxyribonucleosides is 8-oxo-7,8-dihydro-2′-deoxyguanosine (8-oxodG) (18), largely due to its numerous strengths as a biomarker: (i) long-term stability in urine (19); (ii) no artefactual production (17); (iii) multiple methods for its measurement (e.g., chromatography with either mass spectrometric or electrochemical detection, and ELISA); (iv) urine is easily collected, and requires no special treatment or processing prior to storage; (v) only small sample volumes are required; (vi) urine samples from previous studies/biobanks are also suitable for analysis, minimizing the need for new, prospective studies by allowing retrospective analysis of existing cohorts. However, variability within, and in particular between, different chromatographic- and ELISA-based methods of analysis, which together with poorly considered human sources of variation (e.g., urine composition, sampling timepoint, inter- and intra-individual variability), hamper comparisons and the achievement of consistency in results.
The European Standards Committee on Urinary (DNA) Lesion Analysis (ESCULA) was established, in part, to evaluate the differences in reported levels between chromatographic and ELISA techniques, and improve inter-laboratory agreement (6). We recently described the findings of a large multicenter study examining the level of agreement between techniques for measuring urinary 8-oxodG (9). As might be expected, mass spectrometric methods demonstrated the greatest accuracy and sensitivity. Although the study showed greater consensus than previously expected (5, 11, 25, 28, 29), concern remained over ELISA (8); furthermore, all approaches showed significant variability in intra-technique agreement. In a renewed effort to improve inter-assay agreement, we here present a large scale inter-laboratory exercise with 18 laboratories and common calibrants providing 25 data sets on measurement of 8-oxodG in urine by chromatographic and ELISA based assays. It is conceivable that illness may affect the composition of urine, and therefore the analysis. We sought to address this by the analysis of urine samples from both healthy individuals and head and neck cancer patients, the latter being representative of a group in which disease may affect complexity of the matrix.
Despite urinary 8-oxodG having been used as a biomarker for oxidative stress in a large number of human studies, there is limited information published about intra-individual variability, for example, diurnal trends (2) or variability between days (23). Such information is essential when planning a study of 8-oxodG in order to choose the optimal sampling design. While 24 h excretion of 8-oxodG may be considered the gold standard, this is practically and logistically challenging. Consequently, 8-oxodG excretion is usually assessed using spot urine samples, which are then adjusted for urinary flow using creatinine concentration or specific gravity (SG), but there are only two reports in the literature evaluating whether these adjustment techniques are appropriate (23, 24). Therefore, as part of the present study, we also aimed to provide definitive information for the intra-individual variability in 8-oxodG levels in an appreciable number of healthy subjects, from whom repeated samples were collected over 24 h, on 2 different days.
This report is unique in providing a comprehensive statistical evaluation of potential sources of variability, both human and methodological, in the measurement of urinary 8-oxodG, together with recommendations for best practice. Understanding the sources of variability will allow for an improvement in inter- and intra-technique agreement, together with better study design to minimize inter- and intra-individual variability, making the resulting data yet more robust.
Results
Methodological sources of variability
For this inter-laboratory exercise, 26 out of the 31 ESCULA participating laboratories received samples, and 18 laboratories returned results, consisting of 25 data sets (six laboratories provided multiple data sets, due to the use of multiple techniques or calibration methods). Results from Laboratory 7, using HPLC-ECD, did not bear any resemblance to the other data and were excluded from subsequent analyses, but are shown for illustration in Figures 1 and 2. This was a similar result as noted previously for this laboratory (9).
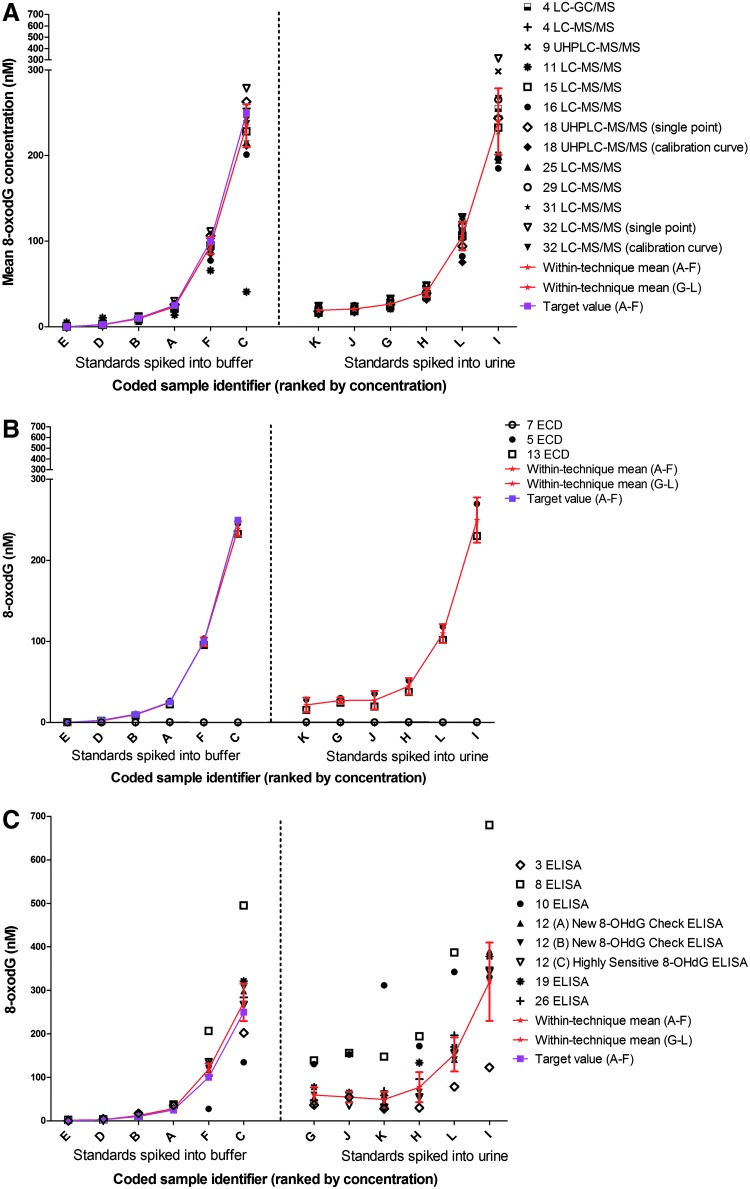
FIG. 1.
Ranked mean concentration of 8-oxodG in samples of phosphate-buffered saline (PBS) and urine, to which exogenous 8-oxodG was added. Determination by (A) mass spectrometric, (B) HPLC-electrochemical, and (C) immunoassay procedures. Each laboratory and technique is denoted by a symbol. Additional information, where relevant, on the method of analysis, such as type of calibration, or a particular kit used, is provided adjacent to the symbol description. All laboratories used the technique-specific calibrants provided by ESCULA. For two of the MS laboratories (18 and 32), two sets of results are presented, which correspond to data derived from more than one method of quantification (‘single point’, internal standard calibration method, or external standard ‘calibration curve’ method). Results for a number of ELISA variants are also shown, representing commercial kits from the Japanese Institute for the Control of Aging: (A) New 8-OHdG Check, (B) Ultrafiltration of samples prior to New 8-OHdG Check, or (C) Highly Sensitive 8-OHdG kit. Samples A–F are 8-oxodG standards in PBS: 0 (negative control), 2.5, 10, 25, 100, and 250 nM (labeled E, D, B, A, F, and C, respectively) and samples G–L are 8-oxodG standards, final concentrations of 0 (negative control), 2.5, 10, 25, 100, and 250 nM, added to a single urine derived from a healthy subject (labeled K, J, G, H, L, and I, respectively). For samples A–F, target values (i.e., known amounts of exogenously added 8-oxodG) are shown as purple-filled squares (■). Also shown are within-technique mean values (★), and SD (red error bars), joined by a connecting line.
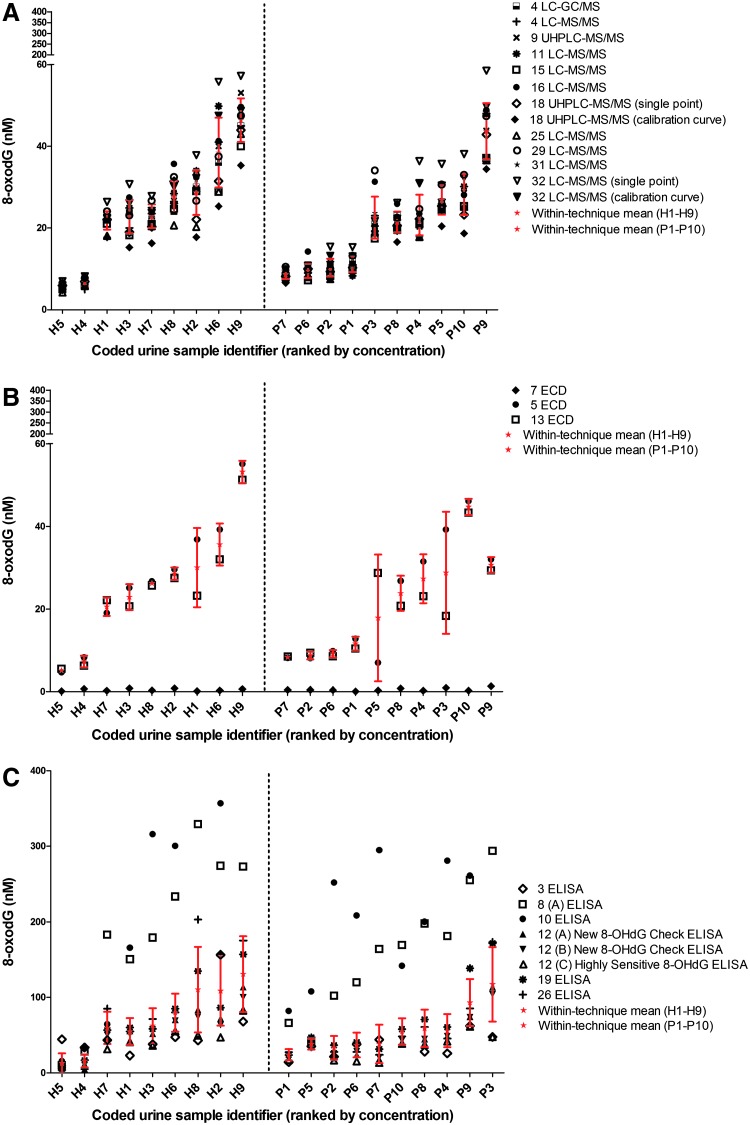
FIG. 2.
