Abstract
Objective:
Epidemiological studies have repeatedly investigated the association between sleep duration and metabolic syndrome. However, the results have been inconsistent. This meta-analysis aimed to summarize the current evidence from cross-sectional and prospective cohort studies that evaluated this.
Data sources:
Relevant studies were identified by systematically searching the PubMed, Cochrane CENTRAL, EMBASE and PsycINFO databases through November 2012 without language restriction.
Study selection:
We identified 12 cross-sectional studies with 76 027 participants including 14 404 cases of metabolic syndrome, and 3 cohort studies with 2055 participants and 283 incident cases of metabolic syndrome.
Results:
For short sleep durations (<5 to 6 h), the odds ratios (OR) was 1.27 (95% confidence interval (CI)=1.10–1.48, I2=75.5%) in the 12 cross-sectional studies and 1.62 (95% CI=0.74–3.55, I2=71.4%) in the 3 cohort studies; for long sleep durations (>8 to 10 h), the OR was 1.23 (95% CI=1.02–1.49, I2=75.8%) in the 11 cross-sectional studies and 1.62 (95% CI=0.86–3.04, I2=0.0%) in the 2 cohort studies.
Conclusions:
Short and long sleep durations are risky behaviors for increasing the risk of metabolic syndrome and thus have important public health implications, as sleep habits are amenable to behavioral interventions. The available data are sparse, and further studies, especially longitudinal studies, are needed to facilitate a better understanding of these associations.
Keywords: sleep duration, metabolic syndrome, epidemiological study, systematic review, meta-analysis
Introduction
The metabolic syndrome is characterized by abdominal adiposity, dyslipidemia, elevated glucose and blood pressure.1 The metabolic syndrome is strongly linked to cardiovascular events,2 cancer3 and mortality.4 Given that the metabolic syndrome is becoming a worldwide pandemic, with prevalence rates between 20 and 30% among the adult population,5 the identification of modifiable risk factors associated with the development of metabolic syndrome is important to public health.
Sleep is a basic human need; it takes up more time in a day than any other activity. Too short or too long a duration of habitual sleep are currently thought to be important lifestyle risk factors for such metabolic diseases such as diabetes,6, 7, 8 obesity8, 9, 10 and cardiovascular disease, (and hypertension in particular11, 12) and sleep duration may also be a significant predictor of all-cause mortality in prospective population studies.13, 14
Several epidemiological studies have been conducted to investigate the association between length of sleep and metabolic syndrome, with inconsistent results. A number of studies in adults have demonstrated an association between short sleep duration and metabolic syndrome.15, 16, 17, 18, 19, 20, 21 Conversely, some of these studies also showed that long sleep duration was associated with metabolic syndrome.22, 23 To our knowledge, there has been no published systematic literature review that has characterized the magnitude of these associations. Therefore, we have summarized published data from cross-sectional and prospective cohort studies, and performed a meta-analysis to obtain a quantitative estimate of the risk.
Materials and methods
We planned, conducted and reported this systematic review according to widely accepted standards of quality for reporting meta-analyses of observational studies in epidemiology (Supplementary Table 1).24
Literature search
A master's level medical librarian with experience in systematic reviews participated in designing the search strategy. We searched PubMed, Cochrane CENTRAL, PsycINFO and EMBASE via Elsevier from the date of inception until 26 November 2012. A PubMed search for studies on sleep and metabolic syndrome was conducted without restrictions by combining synonymous or related search terms for sleep and metabolic syndrome. The keywords used in the PubMed search were converted to search tags for Cochrane CENTRAL, PsycINFO and EMBASE (Supplementary Table 2). In addition, manual searches of the bibliographies of the relevant articles were performed to identify additional studies.
Study selection
Several criteria were used to identify relevant studies for the meta-analysis. The studies included had an observational design, including cross-sectional studies and cohort studies, and were conducted in human adults. The exposure of interest was sleep duration. The outcome of interest was metabolic syndrome. Finally, the adjusted relative risk estimates (odds ratios [OR] in cross-sectional studies) and their corresponding 95% confidence intervals (CI), or sufficient data to calculate these values, were reported. We selected full-length articles without language restriction. Disagreements between the reviewers were resolved by consensus. We also checked the reference lists of included publications using a cross-reference table.
Data extraction
Two reviewers (J-SY and C-WS) independently extracted the data from original reports. The adjusted risk estimates that reflected the most comprehensive control were extracted to avoid potential confounding variables. The following predictors of sleep duration were extracted: study characteristics (study name, authors, publication year, country of data collection, number of participants and follow-up years for cohort studies), participants' characteristics (age range or mean age and sex) and metabolic syndrome measures. The methodological quality of the eligible non-randomized studies reviewed in meta-analyses was assessed according to the seven domains proposed as being fundamental to the methodological quality of the included studies (Supplementary Table 3).25, 26
Statistical analysis
We used adjusted relative risk or OR with the corresponding 95% CI for the meta-analysis. If OR estimates for an outcome were reported in a study, we selected the OR that was adjusted for most covariates and that was derived by using data from the most subjects in the study. Pooled OR estimates were obtained using a random-effect model. For consistency, we chose the group that slept 7 h per day as the reference category. When the reference category differed in each study, we used variances in the independent observations to obtain the CI of OR for each sleep category. When the variables X and Y were considered, the variance was as follows:  or, in another formula:
or, in another formula:  . This correlation coefficient could only be assessed within the studies in which all individual data were presented. By comparing the variance with the reference sleep duration category, we estimated the pooled risk and 95% CI of metabolic syndrome for the short- and long-sleep categories separately. For the meta-analysis, the statistical heterogeneity between the studies was assessed using Q statistics.27 For the Q statistics, heterogeneity was considered to be present if P<0.1. We defined low, moderate and high heterogeneity as I2 values of 25, 50 and 75%, respectively. Publication bias was evaluated using Egger's test and Begg's test. In the presence of publication bias, the P-values for Egger's test and Begg's test are less than 0.05. Subgroup meta-analyses were conducted according to the study design (cross-sectional study or cohort study), the different methods used to measure sleep duration, the definition of metabolic syndrome used and the subject's gender, age and geographic region. We also performed a two-stage random-effects dose-risk meta-analysis to examine a nonlinear dose–response relationship between length of sleep and metabolic syndrome. After modeling the length of sleep using restricted cubic splines with three knots at fixed percentiles (10, 50 and 90%) of the distribution,28, 29, 30 we used a generalized least-squares method31 and a multivariate maximum likelihood method to estimate a summary of nonlinear dose-risk relationship, taking into account random effects.32 All statistical analyses were performed using Stata software Version 12.0 (Stata Corp, College Station, TX, USA).
