Abstract
Background:
Alternate day fasting (ADF) with a low-fat (LF) diet improves brachial artery flow-mediated dilation (FMD). Whether these beneficial effects can be reproduced with a high-fat (HF) diet remains unclear.
Objective:
This study compared the effects of ADF-HF to ADF-LF regimens on FMD. The role that adipokines have in mediating this effect was also investigated.
Methods:
Thirty-two obese subjects were randomized to an ADF-HF (45% fat) or ADF-LF diet (25% fat), consisting of two phases: (1) a 2-week baseline weight maintenance period and (2) an 8-week ADF weight loss period. Food was provided throughout the study.
Results:
Body weight was reduced (P<0.0001) in the ADF-HF (4.4±1.0 kg) and ADF-LF group (3.7±0.7 kg). FMD decreased (P<0.05) by ADF-HF relative to baseline (7±1 to 5±2%) and increased (P<0.05) by ADF-LF (5±1 to 7±2%). Blood pressure remained unchanged in both groups. Adiponectin increased (P<0.05) in the ADF-HF (43±7%) and ADF-LF group (51±7%). Leptin and resistin decreased (P<0.05) in the ADF-HF (32±5% 23±5%) and ADF-LF group (30±3% 27±4%). Increases in adiponectin were associated with augmented FMD in the ADF-LF group only (r=0.34, P=0.03).
Conclusion:
Thus, improvements in FMD with ADF may only occur with LF diets and not with HF diets, and adipokines may not have a significant role in mediating this effect.
Keywords: alternate day fasting, calorie restriction, dietary fat, endothelial function, adipokines, obese humans
Introduction
Obesity is a well-established risk factor for coronary heart disease. Obese individuals often exhibit increased low-density lipoprotein cholesterol and triglyceride concentrations, along with elevated blood pressure.1 Excessive fat mass is also linked to abnormal endothelial function, marked by reduced vasodilation to an increased blood flow (endothelium-dependent flow-mediated dilation; FMD).2 Endothelial dysfunction is an early predictor of future vascular events and most coronary heart disease risk factors are correlated with reduced FMD.3
Weight loss, by means of dietary restriction, has been shown to improve several coronary heart disease risk factors, including FMD. Alternate day fasting (ADF) is a novel diet restriction strategy that has gained considerable popularity over the past decade. ADF consists of a 24-h period of ad libitum food consumption termed the ‘feed day', alternated with a 24-h period of 50–100% energy restriction, termed the ‘fast day'.4, 5 Recent reports indicate that ADF, with a low-fat (LF) diet background (25% of energy as fat), decreases body weight by 5% after 8 weeks of treatment in obese subjects.6 FMD was also increased in this study.6 Although these findings are promising, it is still unclear if the same beneficial increases in FMD would be seen if a high-fat (HF) diet (45% of energy as fat) was used in place of a LF diet during ADF. This question is of importance as the majority of Americans consume 35–45% of their daily calories as dietary fat.7
The underlying mechanisms that link weight loss to improved endothelial function are still uncertain. Accumulating evidence demonstrates that adipokines may have an impact on vascular function. Adiponectin is a fat-cell-derived hormone that increases with weight loss, and protects the endothelium by decreasing oxidative stress.8, 9 Leptin and resistin, in contrast, are adipokines that are positively correlated to body weight and visceral fat mass.10, 11 Leptin and resistin have been shown to cause endothelial dysfunction by promoting oxidative stress.10, 11 In view of this, it can be hypothesized that weight loss strategies, such as ADF, would increase adiponectin and decrease leptin and resistin concentrations. In turn, these improvements in adipokine profiles may have a protective effect on the vascular endothelium resulting in increased FMD.12 However, the effects of ADF-LF versus ADF-HF diets on FMD and adipokines have yet to be tested.
Accordingly, the objective of this study was to compare the effects of ADF-HF to ADF-LF regimens on FMD in obese adults. The role that adipokines have in mediating this effect was also investigated.
Subjects and methods
Subjects and study design
As previously described,13 obese subjects (n=35) were recruited from the Chicago area based on the following inclusion criteria: female, age 25–65 years, body mass index between 30 and 39.9 kg m–2, weight for 3 months before the beginning of the study (that is, <5 kg weight loss or gain), non-diabetic, no history of cardiovascular disease, sedentary or lightly active for 3 months before the beginning of the study (that is, <3 h per week of light-intensity exercise at 2.5–4.0 metabolic equivalents), non-smoker, and not taking weight loss, lipid-lowering, or glucose-lowering medications. The experimental protocol was approved by the Office for the Protection of Research Subjects at the University of Illinois, Chicago, IL, USA, and all volunteers gave written informed consent. Eligible subjects were randomized by way of a stratified random sample based on body mass index (categories: 30–34.9 and 35–39.9 kg m–2) and age (categories: 25–44 and 45–65 years). These strata were counterbalanced between groups. Subjects from each stratum were then randomized to one of two intervention groups: (1) ADF-HF group (n=17) or (2) ADF-LF group (n=18). The 10-week trial consisted of two dietary phases: (1) a 2-week baseline weight maintenance period and (2) an 8-week weight loss ADF period.
