Abstract
The enzyme cyclooxygenase-2 (COX-2) is induced at high levels in tumors, but not in surrounding normal tissues, which makes it an attractive target for molecular imaging of cancer. We evaluated the ability of novel optical imaging agent, fluorocoxib A to detect urinary bladder canine transitional cell carcinomas (K9TCC). Here, we show that fluorocoxib A uptake overlapped with COX-2 expression in primary K9TCC cells in vitro. Using subcutaneously implanted primary K9TCC in athymic mice, we demonstrate specific uptake of fluorocoxib A by COX-2-expressing K9TCC xenograft tumors in vivo. Fluorocoxib A uptake by COX-2 expressing xenograft tumors was blocked by 70% (p<0.005) when pre-treated with the COX-2 selective inhibitor, celecoxib (10 mg/kg), 4 h before intravenous administration of fluorocoxib A (1 mg/kg). Fluorocoxib A was taken up by COX-2-expressing tumors, but not by COX-2 negative human UMUC-3 xenograft tumors. UMUC-3 xenograft tumors with no expression of COX-2 showed no uptake of fluorocoxib A. In addition, fluorocoxib A uptake was evaluated in 5 dogs diagnosed with TCC. Fluorocoxib A specifically detected COX-2-expressing K9TCC during cystoscopy in vivo, but was not detected in normal urothelium. Taken together, our findings show that fluorocoxib A selectively bound to COX-2 expressing primary K9TCC cells in vitro, COX-2 expressing K9TCC xenografts tumors in nude mice and heterogeneous canine TCC during cystoscopy in vivo. Spontaneous cancers in companion animals offer a unique translational model for evaluation of novel imaging and therapeutic agents using primary cancer cells in vitro and in heterogeneous cancers in vivo.
Keywords: COX-2, fluorocoxib A, canine, urinary bladder cancer, cystoscopy
Introduction
Bladder cancer is the fourth most common cancer in men and the eighth most common malignancy in women in the United States according to the American Cancer Society (1). In general, bladder cancer is curable when detected and treated early; therefore, a more accurate early diagnosis of bladder cancer would be advantageous. Bladder cancer in dogs is the most common tumor of the urinary tract and comprises about 1% of all canine cancers (2). Canine transitional cell carcinoma (K9TCC) closely resembles human invasive urinary bladder cancer (3). Urinary bladder tumors in dogs are usually malignant with only 3% of tumors being benign.
Sensitivity and specificity of conventional cystoscopy of bladder tumors can be significantly improved by using tumor-specific agents (4). In contrast to cyclooxygenase (COX) -1, COX-2 is not expressed in most normal tissues, but rather is induced in inflamed tissues and in many carcinomas (5–11). COX-2 expression significantly increases with human urinary bladder tumor stage, comparing muscle-invasive tumors to superficially invasive tumors (12,13). COX-2 is undetectable in human normal urinary bladder 0/10, but COX-2 is expressed in 29/29 of bladder squamous cell carcinomas (SCC), and in 12/35 (34%) of TCC (12,14). Genetically modified COX-2−/− mice exhibit decreased incidence of intestinal and skin tumors (15,16). Numerous pharmacological studies validate COX-2 as a therapeutic target to control the inflammation and tumorigenesis (5,17,18). Thus, newly synthesized non-steroidal anti-inflammatory drugs (NSAIDs) are attractive not only as pharmacological and chemo-preventive agents, but now also as a new family of imaging agents (19,20).
The use of the COX-2 selective inhibitors as a new class of imaging agents is based on the selective uptake by COX-2-expressing neoplastic lesions. The first synthesis and characterization of radioactively-labeled COXIBs as cancer imaging agents have been reported (21,22). As we published previously, promising results were obtained using a derivative of celecoxib as a SPECT radiotracer to identify COX-2- expressing, carcinogen-induced, lung and pancreatic lesions in hamsters (22). Additional novel PET and SPECT imaging radiotracers were later synthesized and evaluated in cancers and inflammation rodent models (23,24). Novel synthesized derivatives of NSAIDs labeled with rhodamine dyes have been evaluated as optical imaging agents for detection of COX-2 expressing inflammation and cancer in rodents (25,26). One of the most promising optical imaging agent that selectively binds to COX-2 called fluorocoxib A, N-[(5-carboxy-X-rhodaminyl)but-4-yl]-2-[1-(4-chlorobenzoyl)-5-methoxy-2-methyl-1 H-indol-3-yl]acetamide (λex = 580 nm andλem = 605 nm) (25). Fluorocoxib A is a weak inhibitor of purified ovine COX-1 and a strong inhibitor of purified human COX-2 enzyme (IC50 =25 μM,IC50 = 0.7 μM; respectively). Fluorocoxib A inhibits LPS- and IFNγ-induced COX-2 activity in murine macrophage-like cells RAW264.7 (IC50 = 0.31 μM) (25,26). Fluorocoxib A was evaluated using carrageenan-induced acute inflammation in the mouse footpad, human tumor xenografts in nude mice, and in mice with spontaneous tumors (25) in vivo. Human colorectal cancer HCT-116 xenografts that do not express COX-2, exhibit minimal fluorescence, whereas the human SCC 1483 xenografts with high levels of COX-2 expression, exhibited bright fluorescence after fluorocoxib A administration (25). Nude mice with 1483 xenografts pretreated with indomethacin (2 mg/kg, i.p.) 2 h before fluorocoxib A administration (2 mg/kg, i.p.) show 92 ± 6% (n = 8) reduction of the fluorocoxib A uptake in these tumors (25). In addition, the COX-2 non-targeted fluorophores, such as 5-ROX and 6-ROX alone, did not accumulate in COX-2 – expressing tumor xenografts, supporting specificity of fluorocoxib A uptake in COX-2-expressing tumors in vivo. We also confirmed specific fluorocoxib A uptake in COX-2-expressing colorectal adenocarcinomas in dogs during endoscopy in vivo (27).
Often, usage of rodent models for testing of new imaging tracers or therapeutic drugs fails in bench-to-bedside translation. To fill this gap, we evaluated fluorocoxib A for the detection of TCC, utilizing not only a rodent model with K9TCC xenografts, but also dogs with naturally-occurring urinary bladder carcinomas during cystoscopy in vivo. In this study, we confirmed selective fluorocoxib A uptake by COX-2-expressing primary K9TCC cells, COX-2-expressing K9TCC xenografts tumors in nude mice, and K9TCC during cystoscopy in dogs.
Material and methods
Fluorocoxib A was synthesized according to the previously described method (25,26). The chemical structure of fluorocoxib A is shown in Figure 1.
Figure 1.
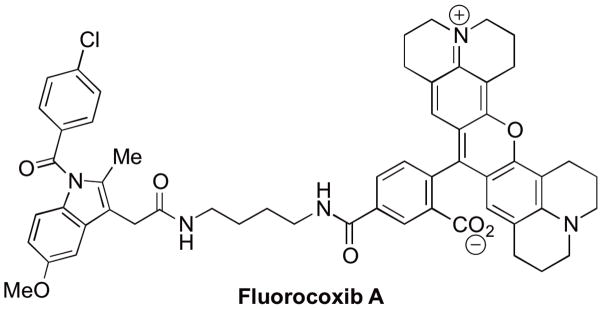
Chemical structure of fluorocoxib A.
