Abstract
Roughly 3 million years ago, an inactivating deletion occurred in CMAH, the human gene encoding CMP-Neu5Ac (cytidine-5′-monophospho-N-acetylneuraminic acid) hydroxylase (Chou HH, Takematsu H, Diaz S, Iber J, Nickerson E, Wright KL, Muchmore EA, Nelson DL, Warren ST, Varki A. 1998. A mutation in human CMP-sialic acid hydroxylase occurred after the Homo-Pan divergence. Proc Natl Acad Sci USA. 95:11751–11756). This inactivating deletion is now homozygous in all humans, causing the loss of N-glycolylneuraminic acid (Neu5Gc) biosynthesis in all human cells and tissues. The CMAH enzyme is active in other mammals, including mice, where Neu5Gc is an abundant form of sialic acid on cellular membranes, including those in cardiac and skeletal muscle. We recently demonstrated that the deletion of mouse Cmah worsened the severity of pathophysiology measures related to muscular dystrophy in mdx mice, a model for Duchenne muscular dystrophy (Chandrasekharan K, Yoon JH, Xu Y, deVries S, Camboni M, Janssen PM, Varki A, Martin PT. 2010. A human-specific deletion in mouse Cmah increases disease severity in the mdx model of Duchenne muscular dystrophy. Sci Transl Med. 2:42–54). Here, we demonstrate similar changes in cardiac and skeletal muscle pathology and physiology resulting from Cmah deletion in α-sarcoglycan-deficient (Sgca−/−) mice, a model for limb girdle muscular dystrophy 2D. These experiments demonstrate that loss of mouse Cmah can worsen disease severity in more than one form of muscular dystrophy and suggest that Cmah may be a general genetic modifier of muscle disease.
Keywords: dystroglycan, limb girdle, muscular dystrophy, sarcoglycan, sialic acid
Introduction
Recent studies have demonstrated that muscle cell surface glycans play important roles, both positive and negative, with regard to muscular dystrophy; loss of function mutations in genes that control the biosynthesis of O-mannosyl-linked glycans on α-dystroglycan give rise to multiple forms of congenital and limb girdle muscular dystrophy (LGMD), termed the dystroglycanopathies (Michele et al. 2002; Martin 2005; Godfrey et al. 2011), whereas the overexpression of terminal β1,4-linked N-acetyl-galactosamine (βGalNAc) by Galgt2 in muscle can inhibit muscular dystrophy in multiple animal models (Nguyen et al. 2002; Xu, Camboni, et al. 2007; Xu, Chandrasekharan, et al. 2007; Xu et al. 2009). Another important terminal glycan modification in muscle is the type of sialic acid that is present. In most mammals, the two most common forms of sialic acid are N-acetylneuraminic acid (Neu5Ac) and Neu5Gc (Varki 2010). Neu5Gc is generated by the cytidine-5′-monophospho-Neu5Ac (CMP-Neu5Ac) hydroxylase or CMAH, which hydroxylates the CMP-Neu5Ac nucleotide sugar to make cytidine-5′-monophospho-Neu5Gc (CMP-Neu5Gc; Shaw et al. 1992; Kawano et al. 1995). CMP-Neu5Ac or CMP-Neu5Gc are then incorporated by the 20 or so known mammalian sialyltransferases into glycoproteins and glycolipids. Humans are unique among mammals studied to date with regard to their sialic acid composition in that all humans lack a functional CMAH gene (Chou et al. 1998; Irie et al. 1998). As such, unlike the muscles of all lower mammals, including the great apes, human muscle has an excess of Neu5Ac and lacks Neu5Gc. We have recently created mice lacking the functional mouse Cmah gene (Hedlund et al. 2007). Modeling this aspect of the human glycome leads to the development of a more severe form of muscular dystrophy in the mdx mouse model for Duchenne muscular dystrophy (DMD; Chandrasekharan et al. 2010). Cmah−/−mdx mice show increased cardiac and skeletal muscle pathology, decreased cardiac and respiratory muscle strength, decreased ambulation and increased mortality. In addition, loss of Cmah in mdx mice alters binding of muscle extracellular matrix proteins to α-dystroglycan, reduces the up-regulation of dystrophin surrogates, such as utrophin, in skeletal muscle and increases the production of serum anti-Neu5Gc antibodies (Chandrasekharan et al. 2010).
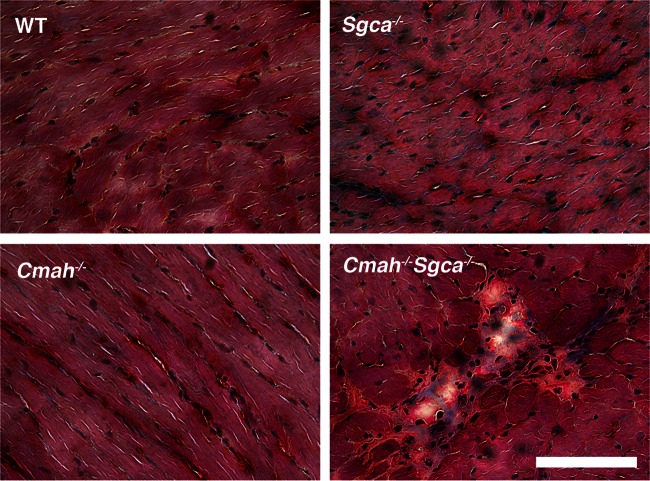
These results leave open the question of whether Neu5Gc deficiency might be a more general cause of human–mouse genotype–phenotype differences in the muscular dystrophies. To test this notion, we have created Cmah-deficient α-sarcoglycan-deficient mice. α-Sarcoglycan (Sgca) is one of four transmembrane proteins (α-, β-, γ- and δ-sarcoglycan) that, as a group, are part of the DAG complex (Miller et al. 2007). Mutations in any of the four proteins leads to the reduced expression of the other three sarcoglycan proteins in the tetrameric complex and gives rise to LGMD 2D, 2C, 2E and 2F, respectively (Straub and Bushby 2006; Guglieri et al. 2008). As with mdx mice relative to human DMD, Sgca−/− mice do not demonstrate all of the disease phenotypes one can observe in human LGMD2D. In particular, Sgca−/− animals show no significant cardiac phenotypes (Duclos et al. 1998), whereas a minority of LGMD2D patients develop cardiomyopathy (Sveen et al. 2008; Ferreira et al. 2011). Here, we show that Cmah−/−Sgca−/− mice demonstrate increased muscle pathology and weakened muscle strength relative to Sgca−/− animals. These findings suggest that humanizing the sialic acid repertoire of Sgca−/− mice by eliminating Cmah function worsens disease severity, providing a more robust animal model for studies of LGMD2D.
