Abstract
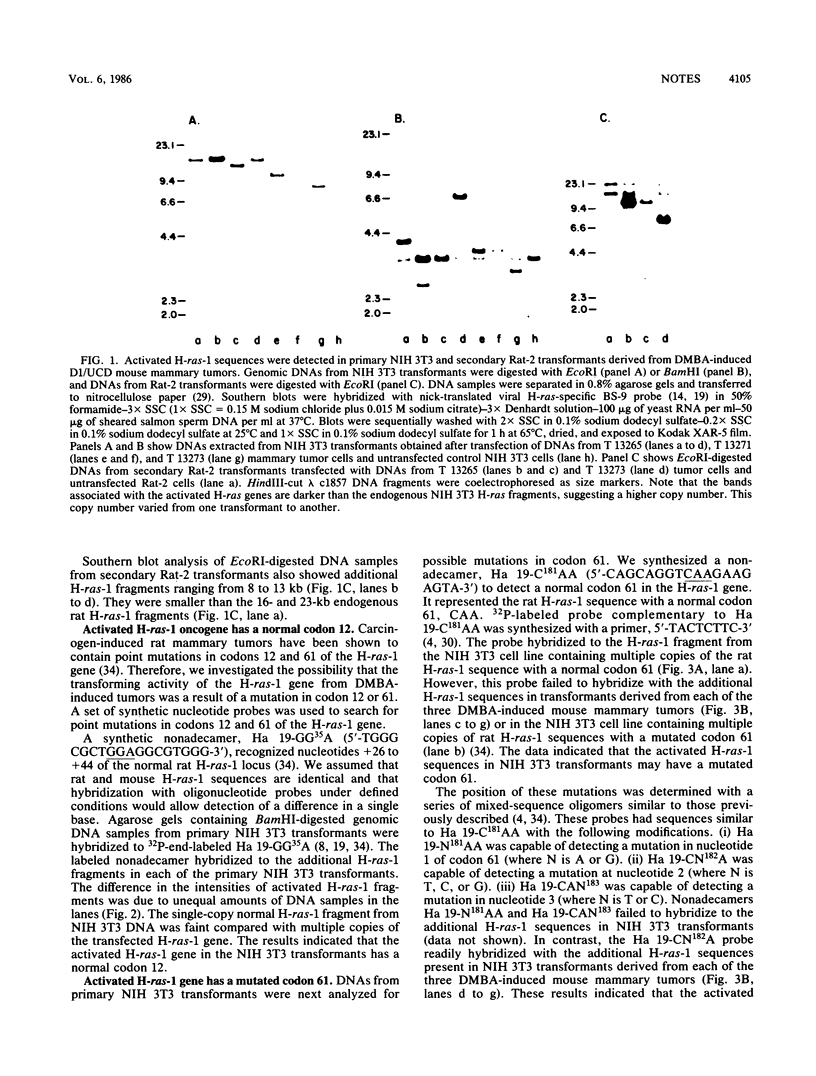
Genomic DNAs from dimethylbenzanthracene-induced BALB/c mouse mammary tumors arising from the transplantable hyperplastic outgrowth (HPO) line designated DI/UCD transformed NIH 3T3 cells upon transfection. Transforming activity was attributed to the presence of activated Harvey ras-1 oncogenes containing an A----T transversion at the middle adenosine nucleotide in codon 61. DNAs from untreated DI/UCD HPO cells and radiation-induced and spontaneous mammary tumors from the DI/UCD HPO line failed to transform NIH 3T3 cells. The results indicated that the mutation activation of Harvey ras-1 oncogenes was specific to dimethylbenzanthracene treatment in the mouse mammary tumor system.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bigger C. A., Sawicki J. T., Blake D. M., Raymond L. G., Dipple A. Products of binding of 7,12-dimethylbenz(a)anthracene to DNA in mouse skin. Cancer Res. 1983 Dec;43(12 Pt 1):5647–5651. [PubMed] [Google Scholar]
- Conner B. J., Reyes A. A., Morin C., Itakura K., Teplitz R. L., Wallace R. B. Detection of sickle cell beta S-globin allele by hybridization with synthetic oligonucleotides. Proc Natl Acad Sci U S A. 1983 Jan;80(1):278–282. doi: 10.1073/pnas.80.1.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel C. W., De Ome K. B., Young J. T., Blair P. B., Faulkin L. J., Jr The in vivo life span of normal and preneoplastic mouse mammary glands: a serial transplantation study. Proc Natl Acad Sci U S A. 1968 Sep;61(1):53–60. doi: 10.1073/pnas.61.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeOme K. B., Medina D. A new approach to mammary tumorigenesis in rodents. Cancer. 1969 Dec;24(6):1255–1258. doi: 10.1002/1097-0142(196912)24:6<1255::aid-cncr2820240632>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Dipple A., Pigott M., Moschel R. C., Costantino N. Evidence that binding of 7,12-dimethylbenz(a)anthracene to DNA in mouse embryo cell cultures results in extensive substitution of both adenine and guanine residues. Cancer Res. 1983 Sep;43(9):4132–4135. [PubMed] [Google Scholar]
- Ellis R. W., DeFeo D., Maryak J. M., Young H. A., Shih T. Y., Chang E. H., Lowy D. R., Scolnick E. M. Dual evolutionary origin for the rat genetic sequences of Harvey murine sarcoma virus. J Virol. 1980 Nov;36(2):408–420. doi: 10.1128/jvi.36.2.408-420.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eva A., Aaronson S. A. Frequent activation of c-kis as a transforming gene in fibrosarcomas induced by methylcholanthrene. Science. 1983 May 27;220(4600):955–956. doi: 10.1126/science.6302839. [DOI] [PubMed] [Google Scholar]
- Faulkin L. J., Mitchell D. J., Cardiff R. D., Rosenblatt L. S., Goldman M. Effects of X irradiation on the growth of normal and hyperplastic mouse mammary gland transplants. Radiat Res. 1983 May;94(2):390–403. [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Guerrero I., Calzada P., Mayer A., Pellicer A. A molecular approach to leukemogenesis: mouse lymphomas contain an activated c-ras oncogene. Proc Natl Acad Sci U S A. 1984 Jan;81(1):202–205. doi: 10.1073/pnas.81.1.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero I., Villasante A., D'Eustachio P., Pellicer A. Isolation, characterization, and chromosome assignment of mouse N-ras gene from carcinogen-induced thymic lymphoma. Science. 1984 Sep 7;225(4666):1041–1043. doi: 10.1126/science.6089339. [DOI] [PubMed] [Google Scholar]