Antibodies and other reagents
Antibody for COX-2 were obtained from Cayman Chemical Corporation (Ann Arbor, MI); E-cadherin and actin were purchased from Santa Cruz Biotechnology (Santa Cruz, CA); F4/80 was obtained from Abcam (Cambridge, MA); phosphorylated p-65 (p-p65) was obtained from BD Biosciences (San Jose, CA); lysozyme and cytokeratin were purchased from Dako (Carpinteria, CA).
Human cell lines
Human transitional cell carcinomas T-24 and UMUC-3 were purchased from American Type Culture Collection (ATCC, Manassas, VA) in 2010. Human T-24 cells were maintained in McCoy’s media, and human UMUC-3 cells were maintained in EMEM media; respectively, supplemented with 10% fetal bovine serum, 100 I.U. penicillin, and 100 μg/ml streptomycin and grown in an atmosphere of 5% COfat 37°C. Cell lines were authenticated via short tandem repeat (STR) DNA profiling by Genetica DNA laboratories (Burlington, NC) justprior manuscript submission.
Isolation of primary K9TCC cells
K9TCC primary cells were isolated from tumor biopsies from client-owned dogs with TCC during cystoscopy procedures according to approved the University of Tennessee Institutional Animal Care and Use Committee (UT IACUC) protocol. The biopsies confirmed by veterinary pathologists were trypsinized and the isolated cells were cultured in RPMI-1640 media supplemented with 10% fetal bovine serum and antibiotics for 24 h. Colonies of K9TCC cells were further expanded and after reaching 80% confluence, the K9TCC cells were sub-cultured and early passages of cells were used for experiments. Primary K9TCC#1Lillie cells were obtained in 2009; K9TCC#2Dakota cells were obtained in 2011, and K9TCC#3Buffy cells were obtained in 2012. Cell lines were tested to prove the epithelial-cell origin by E-cadherin and cytokeratin expressions in vitro and tested for tumorigenic behavior of cells using xenograft mouse model in vivo.
COX-2 Immunofluorescence in K9TCC
Primary K9TCC cells were plated on four-chamber slides (Nalge Nunc, Rochester, NY) and grown for 24 h. Then the cells were treated with 1 μM fluorocoxib A for 1 h, washed and fixed in 4% paraformaldehyde. The non-specific binding of the antibodies was blocked for 30 min at room temperature (r.t.). The cells were incubated with COX-2 primary antibodies overnight at 4°C and incubated with secondary anti-rabbit AlexaFluor 488nm antibodies for 1 h at r.t. in darkness. The nuclei of cells were counter-stained with DAPI (blue color). Slides were evaluated by fluorescence confocal microscopy.
Animals
All animal studies were performed in accordance with the UT IACUC approved protocols and in accordance with the NIH guidelines. Four to five-week-old female athymic nude Foxn1nu mice were purchased from Harlan Laboratories (Indianapolis, IN) and Taconic Laboratories (Hudson, NY). Enrolled client-owned female dogs with bladder TCC with weights of 10 – 20 kg were intravenously (i.v.) injected with fluorocoxib A 1 mg/kg over 20 min and then after 18 – 24 h imaged during cystoscopy using Karl Storz imaging system.
Fluorocoxib A uptake by K9TCC and UMUC-3 xenograft tumors detected by IVIS Lumina system in vivo
Two primary K9TCC cancer cell lines with confirmed COX-2 expression and human UMUC-3 lacking expression of COX-2 were subcutaneously implanted in female athymic mice. All tested mice developed subcutaneous tumors with a size of approximately 0.8–1.5cm3 within three weeks after implantation with K9TCC (1.5x106 mixed 1:1 with Matrigel) and UMUC-3 (3x106 mixed 1:1 with Matrigel). To evaluate the ability of fluorocoxib A to target COX-2 in K9TCC#1Lillie xenografts tumors, mice were injected with fluorocoxib A (1 mg/kg, i.v.) and then up to 24 h imaged using Xenogen IVIS Lumina with DsRed filters with excitation 500–550nm and emission 575–650 nm and background 460–490 nm. Obtained total flux (p/s) and averaged radiance (p/s/cm2/sr) of labeled ROI of selected tissues were analyzed.
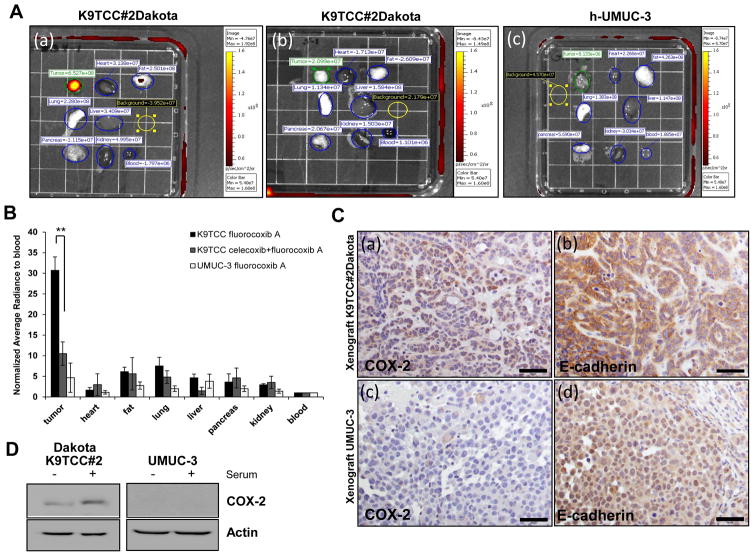
To prove the specificity of fluorocoxib A binding to COX-2 expressing TCC in vivo, we performed two additional experiments using COX-2 expressing K9TCC#2Dakota in xenograft mice pretreated with selective COX-2 inhibitor - celecoxib (10 mg/kg) 4 h before injecting with fluorocoxib A (1 mg/kg). In addition, we used UMUC-3 human TCC with no COX-2 expression. The xenograft tumors were imaged up to 24 h post-injection with fluorocoxib A using IVIS system. The normalized averaged radiance of each tissue to blood was evaluated and compared between groups. After imaging, tissues were formalin-fixed and paraffin-embedded for histological analysis. Histology and expression of COX-2 and E-cadherin in K9TCC#2Dakota and UMUC-3 xenograft tumors were assessed by IHC analysis.
Fluorocoxib A uptake by heterogeneous K9TCC during cystoscopy
All cystoscopy and imaging procedures in dogs were performed by a board-certified veterinary internal medicine specialist (JWB) in accordance with standard veterinary care and the UTIACUC approved protocol as described previously (27). Five client-owned female dogs with bladder cancer lesions were enrolled in our study. The owners signed a consent form to agree enrolling their pets to this study to evaluate fluorocoxib A uptake in TCC during cystoscopy. Fluorocoxib A was administrated i.v. 1mg/kg over 20 min using a catheter, followed by up to 24 h uptake by COX-2 expressing bladder tumor cells. The concentration of fluorocoxib A (1 mg/kg) was determined based on results from our pharmacokinetic and safety studies (27). Cystoscopy was used to evaluate the bladder cancer lesion, specificity of fluorocoxib A uptake, to obtain biopsy samples for diagnosis. The client-owned dogs were returned to owners after complete recovery from the cystoscopic examination and anesthesia.