Diet protocol
All food was provided throughout the 10-week trial to all subjects. During the 2-week baseline weight maintenance period, subjects consumed either the HF or LF diet (providing 100% of their energy needs). Following the baseline period, subjects participated in either an ADF-HF or ADF-LF weight loss intervention for 8 weeks. During the weight loss period, subjects consumed 25% of their energy needs on the fast day (24-h period) and 125% of their energy needs on the feed day (24-h period). The feed/fast days began at midnight each day. Fast day meals were consumed between 1200 and 1400 hours. The same macronutrient composition was used during the weight loss and weight maintenance periods for each group: ADF-HF (45% fat, 40% carbohydrate and 15% protein), ADF-LF (25% fat, 60% carbohydrate and 15% protein), as described previously.13 Fat content of the diets were as follows: ADF-HF (14% saturated fat, 20% monounsaturated fat, 11% polyunsaturated fat and 0% trans fat) and ADF-LF (6% saturated fat, 13% monounsaturated fat, 6% polyunsaturated fat and, 0% trans fat). Energy requirements were calculated using the Mifflin–St Jeor equation for women ((10 × weight (kg))+(6.25 × height (cm))−(5 × age (y))–161).14 An activity factor of 1.2 (sedentary) was then used to calculate total energy expenditure. Energy requirements were calculated at the beginning of the study and at the beginning of the weight loss period. Study diets were formulated for each participant using Nutritionist Pro Software (Axxya Systems LLC, Stafford, TX, USA). Diets were provided as a 3-day rotating menu consisting of typical American foods. All meals were consumed outside of the research center. Participants were requested to eat only the foods provided and to bring back any leftover foods to be weighed and recorded. Subjects were also instructed to maintain their physical activity habits throughout the duration of the study.
Diet compliance, hunger and physical activity maintenance
Subjects were instructed to keep track of all food items consumed using a ‘Food checklist', and to report any extra food item consumed using an ‘Extra food log'. If the log indicated that the subject ate extra food items (totaling >50 kcal) on a feed or fast day, that day was labeled as ‘not adherent'. To measure changes in hunger, satisfaction and fullness, subjects completed a validated visual analog scale on each fast day.15 The form was completed in the evening, approximately 5 min before going to bed. In brief, the visual analog scale consisted of 100-mm lines, and subjects were asked to make a vertical mark across the line corresponding to their feelings from 0 (not at all) to 100 (extremely) for hunger, satisfaction and fullness. The visual analog scale was collected each week and reviewed for completeness. Quantification was performed by measuring the distance from the left end of the line to the vertical mark.15 Free-living physical activity was assessed by a pedometer (Digiwalker SW-200, Yamax Corporation, Tokyo, Japan SW). Subjects wore the pedometer each day throughout the 10-week trial. Number of daily steps was recorded in a pedometer log provided, and the log was collected by study personnel at the weigh-in each week.
Outcome measures
Brachial artery measurements of FMD
Brachial artery FMD was assessed at baseline and week 8. Ultrasound imaging of the brachial artery (MicroMaxx, Sonosite, Seattle, WA, USA) was performed in a longitudinal plane at a site 1–3 cm proximal to the antecubital fossa, with the arm abducted approximately 80 ° from the body and the forearm supinated. All of the ultrasounds were performed by MCK. The ultrasound probe (11 MHz) was positioned to visualize the anterior and posterior lumen-intima interfaces to measure diameter or central flow velocity (pulsed Doppler). After baseline images were recorded, a blood pressure cuff on the forearm was inflated to 200 mm Hg for 5 min. To assess FMD, 10 s of images were captured at a rate of 10 images per second, 30 s, 1 min and 2 min after cuff release. Baseline brachial flow velocity and peak velocity after cuff release were recorded. Images were digitally recorded using Brachial Imager (Medical Imaging, Iowa City, IA, USA) and analyzed. The investigator performing the analyses (EN) was blinded to subject treatment assignment. Percent FMD was calculated using the averaged minimum mean brachial artery diameter at baseline compared with the largest mean values obtained after release of the forearm occlusion. The inter-assay coefficient of variation for FMD was 6.3%. Blood pressure was assessed in triplicate after a 10-min rest each week.
Body weight and body composition assessment
Body weight measurements were taken weekly to the nearest 0.25 kg using a balance beam scale (HealthOMeter; Sunbeam Products, Boca Raton, FL, USA). Height was assessed using a wall-mounted stadiometer to the nearest 0.1 cm. Body mass index was assessed as kg m–2. Fat mass and fat-free mass (FFM) were assessed by dual-energy X-ray absorptiometry at baseline and week 8 (QDR 4500 W, Hologic Inc., Arlington, MA, USA). Fat mass was calculated by multiplying the % fat value from dual-energy X-ray absorptiometry by the metabolic weight recorded the same day. FFM was calculated by subtracting fat mass from body weight. Waist circumference was measured by a flexible tape to the nearest 0.1 cm, midway between the lower costal margin and super iliac crest during a period of expiration.
