Abstract
Members of the galectin family of proteins have been shown to regulate the development and the function of immune cells. We previously identified the increased expression of galectin-1 and galectin-3 mRNA and protein in anergic B cells relative to their naïve counterparts. To investigate the role of these galectins in maintaining B cell tolerance, we crossed mice deficient in galectin-1 or galectin-3 with mice bearing a lupus autoantigen-binding transgenic (Tg) B cell receptor, using a model with a well-characterized B cell tolerance phenotype of deletion, receptor editing and anergy. Here, we present data showing that the global knockout of galectin-1 or galectin-3 yields subtle alterations in B cell fate in autoantibody Tg mice. The absence of galectin-3 leads to a significant increase in the number of Tg spleen B cells, with the recovery of anti-laminin antibodies from a subset of mice. The B cell number increases further in antibody Tg mice with the dual deficiency of both galectin-1 and galectin-3. Isolated galectin-1 deficiency significantly enhances the proliferation of Tg B cells in response to lipopolysaccharide stimulation. These findings add to the growing body of evidence indicating a role for the various galectin family members, and for galectins 1 and 3 in particular, in the regulation of autoimmunity.
Keywords: autoimmunity, CD86, CD62L, humoral immunity, MHCII
Introduction
Autoimmune diseases such as systemic lupus erythematosus are, at their core, the result of failed immunologic tolerance. The dysregulation of autoreactive B cells and/or T cells leads to aberrantly secreted autoantibodies and myriad clinical manifestations. Recent studies have revealed that galectin family members are involved in the development, functional regulation and apoptosis of immune cells (reviewed in van Kooyk and Rabinovich 2008; Rabinovich and Toscano 2009; Rabinovich et al. 2012). Galectin-1 and galectin-3 are implicated in autoimmune disease regulation or pathogenesis, including lupus, and have been proposed as agents or targets, respectively, for therapeutic intervention (Jiang et al. 2009; Kang et al. 2009; Liu et al. 2011). Some insight into the underlying tolerance defects in B and T cells, and the roles for galectin family members in these processes, is emerging from studies in mouse and man, yet much remains to be discovered to assist the rational design of specific, targeted therapies.
To explore the normal fate of autoreactive B cells and to examine molecular events regulating their development in vivo, we previously generated and characterized an immunoglobulin (Ig) transgenic (Tg) murine model. Developing B cells express a Tg Ig heavy chain, LamH, that in conjunction with diverse endogenous Ig light chains binds to laminin, a lupus nephritis-associated self-antigen (Amital et al. 2007; Hanrotel-Saliou et al. 2011). Our studies revealed that LamH Ig Tg B cells are tightly regulated in vivo by three mechanisms: (i) central deletion, due to the induced apoptosis of developing autoreactive B cells in the bone marrow, (ii) receptor editing, the recombination of additional endogenous heavy or light chain gene loci in attempts to generate a non-autoreactive B cell receptor, and (iii) anergy, the functional inactivation of autoreactive cells. These mechanisms to silence autoreactivity are effective in LamH Ig Tg mice, such that serum anti-laminin autoreactivity is rarely recovered (Rudolph et al. 2002; Brady et al. 2004).
Studies using representational difference analysis, microarray and protein blotting further revealed that LamH Ig Tg anergic B cells express higher levels of mRNA and protein for the galactose-binding lectins galectin-1 and galectin-3 relative to their naïve B cell counterparts (Clark et al. 2007). Galectin-1 was also recently demonstrated to be significantly up-regulated in a subset of human anergic B cells (Charles et al. 2011). This led us to hypothesize that galectin-1 and galectin-3 play a role in the induction and/or the maintenance of B cell tolerance. To investigate this possibility, we generated LamH Ig Tg mice genetically deficient (−/−) in galectin-1 and/or galectin-3 and assessed the in vivo fate of resultant Ig Tg B cells.
Here, we present evidence that the global knockout of galectin-1 and/or galectin-3 does not precipitate overt failure of either central deletion or anergy of autoreactive LamH Ig Tg B cells. However, Tg B cells from mice lacking galectin-1 demonstrate enhanced proliferation in response to lipopolysaccharide (LPS) stimulation ex vivo and altered expression of surface CD86. The knockout of galectin-3 prevents the depletion of spleen Tg B cell numbers to the levels observed in galectin-3-sufficient subjects, a finding further exacerbated by the dual deficiency of both galectin-3 and galectin-1, and permits the spontaneous generation of anti-laminin antibodies by Tg B cells in a fraction of subjects. Our results suggest that the genetic loss of galectin-3 and/or galectin-1 in combination with environmental or epigenetic factors can lead to tolerance failure.
Results
Expression of the LamH transgene
The LamH Ig transgene is distinguished from endogenous IgM by IgM-a allotype reagents; endogenous B6 mice are IgM-b allotype. Expression of the transgene was detected on the surface of the majority of B cells from LamH Ig Tg+ mice regardless of galectin status (Table I and Figure 1). Tg IgM-a was also detected in the serum of all Tg+ mice, though at low levels relative to total circulating IgM, similar to the phenotype previously reported for LamH Ig Tg+ B6 mice (Rudolph et al. 2002). Serum Tg IgM-a levels did not differ significantly with the knockout of either galectin-1 or galectin-3 (data not shown).
Table I.
B cell profile and receptor expressiona
| Non-Tg |
LamH Ig Tg+ |
|||||
|---|---|---|---|---|---|---|
| Galectin+ | Galectin-1−/− | Galectin-3−/− | Galectin+ | Galectin-1−/− | Galectin-3−/− | |
| Spleen | ||||||
| Spleen weight (mg) | 0.068 ± 0.011 (16) | 0.075 ± 0.008 (5) | 0.073 ± 0.007 (4) | 0.032 ± 0.013* (20) | 0.042 ± 0.023* (11) | 0.042 ± 0.008*§ (8) |
| Splenocyte count (millions) | 94.0 ± 22.3 (13) | 102.0 ± 14.2 (4) | 100.7 ± 39.0 (4) | 24.7 ± 10.7* (19) | 35.0 ± 21.0* (10) | 30.7 ± 7.4* (6) |
| %B220+ of lymphocytes | 48.8 ± 11.9 (15) | 49.3 ± 13.3 (5) | 56.3 ± 4.6 (4) | 15.6 ± 3.9* (20) | 14.8 ± 4.2* (10) | 20.2 ± 5.8*§ (8) |
| B cell count (millions) | 27.0 ± 11.5 (12) | 36.6 ± 20.7 (4) | 45.6 ± 17.3 (4) | 2.4 ± 1.2* (18) | 3.0 ± 2.1* (9) | 4.1 ± 1.0*§ (6) |
| %IgMa | 2.1 ± 1.8 (14) | 1.9 ± 1.2 (4) | 4.2 ± 5.5 (4) | 69.2 ± 14.6* (18) | 75.7 ± 10.0* (10) | 64.6 ± 8.9* (6) |
| %IgMb | 87.9 ± 7.6 (14) | 92.3 ± 3.4 (4) | 87.8 ± 8.9 (4) | 30.7 ± 17.5* (18) | 26.9 ± 19.0* (10) | 33.5 ± 13.6* (6) |
| %lambda | 5.7 ± 1.0 (11) | 6.5 ± 1.3 (3) | 6.0 ± 1.4 (3) | 10.3 ± 4.3* (15) | 8.2 ± 2.4 (8) | 12.2 ± 1.4* (6) |
| Bone marrow | ||||||
| %B220 | 40.4 ± 11.1 (15) | 35.1 ± 4.1 (5) | 48.0 ± 6.3 (4) | 16.3 ± 6.4* (19) | 18.5 ± 7.1* (10) | 20.1 ± 6.5* (8) |
| %IgM | 50.9 ± 10.4 (14) | 54.0 ± 10.9 (5) | 59.5 ± 9.9 (4) | 20.2 ± 8.9* (18) | 16.5 ± 8.1* (10) | 22.1 ± 3.2* (6) |
| B cell markers of antigen contact | ||||||
| Surface Ig MFIb | 1.00 ± 0.12 (11) | 0.97 ± 0.29 (3) | 1.05 ± 0.09 (3) | 0.61 ± 0.27* (13) | 0.43 ± 0.30 (7) | 0.62 ± 0.11* (6) |
| CD62L MFI | 1.00 ± 0.03 (14) | 1.13 ± 0.08§ (4) | 1.16 ± 0.19 (4) | 1.12 ± 0.30 (17) | 1.11 ± 0.24 (9) | 1.22 ± 0.34 (8) |
| %CD62L of B220+ | 44.3 ± 28.1 (12) | 45.8 ± 37.2 (2) | 69.6 ± 12.3 (3) | 36.6 ± 16.9 (13) | 20.2 ± 13.2 (7) | 55.7 ± 9.8§ (6) |
| MHCII MFI | 1.00 ± 0.04 (14) | 1.12 ± 0.10§ (4) | 1.04 ± 0.17 (4) | 1.12 ± 0.19 (17) | 0.99 ± 0.16 (9) | 1.22 ± 0.21 (8) |
| %MHCII of B220+ | 94.5 ± 2.6 (14) | 95.1 ± 1.7 (4) | 94.9 ± 3.8 (4) | 85.9 ± 5.8* (17) | 86.5 ± 4.9* (9) | 83.6 ± 8.5* (8) |
IgMa, IgMb, lambda and IgM data are shown as the percentage of B220+ gated cells. IgM-a is the allotype of the LamH Ig Tg; any positivity in non-Tg mice reflects background staining.
aShown are the mean ± SD, with the number of subjects in parenthesis.
bMFI=mean fluorescence intensity, normalized per experiment to the non-Tg galectin+ subject(s). All MFI data are lymphocyte gated by forward and side light scatter properties and B cell gated by the B220 marker. Surface Ig=IgM plus IgD expression.