Immunohistochemistry
Xenograft tumors from athymic mice and primary tumor biopsies from dogs with TCC were formalin-fixed paraffin-embedded and sectioned at 7 μm. After de-paraffinization and re-hydration, the sections were stained for COX-2 (1:500 overnight at 4 °C), E-cadherin (1:500 overnight at 4 °C), cytokeratin (1:800 overnight at 4 °C), p-p65 (1:1,000 for 1 h at r.t.), F4/80 (1:500 for 30 min at r.t.), or lysozyme (1:400 for 30 min at r.t.) using IHC protocol as described previously (27). The images were captured by DP71 camera (Hunt Optics and Imaging, Pittsburgh, PA) attached to Olympus microscope BX41 (Pittsburgh, PA) or Leica Leitz DMRB (Buffalo Grove, IL) with microscopic objectives 4x, 20x, and 40x using the CellSens Standard imaging software (HuntOptics and Imaging, Pittsburg, PA) and Adobe Photoshop CS5 (Adobe). The stained slides were reviewed by MC and the percentage of COX-2 positive normal and neoplastic epithelial cells was scored as either absent (-), low (+, less than 20% of positive tumor cells/40x objective), moderate (++, 20–50% positive tumor cells/40x objective), or high (+++, more than 50% positive tumor cells/40x objective).
Western Blotting
Human and canine TCC cells were cultured in media with or without serum for 24 h. The cells were lysed, proteins were extracted and used for detection of COX-2 expression following the WB protocol as described previously (27).
Statistical Analysis
Statistical analyses of obtained data were performed using the Student’s t-test. Results were considered statistically significant at *p<0.05, **p<0.01, and ***p<0.001.
Results
COX-2 expression in canine bladder lesions
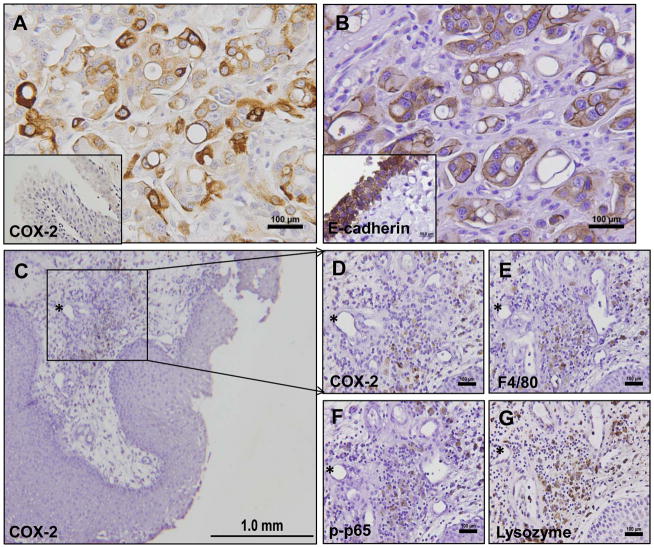
The expression of COX-2 in bladder lesions was assessed by IHC analysis. We evaluated 16 carcinomas and 8 acute and chronic inflammatory lesions of canine urinary bladder specimens obtained from tissue repository bank at UTCVM. IHC analysis of COX-2 expression revealed absent (1/16), low (1/16), moderate (4/16), or high (10/16) levels of COX-2 positive K9TCC cells. The representative image of high levels of COX-2 positive cells in K9TCC (brown color) is shown in Figure 2A. Canine bladder carcinomas showed strong expression of COX-2 in the cytoplasm with perinuclear localization in the tumor cells. The COX-2 positive cells of bladder tumors were predominantly localized at the periphery of K9TCC cancer, whereas the centers of K9TCC tumors contained mostly COX-2 negative cells. The normal urothelium did not show any expression of COX-2 (inset in Figure 2A). The expression of E-cadherin (brown color) is shown in Figure 2B to confirm the epithelial-cell origin of tested K9TCC. K9TCC showed reduced E-cadherin expression as compared to normal urothelium (inset in Figure 2B) confirming one of the characteristic of invasive adenocarcinomas.
Figure 2. COX-2 expression in K9TCC in vitro.
(A) Representative images of COX-2 in K9TCC by IHC (brown color). Inset image show no detection of COX-2 in normal canine urothelium. (B) Expression of E-cadherin confirms the epithelial-cell origin of K9TCC expressing COX-2. (C, D) COX-2, (E) F4/80, (F) p-p65, and (G) lysozyme expressions in macrophages of the submucosa layer of canine bladder inflammation.
We evaluated the expression of COX-2 in the biopsies from dogs with acute and chronic inflammation (n=8). The histology of these tissues revealed mild edema and moderate multifocal inflammatory cell infiltrates that were predominantly composed of perivascular plasma cells, eosinophils, lymphocytes, neutrophils, and macrophages. No COX-2 expression was detected in submucosa and urothelium with neutrophilic and lymphocytic cells inflammation (Supplemental Figure S1). Only the infiltrating perivascular macrophages in the submucosa showed positive COX-2 expression (brown color) as shown in Figure 2C and 2D. We performed additional IHC for detection of F4/80 (macrophage marker), p-p65 (active p-p65, inflammatory marker) and lysozyme (macrophage marker) expressions in order to confirm the presence of inflammatory macrophages in the submucosa as shown in Figure 2E–G.
Fluorocoxib A uptake by K9TCC cells in vitro
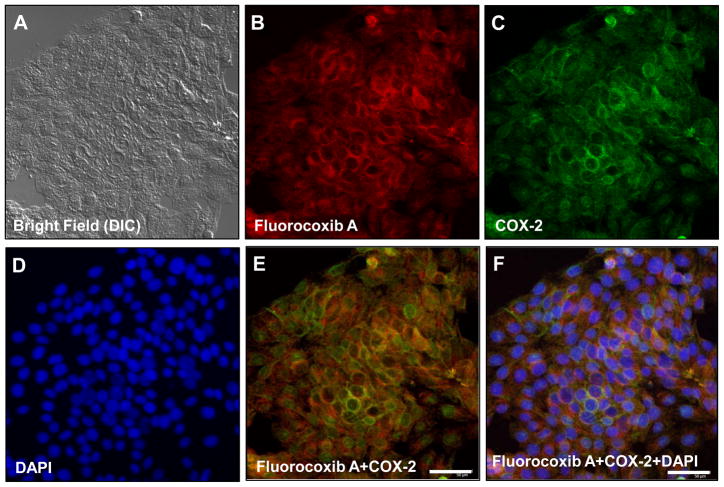
To confirm the reactivity of fluorocoxib A to canine COX-2, we isolated K9TCC primary cancer cells from biopsies obtained during cystoscopy procedures. The primary K9TCC cells (K9TCC#1Lillie Figure 3A, bright field using differential interference contrast microscopy, DIC) were incubated with 1 μM fluorocoxib A for 1 h and stained with COX-2 specific antibodies labeled with Alexa Fluor 488 dye. As shown in Figure 3B fluorocoxib A (red color) penetrated into the cytoplasm of K9TCC and specifically bound to COX-2 (green color) located at perinuclear areas as shown in Figure 3C. The nuclei of K9TCC were counterstained with DAPI staining (blue color) as shown in Figure 3D. Co-localization of COX-2 and fluorocoxib A uptake was confirmed by overlaying the images as shown in Figure 3E (yellow color). The overlaid image of fluorocoxib A, COX-2 and DAPI is shown in Figure 3F.
