Abstract
Manduca sexta, commonly known as the tobacco hornworm, is considered a significant agricultural pest, feeding on solanaceous plants including tobacco and tomato. The susceptibility of M. sexta larvae to a variety of entomopathogenic bacterial species1-5, as well as the wealth of information available regarding the insect's immune system6-8, and the pending genome sequence9 make it a good model organism for use in studying host-microbe interactions during pathogenesis. In addition, M. sexta larvae are relatively large and easy to manipulate and maintain in the laboratory relative to other susceptible insect species. Their large size also facilitates efficient tissue/hemolymph extraction for analysis of the host response to infection.
The method presented here describes the direct injection of bacteria into the hemocoel (blood cavity) of M. sexta larvae. This approach can be used to analyze and compare the virulence characteristics of various bacterial species, strains, or mutants by simply monitoring the time to insect death after injection. This method was developed to study the pathogenicity of Xenorhabdus and Photorhabdus species, which typically associate with nematode vectors as a means to gain entry into the insect. Entomopathogenic nematodes typically infect larvae via natural digestive or respiratory openings, and release their symbiotic bacterial contents into the insect hemolymph (blood) shortly thereafter10. The injection method described here bypasses the need for a nematode vector, thus uncoupling the effects of bacteria and nematode on the insect. This method allows for accurate enumeration of infectious material (cells or protein) within the inoculum, which is not possible using other existing methods for analyzing entomopathogenesis, including nicking11 and oral toxicity assays12. Also, oral toxicity assays address the virulence of secreted toxins introduced into the digestive system of larvae, whereas the direct injection method addresses the virulence of whole-cell inocula.
The utility of the direct injection method as described here is to analyze bacterial pathogenesis by monitoring insect mortality. However, this method can easily be expanded for use in studying the effects of infection on the M. sexta immune system. The insect responds to infection via both humoral and cellular responses. The humoral response includes recognition of bacterial-associated patterns and subsequent production of various antimicrobial peptides7; the expression of genes encoding these peptides can be monitored subsequent to direct infection via RNA extraction and quantitative PCR13. The cellular response to infection involves nodulation, encapsulation, and phagocytosis of infectious agents by hemocytes6. To analyze these responses, injected insects can be dissected and visualized by microscopy13, 14.
Keywords: Infection, Issue 70, Microbiology, Immunology, Bacteriology, Entomology, Bacteria, injection, pathogenesis, insect larvae, instar, Manduca sexta, tobacco hornworm, animal model, host pathogen interactions
Protocol
1. Insect Egg Sterilization and Rearing
Prepare diet by first autoclaving 15 g of the provided agar in 900-1,000 ml H2O. Immediately after autoclaving, mix with 166 g wheat germ diet and blend well in a laboratory blender. Pour into a dish (or dishes) to cool, then transfer diet to aluminum foil, wrap tightly, and store at 4 °C.
Upon arrival, sterilize M. sexta eggs with 250 ml of 0.6% bleach solution for 2-3 min in a glass filter holder and vacuum flask apparatus with a 90 mm filter paper, stirring occasionally.
Turn on vacuum to drain bleach solution and wash eggs 3-4 times with 250 ml sterile distilled H2O per wash.
Transfer eggs to fresh 90 mm filter paper placed in a Petri plate lid and allow them to dry until they no longer stick together (about 20-30 min).
Transfer eggs to the bottom of plastic containers with insect diet, placing approximately 40 eggs in each container (Figure 1A). Eggs are separated from diet to prevent moisture-induced fungal contamination. Maintain eggs at 26 °C with a 16 hr light: 8 hr dark photoperiod. Eggs will lighten to a yellow-white color prior to hatching.
When hatching is complete, carefully transfer approximately 25 larvae each to new containers with diet on the bottom (Figure 1B). It is important to pick up each larva by the long black horn using forceps to avoid harming them during their fragile early developmental stages. Incubate for 2 days as above. While larval death is unusual at this stage, some dead or, more likely, developmentally stunted larvae may be observed. Such insects should be excluded from further study and sacrificed by freezing.
To avoid cannibalistic behaviors, transfer larvae to individual containers with small pieces of food (Figure 1C) and incubate as above. Monitor insect development, replacing food and cleaning feces out of containers every other day until they undergo the third larval molt (usually 6-9 days after hatching). This is the 4th instar larval stage, characterized by the appearance of black hook-like crochets on each proleg and increased prominence of stripes along the insect body (Figure 1D). The larvae may vary considerably in size, but typically weigh 0.15-0.4 g upon entering the 4th instar stage. Larvae of similar size should be chosen for injection.
