Abstract
Understanding the mechanisms leading from DNA to molecules to neurons to networks to behavior is a major goal for neuroscience, but largely out of reach for many fundamental and interesting behaviors. The neural control of breathing may be a rare exception, presenting a unique opportunity to understand how the nervous system functions normally, how it balances inherent robustness with a highly regulated lability, how it adapts to rapidly and slowly changing conditions, and how particular dysfunctions result in disease. Why can we assert this? First and foremost, the functions of breathing are clearly definable, starting with its regulatory job of maintaining blood (and brain) O2, CO2 and pH; failure is not an option. Breathing is also an essential component of many vocal and emotive behaviors including, e.g., crying, laughing, singing, and sniffing, and must be coordinated with such vital behaviors as suckling and swallowing, even at birth. Second, the regulated variables, O2, CO2 and pH (and temperature in non-primate mammals), are continuous and are readily and precisely quantifiable, as is ventilation itself along with the underlying rhythmic motor activity, i.e., respiratory muscle EMGs. Third, we breathe all the time, except for short breaks as during breath-holding (which can be especially long in diving or hibernating mammals) or sleep apnea. Mammals (including humans) breathe in all behavioral states, e.g., sleep-wake, rest, exercise, panic, or fear, during anesthesia and even following decerebration. Moreover, essential aspects of the neural mechanisms driving breathing, including rhythmicity, are present at levels of reduction down to a medullary slice. Fourth, the relevant circuits exhibit a remarkable combination of extraordinary reliability, starting ex utero with the first air breath – intermittent breathing movements actually start in utero during the third trimester – and continuing for as many as ~109 breaths, as well as considerable lability, responding rapidly (in less than one second) and with considerable precision, over an order of magnitude in metabolic demand for O2 (~0.25 to ~5 liters of O2/min). Breathing does indeed persist! Finally, breathing is genetically determined to work at birth, with a well-defined developmental program underlying a neuroanatomical organization with apparent segregation of function, i.e., rhythmogenesis is separate from motor pattern (burst shape and coordination) generation. Importantly, single human gene mutations can affect breathing, and several neurodegenerative disorders compromise breathing by direct effects on brainstem respiratory circuits (See below).
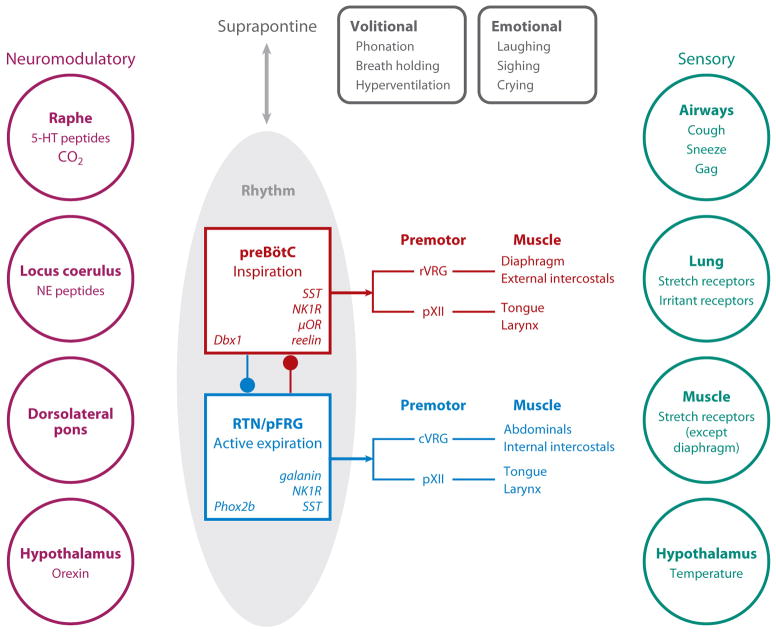
Figure 1 presents a broad overview of the central pattern generator for breathing. For breathing, we distinguish rhythmogenesis from the more complex process of pattern generation, which is the production of precisely coordinated and timed motor nerve burst patterns across a broad array of muscles pumping the lung or controlling airflow resistance. A substantial portion of the nervous system that controls breathing movements is not involved in rhythmogenesis, but instead transforms rhythmic signals into the appropriate pattern of muscle contraction. These critical but non-rhythmogenic structures certainly include motor and premotor neurons. In addition, there are sensory neurons, including mechanoreceptors and chemoreceptors. Here we focus on recent advancements related to two essential sites for rhythmogenesis (Figs 1, 4): i) the preBötzinger Complex (preBötC) in generation of inspiratory rhythm, and; ii) the retrotrapezoid nucleus/parafacial respiratory group (RTN/pFRG) in generation of active expiration. We first discuss their function, anatomy and physiology, providing a foundation for us to then discuss their developmental genetics.
Figure 1. Overview of the central pattern generator for breathing.
Core rhythm generating circuits appear to have two distinct brainstem oscillators, the endogenously active preBötC (red box) driving inspiratory activity by projections to various premotor populations that in turn project to inspiratory muscles that pump air, e.g., diaphragm and external intercostals, and those that modulate airflow resistance, e.g., laryngeal and tongue muscles, and the conditionally active RTN/pFRG (blue box) that have a similar functional path to expiratory muscles. There are numerous influences: i) Neuromodulatory (left). Respiratory pattern is highly labile. When you go from quiet sitting to slow walking, your O2 consumption increases ~3-fold, and if your ventilation does not increase rapidly you probably will pass out within 100 meters. There is widespread agreement that peptides, serotonin, norepinephrine and other endogenous neuromodulators can affect rhythmogenesis (Garcia et al 2011). These actions are essential for normal regulation and may go awry in diseases affecting breathing. ii) Suprapontine inputs (top) related to volition and emotion. iii) Sensory inputs (right) essential for proper regulation of blood gases and for mechanical adjustments related to posture, body mechanics and likely metabolic efficiency. This review focuses on the core oscillators.
Figure 4. Passive expiration transformed into active expiration.
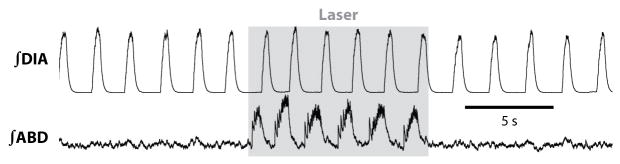
Anesthetized adult rat has active inspiration, reflected in diaphragm EMG (DIA), but passive expiration, reflected in tonic abdominal EMG. Photoactivation of lateral RTN/pFRG neurons transfected with ChR2 (gray) induces active expiration. Redrawn from (Pagliardini et al, 2010).
Caveat lector
For an outsider, and even many insiders, making sense of the literature on respiratory rhythmogenesis is challenging. The ultimate goal is to understand normal and pathological breathing in humans. However, very little experimentation relevant to rhythmogenesis can be done in humans, where the location and relatively small size of the relevant neural structures in the brainstem and prevalence of heart beat- and breathing-induced movements prevent invasive techniques and render noninvasive techniques, such as fMRI or PET, difficult (Pattinson et al 2009). ii) While all non-human in vivo experimental models are studied while breathing or in vitro or in situ while generating breathing-related neural activity, breathing rhythm and pattern are highly dependent on the details of the experimental preparation. In more or less intact mammals, breathing is markedly affected by species (even strain), body temperature, blood gas levels, anesthetic (with different anesthetics having different actions on breathing), paralysis, decerebration, integrity of peripheral nerves such as the carotid sinus and vagus nerves (which can be intact or cut), and sleep-wake state. In in situ preparations, the blood brain barrier is compromised. In rhythmic slice preparations, slight variations in the rostral or caudal borders can markedly affect the behavior, as does the composition of the bathing fluid, which can vary considerably in K+ (3–9 mM) and Ca2+ (1.0–2.5 mM) between different laboratories (Del Negro et al 2009, Ruangkittisakul et al 2011, Ruangkittisakul et al 2008, Ruangkittisakul et al 2007, Smith et al 1991). Unfortunately, we tend to pick and choose amongst results that align with our biases, often ignoring confounds that temper or invalidate the comparisons, and dismiss those that do not agree.
The terminology for breathing patterns, once well-defined and essential for straightforward comparison of experimental results (Feldman 1986), has devolved. During eupnea, commonly understood to mean quiet breathing or resting respiration in fully intact mammals, neural output to respiratory muscles is regular, with rhythmic bursts of motor activity to the diaphragm and external intercostal muscles driving inspiratory airflow, and with expiratory airflow the result of passive recoil of the lung and ribcage. The term eupnea has now been stretched to include the respiratory motor nerve patterns in hypothermic, aortic perfused, vagotomized, blood-brain barrier compromised rodent preparations that breath quite differently from intact rodents at rest (Paton 1996). Further confusing the issue is the addition of the term “fictive”, such as “fictive eupnea”, to describe the pattern of XII nerve activity in rhythmically active slice preparations (Garcia et al 2011, Lieske et al 2000). Gasping, too, is a term that in its longstanding definition described the very stereotyped last breaths in anoxic mammals just before they would otherwise asphyxiate, but now refers to various respiratory motor nerve patterns in highly reduced preparations (Garcia et al 2011, Lieske et al 2000, Paton et al 2006, St-John 2008, Viemari et al 2011), often to dismiss such activity as irrelevant to understanding mechanisms underlying “eupnea”, however defined. We disagree. Experimental limitations and discontinuity of definitions and terminology notwithstanding, advances in the past six years provide considerable new insights into the sites and mechanisms underlying breathing.
preBötzinger Complex and inspiration
There is a broad consensus (with plenty of quibbling over details) for the answer to the age-old question “Where does breathing originate?” The paradigms underlying investigation of the neural control of breathing underwent a shift with the establishment of in vitro experimental models relevant to breathing (Smith et al 1991, Suzue 1984) that led to identification of the preBötC and the hypothesis that it was the kernel for respiratory rhythmogenesis (Feldman et al 1990, Smith et al 1991). In previous reviews (Feldman & Del Negro 2006, Feldman et al 2003, Rekling & Feldman 1998), we discussed key experiments that tested this hypothesis in vivo. To better understand recent work, we note three findings. i) The preBötC is both necessary and sufficient for the generation of inspiratory motor, i.e., hypoglossal nerve (XIIn) output in in vitro rodent slice preparations (Feldman et al 1990, Smith et al 1991). ii) Juvenile rats in vivo with brainstem transection just rostral to the preBötC continue to generate rhythmic inspiratory-dominated breathing patterns (Janczewski & Feldman 2006), as do in situ heart-brainstem preparations (Smith et al 2007). iii) Neurotoxic lesioning of a peptide receptor-defined subset of preBötC neurons results in ataxic breathing in otherwise intact rats. There are ~300 glutamatergic preBötC neurons per side that express the neurokinin-1 receptor (NK1R) for which Substance P (SP) is the ligand. A toxin, saporin, conjugated to SP (SP-SAP), selectively kills, over a period of days, neurons that express NK1R. Injection of SP-SAP into the preBötC in intact adult rats induces, after several days, a disturbed breathing pattern during sleep; with more complete lesions, ataxic breathing occurs during wakefulness (Gray et al 2001) with apneas (complete cessation of breathing) during sleep (McKay et al 2005).
Further (smoking gun) evidence for the preBötC as the kernel for inspiration
Slow neurodegeneration with saporin makes it difficult to separate the specific and immediate effects of the loss of preBötC NK1R+ neurons from secondary effects, including plasticity and bystander death (Loov et al 2012). Severe hypoxia and hypercapnea caused by increasingly long and frequent apneas (Gray et al 2001) can itself cause neuronal death. Moreover, neurotoxin-lesioned rats survive albeit with a markedly pathologic ataxic breathing pattern (McKay et al 2005) for up to 12 days, leaving the unlikely possibility that another site drives breathing under normal conditions. In order to eliminate these confounding effects of slow degeneration, a largely overlapping subpopulation of glutamatergic preBötC neurons that express the neuropeptide somatostatin (Sst; ~300/side) (Llona et al 2004, Stornetta et al 2003) can be transiently suppressed by transfection with the Drosophila allatostatin receptor (AlstR) and subsequent exposure to the ligand allatostatin (AL). AL does not appear to activate any mammalian receptors. Rat neurons made to express AlstRs hyperpolarize when exposed to AL. When AL is introduced into the brainstem of control adult rats, there is no effect on breathing. However, in anesthetized or awake adult rats whose preBötC Sst neurons express AlstR, AL rapidly induces a remarkably persistent and profound apnea that requires mechanical ventilation to prevent asphyxiation (Fig. 2) (Tan et al 2008). Implicit in this observation is the fact that these rats generate no breathing movements at all; there are no volitional or emotive movements, nor sighs or gasps. These are compelling data substantiating the essential role for preBötC neurons in normal breathing, i.e., eupnea.
Figure 2. preBötC is essential for breathing in adult rats.
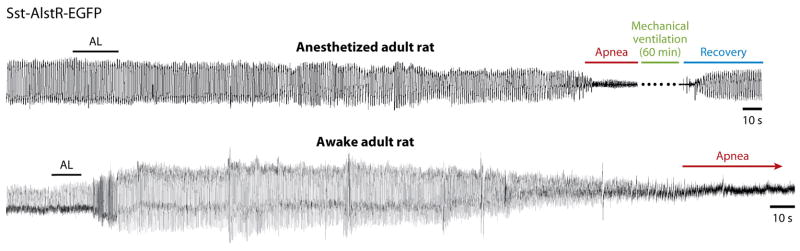
Rapid silencing of AlstR-expressing preBötC SST neurons induces persistent (>45 min when mechanically ventilated) apnea in anesthetized or awake adult rats. Traces are plethysmographic recordings. AL administered intracerebrocisternally induces a gradual decline of frequency and tidal volume until apnea develops after several minutes. After ~60 min mechanical ventilation, rats resume spontaneous breathing. From (Tan et al 2008).
Respiratory rhythm generation in the preBötC
If understanding the neural control of breathing can be broken down into solving a series of problems, foremost is that of delineating the mechanism for rhythmogenesis. More generally, rhythms, oscillations and periodic bursting of various sorts are central to almost all aspects of brain function, ranging from rhythmically patterned movements (Grillner & Jessell 2009), to cortical and cerebellar rhythms associated with signal processing and state (Buzsáki 2006), to slower processes with daily or seasonal rhythms (Foster & Kreitzman 2004). Breathing presents a unique window into neural rhythmogenesis because of a convergence of its essential properties: robustness, lability, a localized and identified rhythm generator (preBötC) (a key piece of the puzzle lacking for locomotion or chewing (Gossard et al 2011)), and various levels of reduction in vitro that provide exceptional access to neurons and networks for imaging and electrophysiology while maintaining spontaneous breathing-related motor output.
We postulate that rhythm is generated at the core of the respiratory central pattern generator and that this core is not primarily concerned with details of the output pattern. Our view is that timing signals normally originate in the preBötC and are broadcast to the rest of the network, perhaps by a subset of glutamatergic neurons with widespread projections (Tan et al 2010), which transforms the timing signals into appropriate patterns of muscle contraction (Fig. 1, red). Adopting this point of view allows one to focus on the oscillation per se and its neural origins. As mentioned above, the highly reduced slice preparation is experimentally optimal to get at such mechanisms. Our explicit assumption is that mechanisms gleaned in slices represent the foundation for rhythmogenic mechanisms in vivo. This point of view is opposite to assertions that rhythm generated in the slice is irrelevant to eupneic breathing in intact mammals (see below, Paton et al 2006, Smith et al 2007). In spite of all of the inherent experimental advantages and the deceptive simplicity, understanding rhythmogenesis in the preBötC in vitro is a tough problem.
Proposals for rhythmogenesis before the preBötC hypothesis focused on building “ball and stick” models consisting of populations of respiratory-modulated spherical neurons, i.e., without dendrites, classified according to firing pattern with simple cellular properties, e.g., no voltage-dependency, no ion selectivity, no metabotropic actions, and straightforward excitatory or inhibitory connections (Bianchi et al 1995, Feldman 1986, Merrill 1974, Richter 1982, von Euler 1986). Once the intrinsic membrane properties of preBötC neurons were measured in vitro, these schematic models were elaborated. Currently, there are two major viewpoints for the mechanism of preBötC rhythm generation based on the widely accepted finding that the preBötC rhythm is generated by glutamatergic interneurons (Funk et al 1993, Wallen-Mackenzie et al 2006). Their distinction lies in whether the inspiratory burst initiates due to a small population of specialized pacemaker neurons (defined below) or results from excitatory interactions among preBötC neurons that express synaptically triggered burst-generating conductances.