. This correlation coefficient could only be assessed within the studies in which all individual data were presented. By comparing the variance with the reference sleep duration category, we estimated the pooled risk and 95% CI of metabolic syndrome for the short- and long-sleep categories separately. For the meta-analysis, the statistical heterogeneity between the studies was assessed using Q statistics.27 For the Q statistics, heterogeneity was considered to be present if P<0.1. We defined low, moderate and high heterogeneity as I2 values of 25, 50 and 75%, respectively. Publication bias was evaluated using Egger's test and Begg's test. In the presence of publication bias, the P-values for Egger's test and Begg's test are less than 0.05. Subgroup meta-analyses were conducted according to the study design (cross-sectional study or cohort study), the different methods used to measure sleep duration, the definition of metabolic syndrome used and the subject's gender, age and geographic region. We also performed a two-stage random-effects dose-risk meta-analysis to examine a nonlinear dose–response relationship between length of sleep and metabolic syndrome. After modeling the length of sleep using restricted cubic splines with three knots at fixed percentiles (10, 50 and 90%) of the distribution,28, 29, 30 we used a generalized least-squares method31 and a multivariate maximum likelihood method to estimate a summary of nonlinear dose-risk relationship, taking into account random effects.32 All statistical analyses were performed using Stata software Version 12.0 (Stata Corp, College Station, TX, USA).
Results
Literature searches and study selection
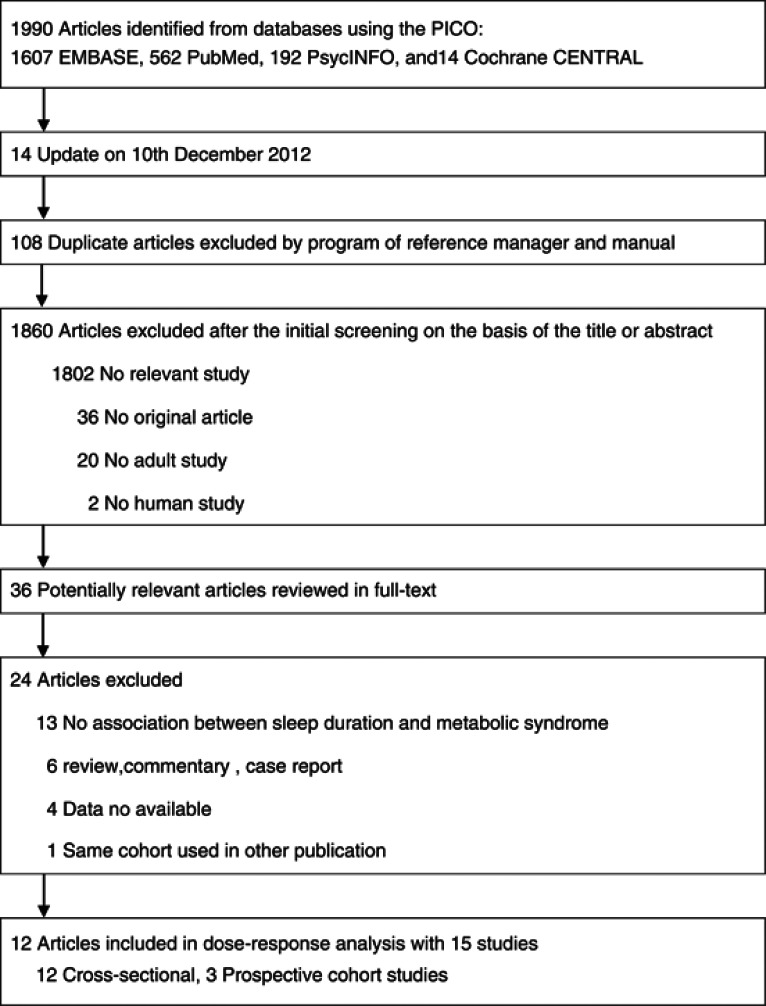
Figure 1 shows a flow diagram of the procedure used to identify the relevant studies. Briefly, we identified 36 potentially relevant articles on sleep duration in relation to metabolic syndrome after an initial screening of titles and abstracts. After we examined the 36 assembled articles, 24 articles were excluded (Supplementary Table 4). Finally, we identified 12 articles including 15 studies that investigated the association between sleep duration and metabolic syndrome risk; 3 articles reported separate results for stratification by gender.15, 20, 23 No additional studies were identified via cross-referencing.
Figure 1.
Flow diagram for search strategy and study selection process.
Study characteristics
Most of studies used multivariate logistic regression to adjust for potential confounders. The 12 cross-sectional17, 19, 20, 21, 22, 23, 33, 34, 35, 36 studies, with 76 027 participants, including 14 404 metabolic syndrome patients and the three cohort15, 18 studies, with 2055 participants, are represented in Table 1. A total of 283 metabolic syndrome cases occurred during follow-up in the three cohort studies. Three studies23, 34, 35 defined metabolic syndrome using the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (ATP-III).1 Four studies15, 20, 22, 33 assessed metabolic syndrome using the modified NCEP ATP-III. Two studies19, 36 used the American Heart Association/National Heart Lung and Blood Institute's (AHA/NHLBI) criteria.37 One study21 used the modified criteria of the AHA/NHLBI and assessed abdominal obesity according to the World Health Organization's definition. Two studies17, 18 used the Japanese criteria for metabolic syndrome. Eight studies17, 18, 19, 20, 21, 23, 33, 34 assessed sleep duration using a questionnaire and four studies15, 22, 35, 36 used an interview. Of the included studies, five were conducted in men,15, 18, 20, 23, 35 four in women15, 20, 23, 35 and eight in both genders;17, 19, 21, 22, 33, 34, 35, 36 seven were conducted in Asia,15, 17, 18, 20, 22, 33, 35 four in the United States19, 21, 34, 36 and one in Europe.23 All of the studies were published in the 2000s. The study periods ranged from 1 to 7 years. The patients' age ranged from ⩾18 to ⩾50 years. The study quality was high in 10 of the 15 included studies (3 cohort and 12 cross-sectional studies).
Table 1. Characteristics of cross-sectional and cohort studies included in the meta-analysis.
