Abstract
Our previous in vitro data have demonstrated that regulatory mechanisms are involved in tolerance of class I-mismatched renal allografts in miniature swine treated with 12 days of high dose Cyclsporin A. In the present study, we attempted to induce tolerance of class I-mismatched kidneys by adoptive transfer of cells and/or kidneys from long-term tolerant animals. Fifteen SLAdd miniature swine received 1.5 Gy whole body irradiation and class I-mismatched (SLAgg) kidneys from naïve pigs with or without co-transplanted kidneys and/or adoptively transferred cells from long-term tolerant (LTT) SLAdd recipients of SLAgg grafts. Additionally, three SLAdd miniature swine received class I mismatched kidney with adoptively transferred cells from LTT SLAdd recipients. Naïve kidneys transplanted without a LTT kidney were rejected within 9 days. All recipients of naive kidneys along with cells and kidney grafts from LTT animals showed markedly prolonged survival of the naive renal grafts (day 28, >150 and >150 days). These studies suggest that (1) tolerated kidneys have potent regulatory effects, and (2) cells from LTT animals infused in conjunction with kidney grafts augment these regulatory effects. To our knowledge, these studies represent the first demonstration of successful adoptive transfer of tolerance in large animals.
Keywords: MGH miniature swine, Adoptive transfer, Kidney transplantation, Tolerance
INTRODUCTION
The induction of tolerance of transplanted organs could avoid the morbidity and mortality currently associated with prolonged immunosuppressive treatment in graft recipients. Understanding the mechanism by which tolerance is induced will aid in the development of clinically applicable tolerance-inducing protocols.
In this laboratory, we have studied tolerance of transplanted kidneys (1–4), islets (5), thymic lobes (6,7) and hearts (8,9), using MGH miniature swine, which allow for the study of transplantation immunity in a genetically defined MHC background (10). We have previously demonstrated that a 12-day course of Cyclosporine uniformly induces tolerance of class I MHC-mismatched kidney allografts (1). Furthermore, when primary grafts were removed and replaced by second, donor-matched grafts on the same day, tolerant recipients accepted the second kidney with stable renal function without further immunosuppression, confirming tolerance. In contrast, primary kidney grafts transplanted without CyA were uniformly rejected within two weeks of transplantation (1,2). Because large animal studies are limited to the use of small numbers of experimental animals, the reproducibility of the “all or none” phenomenon of graft survival in these two protocols using MHC inbred MGH-miniature swine has provided a unique opportunity to study both the induction and maintenance of tolerance in a model where differences in results in even a small number of animals can provide significant information.
Using this established tolerance model (1), we have determined that (1) the presence of an intact thymus is essential in the induction (3), but not the maintenance of tolerance (11); (2) IL-10 is overexpressed in the cell populations that infiltrate the grafts in tolerant animals (12); and (3) peripheral blood lymphocytes (PBL) from tolerant animals can suppress in vitro anti-donor CTL reactivity by naive recipient-matched PBL in a donor-specific manner [3699]. Furthermore, this suppression is dose-dependent and radiation-sensitive, requires cell-to-cell contact, and is not reversed by exogenous IL-2 administration (13–15). While these studies support the hypothesis that regulatory mechanisms play an essential role in the induction and maintenance of tolerance, they provide only indirect evidence of the role of regulatory cells in this process. In this study, we used an adoptive transfer model to investigate whether cells from tolerant animals could induce tolerance of class I MHC-mismatched kidney grafts in naïve recipients.
MATERIALSand METHODS
Animals
Donor animals were SLAgg (class Ic/IId) partially inbred MGH miniature swine. Recipient animals were 4–10 months of age from an inbred line of SLAdd (class Id/IId) MGH miniature swine that are selectively bred to reduce minor antigen differences (16). The immunogenetic characteristics of MGH miniature swine and of the intra-MHC recombinant haplotypes have been described previously (3,17).
Experimental Groups
SLAdd animals received two-haplotype class I mismatched kidneys (SLAgg) with a 12-day course of Cyclosporine A to achieve blood levels of 400–800ng/ml (CyA; Sandimmune, generously provided by Novartis Pharmaceutical Corp., East Hanover, NJ) (3). All animals became long-term tolerant (LTT) animals because they accepted their grafts with stable renal function for at least 90 days(1). The LTT animals were then used as adoptive transfer donors for naïve SLAdd recipients as summarized in Table 1 (Groups A-E). There were three recipients of adoptive transfer in each experimental group, and 6 animals in Group B.
Table 1.
| Group | Treatment to adoptive transfer donor (LTT animal) | Treatment to recipient (SLAdd) of naïve kidney (SLAgg) | Survival (days) | ||
|---|---|---|---|---|---|
| Day -8 | Day -1 | Day -1 | Day 0 | ||
| DST 10 ml/kg | 150 rads WBI | Co-administration of PBMCs transfer 2.5×109 cells/kg | Co-transplantation of tolerated kidney graft transfer | ||
| A | (−) | (−) | (+) | (−) | 7, 7, 7 |
| B | (+) | (+) | (−) (+) |
(−) (−) |
8, 8, 9 7,10,30 |
| C | (+) | (+) | (−) | (+) | 9, 10, 73 |
| D | (−) | (+) | (−) | (+) | 4, 10, 46 |
| E | (+) | (+) | (+) | (+) | 28, >150, >150 |
LTT : long-term tolerant
DST : donor specific transfusion
WBI : whole body irradiation
PBMCs: peripheral blood mononuclear cells
Surgery
The surgical procedures for primary and secondary transplantation have been previously described in detail (18). Both native kidneys were removed on the day of the primary kidney transplant. Indwelling central venous catheters were placed surgically in the external and internal jugular veins of recipient animals to facilitate frequent blood sampling and the administration of fluid, drugs, blood and PBMCs.
