Abstract
Aims
It is necessary to evaluate glucose variability and postprandial hyperglycemia in patients with well-controlled type 2 diabetes mellitus because of the limitations associated with hemoglobin A1c (HbA1c) measurements. We evaluated parameters reflecting postprandial hyperglycemia and glycemic variability in patients with optimal HbA1c.
Patients and Methods
Thirty-nine patients with HbA1c levels below 7% were recruited to the study. A continuous glucose monitoring system (CGMS) was applied for two 72-h periods. 1,5-Anhydroglucitol (1,5-AG) and fructosamine (FA) were measured as parameters for postprandial hyperglycemia and glucose variability. Using CGMS data, the following postprandial hyperglycemia parameters were calculated: mean postprandial maximum glucose (MPMG) and area under the curve for glucose above 180 mg/dL (AUC-180). To measure glycemic variability, we calculated mean amplitude of glucose excursion (MAGE) using a classical (MAGEc) and new method (MAGE group of sign [MAGEgos]).
Results
The baseline HbA1c level was 6.3±0.3%. The mean MPMG was 10.34±1.84 mmol/L, and the mean AUC-180 was 0.17±0.23 mmol/L/day. The mean MAGEgos was 3.27±1.29 mmol/L, and MAGEc was 4.30±1.43 mmol/L, indicating glycemic variability in our patients. The mean levels of 1,5-AG and FA were 16.7±7.4 μg/mL and 273.0±22.5 μmol/L, respectively. In a correlation analysis, FA was significantly correlated with MPMG, AUC-180, MAGEgos, and MAGEc. In contrast, 1,5-AG was only correlated with AUC-180.
Conclusions
This study demonstrated postprandial hyperglycemia and glycemic variability in subjects with well-controlled diabetes. FA may reflect postprandial hyperglycemia and glycemic variability, but 1,5-AG may be of limited value for assessing glucose variability in patients with well-controlled type 2 diabetes mellitus.
Introduction
Glycemic monitoring is essential for the management of type 2 diabetes mellitus, and various glycemic markers are available in clinical practice.1 Hemoglobin A1c (HbA1c) is the most widely used parameter for glycemic monitoring and reflects average glucose levels over 2–3 months.1 However, HbA1c is limited in its ability to reflect short-term glycemic changes, and it cannot reflect postprandial hyperglycemia and fasting hyperglycemia separately.2
A growing body of evidence suggests that postprandial hyperglycemia and glycemic variability may be independent risk factors for macrovascular complications in patients with diabetes.3–5 Even patients with well-controlled diabetes who have HbA1c levels below 7% (53 mmol/mol) may be subject to glycemic variability and postprandial hyperglycemia.4 For more advanced management to prevent chronic complications, it is necessary to monitor various glycemic parameters. However, HbA1c levels may not effectively reflect hyperglycemic excursions that are compensated for by hypoglycemia, something that is overlooked by most clinicians. A continuous glucose monitoring system (CGMS) is the most reliable and precise method for evaluating glycemic variability and postprandial hyperglycemia; however, it is inconvenient and not easily accessible in general practice.
1,5-Anhydroglucitol (1,5-AG) and fructosamine (FA) are circulating biomarkers that reflect short-term glucose control in diabetes mellitus.6 Plasma 1,5-AG is a glucose analog present in healthy subjects that shows extremely stable concentrations, as its intake and excretion are well balanced.7 1,5-AG competes with glucose for reabsorption in the renal tubules.7,8 Plasma 1,5-AG levels therefore decrease in the hyperglycemic state in which glycosuria is present. 1,5-AG is very sensitive, changes rapidly, and is known to reflect glycemic variability within a few days.9 FA is a measure of glycated serum proteins, the most common of which is albumin. FA levels correlate best with average glucose levels during the previous 10–14 days, and it is used clinically as a complementary marker to detect short-term changes in glucose management.6
One previous study reported that 1,5-AG levels well reflected both glycemic variability and postprandial hyperglycemia10; however, another study suggested that 1,5-AG represented mean blood glucose and postprandial hyperglycemia, but not glycemic variability.11 There is no definite evidence for either hypothesis due to differences between the study protocols and subjects. In addition, until now there have been few data regarding 1,5-AG measurements in patients with well-controlled diabetes (HbA1c ≤7%). Some studies reported that glycosylated serum proteins or glycated albumin (GA) is a better marker for evaluating glucose excursion in diabetes than HbA1c.12,13 However, few studies have evaluated the association between FA and postprandial hyperglycemia or glycemic fluctuations.
The aim of this study was to evaluate the glycemic status beyond HbA1c, specifically postprandial hyperglycemia and glycemic variability, in patients with well-controlled type 2 diabetes (HbA1c level <7%) using a CGMS. Furthermore, we evaluated the glycemic biomarkers 1,5-AG and FA and examined the associations between these biomarkers and postprandial hyperglycemia and glycemic variability in patients with well-controlled type 2 diabetes.