| Author study name | Country observation period | No. of participants | No. of cases | Age range mean (s.d.) | Gender population | Metabolic syndrome assessment | Sleeping assessment | Sleep (h) | Odds ratio (95% CI) | Adjusted covariates |
|---|---|---|---|---|---|---|---|---|---|---|
| Cross-sectional studies | ||||||||||
| Santos et al.23 | Portugal 1999–2003 | 832 | 96 | 18–92 | Men | NCEP ATP-III | Questionnaire | ⩽6 | 1.40 (0.76–2.60) | 1, 3, 4, 5 |
| 216 | 7 | 1.00 (Reference) | ||||||||
| 214 | 8 | 1.10 (0.73–1.70) | ||||||||
| 227 | ⩾9 | 1.50 (0.90–2.60) | ||||||||
| 1332 | 153 | Women | ⩽6 | 0.92 (0.55–1.50) | ||||||
| 270 | 7 | 1.00 (Reference) | ||||||||
| 401 | 8 | 1.20 (0.80–1.70) | ||||||||
| 388 | ⩾9 | 2.00 (1.30–3.00) | ||||||||
| Choi et al.33 KNHNS, 2001 | Korea 2001 | 633 | 217 | 44.1 (0.4) | Both | Modified NCEP ATP-III | Questionnaire | ⩽5 | 1.20 (0.87–1.60) | 1, 2, 3, 4, 5, 6, 12 |
| 1056 | 293 | 6 | 1.10 (0.85–1.30) | |||||||
| 1182 | 274 | 7 | 1.00 (Reference) | |||||||
| 1056 | 293 | 8 | 1.30 (1.00–1.70) | |||||||
| 296 | 94 | ⩾9 | 1.70 (1.20–2.50) | |||||||
| Hall et al.36 AHAB | USA | 187 | 58 | 30–54 | Both | AHA/NHLBI | Interview | <6 | 1.80 (1.20–2.80) | 1, 2, 4, 8, 9, 10, 14 |
| 402 | 101 | 6–6.99 | 1.50 (1.10–2.10) | |||||||
| 525 | 100 | 7–8 | 1.00 (Reference) | |||||||
| 100 | 25 | >8 | 1.80 (1.00–3.10) | |||||||
| Not taking antihypertensive medication | <6 | 1.80 (1.10–2.70) | ||||||||
| 6–6.99 | 1.50 (1.00–2.10) | |||||||||
| 7–8 | 1.00 (Reference) | |||||||||
| >8 | 1.60 (0.91–3.00) | |||||||||
| Arora et al.22 GBCS | China | 1142 | 329 | 50–96 | Both | Modified NCEP ATP-III | Interview | <6 | 0.97 (0.88–1.10) | 1, 2, 3, 4, 5, 8, 11 |
| 2020 | 570 | 6–7 | 1.00 (0.93–1.10) | |||||||
| 2303 | 603 | 7–8 | 1.00 (Reference) | |||||||
| 1995 | 575 | 8–9 | 1.20 (1.10–1.30) | |||||||
| 762 | 226 | ⩾9 | 1.20 (1.10–1.30) | |||||||
| Healthy | <6 | 0.93 (0.84–1.00) | ||||||||
| 6–7 | 0.98 (0.90–1.10) | |||||||||
| 7–8 | 1.00 (Reference) | |||||||||
| 8–9 | 1.10 (1.10–1.20) | |||||||||
| ⩾9 | 1.20 (1.10–1.30) | |||||||||
| 51–61 | Middle age | <6 | 1.10 (0.91–1.20) | |||||||
| 6–7 | 1.00 (0.92–1.10) | |||||||||
| 7–8 | 1.00 (Reference) | |||||||||
| 8–9 | 1.10 (1.00–1.30) | |||||||||
| ⩾9 | 1.30 (1.20–1.50) | |||||||||
| >61 | Old age | < 6 | 0.93 (0.83–1.10) | |||||||
| 6–7 | 0.98 (0.89–1.10) | |||||||||
| 7–8 | 1.00 (Reference) | |||||||||
| 8–9 | 1.20 (1.10–1.30) | |||||||||
| ⩾9 | 1.10 (0.95–1.30) | |||||||||
| Kobayashi et al.17 | Japan 2008 | 7295 | 641 | 44.8 (12.8) | Both | JASSO | Questionnaire | <6 | 1.40 (1.20–1.60) | 1, 2, 4, 6, 13 |
| 11355 | 905 | 6–6.99 | 1.10 (0.95–1.30) | |||||||
| 6732 | 592 | 7–7.99 | 1.00 (Reference) | |||||||
| 2410 | 233 | ⩾8 | 0.98 (0.83–1.20) | |||||||
| Najafian et al.35 IHHP | Iran 1999–2006 | 1447 | 485 | 38.89 (14.93) | Both | NCEP ATP-III | Interview | ⩽5 | 1.50 (1.30–1.70) | 1, 2 |
| 2336 | 575 | 6 | 1.20 (1.10–1.40) | |||||||
| 7622 | 1654 | 7–8 | 1.00 (Reference) | |||||||
| 1089 | 223 | ⩾9 | 0.79 (0.68–0.94) | |||||||
| 5976 | Men | ⩽5 | 1.30 (0.99–1.60) | 1 | ||||||
| 6 | 1.10 (0.89–1.30) | |||||||||
| 7–8 | 1.00 (Reference) | |||||||||
| ⩾9 | 0.77 (0.55–1.10) | |||||||||
| 6320 | Women | ⩽5 | 1.70 (1.40–2.00) | |||||||
| 6 | 1.30 (1.10–1.50) | |||||||||
| 7–8 | 1.00 (Reference) | |||||||||
| ⩾9 | 0.81 (0.67–0.98) | |||||||||
| 10699 | <60 | Both | ⩽5 | 1.70 (1.50–2.00) | 2 | |||||
| 6 | 1.20 (1.10–1.40) | |||||||||
| 7–8 | 1.00 (Reference) | |||||||||
| ⩾9 | 0.84 (0.70–1.00) | |||||||||
| 1597 | >60 | Both | ⩽5 | 1.10 (0.84–1.40) | ||||||
| 6 | 1.10 (0.79–1.40) | |||||||||
| 7–8 | 1.00 (Reference) | |||||||||
| ⩾9 | 0.61 (0.40–0.92) | |||||||||
| Sabanayagam et al.19 NHANES | USA 2005–2008 | 935 | 377 | 44.63 (0.46) | Both | AHA-NHLBI | Questionnaire | ⩽5 | 1.20 (0.98–1.60) | 1, 2, 3, 4, 5, 8, 10 |
| 1412 | 548 | 6 | 1.10 (0.93–1.30) | |||||||
| 1777 | 650 | 7 | 1.00 (Reference) | |||||||
| 1621 | 579 | 8 | 0.92 (0.76–1.10) | |||||||
| 377 | 135 | ⩾9 | 0.97 (0.69–1.40) | |||||||
| McCanlies et al.34 | USA | 28 | 7 | 39.61 | Both | NCEP ATP-III | Questionnaire | < 6 | 2.30 (0.81–6.50) | 1, 2, 3, 4 |
| 70 | 7 | Police | ⩾6 | 1.00 (Reference) | ||||||