Results
Expression of Neu5Gc in Cmah−/−Sgca−/− and LGMD2D muscle
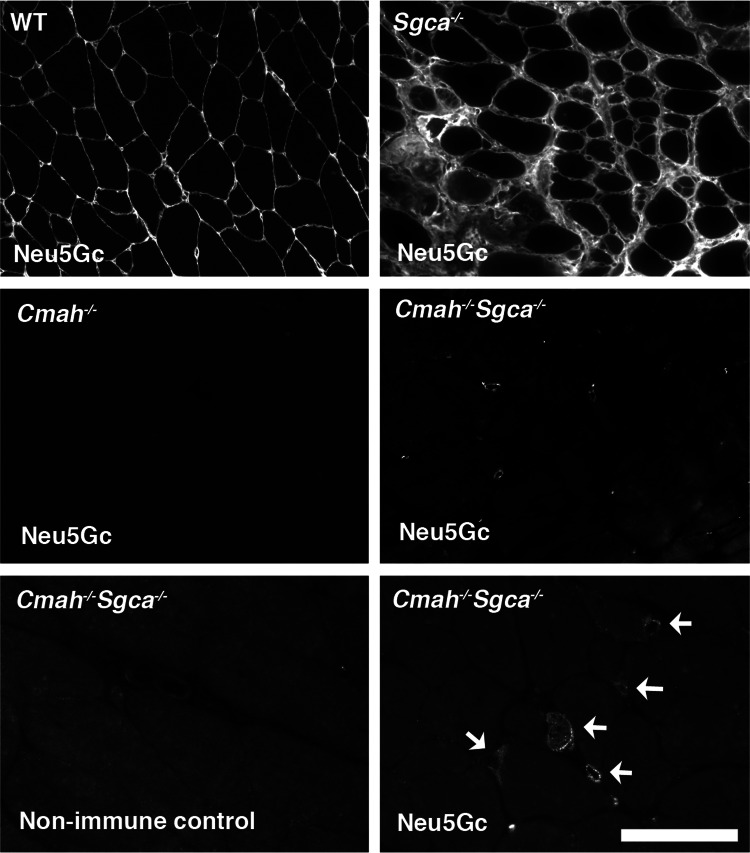
We began by immunostaining wild-type (WT), Cmah-deficient (Cmah−/−), α-sarcoglycan-deficient (Sgca−/−) and Cmah−/−Sgca−/− skeletal muscle with a polyclonal affinity purified IgY that has been shown to be highly specific for Neu5Gc-containing glycans (Diaz et al. 2009; Figure 1). Mice normally express Cmah and abundantly synthesize and incorporate Neu5Gc in skeletal muscle (Hedlund et al. 2007; Chandrasekharan et al. 2010). Therefore, both WT and Sgca−/− muscles showed the high expression of Neu5Gc on all cellular membranes as well as in the extracellular matrix. Neu5Gc staining in Sgca−/− appeared brighter than that in WT muscle. This is likely due to the increased presence of small regenerating muscles and the extracellular matrix in Sgca−/− muscles as a result of muscular dystrophy. A comparison of Neu5Gc levels on total glycoproteins in Sgca−/− and WT muscles by western blotting (Supplementary data, Figure S1), however, showed only a modest increase in Sgca−/− muscle (a 36 ± 2% increase, P < 0.01 for n = 4 samples per comparison). The majority of muscle cells in both Cmah−/− and Cmah−/−Sgca−/− animals showed no Neu5Gc staining (Figure 1), though staining for sialic acid (e.g. with Maackia amurensis agglutinin, MAA) was abundant (not shown). As with our studies in Cmah−/−mdx muscle (Chandrasekharan et al. 2010), we could identify individual mononuclear cells, presumably satellite cells, that stained for Neu5Gc in Cmah−/−Sgca−/− muscle (Figure 1). Small puncta of Neu5Gc immunostaining could also be seen in a minority of skeletal myofibers. All such staining was specific for Cmah−/−Sgca−/− muscle and was not identified in Cmah−/− muscle. This is consistent with our previous studies showing Neu5Gc expression in dystrophic skeletal muscle (Chandrasekharan et al. 2010). This expression most likely arises from the incorporation of Neu5Gc from dietary sources, as the elimination of dietary Neu5Gc causes loss of all Neu5Gc immunoreactivity in Cmah−/− animals (Hedlund et al. 2007).
Fig. 1.
Neu5Gc immunostaining in Cmah-deficient α-sarcoglycan (Sgca)-deficient mouse skeletal muscle. Affinity purified chicken IgY-specific for Neu5Gc was used to immunostain gastrocnemius skeletal muscle from 6-week-old WT, Cmah−/−, Sgca−/−, and Cmah−/−Sgca−/− mice. Non-immune IgY control antisera were used to demonstrate specificity for Neu5Gc. Arrows show the rare cells stained for Neu5Gc in Cmah−/−Sgca−/− muscle. Bar is 100 μm for top four panels and 50 μm for bottom two panels.
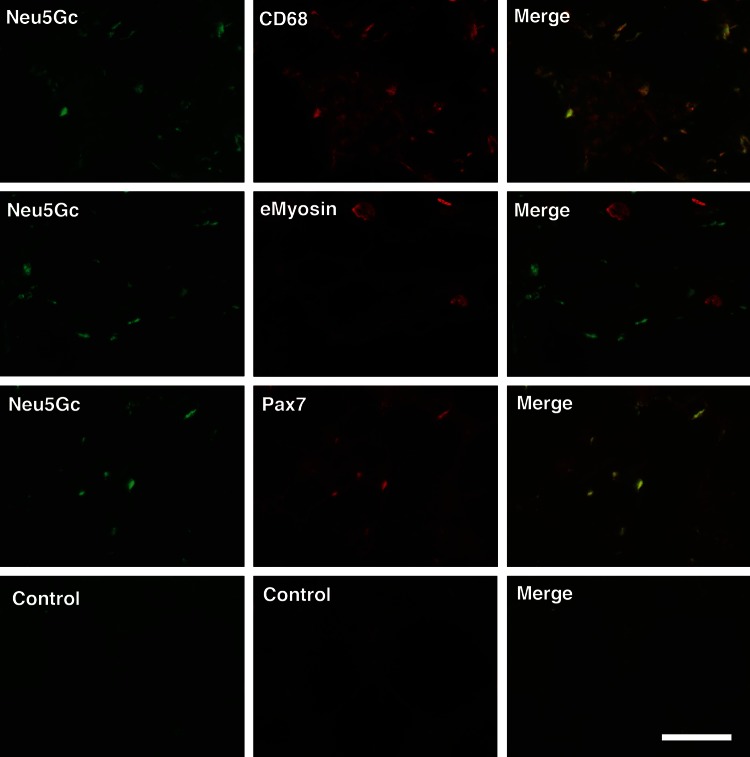
To confirm the expression of Neu5Gc in specific cell types, we double immunostained Cmah−/−Sgca−/− skeletal muscle with antibody to Neu5Gc and antibody to either CD68, a marker for macrophages, eMyosin, a marker for regenerating skeletal myofibers, or Pax7, a marker for satellite cells (Figure 2). Neu5Gc did co-stain with some CD68 in macrophages, particularly those with high CD68 expression. Many macrophages, however, showed no Neu5Gc expression. Similarly, most small regenerating eMyosin-positive muscles did not co-stain with Neu5Gc; however, many Pax7-positive satellite cells did. Thus, some actively dividing cells in dystrophic Cmah−/−Sgca−/− muscles may take up Neu5Gc from dietary sources, including satellite cells, the predominant stem cells of adult skeletal muscle (Seale et al. 2000; Wang and Rudnicki 2012).
Fig. 2.
Expression of Neu5Gc in macrophages and satellite cells of Cmah−/−Sgca−/− muscle. Neu5Gc was co-stained with CD68, a marker of macrophages, embryonic myosin (eMyosin), a marker of regenerating muscle and Pax7, a marker of satellite cells, in Cmah−/−Sgca−/− muscle. Control muscle shown is stained with non-immune chicken IgY (for Neu5Gc) and secondary antibody alone (anti-mouse) and is representative of all control conditions. Bar is 100 μm for all panels.