- Kidd V. J., Wallace R. B., Itakura K., Woo S. L. alpha 1-antitrypsin deficiency detection by direct analysis of the mutation in the gene. Nature. 1983 Jul 21;304(5923):230–234. doi: 10.1038/304230a0. [DOI] [PubMed] [Google Scholar]
- Kraus M. H., Yuasa Y., Aaronson S. A. A position 12-activated H-ras oncogene in all HS578T mammary carcinosarcoma cells but not normal mammary cells of the same patient. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5384–5388. doi: 10.1073/pnas.81.17.5384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall C. J., Vousden K. H., Phillips D. H. Activation of c-Ha-ras-1 proto-oncogene by in vitro modification with a chemical carcinogen, benzo(a)pyrene diol-epoxide. Nature. 1984 Aug 16;310(5978):586–589. doi: 10.1038/310586a0. [DOI] [PubMed] [Google Scholar]
- Medina D., DeOme K. B. Influence of mammary tumor virus on the tumor-producing capabilities of nodule outgrowth free of mammary tumor virus. J Natl Cancer Inst. 1968 Jun;40(6):1303–1308. [PubMed] [Google Scholar]
- Padhy L. C., Shih C., Cowing D., Finkelstein R., Weinberg R. A. Identification of a phosphoprotein specifically induced by the transforming DNA of rat neuroblastomas. Cell. 1982 Apr;28(4):865–871. doi: 10.1016/0092-8674(82)90065-4. [DOI] [PubMed] [Google Scholar]
- Quintanilla M., Brown K., Ramsden M., Balmain A. Carcinogen-specific mutation and amplification of Ha-ras during mouse skin carcinogenesis. Nature. 1986 Jul 3;322(6074):78–80. doi: 10.1038/322078a0. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Singer B., Kuśmierek J. T. Chemical mutagenesis. Annu Rev Biochem. 1982;51:655–693. doi: 10.1146/annurev.bi.51.070182.003255. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sukumar S., Pulciani S., Doniger J., DiPaolo J. A., Evans C. H., Zbar B., Barbacid M. A transforming ras gene in tumorigenic guinea pig cell lines initiated by diverse chemical carcinogens. Science. 1984 Mar 16;223(4641):1197–1199. doi: 10.1126/science.6322298. [DOI] [PubMed] [Google Scholar]
- Tabin C. J., Bradley S. M., Bargmann C. I., Weinberg R. A., Papageorge A. G., Scolnick E. M., Dhar R., Lowy D. R., Chang E. H. Mechanism of activation of a human oncogene. Nature. 1982 Nov 11;300(5888):143–149. doi: 10.1038/300143a0. [DOI] [PubMed] [Google Scholar]
- Wigler M., Silverstein S., Lee L. S., Pellicer A., Cheng Y. c., Axel R. Transfer of purified herpes virus thymidine kinase gene to cultured mouse cells. Cell. 1977 May;11(1):223–232. doi: 10.1016/0092-8674(77)90333-6. [DOI] [PubMed] [Google Scholar]
- Zarbl H., Sukumar S., Arthur A. V., Martin-Zanca D., Barbacid M. Direct mutagenesis of Ha-ras-1 oncogenes by N-nitroso-N-methylurea during initiation of mammary carcinogenesis in rats. 1985 May 30-Jun 5Nature. 315(6018):382–385. doi: 10.1038/315382a0. [DOI] [PubMed] [Google Scholar]