Patients and Methods
Participants
We recruited 53 patients 18–65 years old with type 2 diabetes mellitus, HbA1c levels <7% (53 mmol/mol), and stable glycemic control who visited the Kyung Hee University Medical Center (Seoul, Korea) between February 2008 and June 2010. Other inclusion criteria were an HbA1c change of <0.5% (18 mmol/mol), no recent addition of oral hypoglycemic medications, and no change in insulin dose of >10% in the previous 3 months. Exclusion criteria were pregnancy, anemia (hemoglobin <10.0 g/dL), liver disease (alanine aminotransferase more than twice the upper normal limit), hypoalbuminemia (albumin <3.5 g/dL), serum creatinine ≥1.3 mg/dL, acute or chronic renal tubulointerstitial disease, and severe medical illness.
Study design
On the day of screening, patients were educated about the CGMS (Medtronic MiniMed, Northridge, CA), which was used to record glucose levels every 5 min for 72 h consecutively. Patients were also instructed to self-monitor their blood glucose levels (fasting glucose and postprandial glucose) several times per day, to keep a diary of meals (content and times), and to continue their usual lifestyle. In addition, patients were not allowed to change their diabetes treatment during the study period. Two CGMS studies were consecutively performed for 1 week in all participants. Data from the two CGMS runs were analyzed using MiniMed Solutions software. On the day of screening, levels of HbA1c, 1,5-AG, and FA were measured; 1,5-AG and FA levels were measured again at the end of each CGMS study. We checked dietary patterns using 3-day diet records for each CGMS study period in all patients. Food intake (amount and the frequency) was analyzed by the Computer Aided Nutrient Analysis program version 3.0 (CAN-Pro version 3.0; The Korean Nutrition Society, Seoul). This study was approved by the Kyung Hee University Ethical Review Board (protocol number KMC IRB 0758-05), and written informed consent was obtained from all participants.
Analysis of glycemic parameters using CGMS data
Using the CGMS software, we calculated mean glucose levels and the area under the curve for glucose levels above 180 mg/dL (10 mmol/L) (AUC-180) during 24 h and mean postprandial maximum glucose (MPMG) (the mean maximal glucose level of postmeal glucose excursion after breakfast, lunch, and dinner) as parameters of postprandial hyperglycemia.
As a measurement of glycemic variability, we calculated the mean amplitude of glucose excursion (MAGE) using a new method: group of signs (GOS).14 MAGE is the mean absolute difference in the peak-to-nadir or nadir-to-peak direction in glycemic excursions more than 1 SD during days. Most researchers would calculate MAGE by hand using graphical approaches. However, the definitions of glycemic peaks and nadirs are arbitrary or subjective and not based on a standardized algorithm; this is the main limitation of its use in ambulatory, noncontrolled CGMS analyses.15–17 The GOS method is a new objective algorithm proposed by Zaccardi et al.14 for calculating MAGE. It was reported that MAGE values calculated using the GOS algorithm are highly correlated with MAGE values calculated by the physician (correlation coefficient of 96%).14 We calculated MAGE_abs_gos (abbreviated to MAGEgos in Results) using this algorithm as an alternative to calculation of classical MAGE (MAGEc) by the physician. Because there have been few studies that have investigated MAGEgos, we performed the validation study for MAGEgos. For measuring MAGEc, we modified the graphical method of Service et al.15 so that MAGEc was calculated by averaging both peak-to-nadir (downstroke) and nadir-to-peak (upstroke), which had been originally used in the program developed by Baghurst.18 MAGEgos and MAGEc showed a good correlation with r=0.94 (P<0.001) according to Pearson's correlation analysis.
Glycemic biomarker assays
HbA1c levels were measured with a G7 high-performance liquid chromatography analyzer (Tosoh Corp., Chuo-ku, Japan), using a method certified by the National Glycohemoglobin Standardization Program. Fructosamine levels were measured using a colorimetric assay (BT 1000; Biotechnica, Rome, Italy). Levels of 1,5-AG were measured by automated 1,5-AG assay (Lana 1,5-AG Auto Liquid; Nippon Kayaku, Tokyo, Japan) using a two-step enzymatic method.19 The intra-assay coefficient of variation (CV) ranged from 1.3% to 3.8%, and the inter-assay CV ranged from 0.8% to 3.8%. All biochemical assays were performed in the endocrine research laboratory at Kyung Hee Medical Center.