Ranked mean concentration of urinary 8-oxodG in samples from 9 healthy individuals (samples H1–9) and 10 head and neck cancer (samples P1–10) patients, analyzed by: (A) mass spectrometric detection; (B) electrochemical detection; (C) immunoassay. Each symbol represents a laboratory determination, with method of calibration, where appropriate, in parentheses. Also shown are within-technique mean values (★) and SD (red error bars).
Analysis of 8-oxodG standard added to PBS
Figures 1A, B, and C [mass spectrometry (MS), HPLC ECD (ECD), and ELISA, respectively] show the data for 8-oxodG standards dissolved in PBS (samples A-F) or urine (samples G–L), expressed as nM 8-oxodG. Samples E and K contained no exogenously added 8-oxodG, hence the values obtained for sample K represent the endogenous levels of 8-oxodG present in the urine. Also shown on each plot, for samples A–F, are ‘target values’ (known concentration of 8-oxodG in the sample). For samples A–L, we also present a mean value for the level of 8-oxodG derived from individual laboratory measurements, and the accompanying error bars indicate SD.
Both the MS and ECD technique groups clearly detected successive increases in 8-oxodG spiked into buffer (Fig. 1A and B), although some ELISA approaches failed to discriminate between 0 and 2.5 nM. There was close agreement in the values for the MS and ECD approaches, whereas the 8-oxodG values for the chromatographic assays and ELISAs were substantially different. There was a significant variation in levels between methods and within the ELISA approaches (p<0.001, ANOVA with the laboratory nested in the type of method and spiked 8-oxodG as dependent variable).
For the standards spiked into buffer, the consensus mean curves and the target value curves were practically superimposed at all concentrations, and for all methods of analysis (Fig. 1A–C). Laboratory 11 (LC-MS/MS) showed a single discrepancy with the other MS laboratories for sample C. As this was an isolated incident, we attributed this to a single, undefined, technical error, and excluded this point from the calculation of the mean±SD. For samples F and C, Laboratories 8 and 10 (ELISA) over- and underestimated (i.e., lay outside the SD error bars), respectively—this may be attributed to the levels of 8-oxodG in the samples saturating the assays, as they give values outside the range of their calibration curves. The values for samples F and C derived from these laboratories were similarly excluded from the calculation of the mean±SD.
There was rank-order agreement for all these samples, between all three methods of analysis (Fig. 1A–1C).
Analysis of 8-oxodG standard added to urine
For the urine spiked with increasing concentrations of 8-oxodG standard (samples G–L), there was evidence of some suppression of the concentration dependent trend at lower concentrations, compared to standards in PBS, for each technique, but particularly ELISA (Fig. 1A–C). On the whole, the individual determinations of 8-oxodG were within one SD of the group mean, although there were some notable exceptions with Laboratories 3, 8, and 10 either under- or overestimating the 8-oxodG levels in the majority of samples, although Laboratories 3 and 8 did follow the general trend (Fig. 1C). It should be noted that these laboratories did not use a commercial ELISA kit but their own in-house assays. Examination of the ELISA methodologies used revealed that Laboratory 3 was the only laboratory to perform the primary antibody incubation step at 4°C overnight, together with a clean-up step. Incubation of the primary antibody at 4°C overnight has been reported previously to improve the agreement between chromatographic and ELISA methods (10), decreasing overestimation. The cause of the overestimation by Laboratory 8 is not immediately evident, but this may be due to the relatively narrow range of the calibration curve. Laboratory 10 used a different primary antibody (clone 1F7), compared to the other participants, who used clone N45.1, in conjunction with an in-house assay. 1F7 has a lower specificity for 8-oxodG (31), compared to N45.1, an effect which is particularly evident in spiked urine samples. Consequently, the values from Laboratories 8 and 10 were excluded from the calculation of the mean±SD. Only at the highest 8-oxodG concentrations [i.e., above levels reported to be present in urine (samples L and I)], did a few MS laboratories (Laboratories 9, 16, 18, and 32) return values which lay outside the error bars. This indicates that such concentrations exceed the dynamic range of the MS assays. There was little difference in the values between MS and EC, whereas the ELISA values ranged from being two- to four-fold greater than MS or EC values. On average, the ELISA values were 4.2-fold (95% CI: 2.3, 6.0-fold) higher than the chromatographic techniques, based on intercepts from linear regression analysis.
All laboratories, except Laboratory 10, had significant regression slopes of the measured 8-oxodG concentration in the spiked urine samples (Table 1). In addition, there were generally high R2-values (0.97–0.99, except Laboratory 10 with R2=0.43; Table 1), indicating that the concentration of spiked 8-oxodG in the urine samples was the predominant contributor to the overall variation. The slope of the concentration-response curve did not differ between the chromatographic assays and ELISA (p=0.62, general regression model). The ELISA techniques had the same slope (slope±SE: 0.95±0.13, p<0.001) as the chromatographic techniques (slope±SE: 0.90±0.02, p<0.001). These data demonstrated the ability of all laboratories, with the exception of Laboratory 10, to detect a concentration-response in an authentic matrix.
Table 1.
Linear Regression Analysis of Urine Samples Spiked with Known Concentrations of 8-oxodG
| |
Results from 8-oxodG spiked urine samples |
|||
|---|---|---|---|---|
| Laboratory | Intercept±SE | Slope±SE | R2Value | SDres (nM) |
| ELISA | ||||
| Lab 3 | 34.4±6.7* | 0.36±0.06* | 0.90 | 12.0 |
| Lab 8 | 142.6±8.4* | 2.19±0.08* | 0.99 | 14.9 |
| Lab 10 | 197.4±41.5* | 0.66±0.38 | 0.43 | 73.8 |
| Lab 12 (High sensitivity) | 43.2±6.0* | 1.10±0.05* | 0.99 | 7.81 |
| Lab 12 (A New check) | 34.9±10.4* | 1.37±0.09* | 0.98 | 10.7 |
| Lab 12 (B New check) | 28.7±4.4* | 1.25±0.04* | 0.99 | 18.5 |
| Lab 19 | 68.4±12.0* | 1.21±0.11* | 0.97 | 21.3 |
| Lab 26 | 64.6±6.7* | 1.18±0.06* | 0.99 | 11.8 |
| HPLC-ECD | ||||
| Lab 5 | 26.5±1.4* | 0.96±0.01* | 0.99 | 2.51 |
| Lab 13 | 16.2±2.0* | 0.86±0.02* | 0.99 | 3.62 |
| MS | ||||
| Lab 4 (LC-MS/MS) | 20.6±3.0* | 0.92±0.03* | 0.99 | 5.35 |
| Lab 9 (LC-MS/MS) | 18.1±0.9* | 1.12±0.01* | 0.99 | 1.55 |
| Lab 11 (LC-MS/MS) | 22.4±3.4* | 0.73±0.03* | 0.99 | 6.11 |
| Lab 15 (LC-MS/MS) | 15.8±1.0* | 0.87±0.01* | 0.99 | 1.80 |
| Lab 16 (LC-MS/MS) | 18.1±0.7* | 0.66±0.01* | 0.99 | 1.22 |
| Lab 18 (UHPLC-MS/MS single point calibration) | 15.9±3.2* | 0.89±0.03* | 0.99 | 5.66 |
| Lab 25 (LC-MS/MS) | 16.0±1.3* | 0.72±0.01* | 0.99 | 2.26 |
| Lab 29 (LC-MS/MS) | 21.7±0.04* | 0.79±0.01* | 0.99 | 0.69 |
| Lab 31 (LC-MS/MS) | 19.2±1.0* | 0.83±0.01* | 0.99 | 2.06 |
| Lab 32 (LC-MS/MS, single point internal calibration) | 19.9±2.5* | 1.16±0.02* | 0.99 | 4.48 |
| Lab 32 (LC-MS/MS, external calibration) | 16.9±2.1* | 0.99±0.02* | 0.99 | 3.81 |
The results were obtained by linear regression analysis of datasets from separate laboratories. The asterisks indicate that the intercept or slope is statistically significantly different from zero (p<0.05, linear regression). The SDres indicates the absolute level of unexplained variation in the regression analysis. The intercept represents the endogenous level of 8-oxodG in the urine sample, and the slope is an indication of the efficiency of target recovery.
Innovation.
Although the measurement of urinary 8-oxodG is widespread, little attention has been paid to the potential sources of variability derived from sampling, subjects, and method of analysis. Our findings, supported by comprehensive statistical analysis, can be directly translated into best practice for study design (timing of sample collection, correction for urine dilution, or flow rate), and method of analysis. The arising data will be more robust, with greater inter-laboratory agreement and support the discovery of clinical applications of urinary DNA damage measurements.
Possible effect of inter-individual variation and urine composition on 8-oxodG measurement
To investigate a possible effect of disease on urine composition, and hence 8-oxodG measurement, a number of urine samples were studied from healthy individuals and patients with cancer. The rank order of 8-oxodG results for the nine urine samples from healthy individuals, and ten cancer patients are shown in Figure 2 A–C, representing results from MS, ECD, and ELISA, respectively. The majority of MS results lay within one SD of the group mean, indicating good intra-technique agreement, although it was noted that results from Laboratories 18 (calibration curve) and 32 (single point) had a tendency to under- and overestimate the mean value, respectively. In contrast, the majority of results from the same laboratories, 18 (single point) and 32 (calibration curve), lay within two SDs of the mean, demonstrating that these laboratories can produce acceptable results.
Rank order agreement between MS and ECD was achieved for five out of nine of the healthy urines and six out of ten of the disease urines. The two ECD methods (Laboratories 5 and 13) showed good agreement, for the urines from healthy individuals, although there were large discrepancies for two of the patient samples. Generally, Laboratory 13 showed better agreement with LC-MS/MS laboratories. Rank order agreement between ECD and ELISA was achieved for four out of nine healthy samples, and one out of ten disease samples. The error bars were substantially wider for the ELISA determinations, in urine samples from both healthy subjects and cancer patients. Supporting these data, Table 2 indicates the level of variability, and suggests that it is derived from the disease state. In general, there was more residual (unexplained) variation in the ELISA techniques compared to the chromatographic methods, although it is clear that the urine matrix itself is a contributor to the ELISA variability.
Table 2.