*P < 0.05 vs non-Tg group with identical galectin status.
§P < 0.05 vs galectin+ group with same LamH Ig Tg status.
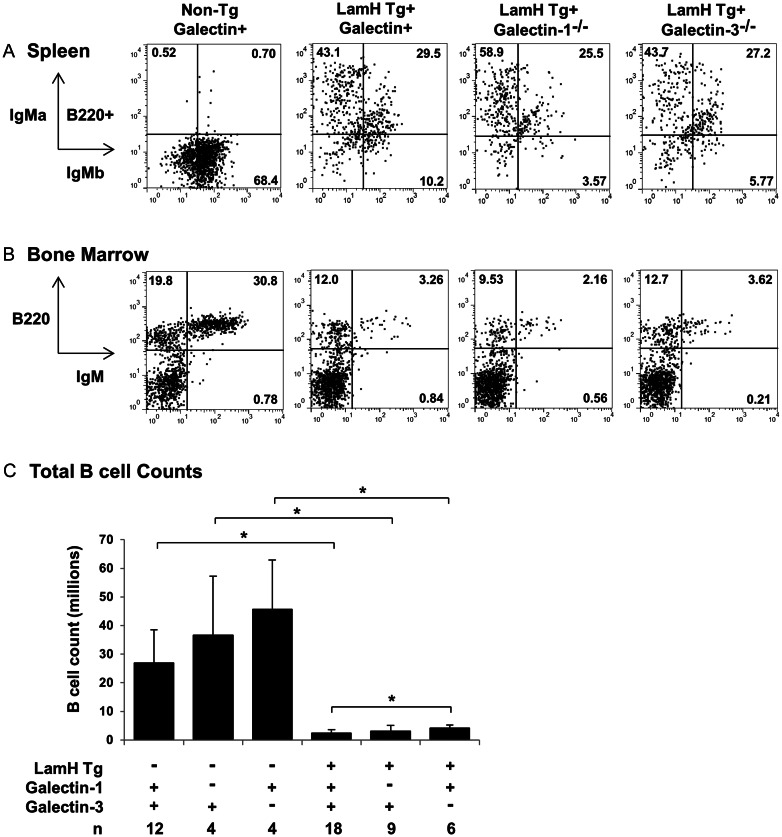
Fig. 1.
B cell profiles. Representative flow cytometry plots of (A) splenocytes and (B) bone marrow from anti-laminin Ig transgene (LamH Tg+) galectin knockout (−/−) mice and non-Tg or galectin sufficient (+) controls. Cells were gated on lymphocytes based on FSC and SSC properties, with further gating on B220+ B cells when indicated. IgM-a, transgene-encoded IgM; IgM-b, endogenous IgM; IgM, total IgM (IgM-a + IgM-b). (C) Total splenic B cell count, in millions, by genotype for galectin knockout mice (−), with (+) or without (−) the LamH Ig Tg, and galectin-sufficient controls (+). *P < 0.05 for pairwise comparison as noted.
Markers of B cell receptor editing in LamH Ig Tg+ mice did not differ with galectin status. The frequency of coexpression of endogenous heavy chain (b-allotype IgM), indicating the secondary rearrangement at the endogenous loci despite the presence of the rearranged Tg H chain, did not differ in LamH Ig Tg+ subjects with and without galectin deficiency (Figure 1 and Table I). Similarly, the frequency of lambda light chain expression, which suggests multiple attempts at light chain rearrangement to replace an autoreactive receptor, did not differ between Tg+ groups (Table I). LamH Ig Tg+ galectin-3−/− mice had a significantly larger proportion of lambda+ B cells when compared with their non-Tg galectin-3−/− counterparts, similar to the previously reported phenotype in galectin-sufficient LamH Ig Tg+ B6 mice (shown in Table I and in Brady et al. 2004), suggesting enhanced editing of LamH Ig Tg+ cells in these strains.
Deletion of Tg B cells
A significant reduction in the number of splenic B cells was seen in all LamH Ig Tg+ groups compared with their respective non-Tg controls, regardless of the presence or the knockout of either galectin-1 or galectin-3 (Figure 1C).
The B cell count in Ig Tg+ galectin-3−/− mice was significantly higher (71% increase) than in Tg+ galectin+ subjects (Figure 1C and Table I). A significant increase in spleen weight was also noted in Tg+ galectin-3−/− vs Tg+ galectin+ mice (Table I). No significant differences were observed between non-Tg galectin+ and galectin−/− mice, although there was a trend in the increased B cell count in non-Tg galectin-3−/− mice vs the non-Tg galectin+ group (P = 0.0523).
The overall decrease in B cells in LamH Ig Tg+ mice is also evident in the central compartment as a reduction in the percentage of bone marrow B220+ lymphocytes and of IgM+ bone marrow B cells in LamH Ig Tg+ mice relative to non-Tg mice of the same galectin status (Table I and Figure 1B).
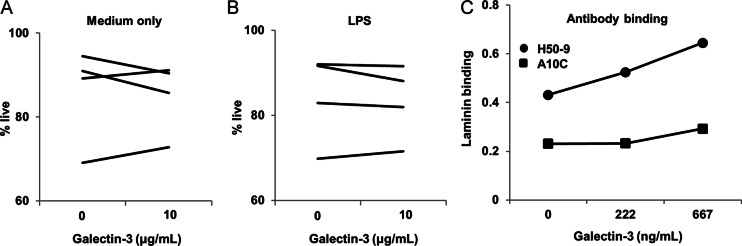
To investigate whether galectin-3 impacts the survival of LamH Ig Tg B cells, which could alter the total B cell number as seen in LamH Ig Tg+ galectin-3−/− subjects, we examined whether addition of exogenous galectin-3 to overnight cultures of LamH Ig Tg+ galectin3−/− B cells impacted cell survival. Figure 2A and B shows that addition of recombinant mouse galectin-3 did not consistently increase the percentage of live B cells in culture, either in the presence or in the absence of LPS.
Fig. 2.
Galectin-3 effects on cell survival and autoantibody laminin binding. (A and B) Purified B cells from 4 Tg+ galectin-3−/− mice (one of which was galectin-1+/−) were cultured for 24 h in the presence of media alone (A) or LPS (B) and the indicated amount of recombinant mouse galectin-3. Shown are the % live cells, which are Annexin-V negative and 7-AAD negative, from lymphocyte-gated CD19+ B cells. (C) Two anti-laminin autoantibodies, H50-9 and A10C, were incubated with increasing concentrations of recombinant mouse galectin-3 then assayed for laminin binding as described, reported as OD405 for binding to laminin minus binding to diluent (PBS) only.
Another potential mechanism by which galectin-3 could impact B cell number is via interaction in vivo with either laminin or laminin-specific B cell receptors. Galectin-3 has been shown to bind to laminin (our data not shown, and Barboni et al. 1999).To investigate whether galectin-3 and lupus anti-laminin autoantibodies bind the same epitope(s) on laminin, two laminin-reactive antibodies, IgG H50-9 (Foster et al. 1993) and IgM A10C (Fitzsimons et al. 2000), were incubated with various concentrations of galectin-3 prior to assay for laminin binding. Addition of exogenous galectin-3 did not inhibit the binding of either antibody to laminin (Figure 2C). Rather, increasing amounts of galectin-3 enhanced Ig binding to laminin, suggesting that galectin-3/laminin interactions facilitate the exposure of distinct laminin epitopes recognized by anti-laminin Ig. Anti-Ig detection reagent did not identify deposited Ig in control wells incubated with galectin-3 alone (not shown), ruling out physical interaction between the anti-laminin Ig and the galectin-3.