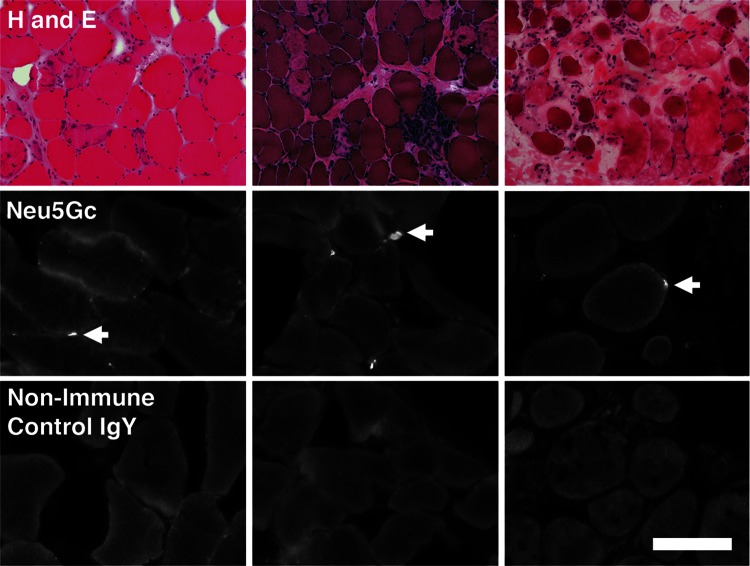
We next assessed Neu5Gc expression in de-identified muscle biopsies from patients with LGMD2D (Figure 3). As with skeletal muscle from patients with DMD (Chandrasekharan et al. 2010), Neu5Gc staining was largely confined to mononuclear cells, consistent with incorporation into satellite cells, in LGMD2D muscle. This staining was more focal than Neu5Gc staining previously seen on DMD biopsies (Chandrasekharan et al. 2010), but this may be due to the relatively sparse number of myofibers available for staining in the LGMD2D biopsies studied. Occasionally, small puncta of Neu5Gc immunostaining could also be seen in LGMD2D skeletal myofibers. These data suggest that both human and mouse muscle deficient in α-sarcoglycan can incorporate Neu5Gc, in the absence of functional Cmah, into regions where muscle regeneration is occurring.
Fig. 3.
Staining of muscle biopsies from LGMD2D patients. Three different LGMD2D biopsies were stained with hematoxylin and eosin (H and E), with affinity purified chicken IgY-specific for Neu5Gc (Neu5Gc) or with non-immune chicken IgY control antiserum (Control). Arrows indicate staining with the Neu5Gc antiserum. Bar is 200 μm for upper row and 100 μm for bottom two rows.
Increased cardiac and skeletal muscle pathology in Cmah−/−Sgca−/− mice
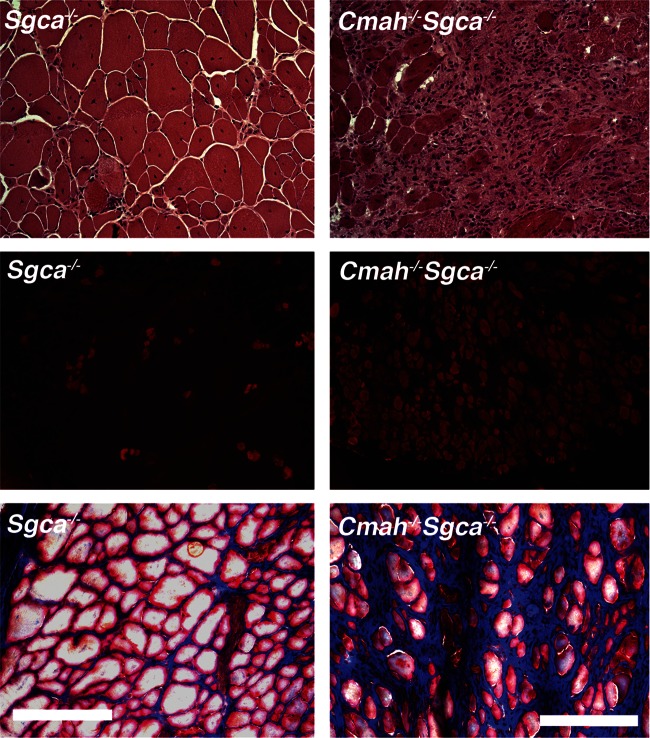
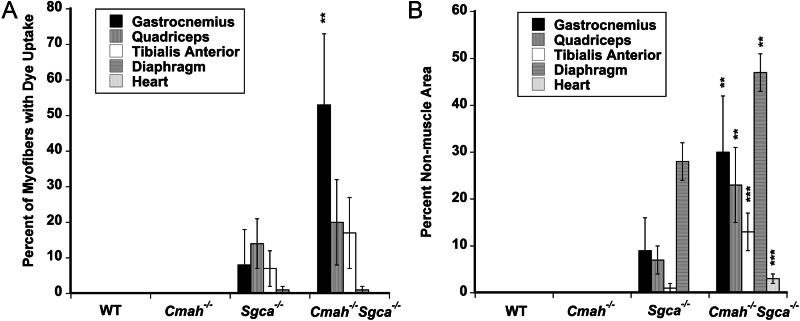
We had previously observed changes in muscle pathology and physiology resulting from the deletion of Cmah in mdx mice (Chandrasekharan et al. 2010). This was particularly true with regard to increased weakness in cardiac and diaphragm muscle strength and variably increased fibrosis in skeletal muscles. Here, we performed similar studies comparing Sgca−/− and Cmah−/−Sgca−/− muscles, using WT and Cmah−/− muscles as controls. We began by assessing skeletal and cardiac muscle histopathology (Figures 4–6 and Table I). As expected as a result of muscular dystrophy, Sgca−/− mice had increased percentages of skeletal myofibers with centrally located nuclei, a result of continued cycles of muscle degeneration and regeneration, when compared with WT mice (Table I). This was true in all skeletal muscles studied (diaphragm, gastrocnemius, quadriceps and tibialis anterior). In addition, the average myofiber area for these same muscles was reduced and the variability in the myofiber diameter was increased, again the result of the presence of smaller regenerating myofibers in dystrophic muscles (Table I). Uptake of Evan's blue dye into skeletal myofibers after a 45-min period of exercise on a treadmill was also increased in almost all Sgca−/− muscles relative to WT, indicating the increased perforation of myofiber membranes in Sgca−/− animals (Figures 4 and 6A). Increased serum creatine kinase (CK) activity in Sgca−/− mice was also indicative of such increased membrane damage (Table I). For almost all of these measures, Cmah−/−Sgca−/− animals also showed dystrophic changes, but these most often were not significantly changed relative to Sgca−/− animals. One exception was the percentage of myofibers with Evan's blue dye uptake in the gastrocnemius muscle, where Cmah−/−Sgca−/− animals were significantly increased (P < 0.01) relative to Sgca−/− (Figure 6A). A measure where both cardiac and skeletal muscles were uniformly different in Cmah−/−Sgca−/− animals relative to Sgca−/− was the percentage of the muscle area with non-muscle tissue (Figures 5 and 6B). This non-muscle tissue can arise from the presence of necrotic foci of dividing or immune cells as well as from the presence of fibrosis due to extracellular matrix (or fat) deposition. The loss of muscle tissue within muscle typically is the major driver of increased muscle weakness in the muscular dystrophies. There was a significant increase in the area of muscle containing non-muscle tissue in Cmah−/−Sgca−/− compared with Sgca−/− in the gastrocnemius, quadriceps, tibialis anterior, diaphragm and heart (Figure 6B). For the heart, both the total area with muscle tissue loss (Figure 6B) and the total number of necrotic/fibrotic lesions per unit area were increased (2.2 ± 0.3 lesions per 1.3 × 106 μm2 in Cmah−/−Sgca−/− compared with 0.8 ± 0.1 lesions in Sgca−/−, P < 0.001). This finding was particularly evident because as the Sgca−/− heart does not normally have significant histopathology (Duclos et al. 1998). Thus, much like the comparison of Cmah−/−mdx mice relative to mdx, the deletion of Cmah in Sgca−/− animals led to increased loss of muscle tissue, or wasting, for skeletal and cardiac muscle.
Fig. 4.
Altered skeletal muscle pathology in Cmah−/−Sgca−/− mice. Hematoxylin and eosin (H and E) staining of the gastrocnemius muscle (Gastroc) in 8-month-old (mo) Sgca−/− and Cmah−/−Sgca−/ mice (upper panels). EBD uptake after a 45-min period of exercise (middle panels). Mason's trichrome staining of the diaphragm muscle (lower panels). Collagen stains blue with trichrome stain. Bar is 50 μm in upper and lower panels and 200 μm in middle panels.
Fig. 5.