Donor-Specific Transfusion (DST)
SLAdd LTT animals that served as adoptive transfer donors to Groups B, C and E were given an intravenous transfusion of 10 ml/kg (body weight) non-irradiated SLAgg whole blood one week prior to leukapheresis. The dose of blood for the DST was determined by extrapolation from rodent allotransplantation models (19) (20).
Transfer of Peripheral Blood Mononuclear Cells (PBMCs)
2.5×109 peripheral blood mononuclear cells (PBMCs)/kg (recipient body weight) were collected by leukapheresis (COBE BCT Inc., Lakewood, Colorado, USA) from LTT animals and then infused intravenously to recipients in groups A, B and E one day prior to transplantation. The appropriate number of tolerant PBMCs used for this adoptive transfer was based on studies in mouse models of the adoptive transfer of tolerant spleen cells (21) (22).
Transfer of Long-Term Tolerated Kidney Allografts
Long-term tolerated SLAgg kidney grafts were harvested from LTT SLAdd animals and transplanted into SLAdd recipients in groups C, D and E at the same time as the naïve SLAgg kidney grafts. Following donation of the tolerated kidney allograft, LTT animals received a second naïve kidney graft from an SLAgg animal.
Histological Analysis of Long-Term Tolerated Kidney Allografts
Immunohistochemical analysis of frozen sections of long-term tolerated kidney grafts was performed using the standard avidin-biotin horseradish-peroxidase complex (ABC) technique to detect the phenotype of graft infiltrating cells. For the detection of proliferating cell nuclear antigen (PCNA), sections of 10% buffered formalin-fixed, paraffin-embedded tissue blocks were stained using the ABC technique (23).
Quantitative analysis of Tregs in kidney grafts
Two LTT kidneys and one rejected kidney from Group b were double-stained with CD25 (FITC) and FoxP3 (Bio-PEAV) for quantitative analysis of the Treg population. Percent of FoxP3 and CD25 double positive cells (out of all CD 25 positive cells) in each x100 magnification view were counted and the average +/− SD of 9 views was calculated.
Monitoring of Rejection
Rejection of kidney grafts was monitored by plasma creatinine levels and by histological examination of kidney biopsies. Renal open-wedge biopsies were performed through a flank incision. Tissues were stained using hematoxylin-eosin and periodic acid-Schiff, and coded slides were examined by light microscopy by a pathologist. Graft rejection was scored according to a standardized grading system of pathological specimens (24).
Preparation of Peripheral Blood Mononuclear Cells (PBMCs)
For the separation of PBMCs, freshly heparinized whole blood was diluted 1:2 with Hank’s balanced salt solution (HBSS) (GIBCO BRL, Gaithersburg, MD) and the mononuclear cells were obtained by gradient centrifugation using lymphocyte separation medium (Organon Teknika, Durham, NC) as previously described (3).
Cell-Mediated Lympholysis (CML) Assays
The procedure for CML assays has been described elsewhere (3). 51Cr release was determined on a gamma counter. The results were expressed as:
Co-culture Assays
Peripheral regulatory mechanisms were investigated by in vitro co-culture assays (14). The primary culture was set up as in CML assays. These primed cells were then harvested and rested overnight at 4°C. The resulting effector cells were co-incubated with naïve SLA matched PBMCs and irradiated donor-type or third party PBMCs for an additional 5 days.
Antibodies and Flow Cytometry
The presence of anti-donor class I (MHC class Ic) IgM and IgG in the serum of recipient swine was detected by indirect flow cytometry. Fluorescence-activated cell sorting (FACS) was performed using a Becton Dickinson FACScan microfluorometer (Sunnyvale, CA) and recombinant SLA PBMCs to determine the SLA-binding specificity of the antibody as previously described (18).
RESULTS
Adoptive transfer of a mega-dose of tolerant cells had a marginal effect on graft survival
A. Infusion of cells from the LTT animal was not sufficient to prolong graft survival of naïve kidneys in a naive recipient across a class I mismatch
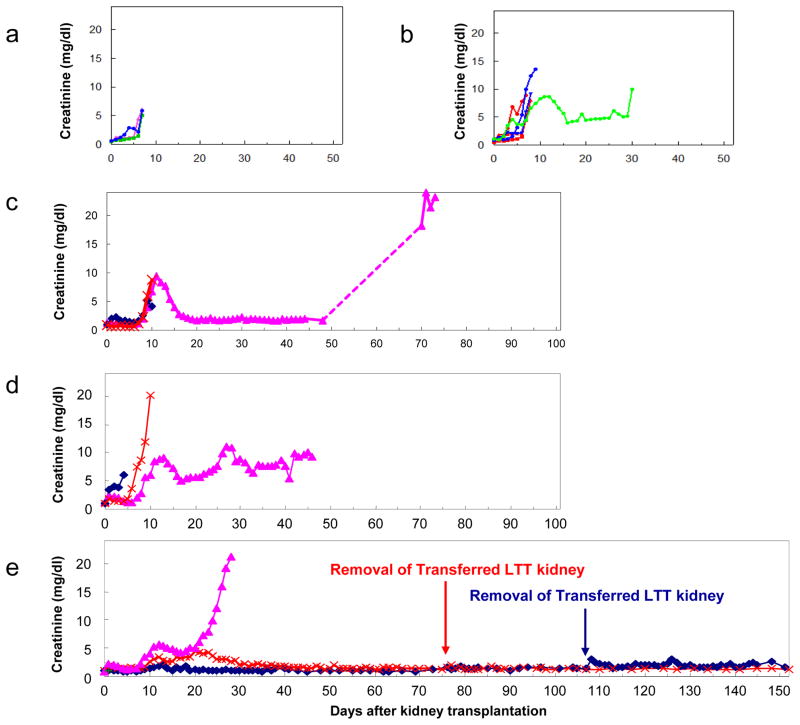
We first tested whether the adoptive transfer of a mega-dose of PBMCs (2.5×109 unprimed PBMCs/recipient kg) from LTT animals one day prior to kidney transplantation could induce graft acceptance of class I mismatched kidneys in naïve animals without any addition treatment (Group A, see Table 1). The transplanted kidneys (n=3) were completely rejected at day 7. The graft survival was similar to that of class I mismatched kidneys transplanted without immunosuppression (25), indicating that the infusion of PBMCs from the LTT animal was not sufficient to prolong graft survival in a naive recipient (Fig. 2a). This result suggested that further manipulation of the recipient or adoptive donor treatment would be necessary to prolong graft survival.