Partitioning of the Total Variation into Differences Between Patients/Controls and Unexplained (Residual) Variation in Results Stratified for Individual Laboratories and Methods
| |
Results from analysis of patients/control samples |
||
|---|---|---|---|
| |
% of total variation |
|
|
| Laboratory/technique | Disease status | Residual | SDres (nM) |
| ELISA | |||
| Lab 3 | 11.1 | 88.9 | 29.1 |
| Lab 8 | 16.2 | 83.8 | 91.8 |
| Lab 10 | 0.3 | 99.7 | 187.9 |
| Lab 12 (High sensitivity) | 3.4 | 96.6 | 19.2 |
| Lab 12 (A New check) | 3.5 | 96.5 | 31.3 |
| Lab 12 (B New check) | 2.4 | 97.6 | 26.9 |
| Lab 19 | 0.4 | 99.6 | 47.3 |
| Lab 26 | 11.2 | 88.8 | 56.2 |
| HPLC-ECD | |||
| Lab 5 | 3.0 | 97.0 | 14.7 |
| Lab 13 | 2.4 | 97.6 | 12.2 |
| MS | |||
| Lab 4 (LC-MS/MS) | 3.1 | 96.9 | 12.9 |
| Lab 9 (LC-MS/MS) | 5.3 | 94.7 | 12.7 |
| Lab 11 (LC-MS/MS) | 10.1 | 89.9 | 12.3 |
| Lab 15 (LC-MS/MS) | 3.4 | 96.6 | 10.1 |
| Lab 16 (LC-MS/MS) | 2.9 | 97.1 | 12.9 |
| Lab 18 (UHPLC-MS/MS single point internal calibration) | 2.4 | 97.6 | 11.3 |
| Lab 25 (LC-MS/MS) | 4.6 | 95.4 | 10.7 |
| Lab 29 (LC-MS/MS) | 0.1 | 99.9 | 12.5 |
| Lab 31 (LC-MS/MS) | 6.7 | 93.3 | 11.3 |
| Lab 32 (LC-MS/MS, single point internal calibration) | 2.0 | 98.0 | 16.0 |
| Lab 32 (LC-MS/MS, external calibration) | 2.0 | 98.0 | 13.6 |
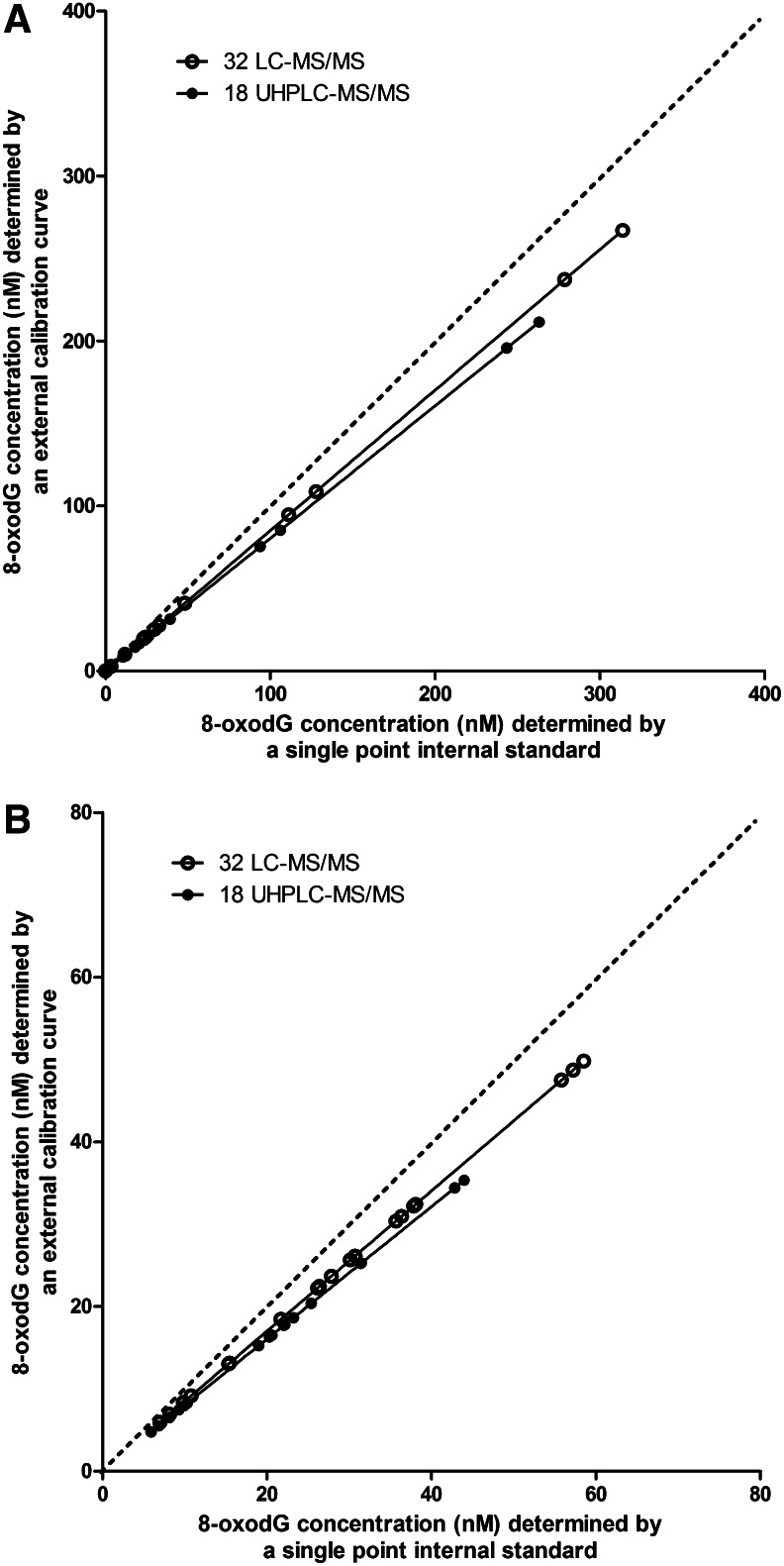
The results from Laboratories 18 (calibration curve) and 32 (single point) shown in Figure 2A are not easily explained, as they indicate some small degree of under-/overestimation, depending upon calibration method and laboratory. However, they do raise the important issue of whether single point internal calibration or multiple point, external calibration is the more accurate approach for quantification using MS–a subject for which there is no discussion, and therefore no agreement, within the literature. In our study, the majority of laboratories used external calibration curves, combined with a fixed concentration of stable isotope-labeled internal standard. Fewer laboratories used a single amount of labeled internal standard for quantification. Two laboratories (18 and 32) returned results using both of these calibration approaches, allowing direct comparison using samples spiked with standards (Fig. 3A) and urine samples (Fig. 3B). The greatest agreement between the two calibration approaches was at lower concentrations of 8-oxodG, such as those commonly seen in urine (Fig. 3B), compared with at higher concentrations (Fig. 3A), and showed only marginal deviation from the line of identity at the lower concentrations. For both laboratories (18 and 32), single point, internal calibration gave higher values than external calibration. Whether the test samples were standards spiked into buffer/urine, or urine samples appear to have little effect on the results.
FIG. 3.
Comparison of single point internal and external standard calibration of mass spectrometric methods (Laboratories 18 and 32) using: (A) standards spiked into PBS and urine; and (B) urine samples from healthy individuals and patients with head and neck cancer. Lines of regression (solid connecting lines) and perfect agreement (………) are shown.
Assessment of the sources of variability
The sources of variation in our dataset may originate from differences in techniques, laboratory-specific procedures in the same techniques, inter-individual variability, disease status and residual variation. Table 3 shows the results following the partitioning of the overall variation into differences between techniques, inter-laboratory variation, disease status, and inter-individual variation. The major difference in values of 8-oxodG appeared to originate from the use of ELISA methods compared to chromatographic techniques, whereas the use of MS or ECD for the chromatographic methods did not appear to be a strong contributor to the overall variation. We therefore compared the chromatographic techniques against ELISA methods. The differences between (i) techniques, (ii) inter-laboratory variation, (iii) disease status, (iv) inter-individual and unexplained variation correspond to 21.6%, 28.9%, 0.6%, 13.7%, and 35.8% of the overall variation, respectively (Table 3, first row). The residual variation was 40.9 nM (n=337). Within the chromatographic methods, the most important determinant for the levels of 8-oxodG excretion was difference between subjects (83.9% of the overall variation), whereas the residual variation accounted for only 9.2% (3.9 nM; n=226) of the overall variation. The inter-laboratory variation accounted for 3.7% of the total variation and disease status 3.2% (Table 3, second row). Amongst the ELISA methods, the most important contributors to the overall variation were: inter-laboratory variation (38.6% of the total variation) and residual variation (34.9% of the total variation). The inter-individual variation (25.2% of the total variation) and disease status (1.3% of the total variation) accounted for less of the total variation (Table 3, third row). The residual variation was 62.0 nM (n=111). Clearly, the residual variation is much larger for the ELISA results than the chromatographic (SDres=3.6 nM). The effect of the disease status was further analyzed in separate datasets for the chromatographic techniques and ELISA (Table 3, fourth row). In general, there was no difference between the techniques with regard to the percentage of the total variation that was explained by the disease status; the mean was 3.8% and 7.1% for the chromatographic techniques and ELISA, respectively. However, there were higher absolute values of the residual variation for the ELISA methods (mean SDres=73.0 nM), compared to the chromatographic methods (mean SDres=12.5 nM). Collectively, these data indicate a high level of inter-laboratory agreement across urines from different subjects by chromatographic assays, whereas ELISA assays showed far more inter-laboratory variation. The same trend is obvious in the urine samples that were spiked with 8-oxodG where the mean residual variation (SDres) was 3.3 and 23.6 nM for the chromatographic techniques and ELISA methods, respectively. The SDres in the spiked urine samples is probably close to the actual assay variation.
Table 3.
Partitioning of Total Variation into Differences Between Methods (Chromatographic Versus ELISA), Inter-Laboratory Variation, Disease Status, Inter-Individual Variation, and Unexplained (Residual Variation)
| |
% of Total Variation |
|
||||
|---|---|---|---|---|---|---|
| Analysis | Methods | Inter-lab variation | Disease status | Inter-individual variation | Residual | SDres (nM) |
| Overalla | 21.6 | 28.9 | 0.6 | 13.7 | 35.8 | 40.9 |
| Chromatographic assaysb | NA | 3.7 | 3.2 | 83.9 | 9.2 | 3.9 |
| ELISAb | NA | 34.9 | 1.3 | 25.2 | 38.6 | 62.0 |
| Disease status | ||||||
| Chromatographic assaysc | NA | NA | 3.8 | NA | 96.2 | 12.5 |
| ELISAc | NA | NA | 7.1 | NA | 92.9 | 73.0 |
Based on ANOVA analysis with the laboratory nested in the type of method (chromatographic technique or ELISA) and the subject nested in the disease status as an independent categorical variable.