Role of glutamatergic pacemaker neurons in respiratory rhythm generation
The reader may note that this section is quite long given that we conclude it is unlikely that pacemaker neurons play an essential role in rhythmogenesis. We evaluated the evidence for and against possible roles of pacemaker neurons over a decade ago in a previous Annual Review (Rekling & Feldman 1998). These critiques, however, remain relevant to more recent work that pertains to the preBötC, as well as pacemaker-driven models of locomotor and oral-motor rhythm generation (Brocard et al 2006, Gossard et al 2010, Gossard et al 2011, Tazerart et al 2008). The heuristic power of the relatively straightforward “pacemaker hypothesis” may, in part, explain its persistence in the face of significant conflicting data. Over the past decade, some investigators have moderated their view, interpreting newer data as demonstrating that pacemakers and their underlying intrinsic conductances can contribute to rhythmogenesis but conceding that they are not essential (Best et al 2005, Koizumi & Smith 2008, Purvis et al 2007, Smith et al 2007). Yet, many authors continue to uncritically assert that preBötC rhythm is pacemaker driven, and interpret their data or build their models in that framework (Ben-Mabrouk & Tryba 2010, Pena et al 2004, Pena & Ramirez 2004, Ramirez et al 2011, Tryba et al 2008, Viemari et al 2011, Wittmeier et al 2008, Zavala-Tecuapetla et al 2008).
PreBötC rhythms in vitro continue without significant changes in frequency after blockade of chloride-mediated inhibition (Brockhaus & Ballanyi 1998, Feldman & Smith 1989, Ren & Greer 2006), which rules out mechanisms predicated on phase transitions that require conventional postsynaptic inhibition. The original observation catalyzed a search for and subsequent identification of preBötC neurons with bursting pacemaker properties that may best be defined by the ability to generate: i) rhythmic membrane polarization absent rhythmic input, and/or ii) “ectopic” bursts of rhythmic activity when depolarized (Del Negro et al 2002a, Del Negro et al 2005, Smith et al 1991, Thoby-Brisson & Ramirez 2001). Bursting in preBötC neurons has two underlying ionic mechanisms. The first is voltage-sensitive and depends on subthreshold activation of persistent Na+ current (INaP) in neurons with sufficiently low leakage-like K+ current (IK-Leak) (Butera et al 1999, Del Negro et al 2010, Del Negro et al 2002a, Koizumi & Smith 2008); INaP-dependent bursting is present in a subset of preBötC neurons at least from birth as well as throughout the medulla postnatally; it is not a specialized property of preBötC neurons (Del Negro et al 2010, Johnson et al 1994, q.v. Koizumi et al 2008). The second mechanism depends on a Ca2+-activated nonspecific cationic current (ICAN) (Thoby-Brisson & Ramirez 2001), whose activation mechanism in the absence of synaptic input depends on voltage-gated Ca2+ channels. Bursting of this type is less voltage-sensitive (Del Negro et al 2005, Ramirez et al 2011 [their Fig. 4c]) and emerges in a subset of preBötC neurons after P4 (Del Negro et al 2005, Pena et al 2004) with its relative prevalence influenced by neuromodulation (Ramirez et al 2011, Ramirez et al 2004, Tryba et al 2008, Viemari et al 2011, Viemari & Ramirez 2006). Under most experimental conditions in slices from neonatal rodents, ~5–25% of all preBötC inspiratory neurons exhibit bursting pacemaker properties, regardless of transmitter phenotype, i.e., glutamatergic or glycinergic (Del Negro et al 2005, Koizumi & Smith 2008, Morgado-Valle et al 2010, Pagliardini et al 2005, Pena et al 2004). However, the logic in the literature gets murky as one proceeds from the observation that pacemakers are present to conclusions that these pacemaker neurons generate the rhythm.
Arguments for the pacemaker hypothesis generally hinge on two observations. First, pacemaker neurons oscillate with a period and burst duration that matches the duty cycle of the respiratory rhythm in vitro. Second, conditions that regulate the period or burst duration of synaptically isolated pacemaker neurons, e.g., high K+, neuromodulators, or ion channel agonists/antagonists, have similar effects on respiratory rhythm in vitro. Both of these arguments assume pacemaker neurons are glutamatergic/excitatory, which, alas, is not the case. In transgenic mice that express enhanced green fluorescent protein (EGFP) in glycinergic neurons, where ~50% of preBötC neurons fluoresce (Winter et al 2009), ~23% of inspiratory glycinergic preBötC neurons have voltage-dependent pacemaker properties (Morgado-Valle et al 2010).
Tests of the pacemaker hypothesis are straightforward neither in execution nor in interpretation. Antagonists of INaP or ICAN such as riluzole or flufenamic acid invariably preclude intrinsic bursting in isolated pacemaker neurons, but only in some cases perturb or stop the rhythm when bath-applied or locally infused into the preBötC of slices, en bloc preparations, or in situ (Del Negro et al 2002b, Del Negro et al 2005, Fong et al 2009, Koizumi & Smith 2008, Pace et al 2007b, Pena et al 2004, St-John 2008, St-John et al 2007). Nevertheless, under the best of circumstances (which is likely never the case), rhythm cessation after drug exposure is a necessary but not sufficient condition if pacemakers are essential for rhythmogenesis. That is because INaP and ICAN antagonists markedly affect other membrane properties and depress excitatory transmission in all neurons. Their net effect is a widespread decrease in neuronal excitability, not limited to particular respiratory or even non-respiratory nuclei (Del Negro et al 2002b, Doble 1996, Ptak et al 2005). Additionally, local infusion of an INaP antagonist into the midline nucleus raphe obscurus also stops the rhythm (Pace et al 2007b, Ptak et al 2009). Therefore, rhythm cessation by drug application cannot be attributed to effects solely on preBötC neurons, and thus the results of pharmacological tests are at best equivocal in support of the pacemaker hypothesis. The conclusions that INaP pacemaker neurons drive gasping-like behavior in reduced in vitro and in situ preparations (Paton et al 2006, Pena et al 2004), and that INaP-dependent pacemaker activity drives spinal locomotor patterns (Brocard et al 2010, Tazerart et al 2008), are similarly unconvincing.
Can respiratory rhythm be generated without pacemaker neurons and without synaptic inhibition?
Previously (Feldman & Del Negro 2006, Rekling & Feldman 1998) we described a schematic “group-pacemaker” mechanism (Rekling et al 1996a, Rekling et al 1996b) that subsequently gave rise to explicit models for respiratory rhythm generation (Mironov 2008, Rubin et al 2009a). Before explaining how a group pacemaker works, we should note that there are few definitive data in support of the group-pacemaker model. It is at present a working hypothesis and framework for further testing. In the model, a fraction of glutamatergic preBötC neurons fire tonically at a low rate (<1 Hz) between breaths. Percolating activity in this interconnected subset of respiratory neurons (Rekling et al 2000) increases via positive feedback. The part of the cycle in which positive feedback dominates other network constituents is properly considered the preinspiratory phase because it precedes the inspiratory burst by 300–400 ms. According to the group pacemaker, recurrent excitation builds up during the preinspiratory phase and leads to a network-wide synchronous inspiratory burst phase. Consistent with this model, a ramp-like increase in the baseline membrane potential accompanied by low-rate spontaneous spiking during the preinspiratory phase is observed in so-called “Type 1” neurons, which are thought to be rhythmogenic based on expression of NK1 receptors (see above) and physiologically verified excitatory synaptic interconnections in the preBötC (Rekling et al 2000).
The termination of the inspiratory burst is still unresolved. Synaptic depression is a possible mechanism that would halt recurrent excitation (Rubin et al 2009a). Burst termination may also involve “activity-dependent” outward currents evoked by intense spiking, including: Na+/K+ ATPase electrogenic pump current, Na+-dependent K+ current, and ATP-dependent K+ channels (Del Negro et al 2009, Haller et al 2001, Krey et al 2010, Mironov et al 1998, Mironov & Skorova 2011).
ICAN, burst generation, and dendritic excitability
Active dendritic membrane properties, in concert with intracellular signaling, can couple synaptic input to burst generation. Because inspiratory bursts are much larger in magnitude (and intraburst spiking is more intense) compared to preinspiratory activity, the onset of the inspiratory burst reflects the recruitment of postsynaptic conductances that amplify synaptic drive, but are unrelated to INaP (Pace et al 2007b). Morphology may play a role in synaptic amplification. Rall’s analyses of dendritic properties, e.g., (Rall et al 1967), the advent of dendritic patch-clamp recordings, and new laser-imaging technologies demonstrate a wide array of excitable properties in dendrites that significantly influence synaptic integration, specifically the spatiotemporal summation of EPSPs (Branco & Hausser 2010, Branco & Hausser 2011). In preBötC neurons, activation of AMPA receptors as well as group I metabotropic glutamate receptors (mGluRs) evoke postsynaptic Ca2+ transients that evoke ICAN, either locally at the dendritic input site (Pace & Del Negro 2008, Pace et al 2007a) or via propagating Ca2+ waves (Mironov 2009, Mironov 2008). The net result is that excitatory synaptic inputs evoke inward currents during the inspiratory burst phase that promote robust inspiratory burst generation (Fig. 3A,B). Somatic Ca2+ transients, on the other hand, do not appear to play a critical role (Morgado-Valle et al 2008). The underlying ion channels appear to be members of the transient receptor potential (trp) family, most likely TRPM4 or TRPM5 (Crowder et al 2007, Mironov 2008, Mironov & Skorova 2011), or possibly TRPC3 or TRPC7 (Ben-Mabrouk & Tryba 2010). Dendritic excitability is critically important in understanding neuron computation in general (London & Hausser 2005, Stuart et al 2007). In a rhythmic central pattern generator, the ability to summate and synchronize excitatory inputs in support of periodic burst generation may be a function particularly well suited for dendrites, which we have just begun to exploit in order to elucidate key aspects of respiratory rhythmogenesis (Del Negro et al 2011, Mironov 2009).
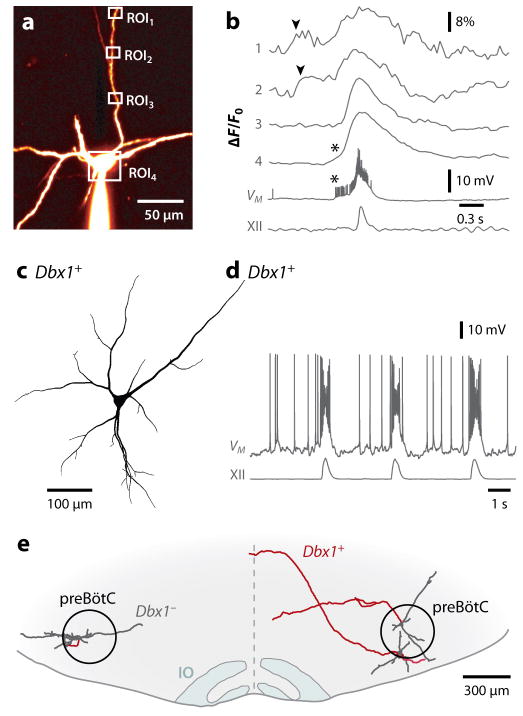
Figure 3. Inspiratory burst generation: role of dendrites and properties of Dbx1+ preBötC neurons.
A and B, dendritic two-photon Ca2+ imaging and somatic patch clamp. A, preBötC neuron filled with fluorescent dye from somatic whole-cell recording. Regions of interest (ROIs) shown, which correspond to B. B, dendritic Ca2+ transients (arrowheads) precede somatic bursts and XII motor output. Asterisks indicate somatic spike-driven Ca2+ transients (Del Negro et al 2011). C and D, morphology (C) and physiology (D) of a Dbx1+ preBötC neuron. Drive potentials of ~25 mV amplitude and depolarization block of spiking indicative of ICAN activation during the inspiratory burst. E, transverse view of a mouse slice (ventral) showing two Dbx1+ neurons (right) and a Dbx1− neuron (left) recorded and biocytin reconstructed. All three were inspiratory neurons. Dbx1+ neurons are commissural (axons in red). IO is inferior olive.
Role of inhibition in rhythmogenesis and pattern formation
Inhibition is an essential element that underlies rhythmic movements in invertebrates, where most central pattern generator neurons are inhibitory (Orlovsky et al 1999). For locomotion in mammals, reciprocal inhibition was first proposed as the mechanism for the alternating rhythm around each joint (Brown 1911, Brown 1914, Sherrington 1906). While this remains hypothetical, inhibition clearly underlies left-right limb alternations (Goulding 2009, Kiehn 2006). For breathing, postsynaptic inhibition is postulated, sometimes stipulated, as an essential element for rhythmogenesis (Büsselberg et al 2001, Büsselberg et al 2003, Richter & Spyer 2001). Inhibitory currents and potentials are readily observed in brainstem respiratory-modulated neurons in intact mammals (Richter 1982), and are quite prominent when recorded under barbiturate anesthesia (ibid). The core mechanism for rhythm generation in one currently widely disseminated model is an “inhibitory ring” of three distinct neuronal populations of preBötC and BötC neurons that sequentially inhibit each other and transform a presumptive tonically active subpopulation of excitatory preBötC neurons into an inspiratory-modulated one (Molkov et al 2010, Rubin et al 2009b, Smith et al 2007). Block of synaptic inhibition throughout the entire neuraxis of a reduced in situ perfused rat preparation produces apnea with tonic firing of preBötC and BötC neurons (Smith et al 2007). Under certain pathological conditions, such as when the hindbrain is transected just rostral to preBötC in this in situ preparation, the inhibitory ring is presumably broken and according to this model preBötC pacemaker neurons are released from tonic excitation and endogenously burst, driving a respiratory rhythm by a completely different mechanism. This presumptively pacemaker-driven rhythm is strikingly different from that presumably produced by the inhibitory ring: i) inspiratory and expiratory outputs discharge synchronously; ii) inspiratory bursts change shape from incrementing to decrementing; and iii) respiratory frequency drops. According to the model removing the inhibitory ring should produce apnea (Molkov et al 2010, Paton et al 2006, Rubin et al 2009b, Smith et al 2007). This does not appear to be the case. In anesthetized adult spontaneously breathing vagus-intact rats, effectively complete antagonism of GABAA and glycine receptors in the preBötC and BötC slows down the rhythm to that equivalent to a vagotomized rat, i.e., no apnea is induced (Janczewski et al. in preparation) but does abolish a key and powerful pulmonary reflex, the Breuer-Hering inspiratory-inhibitory inflation reflex. This result suggests that postsynaptic inhibition within the preBötC (and BötC) is not essential for generation of respiratory rhythm, which is consistent with the group-pacemaker model. The primary role of inhibition appears to be in shaping the pattern of respiratory motor output and assuring its stability, but not in the generation of rhythm per se.
Opiates and the preBötC
Opiates are a class of compounds that can depress breathing, and at too high a concentration, can do so sufficiently to cause asphyxiation, as can happen when administered as an analgesic or taken recreationally. μ-opioid receptors are present on preBötC neurons and when activated reduce their excitability (Gray et al 1999). These preBötC μ-opioid receptors appear responsible for respiratory depressive effects of opiates (Montandon et al 2011). A functional antagonist of the μ-opioid receptor-signaling pathway in preBötC neurons is the 5HT4 receptor, which does not appear to be present in pain pathways (Manzke et al 2003). In adult rat: i) systemic administration of a 5HT4 agonist reverses the respiratory depressive effects of systemic administration fentanyl (a potent μ-opioid agonist) without a significant reduction in analgesia. ii) More to the point, when breathing is depressed by systemic administration of fentanyl, this is fully reversed by focal injection into the preBötC of naloxone, a μ-opioid antagonist (Montandon et al 2011). These observations provide further proof of the importance of the preBötC in assuring that we breathe, and give promise of protocols to reduce the dangers of opiate analgesics.
Rediscovery of expiration
Until recently, the paradigm for rhythmogenesis was that regardless of the underlying mechanism, all phases of respiratory motor outflow originate from the same source(s). Thus, the preBötC was envisioned to sequentially parcel out signals to produce the alternating rhythm of inspiratory and expiratory movements. This does not appear to be the case. The diaphragm, an extraordinarily powerful muscle for inspiratory movements, is a defining characteristic of mammals. Breathing is the only mammalian behavior requiring continuous movement of skeletal muscles 24/7/365; it consumes ~7% of metabolic output at rest, and much more in pulmonary disease (Bell et al 1996, Peters 1969). In mammals, the apparently most efficient pattern for rhythmic breathing at rest is active inspiration, generated by forces resulting from diaphragmatic and external intercostal muscle contraction acting to expand the lung, alternating with passive expiration due to the forces generated by the elastic recoil of the inspiratory muscles, lung and ribcage. Modest increases in ventilation near resting values, such as during slow walking in humans, are typically accomplished by increasing inspiratory (tidal) volume, combined with decreasing expiratory duration, with expiration remaining (predominately) passive (Gardner 1980). During exercise, e.g., when chasing prey or fleeing predators, there is a transition from passive to active expiration (Aliverti et al 1997) due to the onset of contraction of abdominal and internal intercostal muscles. In the past decade there has been a significant paradigm shift, with the current view that the rhythm originates from two distinct but coupled oscillatory populations with segregated function, i.e., one for inspiration and one for expiration (Fig. 1).