Figure 2.
Plasma creatinine levels (mg/dl) of recipients of class I mismatched kidneys treated with (a) infusion of tolerant PBMCs alone (Group A), (b) DST and a 150 rad WBI with/without from LTT animals (Group B), (c) transfer of tolerated kidney grafts from LTT animals treated with DST and a 150 rad WBI (Group C), (d) transfer tolerated kidney grafts from LTT animals with a 150 rad WBI, without DST (Group D), and (e) transfer of both PBMCs and tolerated kidneys from LTT animals treated with DST and a 150 rad WBI (Group E).
B. DST augmented the in vitro suppressive effects of regulatory cells from LTT animals
We hypothesized that a DST could augment the number and/or potency of regulatory T cells (Tregs) in vivo in LTT animals (20,26). These Tregs might then prolong graft survival in naïve recipients of a mega-dose of PBMCs from LTT animals. We first tested whether a donor-specific DST in an LTT animal would increase the suppression of the normal immunologic response to donor stimulation in vitro using co-culture assays that have been well established in our laboratory (13,14). We administered about 800ml (10ml/kg) of donor-matched, non-irradiated whole blood to two LTT animals, and measured the plasma creatinine twice weekly to assess kidney graft function. CML and co-culture assays were set up prior to the DST and 1 week after the DST.
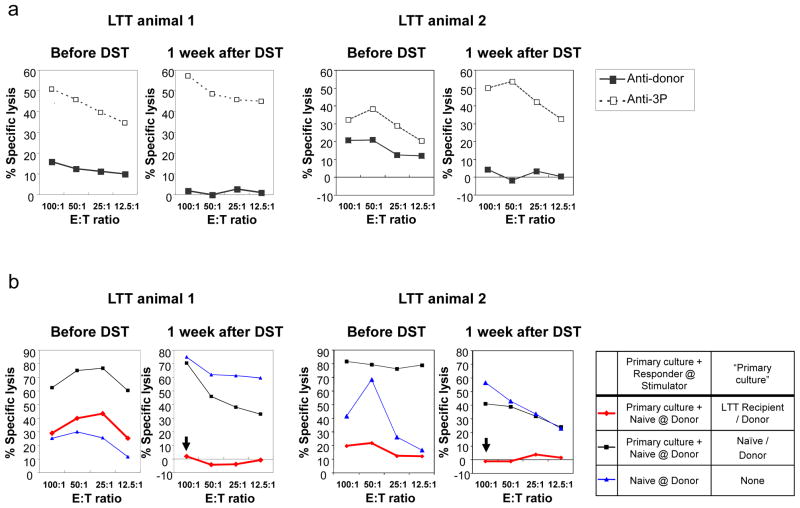
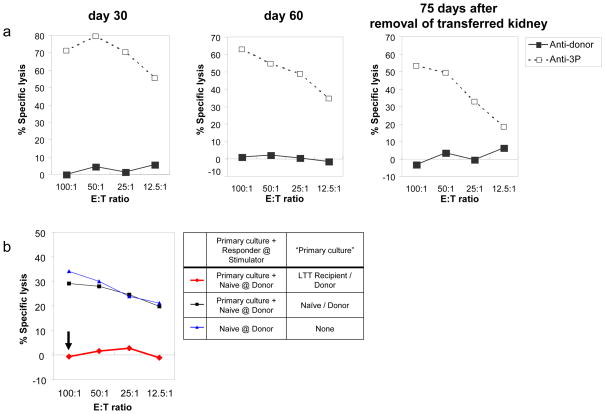
Both animals maintained renal function with plasma creatinine levels around 1.4 mg/dl before and after the DST, indicating that the DST had not compromised the function of the renal graft. Furthermore, the inhibitory effects of the Tregs increased after the DST. The anti-donor CTLs were 16% and 20% respectively (at 100:1 of E:T ratio) before the DST, and were absent one week after the DST (Fig. 1a). Co-culture assays showed that cells from the LTT animals primed by donor antigens suppressed the naïve CTL responses against donor-type cells 70% and 80% respectively before the DST and completely after the DST (Fig. 1b).
Figure 1.
CML and co-culture CMLs demonstrated that DST in LTT animals increased suppression of the anti-donor response in vitro. CTL and co-culture CTL assays were set up one week before and one week after the DST. Recipient responses to donor stimulation are shown with solid lines and boxes; third party stimulation are shown with dotted lines and striped boxes. (a) While the anti-donor CTLs were 16% and 20% respectively before the DST, there were no anti-donor CTL responses 1 week after the DST. (b) Cells from the LTT animal primed by donor antigens specifically suppressed the naïve CTL responses against donor-type cells 70% and 80% respectively before the DST, and completely (red line with an arrow) after the DST.
C. The effect of DST on regulatory cells in vivo was marginal
The second group (Group B), 6 LTT donors were primed with a DST 8 days prior to adoptive transfer of cells in an attempt to expand the number or potency of regulatory T cells in vivo. In addition, recipients received 150 rads of whole body irradiation (WBI) in an attempt to reduce the immunologic effects of alloreactive precursor T cells (Table 1 Group B). Three of 6 recipients received a mega-dose of PBMCs (2.5×109 unprimed PBMCs/recipient kg) from an LTT animal one day prior to kidney transplantation.
