Abstract
Background. A previous analysis of children infected with human immunodeficiency virus (HIV) in the Women and Infants Transmission Study showed a strong correlation between low activated CD8+ T lymphocytes in the first 2 months of life and good immunological prognosis. We sought to extend these observations to neurodevelopmental prognosis.
Methods. Ninety-eight HIV-infected children born before 1994 with flow cytometric data from the first 2 months of life and adequate neurodevelopmental testing through age 30 months were studied. Children were divided into those with low (⩽5% CD8+HLA-DR+ cells or ⩽25% CD8+CD38+ cells) or high (>5% CD8+HLA-DR+ cells or >25% CD8+CD38+ cells) immune activation at 1 and/or 2 months of age. Analysis was performed using survival analysis, Cox's proportional hazard regression, and longitudinal regression models.
Results. Absence of immune activation, measured as ⩽5% CD8+HLA-DR+ cells, was strongly associated with better performance on the psychomotor developmental index of the Bayley scales of infant development through the third year of life. This association persisted after adjustment for CD4 cell count, viral load, and progression to acquired immunodeficiency syndrome (P=.005). An association with the mental development index was also present (P=.048). Significant association between neurodevelopmental outcomes and ⩽25% CD8+CD38+ cells was not seen.
Conclusions. In this prospective cohort study of HIV-infected children, there was a significant favorable association of low immune activation in peripheral T cells at age 1 or 2 months, measured by a low percentage of CD8+HLA-DR+ cells, with subsequent psychomotor and mental development. This association was independent of other indices of severity and progression of HIV infection.
Neurodevelopmental deficits are frequent in children who acquire HIV infection through mother-to-child transmission [1]. The severity of HIV-associated CNS disease ranges from subtle neurobehavioral abnormalities to frank encephalopathy [2]. The prevalence of encephalopathy among HIV-infected children has diminished from an estimated 50%–90% early in the AIDS epidemic to recent estimates of 20%–40% [3]. The rate of progression of HIV encephalopathy is variable, but the prognosis is poor without receipt of HAART [4–7].
Pediatric HIV-associated CNS disease can be independent of systemic HIV disease and has been reported both as the first AIDS-defining symptom [8, 9] and later in the course of disease [10]. Clinical associations with encephalopathy have included hepatosplenomegaly or lymphadenopathy in the first 3 months of life [5] and decreased weight and head circumference at birth [9, 11]. HIV-infected infants with cytomegalovirus infection developed HIV-associated CNS disease more frequently than did those infected with HIV alone [12].
In the Women and Infants Transmission Study (WITS) cohort, a culture result positive for HIV within the first 48 h of life (indicating in utero infection) was associated with a more rapid decline in neurobehavioral functioning, compared with a perinatal infection [13]. Other virological factors associated with HIV-associated CNS disease were detectable p24 antigen in mothers and infants [9], elevated plasma HIV RNA loads in the first year of life [5], and, for later-onset encephalopathy, high HIV RNA loads in CSF [9, 14, 15].
Results of immunological studies have been inconsistent [14]. In general, higher CD4 cell counts, lower CD8 cell counts, and higher CD4:CD8 ratios have been associated with better neurobehavioral functioning [16]. However, contradictory data for adults [17] and children [18] have been presented. Tardieu et al. [9] found that encephalopathy in the first year of life was associated with normal or high levels of CD4+ T lymphocytes, in contrast to later-onset encephalopathy, in which CD4+ T lymphocytes were deficient. In the WITS, CD4 cell counts varied widely at the time of encephalopathy onset [5].
In general, elevated CD8+HLA-DR+, CD8+CD38+, and CD8+HLA-DR+CD38+ subsets, markers of activated CD8+ cells, are seen in more advanced disease [19–22]. Moreover, in a recent study of HIV-infected children, we showed that slow immunological progression up to age 8 years was predicted by a CD8+HLA-DR+ T lymphocyte percentage <5% at age 1–2 months [23]. This study did not address CNS manifestations.
The aim of the current study was to investigate the relationship between CD8+HLA-DR+ or CD8+CD38+ lymphocyte percentage, measured at age 1–2 months, and neurodevelopmental outcomes in the first 3 years of life.
Patients and Methods
Patient population. The WITS was a multicenter, prospective, longitudinal study of HIV-infected women and their children [24]. Study sites included eastern Massachusetts; New York, New York; Houston, Texas; San Juan, Puerto Rico; and Chicago, Illinois. HIV-infected, pregnant women aged 15–44 years consented to the study after approval from each site's institutional review board in accordance with federal guidelines and regulations. Consent for children was obtained from the children's guardians at birth.
Pediatric study visits occurred at birth; at age 7 days; at ages 1, 2, 4, 6, 9, and 12 months; and every 6 months thereafter. Physical examinations were performed, samples were obtained, and histories were recorded at every visit. Gestational age was determined by a combination of prenatal ultrasonographic findings, fundal height, and menstrual history. Maternal hard drug use (cocaine, opiates, other injectable drugs, and/or methadone) was assessed by urine toxicological analysis and/or self-reporting [25]. Children were considered to be infected with HIV when ⩾2 peripheral blood cultures were positive at any age [26]. Children born between 1989 and 1994 were considered in this analysis.