Altered cardiac muscle pathology in Cmah−/−Sgca−/− mice. Mason's trichrome staining of WT, Cmah−/−, Sgca−/− and Cmah−/−Sgca−/− heart muscles at 4 months of age. Collagen stains blue with trichrome stain, whereas muscle stains red. Bar is 50 μm for all panels.
Fig. 6.
Increased dye uptake and loss of muscle tissue in Cmah−/−Sgca−/− mice. (A) Mice were injected with Evan's blue dye and subjected to walking for 45 min prior to analysis of muscles for dye uptake. The percentage of myofibers or cardiomyocytes with dye was compared with the total number of myofibers or cardiomyocytes present. (B) The percentage of the muscle area taken up by non-muscle tissue was quantified. Such non-muscle tissue resulted from the presence of necrotic foci of mononuclear cells as well as fat and extracellular matrix deposition. Errors are SD for n = 5–6 animals per condition in (A) and (B). **P < 0.01, ***P < 0.001, comparing Sgca−/−Cmah−/− to Sgca−/−.
Table I.
Muscle and serum histopathology measures comparing WT, Cmah−/−, Sgca−/− and Cmah−/−Sgca−/− mice
| Measure | WT | Cmah−/− | Sgca−/− | Cmah−/−Sgca−/− |
|---|---|---|---|---|
| Mouse weight (g) | 30 ± 3 | 32 ± 6 | 27 ± 3 | 31 ± 4 |
| Muscle weight (mg) | ||||
| Gastrocnemius | 303 ± 13 | 342 ± 23 | 278 ± 30 | 308 ± 38 |
| Quadriceps | 351 ± 25 | 326 ± 20 | 409 ± 41 | 409 ± 33 |
| Tibialis anterior | 123 ± 12 | 112 ± 10 | 157 ± 22 | 173 ± 12 |
| Diaphragm | 58 ± 2 | 53 ± 2 | 57 ± 3 | 58 ± 2 |
| Heart | 113 ± 7 | 121 ± 15 | 117 ± 4 | 135 ± 11 |
| Myofiber diameter (μm) | ||||
| Gastrocnemius | 45 ± 11 | 45 ± 11 | 27 ± 12 | 26 ± 12 |
| Quadriceps | 53 ± 10 | 48 ± 9 | 30 ± 14 | 31 ± 14 |
| Tibialis anterior | 41 ± 10 | 45 ± 10 | 35 ± 13 | 38 ± 15 |
| Diaphragm | 30 ± 6 | 29 ± 6 | 25 ± 9 | 23 ± 8 |
| % Central nuclei | ||||
| Gastrocnemius | 1 ± 1 | 3 ± 2 | 48 ± 11 | 40 ± 12 |
| Quadriceps | 1 ± 1 | 3 ± 2 | 44 ± 9 | 40 ± 12 |
| Tibialis anterior | 1 ± 1 | 2 ± 2 | 79 ± 8 | 62 ± 11 |
| Diaphragm | 1 ± 1 | 1 ± 1 | 30 ± 7 | 39 ± 8 |
| Serum CK | ||||
| Activity (IU/mL) | 217 ± 41 | 167 ± 45 | 8500 ± 2000 | 7500 ± 1600 |
All measures were analyzed in 8-month-old animals. Errors for mouse and muscle weights are SEM for n = 6–8 per condition. Errors for serum CK activity are SEM for n = 15–43 per condition. Errors for myofiber diameters and % central nuclei are SD for n = 5–6 muscles per condition.
Increased loss of cardiac and skeletal muscle force in Cmah−/−Sgca−/− mice
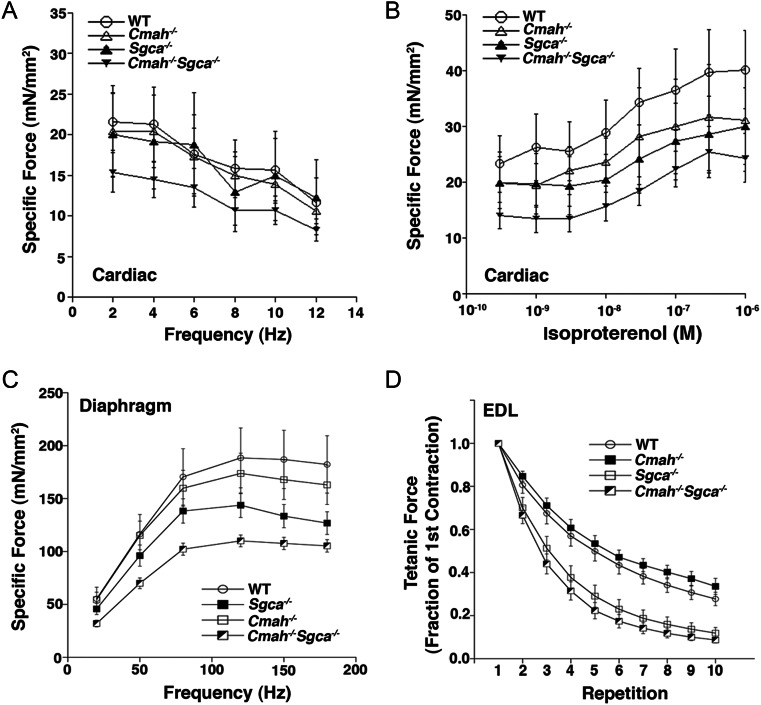
We next subjected muscles isolated from the leg (extensor digitorum longus, EDL), diaphragm or heart to a variety of force measurements to determine if loss of Cmah in Sgca−/− mice resulted in altered muscle function (Figure 7). For cardiac papillary and trabecular muscles, force was generally decreased for Cmah−/−Sgca−/− muscles relative to WT, Cmah−/− and Sgca−/−. When measures were pooled over all frequencies studied (2–12 Hz), this difference reached statistical significance between Sgca−/− and Cmah−/−Sgca−/− [analysis of variance (ANOVA), P < 0.05]. Similarly, cardiac muscle force in response to the β-agonist isoproterenol was significantly lower for Cmah−/−Sgca−/− relative to Sgca−/− (again pooled over all frequencies; ANOVA, P < 0.05). For diaphragm, force in Cmah−/−Sgca−/− muscles was significantly reduced relative to Sgca−/− at all individual frequencies tested (20–180 Hz; ANOVA, P < 0.05). Sgca−/− diaphragm muscle also showed reduced strength relative to WT at higher frequencies (150–180 Hz; P < 0.05 for each vs WT). For the EDL muscle, maximal specific force was significantly reduced in Cmah−/−Sgca−/− relative to both WT and Sgca−/− (by 25 and 20%, respectively; P < 0.05 for both comparisons). Force drop during repeated eccentric contractions was significantly reduced for both Sgca−/− and Cmah−/−Sgca−/− relative to WT and Cmah−/ (ANOVA, P < 0.05 for both vs WT). Here, Cmah−/−Sgca−/− muscle did not differ significantly from Sgca−/−, but was generally lower at all repetitions. These data demonstrate that muscle force was generally reduced in cardiac and skeletal muscles of Cmah−/−Sgca−/− mice relative to Sgca−/−, with a particularly strong decrement occurring in the diaphragm muscle.
Fig. 7.
Altered cardiac and skeletal muscle physiology in Cmah−/−Sgca−/− mice. (A) Maximal developed force of isolated cardiac trabecular muscles, relative to the cross-sectional area, at various frequencies within the physiological range. (B) Developed force of isolated cardiac trabecular muscles in response to different doses of the β-agonist isoproterenol. (C) Specific force of isolated diaphragm muscle. (D) Relative force loss during repeated eccentric contractions in isolated EDL muscles over 10 repetitive stimulations. WT, CMP-Neu5Ac hydroxylase-deficient (Cmah−/−), α-sarcoglycan-deficient (Sgca−/−) and CMP-Neu5Ac hydroxylase-deficient and α-sarcoglycan-deficient (Cmah−/−Sgca−/−). Errors are SEM for n = 6–12 muscles per condition.