2. Preparation of Bacteria for Injection
Grow the bacterial strain(s) to be tested according to standard procedures to the growth phase you wish to test.
Pellet 500 μl of each bacterial strain to be tested by spinning for 2 min in a microcentrifuge at 13,000 rpm at room temperature.
Resuspend cells in 1 ml sterile 1x phosphate buffered saline (PBS) and pellet again as above.
Resuspend cells in 0.5 ml sterile 1x PBS and measure the optical density (OD) of the suspension. Dilute all suspensions to the same OD, if necessary. Sterile growth media may also be used for cell resuspension and dilution in place of 1x PBS, if desired.
3. Injection of 4th Instar Larvae
Prepare serial 10-fold dilutions of the first bacterial strain in 6 wells of a 96-well microtiter plate in sterile 1x PBS, using a fresh pipette tip for each dilution (Figure 2A). The final volume of cell suspension in each well should exceed the volume required for injection (10 μl per insect) plus 20 μl to plate the inoculum (see steps 3.2 and 3.7).
Spot 10 μl each for 5 dilutions (10-2 through 10-6) along the top of a Petri plate containing an appropriate growth medium. Tilt the plate so that the spots spread to the center (Figure 2B).
Remove insect diet and feces and place insects in containers on ice for approximately 5 min.
Sterilize the syringe needle by rinsing 3 times each in 2 tubes of 70-100% ethanol, followed by one tube of sterile H2O (Figure 2C). The volume of liquid must be sufficient to submerge the needle.
Quantitate the dilute cell suspensions under a microscope using a hemocytometer. Depending on the desired inoculum, draw 10 μl from the appropriate microtiter well (Figure 2D).
Swab the insect surface with 95% ethanol, and inject the 10 ml cell suspension into the insect at an angle (less than 45°) behind the one of the abdominal prolegs. Take care not to puncture the gut and inject the contents just underneath the epidermal layer (Figure 2E). While some loss of hemolymph (clear, green-blue liquid) is normal, particulate matter and yellow liquid emerging from the injection site are indicative of gut puncture. If this occurs, sacrifice the insect and obtain a new larva to replace it.
Repeat step 3.6 for each remaining insect in the first injection group. Needle sterilization is not necessary between each insect injected with the same dilution and strain of bacteria unless contamination occurs. Alternatively, a repeating dispenser may be used for greater consistency in injection volume among samples.
After injecting each insect with the first strain of bacteria, repeat step 3.2, this time spotting cell suspensions in the middle of the plate and letting them flow toward the bottom (Figure 2F). Incubate plates at appropriate temperature and count colonies to enumerate the inoculum. This second plating step is used to verify that the samples were not contaminated during the injection process, as this would result in disparate colony numbers between the first and second platings.
Repeat steps 3.1 through 3.8 for each strain of bacteria in the experiment. Finally, inject 3-5 insects with sterile 1x PBS using a sterile needle as a negative control group. Incubate insects at 26 °C with a 16 hr light: 8 hr dark photoperiod.
Monitor insect survival over time after injection. Insect death is characterized as a lack of voluntary movement upon stimulation. Insects often exhibit appetite loss, diarrhea (watery, yellow excrement), and/or water loss ("sweating") during infection prior to death; these characteristics may be worth noting as well.
Representative Results
A representative example of an insect mortality assay is depicted in Figure 3. In this experiment, insects were injected with approximately 50 colony forming units (CFU) of either wild type (ATCC19061) or an attenuated mutant strain (lrp13) of Xenorhabdus nematophila grown to mid-log phase (n=6 insects per strain). Insects were observed for approximately 72 hr, and the percent of injected insects still alive at each timepoint recorded. In this case, the attenuated strain exhibited a clear delay in insect killing; the wild-type strain killed all 6 larvae within 30 hr post-injection, prior to the death of any mutant-infected larva.
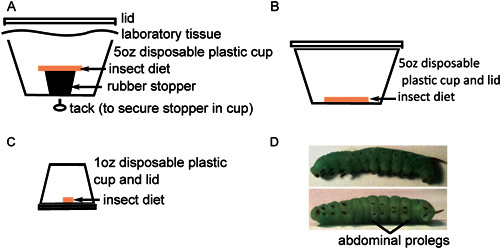 Figure 1. Insect rearing in preparation for injection. A) About 40 surface-sterilized eggs are placed at the bottom of a 5 oz cup with sterile insect diet resting on a rubber stopper. B) Twenty newly hatched insects are transferred to 5 oz cups with sterile insect diet on the bottom and incubated for 2 days. C) Insects are next transferred individually to 1oz cups with sterile diet on the bottom and incubated until they mature. D) Fourth instar M. sexta larvae with prominent stripes along the body (top) and black crochets on the abdominal prolegs (bottom).