The above paradigm shift began with the observation that both in vitro and in vivo, low dose opioids could produce an occasional dropout of the inspiratory phase, i.e., quantal slowing (Mellen et al 2003), which requires structures rostral to the preBötC (Janczewski and Feldman, 2006). This led to a hypothesis of an opiate-insensitive second oscillator that drives active expiration (Janczewski & Feldman 2006, Mellen et al 2003). The location of this second oscillator was suggested by observations in in vitro en bloc preparations, where a population of neurons rostral to the preBötC and mostly ventral to the facial nucleus (VIIn), dubbed the parafacial respiratory group (pFRG), was active prior to the onset of inspiratory activity (Onimaru & Homma 2003). The pFRG overlaps (and could even be identical) with the retrotrapezoid nucleus (RTN, see below), postulated to be a site for central chemoreception for CO2 (Guyenet et al 2009, Smith et al 1989, Stornetta et al 2006). Pending resolution as to their unique identity, we refer to this region as RTN/pFRG (Feldman et al 2003, Onimaru et al 2006). The non-chemosensitive function of the RTN/pFRG was initially hypothesized to be the primary source of inspiratory rhythm (Onimaru & Homma 2003). However, in juvenile rats generating active expiration, transection of the brainstem just caudal to the RTN/pFRG abolishes active expiration without much effect on inspiratory motor activity (Janczewski & Feldman 2006). The RTN/pFRG is postulated as a conditional oscillator for active expiration that is quiescent under certain conditions (Janczewski & Feldman 2006). Reduced preparations containing the RTN/pFRG, but lacking the preBötC generate rhythmic output from VII motor roots under opioid inhibition (Onimaru & Homma 2008), consistent with the RTN/pFRG as an independent oscillator. The absence of significant expiratory neuronal activity in the RTN/pFRG of normocapnic, normoxic adult anesthetized mechanically ventilated rats (Fortuna et al 2008, Mulkey et al 2007) reflects the absence of active expiration (Feldman & Del Negro 2006). Thus, stimulating the lateral RTN/pFRG via local disinhibition or photoactivation with channelrhodopsin transforms a “resting” breathing pattern with no active expiration into one with active expiration associated with activation of late expiratory RTN/pFRG neurons (Fig. 4)(Pagliardini, 2010). These results are consistent with the presence of an expiratory oscillator in the RTN/pFRG that when turned on promotes active expiration. While elevated CO2 stimulates active expiration, this is not a prevalent stimulus in the normal life of most mammals. Rather, we suggest descending signals related to exercise (Eldridge et al 1985) or to a powerful emotional response, e.g., fear, are likely to more commonly turn on the RTN/pFRG oscillator to produce active expiration. Moreover, active expiration can sometimes be seen in anesthetized mammals (De Almedia 2010 a,b, Iizuka 2010) or in highly reduced preparations (Abdala 2009, Iizuka 2004, Iizuka 2011, Taccola 2007) suggesting multiple mechanisms may gate its activity.
Expiration, like Gaul, is divided into 3 parts
Regardless of its origin, expiration has subphases, and in particular the transitions at the beginning and end of the expiratory phase are referred to as the postinspiratory (early expiratory) and preinspiratory (late expiratory) periods. In the postinspiratory period following a sharp decline in phrenic nerve activity, inspiratory airflow stops and expiratory airflow begins. Often just after this point, there is a small, postinspiratory burst of phrenic nerve activity, which causes a lengthening contraction of the diaphragm that brakes expiratory airflow. During the preinspiratory phase (defined above): i) Type 1, putatively rhythmogenic, preBötC neurons exhibit a ramp-like slow depolarization in vitro (Rekling et al 1996a) and ii) in vivo when stimuli that when delivered earlier in expiration affect expiratory duration (TE), are now ineffective, e.g., Figs. 8 & 10 in (Cohen & Feldman 1978, Feldman & Gautier 1976). Whether postinspiratory output constitutes a distinct phase of breathing rhythmogenesis (3-phase model) (Molkov et al 2010, Smith et al 2007) or a motor subcomponent of the expiratory phase (2-phase model) (Janczewski & Feldman 2006) is controversial. The timing of abdominal activity is, in part, dependent upon the experimental condition with purely preinspiratory (de Almeida et al 2010, Iizuka 2009), or a mixture of pre- and postinspiratory (Abdala et al 2009, Janczewski & Feldman 2006) activity reported in some experiments. Similar flexibility in motor patterns is present in reduced neonatal preparations (Ruangkittisakul et al 2007, Taccola et al 2007).
Respiratory connectome
preBötC intrinsic connections
Regardless of the critical cellular properties, the connectivity amongst preBötC neurons must play an essential role in the stability and lability of the rhythm, and perhaps in rhythmogenesis itself. This information has been challenging to obtain. Type 1 inspiratory preBötC neurons, based on a small sample in rhythmically active mouse slices, synaptically project to one out of six neurons that also express Type 1-like properties and also form electrical synapses with one out of six like neurons, but not the same ones (Hayes & Del Negro 2007, Rekling et al 2000). Putative rhythm-generating neurons in organotypic cultures of the preBötC form clusters of ~7 neurons, each projecting to four neighbors. Therefore connectivity estimates range from a low of 15% to as high as 67%. The topological pattern of connectivity in neural networks (like all networks) influences whether and how its elements can synchronize their activity (Bogaard et al 2009), and therefore, will prove to be at least as important as a simple tally or an estimate of connection probability when it comes to understanding the relationship between connectivity and important metrics of network function. For example, the robustness of network function under “attack”, i.e., destruction of its constituent nodes, depends on topology (Barabási 2003). Cumulative single-neuron laser ablation of ~18% of inspiratory preBötC neurons causes irreversible cessation of rhythm in slices (Hayes et al 2012), but it remains unknown to what extent the underlying details of the wiring diagram determine the robustness (fragility) of the rhythmogenic network. Robustness is an important characteristic essential both for understanding the neural basis for periodic behaviors like breathing as well for interpreting and treating neurodegenerative disorders (Alheid et al 2004).
preBötC and the RTN/pFRG interconnect
Glutamatergic Sst inspiratory-modulated preBötC neurons project to the RTN/pFRG (Tan et al 2010). Glutamatergic, galanin-positive RTN/pFRG neurons project to the preBötC (Bochorishvili et al 2012); whether these neurons are involved in the rhythmogenic aspects of RTN/pFRG remain to be established. What is curious is the lack of identification of inhibitory connections between these two structures, since preBötC and the RTN/pFRG oscillate in anti-phase postnatally. A polysynaptic inhibitory pathway is possible, perhaps involving a relay in the BötC. Significant resources are being devoted to determining the connectome for cortical columns, where the challenges are heightened by the paucity of information concerning the sophisticated signal processing that is occurring. We suggest that the preBötC presents a much more straightforward challenge of considerably less complexity, as the signal processing and output is well understood, and may be useful as an intermediate step in the development of brain connectomics.
Genetics of the respiratory network
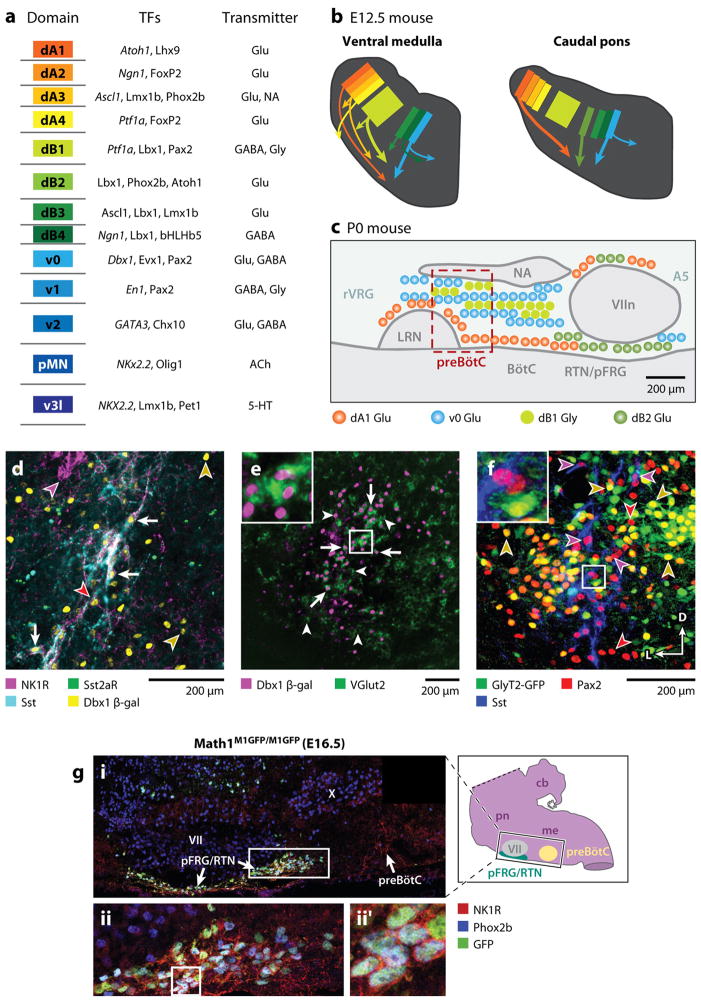
The understanding of the developmental genetics of brainstem circuits underlying the neural control of breathing is rapidly advancing, with considerable potential to inform our understanding for the preBötC and RTN/pFRG. The organization of the brainstem is remarkably consistent amongst vertebrates (Ma et al 2010, Straka et al 2006), a consequence of conserved developmental programs that control stereotyped patterns of gene expression, including, critically, transcription factors (TFs) (Gray 2008, Gray et al 2004). At first approximation, every brainstem neuron results from a specific sequence and combination of TF expression based on its physical location during neurogenesis (Briscoe et al 2000). Rostrocaudally, the brainstem is organized into 7–8 domains (rhombomeres) associated with conserved and discrete patterns of TF expression (Lumsden 1990). Medullary and caudal pontine respiratory populations are distributed, more or less, across rhombomeres 5–7. Dorsoventrally, the brainstem neural tube can be divided into at least 13 distinct dorsoventral domains based on the expression of TFs (Fig. 5a) (Sieber et al 2007). Each of these domains produces distinct classes of neurons with their own neurotransmitter identity (Fig. 5a), morphology and migratory pattern (Fig. 5b). Neurons of ventral respiratory column (Alheid et al 2004) and adjacent RTN/pFRG are predominantly derived from four distinct progenitor domains (Fig. 5b,c).
Figure 5. Genetic organization of brainstem respiratory regions.
A. Schematized description of brainstem progenitor domains for 8 dorsal (dA1–dB4) and 5 ventral progenitor populations (left) based on their relative location within brainstem progenitor region. Partial list of transcription factors expressed at some point within progenitors (italics) or post-mitotic neurons within each domain (middle). Neurotransmitter(s) identity of neurons derived from each domain. Adapted from (Gray 2008) B. Cartoon showing partial migratory path of ventral medulla (left) and caudal pons (right) neurons in embryonic mouse brainstem. Colors correspond to domains in (A). Thick arrows correspond to populations important for breathing. Note dB2 population (light green) is present only in caudal pons. C. Cartoon of developmental origin and approximate anatomical locations of respiratory-related populations in sagittal plane within ventral medulla and caudal pons. Colors correspond to developmental progenitor domain from A. Legend describes transmitter released by these neurons. Within the ventral respiratory column, nearly all respiratory-related glutamatergic neurons are Dbx1-derived. Magenta box outlines the location of preBötC SST-expressing neurons. RTN/pFRG, in contrast, contains Dbx1, Atoh1, and Phox2b glutamatergic populations. Ventral medulla also contains a large number of dB1 derived glycinergic neurons. B. PreBötC neurons are derived from Dbx1-expressing progenitors. Four color confocal image showing coexpression of NK1R (magenta), SST2aR (green), SST (cyan), and β-gal (yellow) in P0 Dbx1 β-gal mouse (adapted from (Gray et al 2010). Arrows indicate coexpression of all 4 genes. Images to right show single channel expression. Red arrowhead indicates Dbx1-derived NK1R/SST2aR-expressing neuron that lacks SST. Yellow arrowheads indicate Dbx1 derived neurons lacking coexpression. Magenta arrowhead indicates NK1R expressing nucleus ambiguus neuron. E. preBötC Dbx1 neurons are glutamatergic. Image showing β-gal (magenta) expression within the majority of VGlut2 (green) expressing preBötC neurons (adapted from (Gray et al 2010)). Inset is enlarged from central square. F. preBötC contains glycinergic neurons. Three color confocal image showing Pax2 (red) and Sst (blue) immunoreactivity with intrinsic GFP from a P0 GlyT2-GFP transgenic mouse (Morgado-Valle et al 2010). Arrows show Pax2 co-localization with Sst or GFP but no GFP expression in SST neurons. Inset is enlarged from central square. Scale bars = 200 μm. D – dorsal, L – lateral. G. RTN/pFRG Phox2b neurons express Phox2b and Atoh1. (A) Magnification of ventral respiratory column from E16.5 Math1M1GFP/M1GFP hindbrain, as indicated by the black rectangle on inset model hindbrain (black ventral region is pFRG/RTN while yellow circle indicates preBötC), showing NK1R (red), Phox2b (blue), and Math1-EGFP (green) expression. NK1R labeled both the pFRG/RTN and 17 preBötC neurons. Magnified pFRG/RTN neurons from the caudal pole of VII (solid white rectangle in A) showing co-localization of Math1EGFP with Phox2b and NK1R. (B) shows the three markers merged. Further magnification from white boxes in B is shown to right of each panel in (B′). Image from (Rose et al 2009b).
Genetics of the preBötC
We made the case that the preBötC is the rhythmogenic source of inspiratory drive. Yet it is a small portion of the rostral ventral respiratory column (Alheid et al 2004), which mostly contains bulbospinal premotoneurons receiving inspiratory drive from the preBötC. What is the developmental mechanism underlying this ventral respiratory column organization, and, in particular, what makes the preBötC special? Are its constituent neurons genetically distinct from adjacent respiratory premotoneurons? The expression in preBötC neurons of the receptor NK1R (Gray et al 1999, Guyenet et al 2002, Guyenet & Wang 2001, Wang et al 2001), the peptide Sst (Stornetta et al 2003, Tan et al 2008), and the glycoprotein reelin (Tan et al 2011) distinguishes this structure from surrounding regions (Fig. 5d) ; yet, none of these markers appear to endow the preBötC with rhythmogenic properties, per se. In mice, these genes are expressed on only a partially overlapping minority of preBötC excitatory neurons (~1000 per side) (Gray et al 2010). The role that different genetically defined subclasses of preBötC neurons play in rhythmogenesis is unknown. Genetic ablation of Sst or NK1R genes, but not the neurons that would otherwise express these genes, does not appear to affect the formation of the preBötC and produces only mild respiratory phenotypes (Berner et al 2007, Doi & Ramirez 2008, Shvarev et al 2010, Telgkamp et al 2002). No intrinsic membrane properties, e.g., INaP or ICAN, appear uniquely expressed in subsets of preBötC neurons.
The ventrolateral medulla (VLM) contains both excitatory (glutamatergic) and inhibitory, mostly glycinergic, respiratory neurons (Morgado-Valle et al 2010, Schreihofer et al 1999, Tanaka et al 2003, Winter et al 2009, Winter et al 2010). Glutamate is the essential excitatory neurotransmitter for preBötC rhythmogenesis (Funk et al 1993, Greer et al 1991). Genetic ablation of the vesicular glutamate transporter 2 (VGlut2, Slc17a6) completely eliminates respiratory-related motor activity both in vitro and in vivo (Wallen-Mackenzie et al 2006). Respiratory dysfunction leading to death at or near birth is a consequence of the genetic ablation of a number of transcription factors (Blanchi & Sieweke 2005, Gray 2008). In some cases (see below) these TFs play a role in the specification of neurons that modulate preBötC function, but not preBötC neurons per se. These include TFs important for the specification of ventral medullary and pontine glycinergic (Lbx1, Ptf1a) or glutamatergic (Atoh1, Krox20/Egr2, Lbx1, Lmx1b, MafB/Kreisler, Phox2b, Tlx3, Tshz3) neurons (Blanchi et al 2003, Blanchi & Sieweke 2005, Borday et al 2005, Caubit et al 2010, Dubreuil et al 2009, Glasgow et al 2005, Goridis et al 2010, Guyenet et al 2009, Hirsch et al 1998, Hoshino et al 2005, Nsegbe et al 2004, Pagliardini et al 2008, Pattyn et al 2000a, Qian et al 2001, Rose et al 2009b, Sieber et al 2007, Thoby-Brisson et al 2009, Yamada et al 2007). None of these mutations, however, affect the specification of preBötC NK1R/Sst neurons. For example, Lmx1b and Tlx3 are expressed in and essential for the formation of ventral medullary catecholaminergic neurons that partially intermingle with preBötC neurons (Cheng et al 2004, Qian et al 2001). Tlx3 mutation leads to respiratory instability in vitro that is alleviated by disinhibition. Similarly, subsets of Atoh1-derived neurons are present near the ventral medullary surface (Rose et al 2009a). Atoh1 mutant mice do not breathe in vivo at birth; yet, a functional preBötC and rhythmic activity persists in vitro (Rose et al 2009b).