Based on ANOVA with the subject nested in the disease status and the laboratory as an independent categorical variable.
Based on ANOVA in datasets from individual laboratories with the disease status as categorical variable.
NA, not applicable because the variable has not been included in the statistical analysis.
Sampling and human sources of variability in urinary 8-oxodG
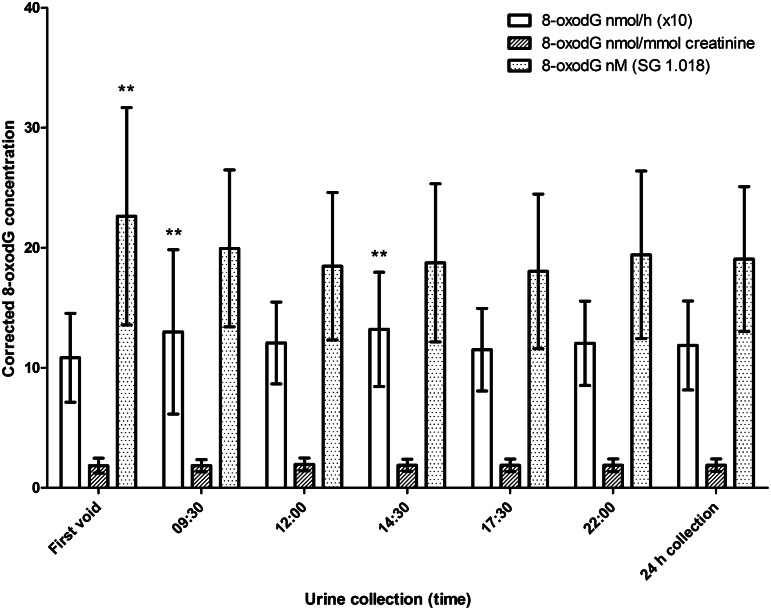
Table 4 contains some demographic and 8-oxodG data for the study population of 26 healthy individuals participating in the study of ‘human variability’, subdivided by sex. Unsurprisingly, women had significantly lower values for weight, BMI, and creatinine excretion. However, only the uncorrected urinary 8-oxodG concentration was significantly lower in women, with no statistical difference between the sexes for the corrected 8-oxodG values. The mean excretion of 8-oxodG was 28.5 nmol/24 h, 1.88 nmol/mmol creatinine, or 19.1 nM when standardized at SG 1.018 (nM-SG 1.018), based upon UHPLC-MS/MS-derived values. Diurnal variation in urinary 8-oxodG is shown in Figure 4. We noted that the rate of excretion (nmol/h) was slightly higher during the day, compared to the first morning void, with statistically significant differences (20% higher; p<0.01) at 09.30 and 14.30, compared to first void. For creatinine-adjusted results (nmol/mmol creatinine) the differences in 8-oxodG concentration between different timepoints were small (maximum 6%) and there was no overall statistically significant impact of time. After 8-oxodG concentrations were adjusted for SG (nM-SG 1.018), the first void 8-oxodG concentration was significantly higher (13%–26%; p<0.01) than at all other points of time.
Table 4.
Characteristics of Study Participants and Levels of 8-oxodG, Determined by UHPLC-MS/MS (Performed by Laboratory 9)
| Mean | Range | Men | Women | |
|---|---|---|---|---|
| Age (years) | 38 | 23–59 | 39 | 38 |
| Weight (kg) | 74 | 53–97 | 82 | 63** |
| BMI | 24 | 19–29 | 25 | 23** |
| Creatinine mmol/24 h | 15.5 | 8.5–25.3 | 17.9 | 12.3** |
| Urinary volume (L/24 h) | 1.97 | 0.85–4.57 | 1.82 | 2.18 |
| UHPLC-MS/MS | ||||
| 8-oxodG (nM) | 16.7 | 5.8–33.4 | 19.2 | 13.2* |
| 8-oxodG/SG 1.018 (nM) | 19.1 | 11.0–34.6 | 19.4 | 18.6 |
| 8-oxodG (nmol/24 h) | 28.5 | 12.7–49.7 | 31.2 | 24.7a |
| 8-oxodG (nmol/mmol creatinine) | 1.88 | 0.95–3.10 | 1.79 | 2.00 |
Study participants were 26 healthy nonsmoking Swedish subjects (11 women and 15 men). The means of two separate 24 h urine samples (from n=26 individuals) are given. Men vs. women: *p<0.05; **p<0.01; ap=0.06.
FIG. 4.
Excretion rate of 8-oxodG (nmol/h; determined by UHPLC-MS/MS, Laboratory 9), and excretion corrected for creatinine (nmol/mmol creatinine), or specific gravity (nM-SG), in timed urine samples at 6 fixed times of the day, and in a corresponding 24 h urine collection, in 26 healthy subjects. Results are means (±SD) of 2 different days, approximately 1 week apart; **p<0.01, compared to first void. **p<0.01 for SG is compared to all other times.
Associations between 8-oxodG excretion rates, 8-oxodG concentration (adjusted for creatinine or SG) in timed spot samples, and the 24 h excretion of 8-oxodG on day “A” are shown in Table 5. The correlations with 24 h excretion were highest for 8-oxodG excretion rates (rp 0.67–0.90). The results using day “B” samples were similar to those for day “A” (data not shown).
Table 5.
Pearson (r) Correlation Coefficients Between 24 h 8-oxodG Excretion and 8-oxodG Levels
| |
8-OxodG Levels |
||
|---|---|---|---|
| Sample collection time | nmol/h | nmol/mmol creatinine | nM-SG 1.018 |
| First morning (FM) | 0.90 | 0.60 | 0.59 |
| FM–09.30 | 0.80 | 0.48 | 0.68 |
| 09.30–12.00 | 0.83 | 0.43 | 0.47 |
| 12.00–14.30 | 0.67 | 0.57 | 0.63 |
| 14.30–17.30 | 0.80 | 0.55 | 0.59 |
| 17.30–22.00 | 0.76 | 0.59 | 0.58 |
Results determined by UHPLC-MS/MS (Laboratory 9), were expressed as (i) excretion rate (nmol/h), or adjusted for (ii) creatinine (nmol/mmol creatinine), or (iii) specific gravity (nM-SG; standard SG=1.018) in timed spot urine samples within the same day (day “A”) in 26 volunteers. All correlation coefficients are statistically significant (p<0.05).
The intra-individual variability in 8-oxodG excretion between days (calculated for day “A” and “B”), expressed as CV, was 17% for 24 h excretion and 20% for creatinine-adjusted first void samples, and 10% for SG-adjusted first void samples. For all spot samples on day “A”, the intra-individual CV was 51% for unadjusted 8-oxodG concentrations (nM), 36% for excretion rate (nmol/h), 24% for nM-SG 1.018, and 12% for excretion in nmol/mmol creatinine. Results for day “B” were similar. The intra- and inter-individual variability in 24h excretion was 24% and 76% of the total variability, respectively. Thus, the intra-class correlation coefficient (ICC) was 0.76. For creatinine-corrected first void these figures were 32% and 68%, respectively (ICC 0.68).
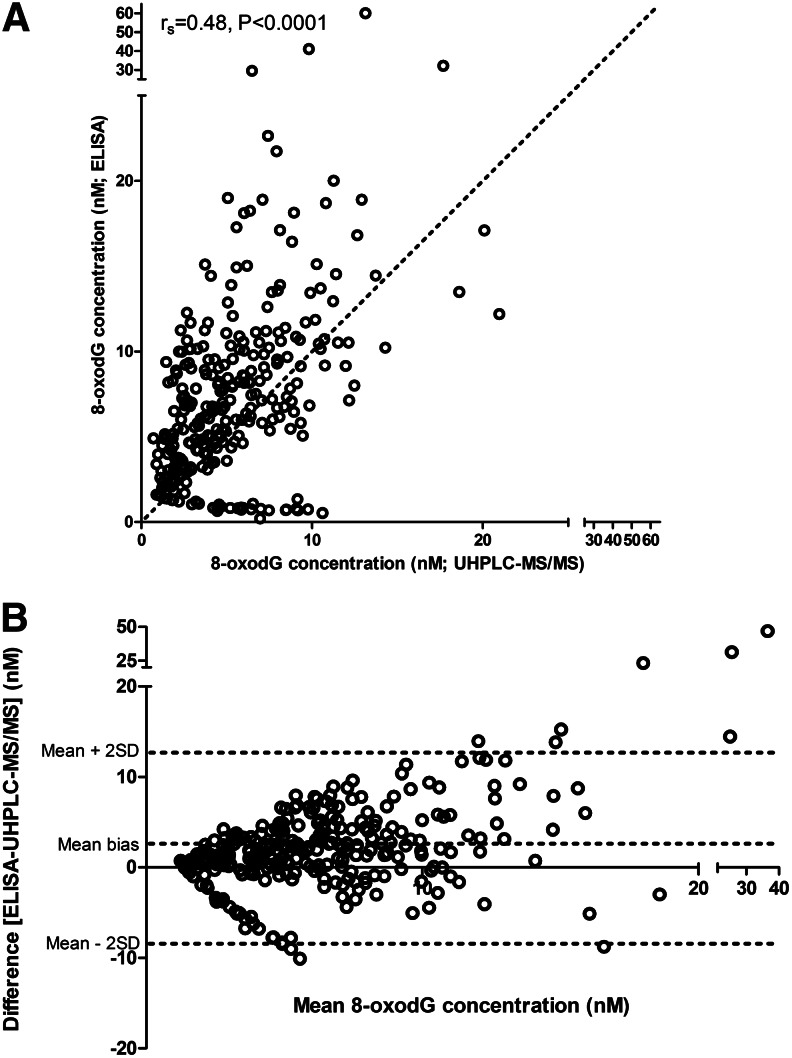
For completeness, we compared 8-oxodG values in these 26 individuals determined by ELISA with those by UHPLC-MS/MS. Mean values by ELISA were significantly higher than those by UHPLC-MS/MS, 27.1 nM vs. 16.7 nM, respectively (p<0.0001), and although there was a significant correlation between the two methods, the level of agreement was not high (rs=0.48, p<0.0001; Fig. 5A), which is evident from the Bland-Altman plot. The correlation between MS and ELISA improved when a comparison was made using the data in Figures 1 and 2 from Laboratory 9 (UHPLC-MS/MS) and Laboratory 3 (ELISA), which used overnight incubation of the primary antibody at 4°C (rs=0.67, p<0.0001; data not shown). A Bland-Altman plot, representing the difference between paired measurements plotted against the corresponding mean value, derived from both techniques, revealed relatively wide variability in values, indicated by a wide range for the 2 SD (Fig. 5B). In particular, as levels of 8-oxodG increase the agreement between chromatographic techniques and ELISA becomes poorer.