Statistical analysis
Statistical analysis and data management were conducted using the Statistical Package for the Social Sciences software (SPSS version 16.0; SPSS Inc., Chicago, IL). Mean values of 1,5-AG, FA, and glycemic parameters were calculated using values from two CGMS studies. Values for continuous variables in the first and second CGMS studies were compared using a paired t test. As for the correlation between parameters of CGMS and biomarkers, we performed Pearson's correlation analysis at each visit. In addition, we used a mixed model for analyzing the correlation of all repeatedly measured data from visit 1 and 2. A mixed model addresses the dependence of observations in a repeated measurement design by modeling the within-person and between-person variances simultaneously.20,21 It allows for an analysis of repeated measures with unbalanced times of measurement.22 We analyzed the correlations of 1,5-AG and FA with other glycemic parameters calculated from CGMS data for the two visits using a mixed model with AR(1) covariance between the two visits.20–23 Statistical significance was established as P<0.05.
Results
Baseline characteristics of the study subjects
Of the 53 patients who were originally enrolled, 14 dropped out, and 39 completed the study. All of those who completed the study had stable, well-controlled type 2 diabetes mellitus. The clinical characteristics of the subjects are summarized in Table 1. In total, 27 patients were male, and 12 were female; the mean age was 56±9.6 years; and mean body mass index was 25.4±3.6 kg/m2. Nearly one-quarter of the patients (23.1%) received no diabetes medication and controlled their disease only by modifying their lifestyles. In total, 26 patients were treated with antidiabetes agents, and four were taking a long-acting basal insulin analog. The detailed drug regimens are shown in Table 1. Baseline clinical parameters were as follows: HbA1c, 6.3±0.3% (45±20 mmol/mol); 1,5-AG, 16.2±7.4 μg/mL; and FA, 276.9±22.1 μmol/L.
Table 1.
Baseline Characteristics of the Subjects
| Characteristic | Value |
|---|---|
| Number (male/female) | 39 (27/12) |
| Age (years) | 56±9.6 |
| BMI (kg/m2) | 25.4±3.6 |
| DM duration (years) | 4.9±5.0 |
| Protein (g/dL) | 7.3±0.4 |
| Albumin (g/dL) | 4.4±0.2 |
| Creatinine (mg/dL) | 0.8±0.2 |
| Urinary albumin:creatinine ratio (μg/mg of creatinine) | 4.8±2.6 |
| HbA1c [% (mmol/mol)] | 6.3±0.3 (45±3) |
| Diabetes therapy | |
| No medication (n) | 9 |
| Oral antidiabetes agents (n)a | 26 |
| Insulin (n) | 4 |
| Glycemic biomarkers | |
| Fructosamine (μmol/L) | 276.9±22.1 |
| 1,5-AG (μg/mL) | 16.2±7.4 |
Data are mean±SD values.
Oral antidiabetes agents were as follows: sulfonylurea (n=1), metformin (n=6), sulfonylurea or meglitinide and metformin (n=9), thiazolidinediones and metformin (n=6), thiazolidinediones and sulfonylurea (n=2), metformin and acarbose (n=1), and metformin and vildagliptin (n=1).
1,5-AG, 1,5-anhydroglucitol; BMI, body mass index; DM, diabetes mellitus; HbA1c, hemoglobin A1c.
Glycemic parameters calculated from CGMS and glycemic biomarkers
Values for short-term biomarkers of glycemic control and glycemic parameters calculated from two CGMS periods are shown in Table 2. The glycemic values determined by CGMS did not differ significantly between the two CGMS periods. In addition, there were no significant differences in the ratios of the three major nutrients between CGMS visit 1 and CGMS visit 2 in the patients who completed the study. Mean calorie intake was 1,679.3±297.3 kcal/day, and the proportions of carbohydrates, proteins, and fats were 58.9±7.9%, 17.5±2.4%, and 22.2±5.4%, respectively.
Table 2.
Glycemic Characteristics Based on Continuous Glucose Monitoring System Data and Biomarker Assays
| CGMS visit 1 | CGMS visit 2 | Mean | |
|---|---|---|---|
| CGMS data | |||
| Mean glucose (mmol/L) | 7.27±0.94 | 7.29±1.03 | 7.28±0.97 |
| SD (mmol/L) | 1.77±0.56 | 1.78±0.58 | 1.77±0.46 |
| Coefficient of variance (%) | 23.85±6.56 | 24.66±7.24 | 24.31±4.75 |
| AUC-180 (mmol/L/day) | 0.17±0.23 | 0.18±0.24 | 0.17±0.23 |
| MPMG (mmol/L) | 10.33±1.67 | 10.34±1.84 | 10.33±1.71 |
| MAGEgos (mmol/L) | 3.29±1.31 | 3.26±1.32 | 3.27±1.29 |
| MAGEc (mmol/L) | 4.36±1.68 | 4.41±1.67 | 4.30±1.43 |
| Glycemic biomarker | |||
| 1,5-AG (μg/mL) | 16.7±7.3 | 16.7±7.3 | 16.7±7.4 |
| FA (μmol/L) | 273.2±21.3 | 273.5±22.5 | 273.0±22.5 |
Data are mean±SD values.