Figure 3. Fluorocoxib A uptake by COX-2-expressing K9TCC#1Lilie cells in vitro.
(A) DIC image of primary K9TCC#1Lillie cells. (B) Specific uptake of fluorocoxib A (1 μM; 1 h) by K9TCC#1Lillie (red color) (C) with COX-2 expression detected by Alexa Fluor488nm (green color) (D) and counterstained with DAPI for nuclei detection (blue color). (E) Merged (B and C) images to confirm overlap of fluorocoxib A uptake with COX-2 expression. (F) Merged (B, C and D) images.
Fluorocoxib A uptake by K9TCC xenograft tumors in vivo
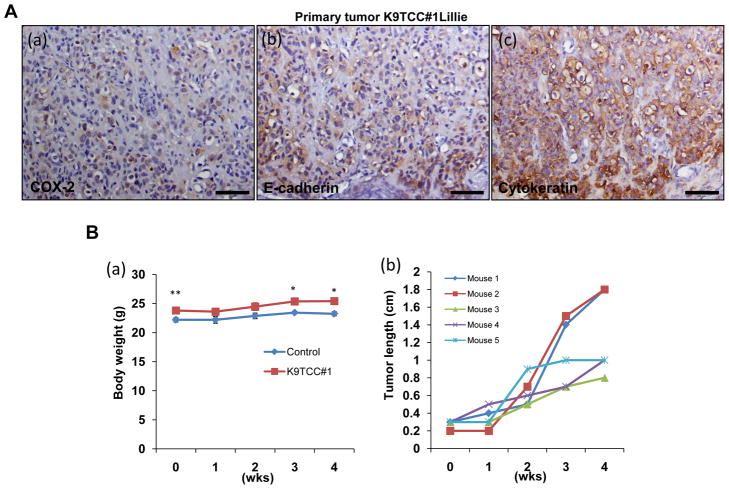
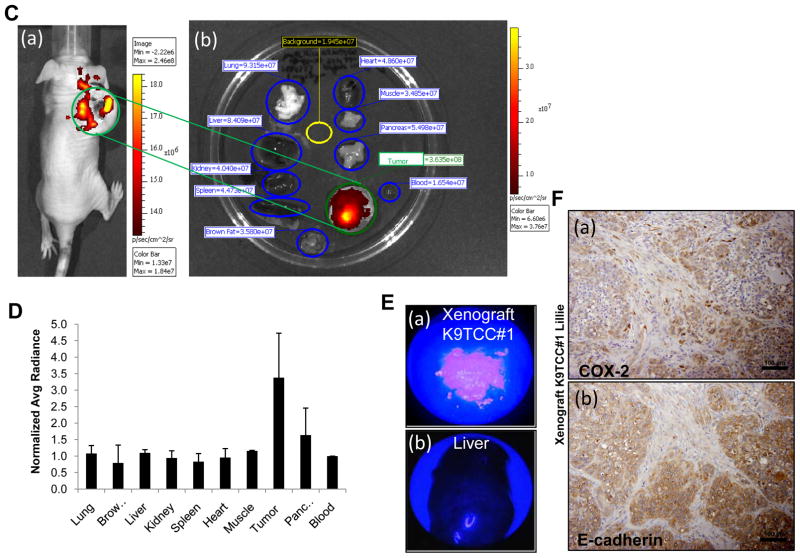
Canine TCC#1Lillie cells were isolated from tumor with confirmed COX-2, E-cadherin and cytokeratin expressions as shown in Figure 4A by IHC. The isolated K9TCC#1Lillie cells were subcutaneously implanted in female nude mice. The body weights of mice bearing the tumors were shown in Figure 4B(a). Tested nude mice (n = 5) developed subcutaneous tumors with 100% incidence with a size of approximately 1–1.8cm3 within three weeks after injection with K9TCC (1.5x106 with matrigel/mouse) as shown in Figure 4B(b). To evaluate the ability of fluorocoxib A to target COX-2 in K9TCC#1Lillie xenograft tumors, four mice were injected with fluorocoxib A (1 mg/kg, i.v.) and after up to 24 h imaged using IVIS Lumina System. K9TCC#1Lillie xenograft tumors exhibited bright fluorescence signal (intensive red-yellow color in ROI green circle) as shown in Figure 4C(a). Specific and selective uptake of fluorocoxib A by K9TCC#1Lillie xenograft tumors were confirmed by comparing the total flux of dissected tissues (in blue circles ROI), such as lung, liver, kidney, spleen, brown fat, heart, muscle, pancreas, blood, and background (ROI in yellow circle) ex vivo as shown in Figure 4C(b). The averaged radiance (p/s/cm2/sr) of each tissue was normalized to blood as shown in Figure 4D. K9TCC#1Lillie xenograft tumors showed 3.5-fold increased fluorocoxib A uptake as compared to blood. Specific uptake of fluorocoxib A in K9TCC#1Lillie tumor was also confirmed by Karl Storz PDD imaging system ex vivo. Fluorocoxib A was detected in xenograft tumors as shown in upper image of Figure 4E(a) with pink color and no detection of fluorocoxib A uptake was detected in liver as shown in the lower image of Figure 4E(b). To confirm specific uptake of fluorocoxib A by COX-2-expressing K9TCC#1Lillie, we performed histological evaluation of dissected K9TCC#1Lillie xenograft tumors for COX-2 expression. Histological analysis of dissected K9TCC#1Lillie xenograft tumors stained with hematoxylin-eosin revealed that tumors were encapsulated with partial central necrosis and with moderate number of mitotic figures. K9TCC#1Lillie cells showed positive perinuclear COX-2 expression (brown color) as shown in Figure 4F(a). In addition, infiltrated macrophages in stroma expressed COX-2 enzyme (brown color). Membrane E-cadherin expression (brown color) in K9TCC#1Lillie xenograft tumors confirmed the epithelial-cell origin of K9TCC#1Lillie as shown in Figure 4F(b).
Figure 4. Detection of COX-2-expressing K9TCC#1Lillie xenograft tumors by fluorocoxib A in vivo.
(A) Primary K9TCC#1Lillie tumors show positive (a) COX-2, (b) E cadherin, and (c) cytokeratin expressions (brown color). (B) Confirmed tumorigenic behavior of established primary K9TCC#1Lillie cells with 100% incidence (n = 5). (a) A graph showed slightly higher body weight of K9TCC#1 tumor bearing mice vs control mice (n = 5). Values represent mean ± S.E., paired Student’s t-test p<0.05*, p<0.01**. (b) A graph shows the increase of the length of tumor (cm) over 5 weeks. (C) (a) Whole body image of mouse with COX-2 positive K9TCC#1Lillie xenograft tumor (ROI, green circle) 24 h after i.v. injection of 1 mg/kg fluorocoxib A. (b) Specific uptake of fluorocoxib A by K9TCC#1Lillie tumor (ROI, green circle) as compared to other dissected tissues imaged ex vivo (from the top on left side: lung, liver, kidney, spleen, brown fat; from top on right side: heart, muscle, pancreas, K9TCC#1Lillie tumor without skin, blood; background ROI in yellow circle) using IVIS system. (D) Averaged radiance (p/s/cm2/sr) of each tissue was normalized to blood (n = 4). (E) Specific uptake of fluorocoxib A detected (a) in K9TCC#1Lillie tumor (pink color) as compared to (b) liver after dissection from mouse using Karl Storz PDD imaging system ex vivo. (F) (a) COX-2 and (b) E-cadherin expressions (brown color) in K9TCC#1Lillie xenograft tumors counterstained with hematoxylin (nuclei, blue color).