Neurodevelopmental assessments. Neurobehavioral data were collected through use of the Bayley scales of infant development (BSID) [27] at ages 4, 9, 12, 15, 18, 24, and 30 months. Assessments were conducted in the child's primary language by the examiner or through an interpreter. Children were not tested when febrile or after invasive procedures. Although a revised edition of the BSID became available in 1993, the first edition was used to ensure consistency [28]. The assessment was administered by psychometricians or pediatric psychologists after centralized training in test administration and scoring. Intertester reliability was established. Scheduled quality-assurance calls helped to maintain reliability. Summary scores of the mental developmental index (MDI) and psychomotor developmental index (PDI) are expressed as standard scores with a mean value of 100 and an SD of 16 [27]. Children with standard scores below the lowest possible age-normed score were assigned a standard score of 49. This is the convention in neurodevelopmental studies but may result in an overestimate of performance for these children [29]. However, this approach reduced the influence of outliers and may have resulted in a better estimate of the true mean than would an estimate that includes these values as missing or zero. Of 105 children with PDI data, 19 had at least 1 PDI score assigned to 49 (a total of 45 of 518 person-visits with PDI data). Of 100 children with MDI data, 17 had at least 1 MDI score assigned to 49 (a total of 40 of 529 person-visits with MDI data). Children were tested on a mean of 5 occasions.
Laboratory tests and flow cytometry. Routine blood tests were performed at hospital laboratories certified by the College of American Pathologists or other recognized quality-assurance programs. Flow cytometric analysis and HIV culture were performed at each site. All laboratories participated in quality-assurance programs of the AIDS Clinical Trials Group [30].
For dual-color flow cytometric analysis, samples were prepared by whole-blood lysis [31]. The same reagent lots were used at all sites (Becton-Dickinson) and consisted of an isotype control (IgG1 and IgG2a), CD45/CD14 (gating reagent), CD4/CD8 (compensation reagent), CD3/CD4 (CD4+ T lymphocytes), CD3/CD8 (CD8+ T lymphocytes), CD8/CD38 (activated CD8+ T lymphocytes), CD8/HLA-DR (activated CD8+ T lymphocytes), CD3−/CD16+CD56+ (natural killer cells), and CD3−/CD19 (B lymphocytes), conjugated to fluorescein isothiocyanate and phycoerythrin, respectively [32]. Results were expressed as a percentage of total lymphocytes, except for CD8+HLA-DR+ T lymphocytes, which were expressed as the calculated subpopulation of CD8+ T lymphocytes. Conversion to a subpopulation of CD8+ T lymphocytes was not possible for CD8/CD38 because of the extensive error expected when gating for the CD38 fluorochrome.
Definitions. Preterm birth was defined as birth before 37 gestational weeks. Maternal antiretroviral drug use during pregnancy included treatment or prophylaxis. Rapid disease progression was defined as a category C event or death before age 18 months [33].
Intrauterine infection was defined by a culture result positive for HIV from blood drawn within 48 h after birth. Intrapartum transmission was defined by a culture result negative for HIV during the first 48 h and positive results of culture thereafter.
Encephalopathy was defined by the presence for at least 2 months, in the absence of an etiology other than HIV-1 infection, of at least 1 of the following: (1) failure to attain or loss of developmental milestones or loss of intellectual ability, verified by standard neuropsychological tests; (2) impaired brain growth, acquired microcephaly (determined by head circumference), or brain atrophy determined by CT or MRI; and (3) acquired symmetric motor deficit manifested by ⩾2 of the following: paresis, pathological reflexes, ataxia, or gait disturbances [5].
Following the definitions derived from receiver operating characteristic curves from a WITS study of pediatric long-term immunological nonprogressors [23], infants with ⩽5% CD8+HLA-DR+ cells or ⩽25% CD8+CD38+ cells at age 1 or 2 months were defined as having early low immune activation, and infants with >5% CD8+HLA-DR+ cells or >25% CD8+CD38+ cells were defined as having early high immune activation. For children with 2 separate flow cytometry measurements at these ages, the results were averaged.
Statistical analysis. Statistical analyses were performed with SAS software, version 9.1 (SAS). Differences in the categorized variables between groups with early low immune activation and high immune activation were assessed by Pearson's χ2 test or Fisher's exact test. Mean values of continuous variables between the 2 groups were compared using Student's t test. All P values were 2 tailed.
Survival analysis and Cox's proportional hazard regression were used to evaluate the relationship between early immune activation and time to the first occurrence of either the MDI or PDI being >1 SD below the mean (i.e., a BSID score of <84) [2]. Infants without an event at the end of follow-up contributed to censored observations. Time to lost to follow-up did not differ between early low and early high immune activation groups. Differences in Kaplan-Meier estimates of the cumulative probability of scoring >1 SD below the mean for each of the 2 developmental indices were assessed using the log-rank test.
Longitudinal regression analyses were used to model the changes in MDI and PDI, accounting for correlation between repeated observations for the same infant. Our models included terms representing random intercepts, autocorrelation, and measurement error, following the approach proposed by Diggle [34]. The unadjusted model included the CD8+HLA-DR+ or CD8+CD38+ group (early low vs. early high immune activation), age (continuous), and the interaction between immune activation groups and age. The effect of immune activation on changes in BSID score is represented by the interaction term in these models. The adjusted models included adjustment for timing of infection (in utero vs. intrapartum), maternal hard drug use, rapid disease progression, prematurity, plasma HIV RNA load during the first 2 months of life, and CD4+ lymphocyte counts during the first 2 months of life. Variables used for adjustment were chosen a priori on the basis of 2 previous studies from the WITS [11, 23].