1,5-AG, 1,5-anhydroglucitol; AUC-180, area under the curve for glucose above 180 mg/dL (10 mmol/L); CGMS, continuous glucose monitoring system; FA, fructosamine; MAGEc, classical mean amplitude of glucose excursion; MAGEgos, groups of signs mean amplitude of glucose excursion _abs_gos; MPMG, mean postprandial maximum glucose.
Analysis of the two CGMS datasets showed that the mean blood glucose level was 7.28±0.97 mmol/L. The mean AUC-180 was 0.17±0.23 mmol/L/day, the mean MPMG level was 10.33±1.71 mmol/L, mean MAGEgos was 3.27±1.29 mmol/L, and MAGEc was 4.30±1.43 mmol/L. These results indicate the existence of postprandial hyperglycemia and glycemic variability in patients with well-controlled type 2 diabetes (HbA1c <7%).
Levels of FA and 1,5-AG did not differ significantly between CGMS visit 1 and visit 2. The mean levels of 1,5-AG and FA were 16.7±7.4 μg/mL and 273.0±22.5 μmol/L, respectively. This result demonstrates that there are various glycemic control statuses beyond HbA1c in well-controlled diabetes.
Correlations between glycemic biomarkers and CGMS parameters
First, Pearson's correlation analysis was performed to observe how CGMS parameters were correlated with FA and 1,5-AG at visits 1 and 2, respectively (Table 3). At visit 1, FA showed significant correlations with mean glucose, MPMG, AUC-180, and MAGEc. Also, FA showed a correlation with SD and MAGEgos but with borderline significance. 1,5-AG was significantly correlated with mean blood glucose and also showed a correlation with AUC-180 but with borderline significance. Other variables did not show any significant correlation with 1,5-AG.
Table 3.
Pearson's Correlation Coefficient Between Glycemic Biomarkers and Continuous Glucose Monitoring System Parameters
| Fructosamine | 1,5-AG | |
|---|---|---|
| CGMS visit 1 | ||
| Mean glucose | 0.512c | −0.361b |
| SD | 0.307a | −0.142 |
| MAGEgos | 0.273a | −0.268 |
| MAGEc | 0.454c | −0.195 |
| MPMG | 0.525c | −0.227 |
| AUC-180 | 0.437c | −0.308a |
| CGMS visit 2 | ||
| Mean glucose | 0.598c | −0.321a |
| SD | 0.271 | −0.179 |
| MAGEgos | 0.312a | −0.304a |
| MAGEc | 0.310a | −0.251 |
| MPMG | 0.463c | −0.371b |
| AUC-180 | 0.581c | −0.271 |
P<0.1, bP<0.05, cP<0.01.
1,5-AG, 1,5-anhydroglucitol; AUC-180, area under the curve for glucose above 180 mg/dL (10 mmol/L); MAGEc, classical mean amplitude of glucose excursion; MAGEgos, groups of signs mean amplitude of glucose excursion _abs_gos; MPMG, mean postprandial maximum glucose.
At visit 2, FA showed significant correlations with mean glucose, MPMG, and AUC-180 but was correlated with borderline significance in the case of MAGEgos and MAGEc. 1,5-AG was significantly correlated with MPMG and also showed a correlation with mean glucose and MAGEgos but with borderline significance.
In order to investigate the correlation between all variables that were repeatedly measured during both visits 1 and 2, we analyzed the relationships of 1,5-AG and FA with the glycemic parameters calculated from the CGMS data using a mixed model with an AR(1) covariance structure (Table 4). FA was significantly correlated with mean glucose, MPMG level, AUC-180, MAGEgos, and MAGEc. In contrast, 1,5-AG was only significantly correlated with mean glucose level and AUC-180.
Table 4.
Correlations Between Glycemic Markers and Continuous Glucose Monitoring System Parameters in a Mixed Model
| Dependent variable, parameter | Estimate | SE | df | t | P value |
|---|---|---|---|---|---|
| Fructosamine | |||||
| Mean glucoseb | 0.23 | 0.11 | 54.6 | 2.05 | 0.045 |
| MPMGb | 0.13 | 0.05 | 40.4 | 2.37 | 0.023 |
| AUC-180b | 0.99 | 0.45 | 53.0 | 2.23 | 0.030 |
| SDa | 0.25 | 0.13 | 35.6 | 1.93 | 0.061 |
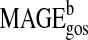
|
0.14 | 0.06 | 42.9 | 2.28 | 0.028 |
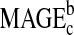
|
0.12 | 0.04 | 35.9 | 2.88 | 0.007 |
| 1,5-Anhydroglucitol | |||||
| Mean glucoseb | −0.03 | 0.01 | 35.6 | −2.49 | 0.018 |
| MPMG | −0.01 | 0.01 | 31.9 | −1.18 | 0.247 |
| AUC-180b | −0.11 | 0.05 | 35.5 | −2.43 | 0.021 |
| SD | −0.01 | 0.02 | 30.6 | −0.60 | 0.555 |
| MAGEgos | −0.00 | 0.01 | 34.8 | −0.68 | 0.501 |
| MAGEc | −0.00 | 0.01 | 30.6 | −0.58 | 0.569 |
P<0.1, bP<0.05.