Serum anti-laminin autoreactivity
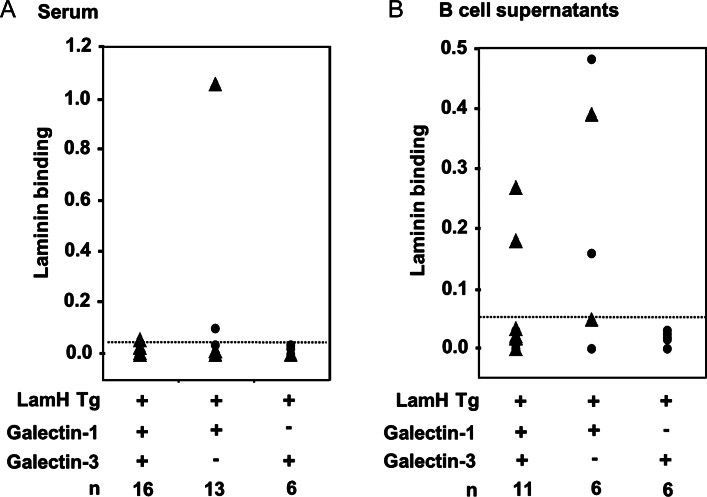
We previously reported strict regulation of the LamH Ig Tg B cells in the B6 background, such that spontaneous serum anti-laminin autoreactivity is rarely recovered (Rudolph et al. 2002; Brady et al. 2004). Superimposition of galectin-1−/− or galectin-3−/− mutations onto LamH Ig Tg+ B6 mice did not alter this phenotype in the majority of mice studied. However, one of 13 LamH Ig Tg+ galectin-3−/− mice had high serum levels of anti-laminin autoreactivity (Figure 3A).
Fig. 3.
Anti-laminin antibody in (A) serum or (B) LPS-stimulated supernatants of purified B cells from LamH Ig Tg+ mice cultured in the presence of LPS. Laminin binding is shown as OD405 for binding to laminin minus binding to diluent only. Line denotes arbitrary cutoff for laminin-binding positivity at an OD of 0.050. Triangles represent subjects heterozygous (+/−) at the galectin+ locus.
B cell response to stimulus
When purified B cells were stimulated in culture with LPS, anti-laminin IgM antibodies were occasionally generated. Shown in Figure 3B, anti-laminin autoreactivity was detected in supernatants of mitogen-stimulated B cells from 2 of 11 (18%) LamH Ig Tg+ galectin+ mice. Three of six (50%) LamH Ig Tg+ galectin 3−/− B cell culture supernatants also contained laminin autoreactivity, whereas none of the LamH Ig Tg+ galectin-1−/− cultures generated substantial levels of anti-laminin reactivity. Similar results were observed when whole splenocytes were cultured with mitogen (not shown).
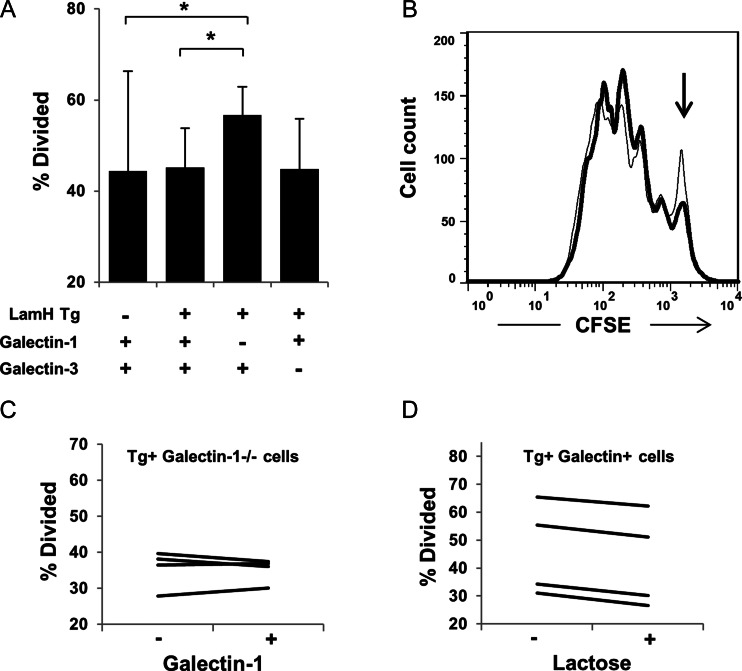
To assess proliferation, purified B cells were labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE) and cultured in the presence of either LPS or B cell receptor cross-linker anti-IgM F(ab')2. Following 2.5 days of stimulation, proliferation was assessed by flow cytometry. In response to LPS stimulation, a significantly higher percentage of B cells from LamH Ig Tg+ galectin-1−/− mice proliferated compared with B cells from either non-Tg or Tg+ galectin+ mice (Figure 4A and B). There was a trend to higher LPS-induced proliferation of LamH Ig Tg+ galectin-1−/− when compared with LamH Ig Tg+ galectin-3−/− B cells (P = 0.0588). No difference in response to receptor cross-linking was detected.
Fig. 4.
B cell proliferation. Purified B cells are loaded initially with non-toxic vital dye CFSE and cultured in the presence of 50 µg/mL of LPS. As the parent cell divides, the stain is equally distributed between the daughter cells, such that each generation has a fluorescence peak approximately half that of the parents. (A) The % of cells that divided ±SD, as calculated using the FlowJo proliferation platform, n = 4–9 subjects per group. *P < 0.05 for indicated comparison. (B) Representative histograms of CFSE staining for Tg+ galectin+ (thin line) relative to Tg+ galectin-1−/− (thick line). The peak of undivided cells is noted with an arrow. (C) B cells from four LamH Ig Tg+ galectin-1−/− subjects (three of whom were +/− at the galectin-3 locus) were cultured with LPS +/− the addition of 10 µg/mL exogenous galectin-1. Each line represents the % of divided cells from an individual subject across both conditions. (D) B cells from four LamH Ig Tg+ galectin+ subjects were cultured in the presence of LPS ± 10 mM lactose. Each line represents the % of divided cells from an individual subject across both conditions.
To investigate whether the enhanced LPS-induced proliferation of LamH Ig Tg+ galectin-1−/− B cells could be reversed by addition of exogenous galectin-1, proliferation assays were performed with LPS +/− 10 µg/mL recombinant galectin-1. Addition of galectin-1 had only a minor and non-uniform effect on the percent of divided cells (Figure 4C).
Conversely, to investigate whether the level of LPS-induced proliferation of LamH Ig Tg+ galectin+ B cells could be increased by blocking exogenous galectin binding, proliferation assays were performed with LPS +/− 10 µM lactose. Addition of lactose failed to enhance B cell proliferation (Figure 4D).
Laminin immunization of galectin-1-deficient Tg+ mice
Because spontaneous anti-laminin autoreactivity was not recovered from serum- or mitogen-stimulated B cells of any LamH Ig Tg+ galectin-1−/− mice, three Tg+ mice with galectin-1 deficiency were immunized with laminin in complete Freund's adjuvant in an attempt to induce an anti-laminin response. Seventeen days following immunization, little to no Tg anti-laminin autoreactivity was recovered from the serum of any of the three subjects. However, in contrast to results using B cells from unimmunized mice anti-laminin Tg Ig were detected in supernatants of mitogen-stimulated B cells from two of three immunized Tg+ galectin-1−/− mice (Table II).
Table II.
Anti-laminin Ig from LamH Ig Tg+ galectin-1−/− subjects with or without immunization
| Subject(s) | Serum (OD405) | B cell supernatant (OD405) |
|---|---|---|
| Laminin immunization | ||
| Mouse 1 | 0.000 | 0.000 |
| Mouse 2 | 0.002 | 0.249 |
| Mouse 3 | 0.008 | 0.048 |
| Unimmunized | ||
| Mean OD ± SD (n = 4–6) | 0.016 ± 0.019 | 0.019 ± 0.011 |
Shown is OD405 for Ig binding on laminin-coated wells minus binding on diluent-coated wells. Sera were assayed at 1:20 dilution, supernatants from LPS-stimulated B cells were assayed undiluted for n = 3 LamH Ig Tg+ galectin-1−/− galectin-3+/− mice. Summary values for unimmunized LamH Ig Tg+ Gal-1−/− mice are shown for reference.
Surface marker expression
A number of B cell surface markers that were previously determined to have altered expression on LamH Ig Tg+ anergic B cells relative to their naïve B cells counterparts were examined. These differences included increased surface expression of CD86, decreased percentages of CD62L- and MHCII-positive cells and decreased total surface Ig density on LamH Ig Tg+ B cells (Brady et al. 2004). In this study, the expression of multiple markers was altered on B cells of LamH Ig Tg+ mice with deficiency of either galectin-1 or galectin-3 in a pattern similar to that reported previously (Table I), suggesting that the anergic phenotype was intact. This includes a trend toward decreased total surface Ig in LamH Ig Tg+ galectin-1−/− relative to non-Tg galectin-1−/− mice (P = 0.053).
To further assess the impact of galectin deficiency on surface markers, LamH Ig Tg+ B cells from galectin-deficient and galectin+ mice were compared. For the most part, these groups were similar in surface marker expression (Table I). However, two differences were noted: LamH Ig Tg+ galectin-1−/− B cells are unique in that they do not show the increase in the surface density of CD86 that is observed on anergic LamH Ig Tg+ B cells from mice with an intact galectin-1 gene (Figure 5). Additionally, LamH Ig Tg+ galectin-3−/− mice have a higher percentage of CD62L+ B cells than LamH Ig Tg+ galectin+ controls (Table I).