Results
HIV-infected singleton children born to HIV-infected women enrolled in the WITS between 1989 and 1994 who had completed at least 1 BSID assessment were included in the analysis. Of 1080 women, 1039 delivered 869 liveborn infants. Of these 869 infants, 836 were singletons. Of these 836 infants, 137 were infected with HIV, and 100 of the HIV-infected infants had flow cytometry data at 1 and/or 2 months of age that included CD8+HLA-DR+ cell measurements, and an additional 5 children had data that included CD8+CD38+ cell measurements. Of the 100 infants with early CD8+HLA-DR+ cell measurements, 94 (94%) had at least 1 completed BSID score. Of 105 infants with early CD8+CD38+ cell measurements, 98 (93%) had at least 1 BSID score.
On the basis of flow cytometry results at ages 1 and 2 months, infants were divided into 2 groups, with infants with high immune activation separated from those with low immune activation, measured by the percentage of CD8+HLA-DR+ cells (high activation, n=70; low activation, n=24) or CD8+CD38+ cells (high activation, n=51; low activation, n=47), with use of the criteria given above [23]. A univariate analysis of maternal and infant characteristics that compared groups with high and low immune activation was performed. This analysis included maternal education (completed to grade <12 vs. grade ⩾12); ethnicity; alcohol, cigarette or hard drug use during pregnancy; maternal antiretroviral drug receipt during pregnancy; maternal age; mode of delivery (elective cesarean vs. vaginal or other cesarean delivery); infant sex; birth weight; head circumference; plasma HIV RNA loads at 1 and/or 2 months of age; timing of infant infection (in utero vs. intrapartum); and infant antiretroviral treatment during the first 30 months of life. None of these variables was significantly related to immune activation (all P values >.10). In contrast, significant associations were found between low immune activation and high CD4+ lymphocyte concentration at 1 and/or 2 months of age (P=.02 and P=.01 for low immune activation measured by CD8+HLA-DR+ cells and CD8+CD38+ cells, respectively), a higher incidence of preterm birth (P=.04 and P=.03), and a lower incidence of rapid disease progression (P=.05 and P=.33).
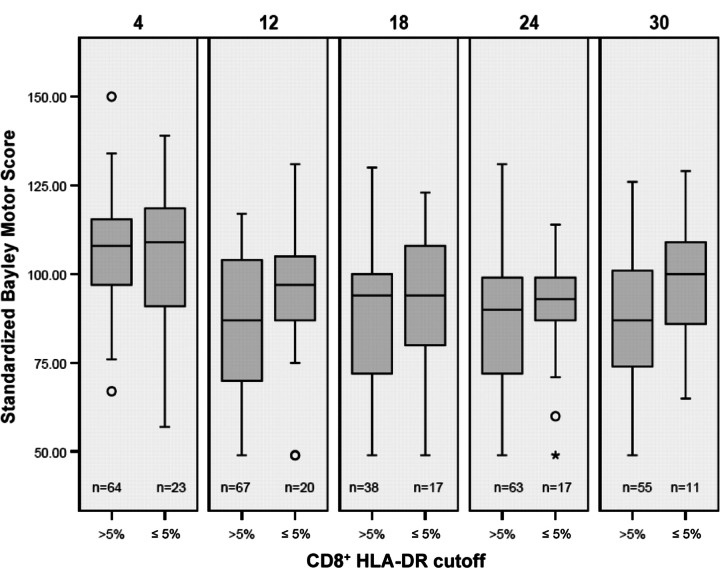
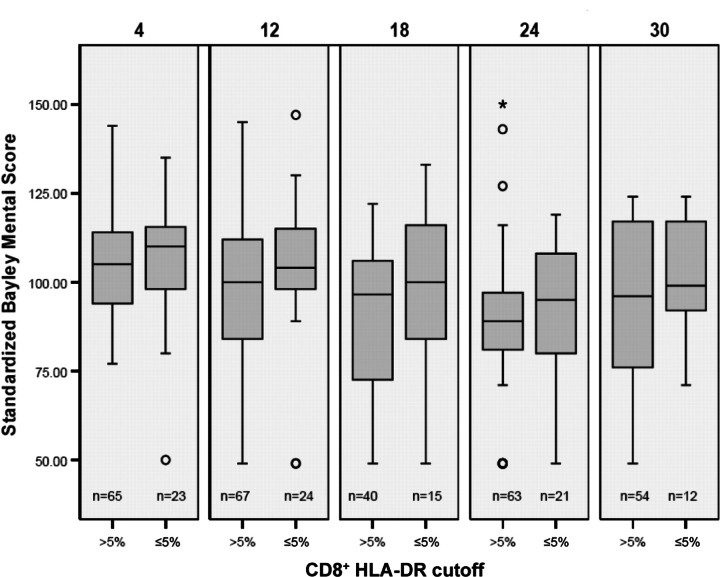
The associations between early immune activation markers and subsequent neurodevelopmental scores were then examined. Figures 1 and 2 show box plots of BSID scores, comparing infants with high and low immune activation markers, measured by percentage of CD8+HLA-DR+ cells at age 1–2 months. There is a trend toward decreasing standard scores as infants grow older, with a nonsignificant separation between the 2 immune activation groups. Further analysis of these trends with univariate regression modeling indicated a clear downward progression for infants in the group with a low percentage of CD8+HLA-DR+ cells, compared with the group with a high percentage (P<.001). Similar trends, although less consistent, were seen for CD8+CD38+ percentage groups.
Figure 1.
Box plots showing range (whiskers), 25th–75th percentiles (shaded box), median (horizontal line), and outliers (open circles and asterisk) of psychomotor developmental index at ages 4, 12, 18, 24, and 30 months, in relation to immune activation as measured by the percentage of CD8+HLA-DR+ cells. The number of children with tests at each age (n) is shown below each plot.
Figure 2.