| Wu et al.20 | Taiwan | 954 | 198 | 47.1 (12.0) | Men | Modified NCEP ATP-III | Questionnaire | <6 | 1.30 (1.00–1.60) | 1, 3, 4, 5, 7 |
| 2006–2009 | 3202 | 535 | 44.8 (11.1) | 6–8 | 1.00 (Reference) | |||||
| 142 | 27 | 52.3 (12.2) | > 8 | 1.40 (0.82–2.50) | ||||||
| 794 | 94 | Women | < 6 | 1.00 (0.72–1.50) | ||||||
| 1909 | 172 | 6–8 | 1.00 (Reference) | |||||||
| 99 | 9 | > 8 | 0.90 (0.32–2.50) | |||||||
| Yoo et al.21 | USA | 32 | 10 | 22–60 | Both | Modified AHA-NHLBI | Questionnaire | ⩽6 | 2.30 (0.71–7.50) | 1, 2, 4, 8, 17 |
| 53 | 13 | Police | >6–<8 | 1.00 (Reference) | ||||||
| 21 | 12 | ⩾8 | 1.90 (1.30–18.0) | |||||||
| Cohort studies | ||||||||||
| Choi et al.15 KGRC | Korea 2005–2009 | 27 | 5 | 40–70 | Men | Modified NCEP ATP-III | Interview | < 6 | 0.62 (0.24–1.60) | 1, 4, 5, 7, 8 |
| 209 | 49 | 6–7.9 | 1.00 (Reference) | |||||||
| 132 | 23 | 8–8.9 | 0.74 (0.44–1.20) | |||||||
| 18 | 5 | ≥10 | 1.60 (0.61–4.00) | |||||||
| 68 | 20 | Women | <6 | 1.80 (1.10–3.00) | 1, 4, 5, 7, 8, 15 | |||||
| 403 | 61 | 6–7.9 | 1.00 (Reference) | |||||||
| 226 | 34 | 8–8.9 | 0.91 (0.59–1.40) | |||||||
| 24 | 7 | ⩾10 | 1.70 (0.71–3.90) | |||||||
| Otsuka et al.18 | Japan 2005–2009 | 120 | 20 | 35–63 | Men | The Japanese criteria | Questionnaire | ⩽5 | 3.20 (1.50–6.60) | 1, 4, 5, 6, 16 |
| 559 | 46 | 5–6 | 1.80 (0.96–3.50) | |||||||
| 269 | 13 | >6 | 1.00 (Reference) | |||||||
Abbrevations: AHA-NHLBI, American Heart Association/National Heart Lung and Blood Institute; AHAB, Adult Health and Behavior; CES-D, Center for Epidemiological Studies Depression Scale; CI, confidence interval; GBCS, The Guangzhou Biobank Cohort Study; IHHP, Isfahan Healthy Heart Program; JASSO, Japan Society for the Study of Obesity; KGRC, The Korean Genomic Rural Cohort; KNHNS, Korean National Health and Nutrition Survey; NCEP ATP-III, National Cholesterol Education Adult Treatment Panel III; PSQI, Pittsburgh Sleep Quality Index; PSS, Perceived Stress Scale; 1, age; 2, sex; 3, education; 4, smoke; 5, alcohol; 6, exercise; 7, body mass index; 8, physical activity; 9, race; 10, depression; 11, diagnosed mental illness, insomnia, use of hypnotics, daytime sleepiness, snoring, mean systolic pressure, glucose, total cholesterol and triglycerides; 12, family history of hypertension or diabetes, residential area and monthly income; 13, myocardial infarction and cerebral infarction; 14, low-density lipoprotein cholesterol; 15, menopause; 16, frequency of vegetable intake, frequency of oily food intake and frequency of salty food intake; 17, burnout, Center for Epidemiological Studies Depression Scale and Perceived Stress Scale.
Short duration of sleep
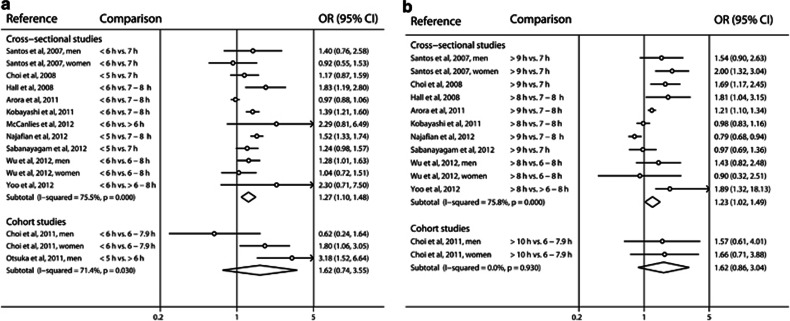
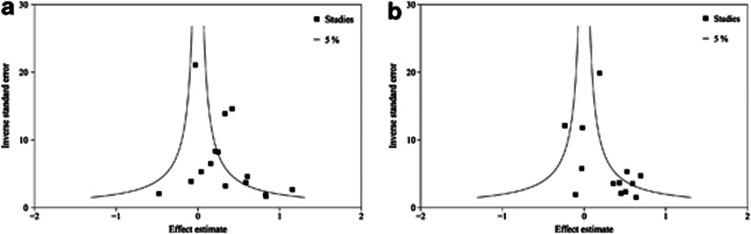
Short duration of sleep was significantly associated with a greater risk of developing metabolic syndrome in four of the twelve cross-sectional studies and two of the three cohort studies. The pooled OR between short sleep and metabolic syndrome was 1.27 (95% CI=1.10–1.48, I2=75.5%) in the 12 cross-sectional studies and 1.62 (95% CI=0.74–3.55, I2=71.4%) in the 3 cohort studies (Figure 2). Significant heterogeneity was observed (P<0.1). No publication bias was detected with Egger's test (P=0.17) or Begg's test (P=0.92) (Figure 3).
Figure 2.
Forest plots of the risk of metabolic syndrome associated with (a) short duration of sleep compared with the reference group, and (b) long duration of sleep compared with the reference group. The results are expressed as odds ratio (relative risk in cohort studies) and 95% confidence intervals.
Figure 3.
Inverse funnel plot with 95% CIs of the odds ratio of metabolic syndrome according to (a) short duration of sleep compared with the reference group (Egger's test, P=0.17; Begg's test, P=0.92), and (b) long duration of sleep compared with the reference group (Egger's test, P=1.33; Begg's test, P=0.95).
Subgroup analyses of the cross-sectional studies are shown in Table 2. There were significant differences in the mean age, cohort-based group, definition of metabolic syndrome, quality of study and sleeping measure (P for heterogeneity <0.1). No significant group difference was found for gender or location.
Table 2. Subgroup meta-analyses of cross-sectional studies to explore sources of heterogeneity.