Animals receiving only 150-rad of WBI rejected their kidney grafts by day 9 (Fig 2b red lines, n=3), indicating that this treatment alone can not prolong survival of allogeneic kidneys. Two of three animals that received PBMCs from LTT animals rejected at time points (Fig. 2b blue lines) similar to those treated with 150-rad WBI without PBMC from LTT animals (Fig 2b red) or animals in Group A (Fig. 2a). The one remaining animal had renal graft function to day 30, however creatinine levels fluctuated and the graft was eventually rejected (Fig 2b green line).
Adoptive transfer of long-term tolerated kidney allografts has immunoregulatory effects
A. Histologic examination showed presence of Foxp3 positive/CD25 positive cells in LTT kidneys
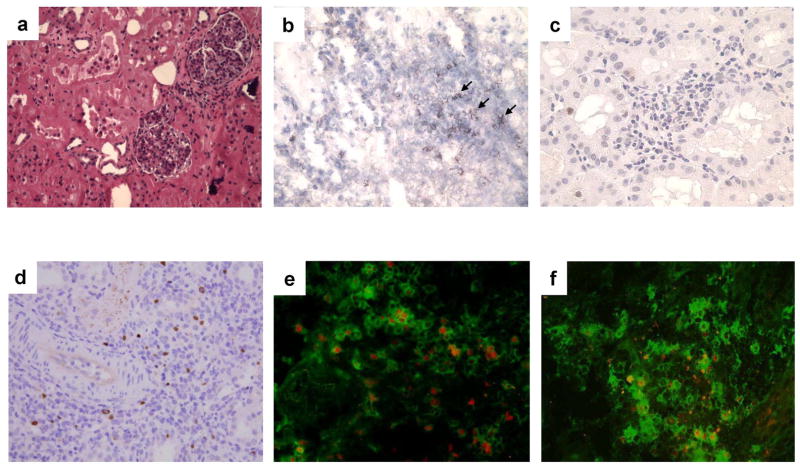
Histological examination of LTT kidneys in this class I mismatched CyA model uniformly revealed focal mononuclear cell infiltrates around vessels (Fig. 3a). We have previously reported that these cells are CD4/CD8 double positive cells while cells in rejectors are predominantly CD4 single positive (25). As IL-10 is expressed in the cell populations that infiltrate the grafts in tolerant animals (12), we therefore determined if there are Foxp3 positive T-reg cells in the GILs.
Figure 3.
Histological analysis of representative long-term tolerated kidney allografts. (a) Minimal cell infiltrate was seen in the tolerated kidney graft (H&E, x200). (b) Several of the cells in the graft were CD25 positive (black arrows); (c) most cells were PCNA negative (x400). (d) Foxp3 positive cells, shown with arrows, were found in the tolerated kidney graft (x400). (e) Some cells in the infiltrate were CD25 (green)/Foxp3 (red) double-positive. (f) As controls, many CD25 positive (green) cells were found within a class I mismatched kidney graft that was rejected in a cellular manner, but most of them were FoxP3 negative (red).
The majority of graft infiltrating cells in LTT kidneys were T cells expressing CD25 (Fig. 3b) but not PCNA (Proliferating cell nuclear antigen) (Fig. 3c), a phenotype that identifies these cells as either activated or regulatory T cells. Staining of the cells in the grafts revealed that one third of the infiltrating cells were Foxp3-positive (Fig. 3d), and that some of these cells were double-positive for both CD25 and Foxp3 (Fig. 3e). As controls, we also stained a class I mismatched kidney that was rejected in a cellular manner (30-day survivor from Group B), with anti-pig CD25 and Foxp3 antibodies (Fig. 3f). There were many CD25 positive cells infiltrated within the graft (green), but most of them were FoxP3 negative (red). Quantitative analysis of these cellular populations within the kidneys of two LTT animals demonstrated that 45.8 ± 10.6 % (60, 52, 42, 38, 47, 36, 62, 31, 45%) of CD25 positive cells/field expressed FoxP3 in LTT grafts while only 10.3 ± 6.2 % (9.6, 7.1, 2.1, 19.7, 10.0, 8.2, 21.2, 6.2, 8.6%) of CD25 positive cells/field were FoxP3 positive in the graft from the 30 days survivor in Group B (t test: p<0.001). The presence of a high percentage of CD25+/Foxp3+ cells indicated the presence of Tregs in the LTT kidney grafts in this model.
B. Long-term tolerated kidney grafts have potent regulatory effects (Groups C and D)
We next examined whether the transfer of LTT kidneys in which the GILs included T-regs, prolonged graft survival in naïve recipients. Six SLAdd animals, treated with 150-rads WBI one day prior to kidney transplantation, each received a naïve SLAgg kidney cotransplanted with an SLAgg tolerated kidney from a LTT SLAdd animal. Three of these LTT donors were treated with a DST one week before transplantation (Group C) in the same manner as in Group B, while the remaining three were not (Group D).