Box plots showing range (whiskers), 25th–75th percentiles (shaded box), median (horizontal line), and outliers (open circles and asterisk) of mental developmental index at ages 4, 12, 18, 24, and 30 months, in relation to immune activation as measured by the percentage of CD8+HLADR+ cells. The number of children with tests at each age (n) is shown below each plot.
Kaplan-Meier estimates of the time to below-normal performance (<84 on the MDI or PDI) are plotted in figures 3 and 4 and show a higher cumulative probability of below-normal BSID scores for infants with early high immune activation, particularly for PDI scores and for immune activation measured by CD8+HLA-DR+ cells.
Figure 3.
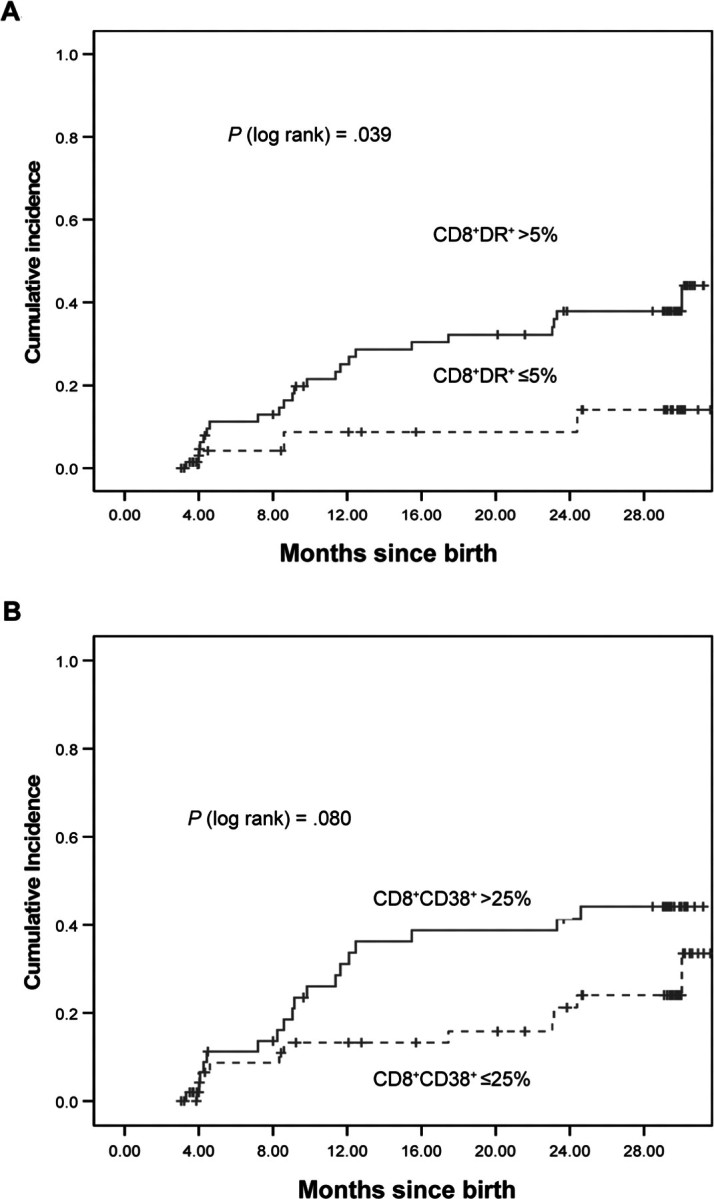
Relationship between immune activation—as measured by CD8+HLA-DR+ cell percentage of>15% or ⩽5% at age 1–2 months (A) or as measured by CD8+CD38+ cell percentage of >25% or ⩽25% at age 1–2 months (B)—and Kaplan-Meier estimate of time to below-normal score (11 SD below the mean) on the psychomotor developmental index of the Bayley scales of infant development. Solid lines, high immune activation; dotted lines, low immune activation.
Figure 4.
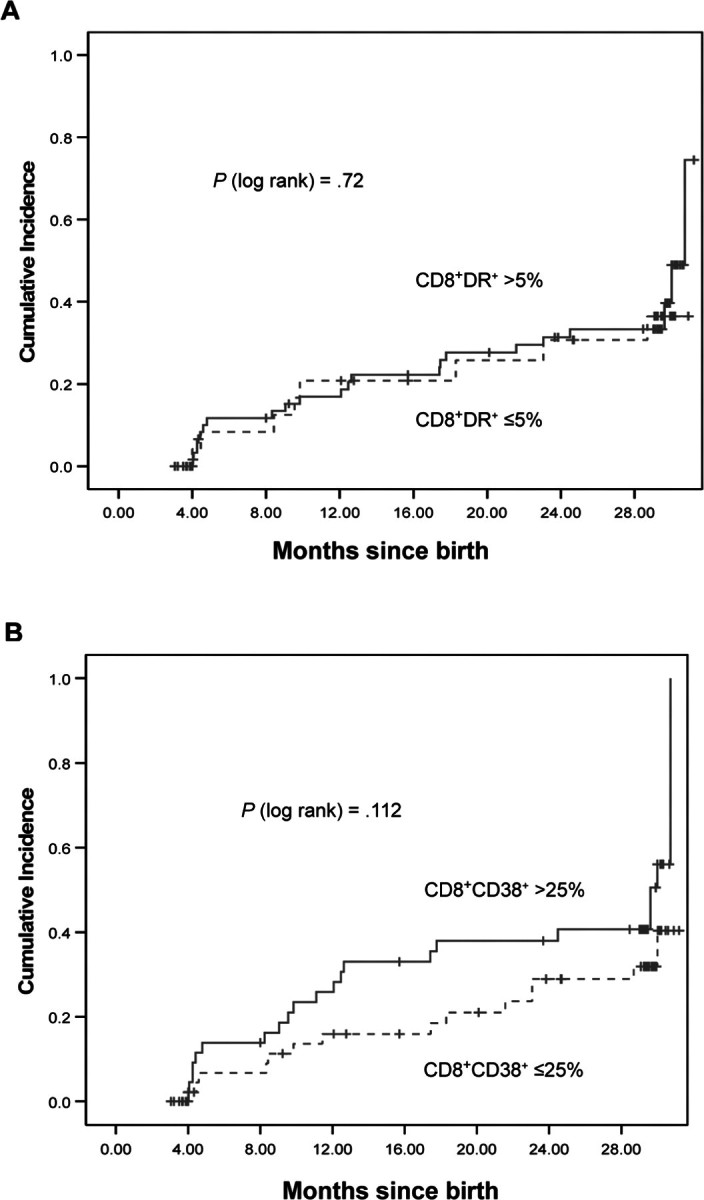
Relationship between immune activation—as measured by CD8+HLA-DR+ cell percentage of >5% or ⩽5% at age 1–2 months (A) or as measured by CD8+CD38+ cell percentage of 125% or 25% at age 1–2 months (B)—and Kaplan-Meier estimate of time to below-normal score (>1 SD below the mean) on the mental developmental index of the Bayley scales of infant development. Solid lines, high immune activation; dotted lines, low immune activation.