| Subgroups |
Short sleep |
Long sleep |
||
|---|---|---|---|---|
| No.of studies | OR (95% CI) P for heterogeneity | No. of studies | OR (95% CI) P for heterogeneity | |
| Gender | ||||
| Men | 3 | 1.27 (1.08, 1.49) | 3 | 1.03 (0.80, 1.33) |
| Women | 3 | 1.49 (1.29, 1.72) | 3 | 0.94 (0.80, 1.12) |
| P=0.156 | P=0.588 | |||
| Mean age (years) | ||||
| aMiddle | 5 | 1.34 (1.21, 1.48) | 4 | 1.15 (1.04, 1.28) |
| bElderly | 2 | 0.96 (0.86, 1.06) | 2 | 1.03 (0.90, 1.18) |
| P<0.1 | P=0.210 | |||
| Location | ||||
| Asia | 6 | 1.18 (1.11, 1.26) | 6 | 1.08 (1.01, 1.16) |
| Europe | 2 | 1.09 (0.74, 1.61) | 2 | 1.81 (1.30, 2.52) |
| USA | 4 | 1.40 (1.15, 1.72) | 3 | 1.18 (0.89, 1.56) |
| P=0.237 | P<0.1 | |||
| Cohorts-based | ||||
| Community | 6 | 1.15 (1.07, 1.23) | 6 | 1.12 (1.03, 1.21) |
| Hospital | 3 | 1.33 (1.18, 1.49) | 3 | 1.01 (0.86, 1.18) |
| P<0.1 | P=0.263 | |||
| Measure of sleeping | ||||
| Interview | 3 | 1.14 (1.06, 1.23) | 3 | 1.09 (1.00, 1.19) |
| Questionnaire | 9 | 1.29 (1.18, 1.42) | 8 | 1.16 (1.03, 1.31) |
| P<0.1 | P<0.1 | |||
| Metabolic syndrome | ||||
| NCEP ATP-III | 4 | 1.48 (1.30, 1.68) | 4 | 0.93 (0.80, 1.07) |
| Modified NCEP ATP-III | 4 | 1.02 (0.94, 1.11) | 4 | 1.24 (1.13, 1.36) |
| AHA-NHLBI | 2 | 1.36 (1.10, 1.67) | 2 | 1.15 (0.86, 1.54) |
| Modified AHA-NHLBI | 1 | 2.30 (0.71, 7.50) | 1 | 1.89 (0.51, 7.00) |
| JASSO | 1 | 1.39 (1.21, 1.60) | 1 | 0.98 (0.83, 1.16) |
| P<0.1 | P<0.1 | |||
| Quality of study | ||||
| High | 7 | 1.06 (0.99, 1.15) | 6 | 1.23 (1.13, 1.34) |
| Low | 5 | 1.44 (1.31, 1.58) | 5 | 0.96 (0.86, 1.07) |
| P<0.1 | P<0.1 | |||
Abbrevations: AHA-NHLBI, American Heart Association/National Heart Lung and Blood Institute; CI, confidence interval; JASSO, Japan Society for the Study of Obesity; NCEP ATP-III, National Cholesterol Education Adult Treatment Panel III; OR, odds ratios.
Middle, <60 or ⩽61.
Elderly, >60 or ⩾61.
Long duration of sleep
Long duration of sleep was associated with a greater risk of developing metabolic syndrome in five of the eleven cross-sectional studies and none of the two cohort studies. The pooled OR between long sleep and metabolic syndrome was 1.23 (95% CI=1.02–1.49, I2=75.8%) in the 11 cross-sectional studies and 1.62 (95% CI=0.86–3.04, I2=0.0%) in the 2 cohort studies. Significant heterogeneity was observed between the 11 cross-sectional studies (P<0.1). No publication bias was detected with Egger's test (P=1.33) or Begg's test (P=0.95) (Figure 3).
Subgroup analyses of the cross-sectional studies are shown in Table 2. There were significant differences in location, definition of metabolic syndrome, quality of study and sleeping measure (P for heterogeneity <0.1). No significant group difference was found for gender, age or cohort-based group.
Dose–response relationship
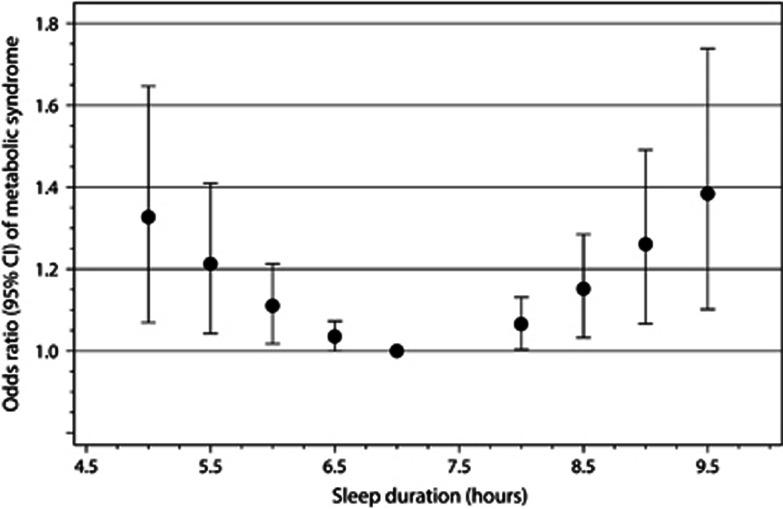
When we assessed the dose–response relationship between sleep duration and metabolic syndrome, we found some evidence of a statistically significant departure from linearity (P<0.001; Figure 4). Among the shorter and longer sleepers, the risk of metabolic syndrome might increase gradually compared with individuals who report sleeping 7 h per day. Compared with the reference level, the combined odds ratios of metabolic syndrome were 1.33 (95% CI=1.07–1.65) for ⩽5 h per day, 1.21 (95% CI=1.04–1.41) for 5.5 h per day, 1.11 (95% CI=1.02–1.21) for 6 h per day, 1.04 (95% CI=1.00–1.07) for 6.5 h per day, 1.07 (95% CI=1.00–1.13) for 8 h per day, 1.15 (95% CI=1.03–1.28) for 8.5 h per day, 1.26 (95% CI=1.07–1.49) for 9 h per day and 1.38 (95% CI=1.10–1.74) for ⩾9.5 h per day in the eight cross-sectional studies.17, 19, 22, 23, 33, 35, 36 There was significant between-study heterogeneity among study-specific trends defined by the coefficient of the first and second spline transformations of sleep duration (P<0.1).
Figure 4.
The odds ratio (filled circle) with 95% CI (solid longitudinal line) for the dose–response relationship between sleep duration and the risk of metabolic syndrome in the cross-sectional studies. The reference duration of 7 h per day was used to estimate all odds ratios. The eight cross-sectional studies (out of 12) with at least four levels of sleep categories were used in a restricted cubic spline random-effects meta-analysis.
Sensitivity analyses in the cross-sectional studies
We included five studies of healthy populations and participants not taking antihypertensive medication for our sensitivity analyses.17, 20, 22, 36 The sensitivity meta-analyses yield somewhat weakened summary ORs of 1.22 (95% CI=0.96–1.54) for short sleep durations and 1.14 (95% CI=0.98–1.32) for long sleep durations. There was heterogeneity for short sleep (I2=33%, P=0.20) and long sleep (I2=84.6%, P<0.1). We also excluded two studies from our sensitivity analyses, as they reported the association between sleep duration and metabolic syndrome in police officers. The sensitivity meta-analyses yielded nearly identical summary ORs of 1.25 (95% CI=1.07–1.46; I2=78.6%) for short sleep and 1.22 (95% CI=1.22–1.48; I2=77.9%) for long sleep.
Discussion
To our knowledge, this is the first quantitative systematic review of observational studies investigating the effect of sleep duration on the risk of metabolic syndrome using data from cross-sectional and cohort studies. This study shows a significant increased risk of metabolic syndrome on either end of the distribution of sleep duration in the cross-sectional studies, but not in the cohort studies. Pooled analyses of cross-sectional studies indicate that short sleepers have a greater risk of metabolic syndrome compared with those who sleep 7–8 h per night. Longer sleepers also show an increased risk for metabolic syndrome, confirming the presence of a U-shaped association in the cross-sectional studies. There was some heterogeneity between studies, no publication bias and no differences between men and women. Furthermore, compared with the 7-h sleep duration, U-shaped associations between sleep duration and the risk of metabolic syndrome were observed in a dose–response meta-analysis of eight cross-sectional studies. This complementary investigation may strengthen the plausibility of a causal association, and allow us to draw an overview of the nonlinear relationship between sleep duration and metabolic syndrome risk.