Fig. 5.
B cell surface expression of CD86. (A) Expression is shown as the mean ± SD for normalized mean fluorescence intensity as determined by flow cytometry. Raw CD86 MFI data for each subject were normalized to the MFI of non-Tg galectin+ subjects from the same experiment to account for cross-experimental differences in staining intensity. The value for the non-Tg galectin+ subject (or the mean in cases where multiple non-Tg galectin+ subjects were used) was set to 1.0. *P < 0.05. (B) Representative histogram of CD86 MFI for B220+ gated B cells. Shown are LamH Ig Tg+ galectin+ (thin line) relative to LamH Ig Tg+ galectin-1−/− (thick line).
Effect of galectin-1 or galectin-3 knockout on B cells from non-Tg B6 mice
Overall, the examination of non-Tg mice with a broad polyclonal B cell repertoire revealed that relatively few variables measured in this study were altered in mice with the knockout of galectin-1 or galectin-3 compared with galectin-sufficient mice (Table I). There were no significant differences in the total splenocyte count, B cell surface Ig expression or serum total IgM levels (data not shown). Table I shows the few surface markers for which differential expression was noted: spleen B cell CD62L and MHCII surface density were significantly increased with the knockout of galectin-1 in non-Tg mice. Additionally, the surface density of CD86 was significantly decreased in non-Tg galectin-3−/− mice relative to non-Tg galectin-3+ (mean normalized mean fluorescence intensity (MFI) of 0.73 ± 0.24, n = 3, vs 0.99 ± 0.14, n = 13, respectively, P < 0.05).
Phenotype of LamH Ig Tg+ galectin-1−/− galectin-3−/− double-knockout mice
Functional redundancy among galectin family members is common. To elucidate phenotypic changes that might be exacerbated by loss of both galectin-1 and galectin-3, the effect of galectin-1−/− galectin-3−/− double knockout on the in vivo fate of LamH Ig Tg+ B cells was examined. The total splenic B cell count of LamH Ig Tg+ galectin-1−/− galectin-3−/− double-knockout subjects was significantly higher than concurrently assayed Tg+ galectin-3−/− single-knockout subjects (Table III). Normalized CD86 MFI levels did not differ significantly between groups. The Tg+ galectin-1−/− galectin-3−/− double-knockout mice also had no detectable spontaneous serum anti-laminin autoantibodies. No other significant differences were noted between these groups in the measured B cell parameters, though it is interesting that double-knockout LamH Ig Tg+ B cells appear to hyperproliferate to B cell receptor cross-linking (mean % divided, 70.4 ± 6.9 vs 42.6 ± 8.8 in LamH Ig Tg+ galectin+ mice, n = 2 per group).
Table III.
Phenotype of LamH Ig Tg+ galectin-1−/− galectin-3−/− double-knockout micea
| LamH Ig Tg+ |
||
|---|---|---|
| Galectin-1+/− Galectin-3−/− | Galectin-1−/− Galectin-3−/− | |
| B cell count (millions) | 3.5 ± 1.3 (5) | 6.5 ± 3.3* (5) |
| %IgMa of B220 | 71.6 ± 2.4 (5) | 73.1 ± 7.4 (5) |
| %IgMb of B220 | 28.2 ± 22.5 (5) | 17.9 ± 12.9 (5) |
| Surface Ig MFIb | 0.59 ± 0.05 (3) | 0.77 ± 0.16 (4) |
| CD86 MFIb | 1.2 ± 0.2 (3) | 1.1 ± 0.1 (4) |
| Serum autoAb | 0.0 ± 0.0 (4) | 0.0 ± 0.0 (4) |
aShown are the mean ± SD, with the number of subjects in parenthesis.
bMFI=mean fluorescence intensity, normalized per experiment to the non-Tg galectin+ subject(s). All MFI data are lymphocyte gated by forward and side light scatter properties and B cell gated by the B220 marker.
*P < 0.05 vs galectin-1+/− galectin-3−/− group.
Discussion
We have shown that the global knockout of galectin-1 or galectin-3 yields subtle alterations in the B cell fate in an anti-laminin autoantibody Tg model. In LamH Ig Tg+ mice, galectin-3 deficiency significantly increases the total number of spleen Tg B cells and supports the secretion of anti-laminin Ig, either spontaneously or after endotoxin stimulation, whereas galectin-1 deficiency enhances B cell proliferation in response to a Toll-like receptor (TLR)4 ligand. These findings add to the growing body of evidence, indicating a role for the various galectin family members, and for galectin-1 and galectin-3 in particular, in the regulation of autoimmunity (reviewed in van Kooyk and Rabinovich 2008; Rabinovich and Toscano 2009; Rabinovich et al. 2012).
Systemic galectin-3 deficiency was previously shown to lessen clinical manifestations of experimental autoimmune encephalomyelitis, antigen-induced arthritis and concanavalin A-induced hepatitis relative to disease in galectin-3 sufficient controls (Jiang et al. 2009; Forsman et al. 2011; Volarevic et al. 2012). In these models, galectin-3 promotes the activation of T cells and the maturation of dendritic cells and alters the inflammatory cytokine profile. Conversely, exogenous galectin-1 administered parenterally or via genetically engineered fibroblasts ameliorates disease in several models of induced autoimmunity and in spontaneous BWF1 murine lupus (Rabinovich et al. 1999; Santucci et al. 2000, 2003; Baum et al. 2003; Liu et al. 2011). The therapeutic efficacy of galectin-1 has been attributed at least in part to the induction of apoptosis in activated T cells and altered cytokine secretion. In addition to their effects on cellular immunity, galectin-1 and galectin-3 have been shown to modulate development, localization and differentiation of B cells (Acosta-Rodriguez et al. 2004; Clark et al. 2007; Oliveira et al. 2009, 2011; Rabinovich and Toscano 2009; Tabrizi et al. 2009; Mourcin et al. 2011; Tsai et al. 2011); however, their involvement in the regulation of humoral autoimmunity and the maintenance of B cell tolerance has not been previously well characterized.
Our results indicate that galectin-3 and galectin-1 both play a role in promoting B cell tolerance, a finding previously suggested by the discovery of their up-regulation in anergic B cells (Clark et al. 2007; Charles et al. 2011). In our model system, galectin-3 contributes to the control of autoreactive B cell numbers and functional inactivation, such that its deficiency can lead to overt loss of tolerance to laminin. Knockout of galectin-3 in LamH Ig Tg+ mice was associated with the presence of anti-laminin autoantibodies in the serum of one mouse and in the supernatants of mitogen-stimulated B cells from 50% of mice. In contrast, anti-laminin Ig rarely appear in the serum or B cell culture supernatants in galectin-3-sufficient B6 LamH Ig Tg+ mice (herein and Rudolph et al. 2002). In the context of the concurrent significantly increased number of peripheral B cells observed in galectin-3-deficient Tg mice, one possible explanation is defective deletion of laminin-reactive B cells. Such a defect could occur due to a requirement for galectin-3 for the induction of apoptosis in developing autoreactive B cells in the bone marrow, a compartment in which galectin-3 is known to be expressed (Oliveira et al. 2007). While galectin-3 is generally considered to be anti-apoptotic (Hernandez and Baum 2002; Hoyer et al. 2004; Liu and Rabinovich 2005), it has been shown to induce apoptosis in activated T cells (Stillman et al. 2006; Stowell et al. 2008); its expression and role in apoptosis in developing B cells has not been well characterized. Alternatively, galectin-3 could facilitate B cell receptor signaling after the engagement of self-antigen by developing B cells, such that a signal threshold that triggers deletion is surpassed. Support for this mechanism is garnered by our finding that addition of galectin-3 increased, rather than inhibited, binding of anti-laminin autoantibodies to self-antigen. In this scenario, galectin-3 deficiency would permit the survival of autoreactive cells that would otherwise not escape the bone marrow. If fewer autoreactive B cells are deleted during development, a heavier burden is placed on editing or maintenance of anergy to prevent autoimmunity. Anergy can be circumvented in some conditions of B cell culture (Hartley et al. 1993), which could explain the increased rate of recovery of laminin autoantibodies in galectin-3−/− B cell supernatants.