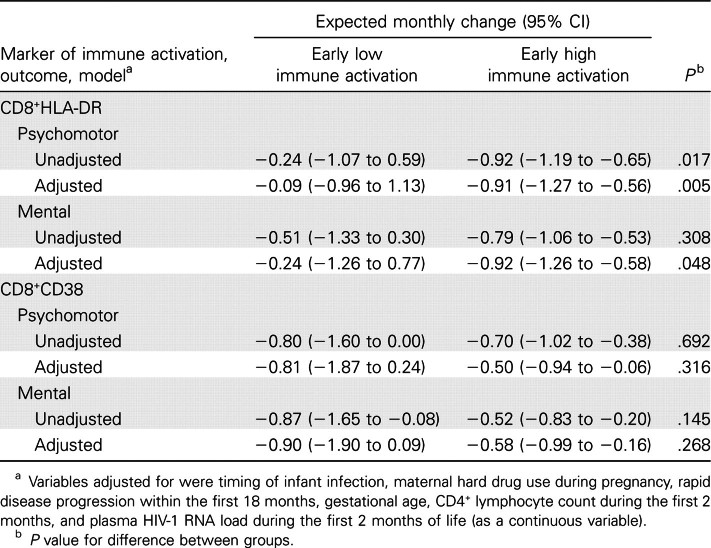
In table 1, we modeled the slope of BSID scores over time and adjusted for the maternal and infant variables that have been associated with changes in neurodevelopmental prognosis in other WITS studies (CD4+ cell count at age 1–2 months, rapid disease progression, gestational age at birth, timing of infant infection, maternal hard drug use during pregnancy, and plasma HIV RNA loads at age 1–2 months). Infants with low immune activation (<5% CD8+HLA-DR+ cells) at 1 and/or 2 months of age had significantly better neurodevelopment than did those with high immune activation, which was reflected in both the PDI (adjusted-model P=.005) and the MDI (adjusted-model P=.048). Low percentage of CD8+HLA-DR+ cells was a superior marker of prognostic outcome, compared with low percentage of CD8+CD38+ cells.
Table 1.
Estimates of the expected slope of scores on Bayley scales of infant development, according to early markers of immune activation, based on longitudinal regression models.
We also examined both death and development of encephalopathy in children with low and high immune activation. In neither instance were significant associations found, although trends for encephalopathy were in the same direction as for lower BSID scores. For immune activation measured by CD8+HLA-DR+ cells, 2 of 24 children with low activation died, compared with 15 of 76 children with high activation (Fisher's exact test P=.35), and 2 of 24 children with low activation developed encephalopathy, compared with 19 of 76 children with high activation (P=.09). Time-to-event analyses also failed to show significant differences (log-rank P=.20 for death and P=.10 for encephalopathy). For separation of groups by percentage of CD8+CD38+ cells, the associations were even less clear—8 of 49 children with low activation died, compared with 9 of 56 children with high activation, and 8 of 49 children with low activation developed encephalopathy, compared with 15 of 56 children with high activation (Fisher's exact test P=1.00 and P=.24, respectively; log-rank P=.97 and P=.22, respectively).
Discussion
We have shown that, in infants with HIV infection acquired through mother-to-child transmission, a relative absence of activated CD8+ (CD8+HLA-DR+) cells in the first few months of life is strongly associated with good neurodevelopmental outcomes in the first years of life, particularly on tests of psychomotor development. This parallels a similarly good prognosis for the preservation of immunological markers—specifically, circulating CD4+ cells—until at least age 8 years [23]. The instrument that we used, the BSID, is valid and normed for testing only until age 30 months, and it is possible that the association with better neurodevelopment may extend for longer periods. However, longitudinal analyses spanning different neurodevelopmental tests present substantial methodological problems [29]. In any case, natural history studies have indicated that the highest incidence of onset of HIV-associated CNS disease is in the first 2 years of life [9]. The stronger association with psychomotor development than with mental development in the prelanguage stages of development is consistent with previous findings for HIV-infected infants [2, 7, 13]. It is worth noting that the association of a lower percentage of activated CD8+ cells with better neurodevelopmental outcome remained significant even after adjustment for overall clinical prognosis [33], as well as for viral load and CD4+ cell count. Evidence of a significant role of CD8+ lymphocytes in HIV neuropathology has been implied for adults, in whom higher numbers of activated CD8+ lymphocytes in the brain were associated with HIV-associated CNS disease [35].