AUC-180, area under the curve for glucose above 180 mg/dL (10 mmol/L); MAGEc, classical mean amplitude of glucose excursion; MAGEgos, groups of signs mean amplitude of glucose excursion _abs_gos; MPMG, mean postprandial maximum glucose.
Discussion
It is currently emphasized that clinicians should consider other glycemic factors beyond HbA1c for the prevention of cardiovascular and microvascular complications and to improve quality of life.3,5
We demonstrated glucose variability and postprandial hyperglycemia in patients with well-controlled type 2 diabetes mellitus by using a CGMS. There is a lack of studies examining postprandial hyperglycemia and glycemic variability in healthy Koreans; however, we can compare our results with data for normal Asian subjects.24,25 Compared with normal Asian subjects, our results showed not only high mean blood glucose levels, but also high MPMG and MAGE values. These results indicate the presence of postprandial hyperglycemia and glycemic variability in well-controlled diabetes.24,25 This suggests that it is important to recognize that despite having “normal” HbA1c values, patients may not have optimal control of their diabetes.
The levels of 1,5-AG in this study were outside the normal range for healthy subjects (range, 23.16–69.85 μg/mL),26 and the levels of FA (1.2–2.1 mmol/L, in subjects with HbA1c <6%) were higher than that of subjects with similar HbA1c values in another study.27 It is already well established that there is a good correlation between HbA1c and short-term glycemic biomarkers such as 1,5-AG and FA; however, this study showed that there are various glycemic statuses through a diverse range of short-term glycemic biomarkers even in well-controlled patients with similar HbA1c values.
Several methods can clinically assess postprandial hyperglycemia and glucose variability. Although CGMS is a precise method, it is an invasive and uncomfortable procedure and has limitations in terms of cost and the reliability. 1,5-AG or FA monitoring may be a convenient method for evaluating short-term glycemic excursion. These biomarkers may be useful parameters for tight glucose control and for detecting postprandial and glycemic variability in patients with well-controlled diabetes mellitus.
In this study, plasma levels of both 1,5-AG and FA were correlated with mean glucose levels and AUC-180. In addition, FA was correlated with MPMG, MAGEgos, and MAGEc in patients with well-controlled diabetes (HbA1c ≤7%).
Few reports have compared short-term glycemic markers such as 1,5-AG and FA within groups with similar HbA1c levels.6 Dungan et al.10 performed a study in 40 patients with type 1 and type 2 diabetes mellitus that revealed a significant negative correlation between 1,5-AG and postprandial hyperglycemia in patients with moderate glycemic control. Furthermore, 1,5-AG was significantly correlated with glycemic variability under various study conditions, including well-controlled diabetes.28–30 In contrast, Kim et al.11 suggested that 1,5-AG did not correlate with glycemic variability and was only correlated with mean glucose and postprandial hyperglycemia in patients with moderately controlled diabetes (HbA1c ≤8%).
Until now, there has been no conclusive evidence in well-controlled diabetes. Our results show that the plasma level of FA, but not that of 1,5-AG, is correlated with MAGEgos and MAGEc. In patients with moderate to poor diabetes control, mean levels of glucose, HbA1c, and FA may all reflect the fasting state, postprandial state, and variability. Therefore, the importance of 1,5-AG, which most sensitively responds to postprandial hyperglycemia or variability, can be further emphasized. As seen in the study by Dungan et al.,10 FA and HbA1c were better correlated with mean glucose than 1,5-AG, whereas 1,5-AG reflects postprandial hyperglycemia more robustly than FA or HbA1c. Glycemic variability is defined as the frequency or amplitude of glucose excursion, or as a combination of glucose excursions in the hyperglycemic and hypoglycemic ranges. Considering our results and the results of previous studies, 1,5-AG may be particularly suited for monitoring hyperglycemic excursions. However, it may have limited ability to reflect glycemic variability in well-controlled patients with glycemic excursions with narrow ranges.