Figure 1. Insect rearing in preparation for injection. A) About 40 surface-sterilized eggs are placed at the bottom of a 5 oz cup with sterile insect diet resting on a rubber stopper. B) Twenty newly hatched insects are transferred to 5 oz cups with sterile insect diet on the bottom and incubated for 2 days. C) Insects are next transferred individually to 1oz cups with sterile diet on the bottom and incubated until they mature. D) Fourth instar M. sexta larvae with prominent stripes along the body (top) and black crochets on the abdominal prolegs (bottom).
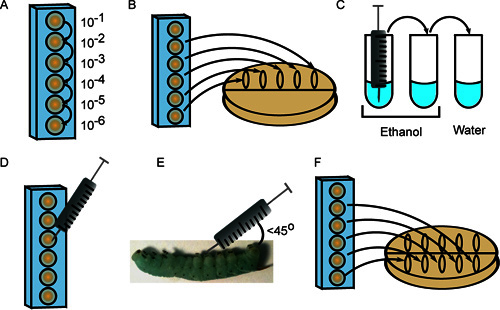 Figure 2. Injection of 4th instar M. sexta larvae. A) Bacteria are serially diluted in a 96-well plate. B) Ten microliters of multiple dilutions are plated to enumerate the inoculum. C) The syringe is sterilized with 3 rinses in ethanol (2x) and sterile water. D) Ten microliters from the appropriate dilution are drawn into the syringe. E) The cell suspension is injected at a 45° angle behind the first abdominal proleg. F) Dilutions are again plated to provide a second measure of the inoculum.
Figure 2. Injection of 4th instar M. sexta larvae. A) Bacteria are serially diluted in a 96-well plate. B) Ten microliters of multiple dilutions are plated to enumerate the inoculum. C) The syringe is sterilized with 3 rinses in ethanol (2x) and sterile water. D) Ten microliters from the appropriate dilution are drawn into the syringe. E) The cell suspension is injected at a 45° angle behind the first abdominal proleg. F) Dilutions are again plated to provide a second measure of the inoculum.
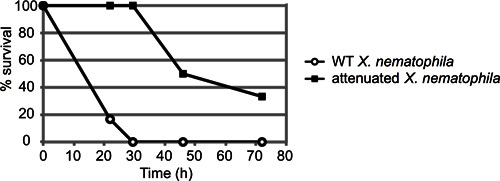 Figure 3. Representative result of M. sexta injection assay. About 50 colony-forming units (CFU) of Xenorhabdus nematophila cells in stationary phase (10 μl from the 10-4 dilution) were injected into six 4th instar M. sexta larvae per strain. Both wild type and a mutant strain (lrp) with an established virulence defect were injected and the insects monitored for mortality over time. Results are reported as percent surviving insects over time (in hours). These curves are statistically distinct, with a p-value of 0.000458 via log-rank analysis.
Figure 3. Representative result of M. sexta injection assay. About 50 colony-forming units (CFU) of Xenorhabdus nematophila cells in stationary phase (10 μl from the 10-4 dilution) were injected into six 4th instar M. sexta larvae per strain. Both wild type and a mutant strain (lrp) with an established virulence defect were injected and the insects monitored for mortality over time. Results are reported as percent surviving insects over time (in hours). These curves are statistically distinct, with a p-value of 0.000458 via log-rank analysis.
Discussion
The direct injection of M. sexta larvae with entomopathogenic bacteria, as described here, serves as a simple and effective means to analyze bacterial virulence. The method is also highly adaptable to suit different experimental subjects and/or conditions. Bacteria can be prepared in various ways prior to injection. In the case of X. nematophila, wild type cells grown in nutrient-rich Luria-Bertani (LB) medium to mid-log phase are typically the most virulent, killing most or all insects within 30 hr subsequent to injection. Cells in stationary phase often take 5-10 hr longer to kill the larvae. Though growth phase impacts virulence, the total number of cells injected appears to be less important16, with typical inocula ranging from 20 to 20,000 CFU. In fact, in the case of Xenorhabdus and Photorhabdus species, as few as 5 CFU are sufficient to kill the insect host17. In order to assess the virulence properties of bacterial species that are resistant to ethanol sterilization (e.g. Bacillus species), disposable needles can be used to inject each unique strain in place of ethanol sterilization (step 3.4).