In unaffected infants in the first months of life, the CD38 marker is found on >95% of CD8+ T cells and is a marker of both lymphocyte immaturity and activation [36, 37]. In the WITS, the association of early low percentage of CD8+CD38+ cells with immunological progression was weaker than that of CD8+HLA-DR+ cells [23]. In adults, in contrast, the CD38 marker is considered to be a more reliable index of activation than is HLA-DR, and association with progression to AIDS has been described [38].
The mechanism of the association is not clear. It might be that low CD8+ cell activation predicts some third factor that, in turn, influences neurological development. Viral load during development could play a role; we did find that high CD8+ activation predicted a small but statistically significant increase in viral load between ages 6 months and 30 months (data not shown). Alternatively, the mechanism may be related to some external factor that simultaneously activates CD8+ cells and worsens the neurodevelopmental prognosis. Cytomegalovirus or other virus coinfection would fit this role. Cytomegalovirus infection is common in the first months of life, acute infection stimulates the production of total CD8+ cells in infants [12] and both total and activated CD8+ cells in adults [39], and coinfection leads to worse overall and neurological prognoses [12]. It is also possible that >1 mechanism was at work.
This study has limitations. First, subjects were selected in part on the basis of the availability of flow cytometry data during the first 2 months of life, and this could have been a source of bias. Second, neurodevelopmental test results were not available for every child at every visit. Third, as in any such natural history study, confounding from other variables is possible. We adjusted our analyses for important covariates measured as part of the WITS protocol (CD4 cell counts at ages 1 and 2 months, prematurity, and rapid disease progression), and we still found a strong association of neurodevelopmental outcome with CD8+HLA-DR+ cells measured in the first 2 months of life. Nevertheless, there may be unmeasured variables that were important confounders.
In conclusion, we found that HIV-infected infants with low percentages of activated CD8+ T cells at ages 1 and 2 months had a better neurodevelopmental prognosis during the first 3 years of life than did infants with high percentages of activated CD8+ cells and that this association was independent of several variables that predict outcome of HIV infection, as well as overall clinical prognosis. The implication of these findings is that the activation state of CD8+ cells is important for the effects of HIV infection on CNS development in infants. Efforts to influence this activation might have preventive or therapeutic effects.
WITS Group
Principal investigators, study coordinators, and program officers of the WITS include the following: Clemente Diaz and Edna Pacheco-Acosta (University of Puerto Rico, San Juan); Ruth Tuomala, Ellen Cooper, and Donna Mesthene (Boston/Worcester site, Massachusetts); Phil LaRussa and Alice Higgins (Columbia Presbyterian Hospital, New York, NY); Sheldon Landesman, Hermann Mendez, Edward Handelsman, and Ava Dennie (State University of New York at Downstate, Brooklyn); Kenneth Rich and Delmyra Turpin (University of Illinois at Chicago, Chicago); William Shearer and Norma Cooper (Baylor College of Medicine, Houston, TX); Joana Rosario (National Institute of Allergy and Infectious Diseases, Bethesda, MD); Kevin Ryan (National Institute of Child Health and Human Development, Bethesda, MD); Vincent Smeriglio and Katherine Davenny (National Institute on Drug Abuse, Bethesda, MD); and Bruce Thompson (Clinical Trials and Surveys Corporation, Baltimore, MD). The Scientific Leadership Core includes Kenneth Rich (principal investigator) and Delmyra Turpin (study coordinator).
Acknowledgments
Financial support. Funding for the Women and Infants Transmission Study includes grants U01 AI 34858 (to University of Puerto Rico), 9U01 DA 15054 (to Boston/Worcester site), U01 DA 15053 (to Columbia Presbyterian Hospital), HD-3–6117 (to State University of New York at Downstate), U01 AI 34841 (to University of Illinois at Chicago), U01 HD 41983 (to Baylor College of Medicine), N01 AI 085339 (to Clinical Trials and Surveys Corporation), and U01 AI 50274–01 (to study coordinator Delmyra Turpin). Additional support was provided by local clinical research centers: Children's Hospital Boston (National Institutes of Health [NIH] General Clinical Research Center [GCRC] RR02174), Baylor College of Medicine (NIH GCRC RR00188), and Columbia University (NIH GCRC RR00645).
Potential conflicts of interest. K.M. has received legal consulting fees from Pfizer. All other authors: no conflicts.