Assessment of anti-Neu5Gc serum antibody titers in Cmah−/−Sgca−/− mice
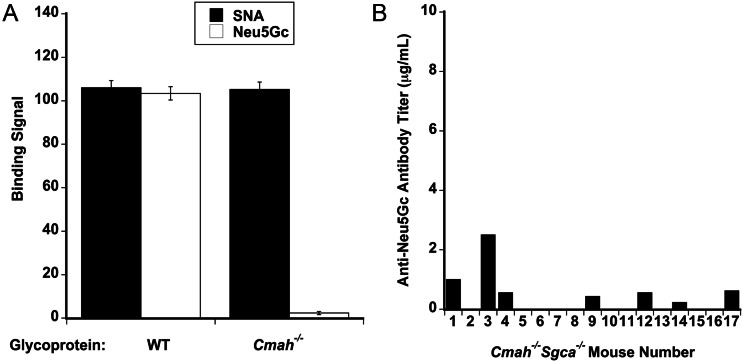
We had previously observed that about half of Cmah−/− mdx animals developed serum antibody titers to Neu5Gc as they aged, which theoretically could contribute to altered muscle histopathology (Chandrasekharan et al. 2010). We therefore also screened WT, Cmah−/−, Sgca−/− and Cmah−/−Sgca−/− animals for titers to Neu5Gc by comparing serum titers to glycoproteins from WT muscle, which is ∼50% Neu5Gc and 50% Neu5Ac, and Cmah−/− muscle, where proteins have no Neu5Gc and more abundant Neu5Ac, much as we had done previously (Chandrasekharan et al. 2010; Figure 8). Use of sialic acid-binding lectins such as Sambucus nigra agglutinin (SNA) showed equivalent sialic acid levels in immobilized WT and Cmah−/− glycoprotein samples, whereas the WT muscle had strong Neu5Gc reactivity and Cmah−/− muscle had essentially none. Serum from Cmah−/−Sgca−/− animals showed that only 7 of 17 animals had measurable anti-Neu5Gc antibody titers (Figure 8). Overall, the average titer for all animals was very low (290 ± 100 ng/mL), a level roughly one log lower than that previously observed in Cmah−/− mdx mice (Chandrasekharan et al. 2010). In addition, we did not identify any increased deposition of the mouse antibody or the activated (C5b-9) complement in Cmah−/−Sgca−/− muscles relative to Sgca−/− (not shown). Thus, serum anti-Neu5Gc antibodies did not appear to be significant in Cmah−/−Sgca−/− muscles.
Fig. 8.
Anti-Neu5Gc serum antibody titers in Cmah−/−Sgca−/− mice. Neu5Gc-specific antibody titers were measured by ELISA, comparing 1 μg spots of Cmah−/− (Neu5Gc-free) muscle glycoprotein and WT (Neu5Gc-rich) muscle glycoprotein, along with mouse Ab antibody standard curves. (A) SNA, a sialic acid binding lectin, bound glycoproteins from WT and Cmah−/− muscle glycoproteins equally well, whereas an anti-Neu5Gc-specific antibody bound only WT muscle glycoprotein and not Cmah−/− muscle glycoprotein. Errors are SD for n = 3–4 mice per condition. (B) Serum anti-Neu5Gc-specific antibody titers in individual Cmah−/−Sgca−/− mice.
Discussion
We have previously utilized Cmah-deficient mice to study the role of loss of Neu5Gc in the mdx mouse model of DMD (Chandrasekharan et al. 2010). Loss of Cmah in mdx animals led to more severe disease phenotypes that better approximated the conditions found in the human disease. If this is an effect on muscle biology in general, one might expect that loss of Cmah would increase disease severity in other forms of muscular dystrophy. Here, we have tested that notion by creating Cmah-deficient α-sarcoglycan-deficient mice (Cmah−/−Sgca−/−). Sgca−/− is a true genetic model for LGMD2D, eliminating all α-sarcoglycan from muscle cells (Duclos et al. 1998), much as mdx mice are a true genetic deficiency model for DMD, eliminating (almost all) dystrophin (Hoffman et al. 1987). Like mdx mice, however, Sgca−/− animals lack, or have muted, LGMD2D disease phenotypes. Perhaps most importantly in this regard, although a fraction of LGMD2D patients develop cardiomyopathy, Sgca−/− animals show no significant cardiac pathology (Duclos et al. 1998). Our findings suggest that loss of Cmah generally increases loss of muscle tissue within skeletal muscles and heart and increases histopathology. In addition, cardiac and skeletal muscle strength is generally reduced as the result of Cmah deletion. Although these effects are not always dramatic, they are all consistent, both in extent and direction, with the notion that Cmah modulates disease severity in Sgca−/− animals.
Future work will be required to determine the mechanisms by which loss of Cmah increases muscle disease severity; however, the combined effects shown here and previously (Chandrasekharan et al. 2010) point to a loss of function phenotype. This is evidenced by the increased uptake of Evans blue dye (EBD) uptake after exercise and the increased muscle wasting in skeletal and cardiac muscles in the absence in serum Neu5Gc antibodies for the majority of Cmah−/−Sgca−/− mice. Although the deletion of Cmah only leads to loss of a single oxygen atom at the 5-N-acyl position of sialic acid, the composition of many sialylated glycoconjugates on the cellular membrane are affected by this single genetic change. Sialic acid is an essential glycan in mammals (Schwarzkopf et al. 2002), and both reductions (e.g. in hereditary inclusion body myopathy 2, HIBM2) and increases (e.g. in sialuria) in sialic acid levels can cause human disease (Freeze 2006). For skeletal muscle, this is perhaps best exemplified by the fact that autosomal recessive mutations in the UDP-GlcNAc-2-epimerase/ManNAc kinase, GNE, the rate-limiting step in sialic acid biosynthesis, cause HIBM (Eisenberg et al. 2001). Removal of all sialic acids from cardiac or skeletal muscle membranes using neuraminidase treatment also causes dramatic changes in the conduction properties of voltage-gated sodium and voltage-gated calcium channels (Recio-Pinto et al. 1990; Fermini and Nathan 1991; Bennett et al. 1997). Although we have no evidence here, a second mechanism by which the deletion of Cmah may increase disease severity is the induction of autoimmune responses to Neu5Gc taken up by intramuscular cells.
Although less than half of Cmah−/−Sgca−/− animals showed measurable anti-Neu5Gc serum antibodies, such antibodies may nevertheless contribute in some way to the phenotypes observed. The deletion of CMAH eliminates the biosynthesis of all Neu5Gc from the human body, thereby making Neu5Gc a foreign antigen in human tissues (Irie et al. 1998; Hedlund et al. 2007). Humans take up Neu5Gc from dietary sources, primarily meat and dairy products, resulting in Neu5Gc expression in tissues (Tangvoranuntakul et al. 2003). Neu5Gc can be incorporated into human cells through a salvage pathway, and this pathway likely mimics the Neu5Gc-uptake mechanism that occurs in vivo (Bardor et al. 2005). Bacteria in the human microbiome, e.g. Haemophilus influenzae, also ingest Neu5Gc and can incorporate this sugar onto their cell surface, perhaps priming immune responses to Neu5Gc (Taylor et al. 2010). Neu5Gc is also found in human cells in other pathological states, e.g. cancer, where, like regenerating muscle, cells are dividing rapidly. Neu5Gc is present in well-known tumor biomarkers (Malykh et al. 2001) and antibodies to Neu5Gc can increase inflammation and alter tumor behavior (Hedlund et al. 2008). Another aspect to consider is the finding of Neu5Gc expression in macrophages and satellite cells within Cmah−/−Sgca−/− muscles. This expression likely results from dietary sources, and it remains to be determined whether such uptake would impact satellite cell function during the chronic regeneration that occurs in dystrophic muscles. Regardless of the mechanisms ultimately found to be involved, these results support the contention that Cmah is a modifier of disease severity for the muscular dystrophies.