Specificity of fluorocoxib A uptake by COX-2-expressing TCC xenograft tumors in vivo
To prove specific uptake of fluorocoxib A by COX-2-expressing tumors in vivo, we used K9TCC#2Dakota xenograft tumor mice that were pretreated with the selective COX-2 inhibitor, celecoxib (10 mg/kg, i.v.) 4 h before i.v. administration of fluorocoxib A (1 mg/kg). In addition, we used UMUC-3 human TCC cells that have no expression of COX-2 as shown in Figure 5. K9TCC#2Dakota xenograft tumors expressing COX-2 had strong specific uptake of fluorocoxib A as compared to other organs as shown in Figure 5A(a). Fluorocoxib A uptake by K9TCC#2Dakota xenograft tumors was significantly reduced by approximately 70% (p<0.005) when mice (n=3/group) were pretreated with celecoxib (4 h before injecting with fluorocoxib A) as shown in Figures 5A(b) and 5B. Furthermore, UMUC-3 xenograft tumors showed no uptake of fluorocoxib A by tumors due to lack of COX-2 expression as shown in Figure 5A(c) and 5B. K9TCC#2Dakota xenograft tumors had positive perinuclear COX-2 expression in cells by IHC (brown color) as shown in Figure 5C(a). Positive E-cadherin expression confirmed epithelial cell origin of TCC as shown in Figure 5C(b). Primary K9TCC#2Dakota tumor with confirmed COX-2 and E-cadherin expressions by IHC is shown in Supplemental Figure S2A(a, b). The UMUC-3 xenograft tumors had no expression of COX-2, but had a positive E-cadherin expression as shown in Figure 5C(c, d). The size of developed xenograft tumors using K9TCC#2Dakota and UMUC-3 in athymic mice over a 3-week period are shown in Supplemental Figure S2B(a, b). In addition, we observed that UMUC3 tumors of approximately the same size were more vascularized than K9TCC xenograft tumors as shown in enlarged images of xenograft tumors in Supplemental Figure S2B. The expression of COX-2 was confirmed by western blot in primary K9TCC#2Dakota, but not in UMUC-3 cells as shown in Figure 5D. Taken together, these data showed that fluorocoxib A uptake was specific for COX-2 expressing tumors and fluorocoxib A was not accumulated in tumors due to increased vascular permeability in tumors.
Figure 5. Specific uptake of fluorocoxib A by TCC in vivo.
(A)(a) Specific uptake of fluorocoxib A by K9TCC#2Dakota xenograft tumor (ROI, green circle) 20–24h after i.v. injection with fluorocoxib A (1 mg/kg) (n = 3) (b) K9TCC#2Dakota tumors (ROI, green circle) pretreated 4 h with celecoxib (10 mg/kg, i.v.) before fluorocoxib A injection (n = 3); (c) UMUC-3 xenograft tumors (ROI, green circle) as compared to other dissected tissues from athymic mice (n = 3). Dissected tissues imaged ex vivo (from the top on left side: tumor, lung, pancreas; from top in middle row: heart, liver, kidney; from the top on right side: fat and blood; background ROI in yellow circle) using IVIS System. (B) Averaged radiance (p/s/cm2/sr) of each tissue was normalized to blood. The fluorocoxib A uptake by K9TCC#2Dakota xenograft tumors were significantly blocked by 70% by pretreatment with celecoxib. Values represent mean ± S.E. (n=3), paired Student’s t-test p<0.005. The UMUC-3 xenograft tumors with no COX-2 expression showed no uptake of fluorocoxib A. (C) (a) COX-2 and (b) E-cadherin expressions (brown color) in K9TCC#2Dakota xenograft tumors and (c, d) UMUC-3 xenograft tumors counterstained with hematoxylin (nuclei, blue color). (D) Confirmed COX-2 expression in established primary K9TCC#2Dakota and COX-2 negative UMUC-3 TCC cells in presence or absence of serum in culture media for 24 h using WB analysis. Actin was used as loading control.
Detection of COX-2-expressed K9TCC by fluorocoxib A during cystoscopy using Karl Storz imaging system
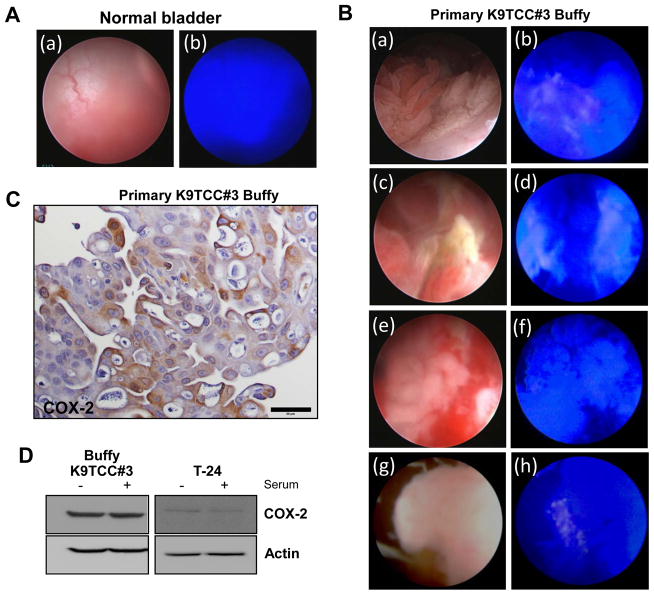
We evaluated this novel optical agent, fluorocoxib A, in dogs with naturally-occurring heterogeneous bladder tumors as a more realistic and suitable translational animal model than rodents for human cancer. Five client-owned female dogs with bladder lesions were enrolled in our study for cystoscopy. Representative images of normal urothelium of canine bladder wall under bright light and fluorescence light during cystoscopy procedure are shown in Figure 6A(a and b; respectively). No uptake of fluorocoxib A was detected in normal urothelium 24 h after 1 mg/kg i.v. administration during cystoscopy as shown in Figure 6A(b). Specific uptake of fluorocoxib A by COX-2 expressing K9TCC#3Buffy was visualized by bright pink color as shown in representative images of heterogeneous K9TCC#3Buffy using bright light (left images Figure 6B a, c, e, and g) and fluorescence light (right images of Figure 6B b, d, f, h) during cystoscopy. The similar heterogeneous expression profile of COX-2 detected by fluorocoxib A was observed in additional K9TCC cases as shown in the representative images of Figure 6B.
Figure 6. Fluorocoxib Auptake by canine COX-2-expressing TCC during cystoscopy.
(A)No uptake of fluorocoxib A was detected in normal urothelium 24 h after 1 mg/kg i.v. administration of fluorocoxib A using bright light and fluorescence light during cystoscopic evaluation. (B) K9TCC#3Buffy using bright (a, c, e, and g left images) and fluorescence light (b, d, f, and h right images) during cystoscopy. Specific uptake of fluorocoxib A by COX-2-expressing heterogeneous K9TCC#3Buffy is shown by bright pink color. (C) COX-2 expression (brown color) from obtained biopsy K9TCC#3Buffy sample using IHC. (D) COX-2 expression in established primary K9TCC#3Buffy and human T-24 TCC in presence or absence of serum in culture media for 24 h using WB analysis. Actin was used as loading control.