FIG. 5.
(A) Comparison on 8-oxodG concentrations (nM), determined by a commercial ELISA from the Japanese Institute for the Control of Aging (performed by Laboratory 19) and UHPLC-MS/MS (Laboratory 9) in 281 urine samples from 26 healthy subjects. In 31/312 available samples, 8-oxodG could not be quantified by ELISA. Also shown is the line of identity (……). (B) Bland-Altman plot of the data in (A), comparing ELISA and UHPLC-MS/MS analysis of 8-oxodG. The 95% limits of agreement are shown (mean bias±2SD).
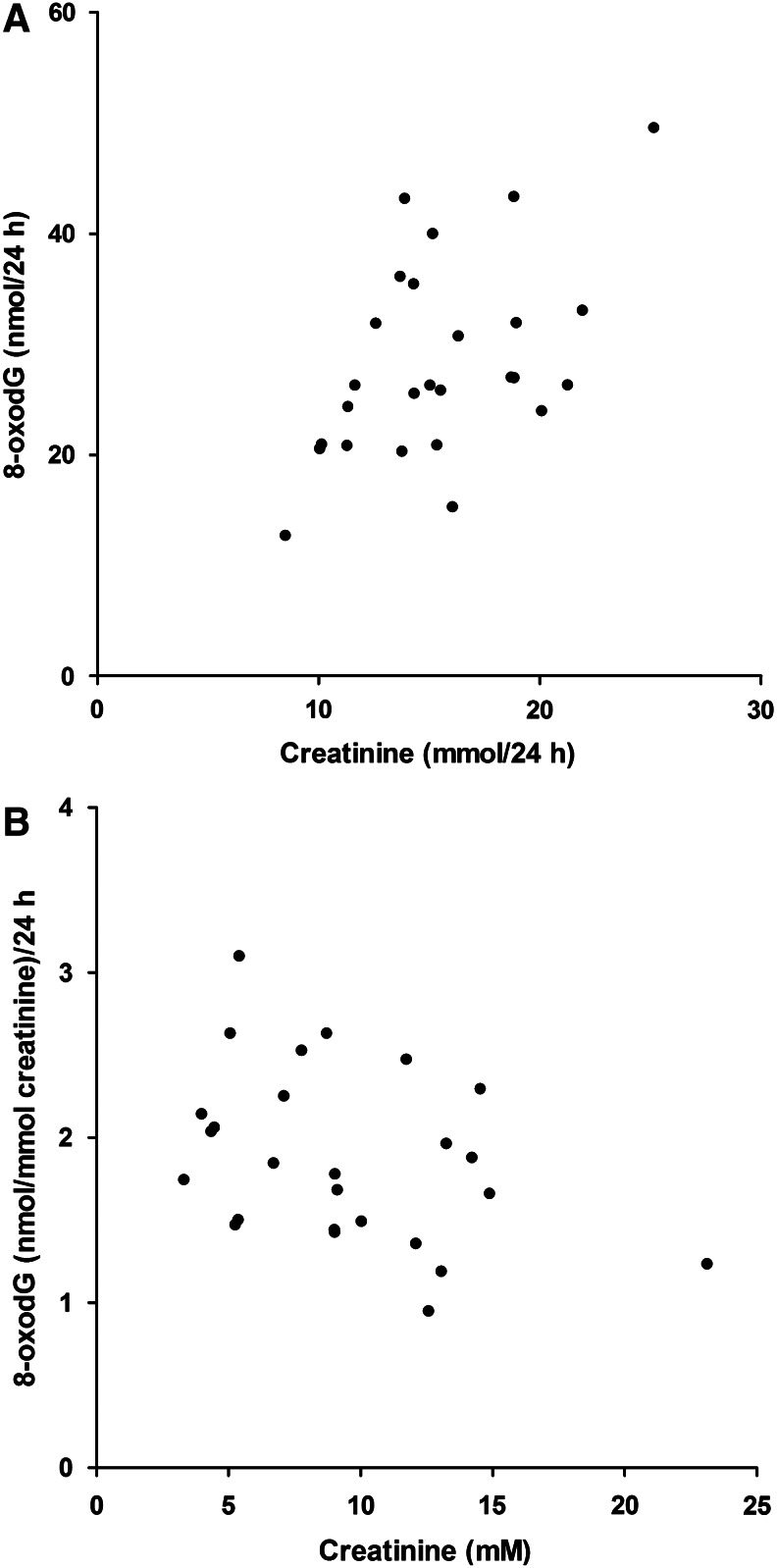
When sources of inter-individual variability were examined, the 24 h excretion of 8-oxodG on the two days (mean of day “A” and mean of day “B”) was found to be significantly, positively associated with the 24 h creatinine excretion and BMI, but not age, weight or sex (Table 6), and tended to be lower in women (Table 4). However, BMI, sex, and creatinine excretion were inter-correlated. In a stepwise multiple regression model, only the association with creatinine excretion remained significant, and explained 26% of the inter-individual variability (Fig. 6A). There was no significant impact of urinary flow or age. The regression model was as follows: 24 h excretion of 8-oxodG (in nmol)=11.3+1.1×24 h creatinine excretion (in mmol) As shown in Table 4, the mean 24 h creatinine excretion was 15.5 mmol. The regression equation shows that an increase in 24 h creatinine excretion from 10 to 20 mmol would appear to increase the 24 h excretion of 8-oxodG from 22 nmol to 33 nmol, (i.e., a 50% increase). There was also a tendency (p=0.06) towards an association between 24 h creatinine concentration and 24 h 8-oxodG excretion (in nmol/mmol creatinine), but with a negative beta coefficient, indicating a slight ‘overadjustment’ when adjusting for creatinine (Fig. 6B). In first morning samples, the point estimate was a 16% decrease of creatinine-adjusted 8-oxodG when the creatinine concentration of the sample increased from 10 mmol/L to 20 mmol/L (p=0.02).
Table 6.
Pearson (r) Correlation Coefficients Between 24 h 8-OxodG Excretion Determined by UHPLC-MS/MSa, and Age, Body Weight, BMI, Sex and 24 h Creatinine Excretiona
| Age | Weight | BMI | Sex | Creatinine (mmol/24h) | |
|---|---|---|---|---|---|
| 8-oxodG (nmol/24h) | −0.16 | 0.34 | 0.43* | −0.37 | 0.51** |
| Age | −0.12 | −0.19 | −0.05 | −0.41* | |
| Weight | 0.85** | −0.70** | 0.75** | ||
| BMI | −0.48** | 0.59** | |||
| Sex (female=1) | −0.69** |
By Laboratory 9; mean of 2 days, about 1 week apart; in 26 volunteers; *p<0.05; **p<0.01.
FIG. 6.
Excretion of 8-oxodG (determined by UHPLC-MS/MS; Laboratory 9) in 24 h urine samples as function of creatinine. (A) 8-oxodG (nmol) versus creatinine (mmol). (B) creatinine-adjusted 8-oxodG (nmol/mmol creatinine) vs. creatinine concentrations (mM). Results are the means (±SD) of 2 different days, approximately 1 week apart.
Discussion
Inter-laboratory sources of variability
The variation in our inter-laboratory validation trial may originate from differences in assays, laboratory protocols, inter-individual variation, disease status, and unexplained variation. Overall, it was clearly shown that the variation between ELISA and chromatographic assays was an important contributor to the overall variation, whereas there was high level of agreement across urine samples from different subjects within the chromatographic assays. The partitioning of the overall variation was based on relative differences because of the large difference in absolute values that were obtained by the ELISA and chromatographic assays. It appears that sample handling and work-up make minimal contribution to within-technique variability, most likely because urine, unlike DNA, requires relatively little manipulation prior to analysis. Overall, this inter-laboratory validation trial shows that it is possible to compare the results from the chromatographic assays in different laboratories and even integrate the results in a pooled analysis, especially if inter-laboratory variation is reduced by common calibrants. This is an important finding, with relevance for large molecular epidemiology studies where many thousands of samples might be available. Moreover, the correct absolute values are more likely to be determined by chromatographic techniques.
For the first time, we considered the comparison between single point, internal, and multiple point, external calibration, both commonly used in MS. Whilst agreement between the two approaches was strong, particularly at levels of 8-oxodG typically seen in urine, perfect agreement was not achieved. This was to be expected, because the external calibration curve was not generated in authentic matrix, therefore any matrix effects, such as ion suppression will not be taken into account, leading to a potential over-estimation—which was seen in our results, although largely at higher concentrations of 8-oxodG. Therefore, across the range of concentrations of urinary 8-oxodG reported in the literature, it would appear that either calibration method is acceptable.
It has been reported previously that constituents of urine, such as urea, influence the quantification by ELISA, because urea cross-reacts with the anti-8-oxodG monoclonal antibody frequently used (N45.1) (29), hence urine composition may contribute to the variability and leveling of the increase in the concentration-response of the ELISA. The large residual variation is a concern and the absolute values and variation in 8-oxodG between urine samples, obtained by ELISA, have little meaning. Of course, it might be possible to use the ELISA with greater accuracy in matrices that are less complex than urine. These data clearly indicate that the ELISA method shows high variability, overestimates the true values, and is not sufficiently sensitive to detect small changes in levels of 8-oxodG. However, we note two sources of inter-laboratory variability that potentially could be controlled for: (i) the use of common primary antibodies (the clone N45.1 is a better alternative to 1F7); and (ii) the use of a commercial kit versus in-house assays. It can be concluded that despite ELISA shortcomings, commercially available kits have some of the acceptable precision, with data consistently falling within the SD of the mean. The results from Laboratory 3 highlight the possibility of improvements in ELISA accuracy from incubating the primary anti-8-oxodG antibody at 4°C overnight, as recommended elsewhere (10). Taken together, these data do not rule out the use of the ELISA entirely, but do urge caution in interpreting the quantification of what is thought to be, but is clearly not entirely, 8-oxodG.