references
- 1.Wolters P, Brouwers P. Evaluation of neurodevelopmental deficits in children with HIV infection. In: Gendelman HE, Grant I, Lipton SA, Everall I, Swindells S, editors. The neurology of AIDS. 2nd ed. London: Oxford University Press; 2005. pp. 667–82. [Google Scholar]
- 2.Chase C, Ware J, Hittelman J, et al. Early cognitive and motor development among infants born to women infected with human immunodeficiency virus. Women and Infants Transmission Study Group. Pediatrics. 2000;106:25. doi: 10.1542/peds.106.2.e25. [DOI] [PubMed] [Google Scholar]
- 3.Weinberg GA, Burchett SK. Pediatric human immunodeficiency virus infection. In: Mandell GL, Bennett JE, Dolin R, editors. Mandell, Douglas, and Bennett's principles and practice of infectious diseases. 6th ed. Philadelphia: Churchill Livingstone; 2005. pp. 1638–54. [Google Scholar]
- 4.Belman AL, Muenz LR, Marcus JC, et al. Neurologic status of human immunodeficiency virus 1-infected infants and their controls: a prospective study from birth to 2 years. Mothers and Infants Cohort Study. Pediatrics. 1996;98:1109–18. [PubMed] [Google Scholar]
- 5.Cooper ER, Hanson C, Diaz C, et al. Encephalopathy and progression of human immunodeficiency virus disease in a cohort of children with perinatally acquired human immunodeficiency virus infection. Women and Infants Transmission Study Group. J Pediatr. 1998;132:808–12. doi: 10.1016/s0022-3476(98)70308-7. [DOI] [PubMed] [Google Scholar]
- 6.Nozyce M, Hittelman J, Muenz L, Durako SJ, Fischer ML, Willoughby A. Effect of perinatally acquired human immunodeficiency virus infection on neurodevelopment in children during the first two years of life. Pediatrics. 1994;94:883–91. [PubMed] [Google Scholar]
- 7.Lindsey JC, Malee KM, Brouwers P, Hughes MD. Neurodevelopmental functioning in HIV-infected infants and young children before and after the introduction of protease inhibitor-based highly active antiretroviral therapy. Pediatrics. 2007;119:e681–93. doi: 10.1542/peds.2006-1145. [DOI] [PubMed] [Google Scholar]
- 8.Scott GB, Hutto C, Makuch RW, et al. Survival in children with perinatally acquired human immunodeficiency virus type 1 infection. N Engl J Med. 1989;321:1791–6. doi: 10.1056/NEJM198912283212604. [DOI] [PubMed] [Google Scholar]
- 9.Tardieu M, Le Chenadec J, Persoz A, Meyer L, Blanche S, Mayaux MJ. HIV-1-related encephalopathy in infants compared with children and adults. French Pediatric HIV Infection Study and the SEROCO Group. Neurology. 2000;54:1089–95. doi: 10.1212/wnl.54.5.1089. [DOI] [PubMed] [Google Scholar]
- 10.Brouwers P, Tudor-Williams G, DeCarli C, et al. Relation between stage of disease and neurobehavioral measures in children with symptomatic HIV disease. AIDS. 1995;9:713–20. doi: 10.1097/00002030-199507000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Macmillan C, Magder LS, Brouwers P, et al. Head growth and neurodevelopment of infants born to HIV-1-infected drug-using women. Neurology. 2001;57:1402–11. doi: 10.1212/wnl.57.8.1402. [DOI] [PubMed] [Google Scholar]
- 12.Kovacs A, Schluchter M, Easley K, et al. Cytomegalovirus infection and HIV-1 disease progression in infants born to HIV-1-infected women. Pediatric Pulmonary and Cardiovascular Complications of Vertically Transmitted HIV Infection Study Group. N Engl J Med. 1999;341:77–84. doi: 10.1056/NEJM199907083410203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith R, Malee K, Charurat M, et al. Timing of perinatal human immunodeficiency virus type 1 infection and rate of neurodevelopment. The Women and Infant Transmission Study Group. Pediatr Infect Dis J. 2000;19:862–71. doi: 10.1097/00006454-200009000-00010. [DOI] [PubMed] [Google Scholar]
- 14.Sei S, Stewart SK, Farley M, et al. Evaluation of human immunodeficiency virus (HIV) type 1 RNA levels in cerebrospinal fluid and viral resistance to zidovudine in children with HIV encephalopathy. J Infect Dis. 1996;174:1200–6. doi: 10.1093/infdis/174.6.1200. [DOI] [PubMed] [Google Scholar]
- 15.Brouwers P, Civitello L, DeCarli C, Wolters P, Sei S. Cerebrospinal fluid viral load is related to cortical atrophy and not to intracerebral calcifications in children with symptomatic HIV disease. J Neurovirol. 2000;6:390–7. doi: 10.3109/13550280009018303. [DOI] [PubMed] [Google Scholar]
- 16.Marcotte TD, Deutsch R, McCutchan JA, et al. Prediction of incident neurocognitive impairment by plasma HIV RNA and CD4 levels early after HIV seroconversion. Arch Neurol. 2003;60:1406–12. doi: 10.1001/archneur.60.10.1406. [DOI] [PubMed] [Google Scholar]
- 17.Karlsen NR, Froland SS, Reinvang I. HIV-related neuropsychological impairment and immunodeficiency: CD8+ lymphocytes and neopterin are related to HIV-encephalopathy. Scand J Psychol. 1994;35:230–9. doi: 10.1111/j.1467-9450.1994.tb00947.x. [DOI] [PubMed] [Google Scholar]
- 18.Sanchez-Ramon S, Bellon JM, Resino S, et al. Low blood CD8+ T-lymphocytes and high circulating monocytes are predictors of HIV-1-associated progressive encephalopathy in children. Pediatrics. 2003;111:e168–75. doi: 10.1542/peds.111.2.e168. [DOI] [PubMed] [Google Scholar]