Confirmed COX-2 expression in K9TCC biopsy samples after fluorocoxib A uptake
Small tissue K9TCC samples were obtained during cystoscopy of dogs with K9TCC imaged using fluorocoxib A. The biopsy samples were divided for histology analysis and for isolation of primary cancer K9TCC cells. COX-2 expression (brown color) was confirmed in fluorocoxib A-uptaking K9TCC#3Buffy using IHC as shown in Figure 6C. We successfully isolated primary K9TCCs from obtained biopsy samples from dogs after fluorocoxib A imaging and we confirmed COX-2 expression in established primary K9TCC#3Buffy in the presence or absence of serum in the culture medium for 24 h using western blotting analysis as shown in Figure 6D. Both K9TCC primary cells showed higher levels of COX-2 expression than human T-24 or COX-2-negative UMUC-3 TCC cell lines (Figures 5D, 6D). Increased expression of COX-2 was detected in K9TCC#2Dakota when cultured in medium containing serum.
Discussion
Because immunodeficient rodent models cannot accurately simulate the in vivo behavior patterns of heterogeneous human tumors, many new imaging agents fail in bench-to-bedside translation. Dogs with naturally-occurring tumors provide a large animal model that is more translatable; can better assess the efficacy of the molecular imaging agents to visualize heterogeneous tumors; are more applicable because the dog’s larger body size allows endoscopy/cystoscopy procedures; and are more similar to human cancers with regards to histologic appearance and biologic behavior rather than chemically- or genetically-induced rodent cancer models (28–30). Usage of primary canine cancer cells isolated from spontaneously-occurring tumors is a more suitable model than stable cell lines. In our study, we isolated primary K9TCC to verify the specificity of fluorocoxib A binding to canine COX-2 in vitro and in vivo.
COX-2-positive bladder tumors in dogs show strong expression of COX-2 in cytoplasm with perinuclear localization in the tumor and surrounding stromal cells that mimics the pattern seen in human tumors (2,29,31–33). COX-2 expression shows a significantly proportional increase (p < 0.05) with advanced stage bladder carcinomas (12,13,34). The COX-2 positive cells in K9TCC were predominantly localized at the tumor edges supporting the evidence that COX-2 is involved in tumor invasiveness and metastasis (34–36). The epidemiological studies indicate that the development of the bladder carcinomas is closely associated with chronic inflammation of the urinary tract, but the underlying mechanisms are still unknown (14,37). COX-2 is markedly expressed in K9TCC, suggesting that chronic inflammation stimulates the production of COX-2 leading to the development of bladder carcinomas. We evaluated the expression of COX-2 in the biopsy samples obtained during cystoscopy in dogs (n=8) diagnosed with acute and chronic inflammation. We included bladder inflammation to check the COX-2 expression, which might create false positive uptake of fluorocoxib A during examination of neoplastic lesions in the bladder. As shown in Supplemental Figure S1, no COX-2 expression was detected in neutrophilic and lymphocytic inflammation regions. Only the infiltrated perivascular macrophages in the submucosa showed positive COX-2 expression (Figure 2C, D), suggesting that only the presence of macrophages might give a false positive signal from fluorocoxib A uptake. However; the limited number of macrophages and the location in the submucosa in contrast to COX-2-expressing tumor cells at the periphery of the tumor might not be sufficient for the fluorescence signal from fluorocoxib A in macrophages to be seen. Additional studies using fluorocoxib A uptake in dogs diagnosed with bladder inflammation are planned for further investigation.
To prove fluorocoxib A selective binding to COX-2 expressing tumors in vivo, we pre-treated mice with the COX-2 selective inhibitor, celecoxib (10 mg/kg) 4 h before injecting fluorocoxib A. The specific uptake of fluorocoxib A was significantly reduced by 70% (p<0.005) in COX-2 expressing tumors as shown in Figure 5. Our preliminary data using 2 mg/kg of celecoxib for 2 h showed only 50% reduction (data not shown) of fluorocoxib A uptake by COX-2 expressing xenograft tumors confirming the dose- and time-dependent competition of celecoxib with fluorocoxib A binding to COX-2. In addition, to prove the specificity of the fluorocoxib A uptake by COX-2 expressing xenograft tumors, we used human UMUC3 xenograft tumors that are negative for COX-2. We did not detected fluorocoxib A uptake by these COX-2 negative UMUC xenograft tumors. We also observed that UMUC3 tumors were more vascularized than K9TCC xenograft tumors as shown in enlarged images of xenograft tumors in Supplemental Figure S2B. Taken together, our data confirmed that fluorocoxib A uptake is specific for COX-2 expressing tumors and fluorocoxib A is not accumulated in tumors due to increased vascular permeability in tumors. Our findings are in agreement with a previously published study using human colorectal cancer HCT-116 xenograft tumors with no expression of COX-2, which exhibited minimal uptake of fluorocoxib A, whereas the human head-and-neck SCC 1483 xenografts with high levels of COX-2 expression, exhibited high uptake of fluorocoxib A (25). The tumor-to-normal tissues ratio of fluorocoxib A was up to 5:1 that make this imaging agent highly sensitive and selective for detection of COX-2-expressing tumors. Fluorocoxib A specifically bound to COX-2-expressing K9TCCs and allowed better visualization and identification of the COX-2 positive K9TCC as shown in Figure 6B (pink color). Additional approaches to prove the specificity of the fluorocoxib A uptake by COX-2 expressing K9TCC in vivo will be undertaken in future studies.
Tumor detection and characterization of COX-2 expression might be useful for COX-2-targeted treatments to increase the survival of canine (38) and human patients. Numerous studies have provided evidence that inhibition of COX-2 pathways may have significant benefits for cancer treatment and prevention (19,28,31,39–42), particularly in bladder cancer (41,43–45). Interestingly, patients with COX-2 negative, non-small-cell lung cancer (NSCLC) fared worse if treated with celecoxib as adjuvant treatment to carboplatin and gemcitabine than patients given chemotherapy alone (46). None of the currently used imaging agents in clinic have the ability to detect COX-2-expressing tumors.
Although we only evaluated fluorocoxib A in bladder cancer; data obtained from this study can be useful for testing of fluorocoxib A in other types of COX-2-expressing cancers, such as breast, lung, pancreas, head and neck, and prostate carcinomas in dogs and people. In our previously published study, we have shown a specific uptake of fluorocoxib A in canine colorectal adenocarcinomas during colonoscopy in vivo (27). Exploration of this approach in the dog model provides an opportunity to assist with more rapid translation of fluorocoxib A to clinical use in people for detection of COX-2-expressing tumors. Fluorocoxib A may help to improve the visualization of COX-2-expressed cancers and help to identify appropriate patients for NSAIDs treatment.
Supplementary Material
Acknowledgments
Grant Supports: The University of Tennessee Center of Excellence in Livestock Diseases and Human Health (COE) grants R181721223, R181721257, R181721276 and research grants to Vanderbilt University VUIIS NIH CA128323 and CA136465.
We thank Dr. Daniel Close (UT) for assistance with imaging analysis using IVIS system, Dr. John R. Dunlap (UT) for assistance with confocal microscopy, Dr. Robert Donnell (UTCVM) for assistance with capturing some IHC images. We thank Gina Galyon (UT) for her technical assistance with nude mice experiments.