Causal mechanisms relating the short duration of sleep to adverse health outcomes include changes in circulating levels of leptin and ghrelin, which in turn would increase appetite and calorie intake, reduce energy expenditure and impair glycemic control.38, 39 Increased cortisol secretion and altered growth hormone metabolism have also been implicated.40 Low-grade inflammation is also activated during short sleep.41 Conversely, the association between a long duration of sleep and metabolic syndrome may be explained by residual confounding factors and comorbidity. Unrecognized confounders such as sleep fragmentation, fatigue and depression42 could lead to both metabolic syndrome and an increased need for sleep.43 Long sleepers have less waking time to undertake physical activity, which may contribute to this association.44 Lifestyle interventions that aim to reduce weight and physical activity may ameliorate the risk of diabetes in long sleepers.45 Furthermore, obesity itself is regarded as a chronic inflammatory condition. The elevated levels of proinflammatory cytokines have been considered as factors that contribute to increased sleep durations,46 which promotes insulin resistance.
There are several possible reasons for the risk of metabolic syndrome according to sleep duration. Hypothalamic–pituitary–adrenal hyperactivity has a role in the pathogenesis of the metabolic syndrome,47 and activation of the hypothalamic–pituitary–adrenal axis can lead to sleeplessness.14 Sleep fragmentation or restriction leads to insulin resistance,48 which appears to have a key role in the pathophysiology of metabolic syndrome.49 In addition, longer sleep could be associated with circadian and hormonal alternations.50 A previous report suggested that the nocturnal intervals of high plasma melatonin levels, increasing cortisol levels, low body temperature and increasing sleepiness are longer in long sleepers (>9 h) than short sleepers (<6 h).51 However, the mechanisms that underline these associations are not fully understood. An investigation reporting an association between variants of the human CLOCK gene and sleep duration may prove fruitful in this regard.52 Some evidence has suggested that the covariation among sleep parameters and clusters of metabolic syndrome may be partially related to genetic influences. For example, disruptions of the core CLOCK genes that regulate endogenous circadian rhythmicity are linked to perturbations in glucose metabolism, adipocyte and vascular function,53 obesity and metabolic syndrome.54 Thus, it is possible that short and long sleep are linked to metabolic syndrome risk through different pathways.
There were significant differences in the relationship between sleep duration and metabolic syndrome in different groups based on age, location, type of cross-sectional study (community based or hospital based), sleep measure, quality of study and definition of metabolic syndrome. However, due to the limited number of studies within several groups, these results should be interpreted cautiously. For short duration of sleep, a significant 33% increase in metabolic syndrome was observed in hospital-based studies. Not surprisingly, hospital-based samples of patients suffering from disease are at a higher risk of developing metabolic syndrome, as metabolic syndrome itself is a cluster of risk factors of metabolic abnormalities, and short sleep duration is a risk behavior for the development of chronic disease, particularly stroke and cancer,55 which can strengthen the relationship between short sleep and metabolic syndrome.
We found that the association was stronger for both long and short sleep in cross-sectional studies that identified habitual sleep duration using questionnaires rather than interviews. One possible explanation is that the cross-sectional studies could contain sources of bias, such as recall bias. Therefore, overestimation or underestimation of sleep duration per night may be caused by a single self-reported survey item that may not capture the subject's actual amount of sleep per night. For short sleep, there was a significant association with middle age. This finding may be explained by the distribution of weekly averaged sleep duration. Sleep duration decreases steadily in both genders from the end of adolescence to the age of menopause. From that age on, sleep duration increases slightly with age.52 This distribution would implicitly allow the inclusion of many people with short sleep. Thus, the inclusion of only middle-aged people may have led to the stronger estimates.
Although our meta-analysis included only multivariable adjusted ORs, there is evidence of heterogeneity across the studies in both study designs. This heterogeneity may be attributable to differences in study design, sample size, analytic strategies, participant characteristics, the diagnostic criteria for metabolic syndrome, study quality and the measurement of sleep duration. To account for this heterogeneity, we used random-effects models of the meta-analyses, and conducted subgroup analyses using fixed-effects models; however, the results were not significantly changed.
This meta-analysis has several strengths and limitations. The primary strength is that this is the first meta-analysis that examines the relationship between sleep duration and metabolic syndrome based on a comprehensive literature search. In addition, a dose–response meta-analysis using restricted cubic splines revealed a U-shaped relationship between sleep duration and the risk of metabolic syndrome, despite the heterogeneous categorization of sleep duration in the individual studies. A limitation of this approach is that it depends on means, median or midpoints of the sleep-duration categories. The estimates of risk in this approach are therefore slightly less accurate than in the individual patient data meta-analyses. We found no publication bias in any of the analyses. Despite the calculation of risk estimates that reflected the greatest degrees of controlling for potential confounders, some limitations must be considered when interpreting the meta-analyses results. As it is impossible to completely adjust for general health status, residual confounders must always be considered while interpreting results from observational studies. Finally, a major limitation was the evidence of heterogeneity across the studies, especially that resulting from heterogeneous sleep-measure methods, quality of study and variations in the definition of metabolic syndrome. Thus, the results of this analysis should be interpreted cautiously.
In this systemic review and meta-analysis of epidemiological studies of the association between sleep duration and metabolic syndrome, both short sleepers and long sleepers are at risk of metabolic syndrome in cross-sectional studies, but not in cohort studies; this is consistent with the results from an analysis using nonlinear dose–response method in the cross-sectional studies. Therefore, short and long sleep durations are risky behaviors for increasing the risk of metabolic syndrome and thus have important public health implications, as sleep habits are amenable to behavioral interventions. However, the available data are sparse, and further studies, especially longitudinal studies, are needed to enable more in-depth analyses, more precise and stable estimates of the association and a better understanding of the potential role of sleep duration in metabolic syndrome risk. Further studies could also shed light on possible sources of heterogeneity across the studies included in this meta-analysis.
Acknowledgments
We thank Jeong So-Na for reviewing and editing this manuscript. This work was supported by research grants from the Catholic Medical Center Research Foundation in the 2012 program year.
Disclaimer
The funding sources had no role in the design or execution of the study; the collection, management, analysis or interpretation of the data; or the preparation, review or approval of the manuscript.