Plasma lipid assessment
Plasma total cholesterol, high-density lipoprotein cholesterol and triglyceride concentrations were measured in duplicate by using enzymatic kits (Biovision Inc., Mountain view, CA, USA) and analyzed using a microplate reader (iMark Microplate Reader; Bio-Rad Laboratories Inc., Richmond, CA, USA). The concentration of low-density lipoprotein cholesterol was calculated using the Friedewald, Levy and Fredrickson equation. The inter-assay coefficient of variation for total cholesterol, high-density lipoprotein cholesterol and triglyceride concentrations were 2.9%, 1.8% and 2.4%, respectively.
Plasma adipokine determination
Twelve-hour fasting blood samples were collected between 0600 and 1000 hours at baseline and week 8. Subjects were instructed to avoid exercise, alcohol and coffee for 24 h before each visit. Blood was centrifuged for 10 min at 1000 g and 4 °C to separate plasma from red blood cell and was stored at −80 °C until analyzed. Plasma adiponectin, leptin and resistin were measured using high sensitivity enzymatic kits (R&D Systems, Minneapolis, MN, USA). The inter-assay coefficient of variation for adiponectin, leptin and resistin were 4.0%, 3.7% and 3.6%, respectively.
Statistics
Values are presented as mean±s.e.m. An independent samples t-test was used to test baseline differences between groups. A paired t-test was used to assess changes from baseline to post-treatment within-group, and an independent samples t-test was used to assess differences between groups post-treatment. Pearson correlations were used to evaluate how FMD related to adipokines. Post hoc analyses were performed using the Tukey's test. Differences were considered significant at P<0.05. All data were analyzed using SPSS software (version 20.0 for Mac, SPSS Inc., Chicago, IL, USA).
Results
Subject dropout and baseline characteristics
Thirty-two out of the initial 35 subjects completed the study. Two subjects in the ADF-HF group dropped out because of an inability to comply with the ADF protocol (n=1) and scheduling conflicts (n=1). One subject dropped out of the ADF-LF group because of an inability to follow the diet protocol. Thus, there were n=15 completers in the ADF-HF group and n=17 completers in the ADF-LF group. Baseline characteristics of the ADF-HF and ADF-LF groups are reported in Table 1. Subjects were all female, with no differences between groups for age, weight, body mass index, body composition or adipokines.
Table 1. Subject baseline characteristicsa.
| Characteristic | ADF-HF | ADF-LF |
|---|---|---|
| n | 15 | 17 |
| Age (years) | 43±11 | 43±9 |
| Body weight (kg) | 92±10 | 92±11 |
| Height (cm) | 161±6 | 160±6 |
| Body mass index (kg m–2) | 35±3 | 35±3 |
| Fat mass (kg) | 44±6 | 42±5 |
| Fat-free mass (kg) | 46±6 | 46±6 |
| Waist circumference (cm) | 98±7 | 99±8 |
| Adiponectin (ng ml–1) | 7319±8955 | 10 259±10 263 |
| Leptin (ng ml–1) | 41±33 | 41±29 |
| Resistin (ng ml–1) | 14±15 | 14±10 |
Abbreviations: ADF-HF, alternate day fasting high-fat diet; ADF-LF, alternate day fasting low-fat diet.
Values reported as mean±s.d. No differences between groups for any parameter (independent samples t-test).
Diet compliance, hunger and physical activity maintenance
ADF-HF and ADF-LF subjects were 96% and 94% compliant, respectively, with the prescribed diet during the weight maintenance period. However, during the weight loss period, the ADF-HF group had higher (P<0.05) percent adherence (87±2%) versus the ADF-LF group (77±2%). Changes in hunger and physical activity are reported in Table 2. Hunger decreased (P<0.05) by 44±5 and 52±2 mm in the ADF-HF and ADF-LF groups, respectively, during the weight loss period. Satisfaction with the diet remained elevated throughout the trial in the ADF-HF diet. Satisfaction in the ADF-LF group started out low (35±5 mm), but gradually increased (P<0.05) from baseline to week 8 (61±5 mm). Fullness decreased (P<0.05) in the ADF-HF group by 17±6 mm and increased in the ADF-LF group by 20±10 mm during the weight loss phase. Physical activity, measured in steps per day, remained unchanged throughout the course of the study in both groups.