Materials and methods
Mice
Sgca−/− mice were originally made by Kevin Campbell (Duclos et al. 1998) and were obtained from Jackson Laboratories (Bar Harbor, ME). Cmah−/− mice were originally made as described and have the identical exon deletion in the mouse Cmah gene that humans have (Hedlund et al. 2007). Cmah−/− and Sgca−/− were bred to obtain Cmah−/−Sgca−/− animals. All lines were maintained on a congenic C57Bl/6 background. Mice were allowed to eat and drink ad libitum and were fed standard mouse chow, which contains Neu5Gc at amounts increased roughly 6-fold on a per weight basis relative to predicted human consumption in a Western diet (A.Varki, unpublished observation). All experiments involving animals were done in accordance with protocols approved by the Institutional Animal Use and Care Committees at the Research Institute at Nationwide Children's Hospital and the Ohio State University.
Histology and immunostaining
Skeletal muscles were dissected and snap-frozen in liquid nitrogen-cooled isopentane. The hearts were dissected, washed in phosphate-buffered saline (PBS), embedded in the optimal cutting temperature freezing medium and frozen in dry ice-cooled isopentane. All tissues were sectioned at 8–10 μm on a cryostat. Sections used for the quantification of histopathology were stained with hematoxylin (72,404; Richard Allan Scientific, Kalamazoo, MI) and eosin (318,906; Sigma, St. Louis, MO) or Mason's modified trichrome (HT15-1KT; Sigma).
Immunostaining for Neu5Gc involved the use of an affinity purified chicken anti-Neu5Gc-specific IgY along with a non-immune control (Diaz et al. 2009). Sections were blocked in PBS with 10% (Neu5Gc-free) human serum, and control immunostaining was done by incubating sections with the non-immune chicken IgY control antibody (both at 1:1000 dilution), as described previously (Chandrasekharan et al. 2010). Staining for α2-3-linked sialic acid was done using fluorescein isothiocyanate-conjugated Maackia amurensis lectin (MAA, EY Labotatories, San Mateo, CA; 10 μg/mL) in Cmah−/− and Cmah−/−Sgca−/− muscles. Here, sections were blocked in 3% (v/v) bovine serum albumin or with 1% fish gelatin. For double immunostaining, sections were first stained for 1 h at room temperature with anti-Neu5Gc or control-specific chicken antiserum after blocking in 10% human serum. Sections were washed in PBS and fixed in 2% paraformaldehyde, washed in PBS and blocked in Mouse-on-Mouse (DAKO, Carpinteria, CA) followed by 10% human serum. Sections were then incubated overnight with rat anti-CD68 (MCA1957GA, AbD Serotech), mouse anti-embryonic myosin (eMyosin; NCL-MHCd, NovaCastra, Newcastle Upon Tyne, UK) or mouse-anti Pax7 (clone P3U1, Developmental Studies Hybridoma Bank). The Pax7 antibody was a generous gift from Michael Rudnicki (Ottawa Health Research Institute). Sections were then stained with Cy2 anti-chicken IgY or rhodamine anti-rat IgG + IgM or anti-mouse IgG1 secondary antibodies (all from Jackson Immunoresearch). Staining of muscle for anti-mouse IgG + IgM or C5b-9 complement was done as described previously (Chandrasekharan et al. 2010). Staining was visualized on a Zeiss Axiophot epifluoresence microscope using rhodamine- or fluorescein-specific optics.
EBD uptake
Eight-month-old WT, Cmah−/−, Sgca−/− and Cmah−/−Sgca−/− mice were injected intraperitoneally with EBD (E2129, Sigma) at a concentration of 50 μg/g of body weight in 100 μL of sterile PBS. Five hours later, mice were normalized for activity by subjecting them to 45 min exercise on a horizontal treadmill at a constant speed of 12 m/min for 15 min and then 24 m/min for 30 min. Thirty-six hours after EBD injection, mice were sacrificed and skeletal and heart muscles were frozen and sectioned. EBD uptake was visualized using rhodamine-specific optics on a Zeiss Axiophot epifluorescence microscope. Dye uptake was quantified using Zeiss AxioVision LE 4.1 software as described previously (Xu et al. 2009).
Serum CK activity
Blood was collected from the tail vein and allowed to clot for 1 h at 37°C. Clotted cells were centrifuged at 1500 × g for 3 min, and the serum was collected and analyzed without freezing. CK activity assays were done using an enzyme-coupled absorbance assay kit (326-10, SCKISUI Diagnostics, Prince Edward Island, Canada) following the manufacturer's instructions. Absorbance was measured at 340 nm every 30 s for 4 min at 25°C to calculate enzyme activity. All measurements were done in triplicate.
Cardiac and skeletal muscle pathology
Quantification of mouse weight, muscle weight, myofiber diameter, percentage of myofibers with central nuclei, percentage of muscle necrotic area, number of necrotic foci and percentage of muscle with EBD uptake were all done as previously described (Xu et al. 2009; Chandrasekharan et al. 2010) using sections stained with hematoxylin and eosin or using rhodamine-specific optics on a Zeiss epifluorescence microscope to visualize EBD uptake. All quantified histopathology measures were analyzed using ANOVA, followed by the post hoc t-test where applicable.
Cardiac and skeletal physiology
All physiology measures were done on blinded samples. Contractile function was assessed in isolated papillary muscles and trabeculae (1–2/mouse) as described previously (Chandrasekharan et al. 2010; Janssen 2010). Briefly, after the isolation of suitable muscles from the hearts, muscles were mounted between a force transducer and a length-displacement device. After equilibration at 37°C and 4 Hz stimulation frequency, contractile force and kinetics were assessed at four different lengths ranging from end-diastolic length to end-systolic length, at frequencies spanning the in vivo range (up to 12 Hz), followed by a concentration-response curve to the β-adrenergic agonist isoproterenol (1 nM–1 µM). All forces were normalized to the cross-sectional area of the muscles.
Diaphragm contractility was performed in isolated strips (1–2/mouse) of 2–3 mm in width as previously described (Chandrasekharan et al. 2010), with the modification that the tetanus duration was reduced to 300 ms. In isolated diaphragm strips, the optimal length was determined by stretching the muscle and record twitch developed tension. At this optimal length, a series of tetani, each 5 min apart, was given at 20, 50, 80, 120, 150 and 180 Hz. To optimize the translation of the results to in vivo conditions, experiments were performed at 37°C (Murray et al. 2012). Muscles were weighed and specific force measured and calculated as before (Chandrasekharan et al. 2010).
Contraction of the EDL muscle was done similar to as described previously (Chandrasekharan et al. 2010). After the isolation of the muscle, it was mounted ex vivo in the experimental set-up, and tetanic contractions at optimal length were recorded (30°C, 250 Hz, 700 ms duration). Thereafter, a series of 10 eccentric contractions were performed where the muscle was stretched to 105% of its optimal length over the final 200 ms of the tetanus. After the cessation of stimulation at t = 700 ms, the muscle was returned to optimal length, and given 2 min rest prior to the next contraction. All muscles were weighed and specific force measures calculated as before (Chandrasekharan et al. 2010). All contractile data were analyzed using ANOVA, followed by the post hoc t-test where applicable.
Western blotting
The gastrocnemius muscle from age-matched adult WT, Cmah−/−, Sgca−/− and Cmah−/−Sgca−/− mice was minced and solubilized in NP-40-containing buffer (75 mM Tris, pH 6.8, 1% NP-40, 1 mM ethylenediaminetetraacetic acid and complete protease inhibitor cocktail; Roche, Indianapolis, IN) overnight at 4°C on a platform shaker. Protein levels were measured using a BCA kit (ThermoScientific, 23235) and 20 μg of protein loaded per lane and separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis on a 4–12% gradient gel (Novex, NP0321), as before (Yoon et al. 2009). Proteins were transferred to nitrocellulose, blocked in 0.5% fish gelatin in Tris-buffered saline, pH 7.2, with 0.1% Tween 20 (TBST, San Diego, CA) and probed with chick anti-Neu5Gc antibody (Sialix, 1:25,000) and anti-Chick IgY-horseradish peroxidase (HRP) secondary to detect Neu5Gc, as before (Chandrasekharan et al. 2010). Blots were stripped and re-probed with anti-glyceraldehyde 3-phosphate dehydrogenase (Millipore, Billerica, MA, mAb 374), coupled with an appropriate secondary HRP reagent, to control for protein loading and transfer. Protein signals were quantified as before (Hoyte et al. 2004).