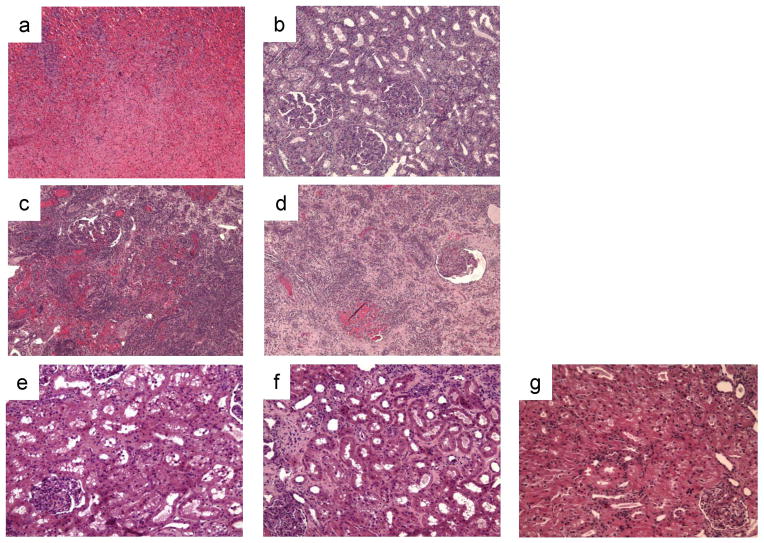
Two animals in Group C and two animals in Group D rejected their grafts within 10 days of transplantation. One animal in Group C, however, maintained stable renal function for at least 50 days and lost the graft at day 73 (Fig. 2c). This extended graft survival was shortened when the adoptive transfer donors did not receive a DST prior to donation of the tolerated kidney - one animal in Group D had renal function for up to 46 days but it was very unstable (Fig. 2d). Interestingly, analysis of the kidney biopsies from these two recipients (Group C: 73 day survival; Group D: 46 day survival) revealed differences between the naïve kidney grafts and the grafts from the LTT animals. Figures 4a and b show the histological analysis of kidney graft biopsies at day 22 post-transplantation in the 73-day Group C survivor. At this time point, the naïve kidney graft was rejected (Fig. 4a), while the graft from the LTT animal contained only minimal cellular infiltrates (Fig. 4b), suggesting that the pre-existing Foxp3+/CD25+ intra-graft passenger leukocytes had protective effects. Furthermore, despite the severe rejection of the naïve kidney graft, no anti-donor (SLAgg) class I IgG was detected in either graft. In addition, CML data for the 73-day survivor at day 48 showed donor specific hyporesponsiveness while the 46-day survivor maintained a high CML response to donor at day 36. These data suggest that the tolerated kidney graft regulated not only local effects, but also the systemic immunologic response.
Figure 4.
Histological analysis of renal allografts. Biopsy specimens on day 22 from the 73-day survivor in Group C showed severe rejection in the kidney from a naïve animal (a, H&E x100), but only minimal cell infiltrate in the kidney from an LTT animal (b, PAS x100). Day 28 biopsy specimens from the 28-day survivor in Group E showed severe diffuse interstitial hemorrhage and cellular infiltrate in the kidney from a naïve animal (c, H&E x100) and glomerular changes but much less interstitial hemorrhage in the kidney graft from an LTT animal (d, H&E x100). Representative histological findings on day 60 from long-term acceptors in Group E showed minimal mononuclear cell infiltrate without chronic vasculopathy in the kidney grafts from both the naïve animal (e, H&E x200) and the LTT animal (f, H&E x200). Biopsies of the naive graft in long-term acceptors in Group E 21 days after the removal of the tolerated graft showed minimal cell infiltrates with no evidence of glomerulitis or vasculitis (g, H&E x200).
Successful induction of tolerance of naive class I mismatched kidneys by adoptive transfer of donor-kidney matched cells and kidneys from LTT animals (Group E)
We next assessed whether both DST-priming of the donor and the transfer of PBMCs along with the tolerated kidney grafts from LTT animals could further prolong the survival of kidney grafts or induce tolerance in recipients that had received a 150-rad WBI (Group E). The experimental protocol for Group E is shown in detail in Figure 5. All three animals in this group received both naïve and tolerated class I mismatched kidneys. Two of three animals underwent graftectomy of the tolerant grafts from LTT donors to assess graft function of the SLAgg kidneys grafts from naïve animals on days 76 and 107, respectively.
Figure 5.
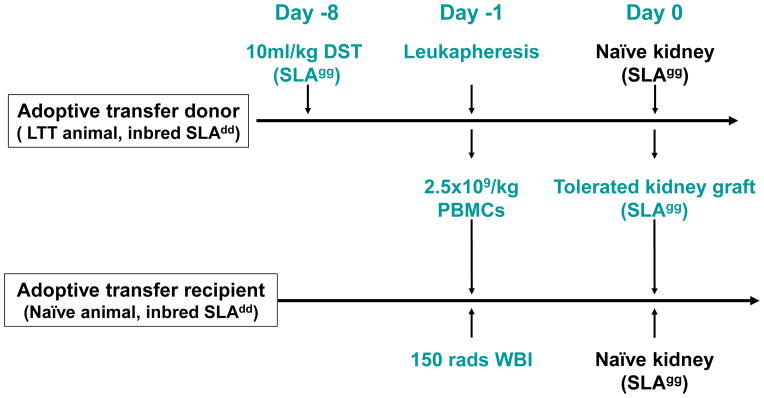
Experimental protocol for Group E. The adoptive transfer donors (LTT animals) received 10ml DST/kg body weight one week prior to leukapheresis. The adoptive transfer recipients were treated with 150 rads WBI and given 2.5×109 PBMCs/kg (recipient body weight) from the adoptive transfer donor one day before kidney transplantation. The recipient then received both a class I mismatched kidney from a naïve SLAgg pig and a long-term tolerated kidney allograft (SLAgg) from the adoptive transfer donor without further immunosuppresssion. In order to assess graft function of SLAgg kidneys grafts from naïve animals, two of three animals received graftectomy of the tolerant grafts from LTT donors on days 76 and 107, respectively.
One of the three animals in this group rejected both grafts by day 28 (Fig. 2e). Histology of the naïve graft on day 28 showed severe diffuse interstitial hemorrhages and mononuclear cell infiltrates (Fig. 4c), while biopsy samples from the LTT graft showed similar diffuse mononuclear cell infiltrates and glomerular changes, but only mild interstitial hemorrhages (Fig. 4d). As in the grafts from Group C and D animals, the tolerated kidney graft may have been protected to some extent by pre-existing intra-graft passenger leukocytes.