Author Contributions
Study concept and design: Ju, Choi. Acquirement of the data: Ju, Choi. Analysis and interpretation of the data: Ju. Drafting the manuscript: Ju. Critical revision of the manuscript for important intellectual content: Choi. Statistical analyses: Ju. Obtaining of funds: Choi. Providing of administrative, technical, or material support: Ju and Choi. Supervision of the study: Choi.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on the Nutrition & Diabetes website (http://www.nature.com/nutd)
Supplementary Material
References
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- Mottillo S, Filion KB, Genest J, Joseph L, Pilote L, Poirier P, et al. The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J Am Coll Cardiol. 2010;56:1113–1132. doi: 10.1016/j.jacc.2010.05.034. [DOI] [PubMed] [Google Scholar]
- Esposito K, Chiodini P, Colao A, Lenzi A, Giugliano D. Metabolic syndrome and risk of cancer: a systematic review and meta-analysis. Diabetes Care. 2012;35:2402–2411. doi: 10.2337/dc12-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SH, Liu Z, Ho SC. Metabolic syndrome and all-cause mortality: a meta-analysis of prospective cohort studies. Eur J Epidemiol. 2010;25:375–384. doi: 10.1007/s10654-010-9459-z. [DOI] [PubMed] [Google Scholar]
- Grundy SM. Metabolic syndrome pandemic. Arterioscler Thromb Vasc Biol. 2008;28:629–636. doi: 10.1161/ATVBAHA.107.151092. [DOI] [PubMed] [Google Scholar]
- Chaput JP, Despres JP, Bouchard C, Tremblay A. Association of sleep duration with type 2 diabetes and impaired glucose tolerance. Diabetologia. 2007;50:2298–2304. doi: 10.1007/s00125-007-0786-x. [DOI] [PubMed] [Google Scholar]
- Gottlieb DJ, Punjabi NM, Newman AB, Resnick HE, Redline S, Baldwin CM, et al. Association of sleep time with diabetes mellitus and impaired glucose tolerance. Arch Intern Med. 2005;165:863–867. doi: 10.1001/archinte.165.8.863. [DOI] [PubMed] [Google Scholar]
- Kim J, Kim HM, Kim KM, Kim DJ. The association of sleep duration and type 2 diabetes in Korean male adults with abdominal obesity: the Korean National Health and Nutrition Examination Survey 2005. Diabetes Res Clin Pract. 2009;86:e34–e36. doi: 10.1016/j.diabres.2009.06.027. [DOI] [PubMed] [Google Scholar]
- Hitze B, Bosy-Westphal A, Bielfeldt F, Settler U, Plachta-Danielzik S, Pfeuffer M, et al. Determinants and impact of sleep duration in children and adolescents: data of the Kiel Obesity Prevention Study. Eur J Clin Nutr. 2009;63:739–746. doi: 10.1038/ejcn.2008.41. [DOI] [PubMed] [Google Scholar]
- Park SE, Kim HM, Kim DH, Kim J, Cha BS, Kim DJ. The association between sleep duration and general and abdominal obesity in Koreans: data from the Korean National Health and Nutrition Examination Survey, 2001 and 2005. Obesity (Silver Spring) 2009;17:767–771. doi: 10.1038/oby.2008.586. [DOI] [PubMed] [Google Scholar]
- Cappuccio FP, Stranges S, Kandala NB, Miller MA, Taggart FM, Kumari M, et al. Gender-specific associations of short sleep duration with prevalent and incident hypertension: the Whitehall II Study. Hypertension. 2007;50:693–700. doi: 10.1161/HYPERTENSIONAHA.107.095471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardi C, Hedner J, Parati G. Sex and age differences in the relationship between sleep duration and hypertension. J Hypertens. 2010;28:883–886. doi: 10.1097/HJH.0b013e3283383d73. [DOI] [PubMed] [Google Scholar]
- Cappuccio FP, D'Elia L, Strazzullo P, Miller MA. Sleep duration and all-cause mortality: a systematic review and meta-analysis of prospective studies. Sleep. 2010;33:585–592. doi: 10.1093/sleep/33.5.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vgontzas AN, Liao D, Pejovic S, Calhoun S, Karataraki M, Basta M, et al. Insomnia with short sleep duration and mortality: the Penn State cohort. Sleep. 2010;33:1159–1164. doi: 10.1093/sleep/33.9.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JK, Kim MY, Kim JK, Park JK, Oh SS, Koh SB, et al. Association between short sleep duration and high incidence of metabolic syndrome in midlife women. Tohoku J Exp Med. 2011;225:187–193. doi: 10.1620/tjem.225.187. [DOI] [PubMed] [Google Scholar]
- Katano S, Nakamura Y, Nakamura A, Murakami Y, Tanaka T, Takebayashi T, et al. Relationship between sleep duration and clustering of metabolic syndrome diagnostic components. Diabetes Metab Syndr Obes. 2011;4:119–125. doi: 10.2147/DMSO.S16147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi D, Takahashi O, Deshpande GA, Shimbo T, Fukui T. Relation between metabolic syndrome and sleep duration in Japan: a large scale cross-sectional study. Intern Med. 2011;50:103–107. doi: 10.2169/internalmedicine.50.4317. [DOI] [PubMed] [Google Scholar]
- Otsuka T, Kawada T, Yanai M, Kitagawa Y, Kan H. (Incidence of metabolic syndrome and associated lifestyle factors in a worksite male population) Sangyo Eiseigaku Zasshi. 2011;53:78–86. doi: 10.1539/sangyoeisei.b10013. [DOI] [PubMed] [Google Scholar]
- Sabanayagam C, Zhang R, Shankar A. Markers of sleep-disordered breathing and metabolic syndrome in a multiethnic sample of US adults: Results from the National Health and Nutrition Examination Survey 2005-2008. Cardiol Res Pract. 2012;2012:630802. doi: 10.1155/2012/630802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu MC, Yang YC, Wu JS, Wang RH, Lu FH, Chang CJ. Short sleep duration associated with a higher prevalence of metabolic syndrome in an apparently healthy population. Prev Med. 2012;55:305–309. doi: 10.1016/j.ypmed.2012.07.013. [DOI] [PubMed] [Google Scholar]
- Yoo H, Franke WD. Sleep habits, mental health, and the metabolic syndrome in law enforcement officers. J Occup Environ Med. 2012;55:99–103. doi: 10.1097/JOM.0b013e31826e294c. [DOI] [PubMed] [Google Scholar]
- Arora T, Jiang CQ, Thomas GN, Lam KB, Zhang WS, Cheng KK, et al. Self-reported long total sleep duration is associated with metabolic syndrome: the Guangzhou Biobank Cohort Study. Diabetes Care. 2011;34:2317–2319. doi: 10.2337/dc11-0647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos AC, Ebrahim S, Barros H. Alcohol intake, smoking, sleeping hours, physical activity and the metabolic syndrome. Prev Med. 2007;44:328–334. doi: 10.1016/j.ypmed.2006.11.016. [DOI] [PubMed] [Google Scholar]
- Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- Panagioti M, Gooding PA, Tarrier N. A meta-analysis of the association between posttraumatic stress disorder and suicidality: the role of comorbid depression. Compr Psychiatry. 2012;53:915–930. doi: 10.1016/j.comppsych.2012.02.009. [DOI] [PubMed] [Google Scholar]