The transcription factor Dbx1 is essential for preBötC development and respiratory function. Dbx1 is expressed throughout the brainstem and spinal cord in V0 neural progenitor cells (Fig. 5a). The V0 domain consists of at least two subdivisions, dorsal and ventral (V0d and V0v) (Lanuza et al 2004, Pierani et al 2001). V0d neurons are predominantly GABAergic, located near the dorsal midline. V0v postmitotic neurons express the TFs Evx1 and Evx2, and a subset also expresses VGlut2. In spinal cord, V0v neurons are a small population that migrates towards the ventral midline. Within the medulla, however, the V0v glutamatergic subpopulation greatly expands and migrates laterally to the ventral medullary surface producing a column extending the full length of the ventral respiratory column including the RTN/pFRG, BötC, preBötC, and rVRG (Figs. 5b–e).
Neurons with high levels of Sst in the neonatal mouse ventral medulla are limited to the (approximate boundaries of the) preBötC, and are derived from Dbx1-expressing progenitors (Fig. 5d) (Bouvier et al 2010, Gray et al 2010). These cells are ~20% of preBötC Dbx1-derived neurons. The function and developmental relationship between preBötC Sst and other neurons within and adjacent to the preBötC are unknown. NK1Rs and Sst2aRs are co-expressed on the majority of preBötC Sst neurons, but are more broadly expressed within and adjacent to the preBötC (Fig 5d). In addition, Dbx1 defines not only preBötC NK1R/Sst expressing neurons, but essentially all glutamatergic neurons of the ventral respiratory column (Fig. 5e). This suggests that, for breathing, rhythm generating and (a significant proportion of the) downstream premotor neurons are derived from a single developmental progenitor domain. This organization is in contrast to that proposed for models of locomotion in which rhythms are generated by the interactions of multiple developmental populations (Gosgnach 2011, Goulding 2009). The genetic mechanisms specifying preBötC neurons from the larger Dbx1-derived population are unknown, but they may induce signaling cascades similar to those responsible for segmental patterning of homeobox genes in brainstem and spinal cord (Champagnat et al 2009).
The elimination of the commissural projections from only Dbx1-derived neurons by deletion of the axon guidance receptor Robo desynchronizes respiratory outflow between left and right sides (Bouvier 2010). Genetic deletion of Dbx1 eliminates all preBötC glutamatergic respiratory neurons, including those expressing NK1R and Sst, with consequent complete elimination of inspiratory activity, both in vitro and in vivo (Bouvier et al 2010, Gray et al 2010). Additionally, preBötC Dbx1-derived neurons are predominately inspiratory-modulated, at least in vitro (Bouvier et al 2010, Gray et al 2010) (Fig. 3D). Together, these data indicate that both the generation and coordination of inspiratory output depends on Dbx1-derived neurons, presumably within the preBötC, which are commissural (Fig. 3E).
The mouse in the room
In vivo experiments targeting respiratory neurons in goats suggest that under certain conditions, breathing does not require preBötC neurons (Krause et al 2009, Neumueller et al 2011, Wenninger et al 2004a, Wenninger et al 2004b). Here, a non-specific excitotoxin (ibotenic acid (IA)) is injected in slowly increasing quantities over 5 weeks (to avoid bilateral application of high dosages that can cause acute cardiac and respiratory failure). In all cases, IA affects breathing during injection, but breathing returns to normal by the next day. This results in a substantial, if not complete, loss of neurons within the presumptive preBötC, at least to the degree that markers and neuroanatomy in goats are homologous to rodents. In contrast, goats injected with SP-Saporin into the same region followed 10–14 days later with a single large dose of IA cease breathing for at least 6 hours without recovery either leading to death or necessitating euthanasia. One interpretation is that normal breathing can be generated in the absence of preBötC neurons given modest damage per event and sufficient time for respiratory network reorganization between events, possibly including neurogenesis. Alternatively: i) with sufficient time, regions outside of the preBötC with a different developmental and genetic background can generate normal respiratory output. One possible source would be neurons in the RTN/pFRG (see below). ii) The post-lesion rhythm may be generated by neurons that are genetically related to preBötC neurons, but which do not normally generate inspiratory rhythm, at least in rats (Tan et al 2008), i.e., some subset of the Dbx1-derived glutamatergic neurons that extend the entire length of the ventral respiratory column (Fig. 5b). This explanation is consistent with the observation that saporin ablations that include but extend beyond the preBötC lead more rapidly to death than smaller ablations (Gray et al 2001, Wang et al 2003). iii), In goats, the boundaries of the preBötC, which have only been well-characterized in rat and mouse, extend beyond the IA lesion and that a prolonged recovery is required for the unlesioned remaining neurons to coordinate their activity for effective breathing movements. NK1R expression is not limited to the preBötC in mice and in goats the expression pattern appears to be a more ubiquitous, so the use of NK1R expression to identify preBötC anatomical boundaries is questionable (Wenninger et al 2004a). iv) The rhythm is generated by a population at some distance from the preBötC, perhaps in the rostral pons, although there are currently no data to support this.
Genetics of RTN/pFRG: active expiration, central chemoreception, and CCHS
There are two critical functions associated with the RTN/pFRG: generation of active expiration and central chemoreception. The lateral RTN/pFRG is the most sensitive site for induction of active expiration without a concomitant increase in inspiration (Pagliardini et al 2011); this region contains Phox2b-, Atoh1- and Dbx1-derived neurons (Fig. 5a,c). One of the most essential functions of breathing in mammals is maintenance of the partial pressure of blood/tissue CO2 (~40 mm Hg in arterial blood in humans). Several brainstem populations of neurons, in particular the medial RTN/pFRG and the raphe, are likely to play a role in this “central” chemosensitivity (Feldman et al 2003). Blunted CO2 chemosensitivity in humans is a defining characteristic of Congenital Central Hypoventilation Syndrome (CCHS) (Berry-Kravis et al 2006, Weese-Mayer et al 2010). CCHS is a rare disorder caused by an expansion in the gene encoding Phox2b, a highly conserved and essential TF involved in the specification of brainstem visceral sensory and motoneurons (Pattyn et al 2000a, Pattyn et al 2000b). The size of the Phox2b expansion directly correlates with the severity of CCHS. In rodents, a small population of ~2100 RTN/pFRG glutamatergic neurons are responsive to small changes in pH and express Phox2b (Guyenet et al 2009, Stornetta et al 2006). This identification of a specific population of neurons as putative CO2 sensors is a landmark finding of the first developmental marker for neurons important for breathing whose dysfunction leads to a survivable breathing phenotype (Dubreuil et al 2008).
The case that RTN/pFRG neurons play a role in mediating chemosensitivity is compelling. Briefly, the elimination of ~70% of RTN/pFRG Phox2b neurons, by the use of the aforementioned saporin lesion technique, leads to a ~50% attenuation of the ventilatory response to increases in blood pH (Takakura et al 2008). Activation of medial RTN/pFRG Phox2b neurons directly increases inspiratory output (Abbott et al 2009) and can produce active expiration (Abbott et al 2011). In contrast, stimulation of all neurons in the non-Phox2b expressing lateral RTN/pFRG reliably evokes active expiration but has little effect on inspiratory activity (Pagliardini et al 2011). In the adult rat, medial RTN/pFRG Phox2b neurons are not rhythmically active, even under conditions of strong chemosensitive drive. This is consistent with a role of the medial RTN/pFRG in chemosensitivity but not in rhythmogenesis or production of active expiration (Guyenet et al 2005, Mulkey et al 2004).
Like nearly all TFs, Phox2b is expressed in distinct subpopulations, only some of which maintain postnatal expression (Kang et al 2007, Pattyn et al 1997). RTN/pFRG neurons are generated from a caudal pontine progenitor population that coexpresses the TF Lbx1 as well as Phox2b (Pagliardini et al 2008, Sieber et al 2007). These neurons migrate to their final location in the ventrolateral medulla (dB2, Fig. 5b,f). The RTN/pFRG is absent in Lbx1 mutant mice as well as in Krox20 null mice that lack rhombomeres 3 and 5 (Jacquin et al 1996, Pagliardini et al 2008, Sieber et al 2007). Phox2b gene function itself is necessary for RTN/pFRG formation as its conditional elimination using either Lbx1 (limited to the dB2 domain, Fig. 5a) or Krox20 (limited to rhomobmeres 2 and 5) cre recombinase mice prevents RTN formation, and produces neonatal lethality and blunted chemosensitivity (Dubreuil et al 2008, Ramanantsoa et al 2011).
Mouse mutants that recapitulate the most common CCHS mutation (Berry-Kravis et al 2006), an alanine expansion (Phox2b27Ala/+) in the germline, have a blunted inspiratory response to elevated CO2 in in vitro embryonic preparations and, although they are capable of at least some inspiratory activity, most, but not all, die at or shortly after birth (Dubreuil et al 2008, Ramanantsoa et al 2011). In this mutation, RTN/pFRG Phox2B neurons form dorsally but do not migrate to the ventral surface. This migration normally coincides with the expression of the TF Atoh1 within putative RTN/pFRG neurons, but Atoh1 expression is lost in these mice (Ramanantsoa et al 2011, Rose et al 2009a, Rose et al 2009b). Loss of Atoh1 also prevents RTN/pFRG migration (Ramanantsoa et al 2011, Rose et al 2009a, Rose et al 2009b). Surprisingly, the conditional expression of the Phox2b27Ala mutation within rhombomeres 3 and 5 is not neonatal lethal, but results in the loss of Atoh1 expression and of RTN/pFRG neuron migration, as well as blunted chemosensitivity in neonates. By 4 months of age, however, mice recover 60% of their CO2 sensitivity (compared to wild type controls) and have normal blood CO2 levels at rest (Ramanantsoa et al 2011).
Fetal development of the RTN/pFRG
In utero, rhythmic respiratory motor output in mice begins around embryonic day 15.5 (E15.5) coincident with the onset of respiratory activity in the embryonic preBötC (Thoby-Brisson et al 2005). However rhythmic brainstem activity is present by E14.5, i.e., ~1 day prior to the onset of preBötC activity, within the Phox2b-expressing region near the facial nucleus, called the embryonic parafacial oscillator (ePF), presumably the embryonic equivalent to the RTN/pFRG. In the later fetal period, the initially slow preBötC rhythm is coupled to a faster ePF rhythm (Thoby-Brisson et al 2009). Unlike the preBötC, ePF rhythms do not require glutamatergic neurotransmission as they persist following pharmacological blockade of glutamate receptors or genetic ablation of the glutamate transporter VGlut2 (Thoby-Brisson et al 2009, Wallen-Mackenzie et al 2006). Rhythmically active ePF neurons express Phox2b and NK1R, but not Sst2a or μ-opioid receptors (Gray et al 2010). In neonatal en bloc preparations, the majority of pFRG pre-I and “respiratory-modulated tonic” neurons express Phox2b (Onimaru et al 2008, Onimaru et al 2012). Mutations that eliminate RTN/pFRG Phox2b neurons, such as in Krox20 mutants, eliminate ePF activity and what remains is a slowed fetal preBötC rhythm (Thoby-Brisson et al 2009). Together these data suggest, that ePF and RTN/pFRG contain the same population of Phox2b-expressing neurons that have a vital perinatal role. As the ePF is an intrinsically rhythmic population coupled to breathing movements, these neurons represent excellent candidates for an evolutionarily conserved, independent expiratory oscillator. Significantly, however, fetal RTN/pFRG rhythms persist in Dbx1 mutant mice. These mice do not generate any rhythm activity from VII or other respiratory motoneurons, suggesting RTN/pFRG neurons alone are insufficient to assure generation of active expiratory (or inspiratory) movements (Bouvier et al 2010).
Serotonin, central chemoreception and SIDS
Raphe serotonergic neurons are also proposed to play a role in mediating CO2 chemosensitivity (Hodges & Richerson 2010). Raphe neurons have a discrete pattern of TF expression including the combinatorial expression of the TFs Pet1 and Lmx1b (v3l, Fig. 5a). Pet1 is expressed only in serotonin neurons and its genetic ablation decreases their number by 70% (Hendricks et al 1999, Hendricks et al 2003). Lmx1b is expressed in several brainstem populations, but its conditional elimination from Pet1-expressing neurons completely eliminates all brainstem serotonergic neurons. In adult mice, this produces ~50% reduction in the ventilatory response to high levels of CO2, but no effect on baseline respiration (Hodges 2008). This is the same pattern of chemosensitivity seen in adult mice with Phox2b27Ala/+ RTN/pFRG mutations. Partial serotonin neuron loss produces a similar but smaller effect (Hodges et al 2011). In neonates, the complete loss of serotonin increases and prolongs neonatal apnea and reduces ventilation at rest; ventilation nevertheless recovers to wild type levels by adulthood (Hodges et al 2009). Selective silencing of raphe neurons in adults depresses breathing and blunts chemosensitivity, indicating a continuing role for these neurons in respiratory modulation (Ray et al 2011). In aggregate, these data strongly suggest that both RTN/pFRG and raphe neurons contribute to normal chemosensitivity and that the explicit role of either one may be dependent upon the age and state, e.g., sleep/wake, rest/exercise, of the animal (Huckstepp & Dale 2011). Medullary serotonergic neurons are present in vitro, where they can affect rhythmogenesis and motor output (Ballanyi 2004, Bianchi et al 1995, Doi & Ramirez 2010, Richter et al 2003), representing mechanisms by which serotonin can acts to regulate CO2.
SIDS may be attributable to serotonin dysfunction in many cases (Paterson et al 2006). In vitro, serotonin induces a depolarizing nonselective cation current in preBötC neurons that enhances burst generation (Ptak et al 2009). The same or a very similar nonselective cation current is also a target of Substance P, likely co-released (under unknown conditions) by raphe neurons (Ben-Mabrouk & Tryba 2010, Hayes & Del Negro 2007, Pena & Ramirez 2004). These serotonergic inputs also enhance bursting pacemaker neurons by amplifying INaP and closing leakage K+ currents (Pena & Ramirez 2002, Ptak et al 2009). Although the molecular identity of the nonselective cation current(s) described in these various studies is unknown, it could be related to the Na+ leakage current (Lu et al 2007) or a canonical transient receptor potential, i.e., TRPC, channel, TRPC3 or TRPC7 (Ben-Mabrouk & Tryba 2010).
Astrocytes and chemosensation
The mechanism(s) underlying central chemosensitivity in vivo is incompletely understood. In highly reduced preparations, both raphe and RTN/pFRG neurons are chemosensitive but no protein or pathway within either of these populations has been unequivocally identified as the transducer of pH/CO2 sensitivity. Hence whether these neurons are intrinsic chemosensors or part of a relay pathway is unclear, although the two possibilities are not mutually exclusive and appear to act in concert through multiple transduction mechanisms (Huckstepp & Dale 2011). An alternate hypothesis is that pH/CO2 is intrinsically sensed by a distinct population of astrocytes, which by releasing ATP near the ventral surface, modulate activity of nearby chemosensitive neurons and thus provide a CO2-related stimulus that modulates breathing (Gourine et al 2010). The ventral medullary surface has several sites where local acidification increases ventilation (Nattie & Li 1996, Schlafke et al 1975). At some of these sites, including adjacent to the RTN/pFRG, astrocytes respond to modest acidification (~0.2 pH units) by releasing ATP. Also, there is an increase in ATP release as blood CO2 increases in vivo and local application of ATP receptor agonists increase ventilation (Gourine et al 2005). Optogenetic stimulation of astrocytes in the region of the RTN/pFRG increases ventilation as well (Gourine et al 2010). A subset of brainstem astrocytes, including those adjacent to the RTN/pFRG, express connexin 26 (Cx26). Cx26 hemichannels are directly CO2 sensitive and open to release ATP upon acidification (Huckstepp et al 2010a, Huckstepp et al 2010b) and blocking gap junctions reduces CO2 sensitivity akin to purinergic receptor antagonists (Wenker et al 2012). Astrocytic gliotransmission thus provides a hypothetical mechanism linking CO2 levels to changes in breathing.