It is possible that the effect of galectin-3 deficiency to boost Tg B cell numbers is manifest primarily in the periphery. Potential checkpoints for galectin-3 control of autoreactive B cells include shortening the life span of anergic B cells, excluding autoreactive cells from follicles or limiting homeostatic proliferation or expansion of activated B cells, such that galectin-3 deficiency permits autoreactive cells to accumulate. These possibilities seem at odds with reports of a role of galectin-3 in mediating IL-4 induced B cell survival and differentiation (Acosta-Rodriguez et al. 2004), though galectin-3 functions likely vary with cell subset or differentiation state as well as galectin cellular localization. We found that exogenous galectin-3 did not alter survival of Tg+ galectin-3-deficient B cells in culture. Other potential mechanisms for the galectin-3 modulation of B cell numbers are suggested by observations in T cells. Galectin-3:glycan lattices limit T cell receptor (TCR) clustering, decrease lateral movement across the cell membrane and increase agonist thresholds for TCR signaling (Demetriou et al. 2001). One predicts that galectin-3 deficiency could thus lower activation thresholds in differentiated cells and permit cell expansion. Galectin-3 lattices are also implicated in maintaining murine cytolytic T cell anergy; lattices trap T cell receptors and prevent their colocalization with CD8, whereas galectin removal by competitive binding restores T cell function (Demotte et al. 2008). Future studies can dissect if analogous mechanisms are operative in B cells.
Tg B cells from galectin-1-deficient mice had a distinct tolerance phenotype. Neither serum nor LPS-stimulated B cells of unimmunized LamH Ig Tg+ galectin-1−/− mice yielded anti-laminin Ig, similar to previous results in LamH Ig Tg+ B6 galectin-sufficient mice (Rudolph et al. 2002). However, residual B cells from LamH Ig Tg+ galectin-1−/− mice hyperproliferate in response to LPS, suggesting a role for galectin-1 in dampening B cell TLR4 signaling or responses. This would expand the role for galectin-1 in downmodulating LPS-induced effects in immune cells, as galectin-1 has also been shown to reduce macrophage and dendritic cell responses to endotoxin (Rabinovich et al. 2000; Santucci et al. 2000; Kuo et al. 2011). Galectin-1 control of TLR-induced proliferative responses in B cells may require intracellular actions of galectin-1, as in our system supplementation of exogenous galectin-1 did not alter the proliferation of galectin-deficient cells in culture. B cell receptor (BCR)-stimulated proliferation was not altered in our galectin-1-deficient Tg B cells, suggesting a complex role in antigen receptor signaling, as exogenous galectin-1 was previously shown to decrease mouse primary B cell proliferation in response to anti-IgM-mediated BCR cross-linking (Yu et al. 2006).
It is of note that Tg+ anti-laminin autoantibodies were recovered in culture supernatants of LPS-stimulated B cells derived from LamH Ig Tg+ galectin-1−/− mice that had previously been immunized with laminin in Complete Freund's adjuvant. This finding suggests that some anti-laminin B cells do escape deletion in vivo and can be activated in galectin-1−/− mice under certain conditions, possibly from environmental toxins or microbes. A role for key mechanisms under the redundant control of galectin-1 and galectin-3 cannot be excluded by our data. B cell hyperproliferation to endotoxin in the setting of galectin-1 deficiency could exacerbate autoreactivity in this setting.
Residual spleen B cells in LamH Ig Tg+ galectin-1−/− mice are unique compared with their Tg+ counterparts in galectin-sufficient and galectin-3-deficient mice in that they do not express increased surface CD86 (B7.2). Up-regulation of this costimulatory molecule is typically observed in antigen experienced B cells, as a consequence of B cell receptor cross-linking during either cell activation (Lenschow et al. 1994) or anergy induction in some settings (Brady et al. 2004; Terrier et al. 2011). Its normalization in the galectin-1-deficient LamH Ig Tg+ B cells suggests either lack of prior antigen contact, an unlikely circumstance given the evidence of concurrent marked cell deletion, a shift in the residual B cell repertoire to non-autoreactivity, presumably via receptor editing or dependence on galectin-1 in the signaling networks responsible for CD86 up-regulation. Some support for the latter possibility comes from studies of galectin-1-induced maturation of, and CD86 up-regulation on, human monocyte-derived dendritic cells (Fulcher et al. 2006). CD86 on activated B cells promotes T cell activation via interactions with CD28/CD152 (CTLA-4) (Damle et al. 1992; Freeman et al. 1993), germinal center formation and memory B cell development (Borriello et al. 1997) and transgene-mediated restoration of CD86 expression on anergic B cells with basal down-regulated expression promotes B cell clonal expansion and differentiation (Rathmell et al. 1998). Counteracting functions are possible, however, as CD86 expressed on dendritic cells is also required to maintain regulatory T cells (Ellestad et al. 2009). It is tempting to speculate that reduced CD86 on the galectin-1-deficient Tg B cells provides some protection against autoimmunity; future experiments can determine if this is sustained after antigen engagement analogous to the persistent decrease in B cell CD86 expression associated with the durable inhibition of antibody production in oral tolerance to ovalbumin (Futata et al. 2006).
It is unclear if the observed effects of galectin-1 or galectin-3 deficiency on the B cell fate are due to alterations in B cell intrinsic galectin expression, and if so, the subcellular location of action, or if the effects are due to alterations in galectin expression in other cell types or in certain body compartments. We did not observe significant effects on B cell survival or proliferation ex vivo by the supplementation of culture medium with exogenous galectin-1 or galectin-3, respectively. Also unresolved is whether our findings are due to direct effects of the galectins on B cell function or are mediated indirectly by galectin actions on other cell types, such as T cells, dendritic cells, monocytes or tissue parenchymal cells. Further studies, including the use of the cell-targeted deletion of galectin-1 or galectin-3, can address these possibilities.
It is of note that in non-Tg B6 mice there are few differences in measured variables between galectin-deficient and galectin-sufficient control mouse splenic B cells. Our findings are consistent with earlier reports showing lack of overt B cell phenotypes in galectin-1 (Espeli et al. 2009) or galectin-3 (Colnot et al. 1998; Hsu et al. 2000) knockout mice, a feature attributed at least in part to redundancy within the galectin family in both ligands and function. In this regard, redundancy has been shown between galectin-1 and galectin-8 roles in B cell differentiation into plasma cells (Tsai et al. 2011), galectin-1 and galectin-3 have been shown to have overlapping functions in mRNA splicing (Patterson et al. 2004), and both galectin-1 and galectin-3 are capable of inducing T cell apoptosis (Stillman et al. 2006). Study of B cells from autoantibody Tg mice genetically lacking both galectin-1 and galectin-3 revealed a synergistic effect on the control of spleen autoreactive B cell numbers. Whether this reflects redundant effects on the control of B cell deletion or apoptosis revealed only in the dual knockout or reflects additive independent effects on deletion and proliferation will require further investigation.
We also observed an increase in the B cell surface expression of MHCII and CD62L (L-selectin) in non-Tg galectin-1 knockout mice, compared with their non-Tg galectin-sufficient counterparts. MHCII and CD62L are constitutively expressed on naïve B cells; following antigen activation, surface MHCII is normally up-regulated to facilitate antigen presentation to T cells, whereas surface CD62L decreases to modulate B cell adhesion and trafficking (Reichert et al. 1983). Their increased constitutive expression on resting B cells could modify initiation of adaptive immune responses. There is precedence for an influence of galectin-1 on the biology of both molecules. Exogenous galectin-1 selectively inhibits constitutive and interferon-induced MHCII expression on human monocytes and mouse inflammatory macrophages, resulting in decreased ability to stimulate T cells (Barrionuevo et al. 2007). Conversely, galectin-1 exposure up-regulates Human leukocyte antigen DR on immature human monocyte-derived dendritic cells (Fulcher et al. 2006), suggesting that MHCII modulation by galectin-1 is developmental or differentiation stage-dependent. Inflammatory macrophages from galectin-1−/− mice express increased surface MHCII, an effect that has functional consequences and is largely reversible with addition of recombinant galectin-1 (Barrionuevo et al. 2007). Galectin-1 colocalizes with CD62L in mouse inflammatory neutrophils, and galectin-1 exposure modulates mouse neutrophil CD62L expression (Gil et al. 2011). Future investigation of the role of increased B cell MHCII and CD62L in galectin-1 deficiency could provide information about their physiological significance.
Materials and methods
Animals
All studies and procedures were approved by the Duke University and the Durham Veterans Affairs Medical Center Animal Care and use Committees. Cloning of the anti-laminin LamH Cµ heavy chain construct and characterization of LamH Tg mice is described in Foster and Fitzsimons (1998), Rudolph et al. (2002) and Brady et al. (2004). Galectin-1 knockout (−/−) mice and galectin-3−/− mice were generously provided via the Consortium for Functional Glycomics. Description of the knockouts and initial phenotyping are described in Poirier and Robertson (1993) for galectin-1−/− and Hsu et al. (2000) for galectin-3−/−.