FA primarily represents GA, as it is the most abundant protein present. FA and GA levels were reported to be strongly correlated with each other.31 There have been a few studies outlining the associations between FA and glycemic parameters from CGMS data. A recent study reported that GA may reflect not only the average glucose level, but also glucose fluctuations and postprandial glucose excursions.32 Our findings are consistent with these results. Because GA levels increase as blood glucose levels rise33 and the rate of GA level increase is 10 times faster than that of hemoglobin,34 serum GA levels may also be affected by temporary blood glucose spikes.35 However, the exact reasons why FA and GA are related to daily glycemic excursions remain unclear.36 FA assays are cheap and fast and can be performed in routine laboratories; however, they are currently underutilized.37 Some studies have reviewed the usefulness of FA.37–39 It has been suggested that FA testing should be performed as a routine check in diabetes patients with normal HbA1c concentrations to confirm that their glucose is under control.37
This study measured MAGEc and MAGEgos with CGMS data as markers for glucose variability. In a previously published literature,14 MAGEgos has been suggested as a standardized and objective approach to measure MAGE, and in our study it was further found that MAGEgos showed a significant correlation with MAGEc. However, SD, which is known to be one of the most essential variables reflecting the glucose variability, also showed a higher correlation with MAGEc than MAGEgos, and the correlation analysis between MAGEgos and MAGEc demonstrated a systematic bias. Therefore, we suggest MAGEc should be considered in future studies.
The present study has several limitations. First, the number of patients was small. Studies using CGMS are difficult to carry out with a large number of patients. Even previously published studies were with small numbers of patients,10,11 as was with our study. One study performed two consecutive CGMS studies in 10 patients with type 2 diabetes and 24 with type 1 diabetes.10 The other study was carried out in 60 patients with type 2 diabetes by a single CGMS study.11 Even though the number of patients was small, in order to obtain more reliable data, we performed two consecutive CGMS periods in patients with the same type of diabetes, type 2 diabetes mellitus. Second, the study did not include many patients with large glycemic excursions during the relatively short study period. However, there was no intent to specifically select patients with small glycemic variability, and this might be an accurate reflection of the reality of well-controlled type 2 diabetes mellitus.
In conclusion, we showed the presence of glycemic variability and postprandial hyperglycemia in patients with well-controlled diabetes (HbA1c <7%), which suggests that it is necessary to evaluate glycemic parameters beyond HbA1c in such patients. 1,5-AG and FA are convenient molecules for the evaluation of short-term glycemic changes, even in patients with well-controlled diabetes. Both FA and 1,5-AG are correlated with mean blood glucose and postprandial hyperglycemia. However, our study suggests that, in patients with well-controlled diabetes (HbA1c <7%), FA may reflect postprandial hyperglycemia and glycemic variability, but 1,5-AG may be of limited value for assessing glucose variability. Additional studies are needed to identify appropriate biomarkers for postprandial hyperglycemia and glucose variability and to assess the potential roles of these biomarkers in well-controlled diabetes.
Acknowledgments
This study was supported by a grant from the Korea Healthcare Technology R&D Project, the Ministry of Health and Welfare, Republic of Korea (grant A102065), and the Kyung Hee University Program for Young Researchers in Medical Science (grant KHU-20071473).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Sacks DB. Bruns DE. Goldstein DE. Maclaren NK. McDonald JM. Parrott M. Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Clin Chem. 2002;48:436–472. [PubMed] [Google Scholar]
- 2.Derr R. Garrett E. Stacy GA. Saudek CD. Is HbA1c affected by glycemic instability? Diabetes Care. 2003;26:2728–2733. doi: 10.2337/diacare.26.10.2728. [DOI] [PubMed] [Google Scholar]
- 3.Temelkova-Kurktschiev TS. Koehler C. Henkel E. Leonhardt W. Fuecker K. Hanefeld M. Postchallenge plasma glucose and glycemic spikes are more strongly associated with atherosclerosis than fasting glucose or HbA1c level. Diabetes Care. 2000;23:1830–1834. doi: 10.2337/diacare.23.12.1830. [DOI] [PubMed] [Google Scholar]