Respiratory networks and human disease
Increasingly, researchers working on the neural control of breathing are trying to understand the causes of, and find rational therapies for, diseases that affect breathing. Central respiratory dysfunction is symptomatic of many diseases that can occur at almost any time during life, and unfortunately, we poorly understand the underlying causes (Axelrod et al 2006, Weese-Mayer et al 2008a, Weese-Mayer et al 2006). This is true even for diseases where causative single gene mutations are known and relevant transgenic mice are available such as CCHS (see above), Rett or Joubert Syndromes (Ben-Shachar et al 2009, Parisi et al 2006, Valente et al 2006a, Valente et al 2006b, Wan et al 1999). In Rett Syndrome, due to a mutation in the MeCP2 gene, girls, and much more rarely, boys, show prolonged periods of respiratory dysfunction during wakefulness and milder effects during sleep (Dunn & MacLeod 2001, Weese-Mayer et al 2008b). MeCP2 mutant mice show similar respiratory deficits and dysfunction in several different populations of glutamatergic and noradrenergic neurons are suggested as causative, although the respiratory phenotype may not manifest by effects in the brainstem or glia (Lioy et al 2011, Robinson et al 2012, Viemari et al 2005, Voituron et al 2010, Weese-Mayer et al 2008b). Similarly, breathing pathologies, especially prolonged and frequent apneas during sleep, are present in a number of neurodegenerative diseases such as Parkinson’s, amyotrophic lateral sclerosis, and multiple systems atrophy, where there are losses of preBötC NK1R or Sst neurons (Alheid et al 2004, Benarroch 2003, Benarroch 2007, Benarroch et al 2003, Schwarzacher et al 2010).
More problematic are diseases that likely have a strong, but currently unknown, genetic component(s) such as Rapid Onset Obesity, Hypoventilation, and Hypothalamic Dysfunction (ROHHAD) or diseases where the role of genetics is unknown, such as apnea of prematurity, sudden infant death syndrome (SIDS), Perry syndrome, and sleep apnea (Axelrod et al 2006, Carroll et al 2010, Ize-Ludlow et al 2007, Paterson et al 2009, Rand et al 2011, Tsuboi et al 2008, Weese-Mayer 2008, Weese-Mayer et al 2008a). These last two are the most prevalent diseases with a breathing phenotype (for live births, SIDS which kills ~6 in 1000 infants <1 year of age (http://www.sidscenter.org/Statistics/table1.html), and sleep apnea affects 4–6% of the adult male population at all ages and an equivalent percentage of females postmenopause (http://www.rightdiagnosis.com/s/sleep_disorders/prevalence-types.htm), with significant increases in morbidity and mortality). As mentioned above, a leading hypothesis posits that SIDS is a dysfunction of serotonin in infant brainstem (Paterson et al 2009). The relatively early stage of our knowledge of the respiratory neural control system as it relates to human disease has lead to confusion about the underlying causes, which consequently may overemphasize possible future treatments to the detriment of both scientific progress and patient hopes.
Challenges remaining
While great progress has been made since the discovery of the preBötC, especially as related to key and specific brainstem sites generating and modulating respiratory rhythm and pattern, our understanding of the underlying mechanisms is still at an early stage. Key high priority problems include identifying and understanding neurons important for the generation of expiratory activity, and understanding the mechanisms of rhythm generation within the preBötC. While the problem is still unsolved, of all behaviors of interest, understanding the neural control of breathing may be first to cross the finish line.
Contributor Information
Jack L. Feldman, Email: feldman@ucla.edu.
Christopher A. Del Negro, Email: cadeln@wm.edu.
Paul A. Gray, Email: pgray@pcg.wustl.edu.
References
- Abbott SB, Stornetta RL, Coates MB, Guyenet PG. Phox2b-expressing neurons of the parafacial region regulate breathing rate, inspiration, and expiration in conscious rats. J Neurosci. 2011;31:16410–22. doi: 10.1523/JNEUROSCI.3280-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbott SB, Stornetta RL, Fortuna MG, Depuy SD, West GH, et al. Photostimulation of retrotrapezoid nucleus phox2b-expressing neurons in vivo produces long-lasting activation of breathing in rats. J Neurosci. 2009;29:5806–19. doi: 10.1523/JNEUROSCI.1106-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdala AP, Rybak IA, Smith JC, Paton JF. Abdominal expiratory activity in the rat brainstem-spinal cord in situ: patterns, origins and implications for respiratory rhythm generation. J Physiol. 2009;587:3539–59. doi: 10.1113/jphysiol.2008.167502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alheid GF, Milsom WK, McCrimmon DR. Pontine influences on breathing: an overview. Respir Physiol Neurobiol. 2004;143:105–14. doi: 10.1016/j.resp.2004.06.016. [DOI] [PubMed] [Google Scholar]
- Aliverti A, Cala SJ, Duranti R, Ferrigno G, Kenyon CM, et al. Human respiratory muscle actions and control during exercise. J Appl Physiol. 1997;83:1256–69. doi: 10.1152/jappl.1997.83.4.1256. [DOI] [PubMed] [Google Scholar]
- Axelrod FB, Chelimsky GG, Weese-Mayer DE. Pediatric autonomic disorders. Pediatrics. 2006;118:309–21. doi: 10.1542/peds.2005-3032. [DOI] [PubMed] [Google Scholar]
- Ballanyi K. Neuromodulation of the perinatal respiratory network. Curr Neuropharmacol. 2004;2:221–43. doi: 10.2174/1570159043476828. [DOI] [PubMed] [Google Scholar]
- Barabási A-L. Linked : how everything is connected to everything else and what it means for business, science, and everyday life. New York: Plume; 2003. p. 294. [Google Scholar]
- Bell SC, Saunders MJ, Elborn JS, Shale DJ. Resting energy expenditure and oxygen cost of breathing in patients with cystic fibrosis. Thorax. 1996;51:126–31. doi: 10.1136/thx.51.2.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Mabrouk F, Tryba AK. Substance P modulation of TRPC3/7 channels improves respiratory rhythm regularity and ICAN-dependent pacemaker activity. Eur J Neurosci. 2010;31:1219–32. doi: 10.1111/j.1460-9568.2010.07156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shachar S, Chahrour M, Thaller C, Shaw CA, Zoghbi HY. Mouse models of MeCP2 disorders share gene expression changes in the cerebellum and hypothalamus. Hum Mol Genet. 2009;18:2431–42. doi: 10.1093/hmg/ddp181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benarroch EE. Brainstem in multiple system atrophy: clinicopathological correlations. Cell Mol Neurobiol. 2003;23:519–26. doi: 10.1023/A:1025067912199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benarroch EE. Brainstem respiratory control: substrates of respiratory failure of multiple system atrophy. Mov Disord. 2007;22:155–61. doi: 10.1002/mds.21236. [DOI] [PubMed] [Google Scholar]
- Benarroch EE, Schmeichel AM, Low PA, Parisi JE. Depletion of ventromedullary NK-1 receptor-immunoreactive neurons in multiple system atrophy. Brain : a journal of neurology. 2003;126:2183–90. doi: 10.1093/brain/awg220. [DOI] [PubMed] [Google Scholar]
- Berner J, Shvarev Y, Lagercrantz H, Bilkei-Gorzo A, Hokfelt T, Wickstrom R. Altered respiratory pattern and hypoxic response in transgenic newborn mice lacking the tachykinin-1 gene. J Appl Physiol. 2007;103:552–9. doi: 10.1152/japplphysiol.01389.2006. [DOI] [PubMed] [Google Scholar]
- Berry-Kravis EM, Zhou L, Rand CM, Weese-Mayer DE. Congenital Central Hypoventilation Syndrome: PHOX2B Mutations and Phenotype. Am J Respir Crit Care Med. 2006 doi: 10.1164/rccm.200602-305OC. [DOI] [PubMed] [Google Scholar]
- Best J, Borisyuk A, Rubin J, Terman D, Wechselberger M. The dynamic range of bursting in a model respiratory pacemaker network. SIAM J App Dyn Sys. 2005;4:1107–39. [Google Scholar]
- Bianchi AL, Denavit-Saubie M, Champagnat J. Central control of breathing in mammals: neuronal circuitry, membrane properties, and neurotransmitters. Physiol Rev. 1995;75:1–45. doi: 10.1152/physrev.1995.75.1.1. [DOI] [PubMed] [Google Scholar]
- Blanchi B, Kelly LM, Viemari JC, Lafon I, Burnet H, et al. MafB deficiency causes defective respiratory rhythmogenesis and fatal central apnea at birth. Nat Neurosci. 2003;6:1091–100. doi: 10.1038/nn1129. [DOI] [PubMed] [Google Scholar]
- Blanchi B, Sieweke MH. Mutations of brainstem transcription factors and central respiratory disorders. Trends Mol Med. 2005;11:23–30. doi: 10.1016/j.molmed.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Bochorishvili G, Stornetta RL, Coates MB, Guyenet PG. Pre-Bötzinger complex receives glutamatergic innervation from galaninergic and other retrotrapezoid nucleus neurons. J Comp Neurol. 2012;520:1047–61. doi: 10.1002/cne.22769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogaard A, Parent J, Zochowski M, Booth V. Interaction of cellular and network mechanisms in spatiotemporal pattern formation in neuronal networks. J Neurosci. 2009;29:1677–87. doi: 10.1523/JNEUROSCI.5218-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borday C, Chatonnet F, Thoby-Brisson M, Champagnat J, Fortin G. Neural tube patterning by Krox20 and emergence of a respiratory control. Respir Physiol Neurobiol. 2005;149:63–72. doi: 10.1016/j.resp.2005.02.014. [DOI] [PubMed] [Google Scholar]
- Bouvier J, Thoby-Brisson M, Renier N, Dubreuil V, Ericson J, et al. Hindbrain interneurons and axon guidance signaling critical for breathing. Nat Neurosci. 2010;13:1066–74. doi: 10.1038/nn.2622. [DOI] [PubMed] [Google Scholar]
- Branco T, Hausser M. The single dendritic branch as a fundamental functional unit in the nervous system. Curr Opin Neurobiol. 2010;20:494–502. doi: 10.1016/j.conb.2010.07.009. [DOI] [PubMed] [Google Scholar]
- Branco T, Hausser M. Synaptic integration gradients in single cortical pyramidal cell dendrites. Neuron. 2011;69:885–92. doi: 10.1016/j.neuron.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briscoe J, Pierani A, Jessell TM, Ericson J. A homeodomain protein code specifies progenitor cell identity and neuronal fate in the ventral neural tube. Cell. 2000;101:435–45. doi: 10.1016/s0092-8674(00)80853-3. [DOI] [PubMed] [Google Scholar]
- Brocard F, Tazerart S, Vinay L. Do pacemakers drive the central pattern generator for locomotion in mammals? Neuroscientist. 2010;16:139–55. doi: 10.1177/1073858409346339. [DOI] [PubMed] [Google Scholar]
- Brocard F, Verdier D, Arsenault I, Lund JP, Kolta A. Emergence of intrinsic bursting in trigeminal sensory neurons parallels the acquisition of mastication in weanling rats. J Neurophysiol. 2006;96:2410–24. doi: 10.1152/jn.00352.2006. [DOI] [PubMed] [Google Scholar]
- Brockhaus J, Ballanyi K. Synaptic inhibition in the isolated respiratory network of neonatal rats. Eur J Neurosci. 1998;10:3823–39. doi: 10.1046/j.1460-9568.1998.00396.x. [DOI] [PubMed] [Google Scholar]
- Brown T. The intrinsic factors in the act of progression in the mammal. Proc R Soc Lond B. 1911;84:308–19. [Google Scholar]
- Brown TG. On the nature of the fundamental activity of the nervous centres: together with an analysis of the conditioning of rhythmic activity in progression, and a theory of the evolution of function in the nervous system. J Physiol. 1914;48:18–46. doi: 10.1113/jphysiol.1914.sp001646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büsselberg D, Bischoff AM, Paton JF, Richter DW. Reorganisation of respiratory network activity after loss of glycinergic inhibition. Pflugers Arch. 2001;441:444–9. doi: 10.1007/s004240000453. [DOI] [PubMed] [Google Scholar]
- Büsselberg D, Bischoff AM, Richter DW. A combined blockade of glycine and calcium-dependent potassium channels abolishes the respiratory rhythm. Neurosci. 2003;122:831–41. doi: 10.1016/j.neuroscience.2003.07.014. [DOI] [PubMed] [Google Scholar]
- Butera RJ, Jr, Rinzel J, Smith JC. Models of respiratory rhythm generation in the pre-Botzinger complex. I. Bursting pacemaker neurons. J Neurophysiol. 1999;82:382–97. doi: 10.1152/jn.1999.82.1.382. [DOI] [PubMed] [Google Scholar]
- Buzsáki G. Rhythms of the brain. Oxford ; New York: Oxford University Press; 2006. p. xiv.p. 448. [Google Scholar]
- Carroll MS, Kenny AS, Ramirez J-M, Weese-Mayer DE. Alterations in cardiopulmonary regulation during wakefulness and sleep. Clin Auton Res. 2010;20:325. [Google Scholar]
- Caubit X, Thoby-Brisson M, Voituron N, Filippi P, Bevengut M, et al. Teashirt 3 regulates development of neurons involved in both respiratory rhythm and airflow control. J Neurosci. 2010;30:9465–76. doi: 10.1523/JNEUROSCI.1765-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagnat J, Morin-Surun MP, Fortin G, Thoby-Brisson M. Developmental basis of the rostro-caudal organization of the brainstem respiratory rhythm generator. Philos Trans R Soc Lond B Biol Sci. 2009;364:2469–76. doi: 10.1098/rstb.2009.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L, Arata A, Mizuguchi R, Qian Y, Karunaratne A, et al. Tlx3 and Tlx1 are post-mitotic selector genes determining glutamatergic over GABAergic cell fates. Nat Neurosci. 2004;7:510–7. doi: 10.1038/nn1221. [DOI] [PubMed] [Google Scholar]
- Cohen MI, Feldman JL. Central mechanisms controlling expiratory duration. Adv Exp Med Biol. 1978;99:369–82. doi: 10.1007/978-1-4613-4009-6_40. [DOI] [PubMed] [Google Scholar]
- Crowder EA, Saha MS, Pace RW, Zhang H, Prestwich GD, Del Negro CA. Phosphatidylinositol 4,5-bisphosphate regulates inspiratory burst activity in the neonatal mouse preBotzinger complex. J Physiol. 2007;582:1047–58. doi: 10.1113/jphysiol.2007.134577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Almeida AT, Al-Izki S, Denton ME, Kirkwood PA. Patterns of expiratory and inspiratory activation for thoracic motoneurones in the anaesthetized and the decerebrate rat. J Physiol. 2010;588:2707–29. doi: 10.1113/jphysiol.2010.192518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Negro CA, Hayes JA, Pace RW, Brush BR, Teruyama R, Feldman JL. Synaptically Activated Burst-Generating Conductances Underlie a Group-Pacemaker Mechanism for Respiratory Rhythm Generation in Mammal. In: Gossard J-P, Dubuc R, Kolta A, editors. Prog Brain Res. New York: Elsevier; 2010. pp. 111–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Negro CA, Hayes JA, Rekling JC. Dendritic Calcium Activity Precedes Inspiratory Bursts in preBotzinger Complex Neurons. J Neurosci. 2011;31:1017–22. doi: 10.1523/JNEUROSCI.4731-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Negro CA, Kam K, Hayes JA, Feldman JL. Asymmetric control of inspiratory and expiratory phases by excitability in the respiratory network of neonatal mice in vitro. J Physiol. 2009;587:1217–31. doi: 10.1113/jphysiol.2008.164079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Negro CA, Koshiya N, Butera RJ, Jr, Smith JC. Persistent sodium current, membrane properties and bursting behavior of pre-Bötzinger complex inspiratory neurons in vitro. J Neurophysiol. 2002a;88:2242–50. doi: 10.1152/jn.00081.2002. [DOI] [PubMed] [Google Scholar]
- Del Negro CA, Morgado-Valle C, Feldman JL. Respiratory rhythm: an emergent network property? Neuron. 2002b;34:821–30. doi: 10.1016/s0896-6273(02)00712-2. [DOI] [PubMed] [Google Scholar]
- Del Negro CA, Morgado-Valle C, Hayes JA, Mackay DD, Pace RW, et al. Sodium and calcium current-mediated pacemaker neurons and respiratory rhythm generation. J Neurosci. 2005;25:446–53. doi: 10.1523/JNEUROSCI.2237-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doble A. The pharmacology and mechanism of action of riluzole. Neurol. 1996;47:S233–41. doi: 10.1212/wnl.47.6_suppl_4.233s. [DOI] [PubMed] [Google Scholar]