LamH Tg mice, previously backcrossed 12–14 generations onto the C57BL/6J background (Jackson Labs, Bar Harbor, ME), were bred with galectin-1−/− or galectin-3−/− breeders and resulting progeny were interbred to generate the experimental genotypes. There were 18 different possible genotypes: LamH Ig Tg (+ or -), galectin-1 (+/+, +/− or −/− knockout) and galectin-3 (+/+, +/− or −/− knockout). Data presented herein are limited to the experimental genotypes LamH Tg+ galectin-1+/+ galectin-3−/− (“galectin-3−/−”) and Tg+ galectin-1−/− galectin-3+/+ (“galectin-1−/−”) and their non-Tg counterparts, unless otherwise noted in the text. For control (galectin-sufficient) groups, statistical analysis showed no difference across galectin +/− and galectin +/+ groups in the variables reported, with one exception, and these genotypes are pooled to generate the “galectin+” group. One variable, %lambda+ of B220+, showed a heterozygote effect in the control group, so only galectin-1+/+ galectin-3+/+ subjects were included in presentation of % lambda+ data.
Subjects were genotyped by polymerase chain reaction using the following primers.
LamH Tg:
5′-AGC TGC AAC AGT CTG GAC CTG A-3′ and
5′-GGC CCC AGT AGT CAA AGG TAC CAT C-3′
Galectin-1−/−:
5′-GAC CCC ATC CCT ACA CCC CAG-3′
5′-AAA CTT CAG CCG GGA GAA AGG-3′
5′-CTA TCA GGA CAT AGC GTT GG-3′
Galectin-3−/−:
5′-GTA GGT GAG AGT CAC AAG CTG GAG GCC-3′
5′-CAC TCT CAA AGG GGA AGG CTG ACT GTC-3′
5′-GGC TGA CCG CTT CCT CGT GCT TTA CGG-3′
Cell staining
Single-cell splenocyte and bone marrow suspensions were stained for flow cytometry as described (Clark et al. 2011) using the following fluorescent stains: anti-CD45R/B220-PE and -PerCP, anti-IgD-fluorescein isothiocyanate (FITC), anti-IgMb-FTC, anti-IgMa-PE and anti-CD86-RPE (Becton Dickinson-Pharmingen, San Jose, CA) and anti-IgM-FTC, anti-lambda-FTC, anti-kappa-PE, anti-CD62L-FTC, anti-CD19-FITC and anti-MHCII-FTC (Southern Biotech, Birmingham, Alabama). Cells were analyzed on a FACSCalibur (Becton Dickinson), and results analyzed using FlowJo (Treestar, Ashland, OR). Lymphocytes were gated based on forward scatter and side scatter properties. B cell counts were determined as %B220+ × % lymphocytes × number of splenocytes.
Ig concentration and laminin-binding enzyme-linked immunosorbent assay (ELISA)
Quantitation of LamH Tg IgM-a in serum was as described (Clark et al. 2011). To assay for laminin-binding activity, Immulon II plates were coated overnight at 4°C with 10 µg/mL of laminin Engelbreth-Holm-Swarm (Sigma, St. Louis, MO) in phosphate buffered saline (PBS). Plates were blocked, rinsed, incubated with mouse sera (diluted 1:20) or B cell supernatants (undiluted) and detected using the alkaline phosphatase-conjugated anti-IgM antibody (Southern Biotech). Control Ig is anti-laminin mAb A10C supernatant (Foster and Fitzsimons 1998) and H50-9 antibody (Foster et al. 1993), which is detected with a goat–anti-mouse IgG alkaline phosphatase conjugated antibody. Results were recorded as the mean sample binding OD to laminin after the subtraction of mean OD on diluent (PBS)-coated plates. In cases where binding to laminin was lower than binding to diluent-coated plates, the reported laminin-binding value was zero.
For laminin-binding assays in the presence of exogenous galectin-3, the concentration of H50-9 and A10C control antibodies that generated half-maximal binding (300 ng/mL for H50-9, 1:2 dilution for A10C supernatant) was assayed for laminin binding in the presence of 0, 222 and 667 ng/mL of recombinant mouse galectin-3 (R&D Systems, Minneapolis, MN).
In vitro proliferation and differentiation assays
B cells were purified from single-cell RBC-lysed splenocyte suspensions using CD43 microbead depletion of non-B cells (Miltenyi Biotec, Auburn, CA). Purified B cells were plated in proliferation media (Roswell Park Memorial Institute supplemented with 10% fetal bovine serum, 2 mM l-glutamine, beta-mercaptoethanol and 100 U/mL penicillin–streptomycin), with and without 50 μg/mL of LPS stimulation (#L6386, Sigma) at 1 × 106 cells/mL in 1 mL/well of a 24-well plate. Mature supernatants harvested after 8–10 days of culture were assayed, undiluted, for laminin binding by ELISA.
For cell proliferation assays, purified B cells were labeled with 2.5–5.0 µM CFSE (Molecular Probes, Eugene, OR) as per the manufacturer's protocol. Labeled cells were cultured at 1.25 × 106 cells/200 μL per well, in proliferation media as defined above, in a 96-well plate ±50 μg/mL of LPS (#L6386, Sigma), 80 µg/mL of goat–anti-mouse IgM F(ab')2 (Pierce/Thermo Scientific, Rockford, IL) or LPS as above plus 10 mM lactose (Sigma) or 10 µg/mL recombinant mouse galectin-1 (R&D Systems). Following 2.5 days in culture, cells were collected and stained with B220-PE and assessed by flow cytometry as detailed above. The percent of divided cells was calculated for the B220+ population using the FlowJo proliferation platform.
Viability assay
B cells were purified by negative selection and cultured overnight in proliferation medium with and without 50 µg/mL of LPS and/or 10 µg/mL of recombinant mouse galectin-3 (R&D Systems), at a concentration of 1 × 106 cells/mL in 200 µL/well of a 96-well plate. Following 24 h incubation, cells were assayed for apoptosis/death using Annexin V-FITC and 7-Aminoactinomycin D (AAD) (BD Pharmingen, San Jose, CA) as per the manufacturer's protocol. The percentage of live cells is determined as cells negative for both Annexin V-FITC and 7-AAD.
Immunization
Three LamH Ig Tg+ galectin-1−/− galectin-3+/− mice were immunized subcutaneously with 50 µg laminin EHS (Sigma) in Complete Freund's Adjuvant.
Statistical analysis
Statistical analysis was performed using JMP software (SAS Institute, Cary, NC). Data are shown as mean values ± SD. Comparisons between two groups were performed using the non-parametric Wilcoxon test. A P-value of <0.05 is considered significant.
Funding
This work was supported by Award Number R01DK047424 from the National Institute of Diabetes and Digestive and Kidney Diseases (MHF) and the DVAMC Research Service. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Diabetes and Digestive and Kidney Diseases or the National Institutes of Health.
Conflict of interest
None declared.
Abbreviations
B6, C57BL/6J mouse strain; CFSE, carboxyfluorescein diacetate succinimidyl ester; Ig, immunoglobulin; LPS, lipopolysaccharide; OD, optical density at 405 nm; Tg, transgenic.
Acknowledgements
The authors wish to acknowledge the NIH-sponsored Mutant Mouse Regional Resource Center (MMRRC) National System as the source of genetically altered galectin-1- and galectin-3-deficient mice for use in this study. The mice were produced and deposited to the MMRRC by the Consortium for Functional Glycomics supported by the National Institute of General Medical Sciences (GM62116). We thank the Duke Cancer Institute Flow Cytometry Shared Resource and DNA Analysis/Automated Sequencing & Phosphorimaging Shared Resource.