- 4.Ceriello A. Postprandial hyperglycemia and diabetes complications: is it time to treat? Diabetes. 2005;54:1–7. doi: 10.2337/diabetes.54.1.1. [DOI] [PubMed] [Google Scholar]
- 5.Monnier L. Mas E. Ginet C. Michel F. Villon L. Cristol JP. Colette C. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA. 2006;295:1681–1687. doi: 10.1001/jama.295.14.1681. [DOI] [PubMed] [Google Scholar]
- 6.True MW. Circulating biomarkers of glycemia in diabetes management and implications for personalized medicine. J Diabetes Sci Technol. 2009;3:743–747. doi: 10.1177/193229680900300421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buse JB FJ. Edelman SV. Jovanovic L. McGill JB. Serum 1,5-anhydroglucitol (GlycoMark): a short-term glycemic marker. Diabetes Technol Ther. 2003;5:335–363. doi: 10.1089/152091503765691839. [DOI] [PubMed] [Google Scholar]
- 8.Yamanouchi T. Minoda S. Yabuuchi M. Akanuma Y. Akanuma H. Miyashita H. Akaoka I. Plasma 1,5-anhydro-d-glucitol as new clinical marker of glycemic control in NIDDM patients. Diabetes. 1989;38:723–729. doi: 10.2337/diab.38.6.723. [DOI] [PubMed] [Google Scholar]
- 9.Yamanouchi T. Ogata N. Tagaya T. Kawasaki T. Sekino N. Funato H. Akaoka L. Miyashita H. Clinical usefulness of serum 1,5-anhydroglucitol in monitoring glycaemic control. Lancet. 1996;347:1514–1518. doi: 10.1016/s0140-6736(96)90672-8. [DOI] [PubMed] [Google Scholar]
- 10.Dungan KM. Buse JB. Largay J. Kelly MM. Button EA. Kato S. Wittlin S. 1,5-Anhydroglucitol and postprandial hyperglycemia as measured by continuous glucose monitoring system in moderately controlled patients with diabetes. Diabetes Care. 2006;29:1214–1219. doi: 10.2337/dc06-1910. [DOI] [PubMed] [Google Scholar]
- 11.Kim MJ. Jung HS. Hwang-Bo Y. Cho SW. Jang HC. Kim SY. Park KS. Evaluation of 1,5-anhydroglucitol as a marker for glycemic variability in patients with type 2 diabetes mellitus. Acta Diabetol. 2011 doi: 10.1007/s00592-011-0302-0. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 12.Yoshiuchi K. Matsuhisa M. Katakami N. Nakatani Y. Sakamoto K. Matsuoka T. Umayahara Y. Kosugi K. Kaneto H. Yamasaki Y. Hori M. Glycated albumin is a better indicator for glucose excursion than glycated hemoglobin in type 1 and type 2 diabetes. Endocr J. 2008;55:503–507. doi: 10.1507/endocrj.k07e-089. [DOI] [PubMed] [Google Scholar]
- 13.Beisswenger PJ. Healy JC. Shultz EK. Glycosylated serum proteins and glycosylated hemoglobin in the assessment of glycemic control in insulin-dependent and non-insulin-dependent diabetes mellitus. Metabolism. 1993;42:989–992. doi: 10.1016/0026-0495(93)90011-c. [DOI] [PubMed] [Google Scholar]
- 14.Zaccardi F. Stefano PD. Busetto E. Federici MO. Manto A. Infusino F. Lanza GA. Pitocco D. Ghirlanda G. Group of signs: a new method to evaluate glycemic variability. J Diabetes Sci Technol. 2008;2:1061–1065. doi: 10.1177/193229680800200614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Service FJ. Molnar GD. Rosevear JW. Ackerman E. Gatewood LC. Taylor WF. Mean amplitude of glycemic excursions, a measure of diabetic instability. Diabetes. 1970;19:644–655. doi: 10.2337/diab.19.9.644. [DOI] [PubMed] [Google Scholar]
- 16.Rodbard D. New and improved methods to characterize glycemic variability using continuous glucose monitoring. Diabetes Technol Ther. 2009;11:551–565. doi: 10.1089/dia.2009.0015. [DOI] [PubMed] [Google Scholar]
- 17.Rodbard D. Interpretation of continuous glucose monitoring data: glycemic variability, quality of glycemic control. Diabetes Technol Ther. 2009;11(Suppl 1):S-55–S-67. doi: 10.1089/dia.2008.0132. [DOI] [PubMed] [Google Scholar]
- 18.Baghurst PA. Calculating the mean amplitude of glycemic excursion from continuous glucose monitoring data: an automated algorithm. Diabetes Technol Ther. 2011;13:296–302. doi: 10.1089/dia.2010.0090. [DOI] [PubMed] [Google Scholar]
- 19.Fukumura Y. Tajima S. Oshitani S. Ushijima Y. Kobayashi I. Hara F. Yamamoto S. Yabuuchi M. Fully enzymatic method for determining 1,5-anhydro-d-glucitol in serum. Clin Chem. 1994;40:2013–2016. [PubMed] [Google Scholar]
- 20.Brown H. Prescott R. New York: John Wiley & Sons; 1999. Applied Mixed Models in Medicine. [Google Scholar]
- 21.Littell RC. Milliken GA. Stroup WW. Wolfinger RD. Cary, NC: SAS Institute Incorporated; 1996. SAS System for MIXED Models. [Google Scholar]
- 22.Singer JD. Willett JB. Applied longitudinal data analysis: Modeling change and event occurrence. Oxford: Oxford University Press; 2003. [Google Scholar]