- Doi A, Ramirez JM. Neuromodulation and the orchestration of the respiratory rhythm. Respir Physiol Neurobiol. 2008;164:96–104. doi: 10.1016/j.resp.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi A, Ramirez JM. State-dependent interactions between excitatory neuromodulators in the neuronal control of breathing. J Neurosci. 2010;30:8251–62. doi: 10.1523/JNEUROSCI.5361-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubreuil V, Barhanin J, Goridis C, Brunet JF. Breathing with phox2b. Philos Trans R Soc Lond B Biol Sci. 2009;364:2477–83. doi: 10.1098/rstb.2009.0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubreuil V, Ramanantsoa N, Trochet D, Vaubourg V, Amiel J, et al. A human mutation in Phox2b causes lack of CO2 chemosensitivity, fatal central apnea, and specific loss of parafacial neurons. Proc Natl Acad Sci U S A. 2008;105:1067–72. doi: 10.1073/pnas.0709115105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn HG, MacLeod PM. Rett syndrome: review of biological abnormalities. Can J Neurol Sci. 2001;28:16–29. doi: 10.1017/s0317167100052513. [DOI] [PubMed] [Google Scholar]
- Eldridge FL, Millhorn DE, Kiley JP, Waldrop TG. Stimulation by central command of locomotion, respiration and circulation during exercise. Respir Physiol. 1985;59:313–37. doi: 10.1016/0034-5687(85)90136-7. [DOI] [PubMed] [Google Scholar]
- Feldman JL. Neurophysiology of breathing in mammals. In: Bloom FE, editor. Handbook of Physiology; Section I: The Nervous System; Volume IV: Intrinsic Regulatory Systems of the Brain. Bethesda, MD: Am. Physiol. Soc; 1986. pp. 463–524. [Google Scholar]
- Feldman JL, Connelly CA, Ellenberger HH, Smith JC. The cardiorespiratory system within the brainstem. Eur J Neurosci Suppl. 1990;3:171. [Google Scholar]
- Feldman JL, Del Negro CA. Looking for inspiration: new perspectives on respiratory rhythm. Nat Rev Neurosci. 2006;7:232–42. doi: 10.1038/nrn1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman JL, Gautier H. Interaction of pulmonary afferents and pneumotaxic center in control of respiratory pattern in cats. J Neurophysiol. 1976;39:31–44. doi: 10.1152/jn.1976.39.1.31. [DOI] [PubMed] [Google Scholar]
- Feldman JL, Mitchell GS, Nattie EE. Breathing: Rhythmicity, Plasticity, Chemosensitivity. Annu Rev Neurosci. 2003;26:239–66. doi: 10.1146/annurev.neuro.26.041002.131103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman JL, Smith JC. Cellular mechanisms underlying modulation of breathing pattern in mammals. Ann N Y Acad Sci. 1989;563:114–30. doi: 10.1111/j.1749-6632.1989.tb42194.x. [DOI] [PubMed] [Google Scholar]
- Fong AY, Marshall LH, Milsom WK. Riluzole disrupts autoresuscitation from hypothermic respiratory arrest in neonatal hamsters but not rats. Respir Physiol Neurobiol. 2009;166:175–83. doi: 10.1016/j.resp.2009.03.004. [DOI] [PubMed] [Google Scholar]
- Fortuna MG, West GH, Stornetta RL, Guyenet PG. Botzinger expiratory-augmenting neurons and the parafacial respiratory group. J Neurosci. 2008;28:2506–15. doi: 10.1523/JNEUROSCI.5595-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster RG, Kreitzman L. Rhythms of life : the biological clocks that control the daily lives of every living thing. New Haven, CT: Yale University Press; 2004. p. xiii.p. 276. [Google Scholar]
- Funk GD, Smith JC, Feldman JL. Generation and transmission of respiratory oscillations in medullary slices: role of excitatory amino acids. J Neurophysiol. 1993;70:1497–515. doi: 10.1152/jn.1993.70.4.1497. [DOI] [PubMed] [Google Scholar]
- Garcia AJ, 3rd, Zanella S, Koch H, Doi A, Ramirez JM. Chapter 3--networks within networks: the neuronal control of breathing. Prog Brain Res. 2011;188:31–50. doi: 10.1016/B978-0-444-53825-3.00008-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner WN. The pattern of breathing following step changes of alveolar partial pressures of carbon dioxide and oxygen in man. J Physiol. 1980;300:55–73. doi: 10.1113/jphysiol.1980.sp013151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasgow SM, Henke RM, Macdonald RJ, Wright CV, Johnson JE. Ptf1a determines GABAergic over glutamatergic neuronal cell fate in the spinal cord dorsal horn. Development. 2005;132:5461–9. doi: 10.1242/dev.02167. [DOI] [PubMed] [Google Scholar]
- Goridis C, Dubreuil V, Thoby-Brisson M, Fortin G, Brunet JF. Phox2b, congenital central hypoventilation syndrome and the control of respiration. Semin Cell Dev Biol. 2010 doi: 10.1016/j.semcdb.2010.07.006. [DOI] [PubMed] [Google Scholar]
- Gosgnach S. The role of genetically-defined interneurons in generating the mammalian locomotor rhythm. Integ Comp Biol. 2011;51:903–12. doi: 10.1093/icb/icr022. [DOI] [PubMed] [Google Scholar]
- Gossard JP, Dubuc R, Kolta A, editors. Breathe, Walk and Chew: The Neural Challenge: Part I. 2010/11/30 Vol. 187. New York: Elsevier; 2010. p. 230. [Google Scholar]
- Gossard JP, Dubuc R, Kolta A. Preface. Breathe, walk and chew: the neural challenge: part II. Prog Brain Res. 2011;188:ix. doi: 10.1016/B978-0-444-53825-3.00022-X. [DOI] [PubMed] [Google Scholar]
- Goulding M. Circuits controlling vertebrate locomotion: moving in a new direction. Nat Rev Neurosci. 2009;10:507–18. doi: 10.1038/nrn2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourine AV, Kasymov V, Marina N, Tang F, Figueiredo MF, et al. Astrocytes control breathing through pH-dependent release of ATP. Science. 2010;329:571–5. doi: 10.1126/science.1190721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourine AV, Llaudet E, Dale N, Spyer KM. ATP is a mediator of chemosensory transduction in the central nervous system. Nature. 2005;436:108–11. doi: 10.1038/nature03690. [DOI] [PubMed] [Google Scholar]
- Gray PA. Transcription factors and the genetic organization of brain stem respiratory neurons. J Appl Physiol. 2008;104:1513–21. doi: 10.1152/japplphysiol.01383.2007. [DOI] [PubMed] [Google Scholar]
- Gray PA, Fu H, Luo P, Zhao Q, Yu J, et al. Mouse Brain Organization Revealed Through Direct Genome-Scale TF Expression Analysis. Science. 2004;306:2255–57. doi: 10.1126/science.1104935. [DOI] [PubMed] [Google Scholar]
- Gray PA, Hayes JA, Ling GY, Llona I, Tupal S, et al. Developmental origin of preBötzinger complex respiratory neurons. J Neurosci. 2010;30:14883–95. doi: 10.1523/JNEUROSCI.4031-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray PA, Janczewski WA, Mellen N, McCrimmon DR, Feldman JL. Normal breathing requires preBötzinger complex neurokinin-1 receptor-expressing neurons. Nat Neurosci. 2001;4:927–30. doi: 10.1038/nn0901-927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray PA, Rekling JC, Bocchiaro CM, Feldman JL. Modulation of respiratory frequency by peptidergic input to rhythmogenic neurons in the preBötzinger complex. Science. 1999;286:1566–8. doi: 10.1126/science.286.5444.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer JJ, Smith JC, Feldman JL. Role of excitatory amino acids in the generation and transmission of respiratory drive in neonatal rat. J Physiol. 1991;437:727–49. doi: 10.1113/jphysiol.1991.sp018622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillner S, Jessell TM. Measured motion: searching for simplicity in spinal locomotor networks. Curr Opin Neurobiol. 2009;19:572–86. doi: 10.1016/j.conb.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG, Bayliss DA, Stornetta RL, Fortuna MG, Abbott SB, DePuy SD. Retrotrapezoid nucleus, respiratory chemosensitivity and breathing automaticity. Respir Physiol Neurobiol. 2009;168:59–68. doi: 10.1016/j.resp.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG, Mulkey DK, Stornetta RL, Bayliss DA. Regulation of ventral surface chemoreceptors by the central respiratory pattern generator. J Neurosci. 2005;25:8938–47. doi: 10.1523/JNEUROSCI.2415-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG, Sevigny CP, Weston MC, Stornetta RL. Neurokinin-1 receptor-expressing cells of the ventral respiratory group are functionally heterogeneous and predominantly glutamatergic. J Neurosci. 2002;22:3806–16. doi: 10.1523/JNEUROSCI.22-09-03806.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG, Wang H. Pre-Bötzinger neurons with preinspiratory discharges “in vivo” express NK1 receptors in the rat. J Neurophysiol. 2001;86:438–46. doi: 10.1152/jn.2001.86.1.438. [DOI] [PubMed] [Google Scholar]
- Haller M, Mironov SL, Karschin A, Richter DW. Dynamic activation of K(ATP) channels in rhythmically active neurons. J Physiol. 2001;537:69–81. doi: 10.1111/j.1469-7793.2001.0069k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes JA, Del Negro CA. Neurokinin Receptor-Expressing PreBötzinger Complex Neurons in Neonatal Mice Studied In Vitro. J Neurophysiol. 2007;97:4215–24. doi: 10.1152/jn.00228.2007. [DOI] [PubMed] [Google Scholar]
- Hayes JA, Wang X, Del Negro CA. Cumulative lesioning of respiratory interneurons disrupts and precludes motor rhythms in vitro. Proc Natl Acad Sci U S A. 2012 doi: 10.1073/pnas.1200912109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks T, Francis N, Fyodorov D, Deneris ES. The ETS domain factor Pet-1 is an early and precise marker of central serotonin neurons and interacts with a conserved element in serotonergic genes. J Neurosci. 1999;19:10348–56. doi: 10.1523/JNEUROSCI.19-23-10348.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks TJ, Fyodorov DV, Wegman LJ, Lelutiu NB, Pehek EA, et al. Pet-1 ETS gene plays a critical role in 5-HT neuron development and is required for normal anxiety-like and aggressive behavior. Neuron. 2003;37:233–47. doi: 10.1016/s0896-6273(02)01167-4. [DOI] [PubMed] [Google Scholar]
- Hirsch MR, Tiveron MC, Guillemot F, Brunet JF, Goridis C. Control of noradrenergic differentiation and Phox2a expression by MASH1 in the central and peripheral nervous system. Development. 1998;125:599–608. doi: 10.1242/dev.125.4.599. [DOI] [PubMed] [Google Scholar]
- Hodges MR, Best S, Richerson GB. Altered ventilatory and thermoregulatory control in male and female adult Pet-1 null mice. Respir Physiol Neurobiol. 2011;177:133–40. doi: 10.1016/j.resp.2011.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges MR, Richerson GB. Medullary serotonin neurons and their roles in central respiratory chemoreception. Respir Physiol Neurobiol. 2010;173:256–63. doi: 10.1016/j.resp.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges MR, Wehner M, Aungst J, Smith JC, Richerson GB. Transgenic mice lacking serotonin neurons have severe apnea and high mortality during development. J Neurosci. 2009;29:10341–9. doi: 10.1523/JNEUROSCI.1963-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino M, Nakamura S, Mori K, Kawauchi T, Terao M, et al. Ptf1a, a bHLH transcriptional gene, defines GABAergic neuronal fates in cerebellum. Neuron. 2005;47:201–13. doi: 10.1016/j.neuron.2005.06.007. http://www.rightdiagnosis.com/s/sleep_disorders/prevalence-types.htm. http://www.sidscenter.org/Statistics/table1.html. [DOI] [PubMed] [Google Scholar]
- Huckstepp RT, Dale N. Redefining the components of central CO2 chemosensitivity - towards a better understanding of mechanism. J Physiol. 2011;589:5561–79. doi: 10.1113/jphysiol.2011.214759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huckstepp RT, Eason R, Sachdev A, Dale N. CO2-dependent opening of connexin 26 and related beta connexins. J Physiol. 2010a;588:3921–31. doi: 10.1113/jphysiol.2010.192096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huckstepp RT, id Bihi R, Eason R, Spyer KM, Dicke N, et al. Connexin hemichannel-mediated CO2-dependent release of ATP in the medulla oblongata contributes to central respiratory chemosensitivity. J Physiol. 2010b;588:3901–20. doi: 10.1113/jphysiol.2010.192088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iizuka M. Abdominal expiratory muscle activity in anesthetized vagotomized neonatal rats. J Physiol Sci. 2009;59:157–63. doi: 10.1007/s12576-009-0020-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ize-Ludlow D, Gray JA, Sperling MA, Berry-Kravis EM, Milunsky JM, et al. Rapid-onset obesity with hypothalamic dysfunction, hypoventilation, and autonomic dysregulation presenting in childhood. Pediatrics. 2007;120:e179–88. doi: 10.1542/peds.2006-3324. [DOI] [PubMed] [Google Scholar]
- Jacquin TD, Borday V, Schneider-Maunoury S, Topilko P, Ghilini G, et al. Reorganization of pontine rhythmogenic neuronal networks in Krox-20 knockout mice. Neuron. 1996;17:747–58. doi: 10.1016/s0896-6273(00)80206-8. [DOI] [PubMed] [Google Scholar]
- Janczewski WA, Feldman JL. Distinct rhythm generators for inspiration and expiration in the juvenile rat. J Physiol. 2006;570:407–20. doi: 10.1113/jphysiol.2005.098848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SM, Smith JC, Funk GD, Feldman JL. Pacemaker behavior of respiratory neurons in medullary slices from neonatal rat. J Neurophysiol. 1994;72:2598–608. doi: 10.1152/jn.1994.72.6.2598. [DOI] [PubMed] [Google Scholar]
- Kang BJ, Chang DA, Mackay DD, West GH, Moreira TS, et al. Central nervous system distribution of the transcription factor Phox2b in the adult rat. J Comp Neurol. 2007;503:627–41. doi: 10.1002/cne.21409. [DOI] [PubMed] [Google Scholar]
- Kiehn O. Locomotor circuits in the mammalian spinal cord. Annu Rev Neurosci. 2006;29:279–306. doi: 10.1146/annurev.neuro.29.051605.112910. [DOI] [PubMed] [Google Scholar]
- Koizumi H, Smith JC. Persistent Na+ and K+-dominated leak currents contribute to respiratory rhythm generation in the pre-Bötzinger complex in vitro. J Neurosci. 2008;28:1773–85. doi: 10.1523/JNEUROSCI.3916-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi H, Wilson CG, Wong S, Yamanishi T, Koshiya N, Smith JC. Functional imaging, spatial reconstruction, and biophysical analysis of a respiratory motor circuit isolated in vitro. J Neurosci. 2008;28:2353–65. doi: 10.1523/JNEUROSCI.3553-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause KL, Forster HV, Kiner T, Davis SE, Bonis JM, et al. Normal breathing pattern and arterial blood gases in awake and sleeping goats after near total destruction of the presumed pre-Botzinger complex and the surrounding region. J Appl Physiol. 2009;106:605–19. doi: 10.1152/japplphysiol.90966.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krey RA, Goodreau AM, Arnold TB, Del Negro CA. Outward Currents Contributing to Inspiratory Burst Termination in preBotzinger Complex Neurons of Neonatal Mice Studied in Vitro. Front Neural Circuits. 2010;4:124. doi: 10.3389/fncir.2010.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanuza GM, Gosgnach S, Pierani A, Jessell TM, Goulding M. Genetic identification of spinal interneurons that coordinate left-right locomotor activity necessary for walking movements. Neuron. 2004;42:375–86. doi: 10.1016/s0896-6273(04)00249-1. [DOI] [PubMed] [Google Scholar]
- Lieske SP, Thoby-Brisson M, Telgkamp P, Ramirez JM. Reconfiguration of the neural network controlling multiple breathing patterns: eupnea, sighs and gasps [see comment] Nat Neurosci. 2000;3:600–7. doi: 10.1038/75776. [DOI] [PubMed] [Google Scholar]
- Lioy DT, Garg SK, Monaghan CE, Raber J, Foust KD, et al. A role for glia in the progression of Rett’s syndrome. Nature. 2011;475:497–500. doi: 10.1038/nature10214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llona I, Ampuero E, Eugenin JL. Somatostatin inhibition of fictive respiration is modulated by pH. Brain Res. 2004;1026:136–42. doi: 10.1016/j.brainres.2004.08.028. [DOI] [PubMed] [Google Scholar]
- London M, Hausser M. Dendritic computation. Ann Rev Neurosci. 2005;28:503–32. doi: 10.1146/annurev.neuro.28.061604.135703. [DOI] [PubMed] [Google Scholar]