References
- Acosta-Rodriguez EV, Montes CL, Motran CC, Zuniga EI, Liu FT, Rabinovich GA, Gruppi A. Galectin-3 mediates IL-4-induced survival and differentiation of B cells: Functional cross-talk and implications during Trypanosoma cruzi infection. J Immunol. 2004;172:493–502. doi: 10.4049/jimmunol.172.1.493. [DOI] [PubMed] [Google Scholar]
- Amital H, Heilweil-Harel M, Ulmansky R, Harlev M, Toubi E, Hershko A, Naparstek Y. Antibodies against the VRT101 laminin epitope correlate with human SLE disease activity and can be removed by extracorporeal immunoadsorption. Rheumatology (Oxford) 2007;46:1433–1437. doi: 10.1093/rheumatology/kem181. [DOI] [PubMed] [Google Scholar]
- Barboni EAM, Bawumia S, Hughes RC. Kinetic measurements of binding of galectin 3 to a laminin substratum. Glycoconj J. 1999;16:365–373. doi: 10.1023/a:1007004330048. [DOI] [PubMed] [Google Scholar]
- Barrionuevo P, Beigier-Bompadre M, Ilarregui JM, Toscano MA, Bianco GA, Isturiz MA, Rabinovich GA. A novel function for galectin-1 at the crossroad of innate and adaptive immunity: Galectin-1 regulates monocyte/macrophage physiology through a nonapoptotic ERK-dependent pathway. J Immunol. 2007;178:436–445. doi: 10.4049/jimmunol.178.1.436. [DOI] [PubMed] [Google Scholar]
- Baum LG, Blackall DP, Arias-Magallano S, Nanigian D, Uh SY, Browne JM, Hoffmann D, Emmanouilides CE, Territo MC, Baldwin GC. Amelioration of graft versus host disease by galectin-1. Clin Immunol. 2003;109:295–307. doi: 10.1016/j.clim.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Borriello F, Sethna MP, Boyd SD, Schweitzer AN, Tivol EA, Jacoby D, Strom TB, Simpson EM, Freeman GJ, Sharpe AH. B7–1 and B7–2 have overlapping, critical roles in immunoglobulin class switching and germinal center formation. Immunity. 1997;6:303. doi: 10.1016/s1074-7613(00)80333-7. [DOI] [PubMed] [Google Scholar]
- Brady GF, Congdon KL, Clark AG, Sackey FN, Rudolph EH, Radic MZ, Foster MH. Kappa editing rescues autoreactive B cells destined for deletion in mice transgenic for a dual specific anti-laminin Ig. J Immunol. 2004;172:5313–5321. doi: 10.4049/jimmunol.172.9.5313. [DOI] [PubMed] [Google Scholar]
- Charles ED, Brunetti C, Marukian S, Ritola KD, Talal AH, Marks K, Jacobson IM, Rice CM, Dustin LB. Clonal B cells in patients with hepatitis C virus-associated mixed cryoglobulinemia contain an expanded anergic CD21low B-cell subset. Blood. 2011;117:5425–5437. doi: 10.1182/blood-2010-10-312942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark AG, Chen S, Zhang H, Brady GF, Ungewitter EK, Bradley JK, Sackey FN, Foster MH. Multifunctional regulators of cell growth are differentially expressed in anergic murine B cells. Mol Immunol. 2007;44:1274–1285. doi: 10.1016/j.molimm.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Clark AG, Mackin KM, Foster MH. Genetic elimination of alpha3(IV) collagen fails to rescue anti-collagen B cells. Immunol Lett. 2011;141:134–139. doi: 10.1016/j.imlet.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colnot C, Fowlis D, Ripoche MA, Bouchaert I, Poirier F. Embryonic implantation in galectin 1/galectin 3 double mutant mice. Dev Dyn. 1998;211:306–313. doi: 10.1002/(SICI)1097-0177(199804)211:4<306::AID-AJA2>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Damle NK, Klussman K, Linsley PS, Aruffo A. Differential costimulatory effects of adhesion molecules B6, ICAM-1, LFA-3, and VCAM-1 on resting and antigen-primerd CD4+ T lymphocytes. J Immunol. 1992;148:1985–1992. [PubMed] [Google Scholar]
- Demetriou M, Granovsky M, Quaggin S, Dennis JW. Negative regulation of T-cell activation and autoimmunity by Mgat5 N-glycosylation. Nature. 2001;409:733–739. doi: 10.1038/35055582. [DOI] [PubMed] [Google Scholar]
- Demotte N, Stroobant V, Courtoy PJ, Van Der Smissen P, Colau D, Luescher IF, Hivroz C, Nicaise J, Squifflet J-L, Mourad M., et al. Restoring the association of the T cell receptor with CD8 reverses anergy in human tumor-infiltrating lymphocytes. Immunity. 2008;28:414–424. doi: 10.1016/j.immuni.2008.01.011. [DOI] [PubMed] [Google Scholar]
- Ellestad KK, Tsutsui S, Noorbakhsh F, Warren KG, Yong VW, Pittman QJ, Power C. Early life exposure to lipopolysaccharide suppresses experimental autoimmune encephalomyelitis by promoting tolerogenic dendritic cells and regulatory T cells. J Immunol. 2009;183:298–309. doi: 10.4049/jimmunol.0803576. [DOI] [PubMed] [Google Scholar]
- Espeli M, Mancini SJC, Breton C, Poirier F, Schiff C. Impaired B-cell development at the pre-BII-cell stage in galectin-1-deficient mice dur to inefficient pre-BII/stromal cell interactions. Blood. 2009;113:5878–5886. doi: 10.1182/blood-2009-01-198465. [DOI] [PubMed] [Google Scholar]
- Fitzsimons MM, Chen H, Foster MH. Diverse endogenous light chains contribute to basement membrane reactivity in nonautoimmune mice transgenic for an anti-laminin Ig heavy chain. Immunogenetics. 2000;51:20–29. doi: 10.1007/s002510050004. [DOI] [PubMed] [Google Scholar]
- Forsman H, Islander U, Andreasson E, Andersson A, Onnheim K, Karlstrom A, Savman K, Magnusson M, Brown KL, Karlsson A. Galectin 3 aggravates joint inflammation and destruction in antigen-induced arthritis. Arthritis Rheum. 2011;63:445–454. doi: 10.1002/art.30118. [DOI] [PubMed] [Google Scholar]
- Foster MH, Fitzsimons MM. Lupus-like nephrotropic autoantibodies in nonautoimmune mice harboring an anti-laminin Ig heavy chain transgene. Mol Immunol. 1998;35:83–94. doi: 10.1016/s0161-5890(98)00018-2. [DOI] [PubMed] [Google Scholar]
- Foster MH, Sabbaga J, Line SRP, Thompson KS, Barrett KJ, Madaio MP. Molecular analysis of nephrotropic anti-laminin antibodies from an MRL/lpr autoimmune mouse. J Immunol. 1993;151:814–824. [PubMed] [Google Scholar]
- Freeman GJ, Borriello F, Hodes RJ, Reiser H, Gribben JG, Ng JW, Kim J, Goldberg JM, Hathcock K, Laszlo G., et al. Murine B7–2, an alternative CTLA4 counter-receptor that costimulates T cell proliferation and interleukin-2 production. J Exp Med. 1993;178:2185. doi: 10.1084/jem.178.6.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulcher JA, Hashimi ST, Levroney EL, Pang M, Gurney KB, Baum LG, Lee B. Galectin-1-matured human monocyte-derived dendritic cells have enhanced migration through extracellular matrix. J Immunol. 2006;177:216–226. doi: 10.4049/jimmunol.177.1.216. [DOI] [PubMed] [Google Scholar]
- Futata EA, de Brito CA, Victor JR, Fusar AE, Oliveira CR, Maciel J. M., da Silva Duarte AJ, Sato MN. Long-term anergy in orally tolerized mice is linked to decreased B7.2 expression on B cells. Immunobiology. 2006;211:157–166. doi: 10.1016/j.imbio.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Gil CD, Gullo CE, Oliani SM. Effect of exogenous galectin-1 on leukocyte migration: Modulation of cytokine levels and adhesion molecules. Int J Clin Exp Pathol. 2011;4:74–84. [PMC free article] [PubMed] [Google Scholar]
- Hanrotel-Saliou C, Segalen I, Le Meur Y, Youinou P, Renaudineau Y. Glomerular antibodies in lupus nephritis. Clinic Rev Allerg Immunol. 2011;40:151–158. doi: 10.1007/s12016-010-8204-4. [DOI] [PubMed] [Google Scholar]
- Hartley S, Cooke M, Fulcher D, Harris A, Cory S, Basten A, Goodnow C. Elimination of self-reactive B lymphocytes proceeds in two stages: Arrested development and cell death. Cell. 1993;72:325–335. doi: 10.1016/0092-8674(93)90111-3. [DOI] [PubMed] [Google Scholar]
- Hernandez JD, Baum LG. Ah, sweet mystery of death! Galectins and control of cell fate. Glycobiology. 2002;12:127R–136R. doi: 10.1093/glycob/cwf081. [DOI] [PubMed] [Google Scholar]
- Hoyer KK, Pang M, Gui D, Shintaku IP, Kuwabara I, Liu FT, Said JW, Baum LG, Teitell MA. An anti-apoptotic role for galectin-3 in diffuse large B-cell lymphomas. Am J Pathol. 2004;164:893–902. doi: 10.1016/S0002-9440(10)63177-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu DK, Yang RY, Pan Z, Yu L, Salomon DR, Fung-Leung WP, Liu FT. Targeted disruption of the galectin-3 gene results in attenuated peritoneal inflammatory responses. Am J Pathol. 2000;156:1073–1083. doi: 10.1016/S0002-9440(10)64975-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H-R, Rasebi ZA, Mensah-Brown E, Shahin A, Xu D, Goodyear CS, Fukada SY, Liu F-T, Liew FY, Lukic ML. Galectin-3 deficiency reduces the severity of experimental autoimmune encephalomyelitis. J Immunol. 2009;182:1167–1173. doi: 10.4049/jimmunol.182.2.1167. [DOI] [PubMed] [Google Scholar]