Table 2. Hunger and physical activity maintenancea.
|
ADF-HF |
ADF-LF |
|||||
|---|---|---|---|---|---|---|
| Baseline | Week 8 | Changeb | Baseline | Week 8 | Changeb | |
| Hunger (mm) | 69±3 | 25±4c | −44±5 | 76±3 | 24±4c | −52±2 |
| Satisfaction (mm) | 68±3 | 69±4 | 1±5 | 35±5 | 61±5c | 26±8d |
| Fullness (mm) | 72±3 | 55±5c | −17±6 | 42±6 | 62±6c | 20±10d |
| Steps per day | 5698±502 | 5034+307 | −664±683 | 5531±573 | 5374+650 | −157±934 |
Abbreviations: ADF, alternate day fasting; HF, high-fat diet; LF, low-fat diet.
Values reported as mean±s.e.m. ADF-HF, n=15; ADF-LF, n=17.
Change expressed as the difference between baseline and week 8 absolute values.
Significantly different from baseline, P<0.05 (paired t-test).
Significantly different between groups for absolute change, P<0.05 (independent samples t-test).
Weight loss and body composition
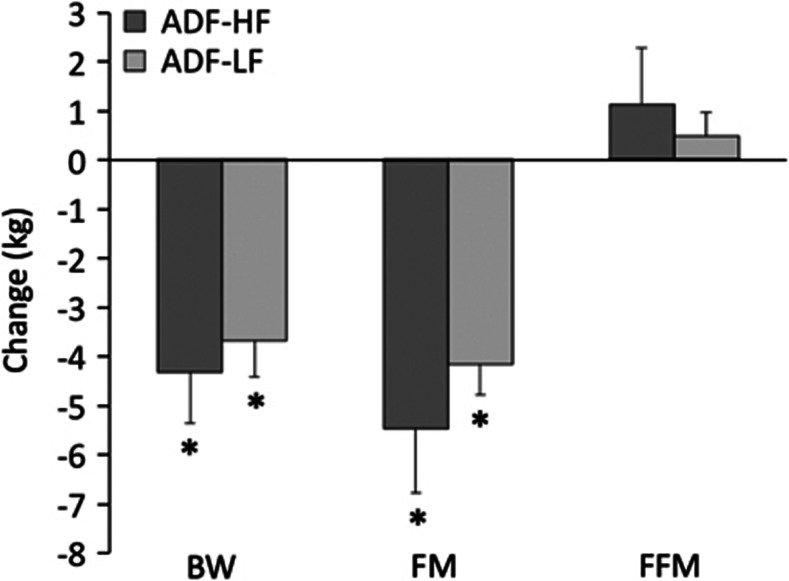
Changes in body weight and body composition are displayed in Figure 1. During the baseline weight maintenance period, body weight decreased (P<0.0001) in the ADF-HF (2.1±0.4 kg) and ADF-LF groups (1.7±0.4 kg), despite being provided with 100% of their energy needs. ADF subjects in the ADF-HF and ADF-LF group reduced body weight (P<0.0001) by 4.4±1.0 and 3.7±0.7 kg, respectively, from baseline to week 8, with no differences between groups. Fat mass also decreased (P<0.0001) in the ADF-HF and ADF-LF groups by 5.4±1.5 and 4.2±0.6 kg, respectively. FFM did not change during the course of the trial. Subjects in the ADF-HF and ADF-LF groups decreased waist circumference (P<0.001) by 7.2±1.5 and 7.3±0.9 cm, respectively, from baseline and week 8.
Figure 1.
Body weight and composition changes during the weight loss period. Values reported as mean±s.e.m. ADF-HF, n=15; ADF-LF, n=17. BW, body weight; FM, fat mass; FFM, fat-free mass. *Week 8 values significantly different (P<0.0001) from baseline values (paired t-test). No differences between groups at any time point (independent samples t-test).
Brachial artery FMD
There were no differences in FMD between groups at baseline (Table 3). At the end of the trial, FMD decreased (P<0.05) in the ADF-HF group relative to baseline (−2% decrease) and increased (P<0.05) in the ADF-LF group (+2% increase). Post-treatment values were higher (P<0.05) in the ADF-LF group compared with the ADF-HF group. Systolic blood pressure remained unchanged in the ADF-HF group (baseline: 111±2 mm Hg, week 8: 109±2 mm Hg) and the ADF-LF group (baseline: 116±3 mm Hg, week 8: 118±3 mm Hg). Similarly, diastolic blood pressure was not affected by the ADF-HF diet (baseline: 77±3 mm Hg, week 8: 75±2 mm Hg) or the ADF-LF diet (baseline: 79±3 mm Hg, week 8: 81±3 mm Hg).
Table 3. Brachial-artery FMDa.
| Baseline | Week 8 | ||
|---|---|---|---|
| Max dilation (%) | Max dilation (%) | FMD change score (%)b | |
| ADF-HF | 6.5±1.3 (−2.4 to 11.8) | 4.7±1.6 (−2.4 to 15.2)c | −1.8±1.7 (−8.6 to 6.8)d |
| ADF-LF | 4.6±0.7 (0 to 9.7) | 6.7±0.1 (0 to 13.4)c | 2.1±1.0 (−4.7 to 8.2) |
Abbreviations: ADF, alternate day fasting; FMD, flow-mediated dilation; HF, high-fat diet; LF, low-fat diet.