- Loov C, Hillered L, Ebendal T, Erlandsson A. Engulfing Astrocytes Protect Neurons from Contact-Induced Apoptosis following Injury. PLoS One. 2012;7:e33090. doi: 10.1371/journal.pone.0033090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B, Su Y, Das S, Liu J, Xia J, Ren D. The neuronal channel NALCN contributes resting sodium permeability and is required for normal respiratory rhythm. Cell. 2007;129:371–83. doi: 10.1016/j.cell.2007.02.041. [DOI] [PubMed] [Google Scholar]
- Lumsden A. The cellular basis of segmentation in the developing hindbrain. Trends Neurosci. 1990;13:329–35. doi: 10.1016/0166-2236(90)90144-y. [DOI] [PubMed] [Google Scholar]
- Ma LH, Gilland E, Bass AH, Baker R. Ancestry of motor innervation to pectoral fin and forelimb. Nat Commun. 2010;1:49. doi: 10.1038/ncomms1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzke T, Guenther U, Ponimaskin EG, Haller M, Dutschmann M, et al. 5-HT4(a) receptors avert opioid-induced breathing depression without loss of analgesia. Science. 2003;301:226–9. doi: 10.1126/science.1084674. [DOI] [PubMed] [Google Scholar]
- McKay LC, Janczewski WA, Feldman JL. Sleep-disordered breathing after targeted ablation of preBotzinger complex neurons. Nat Neurosci. 2005;8:1142–4. doi: 10.1038/nn1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellen NM, Janczewski WA, Bocchiaro CM, Feldman JL. Opioid-induced quantal slowing reveals dual networks for respiratory rhythm generation. Neuron. 2003;37:821–6. doi: 10.1016/s0896-6273(03)00092-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill EG. Finding a respiratory function for the medullary respiratory neurons. In: Bellairs R, Gray EG, editors. Essays on the Nervous System. Clarendon: Oxford; 1974. pp. 451–86. [Google Scholar]
- Mironov S. Respiratory circuits: function, mechanisms, topology, and pathology. Neuroscientist. 2009;15:194–208. doi: 10.1177/1073858408329510. [DOI] [PubMed] [Google Scholar]
- Mironov SL. Metabotropic glutamate receptors activate dendritic calcium waves and TRPM channels which drive rhythmic respiratory patterns in mice. J Physiol. 2008;586:2277–91. doi: 10.1113/jphysiol.2007.149021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mironov SL, Langohr K, Haller M, Richter DW. Hypoxia activates ATP-dependent potassium channels in inspiratory neurones of neonatal mice. J Physiol. 1998;509 (Pt 3):755–66. doi: 10.1111/j.1469-7793.1998.755bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mironov SL, Skorova EY. Stimulation of bursting in pre-Botzinger neurons by Epac through calcium release and modulation of TRPM4 and K-ATP channels. J Neurochem. 2011;117:295–308. doi: 10.1111/j.1471-4159.2011.07202.x. [DOI] [PubMed] [Google Scholar]
- Molkov YI, Abdala AP, Bacak BJ, Smith JC, Paton JF, Rybak IA. Late-expiratory activity: emergence and interactions with the respiratory CpG. J Neurophysiol. 2010;104:2713–29. doi: 10.1152/jn.00334.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montandon G, Qin W, Liu H, Ren J, Greer JJ, Horner RL. PreBötzinger Complex Neurokinin-1 Receptor-Expressing Neurons Mediate Opioid-Induced Respiratory Depression. J Neurosci. 2011;31:1292–301. doi: 10.1523/JNEUROSCI.4611-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgado-Valle C, Baca SM, Feldman JL. Glycinergic Pacemaker Neurons in PreBötzinger Complex of Neonatal Mouse. J Neurosci. 2010;30:3634–9. doi: 10.1523/JNEUROSCI.3040-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgado-Valle C, Beltran-Parrazal L, DiFranco M, Vergara JL, Feldman JL. Somatic Ca2+ transients do not contribute to inspiratory drive in preBötzinger Complex neurons. J Physiol. 2008;586:4531–40. doi: 10.1113/jphysiol.2008.154765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkey DK, Stornetta RL, Weston MC, Simmons JR, Parker A, et al. Respiratory control by ventral surface chemoreceptor neurons in rats. Nat Neurosci. 2004;7:1360–9. doi: 10.1038/nn1357. [DOI] [PubMed] [Google Scholar]
- Mulkey DK, Talley EM, Stornetta RL, Siegel AR, West GH, et al. TASK channels determine pH sensitivity in select respiratory neurons but do not contribute to central respiratory chemosensitivity. J Neurosci. 2007;27:14049–58. doi: 10.1523/JNEUROSCI.4254-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nattie EE, Li A. Central chemoreception in the region of the ventral respiratory group in the rat. J Appl Physiol. 1996;81:1987–95. doi: 10.1152/jappl.1996.81.5.1987. [DOI] [PubMed] [Google Scholar]
- Neumueller S, Hodges MR, Krause K, Marshall B, Bonis J, et al. Anatomic changes in multiple brainstem nuclei after incremental, near-complete neurotoxic destruction of the pre-Botzinger Complex in adult goats. Respir Physiol Neurobiol. 2011;175:1–11. doi: 10.1016/j.resp.2010.06.010. [DOI] [PubMed] [Google Scholar]
- Nsegbe E, Wallen-Mackenzie A, Dauger S, Roux JC, Shvarev Y, et al. Congenital hypoventilation and impaired hypoxic response in Nurr1 mutant mice. J Physiol. 2004;556:43–59. doi: 10.1113/jphysiol.2003.058560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onimaru H, Homma I. A novel functional neuron group for respiratory rhythm generation in the ventral medulla. J Neurosci. 2003;23:1478–86. doi: 10.1523/JNEUROSCI.23-04-01478.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onimaru H, Homma I. Two modes of respiratory rhythm generation in the newborn rat brainstem-spinal cord preparation. Adv Exp Med Biol. 2008;605:104–8. doi: 10.1007/978-0-387-73693-8_18. [DOI] [PubMed] [Google Scholar]
- Onimaru H, Homma I, Feldman JL, Janczewski WA. The para-facial respiratory group (pFRG)/pre Botzinger Complex (preBotC) is the primary site of respiratory rhythm generation in the mammal. J Appl Physiol. 2006;100:2094–98. doi: 10.1152/japplphysiol.00119.2006. [DOI] [PubMed] [Google Scholar]
- Onimaru H, Ikeda K, Kawakami K. CO2-sensitive preinspiratory neurons of the parafacial respiratory group express Phox2b in the neonatal rat. J Neurosci. 2008;28:12845–50. doi: 10.1523/JNEUROSCI.3625-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onimaru H, Ikeda K, Kawakami K. Postsynaptic mechanisms of CO(2) responses in parafacial respiratory neurons of newborn rats. J Physiol. 2012;590:1615–24. doi: 10.1113/jphysiol.2011.222687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlovsky GN, Deliagina TG, Grillner S. Neuronal control of locomotion : from mollusc to man. New York: Oxford University Press; 1999. p. xiv.p. 322. [Google Scholar]
- Pace RW, Del Negro CA. AMPA and metabotropic glutamate receptors cooperatively generate inspiratory-like depolarization in mouse respiratory neurons in vitro. Eur J Neurosci. 2008;28:2434–42. doi: 10.1111/j.1460-9568.2008.06540.x. [DOI] [PubMed] [Google Scholar]
- Pace RW, Mackay DD, Feldman JL, Del Negro CA. Inspiratory bursts in the preBötzinger Complex depend on a calcium-activated nonspecific cationic current linked to glutamate receptors. J Physiol. 2007a;582:113–25. doi: 10.1113/jphysiol.2007.133660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace RW, Mackay DD, Feldman JL, Del Negro CA. Role of persistent sodium current in mouse preBotzinger Complex neurons and respiratory rhythm generation. J Physiol. 2007b;580:485–96. doi: 10.1113/jphysiol.2006.124602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliardini S, Adachi T, Ren J, Funk GD, Greer JJ. Fluorescent tagging of rhythmically active respiratory neurons within the pre-Bötzinger complex of rat medullary slice preparations. J Neurosci. 2005;25:2591–6. doi: 10.1523/JNEUROSCI.4930-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliardini S, Janczewski WA, Tan W, Dickson CT, Deisseroth K, Feldman JL. Active expiration induced by excitation of ventral medulla in adult anesthetized rats. J Neurosci. 2011;31:2895–905. doi: 10.1523/JNEUROSCI.5338-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliardini S, Ren J, Gray PA, Vandunk C, Gross M, et al. Central respiratory rhythmogenesis is abnormal in lbx1- deficient mice. J Neurosci. 2008;28:11030–41. doi: 10.1523/JNEUROSCI.1648-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parisi MA, Doherty D, Eckert ML, Shaw DW, Ozyurek H, et al. AHI1 mutations cause both retinal dystrophy and renal cystic disease in Joubert syndrome. J Med Genet. 2006;43:334–9. doi: 10.1136/jmg.2005.036608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson DS, Hilaire G, Weese-Mayer DE. Medullary serotonin defects and respiratory dysfunction in sudden infant death syndrome. Respir Physiol Neurobiol. 2009;168:133–43. doi: 10.1016/j.resp.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson DS, Trachtenberg FL, Thompson EG, Belliveau RA, Beggs AH, et al. Multiple serotonergic brainstem abnormalities in sudden infant death syndrome. JAMA. 2006;296:2124–32. doi: 10.1001/jama.296.17.2124. [DOI] [PubMed] [Google Scholar]
- Paton JF. A working heart-brainstem preparation of the mouse. J Neurosci Meth. 1996;65:63–8. doi: 10.1016/0165-0270(95)00147-6. [DOI] [PubMed] [Google Scholar]
- Paton JF, Abdala AP, Koizumi H, Smith JC, St-John WM. Respiratory rhythm generation during gasping depends on persistent sodium current. Nat Neurosci. 2006;9:311–3. doi: 10.1038/nn1650. [DOI] [PubMed] [Google Scholar]
- Pattinson KT, Mitsis GD, Harvey AK, Jbabdi S, Dirckx S, et al. Determination of the human brainstem respiratory control network and its cortical connections in vivo using functional and structural imaging. NeuroImage. 2009;44:295–305. doi: 10.1016/j.neuroimage.2008.09.007. [DOI] [PubMed] [Google Scholar]
- Pattyn A, Goridis C, Brunet JF. Specification of the central noradrenergic phenotype by the homeobox gene Phox2b. Mol Cell Neurosci. 2000a;15:235–43. doi: 10.1006/mcne.1999.0826. [DOI] [PubMed] [Google Scholar]
- Pattyn A, Hirsch M, Goridis C, Brunet JF. Control of hindbrain motor neuron differentiation by the homeobox gene Phox2b. Development. 2000b;127:1349–58. doi: 10.1242/dev.127.7.1349. [DOI] [PubMed] [Google Scholar]
- Pattyn A, Morin X, Cremer H, Goridis C, Brunet JF. Expression and interactions of the two closely related homeobox genes Phox2a and Phox2b during neurogenesis. Development. 1997;124:4065–75. doi: 10.1242/dev.124.20.4065. [DOI] [PubMed] [Google Scholar]
- Pena F, Parkis MA, Tryba AK, Ramirez JM. Differential Contribution of Pacemaker Properties to the Generation of Respiratory Rhythms during Normoxia and Hypoxia. Neuron. 2004;43:105–17. doi: 10.1016/j.neuron.2004.06.023. [DOI] [PubMed] [Google Scholar]
- Pena F, Ramirez JM. Endogenous activation of serotonin-2A receptors is required for respiratory rhythm generation in vitro. J Neurosci. 2002;22:11055–64. doi: 10.1523/JNEUROSCI.22-24-11055.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena F, Ramirez JM. Substance P-mediated modulation of pacemaker properties in the mammalian respiratory network. J Neurosci. 2004;24:7549–56. doi: 10.1523/JNEUROSCI.1871-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters RM. The energy cost (work) of breathing. Ann Thorac Surg. 1969;7:51–67. doi: 10.1016/s0003-4975(10)66146-2. [DOI] [PubMed] [Google Scholar]
- Pierani A, Moran-Rivard L, Sunshine MJ, Littman DR, Goulding M, Jessell TM. Control of interneuron fate in the developing spinal cord by the progenitor homeodomain protein Dbx1. Neuron. 2001;29:367–84. doi: 10.1016/s0896-6273(01)00212-4. [DOI] [PubMed] [Google Scholar]
- Ptak K, Yamanishi T, Aungst J, Milescu LS, Zhang R, et al. Raphe neurons stimulate respiratory circuit activity by multiple mechanisms via endogenously released serotonin and substance P. J Neurosci. 2009;29:3720–37. doi: 10.1523/JNEUROSCI.5271-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptak K, Zummo GG, Alheid GF, Tkatch T, Surmeier DJ, McCrimmon DR. Sodium currents in medullary neurons isolated from the pre-Bötzinger complex region. J Neurosci. 2005;25:5159–70. doi: 10.1523/JNEUROSCI.4238-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purvis LK, Smith JC, Koizumi H, Butera RJ. Intrinsic bursters increase the robustness of rhythm generation in an excitatory network. J Neurophysiol. 2007;97:1515–26. doi: 10.1152/jn.00908.2006. [DOI] [PubMed] [Google Scholar]
- Qian Y, Fritzsch B, Shirasawa S, Chen CL, Choi Y, Ma Q. Formation of brainstem (nor)adrenergic centers and first-order relay visceral sensory neurons is dependent on homeodomain protein Rnx/Tlx3. Genes Dev. 2001;15:2533–45. doi: 10.1101/gad.921501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rall W, Burke RE, Smith TG, Nelson PG, Frank K. Dendritic location of synapses and possible mechanisms for the monosynaptic EPSP in motoneurons. J Neurophysiol. 1967;30:1169–93. doi: 10.1152/jn.1967.30.5.1169. [DOI] [PubMed] [Google Scholar]
- Ramanantsoa N, Hirsch MR, Thoby-Brisson M, Dubreuil V, Bouvier J, et al. Breathing without CO2 Chemosensitivity in Conditional Phox2b Mutants. J Neurosci. 2011;31:12880–8. doi: 10.1523/JNEUROSCI.1721-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez JM, Koch H, Garcia AJ, 3rd, Doi A, Zanella S. The role of spiking and bursting pacemakers in the neuronal control of breathing. J Biol Phys. 2011;37:241–61. doi: 10.1007/s10867-011-9214-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez JM, Tryba AK, Pena F. Pacemaker neurons and neuronal networks: an integrative view. Curr Opin Neurobiol. 2004;14:665–74. doi: 10.1016/j.conb.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Rand CM, Patwari PP, Rodikova EA, Zhou L, Berry-Kravis EM, et al. Rapid-onset obesity with hypothalamic dysfunction, hypoventilation, and autonomic dysregulation: analysis of hypothalamic and autonomic candidate genes. Ped Res. 2011;70:375–8. doi: 10.1203/PDR.0b013e318229474d. [DOI] [PubMed] [Google Scholar]
- Ray RS, Corcoran AE, Brust RD, Kim JC, Richerson GB, et al. Impaired respiratory and body temperature control upon acute serotonergic neuron inhibition. Science. 2011;333:637–42. doi: 10.1126/science.1205295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rekling JC, Champagnat J, Denavit-Saubie M. Electroresponsive properties and membrane potential trajectories of three types of inspiratory neurons in the newborn mouse brain stem in vitro. J Neurophysiol. 1996a;75:795–810. doi: 10.1152/jn.1996.75.2.795. [DOI] [PubMed] [Google Scholar]
- Rekling JC, Champagnat J, Denavit-Saubie M. Thyrotropin-releasing hormone (TRH) depolarizes a subset of inspiratory neurons in the newborn mouse brain stem in vitro. J Neurophysiol. 1996b;75:811–9. doi: 10.1152/jn.1996.75.2.811. [DOI] [PubMed] [Google Scholar]
- Rekling JC, Feldman JL. PreBötzinger complex and pacemaker neurons: hypothesized site and kernel for respiratory rhythm generation. Annu Rev Physiol. 1998;60:385–405. doi: 10.1146/annurev.physiol.60.1.385. [DOI] [PubMed] [Google Scholar]
- Rekling JC, Shao XM, Feldman JL. Electrical coupling and excitatory synaptic transmission between rhythmogenic respiratory neurons in the preBotzinger complex. J Neurosci. 2000;20:RC113. doi: 10.1523/JNEUROSCI.20-23-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren J, Greer JJ. Modulation of respiratory rhythmogenesis by chloride-mediated conductances during the perinatal period. J Neurosci. 2006;26:3721–30. doi: 10.1523/JNEUROSCI.0026-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter DW. Generation and maintenance of the respiratory rhythm. J Exp Biol. 1982;100:93–107. doi: 10.1242/jeb.100.1.93. [DOI] [PubMed] [Google Scholar]
- Richter DW, Manzke T, Wilken B, Ponimaskin E. Serotonin receptors: guardians of stable breathing. Trends Mol Med. 2003;9:542–8. doi: 10.1016/j.molmed.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Richter DW, Spyer KM. Studying rhythmogenesis of breathing: comparison of in vivo and in vitro models. Trends Neurosci. 2001;24:464–72. doi: 10.1016/s0166-2236(00)01867-1. [DOI] [PubMed] [Google Scholar]