Intra-individual variability
The present study shows that there is a moderate intra-individual, day-to-day variability in excretion of 8-oxodG measured by UHPLC-MS/MS; 17% (expressed as CV) for 24 h excretion and 20% for creatinine-adjusted first morning samples. Pilger et al. (23) previously found an intra-individual CV of 48% in a study group that included smokers, which might represent a wider range of 8-oxodG values, through possible variation in the amount smoked. Additionally, 24 h samples were collected with intervals of 4–8 weeks (compared to 1 week in the present study). There is also variability between spot samples within a certain day, but for creatinine-adjusted levels, this is small (CV 12%). Thus creatinine-adjusted first morning samples is recommended if the aim is to decrease intra-individual variability, plus first void samples adjusted for creatinine also showed a reasonable correlation (rp 0.60) with 24 h excretion.
Inter-individual variability
Our results showed a relatively strong effect of creatinine on 8-oxodG excretion. The 24 h excretion of 8-oxodG could be 50% higher when the creatinine excretion increases by a factor of two, although still well within the normal range. Pilger et al. (23) also found a positive correlation between the 24 h 8-oxodG excretion and the 24 h creatinine excretion (rp=0.64) (23). It is likely that muscle mass is an underlying factor since metabolic rate is associated with lean body mass, and a high metabolic rate generates a larger amount of modified 2′-deoxyribonucleoside products, excreted in urine (20). Consistent with this, men on average excrete more 8-oxodG per kg body weight than women (20). This suggests that adjustment for creatinine excretion is appropriate. Indeed, this does assist, but creatinine adjustment seems to be not entirely optimal either. As shown in Figure 5B, 8-oxodG levels adjusted for creatinine seem to be higher in samples with low creatinine concentrations than in samples with high creatinine content. This tendency in our small sample could be dismissed as a chance finding, but we believe that the “overadjustment” is a true phenomenon, since we found similar results in other populations (unpublished results). Our recommendation is that in cross-sectional studies, when searching for predictors of 8-oxodG excretion, creatinine concentrations should also be included in the models, when using creatinine-adjusted 8-oxodG. If creatinine or SG values are not available, adjustment for BMI is a suitable alternative (Table 6).
Current status of the validation of urinary 8-oxodG measurement
The present report is the first study to integrate a full inter-laboratory validation trial with an assessment of the variation in urinary excretion of 8-oxodG, together with a comprehensive statistical analysis of variation. Our previous report examining the inter-laboratory variation in measurement of urinary 8-oxodG noted significant differences in the technique-specific group mean and target values of 8-oxodG (9). The present study demonstrates a clear improvement in intra-technique agreement with the use of common calibrants, and clearly demonstrates that diurnal variation in 8-oxodG excretion is not large.
Importantly, the ELISA methods detected the same small contribution to variation of urinary excretion related to the urine donors being cancer patients or healthy subjects as the chromatographic assays, although with substantially higher residual variation. This explains the large number of reports in the literature in which the ELISA has been used, and identified novel findings (26), but now makes important the identification of what precisely the ELISAs are recognizing. Future ELISA studies should focus on further investigation of the source(s) of variability, together with the correlations between spot urine and 24 h collection and improving the agreement between the ELISAs. Whilst the use of common calibrants in our study did not generate complete within-technique concordance, it is clearly useful to include such calibrants to improve comparison between assays/laboratories and facilitate the integration of results for pooled analyses, the latter being vital if very high sample numbers are to be analysed.
Recommendations for the study design and analysis of urinary 8-oxodG
1. Chromatography, coupled with electrochemical or mass spectrometric detection are both acceptable approaches for quantification of urinary 8-oxodG. However, the additional strengths brought to MS by stable isotope internal standardization (accounting for analyte loss during sample workup and analysis, together with calibration in the identical matrix and compensating for ion suppression) makes it the method of choice.
2. Presently, ELISA approaches cannot be recommended for the accurate quantification of 8-oxodG in human urine. Whilst they do appear to detect changes which appear to be associated to oxidative stress, overestimation and issues of cross-reactivity (4, 11) confound comparisons with chromatographic techniques and interpretation.
3. Use of common calibrants improves inter-laboratory agreement, particularly for mass spectrometric methods, and allows data from different laboratories to be pooled. This level of agreement is irrespective of laboratory-specific sample work up and analysis conditions. Greater harmonizing of these latter factors is likely to improve concordance further.
4. For mass spectrometric assays, use of stable isotopically labeled internal standards is essential. The use of single point calibration or multiple point, external calibration curves are both acceptable.
5. Although we consider the 24 h excretion to be the gold standard, spot samples are also acceptable. In this case we recommend timed samples where possible, making it possible to calculate excretion rates (nmol/h). If this is not possible, correction for creatinine is the next preferred option, which also decreases intra-individual variability. However, when comparing populations, or examining predictors or treatment effects, creatinine concentrations should be included in the model for creatinine-adjusted levels. In addition, it should be noted that differences in muscle mass (e.g., due to vastly different ages, or between men and women), may affect creatinine-adjusted levels. The potential for significant differences in urinary creatinine concentrations, in different study populations, should be evaluated before determining the most appropriate correction method and taken into account when interpreting results. If first void samples are used, adjustment for specific gravity is also a good alternative.
6. Further to point 5 (above), the units in which 8-oxodG concentration is expressed will be dependent upon the method of normalizing for urine concentration. We therefore recommend the following: nmol 8-oxodG/mmol creatinine.
7. There appears to be little influence of disease status on the composition of urine, at least with respect to cancer, and the ability of chromatographic or ELISA approaches to measure urinary 8-oxodG.
8. We recommend the establishment of an on-going quality assurance programme, for laboratories performing analysis of oxidatively damaged nucleic acid products in urine.
9. Broadly, we feel that many of these recommendations can apply to other damaged nucleic acid products in urine, until their individual assessment can be achieved.
Materials and Methods
As previously (9), the participating laboratories' techniques were broadly classified as mass spectrometric, HPLC with electrochemical detection, and ELISA.
ESCULA calibrants for the inter-laboratory study
We aimed to improve intra-technique agreement by providing to each laboratory using the same technique a set of calibrants for use in producing a calibration curve, against which further sets of test samples would be determined. The concentrations of the mass spectrometric calibrants were 10.3 μM for [15N5]-8-oxodG and 10.3 μM for 8-oxodG, and diluted appropriately to generate calibration curves as required by each laboratory; the HPLC-EC calibrants were 0, 2.5, 10, 25, 100, and 250 nM, and used undiluted to generate an external calibration curve; and the ELISA calibrants were 0, 1.8, 7.1, 28.2, 70.6, 282.4, and 706 nM.
Test samples for the inter-laboratory study
For test samples spiked with 8-oxodG, a concentrated stock of 8-oxodG (Sigma Chemical Co., Poole, Dorset, UK) was prepared by dissolving the entire 5 mg content, as received, in 5 mL ultrapure water, and the final concentration verified by UV absorbance. Sequential dilutions of this stock were used to make solutions to prepare the test materials described below. The test samples consisted of the following:
Phosphate buffered saline (PBS) samples. To simulate a physiological matrix, PBS was spiked with 8-oxodG to final concentrations of 0 (negative control), 2.5, 10, 25, 100, and 250 nM, and randomly labeled A–F.
Urine samples. A single, spot urine (derived from one healthy individual) was spiked with 8-oxodG to a final concentration of 0 (i.e., endogenous levels of 8-oxodG only), 2.5, 10, 25, 100, and 250 nM and randomly labeled G–L.
Healthy/patient urines. A number of 1 mL aliquots from ‘spot’ urine samples (first void, mid-stream) were prepared from nine healthy adults, labeled H1 to H9, and from 10 head or neck cancer patients, labeled P1-P10. The purpose of these samples was to investigate a potential effect of difference in urine composition, due to disease, upon 8-oxodG analysis. Collection and analysis of samples provided in this part of the study was approved by the medical ethics committee of The Collegium Medicum Nicolaus Copernicus University Bydgoszcz, Poland, No 241/2002; (in accordance with Good Clinical Practice, Warsaw 1998) and all the patients gave informed consent.
Whilst it was evident to the participating laboratories which samples were urine and which were PBS, no indication was given as to the 8-oxodG concentration for those spiked with standard. The code for the samples was unknown to the participants including the sample distribution laboratory (in which sample preparation and analysis was performed by different people), until the analysis was complete. Samples and calibrants for analysis were prepared by the Universities of Copenhagen (DK), Leicester (UK), and Nicolaus Copernicus University (PL). All calibrants and samples were transferred to the University of Leicester before onward distribution to the participating laboratories by courier on dry ice. Samples were kept frozen until analysis.
Analytical procedures for 8-oxodG measurement
Each laboratory used its own method for urinary 8-oxodG determinations, including sample preparation, but using the calibrants provided centrally. The salient features of the methodologies used are given in Table 7.
Table 7.