- Kang EH, Moon KC, Lee EY, Lee YJ, Lee EB, Ahn C, Song YW. Renal expression of galectin-3 in systemic lupus erythematosus patients with nephritis. Lupus. 2009;18:22–28. doi: 10.1177/0961203308094361. [DOI] [PubMed] [Google Scholar]
- Kuo P-L, Hung J-Y, Huang S-K, Chou S-H, Cheng D-E, Jong Y-J, Hung C-H, Yang C-J, Tsai Y-M, Hsu Y-L., et al. Lung cancer-derived galectin-1 mediates dendritic cell anergy through inhibitor of DNA binding 3/IL-10 signaling pathway. J Immunol. 2011;186:1521–1530. doi: 10.4049/jimmunol.1002940. [DOI] [PubMed] [Google Scholar]
- Lenschow D, Sperling A, Cooke M, Freeman G, Rhee L, Decker D, Gray G, Nadler L, Goodnow C, Bluestone J. Differential up-regulation of the B7–1 and B7–2 costimulatory molecules after Ig receptor engagement by antigen. J Immunol. 1994;153:1990–1997. [PubMed] [Google Scholar]
- Liu SD, Lee S, La Cava A, Motran CC, Hahn BH, Miceli MC. Galectin-1-induced down-regulation of T lymphocyte activation protects (NZB x NZW) F1 mice from lupus-like disease. Lupus. 2011;20:473–484. doi: 10.1177/0961203310388444. [DOI] [PubMed] [Google Scholar]
- Liu FT, Rabinovich GA. Galectins as modulators of tumour progression. Nat Rev Cancer. 2005;5:29–41. doi: 10.1038/nrc1527. [DOI] [PubMed] [Google Scholar]
- Mourcin F, Breton C, Tellier J, Narang P, Chasson L, Jorquera A, Coles M, Schiff C, Mancini SJC. Galectin-1-expressing stromal cells constitute a specific niche for pre-BII cell development in mouse bone marrow. Blood. 2011;117:6552–6561. doi: 10.1182/blood-2010-12-323113. [DOI] [PubMed] [Google Scholar]
- Oliveira FL, Brand C, Paula AA, Arcanjo KD, Hsu DK, Liu F-T, Takiya CM, Borojevic R, Chammas R, El-Cheikh MC. Lack of galectin-3 disturbs mesenteric lymph node homeostasis and B cell niches in the course of Schistosoma mansoni infection. PLoS One. 2011;6:e19216. doi: 10.1371/journal.pone.0019216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira FL, Chammas R, Hsu DK, Liu FT, Borojevic R, Takiya CM, El-Cheikh MC. Kinetics of mobilization and differentiation of lymphohematopoietic cells during experimental murine schistosomiasis in galectin-3−/− mice. J Leukocyte Biol. 2007;82:300–310. doi: 10.1189/jlb.1206747. [DOI] [PubMed] [Google Scholar]
- Oliveira FL, Chammas R, Ricon L, Fermino ML, Bernardes ES, Hsu DK, Liu F-T, Borojevic R, El-Cheikh MC. Galectin-3 regulates peritoneal B1-cell differentiation into plasma cells. Glycobiology. 2009;19:1248–1258. doi: 10.1093/glycob/cwp120. [DOI] [PubMed] [Google Scholar]
- Patterson RJ, Wang W, Wang JL. Understanding the biochemical activities of galectin-1 and galectin-3 in the nucleus. Glycoconj J. 2004;19:499–506. doi: 10.1023/B:GLYC.0000014079.87862.c7. [DOI] [PubMed] [Google Scholar]
- Poirier F, Robertson EJ. Normal development of mice carrying a null mutation in the gene encoding the L14 S-type lectin. Development. 1993;119:1229–1236. doi: 10.1242/dev.119.4.1229. [DOI] [PubMed] [Google Scholar]
- Rabinovich GA, Daly G, Dreja H, Tailor H, Riera CM, Hirabayashi J, Chernajovsky Y. Recombinant galectin-1 and its genetic delivery suppress collagen-induced arthritis via T cell apoptosis. J Exp Med. 1999;190:385–398. doi: 10.1084/jem.190.3.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovich GA, Sotomayor CE, Riera CM, Bianco I, Correa SG. Evidence of a role for galectin-1 in acute inflammation. Eur J Immunol. 2000;30:1331–1339. doi: 10.1002/(SICI)1521-4141(200005)30:5<1331::AID-IMMU1331>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Rabinovich GA, Toscano MA. Turning ‘sweet’ on immunity: Galectin-glycan interactions in immune tolerance and inflammation. Nat Rev Immunol. 2009;9:338–352. doi: 10.1038/nri2536. [DOI] [PubMed] [Google Scholar]
- Rabinovich GA, van Kooyk Y, Cobb BA. Glycobiology of immune responses. Ann N Y Acad Sci. 2012;1253:1–15. doi: 10.1111/j.1749-6632.2012.06492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathmell J, Fournier S, Weintraub B, Allison J, Goodnow CC. Repression of B7.2 on self-reactive B cells is essential to prevent proliferation and allow Fas-mediated deletion by CD4(+) T cells. J Exp Med. 1998;188:651–659. doi: 10.1084/jem.188.4.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichert RA, Gallatin WM, Weissman IL, Butcher EC. Germinal center B cells lack homing receptors necessary for normal lymphocyte recirculation. J Exp Med. 1983;157:813–827. doi: 10.1084/jem.157.3.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph EH, Congdon KL, Sackey FN, Fitzsimons MM, Foster MH. Humoral autoimmunity to basement membrane antigens is regulated in C57BL/6 and MRL/MpJ mice transgenic for anti-laminin Ig receptors. J Immunol. 2002;168:5943–5953. doi: 10.4049/jimmunol.168.11.5943. [DOI] [PubMed] [Google Scholar]
- Santucci L, Fiorucci S, Cammilleri F, Servillo G, Federici B, Morelli A. Galectin-1 exerts immunomodulatory and protective effects on concanavalin A-induced hepatitis in mice. Hepatology. 2000;31:399–406. doi: 10.1002/hep.510310220. [DOI] [PubMed] [Google Scholar]
- Santucci L, Fiorucci S, Rubinstein N, Mencarelli A, Palazzetti B, Federici B, Rabinovich GA, Morelli A. Galectin-1 suppresses experimental colitis in mice. Gastroenterology. 2003;124:1381–1394. doi: 10.1016/s0016-5085(03)00267-1. [DOI] [PubMed] [Google Scholar]
- Stillman BN, Hsu DK, Pang M, Brewer CF, Johnson P, Liu FT, Baum LG. Galectin-3 and galectin-1 bind distinct cell surface glycoprotein receptors to induce T cell death. J Immunol. 2006;176:778–789. doi: 10.4049/jimmunol.176.2.778. [DOI] [PubMed] [Google Scholar]
- Stowell SR, Qian Y, Karmakar S, Koyama NS, Dias-Baruffi M, Leffler H, McEver RP, Cummings RD. Differential roles of galectin-1 and galectin-3 in regulating leukocyte viability and cytokine secretion. J Immunol. 2008;180:3091–3102. doi: 10.4049/jimmunol.180.5.3091. [DOI] [PubMed] [Google Scholar]
- Tabrizi SJ, Niiro H, Masui M, Yoshimoto G, Iino T, Kikushige Y, Wakasaki T, Baba E, Shimoda S, Miyamoto T., et al. T cell leukemia/lymphoma 1 and galectin-1 regulate survival/cell death pathways in human naive and IgM+ memory B cells through altering balances in Bcl-2 family proteins. J Immunol. 2009;182:1490–1499. doi: 10.4049/jimmunol.182.3.1490. [DOI] [PubMed] [Google Scholar]
- Terrier B, Joly F, Vazquez T, Benech P, Rosenzwajg M, Carpentier W, Garrido M, Ghillani-Dalbin P, Klatzmann D, Cacoub P., et al. Expansion of functionally anergic CD21-/low marginal zone-like B cell clones in hepatitis C virus infection-related autoimmunity. J Immunol. 2011;187:6550–6563. doi: 10.4049/jimmunol.1102022. [DOI] [PubMed] [Google Scholar]
- Tsai C-M, Guan C-H, Hsieh H-W, Hsu T-L, Tu Z, Wu K-J, Lin C-H, Lin K-I. Galectin-1 and galectin-8 have redundant roles in promoting plasma cell formation. J Immunol. 2011;187:1643–1652. doi: 10.4049/jimmunol.1100297. [DOI] [PubMed] [Google Scholar]
- van Kooyk Y, Rabinovich GA. Protein-glycan interactions in the control of innate and adaptive immune responses. Nat Immunol. 2008;9:593–601. doi: 10.1038/ni.f.203. [DOI] [PubMed] [Google Scholar]
- Volarevic V, Milovanovic M, Ljujic B, Pejnovic N, Arsenijevic N, Nilsson U, Leffler H, Lukic ML. Galectin-3 deficiency prevents concanavalin A-induced hepatitis in mice. Hepatology. 2012;55:1954–1964. doi: 10.1002/hep.25542. [DOI] [PubMed] [Google Scholar]
- Yu X, Siegel R, Roeder RG. Interaction of the B cell-specific transcriptional coactivator OCA-B and galectin-1 and a possible role in regulating BCR-mediated B cell proliferation. J Biol Chem. 2006;281:15505–15516. doi: 10.1074/jbc.M509041200. [DOI] [PubMed] [Google Scholar]