- Robinson L, Guy J, McKay L, Brockett E, Spike RC, et al. Morphological and functional reversal of phenotypes in a mouse model of Rett syndrome. Brain : a journal of neurology. 2012 doi: 10.1093/brain/aws096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose MF, Ahmad KA, Thaller C, Zoghbi HY. Excitatory neurons of the proprioceptive, interoceptive, and arousal hindbrain networks share a developmental requirement for Math1. Proc Natl Acad Sci U S A. 2009a;106:22462–7. doi: 10.1073/pnas.0911579106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose MF, Ren J, Ahmad KA, Chao HT, Klisch TJ, et al. Math1 is essential for the development of hindbrain neurons critical for perinatal breathing. Neuron. 2009b;64:341–54. doi: 10.1016/j.neuron.2009.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruangkittisakul A, Panaitescu B, Ballanyi K. K(+) and Ca(2)(+) dependence of inspiratory-related rhythm in novel “calibrated” mouse brainstem slices. Respir Physiol Neurobiol. 2011;175:37–48. doi: 10.1016/j.resp.2010.09.004. [DOI] [PubMed] [Google Scholar]
- Ruangkittisakul A, Schwarzacher SW, Secchia L, Ma Y, Bobocea N, et al. Generation of eupnea and sighs by a spatiochemically organized inspiratory network. J Neurosci. 2008;28:2447–58. doi: 10.1523/JNEUROSCI.1926-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruangkittisakul A, Secchia L, Bornes TD, Palathinkal DM, Ballanyi K. Dependence on extracellular Ca2+/K+ antagonism of inspiratory centre rhythms in slices and en bloc preparations of newborn rat brainstem. J Physiol. 2007;584:489–508. doi: 10.1113/jphysiol.2007.142760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin JE, Hayes JA, Mendenhall JL, Del Negro CA. Calcium-activated nonspecific cation current and synaptic depression promote network-dependent burst oscillations. Proc Natl Acad Sci U S A. 2009a;106:2939–44. doi: 10.1073/pnas.0808776106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin JE, Shevtsova NA, Ermentrout GB, Smith JC, Rybak IA. Multiple rhythmic states in a model of the respiratory central pattern generator. J Neurophysiol. 2009b;101:2146–65. doi: 10.1152/jn.90958.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlafke ME, Pokorski M, See WR, Prill RK, Loeschcke HH. Chemosensitive neurons on the ventral medullary surface. Bull Physiopathol Respir (Nancy) 1975;11:277–84. [PubMed] [Google Scholar]
- Schreihofer AM, Stornetta RL, Guyenet PG. Evidence for glycinergic respiratory neurons: Bötzinger neurons express mRNA for glycinergic transporter 2. J Comp Neurol. 1999;407:583–97. doi: 10.1002/(sici)1096-9861(19990517)407:4<583::aid-cne8>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Schwarzacher SW, Rub U, Deller T. Neuroanatomical characteristics of the human pre-Botzinger complex and its involvement in neurodegenerative brainstem diseases. Brain : a journal of neurology. 2010 doi: 10.1093/brain/awq327. [DOI] [PubMed] [Google Scholar]
- Sherrington CS. The integrative action of the nervous system. New York: C. Scribner’s sons; 1906. p. xvi.p. 411. [Google Scholar]
- Shvarev Y, Berner J, Bilkei-Gorzo A, Lagercrantz H, Wickstrom R. Acute morphine effects on respiratory activity in mice with target deletion of the tachykinin 1 gene (Tac1−/−) Adv Exp Med Biol. 2010;669:129–32. doi: 10.1007/978-1-4419-5692-7_26. [DOI] [PubMed] [Google Scholar]
- Sieber MA, Storm R, Martinez-de-la-Torre M, Muller T, Wende H, et al. Lbx1 acts as a selector gene in the fate determination of somatosensory and viscerosensory relay neurons in the hindbrain. J Neurosci. 2007;27:4902–9. doi: 10.1523/JNEUROSCI.0717-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC, Abdala AP, Koizumi H, Rybak IA, Paton JF. Spatial and functional architecture of the mammalian brain stem respiratory network: a hierarchy of three oscillatory mechanisms. J Neurophysiol. 2007;98:3370–87. doi: 10.1152/jn.00985.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL. Pre-Bötzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science. 1991;254:726–9. doi: 10.1126/science.1683005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC, Morrison DE, Ellenberger HH, Otto MR, Feldman JL. Brainstem projections to the major respiratory neuron populations in the medulla of the cat. J Comp Neurol. 1989;281:69–96. doi: 10.1002/cne.902810107. [DOI] [PubMed] [Google Scholar]
- St-John WM. Eupnea of in situ rats persists following blockers of in vitro pacemaker burster activities. Respir Physiol Neurobiol. 2008;160:353–6. doi: 10.1016/j.resp.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-John WM, Waki H, Dutschmann M, Paton JF. Maintenance of eupnea of in situ and in vivo rats following riluzole: a blocker of persistent sodium channels. Respir Physiol Neurobiol. 2007;155:97–100. doi: 10.1016/j.resp.2006.04.018. [DOI] [PubMed] [Google Scholar]
- Stornetta RL, Moreira TS, Takakura AC, Kang BJ, Chang DA, et al. Expression of Phox2b by brainstem neurons involved in chemosensory integration in the adult rat. J Neurosci. 2006;26:10305–14. doi: 10.1523/JNEUROSCI.2917-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stornetta RL, Rosin DL, Wang H, Sevigny CP, Weston MC, Guyenet PG. A group of glutamatergic interneurons expressing high levels of both neurokinin-1 receptors and somatostatin identifies the region of the pre-Bötzinger complex. J Comp Neurol. 2003;455:499–512. doi: 10.1002/cne.10504. [DOI] [PubMed] [Google Scholar]
- Straka H, Baker R, Gilland E. Preservation of segmental hindbrain organization in adult frogs. J Comp Neurol. 2006;494:228–45. doi: 10.1002/cne.20801. [DOI] [PubMed] [Google Scholar]
- Stuart G, Spruston N, Häusser M. Dendrites. Oxford ; New York: Oxford University Press; 2007. p. xv.p. 560. [Google Scholar]
- Suzue T. Respiratory rhythm generation in the in vitro brain stem-spinal cord preparation of the neonatal rat. J Physiol. 1984;354:173–83. doi: 10.1113/jphysiol.1984.sp015370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taccola G, Secchia L, Ballanyi K. Anoxic persistence of lumbar respiratory bursts and block of lumbar locomotion in newborn rat brainstem spinal cords. J Physiol. 2007;585:507–24. doi: 10.1113/jphysiol.2007.143594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takakura AC, Moreira TS, Stornetta RL, West GH, Gwilt JM, Guyenet PG. Selective lesion of retrotrapezoid Phox2b-expressing neurons raises the apnoeic threshold in rats. J Physiol. 2008;586:2975–91. doi: 10.1113/jphysiol.2008.153163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan W, Janczewski WA, Yang P, Shao XM, Callaway EM, Feldman JL. Silencing preBötzinger Complex somatostatin-expressing neurons induces persistent apnea in awake rat. Nat Neurosci. 2008;11:538–40. doi: 10.1038/nn.2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan W, Pagliardini S, Yang P, Janczewski WA, Feldman JL. Projections of preBotzinger complex neurons in adult rats. J Comp Neurol. 2010;518:1862–78. doi: 10.1002/cne.22308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan W, Sherman D, Turesson J, Shao XM, Janczewski WA, Feldman JL. Reelin demarks a subset of preBötzinger Complex neurons in adult rat. J Comp Neurol. 2011 doi: 10.1002/cne.22753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka I, Ezure K, Kondo M. Distribution of glycine transporter 2 mRNA-containing neurons in relation to glutamic acid decarboxylase mRNA-containing neurons in rat medulla. Neurosci Res. 2003;47:139–51. doi: 10.1016/s0168-0102(03)00192-5. [DOI] [PubMed] [Google Scholar]
- Tazerart S, Vinay L, Brocard F. The persistent sodium current generates pacemaker activities in the central pattern generator for locomotion and regulates the locomotor rhythm. J Neurosci. 2008;28:8577–89. doi: 10.1523/JNEUROSCI.1437-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telgkamp P, Cao YQ, Basbaum AI, Ramirez JM. Long-term deprivation of substance P in PPT-A mutant mice alters the anoxic response of the isolated respiratory network. J Neurophysiol. 2002;88:206–13. doi: 10.1152/jn.2002.88.1.206. [DOI] [PubMed] [Google Scholar]
- Thoby-Brisson M, Karlen M, Wu N, Charnay P, Champagnat J, Fortin G. Genetic identification of an embryonic parafacial oscillator coupling to the preBotzinger complex. Nat Neurosci. 2009;12:1028–35. doi: 10.1038/nn.2354. [DOI] [PubMed] [Google Scholar]
- Thoby-Brisson M, Ramirez JM. Identification of two types of inspiratory pacemaker neurons in the isolated respiratory neural network of mice. J Neurophysiol. 2001;86:104–12. doi: 10.1152/jn.2001.86.1.104. [DOI] [PubMed] [Google Scholar]
- Thoby-Brisson M, Trinh JB, Champagnat J, Fortin G. Emergence of the pre-Botzinger respiratory rhythm generator in the mouse embryo. J Neurosci. 2005;25:4307–18. doi: 10.1523/JNEUROSCI.0551-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tryba AK, Pena F, Lieske SP, Viemari JC, Thoby-Brisson M, Ramirez JM. Differential modulation of neural network and pacemaker activity underlying eupnea and sigh-breathing activities. J Neurophysiol. 2008;99:2114–25. doi: 10.1152/jn.01192.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuboi Y, Dickson DW, Nabeshima K, Schmeichel AM, Wszolek ZK, et al. Neurodegeneration involving putative respiratory neurons in Perry syndrome. Acta Neuropathol. 2008;115:263–8. doi: 10.1007/s00401-007-0246-1. [DOI] [PubMed] [Google Scholar]
- Valente EM, Brancati F, Silhavy JL, Castori M, Marsh SE, et al. AHI1 gene mutations cause specific forms of Joubert syndrome-related disorders. Ann Neurol. 2006a;59:527–34. doi: 10.1002/ana.20749. [DOI] [PubMed] [Google Scholar]
- Valente EM, Silhavy JL, Brancati F, Barrano G, Krishnaswami SR, et al. Mutations in CEP290, which encodes a centrosomal protein, cause pleiotropic forms of Joubert syndrome. Nat Genet. 2006b;38:623–5. doi: 10.1038/ng1805. [DOI] [PubMed] [Google Scholar]
- Viemari JC, Garcia AJ, 3rd, Doi A, Ramirez JM. Activation of alpha-2 noradrenergic receptors is critical for the generation of fictive eupnea and fictive gasping inspiratory activities in mammals in vitro. Eur J Neurosci. 2011;33:2228–37. doi: 10.1111/j.1460-9568.2011.07706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viemari JC, Ramirez JM. Norepinephrine differentially modulates different types of respiratory pacemaker and nonpacemaker neurons. J Neurophysiol. 2006;95:2070–82. doi: 10.1152/jn.01308.2005. [DOI] [PubMed] [Google Scholar]
- Viemari JC, Roux JC, Tryba AK, Saywell V, Burnet H, et al. Mecp2 deficiency disrupts norepinephrine and respiratory systems in mice. J Neurosci. 2005;25:11521–30. doi: 10.1523/JNEUROSCI.4373-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voituron N, Zanella S, Menuet C, Lajard AM, Dutschmann M, Hilaire G. Early abnormalities of post-sigh breathing in a mouse model of Rett syndrome. Respir Physiol Neurobiol. 2010;170:173–82. doi: 10.1016/j.resp.2009.12.009. [DOI] [PubMed] [Google Scholar]
- von Euler C. Brain stem mechanisms for generation and control of breathing pattern. In: Fishman A, Cherniak N, Widdicombe J, editors. Handbook of Physiology. Bethesda: American Physiological Society; 1986. pp. 1–67. [Google Scholar]
- Wallen-Mackenzie A, Gezelius H, Thoby-Brisson M, Nygard A, Enjin A, et al. Vesicular glutamate transporter 2 is required for central respiratory rhythm generation but not for locomotor central pattern generation. J Neurosci. 2006;26:12294–307. doi: 10.1523/JNEUROSCI.3855-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan M, Lee SS, Zhang X, Houwink-Manville I, Song HR, et al. Rett syndrome and beyond: recurrent spontaneous and familial MECP2 mutations at CpG hotspots. Am J Hum Genet. 1999;65:1520–9. doi: 10.1086/302690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Stornetta RL, Rosin DL, Guyenet PG. Neurokinin-1 receptor-immunoreactive neurons of the ventral respiratory group in the rat. J Comp Neurol. 2001;434:128–46. doi: 10.1002/cne.1169. [DOI] [PubMed] [Google Scholar]
- Wang H, Weston MC, McQuiston TJ, Stornetta RL, Guyenet PG. Neurokinin-1 receptor-expressing cells regulate depressor region of rat ventrolateral medulla. Am J Physiol Heart Circ Physiol. 2003;285:H2757–69. doi: 10.1152/ajpheart.00528.2003. [DOI] [PubMed] [Google Scholar]
- Weese-Mayer DE. Sudden infant death syndrome: the genetic segue? Acta Paediatr. 2008;97:846–7. doi: 10.1111/j.1651-2227.2008.00860.x. [DOI] [PubMed] [Google Scholar]
- Weese-Mayer DE, Berry-Kravis EM, Ceccherini I, Keens TG, Loghmanee DA, Trang H. An official ATS clinical policy statement: Congenital central hypoventilation syndrome: genetic basis, diagnosis, and management. Am J Respir Crit Care Med. 2010;181:626–44. doi: 10.1164/rccm.200807-1069ST. [DOI] [PubMed] [Google Scholar]
- Weese-Mayer DE, Berry-Kravis EM, Ceccherini I, Rand CM. Congenital central hypoventilation syndrome (CCHS) and sudden infant death syndrome (SIDS): kindred disorders of autonomic regulation. Respir Physiol Neurobiol. 2008a;164:38–48. doi: 10.1016/j.resp.2008.05.011. [DOI] [PubMed] [Google Scholar]
- Weese-Mayer DE, Lieske SP, Boothby CM, Kenny AS, Bennett HL, Ramirez JM. Autonomic dysregulation in young girls with Rett Syndrome during nighttime in-home recordings. Pediatr Pulmonol. 2008b;43:1045–60. doi: 10.1002/ppul.20866. [DOI] [PubMed] [Google Scholar]
- Weese-Mayer DE, Lieske SP, Boothby CM, Kenny AS, Bennett HL, et al. Autonomic nervous system dysregulation: breathing and heart rate perturbation during wakefulness in young girls with Rett syndrome. Pediatr Res. 2006;60:443–9. doi: 10.1203/01.pdr.0000238302.84552.d0. [DOI] [PubMed] [Google Scholar]
- Wenker IC, Sobrinho CR, Takakura AC, Moreira TS, Mulkey DK. Regulation of ventral surface CO2/H+-sensitive neurons by purinergic signaling. J Physiol. 2012 doi: 10.1113/jphysiol.2012.229666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenninger JM, Pan LG, Klum L, Leekley T, Bastastic J, et al. Small reduction of neurokinin-1 receptor-expressing neurons in the pre-Bötzinger complex area induces abnormal breathing periods in awake goats. J Appl Physiol. 2004a;97:1620–8. doi: 10.1152/japplphysiol.00952.2003. [DOI] [PubMed] [Google Scholar]
- Wenninger JM, Pan LG, Klum L, Leekley T, Bastastic J, et al. Large lesions in the pre-Bötzinger complex area eliminate eupneic respiratory rhythm in awake goats. J Appl Physiol. 2004b;97:1629–36. doi: 10.1152/japplphysiol.00953.2003. [DOI] [PubMed] [Google Scholar]
- Winter SM, Fresemann J, Schnell C, Oku Y, Hirrlinger J, Hulsmann S. Glycinergic interneurons are functionally integrated into the inspiratory network of mouse medullary slices. Pflugers Arch. 2009;458:459–69. doi: 10.1007/s00424-009-0647-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter SM, Fresemann J, Schnell C, Oku Y, Hirrlinger J, Hulsmann S. Glycinergic interneurons in the respiratory network of the rhythmic slice preparation. Adv Exp Med Biol. 2010;669:97–100. doi: 10.1007/978-1-4419-5692-7_20. [DOI] [PubMed] [Google Scholar]
- Wittmeier S, Song G, Duffin J, Poon CS. Pacemakers handshake synchronization mechanism of mammalian respiratory rhythmogenesis. Proc Natl Acad Sci U S A. 2008;105:18000–5. doi: 10.1073/pnas.0809377105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M, Terao M, Terashima T, Fujiyama T, Kawaguchi Y, et al. Origin of climbing fiber neurons and their developmental dependence on Ptf1a. J Neurosci. 2007;27:10924–34. doi: 10.1523/JNEUROSCI.1423-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavala-Tecuapetla C, Aguileta MA, Lopez-Guerrero JJ, Gonzalez-Marin MC, Pena F. Calcium-activated potassium currents differentially modulate respiratory rhythm generation. Eur J Neurosci. 2008;27:2871–84. doi: 10.1111/j.1460-9568.2008.06214.x. [DOI] [PubMed] [Google Scholar]