Key Properties of Methods of Analysis Used in the Present Study
| Lab ID | Technique | Key properties |
|---|---|---|
| MS | ||
| 4 | LC-GC/MS | Single point internal calibration and external calibration curve. [15N5]-8-oxodG standard added to all samples. HPLC prepurification. |
| 4 | LC-MS/MS | Single point internal calibration and external calibration curve. [15N5]-8-oxodG standard added to all samples. |
| 9 | UHPLC-MS/MS | External calibration using calibrants prepared in milliQ-water. Isotopically-labeled internal standard was added to all samples and calibrants. Urine samples were heated to 37°C. After centrifugation, an aliquot of the supernatant was diluted with buffer (0.1 M LiAc, pH 4) containing [15N5]-8-oxodG (13). |
| 11 | LC-MS/MS | Online SPE with ODS-3 column, 20 μL injected. |
| 15 | LC-MS/MS | Isotope-dilution technique was applied by adding a fixed amount of [15N5]-8-oxodG to all samples. Urine samples were analyzed by on-line SPE LC-MS/MS (14). |
| 16 | LC-MS/MS | 10 μL injected, [15N5]-8-oxodG standard, external calibration curve. |
| 18 | UHPLC-MS/MS | [15N5]-8-oxodG standard, both internal and external calibration curves. Solid phase extraction prior to analysis (16). |
| 25 | LC-MS/MS | Thermo Fisher LC-MS/MS with ESI, without sample extraction, only filtration and dilution in lithium acetate mobile phase. Chromatographic separation is obtained in 35 min using a UNISOL (AGELA Technology) C18 column. |
| 29 | LC-MS/MS | External calibration method on one urine sample randomly selected from H1–H9. Calibration levels were prepared from dilution of ESCULA stock solution. [15N5]-8-oxodG was added to each sample at a fixed concentration. Each urine sample was centrifuged and diluted before the injection (2). |
| 31 | LC-MS/MS | External calibration using calibrants prepared in urine. [15N5]-8-oxodG standard was added as internal standard. Solid phase extraction prior to analysis (7). |
| 32 | LC-MS/MS | [15N5]-8-oxodG internal standard added to all samples and standards, both single point internal standard calibration and external standard calibration curve. Solid phase extraction purification prior to analysis (30). |
| ECD | ||
| 5 | HPLC-ECD | Twin HPLC, carbon column-switching method with electrochemical detection (3). |
| 7 | HPLC-ECD | Sequential HPLC using twin carbon column-switching and electrochemical detection (8). |
| 13 | HPLC-ECD | On-line pre-treatment, anion exchange column (MCI GEL CA08F)(15). |
| ELISA | ||
| 3 | In-house assay, which includes sample enrichment using a Bond Elute filter. Sample volume required: 95 μL; range of calibrants: 0.1–10 ng/mL; primary antibody N45.1, incubated overnight at 4°C (12). | |
| 8 | In-house assay: 50 μL of sample incubated with primary Ab (N45.1, JaICA) for 1.5 h; incubation with secondary Ab for 1.5 h; all incubations at room temperature; the range of the standard curve: 1.25–40 ng/mL (27). | |
| 10 | In-house assay with monoclonal antibody 1F7; range of the standard curve is 1.25–40 ng/mL (31). | |
| 12 | Commercial ELISA kits (New 8-OHdG Check and Highly Sensitive 8-OHdG Check, JaICA) with primary antibody N45.1 (incubated at room temperature for 1 h). | |
| 19 | Commercial assay with monoclonal antibody, incubations at room temperature; the range of the standard curve: 0.5–200 ng/mL (22). | |
| 26 | 8-oxodG was assessed with the Highly Sensitive 8-OHdG Check ELISA kit, using anti-8-oxodG monoclonal antibody clone N45.1 (incubated for 1 h at 37°C). 50 μL of urine per sample, analyzed in duplicate. The standard concentrations provided by ESCULA were used. |
Full details are contained in the cited references.
Creatinine determinations
Urinary creatinine concentration is widely used as a correction factor for urine concentration when measuring urinary 8-oxodG (21, 24). We have previously shown good inter-laboratory and inter-technique agreement in the measurement of urinary creatinine (9), and so this comparison was not performed here. The urinary creatinine measurements, and SG, used here to correct for urine dilution were determined previously (1).
Evaluation of urine sampling regime and method of correction for urine concentration on 8-oxodG values
The population group used for this part of the study has been described elsewhere (1). Briefly, 26 healthy (no diabetes, kidney disease, or other serious disease), nonsmoking (at recruitment) Swedish subjects were recruited. The study population comprised 15 men (mean age 39, range 23–59 years) and 11 women (mean age 38, range 26–56 years), who provided complete urine samples as described below. Subjects were recruited from the staff and students at the University of Gothenburg. The study was approved by the Ethics Committee, University of Gothenburg, and all subjects gave their informed consent.
We examined the 24 h excretion of 8-oxodG on 2 different days (denoted “A” and “B”), 1 week apart, from which 8-oxodG excretion rates (nmol/h) were calculated, as well as 8-oxodG concentration, corrected for creatinine and SG. From each subject, urine samples were collected at six fixed times of the day (first morning void, 09:30, 12:00, 14:30, 17:30, 22:00), generating a total of 312 samples. If urination was necessary between fixed times, the next container was used. All samples were timed, and volumes were recorded. From the urine samples, 5 mL aliquots were transferred to Minisorb tubes (NUNC, Denmark). Samples were immediately frozen at −20°C until analysis 4 years later. For completeness, the above samples were analyzed for 8-oxodG using two distinct analytical methods: UHPLC-MS/MS (by Laboratory 9), representing the chromatographic methods and a commercial ELISA kit (Japanese Institute for the Control of Aging, Fukuroi, Japan), performed by Laboratory 18, according to the manufacturer's instructions.
Statistical analysis
Analysis of the inter-laboratory variation in the urinary excretion of 8-oxodG was assessed by ANOVA analysis with the laboratory nested by type of method (chromatographic technique or ELISA) and the subject nested in disease status as an independent categorical variable. The inter-individual variability was assessed in separate strata for the chromatographic techniques and ELISA methods; the subject was also nested in the disease status and the laboratory was included as an independent categorical variable. The contribution of the disease status to the overall variation was assessed by ANOVA in datasets from individual laboratories. The concentration-response relationship in urine samples spiked with 8-oxodG was analyzed by a general regression model with test for interaction between the spiked concentration of 8-oxodG (continuous variable) and the type of method (categorical variable). The partitioning of the total variability to contributions explained by the type of method, disease status, inter-individual, and residual variation was assessed as relative values calculated from the sum of squares in the statistical analysis. The residual variation was also assessed by the magnitude of the residual variation (SDres). The statistical analysis was carried out in Statistica 5.5 from StatSoft, Inc. (Tulsa, OK).
Excretion rates of 8-oxodG (nmol/h) and creatinine (mmol/h) for each time period were calculated from concentrations, volume, and sampling interval. 8-OxodG was also adjusted for urinary flow rate using creatinine (nmol 8-oxodG/mmol creatinine; nmol/mmol creatinine) and SG (nM-SG). A standard SG of 1.018 was used. Differences between levels of 8-oxodG (nmol/h, nmol/mmol creatinine, or nM-SG-adjusted) at different time periods were assessed using repeated measures mixed effect models (Proc Mixed in SAS), with individual as a random factor (SAS Institute, Cary, NC). The associations between levels of 8-oxodG in the timed individual spot samples and the 24 h excretion in the whole sampling day were examined using the Pearson correlation coefficient (rp). Variability in 8-oxodG levels between days, and within individuals, was calculated from the 24 h samples and expressed as coefficient of variation (CV) for excretion rate (nmol/24 h), and pooled 24 h concentrations adjusted for creatinine or SG. Intra-individual variability was also quantified (expressed as CV) for spot urine samples within a day (day A). The intra-individual variability was compared with variability between individuals. Partitioning of intra- and inter-individual variability was performed using Proc Nested in SAS, and the intra-class correlation (ICC; inter-individual variability/total variability) was calculated. Sources of inter-individual variability were assessed using stepwise multiple linear regression.
All data were plotted using Prism v5.0 (GraphPad Software Inc., La Jolla, CA, USA).
Abbreviations Used
- 8-oxodG
(in a specific instance also abbreviated as 8-OHdG) - 8-oxo-7,8-dihydro-2′-deoxyguanosine
- ELISA
enzyme-linked immunosorbent assay
- ESCULA
European Standards Committee of Urinary (DNA) Lesion Analysis
- HPLC-ECD
high performance liquid chromatography with electrochemical detection
- HPLC-MS/MS
high performance liquid chromatography with tandem mass spectrometry
- SG
specific gravity
- UHPLC-MS/MS
ultra-high performance liquid chromatography with tandem mass spectrometry
Acknowledgments
Some of the authors of this work were partners in, and this work was partly supported by, ECNIS (Environmental Cancer Risk, Nutrition and Individual Susceptibility), a network of excellence operating within the European Union 6th Framework Program, Priority 5:"Food Quality and Safety" (Contract No. FOOD-CT-2005-513943), and also ECNIS2, a coordination and support action within the European Union FP7 Cooperation Theme 2 Food, Agriculture, Fisheries and Biotechnologies.
P Møller and S Loft are supported by CISBO and the Ingeborg and Leo Dannin Foundation.
M-R Chao and C-W Hu acknowledge financial support from the National Science Council, Taiwan (Grants NSC 97-2314-B-040-015-MY3 and NSC 100-2628-B-040-001-MY4).
R Santella acknowledges the contribution of Qiao Wang, and support from US NIH P30ES009089.
G Saez and C Cerda acknowledge financial support from the Instituto Carlos III division of the Government for Clinical Research (Grants PI-10/00802 and RD06/0045/0006) and Grant ACOM/2012/238 from Generalitat Valenciana.
K Broberg, C Lindh, and M Hossain acknowledge financial support from the Swedish Council for Working Life and Social Research
H Orhan and N Senduran acknowledge financial support from TÜBİTAK (Technical and Scientific Research Council of Turkey), grant number 108Y049.
P Rossner, Jr. and RJ Sram acknowledge support from the Grant Agency of the Czech Republic (P503/11/0084).
L Barregard acknowledges financial support from the Sahlgrenska University Hospital, Gothenburg.
MS Cooke acknowledges support from the UK Medical Research Council via a People Exchange Programme Research Leader Fellowship award (G1001808/98136).