Values reported as mean±s.e.m (range). ADF-HF, n=15; ADF-LF, n=17.
Change expressed as the difference between baseline and week 8 absolute values.
Significantly different from baseline, P<0.05 (paired t-test).
Significantly different between groups for absolute change, P<0.05 (independent samples t-test).
Plasma lipids
Total cholesterol concentrations decreased (P<0.0001) in both the ADF-HF group (13.0±1.8%, baseline: 198±11 mg dl–1, week 8: 172±9 mg dl–1) and ADF-LF group (16.3±1.7%, baseline: 193±8 mg dl–1, week 8: 162±7 mg dl–1). Low-density lipoprotein cholesterol concentrations were reduced (P<0.0001) during the weight loss period by the ADF-HF diet (18.3±4.6%, baseline: 109±9 mg dl–1, week 8: 90±7 mg dl–1) and ADF-LF diet (24.8±2.6%, baseline: 113±7 mg dl–1, week 8: 85±7 mg dl–1). Triglyceride concentrations decreased (P<0.001) in the ADF-HF group (13.7±4.8%, baseline: 123±15 mg dl–1, week 8: 108±15 mg dl–1) and ADF-LF group (14.3±4.4%, baseline: 97±11 mg dl–1, week 8: 83±10 mg dl–1) from baseline to week 8. high-density lipoprotein cholesterol concentrations remained unchanged. There were no differences between groups for any plasma lipid parameter.
Plasma adipokines
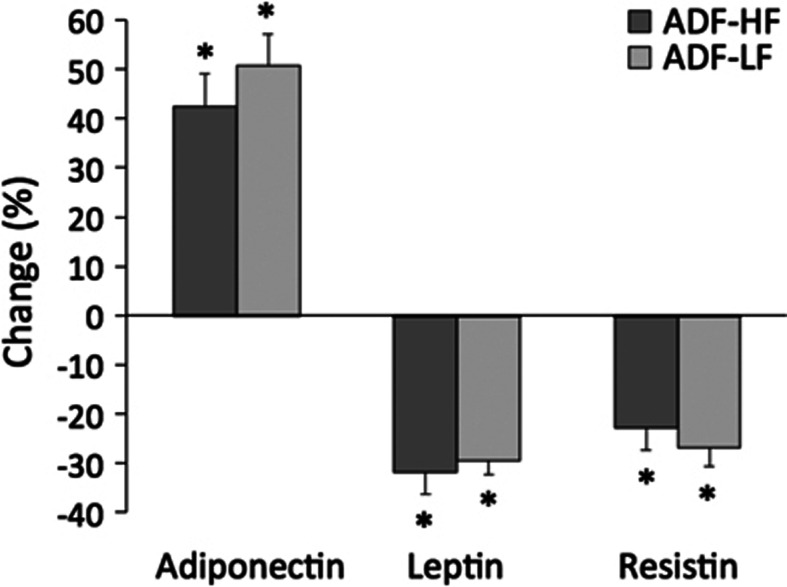
Adiponectin increased (P<0.05) by 43±7% and 51±7% in the ADF-HF and ADF-LF group, respectively. Leptin decreased (P<0.05) in the ADF-HF and ADF-LF group by 32±5% and 30±3%, respectively. Similarly, resistin (P<0.05) decreased by 23±5% and 27±4% in the ADF-HF and ADF-LF groups, respectively (Figure 2). Increases in adiponectin were associated with augmented FMD post-treatment in the ADF-LF group only (r=0.34, P=0.03). Leptin and resistin were not correlated with changes in FMD.
Figure 2.
Plasma adipokines during the weight loss period. Values reported as mean ±s.e.m. ADF-HF, n=15; ADF-LF, n=17. *Week 8 values significantly different (P<0.05) from baseline values (paired t-test). No differences between groups at any time point (independent samples t-test).
Discussion
In this study, we observed an improvement in brachial artery FMD with an ADF-LF (25% fat) diet after 8 weeks of weight loss. In contrast, FMD was impaired with an ADF-HF diet (45% fat). Both intervention groups experienced increases in adiponectin and decreases in leptin and resistin. However, only adiponectin was correlated with FMD in the ADF-LF group.