Serum antibody Enzyme-Linked Immuno-Sorbent Assays
Skeletal muscles from adult WT or Cmah−/− mice were dissected, minced and solubilized in 1% SDS-containing buffer as described previously (Chandrasekharan et al. 2010). Protein levels were measured, as before (Chandrasekharan et al. 2010), diluted into 50 mM Tris, pH 6.8, with 0.1% SDS and spotted at 1 μg well on the nitrocellulose-coated substratum. Mouse antibody concentration curves, ranging from 0.5 to 10 ng of protein, were also spotted, much as described previously (Chandrasekharan et al. 2010). Spots were blocked in TBST with 0.5% fish gelatin and incubated with either sialic acid binding lectins (2 μg/mL of SNA; 2 μg/mL of MAA), 1:25,000 diluted Neu5Gc antibody (Sialix) or 1:100 diluted serum from WT, Cmah−/−, Sgca−/− or Cmah−/−Sgca−/− mice. To visualize signals, SNA and MAA were directly conjugated to HRP, Neu5Gc was washed and incubated with anti-Chick IgY HRP, and mouse sera were washed and incubated with goat anti-mouse IgG + IgM HRP. The same secondary antibody was used to probe mouse Ig standard curves. Binding was developed and quantified as described previously (Chandrasekharan et al. 2010). Neu5Gc titers were defined by subtracting signals for the Cmah−/− protein from the WT protein, as before (Chandrasekharan et al. 2010). The concentration detection limit of serum antibodies in these assays was ∼500 ng/mL. This limit of detection is below the average anti-Neu5Gc antibody concentration previously identified in the Cmah−/−mdx serum (Chandrasekharan et al. 2010) and many Neu5Gc titers identified in the human serum (Padler-Karavani et al. 2008).
Supplementary data
Supplementary data for this article is available online at http://glycob.oxfordjournals.org/.
Funding
This grant was supported by National Institute of Health [AR060949 to P.T.M., P30 NS045758 (Muscle Physiology Core) to P.J. and GM32373 to A.V.].
Conflict of interest
A.V. is a board member and co-founder of Sialix Inc. (formerly Gc-free, Inc.), a biotech company focused on developing therapeutics and reagents related to sialic acid. None of the other authors have any conflict with regard to the publication of this work.
Abbreviations
ANOVA, analysis of variance; CK, creatine kinase; CMP-Neu5Ac, cytidine-5′-monophospho-N-acetylneuraminic acid; CMP-Neu5Gc, cytidine-5′-monophospho-N-glycolylneuraminic acid; DMD, Duchenne muscular dystrophy; EBD, Evans blue dye; EDL, extensor digitorum longus; HIBM, hereditary inclusion body myopathy; HRP, horseradish peroxidase; LGMD, limb girdle muscular dystrophy; MAA, Maackia amurensis agglutinin; Neu5Gc, N-glycolylneuraminic acid; Neu5Ac, N-acetylneuraminic acid; PBS, phosphate-buffered saline; SNA, Sambucus nigra agglutinin; TBST, Tris-buffered saline with Tween 20; WT, wild type.
Supplementary Material
Acknowledgements
We would like to thank Sarah Lewis, Zariffe Sahenk and Jerry Mendell (all at Nationwide Children's Hospital) for assistance with analysis of de-identified human muscle biopsies, Benjamin Martin and Daniel Fenn for technical assistance in morphometric and histologic analysis of mouse tissues and Benjamin Canan, Jason Murray and Jenna Stangland for technical assistance with functional force measurements. Special thanks to Michael Rudnicki (Ottawa Health Research Institute) for Pax7 antibody.
References
- Bardor M, Nguyen DH, Diaz S, Varki A. Mechanism of uptake and incorporation of the non-human sialic acid N-glycolylneuraminic acid into human cells. J Biol Chem. 2005;280:4228–4237. doi: 10.1074/jbc.M412040200. [DOI] [PubMed] [Google Scholar]
- Bennett E, Urcan MS, Tinkle SS, Koszowski AG, Levinson SR. Contribution of sialic acid to the voltage dependence of sodium channel gating. A possible electrostatic mechanism. J Gen Physiol. 1997;109:327–343. doi: 10.1085/jgp.109.3.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekharan K, Yoon JH, Xu Y, deVries S, Camboni M, Janssen PM, Varki A, Martin PT. A human-specific deletion in mouse Cmah increases disease severity in the mdx model of Duchenne muscular dystrophy. Sci Transl Med. 2010;2:42–54. doi: 10.1126/scitranslmed.3000692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou HH, Takematsu H, Diaz S, Iber J, Nickerson E, Wright KL, Muchmore EA, Nelson DL, Warren ST, Varki A. A mutation in human CMP-sialic acid hydroxylase occurred after the Homo-Pan divergence. Proc Natl Acad Sci USA. 1998;95:11751–11756. doi: 10.1073/pnas.95.20.11751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz SL, Padler-Karavani V, Ghaderi D, Hurtado-Ziola N, Yu H, Chen X, Brinkman-Van der Linden EC, Varki A, Varki NM. Sensitive and specific detection of the non-human sialic Acid N-glycolylneuraminic acid in human tissues and biotherapeutic products. PLoS One. 2009;4:e4241. doi: 10.1371/journal.pone.0004241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duclos F, Straub V, Moore SA, Venzke DP, Hrstka RF, Crosbie RH, Durbeej M, Lebakken CS, Ettinger AJ, van der Meulen J. Progressive muscular dystrophy in alpha-sarcoglycan-deficient mice. J Cell Biol. 1998;142:1461–1471. doi: 10.1083/jcb.142.6.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg I, Avidan N, Potikha T, Hochner H, Chen M, Olender T, Barash M, Shemesh M, Sadeh M, Grabov-Nardini G. The UDP-N-acetylglucosamine 2-epimerase/N-acetylmannosamine kinase gene is mutated in recessive hereditary inclusion body myopathy. Nat Genet. 2001;29:83–87. doi: 10.1038/ng718. [DOI] [PubMed] [Google Scholar]
- Fermini B, Nathan RD. Removal of sialic acid alters both T- and L-type calcium currents in cardiac myocytes. Am J Physiol. 1991;260:H735–H743. doi: 10.1152/ajpheart.1991.260.3.H735. [DOI] [PubMed] [Google Scholar]
- Ferreira AF, Carvalho MS, Resende MB, Wakamatsu A, Reed UC, Marie SK. Phenotypic and immunohistochemical characterization of sarcoglycanopathies. Clinics (Sao Paulo) 2011;66:1713–1719. doi: 10.1590/S1807-59322011001000008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeze HH. Genetic defects in the human glycome. Nat Rev Genet. 2006;7:537–551. doi: 10.1038/nrg1894. [DOI] [PubMed] [Google Scholar]
- Godfrey C, Foley AR, Clement E, Muntoni F. Dystroglycanopathies: Coming into focus. Curr Opin Genet Dev. 2011;21:278–285. doi: 10.1016/j.gde.2011.02.001. [DOI] [PubMed] [Google Scholar]
- Guglieri M, Straub V, Bushby K, Lochmuller H. Limb-girdle muscular dystrophies. Curr Opin Neurol. 2008;21:576–584. doi: 10.1097/WCO.0b013e32830efdc2. [DOI] [PubMed] [Google Scholar]