Footnotes
Financial disclosures: Vanderbilt University holds a patent on fluorocoxibs and their use for COX-2-targeted imaging.
Author’s Contributions
Conception and design: M. Cekanova
Development of methodology: M. Cekanova, J. Uddin, J. Barges, A. Legendre, L. Marnett
Acquisition of data: M. Cekanova, J. Bartges, K. Rathore
Analysis and interpretation of data: M. Cekanova, J. Bartges
Writing, review and/or revision of manuscript: M. Cekanova, J. Uddin, A. Legendre, J. Bartges, L. Marnett
Administrative, technical, or material support: A. Callens, L. Wright, A. Carter, K. Rathore
Study supervision: M. Cekanova
References
- 1.American Cancer Society. Cancer Facts and Figures 2013. 2013 www.cancer.org.
- 2.Lee JY, Tanabe S, Shimohira H, Kobayashi Y, Oomachi T, Azuma S, et al. Expression of cyclooxygenase-2, P-glycoprotein and multi-drug resistance-associated protein in canine transitional cell carcinoma. Res Vet Sci. 2007;83:210–6. doi: 10.1016/j.rvsc.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 3.Mutsaers AJ, Widmer WR, Knapp DW. Canine transitional cell carcinoma. J Vet Intern Med. 2003;17:136–44. doi: 10.1892/0891-6640(2003)017<0136:ctcc>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 4.Michalski MH, Chen X. Molecular imaging in cancer treatment. Eur J Nucl Med Mol Imaging. 2011;38:358–77. doi: 10.1007/s00259-010-1569-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marnett LJ. The COXIB experience: a look in the rearview mirror. Annu Rev Pharmacol Toxicol. 2009;49:265–90. doi: 10.1146/annurev.pharmtox.011008.145638. [DOI] [PubMed] [Google Scholar]
- 6.Gallo O, Franchi A, Magnelli L, Sardi I, Vannacci A, Boddi V, et al. Cyclooxygenase-2 pathway correlates with VEGF expression in head and neck cancer. Implications for tumor angiogenesis and metastasis. Neoplasia. 2001;3:53–61. doi: 10.1038/sj.neo.7900127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taketo MM. COX-2 and colon cancer. Inflamm Res. 1998;47 (Suppl 2):S112–6. doi: 10.1007/s000110050295. [DOI] [PubMed] [Google Scholar]
- 8.Eberhart CE, Coffey RJ, Radhika A, Giardiello FM, Ferrenbach S, DuBois RN. Up-regulation of cyclooxygenase 2 gene expression in human colorectal adenomas and adenocarcinomas. Gastroenterology. 1994;107:1183–8. doi: 10.1016/0016-5085(94)90246-1. [DOI] [PubMed] [Google Scholar]
- 9.Abdalla SI, Lao-Sirieix P, Novelli MR, Lovat LB, Sanderson IR, Fitzgerald RC. Gastrin-induced cyclooxygenase-2 expression in Barrett’s carcinogenesis. Clin Cancer Res. 2004;10:4784–92. doi: 10.1158/1078-0432.CCR-04-0015. [DOI] [PubMed] [Google Scholar]
- 10.Liu CH, Chang SH, Narko K, Trifan OC, Wu MT, Smith E, et al. Overexpression of cyclooxygenase-2 is sufficient to induce tumorigenesis in transgenic mice. J Biol Chem. 2001;276:18563–9. doi: 10.1074/jbc.M010787200. [DOI] [PubMed] [Google Scholar]
- 11.Neufang G, Furstenberger G, Heidt M, Marks F, Muller-Decker K. Abnormal differentiation of epidermis in transgenic mice constitutively expressing cyclooxygenase-2 in skin. Proc Natl Acad Sci U S A. 2001;98:7629–34. doi: 10.1073/pnas.121574098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shirahama T. Cyclooxygenase-2 expression is up-regulated in transitional cell carcinoma and its preneoplastic lesions in the human urinary bladder. Clin Cancer Res. 2000;6:2424–30. [PubMed] [Google Scholar]
- 13.Wadhwa P, Goswami AK, Joshi K, Sharma SK. Cyclooxygenase-2 expression increases with the stage and grade in transitional cell carcinoma of the urinary bladder. Int Urol Nephrol. 2005;37:47–53. doi: 10.1007/s11255-004-4699-z. [DOI] [PubMed] [Google Scholar]
- 14.Shirahama T, Sakakura C. Overexpression of cyclooxygenase-2 in squamous cell carcinoma of the urinary bladder. Clin Cancer Res. 2001;7:558–61. [PubMed] [Google Scholar]
- 15.Oshima M, Dinchuk JE, Kargman SL, Oshima H, Hancock B, Kwong E, et al. Suppression of intestinal polyposis in Apc delta716 knockout mice by inhibition of cyclooxygenase 2 (COX-2) Cell. 1996;87:803–9. doi: 10.1016/s0092-8674(00)81988-1. [DOI] [PubMed] [Google Scholar]
- 16.Chulada PC, Thompson MB, Mahler JF, Doyle CM, Gaul BW, Lee C, et al. Genetic disruption of Ptgs-1, as well as Ptgs-2, reduces intestinal tumorigenesis in Min mice. Cancer Res. 2000;60:4705–8. [PubMed] [Google Scholar]
- 17.Vane JR. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol. 1971;231:232–5. doi: 10.1038/newbio231232a0. [DOI] [PubMed] [Google Scholar]
- 18.Talley JJ. Selective inhibitors of cyclooxygenase-2 (COX-2) Prog Med Chem. 1999;36:201–34. doi: 10.1016/s0079-6468(08)70048-1. [DOI] [PubMed] [Google Scholar]
- 19.Dannenberg AJ, Lippman SM, Mann JR, Subbaramaiah K, DuBois RN. Cyclooxygenase-2 and epidermal growth factor receptor: pharmacologic targets for chemoprevention. J Clin Oncol. 2005;23:254–66. doi: 10.1200/JCO.2005.09.112. [DOI] [PubMed] [Google Scholar]
- 20.Marnett LJ, Kalgutkar AS. Cyclooxygenase 2 inhibitors: discovery, selectivity and the future. Trends Pharmacol Sci. 1999;20:465–9. doi: 10.1016/s0165-6147(99)01385-1. [DOI] [PubMed] [Google Scholar]
- 21.Kuge Y, Katada Y, Shimonaka S, Temma T, Kimura H, Kiyono Y, et al. Synthesis and evaluation of radioiodinated cyclooxygenase-2 inhibitors as potential SPECT tracers for cyclooxygenase-2 expression. Nucl Med Biol. 2006;33:21–7. doi: 10.1016/j.nucmedbio.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 22.Schuller HM, Kabalka G, Smith G, Mereddy A, Akula M, Cekanova M. Detection of overexpressed COX-2 in precancerous lesions of hamster pancreas and lungs by molecular imaging: implications for early diagnosis and prevention. ChemMedChem. 2006;1:603–10. doi: 10.1002/cmdc.200500032. [DOI] [PubMed] [Google Scholar]
- 23.Uddin MJ, Crews BC, Blobaum AL, Kingsley PJ, Ghebreselasie K, Saleh SS, et al. Synthesis and evaluation of [123I]-indomethacin derivatives as COX-2 targeted imaging agents. Journal of Labelled Compounds and Radiopharmaceuticals. 2009;52:387–93. [Google Scholar]