The other two animals in Group E, however, maintained stable renal function after small transient rises in creatinine (Fig. 2e). Histological analysis of both the naïve and LTT kidney grafts on day 60 showed minimal mononuclear cell infiltrates without chronic vasculopathy (Fig. 4e and 4f). In order to assess graft function of SLAgg kidneys grafts from naïve animals, we removed the kidney grafts transferred from the LTT animals on days 76 and 107, respectively. Both animals maintained normal renal function via the naïve kidney graft for greater than 90 additional days (Fig. 2e) without histological evidence of acute or chronic rejection (Fig. 4g). These two animals accepted naïve class I MHC-mismatched life-supporting kidney grafts long-term indicating that cotransplantation of both cells and kidneys from tolerant animals confers systemically infectious tolerance in a large animal model.
CML assays revealed donor-specific unresponsiveness in these two long-term acceptors 30 and 60 days after the initial transplant. Furthermore, cells from these animals remained unresponsive to donor stimulation for 75 days after the removal of the LTT kidney graft (Fig. 6a). Co-culture assays to assess regulatory mechanisms showed nearly 100% inhibition of the naïve CTL response against donor antigens (Fig. 6b).
Figure 6.
Representative results of CML and co-culture CML assays for the long-term acceptor animals in Group E. (a) CML assays 30 and 60 days after primary transplant, as well as 75 days after removal of the LTT kidney showed donor-specific unresponsiveness. Recipient responses to donor stimulation are shown with solid lines and boxes, while responses to third party stimulation are shown with dotted lines and striped boxes. (b) Co-culture assay performed 126 days showed that recipient PBMCs inhibited naïve CTL responses against donor antigens nearly 100% (red line with an arrow) when primed with donor antigen.
DISCUSSION
This study demonstrates successful induction of systemic transplant tolerance of naïve class I mismatched kidneys by adoptive transfer of leukapheresed PBMCs and tolerated kidney grafts from LTT animals, SLA matched to the naïve donor kidney. The recipient animals were conditioned with only 150 rads of WBI. To our knowledge, this is the first successful demonstration of adoptive transfer of tolerance in large animals.
Our protocol includes DST treatment in the donors prior to the adoptive transfer. In rodent models, anti-CD4 antibody plus a donor-specific transfusion generated donor-specific regulatory CD25+CD4+ T cells that could suppress graft rejection (27). Adoptive transfer of these cells into syngeneic immunodeficient animals prevented rejection (28). Recent studies have reported that preoperative donor-specific blood transfusion induces the generation of tolerogenic Tregs in the presence of alloantigens (26). In our present study, CML and co-culture assays showed that DST treatment in an LTT animal augmented the suppressive effects. Based on these results, DST treatment was added to our protocol to expand the number and potency of Tregs in LTT animal serving as an adoptive transfer donor. One of three animals in Group C whose LTT donors were treated with DST survived up to 73 days, while one of three animals in Group D whose LTT donors did not receive DST survived up to 46 days. Although slight differences in these two animals were seen, the DST effect was marginal in vivo. Therefore, further investigations will be needed to understand the effect of DST in vivo.
Kidney grafts transferred from tolerant animals (LTT kidney grafts) appear to play an important role in the successful induction of infectious tolerance. In both the 73 and 46-day survivors, the tolerated kidney grafts had less cell infiltration or vasculitis than the naïve kidney grafts until there was a loss of renal function. Three potential major cell components of LTT kidneys may cause the protective effects: (1) passenger GILs, (2) less immunogenic donor APCs, and (3) immunoregulatory renal tubular cells (RTEC). One possible factor is the presence of passenger Tregs, expressing CD25+/Foxp3+ in LTT grafts (29–31). These Tregs in the tolerated kidneys could regulate the systemic immunologic alloresponse following adoptive transfer (32,33). Additionally, the cytokine/chemokine milieu in LTT kidneys facilitates the expansion of Tregs. Foxp3 expression is specifically up-regulated within tolerated allografts and recruitment of Foxp3+ Tregs to allograft tissue is dependent on the chemokine receptor CCR4 in a mouse cardiac allograft model (34). We have also previously reported that the increased expression of IL-10 by cells infiltrating the kidney grafts in LTT animals may lead to the generation of an immunoregulatory milleu (12). Other possible protective components in LTT grafts include donor APCs that are less immunogenic or present in lower numbers and immunoregulatory renal tubular cells (RTECs). Kidney grafts from naïve animals are rich in donor MHC-type dendritic cells (DCs) which serve as professional antigen-presenting cells (APC) and play a key role in the direct alloantigen presentation. In contrast, kidney grafts from LTT animals have few or no donor-type DCs. A reduction or absence of immunoresponses via the direct pathway could diminish the immunogenicity of LTT grafts relative to naïve kidneys. Furthermore, RTECs and other parenchymal cells of the tolerated kidneys may also contribute the protective effects of LTT grafts. Recent studies have demonstrated that RTECs, which inducibly express MHC class II, have the capacity to function as APCs (35,36). The processing of donor antigen and the presentation of peptides bound to MHC class II products on tubular epithelial cells result in the engagement of the T cell receptor. RTECs also express a variety of adhesion molecules, such as intercellular adhesion molecules (ICAM-1) and vascular cell adhesion molecules (VCAM-1), and cytokines including tumor necrosis factor alpha (TNF alpha) which may enhance their interaction with T cells. Another group has reported that interferon-gamma-treated renal tubular epithelial cells induce allospecific tolerance (37). Thus, the kidney grafts from LTT animals may have more tolerogenic RTECs which could function as tolerogenic APCs and more effectively induce systemic tolerance than the kidneys from naïve animals.
Although a large number of cells transferred from LTT animals themselves appear to have minimal effects on the induction of tolerance in this large animal model, adoptive transfer of both cells and kidneys from LTT animals was required for successful induction of tolerance of naive class I mismatched kidneys. Based upon these results, our hypothesis for the synergistic effects of the transferred cells and kidneys leading to successful tolerance induction in this model is that transferred Tregs traffic to the LTT kidney where they undergo quantitative or qualitative expansion of suppressive activity in response to kidney specific antigens via indirect or direct presentation in the donor renal grafts. In order to translate these finding to clinically applicable protocols, we are currently elucidating the immunoregulatory milleu and immunogenic characteristics of tolerated kidney grafts. Defining the tolerogenic mechanisms of the graft infiltrating T-regs and RTECs may enable us to utilize these cells for the induction of tolerance of naïve kidneys without co-transplantation of LTT kidneys.