- Sanderson S, Tatt ID, Higgins JP. Tools for assessing quality and susceptibility to bias in observational studies in epidemiology: a systematic review and annotated bibliography. Int J Epidemiol. 2007;36:666–676. doi: 10.1093/ije/dym018. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989;8:551–561. doi: 10.1002/sim.4780080504. [DOI] [PubMed] [Google Scholar]
- Harrell FE, Jr., Lee KL, Pollock BG. Regression models in clinical studies: determining relationships between predictors and response. J Natl Cancer Inst. 1988;80:1198–1202. doi: 10.1093/jnci/80.15.1198. [DOI] [PubMed] [Google Scholar]
- Smith PL. Splines as a useful and convenient statistical tool. Am Stat. 1979;33:57–62. [Google Scholar]
- Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. 1992;135:1301–1309. doi: 10.1093/oxfordjournals.aje.a116237. [DOI] [PubMed] [Google Scholar]
- Orsini N, Bellocco R, Greenland S. Generalized least squares for trend estimation of summarized dose-response data. Stata J. 2006;6:40–57. [Google Scholar]
- Choi KM, Lee JS, Park HS, Baik SH, Choi DS, Kim SM. Relationship between sleep duration and the metabolic syndrome: Korean National Health and Nutrition Survey 2001. Int J Obes (Lond) 2008;32:1091–1097. doi: 10.1038/ijo.2008.62. [DOI] [PubMed] [Google Scholar]
- McCanlies EC, Slaven JE, Smith LM, Andrew ME, Charles LE, Burchfiel CM, et al. Metabolic syndrome and sleep duration in police officers. Work. 2012;43:133–139. doi: 10.3233/WOR-2012-1399. [DOI] [PubMed] [Google Scholar]
- Najafian J, Toghianifar N, Mohammadifard N, Nouri F. Association between sleep duration and metabolic syndrome in a population-based study: Isfahan Healthy Heart Program. J Res Med Sci. 2011;16:801–806. [PMC free article] [PubMed] [Google Scholar]
- Hall MH, Muldoon MF, Jennings JR, Buysse DJ, Flory JD, Manuck SB. Self-reported sleep duration is associated with the metabolic syndrome in midlife adults. Sleep. 2008;31:635–643. doi: 10.1093/sleep/31.5.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- Nedeltcheva AV, Kilkus JM, Imperial J, Kasza K, Schoeller DA, Penev PD. Sleep curtailment is accompanied by increased intake of calories from snacks. Am J Clin Nutr. 2009;89:126–133. doi: 10.3945/ajcn.2008.26574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel K, Tasali E, Penev P, Van Cauter E. Brief communication: sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med. 2004;141:846–850. doi: 10.7326/0003-4819-141-11-200412070-00008. [DOI] [PubMed] [Google Scholar]
- Vgontzas AN, Chrousos GP. Sleep, the hypothalamic-pituitary-adrenal axis, and cytokines: multiple interactions and disturbances in sleep disorders. Endocrinol Metab Clin North Am. 2002;31:15–36. doi: 10.1016/s0889-8529(01)00005-6. [DOI] [PubMed] [Google Scholar]
- Miller MA, Cappuccio FP. Inflammation, sleep, obesity and cardiovascular disease. Curr Vasc Pharmacol. 2007;5:93–102. doi: 10.2174/157016107780368280. [DOI] [PubMed] [Google Scholar]
- Pan A, Keum N, Okereke OI, Sun Q, Kivimaki M, Rubin RR, et al. Bidirectional association between depression and metabolic syndrome: a systematic review and meta-analysis of epidemiological studies. Diabetes Care. 2012;35:1171–1180. doi: 10.2337/dc11-2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandner MA, Drummond SP. Who are the long sleepers? Towards an understanding of the mortality relationship. Sleep Med Rev. 2007;11:341–360. doi: 10.1016/j.smrv.2007.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranges S, Dorn JM, Shipley MJ, Kandala NB, Trevisan M, Miller MA, et al. Correlates of short and long sleep duration: a cross-cultural comparison between the United Kingdom and the United States: the Whitehall II Study and the Western New York Health Study. Am J Epidemiol. 2008;168:1353–1364. doi: 10.1093/aje/kwn337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuomilehto H, Peltonen M, Partinen M, Lavigne G, Eriksson JG, Herder C, et al. Sleep duration, lifestyle intervention, and incidence of type 2 diabetes in impaired glucose tolerance: The Finnish Diabetes Prevention Study. Diabetes Care. 2009;32:1965–1971. doi: 10.2337/dc08-1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CJ, Hu FB, Patel SR, Mantzoros CS. Sleep duration and snoring in relation to biomarkers of cardiovascular disease risk among women with type 2 diabetes. Diabetes Care. 2007;30:1233–1240. doi: 10.2337/dc06-2107. [DOI] [PubMed] [Google Scholar]
- Cagampang FR, Poore KR, Hanson MA. Developmental origins of the metabolic syndrome: body clocks and stress responses. Brain Behav Immun. 2011;25:214–220. doi: 10.1016/j.bbi.2010.09.005. [DOI] [PubMed] [Google Scholar]
- Buxton OM, Pavlova M, Reid EW, Wang W, Simonson DC, Adler GK. Sleep restriction for 1 week reduces insulin sensitivity in healthy men. Diabetes. 2010;59:2126–2133. doi: 10.2337/db09-0699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornier MA, Dabelea D, Hernandez TL, Lindstrom RC, Steig AJ, Stob NR, et al. The metabolic syndrome. Endocr Rev. 2008;29:777–822. doi: 10.1210/er.2008-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maury E, Ramsey KM, Bass J. Circadian rhythms and metabolic syndrome: from experimental genetics to human disease. Circ Res. 2010;106:447–462. doi: 10.1161/CIRCRESAHA.109.208355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aeschbach D, Sher L, Postolache TT, Matthews JR, Jackson MA, Wehr TA. A longer biological night in long sleepers than in short sleepers. J Clin Endocrinol Metab. 2003;88:26–30. doi: 10.1210/jc.2002-020827. [DOI] [PubMed] [Google Scholar]
- Allebrandt KV, Teder-Laving M, Akyol M, Pichler I, Muller-Myhsok B, Pramstaller P, et al. CLOCK gene variants associate with sleep duration in two independent populations. Biol Psychiatry. 2010;67:1040–1047. doi: 10.1016/j.biopsych.2009.12.026. [DOI] [PubMed] [Google Scholar]
- Prasai MJ, George JT, Scott EM. Molecular clocks, type 2 diabetes and cardiovascular disease. Diab Vasc Dis Res. 2008;5:89–95. doi: 10.3132/dvdr.2008.015. [DOI] [PubMed] [Google Scholar]
- Milagro FI, Gomez-Abellan P, Campion J, Martinez JA, Ordovas JM, Garaulet M. CLOCK, PER2 and BMAL1 DNA methylation: association with obesity and metabolic syndrome characteristics and monounsaturated fat intake. Chronobiol Int. 2012;29:1180–1194. doi: 10.3109/07420528.2012.719967. [DOI] [PubMed] [Google Scholar]
- von Ruesten A, Weikert C, Fietze I, Boeing H. Association of sleep duration with chronic diseases in the European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam study. PLoS One. 2012;7:e30972. doi: 10.1371/journal.pone.0030972. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.