The primary goal of this study was to determine how dietary fat composition affects endothelial function during periods of ADF. Previous work in this field indicates that ADF with a LF background diet increases FMD.6 Although these results are encouraging, it was still unclear if these favorable effects could be reproduced if a HF diet was used in place of a LF diet. Results from this study demonstrate that implementing a HF background diet impairs FMD (–2%) while using a LF diet improves FMD (+2%). These effects on FMD occurred despite similar weight loss (4 kg) and waist circumference reductions (7 cm) in both groups. These findings are similar to what has been reported previously for calorie restriction (CR) diets.16, 17 For instance, obese patients following a CR-LF diet (25% fat) increased FMD after 8 weeks of weight loss in a study by Khoo et al.16 Similarly, Phillips et al.17 report increases in FMD after 6 weeks of a CR-LF diet (25% fat) and decreases in FMD with a CR-HF diet (60% fat). In contrast, Volek et al.,18 reported increases in FMD with a CR-HF (60% fat) diet and decreases with a CR-LF diet (20% fat), while Keogh et al.19, 20 demonstrated no effect of either diet on vascular endothelial function during weight loss. The reason why our findings differ from those of Volek et al.18 and Keogh et al.19, 20 is not clear. However, it should be noted that the studies showing beneficial effects of LF diets and deleterious effects of HF diets,16, 17 all provided food to subjects to ensure that the macronutrient composition of the diets were well controlled. On the other hand, the studies that demonstrate opposite,18 or no effect,19, 20 did not provide food to subjects. Thus, the lack of diet standardization in the Volek et al.18 and Keogh et al.19, 20 studies may partly explain these inconsistent findings. The reason why HF diets impair FMD is not clear, but may involve increased intake of saturated fat. In this study, subjects in the ADF-HF group consumed 14% of total energy as saturated fat. High intakes of saturated fat have been shown to directly impair arterial endothelial function by reducing the anti-inflammatory potential of high-density lipoprotein.21 Therefore, the high saturated fat intake may have contributed to the decreased FMD observed in the ADF-HF group. It should also be noted that the ADF-LF group experienced improvements in FMD despite demonstrating deleterious increases in blood pressure (though nonsignificant). Increases in FMD are generally accompanied by decreases in blood pressure. As such, it is possible that the improvements in FMD by the ADF-LF diet would have been more pronounced if this group also experienced significant decreases in blood pressure. Also of interest, is the increase in FFM in both groups, and how this may impact FMD. To date, there is no evidence to support that increasing FFM may enhance FMD.22 Thus, it is likely that the changes in FFM had no impact on endothelial function.
Our secondary objective was to determine the role that adipokines have in mediating these changes in FMD. Adiponectin increased in both ADF-LF (43%) and ADF-HF (51%) groups after 8 weeks of weight loss. However, increases in adiponectin were only correlated to augmented FMD in the ADF-LF group. Although the precise mechanism by which adiponectin may improve FMD has yet to be established, we speculate that modulations in nitric oxide (NO) may be involved.23 NO, released from the endothelium, is a powerful vasodilator that is important in regulating vascular tone. Plasma adiponectin can stimulate the phosphorylation of endothelial NO synthase (eNOS), thereby increasing NO-dependent endothelial vasodilation.23 As such, increased plasma adiponectin in the LF group may contribute to enhanced endothelial function. The reason why the augmented adiponectin in the HF did not contribute to improvements in FMD is not clear.
Leptin and resistin were also improved during the ADF-LF and ADF-HF regimen. Leptin decreased by 32% and 30% in the ADF-HF and ADF-LF group, respectively, whereas resistin decreased by 23% and 27%, respectively. However, these reductions in leptin and resistin were not correlated to changes in FMD in either group. The role that leptin and resistin have in mediating FMD most likely involves changes in the production of NO. More specifically, leptin and resistin blunt the production of NO, which likely occurs through the stimulation of reactive oxygen species that scavenge NO and impair endothelial NO synthase function.24 As concentrations of these adipokines were reduced in the present trial, we would assume that there would be less leptin and resistin in the circulation to inhibit NO.24 This would lead to a higher production of NO, resulting in an enhancement in endothelium-dependent vasodilation. The reason why these decreases in leptin and resistin did not contribute to increases in FMD is unclear. However, it is possible that greater decreases in leptin (>50%) would be necessary to improve FMD.25
Results from the hunger questionnaire indicate that the ADF-HF and ADF-LF diets were well tolerated. For instance, hunger was elevated at the beginning of the trial in both groups, but quickly decreased within the first 2 weeks (data not shown). Satisfaction and fullness remained relatively high in the ADF-HF group throughout the trial, which is not surprising as HF diets are generally more palatable. In contrast, satisfaction and fullness started out low in the LF group, but gradually increased over 10 weeks. In view of this data, it is possible that the ADF-HF group lost slightly more weight and fat mass (although not significantly) than the ADF-LF group because they found the HF diet more satisfying. However, the difference in weight loss (0.7 kg) between the two groups may have been too small to impact FMD. We also show here that physical activity remained constant throughout the trial, suggesting that the weight loss is primarily because of the decrease in energy intake with the ADF diet. It should be noted, however, that although not significant, the ADF-HF group decreased their activity level by 660 steps per day from baseline to post-treatment. Habitual endurance exercise is associated with enhanced FMD.26 Thus, the decline in habitual exercise by the ADF-HF subjects may have contributed to the decrease in FMD observed here.