Acknowledgments
We thank Dr. Joren C Madsen and Dr. Isabel Hanekamp for their helpful review of this manuscript; and Rebecca Wark for editorial assistance. All experiments were performed in accordance with the NIH Guidelines for Care and Use of Laboratory Animals and were approved by the Massachusetts General Hospital Subcommittee on Research Animal Care.
Funding Source: This work was supported by NIH/NIAID 4R37AI031046-21. We acknowledge 1CO6RR20135-01 for construction of the facility utilized for production and maintenance of miniature swine.
Abbreviations
- CyA
Cyclosporine A
- CML
Cell mediated lympholysis
- DST
donor specific transfusion
- GILs
graft infiltrating cells
- LTT
Long-term tolerant
- MGH
Massachusetts General Hospital
- MHC
Major histocompatibility complex
- SLA
Swine leukocyte antigens
- PBMC
Peripheral blood mononuclear cells
- WBI
whole body irradiation
Footnotes
Disclosure
None of the authors has any conflict of interest to declare with regard to the content of this article.
Reference List
- 1.Rosengard BR, Ojikutu CA, Guzzetta PC, et al. Induction of specific tolerance to class I disparate renal allografts in miniature swine with cyclosporine. Transplantation. 1992;54:490–497. doi: 10.1097/00007890-199209000-00020. [DOI] [PubMed] [Google Scholar]
- 2.Gianello PR, Sachs DH. Effect of major histocompatibility complex matching on the development of tolerance to primarily vascularized renal allografts: a study in miniature swine. Hum Immunol. 1996;50:1–10. doi: 10.1016/0198-8859(96)00059-6. [DOI] [PubMed] [Google Scholar]
- 3.Yamada K, Gianello PR, Ierino FL, et al. Role of the thymus in transplantation tolerance in miniature swine. I. Requirement of the thymus for rapid and stable induction of tolerance to class I-mismatched renal allografts. J Exp Med. 1997;186:497–506. doi: 10.1084/jem.186.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Utsugi R, Barth RN, Lee RS, et al. Induction of transplantation tolerance with a short course of tacrolimus (FK506): I. Rapid and stable tolerance to two-haplotype fully mhc-mismatched kidney allografts in miniature swine. Transplantation. 2001;71:1368–1379. doi: 10.1097/00007890-200105270-00003. [DOI] [PubMed] [Google Scholar]
- 5.Kumagai N, LaMattina JC, Kamano C, et al. Vascularized islet cell transplantation in miniature Swine: islet-kidney allografts correct the diabetic hyperglycemia induced by total pancreatectomy. Diabetes. 2002;51:3220–3228. doi: 10.2337/diabetes.51.11.3220. [DOI] [PubMed] [Google Scholar]
- 6.Kamano C, Vagefi PA, Kumagai N, et al. Vascularized thymic lobe transplantation in miniature swine: Thymopoiesis and tolerance induction across fully MHC-mismatched barriers. Proc Natl Acad Sci U S A. 2004;101:3827–3832. doi: 10.1073/pnas.0306666101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nobori S, Samelson-Jones E, Shimizu A, et al. Long-term acceptance of fully allogeneic cardiac grafts by cotransplantation of vascularized thymus in miniature swine. Transplantation. 2006;81:26–35. doi: 10.1097/01.tp.0000200368.03991.e0. [DOI] [PubMed] [Google Scholar]
- 8.Yamada K, Choo JK, Allan JS, et al. The effect of thymectomy on tolerance induction and cardiac allograft vasculopathy in a miniature swine heart/kidney transplantation model. Transplantation. 1999;68:485–491. doi: 10.1097/00007890-199908270-00007. [DOI] [PubMed] [Google Scholar]
- 9.Yamamoto S, Teranishi K, Kamano C, et al. Role of the thymus in transplantation tolerance in miniature swine: V. Deficiency of the graft-to-thymus pathway of tolerance induction in recipients of cardiac transplants. Transplantation. 2006;81:607–613. doi: 10.1097/01.tp.0000198735.17555.f1. [DOI] [PubMed] [Google Scholar]
- 10.Sachs DH. MHC Homozygous Miniature Swine. In: Swindle MM, Moody DC, Phillips LD, editors. Swine as Models in Biomedical Research. Ames, Iowa: Iowa State University Press; 1992. pp. 3–15. [Google Scholar]
- 11.Vagefi PA, Ierino FL, Gianello PR, et al. Role of the thymus in transplantation tolerance in miniature Swine: IV. The thymus is required during the induction phase, but not the maintenance phase, of renal allograft tolerance. Transplantation. 2004;77:979–985. doi: 10.1097/01.tp.0000116416.10799.c6. [DOI] [PubMed] [Google Scholar]
- 12.Blancho G, Gianello P, Germana S, Baetscher M, Sachs DH, LeGuern C. Molecular identification of porcine interleukin-10: regulation of expression in a kidney allograft model. Proc Natl Acad Sci USA. 1995;92:2800–2804. doi: 10.1073/pnas.92.7.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ierino FL, Yamada K, Lorf T, Arn JS, Sachs DH. Mechanism of tolerance to class I-mismatched allografts in miniature swine: Regulation of interleukin-2 receptor α-chain expression on CD8 peripheral blood lymphocytes of tolerant animals. Transplantation. 1998;66:454–460. doi: 10.1097/00007890-199808270-00007. [DOI] [PubMed] [Google Scholar]
- 14.Ierino FL, Yamada K, Hatch T, Rembert J, Sachs DH. Peripheral tolerance to class I mismatched renal allografts in miniature swine: donor antigen-activated peripheral blood lymphocytes from tolerant swine inhibit antidonor CTL reactivity. J Immunol. 1999;162:550–559. [PubMed] [Google Scholar]