- 24.Uddin MJ, Crews BC, Ghebreselasie K, Tantawy MN, Marnett LJ. [I]-Celecoxib Analogues as SPECT Tracers of Cyclooxygenase-2 in Inflammation. ACS Med Chem Lett. 2011;2:160–4. doi: 10.1021/ml100232q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Uddin MJ, Crews BC, Blobaum AL, Kingsley PJ, Gorden DL, McIntyre JO, et al. Selective visualization of cyclooxygenase-2 in inflammation and cancer by targeted fluorescent imaging agents. Cancer Res. 2010;70:3618–27. doi: 10.1158/0008-5472.CAN-09-2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Uddin MJ, Marnett LJ. Synthesis of 5- and 6-carboxy-X-rhodamines. Org Lett. 2008;10:4799–801. doi: 10.1021/ol801904k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cekanova M, Uddin MJ, Legendre AM, Galyon G, Bartges JW, Callens A, et al. Single-dose safety and pharmacokinetic evaluation of fluorocoxib A: pilot study of novel cyclooxygenase-2-targeted optical imaging agent in a canine model. J Biomed Opt. 2012;17:116002. doi: 10.1117/1.JBO.17.11.116002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Knapp DW, Waters DJ. Naturally occurring cancer in pet dogs: important models for developing improved cancer therapy for humans. Mol Med Today. 1997;3:8–11. doi: 10.1016/s1357-4310(96)20031-0. [DOI] [PubMed] [Google Scholar]
- 29.Spugnini EP, Porrello A, Citro G, Baldi A. COX-2 overexpression in canine tumors: potential therapeutic targets in oncology. Histol Histopathol. 2005;20:1309–12. doi: 10.14670/HH-20.1309. [DOI] [PubMed] [Google Scholar]
- 30.MacEwen EG. Spontaneous tumors in dogs and cats: models for the study of cancer biology and treatment. Cancer Metastasis Rev. 1990;9:125–36. doi: 10.1007/BF00046339. [DOI] [PubMed] [Google Scholar]
- 31.Mohammed SI, Craig BA, Mutsaers AJ, Glickman NW, Snyder PW, deGortari AE, et al. Effects of the cyclooxygenase inhibitor, piroxicam, in combination with chemotherapy on tumor response, apoptosis, and angiogenesis in a canine model of human invasive urinary bladder cancer. Mol Cancer Ther. 2003;2:183–8. [PubMed] [Google Scholar]
- 32.Mohammed SI, Khan KN, Sellers RS, Hayek MG, DeNicola DB, Wu L, et al. Expression of cyclooxygenase-1 and 2 in naturally-occurring canine cancer. Prostaglandins Leukot Essent Fatty Acids. 2004;70:479–83. doi: 10.1016/j.plefa.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 33.Pestili de Almeida EM, Piche C, Sirois J, Dore M. Expression of cyclo-oxygenase-2 in naturally occurring squamous cell carcinomas in dogs. J Histochem Cytochem. 2001;49:867–75. doi: 10.1177/002215540104900707. [DOI] [PubMed] [Google Scholar]
- 34.Shirahama T, Arima J, Akiba S, Sakakura C. Relation between cyclooxygenase-2 expression and tumor invasiveness and patient survival in transitional cell carcinoma of the urinary bladder. Cancer. 2001;92:188–93. doi: 10.1002/1097-0142(20010701)92:1<188::aid-cncr1308>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 35.Tsujii M, DuBois RN. Alterations in cellular adhesion and apoptosis in epithelial cells overexpressing prostaglandin endoperoxide synthase 2. Cell. 1995;83:493–501. doi: 10.1016/0092-8674(95)90127-2. [DOI] [PubMed] [Google Scholar]
- 36.Tsujii M, Kawano S, DuBois RN. Cyclooxygenase-2 expression in human colon cancer cells increases metastatic potential. Proc Natl Acad Sci U S A. 1997;94:3336–40. doi: 10.1073/pnas.94.7.3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.La Vecchia C, Negri E, D’Avanzo B, Savoldelli R, Franceschi S. Genital and urinary tract diseases and bladder cancer. Cancer Res. 1991;51:629–31. [PubMed] [Google Scholar]
- 38.Henry CJ. Management of transitional cell carcinoma. Vet Clin North Am Small Anim Pract. 2003;33:597–613. doi: 10.1016/s0195-5616(03)00032-9. [DOI] [PubMed] [Google Scholar]
- 39.Dannenberg AJ, Subbaramaiah K. Targeting cyclooxygenase-2 in human neoplasia: rationale and promise. Cancer Cell. 2003;4:431–6. doi: 10.1016/s1535-6108(03)00310-6. [DOI] [PubMed] [Google Scholar]
- 40.Knapp DW, Richardson RC, Bottoms GD, Teclaw R, Chan TC. Phase I trial of piroxicam in 62 dogs bearing naturally occurring tumors. Cancer Chemother Pharmacol. 1992;29:214–8. doi: 10.1007/BF00686255. [DOI] [PubMed] [Google Scholar]
- 41.Knapp DW, Richardson RC, Chan TC, Bottoms GD, Widmer WR, DeNicola DB, et al. Piroxicam therapy in 34 dogs with transitional cell carcinoma of the urinary bladder. J Vet Intern Med. 1994;8:273–8. doi: 10.1111/j.1939-1676.1994.tb03232.x. [DOI] [PubMed] [Google Scholar]
- 42.Schmidt BR, Glickman NW, DeNicola DB, de Gortari AE, Knapp DW. Evaluation of piroxicam for the treatment of oral squamous cell carcinoma in dogs. J Am Vet Med Assoc. 2001;218:1783–6. doi: 10.2460/javma.2001.218.1783. [DOI] [PubMed] [Google Scholar]
- 43.Mohammed SI, Knapp DW, Bostwick DG, Foster RS, Khan KN, Masferrer JL, et al. Expression of cyclooxygenase-2 (COX-2) in human invasive transitional cell carcinoma (TCC) of the urinary bladder. Cancer Res. 1999;59:5647–50. [PubMed] [Google Scholar]
- 44.Okajima E, Uemura H, Ohnishi S, Tanaka M, Ohta M, Tani M, et al. Expression of cyclooxygenase-2 in primary superficial bladder cancer tissue may predict risk of its recurrence after complete transurethral resection. Aktuelle Urol. 2003;34:256–8. doi: 10.1055/s-2003-41610. [DOI] [PubMed] [Google Scholar]
- 45.Grubbs CJ, Lubet RA, Koki AT, Leahy KM, Masferrer JL, Steele VE, et al. Celecoxib inhibits N-butyl-N-(4-hydroxybutyl)-nitrosamine-induced urinary bladder cancers in male B6D2F1 mice and female Fischer-344 rats. Cancer Res. 2000;60:5599–602. [PubMed] [Google Scholar]
- 46.Edelman MJ, Watson D, Wang X, Morrison C, Kratzke RA, Jewell S, et al. Eicosanoid modulation in advanced lung cancer: cyclooxygenase-2 expression is a positive predictive factor for celecoxib + chemotherapy--Cancer and Leukemia Group B Trial 30203. J Clin Oncol. 2008;26:848–55. doi: 10.1200/JCO.2007.13.8081. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.