This study has several limitations. First, the sample size of the study was small (n=15–17 per group). This small sample size may have impacted our ability to detect a significant relationship between adipokine concentrations and changes in FMD. Second, we did not assess NO-independent dilations. As such, we cannot confirm that the effects of the diets were specific to the endothelium or altered the smooth muscle sensitivity to NO. Third, the study duration was quite short (8 weeks). It will be of interest in future studies to see if these changes in FMD persist long term (>24 weeks) with ADF-HF and AD-LF diets. Fourth, our body composition data are peculiar in that change in fat mass was larger than the change in body weight. These inaccurate measurements may be the result of improper calibration of the dual-energy X-ray absorptiometry machine at baseline or post-treatment. Fifth, this study did not make any adjustments for multiple comparisons. As we are testing a number of hypotheses, this may increase our likelihood of rejecting the null hypotheses when it is true (type 1 error).
This study suggests that the improvements in endothelial function observed with ADF may only occur with LF background diets and not with HF diets. We also show here that although ADF-LF and ADF-HF diets improved adipokine profile, these improvements may not necessarily impact FMD. Previous reports suggest that an ADF-HF diet has the same cardio-protective effects as an ADF-LF diet, in terms of low-density lipoprotein cholesterol and visceral fat mass reduction.13 Taken together, ADF-HF diets may have favorable lipid-lowering effects, but these benefits may be counteracted by impairments in FMD, a composite physiological marker of cardiovascular risk. As such, the results of this study may suggest that individuals undergoing ADF should be cautioned against the long-term use of HF background diets (45% fat) as this may eventually have harmful effects on cardiovascular health.
Acknowledgments
This work was supported by grants funded by University of Illinois at Chicago, Department of Kinesiology and Nutrition, Departmental funding, National Institutes of Health grants lK23HL85614 and HL095701.
Author contributions
MCK designed the experiment, conducted the clinical trial, analyzed the data and wrote the manuscript. CMK, MG and EN assisted with the conduction of the clinical trial and data analysis. SAP assisted with the data analysis and helped prepare the manuscript. KAV assisted with the design of the experiment and wrote the manuscript.
The authors declare no conflict of interest.
References
- Mannu GS, Zaman MJ, Gupta A, Rehman HU, Myint PK. Evidence of lifestyle modification in the management of hypercholesterolemia. Curr Cardiol Rev. 2012;9:2–14. doi: 10.2174/157340313805076313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borel JC, Roux-Lombard P, Tamisier R. Endothelial dysfunction and specific inflammation in obesity hypoventilation syndrome. PLoS One. 2009;4:e6733. doi: 10.1371/journal.pone.0006733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jambrik Z, Venneri L, Varga A, Rigo F, Borges A, Picano E. Peripheral vascular endothelial function testing for the diagnosis of coronary artery disease. Am Heart J. 2004;148:684–689. doi: 10.1016/j.ahj.2004.04.016. [DOI] [PubMed] [Google Scholar]
- Varady KA, Bhutani S, Church EC, Klempel MC. Short-term modified alternate-day fasting: a novel dietary strategy for weight loss and cardioprotection in obese adults. Am J Clin Nutr. 2009;90:1138–1143. doi: 10.3945/ajcn.2009.28380. [DOI] [PubMed] [Google Scholar]
- Bhutani S, Klempel MC, Berger RA, Varady KA. Improvements in coronary heart disease risk indicators by alternate-day fasting involve adipose tissue modulations. Obesity (Silver Spring) 2010;18:2152–2159. doi: 10.1038/oby.2010.54. [DOI] [PubMed] [Google Scholar]
- Bhutani S, Klempel MC, Kroeger CM, Phillips SA, Varady KA.Alternate day fasting with or without exercise: effects on endothelial function and adipokines in obese humans Nutrition 2013. in press.