- 19.McCloskey TW, Kohn N, Lesser M, Bakshi S, Pahwa S. Immunophenotypic analysis of HIV-infected children: alterations within the first year of life, changes with disease progression, and longitudinal analyses of lymphocyte subsets. Cytometry. 2001;46:157–65. doi: 10.1002/cyto.1100. [DOI] [PubMed] [Google Scholar]
- 20.Paul ME, Shearer WT, Kozinetz CA, Lewis DE. Comparison of CD8+ T-cell subsets in HIV-infected rapid progressor children versus non-rapid progressor children. J Allergy Clin Immunol. 2001;108:258–64. doi: 10.1067/mai.2001.117179. [DOI] [PubMed] [Google Scholar]
- 21.Than S, Kharbanda M, Chitnis V, Bakshi S, Gregersen PK, Pahwa S. Clonal dominance patterns of CD8 T cells in relation to disease progression in HIV-infected children. J Immunol. 1999;162:3680–6. [PubMed] [Google Scholar]
- 22.Sherman GG, Scott LE, Galpin JS, et al. CD38 expression on CD8+ T cells as a prognostic marker in vertically HIV-infected pediatric patients. Pediatr Res. 2002;51:740–5. doi: 10.1203/00006450-200206000-00013. [DOI] [PubMed] [Google Scholar]
- 23.Paul ME, Mao C, Charurat M, et al. Predictors of immunologic long-term nonprogression in HIV-infected children: implications for initiating therapy. J Allergy Clin Immunol. 2005;115:848–55. doi: 10.1016/j.jaci.2004.11.054. [DOI] [PubMed] [Google Scholar]
- 24.Sheon A, Fox H, Rich K. The Women and Infants Transmission Study (WITS) of maternal-infant HIV transmission: study design, methods, and baseline data. J Womens Health. 1996;5:69–78. [Google Scholar]
- 25.Rodriguez EM, Mendez H, Rich K, et al. Maternal drug use in perinatal HIV studies. The Women and Infants Transmission Study. Ann N Y Acad Sci. 1993;693:245–8. doi: 10.1111/j.1749-6632.1993.tb26272.x. [DOI] [PubMed] [Google Scholar]
- 26.McIntosh K, Pitt J, Brambilla D, et al. Blood culture in the first 6 months of life for the diagnosis of vertically transmitted human immunodeficiency virus infection. The Women and Infants Transmission Study Group. J Infect Dis. 1994;170:996–1000. doi: 10.1093/infdis/170.4.996. [DOI] [PubMed] [Google Scholar]
- 27.Bayley N. Manual for the Bayley scales of infant development. San Antonio, TX: Psychological Corporation; 1969. [Google Scholar]
- 28.Mayes SD. Potential problems using Bayley scales of infant development-II mental scale. J Early Infancy. 1997;2:36–44. [Google Scholar]
- 29.Lindsey JC, O'Donnell K, Brouwers P. Methodological issues in analyzing psychological test scores in pediatric clinical trials. J Dev Behav Pediatr. 2000;21:141–51. doi: 10.1097/00004703-200004000-00010. [DOI] [PubMed] [Google Scholar]
- 30.Paxton H, Kidd P, Landay A, et al. Results of the flow cytometry ACTG quality control program: analysis and findings. Clin Immunol Immunopathol. 1989;52:68–84. doi: 10.1016/0090-1229(89)90194-3. [DOI] [PubMed] [Google Scholar]
- 31.Calvelli T, Denny TN, Paxton H, Gelman R, Kagan J. Guideline for flow cytometric immunophenotyping: a report from the National Institute of Allergy and Infectious Diseases, Division of AIDS. Cytometry. 1993;14:702–15. doi: 10.1002/cyto.990140703. [DOI] [PubMed] [Google Scholar]
- 32.Rich KC, Brambilla D, Pitt J, et al. Lymphocyte phenotyping in infants: maturation of lymphocyte subpopulations and the effects of HIV infection. Clin Immunol Immunopathol. 1997;85:273–81. doi: 10.1006/clin.1997.4439. [DOI] [PubMed] [Google Scholar]
- 33.Centers for Disease Control Prevention 1994 Revised classification system for human immunodeficiency virus infection in children less than 13 years of age. MMWR Recomm Rep. 1994;43(RR-12):1–19. [Google Scholar]
- 34.Diggle PJ. An approach to the analysis of repeated measurements. Biometrics. 1988;44:959–71. [PubMed] [Google Scholar]
- 35.McCrossan M, Marsden M, Carnie FW, et al. An immune control model for viral replication in the CNS during presymptomatic HIV infection. Brain. 2006;129:503–16. doi: 10.1093/brain/awh695. [DOI] [PubMed] [Google Scholar]
- 36.McCloskey TW, Cavaliere T, Bakshi S, et al. Immunophenotyping of T lymphocytes by three-color flow cytometry in healthy newborns, children, and adults. Clin Immunol Immunopathol. 1997;84:46–55. doi: 10.1006/clin.1997.4370. [DOI] [PubMed] [Google Scholar]
- 37.Shearer WT, Rosenblatt HM, Gelman RS, et al. Lymphocyte subsets in healthy children from birth through 18 years of age: the Pediatric AIDS Clinical Trials Group P1009 Study. J Allergy Clin Immunol. 2003;112:973–80. doi: 10.1016/j.jaci.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 38.Giorgi JV, Liu Z, Hultin LE, Cumberland WG, Hennessey K, Detels R. Elevated levels of CD38+CD8+ T cells in HIV infection add to the prognostic value of low CD4+ T cell levels: results of 6 years of follow-up. The Los Angeles Center, Multicenter AIDS Cohort Study. J Acquir Immune Defic Syndr. 1993;6:904–12. [PubMed] [Google Scholar]
- 39.Belles-Isles M, Houde I, Lachance JG, Noel R, Kingma I, Roy R. Monitoring of cytomegalovirus infections by the CD8+CD38+ T-cell subset in kidney transplant recipients. Transplantation. 1998;65:279–82. doi: 10.1097/00007890-199801270-00026. [DOI] [PubMed] [Google Scholar]