More information about ESCULA may be found at http://escula.org.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Andersson L. Haraldsson B. Johansson C. Barregard L. Methodological issues on the use of urinary alpha-1-microglobuline in epidemiological studies. Nephrol Dial Transplant. 2008;23:1252–1256. doi: 10.1093/ndt/gfm729. [DOI] [PubMed] [Google Scholar]
- 2.Andreoli R. Manini P. De Palma G. Alinovi R. Goldoni M. Niessen WM. Mutti A. Quantitative determination of urinary 8-oxo-7,8-dihydro-2'-deoxyguanosine, 8-oxo-7,8-dihydroguanine, 8-oxo-7,8-dihydroguanosine, and their non-oxidized forms: Daily concentration profile in healthy volunteers. Biomarkers. 2010;15:221–231. doi: 10.3109/13547500903434501. [DOI] [PubMed] [Google Scholar]
- 3.Bogdanov MB. Beal MF. McCabe DR. Griffin RM. Matson WR. A carbon column-based liquid chromatography electrochemical approach to routine 8-hydroxy-2'-deoxyguanosine measurements in urine and other biologic matrices: A one-year evaluation of methods. Free Radic Biol Med. 1999;27:647–666. doi: 10.1016/s0891-5849(99)00113-6. [DOI] [PubMed] [Google Scholar]
- 4.Cadet J. Poulsen H. Measurement of oxidatively generated base damage in cellular DNA and urine. Free Radic Biol Med. 2010;48:1457–1459. doi: 10.1016/j.freeradbiomed.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 5.Cooke MS. Barregard L. Mistry V. Potdar N. Rozalski R. Gackowski D. Siomek A. Foksinski M. Svoboda P. Kasai H. Konje JC. Sallsten G. Evans MD. Olinski R. Interlaboratory comparison of methodologies for the measurement of urinary 8-oxo-7,8-dihydro-2'-deoxyguanosine. Biomarkers. 2009;14:103–110. doi: 10.1080/13547500802706012. [DOI] [PubMed] [Google Scholar]
- 6.Cooke MS. Olinski R. Loft S. Measurement and meaning of oxidatively modified DNA lesions in urine. Cancer Epidemiol Biomarkers Prev. 2008;17:3–14. doi: 10.1158/1055-9965.EPI-07-0751. [DOI] [PubMed] [Google Scholar]
- 7.Engstrom KS. Vahter M. Lindh C. Teichert F. Singh R. Concha G. Nermell B. Farmer PB. Stromberg U. Broberg K. Low 8-oxo-7,8-dihydro-2'-deoxyguanosine levels and influence of genetic background in an Andean population exposed to high levels of arsenic. Mutat Res. 2010;683:98–105. doi: 10.1016/j.mrfmmm.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 8.Espinosa O. Jimenez-Almazan J. Chaves FJ. Tormos MC. Clapes S. Iradi A. Salvador A. Fandos M. Redon J. Saez GT. Urinary 8-oxo-7,8-dihydro-2'-deoxyguanosine (8-oxo-dG), a reliable oxidative stress marker in hypertension. Free Radic Res. 2007;41:546–554. doi: 10.1080/10715760601164050. [DOI] [PubMed] [Google Scholar]
- 9.Evans MD. Olinski R. Loft S. Cooke MS. Toward consensus in the analysis of urinary 8-oxo-7,8-dihydro-2'-deoxyguanosine as a noninvasive biomarker of oxidative stress. FASEB J. 2010;24:1249–1260. doi: 10.1096/fj.09-147124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Evans MD. Singh R. Mistry V. Sandhu K. Farmer PB. Cooke MS. Analysis of urinary 8-oxo-7,8-dihydro-purine-2'-deoxyribonucleosides by LC-MS/MS and improved ELISA. Free Radic Res. 2008;42:831–840. doi: 10.1080/10715760802506323. [DOI] [PubMed] [Google Scholar]
- 11.Garratt LW. Mistry V. Singh R. Sandhu JK. Sheil B. Cooke MS. Sly PD. Interpretation of urinary 8-oxo-7,8-dihydro-2'-deoxyguanosine is adversely affected by methodological inaccuracies when using a commercial ELISA. Free Radic Biol Med. 2010;48:1460–1464. doi: 10.1016/j.freeradbiomed.2010.02.017. [DOI] [PubMed] [Google Scholar]
- 12.Haghdoost S. Czene S. Naslund I. Skog S. Harms-Ringdahl M. Extracellular 8-oxo-dG as a sensitive parameter for oxidative stress in vivo and in vitro. Free Radic Res. 2005;39:153–162. doi: 10.1080/10715760500043132. [DOI] [PubMed] [Google Scholar]
- 13.Henriksen T. Hillestrom PR. Poulsen HE. Weimann A. Automated method for the direct analysis of 8-oxo-guanosine and 8-oxo-2'-deoxyguanosine in human urine using ultraperformance liquid chromatography and tandem mass spectrometry. Free Radic Biol Med. 2009;47:629–635. doi: 10.1016/j.freeradbiomed.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 14.Hu CW. Chao MR. Sie CH. Urinary analysis of 8-oxo-7,8-dihydroguanine and 8-oxo-7,8-dihydro-2'-deoxyguanosine by isotope-dilution LC-MS/MS with automated solid-phase extraction: Study of 8-oxo-7,8-dihydroguanine stability. Free Radic Biol Med. 2010;48:89–97. doi: 10.1016/j.freeradbiomed.2009.10.029. [DOI] [PubMed] [Google Scholar]
- 15.Kasai H. A new automated method to analyze urinary 8-hydroxydeoxyguanosine by a high-performance liquid chromatography-electrochemical detector system. J Radiat Res (Tokyo) 2003;44:185–189. doi: 10.1269/jrr.44.185. [DOI] [PubMed] [Google Scholar]
- 16.Lam PM. Mistry V. Marczylo TH. Konje JC. Evans MD. Cooke MS. Rapid measurement of 8-oxo-7,8-dihydro-2'-deoxyguanosine in human biological matrices using ultra-high-performance liquid chromatography-tandem mass spectrometry. Free Radic Biol Med. 2012;52:2057–2063. doi: 10.1016/j.freeradbiomed.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin HS. Jenner AM. Ong CN. Huang SH. Whiteman M. Halliwell B. A high-throughput and sensitive methodology for the quantification of urinary 8-hydroxy-2'-deoxyguanosine: Measurement with gas chromatography-mass spectrometry after single solid-phase extraction. Biochem J. 2004;380:541–548. doi: 10.1042/BJ20040004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Loft S. Danielsen P. Lohr M. Jantzen K. Hemmingsen JG. Roursgaard M. Karotki DG. Moller P. Urinary excretion of 8-oxo-7,8-dihydroguanine as biomarker of oxidative damage to DNA. Arch Biochem Biophys. 2012;518:142–150. doi: 10.1016/j.abb.2011.12.026. [DOI] [PubMed] [Google Scholar]
- 19.Loft S. Svoboda P. Kasai H. Tjonneland A. Vogel U. Moller P. Overvad K. Raaschou-Nielsen O. Prospective study of 8-oxo-7,8-dihydro-2'-deoxyguanosine excretion and the risk of lung cancer. Carcinogenesis. 2006;27:1245–1250. doi: 10.1093/carcin/bgi313. [DOI] [PubMed] [Google Scholar]
- 20.Loft S. Vistisen K. Ewertz M. Tjonneland A. Overvad K. Poulsen HE. Oxidative DNA damage estimated by 8-hydroxydeoxyguanosine excretion in humans: Influence of smoking, gender and body mass index. Carcinogenesis. 1992;13:2241–2247. doi: 10.1093/carcin/13.12.2241. [DOI] [PubMed] [Google Scholar]
- 21.Olinski R. Rozalski R. Gackowski D. Foksinski M. Siomek A. Cooke MS. Urinary measurement of 8-OxodG, 8-OxoGua, and 5HMUra: A noninvasive assessment of oxidative damage to DNA. Antioxid Redox Signal. 2006;8:1011–1019. doi: 10.1089/ars.2006.8.1011. [DOI] [PubMed] [Google Scholar]
- 22.Orhan H. van Holland B. Krab B. Moeken J. Vermeulen NP. Hollander P. Meerman JH. Evaluation of a multi-parameter biomarker set for oxidative damage in man: Increased urinary excretion of lipid, protein and DNA oxidation products after one hour of exercise. Free Radic Res. 2004;38:1269–1279. doi: 10.1080/10715760400013763. [DOI] [PubMed] [Google Scholar]
- 23.Pilger A. Germadnik D. Riedel K. Meger-Kossien I. Scherer G. Rudiger HW. Longitudinal study of urinary 8-hydroxy-2'-deoxyguanosine excretion in healthy adults. Free Radic Res. 2001;35:273–280. doi: 10.1080/10715760100300811. [DOI] [PubMed] [Google Scholar]
- 24.Poulsen HE. Loft S. Prieme H. Vistisen K. Lykkesfeldt J. Nyyssonen K. Salonen JT. Oxidative DNA damage in vivo: Relationship to age, plasma antioxidants, drug metabolism, glutathione-S-transferase activity and urinary creatinine excretion. Free Radic Res. 1998;29:565–571. doi: 10.1080/10715769800300601. [DOI] [PubMed] [Google Scholar]
- 25.Prieme H. Loft S. Cutler RG. Poulsen HE. Measurement of oxidative DNA injury in humans: evaluation of a commercially available ELISA assay. In: Kumpulainen JT, editor; Salonen JT, editor. Natural Antioxidants and Food Quality in Atherosclerosis and Cancer Prevention. London: The Royal Society of Chemistry; 1996. pp. 78–82. [Google Scholar]
- 26.Rossner P., Jr Sram RJ. Immunochemical detection of oxidatively damaged DNA. Free Radic Res. 2012;46:492–522. doi: 10.3109/10715762.2011.632415. [DOI] [PubMed] [Google Scholar]
- 27.Rossner P., Jr Svecova V. Milcova A. Lnenickova Z. Solansky I. Santella RM. Sram RJ. Oxidative and nitrosative stress markers in bus drivers. Mutat Res. 2007;617:23–32. doi: 10.1016/j.mrfmmm.2006.11.033. [DOI] [PubMed] [Google Scholar]
- 28.Shimoi K. Kasai H. Yokota N. Toyokuni S. Kinae N. Comparison between high-performance liquid chromatography and enzyme-linked immunosorbent assay for the determination of 8-hydroxy-2'-deoxyguanosine in human urine. Cancer Epidemiol Biomarkers Prev. 2002;11:767–770. [PubMed] [Google Scholar]
- 29.Song MF. Li YS. Ootsuyama Y. Kasai H. Kawai K. Ohta M. Eguchi Y. Yamato H. Matsumoto Y. Yoshida R. Ogawa Y. Urea, the most abundant component in urine, cross-reacts with a commercial 8-OH-dG ELISA kit and contributes to overestimation of urinary 8-OH-dG. Free Radic Biol Med. 2009;47:41–46. doi: 10.1016/j.freeradbiomed.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 30.Teichert F. Verschoyle RD. Greaves P. Thorpe JF. Mellon JK. Steward WP. Farmer PB. Gescher AJ. Singh R. Determination of 8-oxo-2'-deoxyguanosine and creatinine in murine and human urine by liquid chromatography/tandem mass spectrometry: Application to chemoprevention studies. Rapid Commun Mass Spectrom. 2009;23:258–266. doi: 10.1002/rcm.3873. [DOI] [PubMed] [Google Scholar]
- 31.Yin B. Whyatt RM. Perera FP. Randall MC. Cooper TB. Santella RM. Determination of 8-hydroxydeoxyguanosine by an immunoaffinity chromatography-monoclonal antibody-based ELISA. Free Radic Biol Med. 1995;18:1023–1032. doi: 10.1016/0891-5849(95)00003-g. [DOI] [PubMed] [Google Scholar]