- What we eat in America, NHANES 2007–2008, individuals 2 years and over 1 day dietary intake data, weighted2010
- Plant S, Shand B, Elder P, Scott R. Adiponectin attenuates endothelial dysfunction induced by oxidixed low-density lipoproteins. Diab Vasc Dis Res. 2008;5:102–108. doi: 10.3132/dvdr.2008.017. [DOI] [PubMed] [Google Scholar]
- Rasouli N, Kern PA. Adipocytokines and the metabolic complications of obesity. J Clin Endocrinol Metab. 2008;93:S64–S73. doi: 10.1210/jc.2008-1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Procopio C, Andreozzi F, Laratta E. Leptin-stimulated endothelial nitric-oxide synthase via an adenosine 5′-monophosphate-activated protein kinase/Akt signaling pathway is attenuated by interaction with C-reactive protein. Endocrinology. 2009;150:3584–3593. doi: 10.1210/en.2008-0921. [DOI] [PubMed] [Google Scholar]
- Dick GM, Katz PS, Farias M. Resistin impairs endothelium-dependent dilation to bradykinin, but not acetylcholine, in the coronary circulation. Am J Physiol Heart Circ Physiol. 2006;291:2997–3002. doi: 10.1152/ajpheart.01035.2005. [DOI] [PubMed] [Google Scholar]
- Marinou K, Tousoulis D, Antonopoulos AS, Stefanadi E, Stefanadis C. Obesity and cardiovascular disease: from pathophysiology to risk stratification. Int J Cardiol. 2012;138:3–8. doi: 10.1016/j.ijcard.2009.03.135. [DOI] [PubMed] [Google Scholar]
- Klempel MC, Kroeger CM, Varady KA. Alternate day fasting (ADF) with a high-fat diet produces similar weight loss and cardio-protection as ADF with a low-fat diet. Metabolism. 2013;62:137–143. doi: 10.1016/j.metabol.2012.07.002. [DOI] [PubMed] [Google Scholar]
- Mifflin MD, St, Jeor ST, Hill LA, Scott BJ, Daugherty SA, Koh YO. A new predictive equation for resting energy expenditure in healthy individuals. Am J Clin Nutr. 1990;51:241–247. doi: 10.1093/ajcn/51.2.241. [DOI] [PubMed] [Google Scholar]
- Flint A, Raben A, Blundell JE, Astrup A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int J Obes Relat Metab Disord. 2000;24:38–48. doi: 10.1038/sj.ijo.0801083. [DOI] [PubMed] [Google Scholar]
- Khoo J, Piantadosi C, Duncan R, Worthley SG, Jenkins A, Noakes M, et al. Comparing effects of a low-energy diet and a high-protein low-fat diet on sexual and endothelial function, urinary tract symptoms, and inflammation in obese diabetic men. J Sex Med. 2011;8:2868–2875. doi: 10.1111/j.1743-6109.2011.02417.x. [DOI] [PubMed] [Google Scholar]
- Phillips SA, Jurva JW, Syed AQ, Syed AQ, Kulinski JP, Pleuss J, et al. Benefit of low-fat over low-carbohydrate diet on endothelial health in obesity. Hypertension. 2008;51:376–382. doi: 10.1161/HYPERTENSIONAHA.107.101824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volek JS, Ballard KD, Silvestre R. Effects of dietary carbohydrate restriction versus low-fat diet on flow-mediated dilation. Metabolism. 2009;58:1769–1777. doi: 10.1016/j.metabol.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Keogh JB, Brinkworth GD, Clifton PM. Effects of weight loss on a low-carbohydrate diet on flow-mediated dilatation, adhesion molecules and adiponectin. Br J Nutr. 2007;98:852–859. doi: 10.1017/S0007114507747815. [DOI] [PubMed] [Google Scholar]
- Keogh JB, Brinkworth GD, Noakes M, Belobrajdic DP, Buckley JD, Clifton PM. Effects of weight loss from a very-low-carbohydrate diet on endothelial function and markers of cardiovascular disease risk in subjects with abdominal obesity. Am J Clin Nutr. 2008;87:567–576. doi: 10.1093/ajcn/87.3.567. [DOI] [PubMed] [Google Scholar]
- Nicholls SJ, Lundman P, Harmer JA. Consumption of saturated fat impairs the anti-inflammatory properties of high-density lipoproteins and endothelial function. J Am Coll Cardiol. 2006;48:715–720. doi: 10.1016/j.jacc.2006.04.080. [DOI] [PubMed] [Google Scholar]
- Hopkins ND, Green DJ, Tinken TM, Sutton L, McWhannell N, Thijssen DH, et al. Does conduit artery diameter vary according to the anthropometric characteristics of children or men. Am J Physiol Heart Circ Physiol. 2009;297:2182–2187. doi: 10.1152/ajpheart.00228.2009. [DOI] [PubMed] [Google Scholar]
- Chen H, Montagnani M, Funahashi T, Shimomura I, Quon MJ. Adiponectin stimulates production of nitric oxide in vascular endothelial cells. J Biol Chem. 2003;278:45021–45026. doi: 10.1074/jbc.M307878200. [DOI] [PubMed] [Google Scholar]
- Korda M, Kubant R, Patton S, Malinski T. Leptin-induced endothelial dysfunction in obesity. Am J Physiol Heart Circ Physiol. 2008;295:1514–1521. doi: 10.1152/ajpheart.00479.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varady KA, Bhutani S, Klempel MC, Phillips SA. Improvements in vascular health by a low-fat diet, but not a high-fat diet, are mediated by changes in adipocyte biology. Nutr J. 2011;10:8. doi: 10.1186/1475-2891-10-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black MA, Cable NT, Thijssen DH, Green DJ. Impact of age, sex, and exercise on brachial artery flow-mediated dilatation. Am J Physiol Heart Circ Physiol. 2009;297:1109–1116. doi: 10.1152/ajpheart.00226.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]