- 23.Kincaid C. Guidelines for selecting the covariance structure in mixed model analysis. Proceedings of the Thirtieth Annual SAS Users Group International Conference; Cary, NC: SAS Institute Inc; 2005. [Google Scholar]
- 24.Hill NR. Oliver NS. Choudhary P. Levy JC. Hindmarsh P. Matthews DR. Normal reference range for mean tissue glucose and glycemic variability derived from continuous glucose monitoring for subjects without diabetes in different ethnic groups. Diabetes Technol Ther. 2011;13:921–928. doi: 10.1089/dia.2010.0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang C. Lv L. Yang Y. Chen D. Liu G. Chen L. Song Y. He L. Li X. Tian H. Jia W. Ran X. Glucose fluctuations in subjects with normal glucose tolerance, impaired glucose regulation and newly diagnosed type 2 diabetes mellitus. Clin Endocrinol. 2012;76:810–815. doi: 10.1111/j.1365-2265.2011.04205.x. [DOI] [PubMed] [Google Scholar]
- 26.Lee SY LS. Kong SY. Ko SY. Kim JW. 1,5-Anhydroglucitol as a marker of glycemic control [in Korean] Korean J Clin Pathol. 2000;20:157–162. [Google Scholar]
- 27.Ludvigsen CW. Sprague G. Smith KM. Fructosamine clinical usefulness and determination of reference ranges. J Insurance Med. 1989;21:203–208. [Google Scholar]
- 28.Yamanouchi T. Moromizato H. Shinohara T. Minoda S. Miyashita H. Akaoka I. Estimation of plasma glucose fluctuation with a combination test of hemoglobin A1c and 1,5-anhydroglucitol. Metabolism. 1992;41:862–867. doi: 10.1016/0026-0495(92)90168-a. [DOI] [PubMed] [Google Scholar]
- 29.Dworacka M. Winiarska H. The application of plasma 1,5-anhydro-d-glucitol for monitoring type 2 diabetic patients. Dis Markers. 2005;21:127–132. doi: 10.1155/2005/251068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dungan KM. 1,5-Anhydroglucitol (GlycoMark) as a marker of short-term glycemic control and glycemic excursions. Expert Rev Mol Diagn. 2008;8:9–19. doi: 10.1586/14737159.8.1.9. [DOI] [PubMed] [Google Scholar]
- 31.Beck R. Steffes M. Xing D. Ruedy K. Mauras N. Wilson DM. Kollman C. Diabetes Research in Children Network (DirecNet) Study Group: The interrelationships of glycemic control measures: HbA1c, glycated albumin, fructosamine, 1,5-anhydroglucitrol, and continuous glucose monitoring. Pediatr Diabetes. 2011;12:690–695. doi: 10.1111/j.1399-5448.2011.00764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matsumoto H. Murase-Mishiba Y. Yamamoto N. Sugitatsu-Nakatsukasa S. Shibasaki S. Sano H. Terasaki J. Imagawa A. Hanafusa T. Glycated albumin to glycated hemoglobin ratio is a sensitive indicator of blood glucose variability in patients with fulminant type 1 diabetes. Intern Med. 2012;51:1315–1321. doi: 10.2169/internalmedicine.51.7236. [DOI] [PubMed] [Google Scholar]
- 33.Kisugi R. Kouzuma T. Yamamoto T. Akizuki S. Miyamoto H. Someya Y. Yokoyama J. Abe I. Hirai N. Ohnishi A. Structural and glycation site changes of albumin in diabetic patient with very high glycated albumin. Clin Chim Acta. 2007;382:59–64. doi: 10.1016/j.cca.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 34.Day JF. Thornburg RW. Thorpe SR. Baynes JW. Nonenzymatic glucosylation of rat albumin. Studies in vitro and in vivo. J Biol Chem. 1979;254:9394–9400. [PubMed] [Google Scholar]
- 35.Ogawa A. Hayashi A. Kishihara E. Yoshino S. Takeuchi A. Shichiri M. New indices for predicting glycaemic variability. PloS One. 2012;7:e46517. doi: 10.1371/journal.pone.0046517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suwa T. Ohta A. Matsui T. Koganei R. Kato H. Kawata T. Sada Y. Ishii S. Kondo A. Murakami K. Katabami T. Tanaka Y. Relationship between clinical markers of glycemia and glucose excursion evaluated by continuous glucose monitoring (CGM) Endocr J. 2010;57:135–140. doi: 10.1507/endocrj.k09e-234. [DOI] [PubMed] [Google Scholar]
- 37.Youssef D. El Abbassi A. Jordan RM. Peiris AN. Fructosamine—an underutilized tool in diabetes management: case report and literature review. Tenn Med. 2008;101:31–33. [PubMed] [Google Scholar]
- 38.Macdonald DR. Hanson AM. Holland MR. Singh BM. Clinical impact of variability in HbA1c as assessed by simultaneously measuring fructosamine and use of error grid analysis. Ann Clin Biochem. 2008;45:421–425. doi: 10.1258/acb.2008.007259. [DOI] [PubMed] [Google Scholar]
- 39.Mittman N. Desiraju B. Fazil I. Kapupara H. Chattopadhyay J. Jani CM. Avram MM. Serum fructosamine versus glycosylated hemoglobin as an index of glycemic control, hospitalization, and infection in diabetic hemodialysis patients. Kidney Int Suppl. 2010;117:S41–S45. doi: 10.1038/ki.2010.193. [DOI] [PubMed] [Google Scholar]