- 15.Gianello PR, Blancho G, Fishbein JF, et al. Mechanism of cyclosporin-induced tolerance to primarily vascularized allografts in miniature swine. Effect of administration of exogenous IL-2. J Immunol. 1994;153:4788–4797. [PubMed] [Google Scholar]
- 16.Mezrich JD, Haller GW, Arn JS, Houser SL, Madsen JC, Sachs DH. Histocompatible miniature swine: an inbred large-animal model. Transplantation. 2003;75:904–907. doi: 10.1097/01.TP.0000054839.43852.BF. [DOI] [PubMed] [Google Scholar]
- 17.Pennington LR, Lunney JK, Sachs DH. Transplantation in miniature swine. VIII. Recombination within the major histocompatibility complex of miniature swine. Transplantation. 1981;31:66–71. [PubMed] [Google Scholar]
- 18.Griesemer AD, LaMattina JC, Okumi M, et al. Linked suppression across an MHC-mismatched barrier in a miniature swine kidney transplantation model. J Immunol. 2008;181:4027–4036. doi: 10.4049/jimmunol.181.6.4027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Niimi M, Roelen DL, Witzke O, van Rood JJ, Claas FH, Wood KJ. The importance of H2 haplotype sharing in the induction of specific unresponsiveness by pretransplant blood transfusions. Transplantation. 2000;69:411–417. doi: 10.1097/00007890-200002150-00018. [DOI] [PubMed] [Google Scholar]
- 20.Bushell A, Karim M, Kingsley CI, Wood KJ. Pretransplant blood transfusion without additional immunotherapy generates CD25+CD4+ regulatory T cells: a potential explanation for the blood-transfusion effect. Transplantation. 2003;76:449–455. doi: 10.1097/01.TP.0000083043.84630.99. [DOI] [PubMed] [Google Scholar]
- 21.Qin S, Cobbold SP, Pope H, et al. “Infectious” transplantation tolerance. Science. 1993;259:974–977. doi: 10.1126/science.8094901. [DOI] [PubMed] [Google Scholar]
- 22.Bemelman F, Honey K, Adams E, Cobbold S, Waldmann H. Bone marrow transplantation induces either clonal deletion or infectious tolerance depending on the dose. J Immunol. 1998;160:2645–2648. [PubMed] [Google Scholar]
- 23.Shimizu A, Yamada K, Meehan SM, Sachs DH, Colvin RB. Acceptance reaction: intragraft events associated with tolerance to renal allografts in miniature swine. J Am Soc Nephrol. 2000;11:2371–2380. doi: 10.1681/ASN.V11122371. [DOI] [PubMed] [Google Scholar]
- 24.Colvin RB. The renal allograft biopsy. Kidney Int. 1996;50:1069–1082. doi: 10.1038/ki.1996.410. [DOI] [PubMed] [Google Scholar]
- 25.Giangrande I, Yamada K, Arn S, Lorf T, Sachs DH, LeGuern C. Selective increase in CD4-positive graft-infiltrating mononuclear cells among the infiltrates in class I disparate kidney grafts undergoing rejection. Transplantation. 1997;63:722–728. doi: 10.1097/00007890-199703150-00019. [DOI] [PubMed] [Google Scholar]
- 26.Abe Y, Urakami H, Ostanin D, et al. Induction of Foxp3-expressing regulatory T-cells by donor blood transfusion is required for tolerance to rat liver allografts. PLoS One. 2009;4:e7840. doi: 10.1371/journal.pone.0007840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kingsley CI, Karim M, Bushell AR, Wood KJ. CD25+CD4+ regulatory T cells prevent graft rejection: CTLA-4- and IL-10-dependent immunoregulation of alloresponses. J Immunol. 2002;168:1080–1086. doi: 10.4049/jimmunol.168.3.1080. [DOI] [PubMed] [Google Scholar]
- 28.Bushell A, Karim M, Kingsley CI, Wood KJ. Pretransplant blood transfusion without additional immunotherapy generates CD25+CD4+ regulatory T cells: a potential explanation for the blood-transfusion effect. Transplantation. 2003;76:449–455. doi: 10.1097/01.TP.0000083043.84630.99. [DOI] [PubMed] [Google Scholar]
- 29.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- 30.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 31.Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol. 2003;4:337–342. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- 32.Graca L, Cobbold SP, Waldmann H. Identification of regulatory T cells in tolerated allografts. J Exp Med. 2002;195:1641–1646. doi: 10.1084/jem.20012097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cobbold SP, Castejon R, Adams E, et al. Induction of foxP3+ regulatory T cells in the periphery of T cell receptor transgenic mice tolerized to transplants. J Immunol. 2004;172:6003–6010. doi: 10.4049/jimmunol.172.10.6003. [DOI] [PubMed] [Google Scholar]
- 34.Lee I, Wang L, Wells AD, Dorf ME, Ozkaynak E, Hancock WW. Recruitment of Foxp3+ T regulatory cells mediating allograft tolerance depends on the CCR4 chemokine receptor. J Exp Med. 2005;201:1037–1044. doi: 10.1084/jem.20041709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rubin-Kelley VE, Jevnikar AM. Antigen presentation by renal tubular epithelial cells. J Am Soc Nephrol. 1991;2:13–26. doi: 10.1681/ASN.V2113. [DOI] [PubMed] [Google Scholar]
- 36.Kelley VR, Singer GG. The antigen presentation function of renal tubular epithelial cells. Exp Nephrol. 1993;1:102–111. [PubMed] [Google Scholar]
- 37.Frasca L, Marelli-Berg F, Imami N, et al. Interferon-gamma-treated renal tubular epithelial cells induce allospecific tolerance. Kidney Int. 1998;53:679–689. doi: 10.1046/j.1523-1755.1998.00800.x. [DOI] [PubMed] [Google Scholar]