- Hedlund M, Padler-Karavani V, Varki NM, Varki A. Evidence for a human-specific mechanism for diet and antibody-mediated inflammation in carcinoma progression. Proc Natl Acad Sci USA. 2008;105:18936–18941. doi: 10.1073/pnas.0803943105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedlund M, Tangvoranuntakul P, Takematsu H, Long JM, Housley GD, Kozutsumi Y, Suzuki A, Wynshaw-Boris A, Ryan AF, Gallo RL. N-glycolylneuraminic acid deficiency in mice: Implications for human biology and evolution. Mol Cell Biol. 2007;27:4340–4346. doi: 10.1128/MCB.00379-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman EP, Brown RH, Jr., Kunkel LM. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987;51:919–928. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- Hoyte K, Jayasinha V, Xia B, Martin PT. Transgenic overexpression of dystroglycan does not inhibit muscular dystrophy in mdx mice. Am J Pathol. 2004;164:711–718. doi: 10.1016/S0002-9440(10)63158-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irie A, Koyama S, Kozutsumi Y, Kawasaki T, Suzuki A. The molecular basis for the absence of N-glycolylneuraminic acid in humans. J Biol Chem. 1998;273:15866–15871. doi: 10.1074/jbc.273.25.15866. [DOI] [PubMed] [Google Scholar]
- Janssen PM. Myocardial contraction-relaxation coupling. Am J Physiol Heart Circ Physiol. 2010;299:H1741–H1749. doi: 10.1152/ajpheart.00759.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano T, Koyama S, Takematsu H, Kozutsumi Y, Kawasaki H, Kawashima S, Kawasaki T, Suzuki A. Molecular cloning of cytidine monophospho-N-acetylneuraminic acid hydroxylase. Regulation of species- and tissue-specific expression of N-glycolylneuraminic acid. J Biol Chem. 1995;270:16458–16463. doi: 10.1074/jbc.270.27.16458. [DOI] [PubMed] [Google Scholar]
- Malykh YN, Schauer R, Shaw L. N-Glycolylneuraminic acid in human tumours. Biochimie. 2001;83:623–634. doi: 10.1016/s0300-9084(01)01303-7. [DOI] [PubMed] [Google Scholar]
- Martin PT. The dystroglycanopathies: the new disorders of O-linked glycosylation. Semin Pediatr Neurol. 2005;12:152–158. doi: 10.1016/j.spen.2005.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michele DE, Barresi R, Kanagawa M, Saito F, Cohn RD, Satz JS, Dollar J, Nishino I, Kelley RI, Somer H. Post-translational disruption of dystroglycan-ligand interactions in congenital muscular dystrophies. Nature. 2002;418:417–422. doi: 10.1038/nature00837. [DOI] [PubMed] [Google Scholar]
- Miller G, Wang EL, Nassar KL, Peter AK, Crosbie RH. Structural and functional analysis of the sarcoglycan-sarcospan subcomplex. Exp Cell Res. 2007;313:639–651. doi: 10.1016/j.yexcr.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray JD, Canan BD, Martin CD, Stangland JE, Rastogi N, Rafael-Fortney JA, Janssen PM. The force-temperature relationship in healthy and dystrophic mouse diaphragm; implications for translational study design. Front Physiol. 2012;3:422. doi: 10.3389/fphys.2012.00422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen HH, Jayasinha V, Xia B, Hoyte K, Martin PT. Overexpression of the cytotoxic T cell GalNAc transferase in skeletal muscle inhibits muscular dystrophy in mdx mice. Proc Natl Acad Sci USA. 2002;99:5616–5621. doi: 10.1073/pnas.082613599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padler-Karavani V, Yu H, Cao H, Chokhawala H, Karp F, Varki N, Chen X, Varki A. Diversity in specificity, abundance, and composition of anti-Neu5Gc antibodies in normal humans: Potential implications for disease. Glycobiology. 2008;18:818–830. doi: 10.1093/glycob/cwn072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recio-Pinto E, Thornhill WB, Duch DS, Levinson SR, Urban BW. Neuraminidase treatment modifies the function of electroplax sodium channels in planar lipid bilayers. Neuron. 1990;5:675–684. doi: 10.1016/0896-6273(90)90221-z. [DOI] [PubMed] [Google Scholar]
- Schwarzkopf M, Knobeloch KP, Rohde E, Hinderlich S, Wiechens N, Lucka L, Horak I, Reutter W, Horstkorte R. Sialylation is essential for early development in mice. Proc Natl Acad Sci USA. 2002;99:5267–5270. doi: 10.1073/pnas.072066199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seale P, Sabourin LA, Girgis-Gabardo A, Mansouri A, Gruss P, Rudnicki MA. Pax7 is required for the specification of myogenic satellite cells. Cell. 2000;102:777–786. doi: 10.1016/s0092-8674(00)00066-0. [DOI] [PubMed] [Google Scholar]
- Shaw L, Schneckenburger P, Carlsen J, Christiansen K, Schauer R. Mouse liver cytidine-5′-monophosphate-N-acetylneuraminic acid hydroxylase. Catalytic function and regulation. Eur J Biochem. 1992;206:269–277. doi: 10.1111/j.1432-1033.1992.tb16925.x. [DOI] [PubMed] [Google Scholar]
- Straub V, Bushby K. The childhood limb-girdle muscular dystrophies. Semin Pediatr Neurol. 2006;13:104–114. doi: 10.1016/j.spen.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Sveen ML, Thune JJ, Kober L, Vissing J. Cardiac involvement in patients with limb-girdle muscular dystrophy type 2 and Becker muscular dystrophy. Arch Neurol. 2008;65:1196–1201. doi: 10.1001/archneur.65.9.1196. [DOI] [PubMed] [Google Scholar]
- Tangvoranuntakul P, Gagneux P, Diaz S, Bardor M, Varki N, Varki A, Muchmore E. Human uptake and incorporation of an immunogenic nonhuman dietary sialic acid. Proc Natl Acad Sci USA. 2003;100:12045–12050. doi: 10.1073/pnas.2131556100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor RE, Gregg CJ, Padler-Karavani V, Ghaderi D, Yu H, Huang S, Sorensen RU, Chen X, Inostroza J, Nizet V. Novel mechanism for the generation of human xeno-autoantibodies against the nonhuman sialic acid N-glycolylneuraminic acid. J Exp Med. 2010;207:1637–1646. doi: 10.1084/jem.20100575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varki A. Colloquium paper: Uniquely human evolution of sialic acid genetics and biology. Proc Natl Acad Sci USA. 2010;107(Suppl 2):8939–8946. doi: 10.1073/pnas.0914634107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YX, Rudnicki MA. Satellite cells, the engines of muscle repair. Nat Rev Mol Cell Biol. 2012;13:127–133. doi: 10.1038/nrm3265. [DOI] [PubMed] [Google Scholar]
- Xu R, Camboni M, Martin PT. Postnatal overexpression of the CT GalNAc transferase inhibits muscular dystrophy in mdx mice without altering muscle growth or neuromuscular development: Evidence for a utrophin-independent mechanism. Neuromuscul Disord. 2007;17:209–220. doi: 10.1016/j.nmd.2006.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu R, Chandrasekharan K, Yoon JH, Camboni M, Martin PT. Overexpression of the cytotoxic T cell (CT) carbohydrate inhibits muscular dystrophy in the dyW mouse model of congenital muscular dystrophy 1A. Am J Pathol. 2007;171:181–199. doi: 10.2353/ajpath.2007.060927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu R, DeVries S, Camboni M, Martin PT. Overexpression of Galgt2 reduces dystrophic pathology in the skeletal muscles of alpha sarcoglycan-deficient mice. Am J Pathol. 2009;175:235–247. doi: 10.2353/ajpath.2009.080967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon JH, Chandrasekharan K, Xu R, Glass M, Singhal N, Martin PT. The synaptic CT carbohydrate modulates binding and expression of extracellular matrix proteins in skeletal muscle: Partial dependence on utrophin. Mol Cell Neurosci. 2009;41:448–463. doi: 10.1016/j.mcn.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.