Further adjustments to this method may involve changes in rearing and/or manipulation of M. sexta. For example, insects may be reared on tomato or tobacco leaves as a more natural food source. Alternatively, different developmental stages of M. sexta larvae can be assayed by this method. Fourth instar larvae were chosen based on their relatively large size, but smaller larvae may also be injected by this method. Fifth instar larvae can be injected, however the changes to the immune system during this stage of the development render late 5th instar larvae more susceptible than early 5th instar larvae18, potentially complicating data analysis.
Finally, the direct injection method may be adapted for use with other insect species. M. sexta is used as a model host for highly pathogenic species because it is less susceptible to infection than other (more susceptible) model organisms, such as Galleria mellonella. G. mellonella can be injected by the method described in this work19, however, and may be useful to assay bacterial species less virulent than Xenorhabdus and Photorhabdus species.
Disclosures
No conflicts of interest declared.
Acknowledgments
The authors wish to thank past members of the Goodrich-Blair lab: Samantha Orchard, Kimberly Cowles, Erin Herbert-Tran, Greg Richards, Megan Menard, and Youngjin Park for their contributions to the development of this protocol. This work was funded by the National Science Foundation grant IOS-0950873 and the National Institutes of Health NRSA fellowship FAI084441Z.
References
- Bintrim SB, Ensign JC. Insertional inactivation of genes encoding the crystalline inclusion proteins of Photorhabdus luminescens results in mutants with pleiotropic phenotypes. J. Bacteriol. 1998;180:1261–1269. doi: 10.1128/jb.180.5.1261-1269.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schesser JH, Kramer KJ, Bulla LA. Bioassay for homogeneous parasporal crystal of Bacillus thuringiensis using the tobacco hornworm, Manduca sexta. Appl. Environ. Microbiol. 1977;33:878–880. doi: 10.1128/aem.33.4.878-880.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Péchy-Tarr M, Bruck DJ, Maurhofer M, Fischer E, Vogne C, Henkels MD, Donahue KM, Grunder J, Loper JE, Keel C. Molecular analysis of a novel gene cluster encoding an insect toxin in plant-associated strains of Pseudomonas fluorescens. Environ. Microbiol. 2008;10:2368–2386. doi: 10.1111/j.1462-2920.2008.01662.x. [DOI] [PubMed] [Google Scholar]
- Nuñez-Valdez ME, Calderón MA, Aranda E, Hernández L, Ramírez-Gama RM, Lina L, Rodríguez-Segura Z, Gutiérrez Mdel C, Villalobos FJ. Identification of a putative Mexican strain of Serratia entomophila pathogenic against root-damaging larvae of Scarabaeidae (Coleoptera) Appl. Environ. Microbiol. 2008;74:802–810. doi: 10.1128/AEM.01074-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forst SA, Tabatabai N. Role of the histidine kinase, EnvZ, in the production of outer membrane proteins in the symbiotic-pathogenic bacterium Xenorhabdus nematophilus. Appl. Environ. Microbiol. 1997;63:962–968. doi: 10.1128/aem.63.3.962-968.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanost MR, Jiang H, Yu XQ. Innate immune responses of a lepidopteran insect, Manduca sexta. Immunol. Rev. 2004;198:97–105. doi: 10.1111/j.0105-2896.2004.0121.x. [DOI] [PubMed] [Google Scholar]
- Yu XQ, Zhu YF, Ma C, Fabrick JA, Kanost MR. Pattern recognition proteins in Manduca sexta plasma. Insect Biochem. Mol. Biol. 2002;32:1287–1293. doi: 10.1016/s0965-1748(02)00091-7. [DOI] [PubMed] [Google Scholar]
- Eleftherianos I, ffrench-Constant RH, Clarke DJ, Dowling AJ, Reynolds SE. Dissecting the immune response to the entomopathogen Photorhabdus. Trends Microbiol. 2010;18:552–560. doi: 10.1016/j.tim.2010.09.006. [DOI] [PubMed] [Google Scholar]
- Tobacco Hornworm Genome Project [Internet] Houston, TX: Baylor College of Medicine; 2012. Available from: http://www.hgsc.bcm.tmc.edu/content/tobacco-hornworm-genome-project. [Google Scholar]
- Herbert EE, Goodrich-Blair H. Friend and foe: the two faces of Xenorhabdus nematophila. Nat. Rev. Microbiol. 2007;5:634–646. doi: 10.1038/nrmicro1706. [DOI] [PubMed] [Google Scholar]
- D'Argenio DA, Gallagher LA, Berg CA, Manoil C. Drosophila as a model host for Pseudomonas aeruginosa Infection. J. Bacteriol. 2001;183:1466–1471. doi: 10.1128/JB.183.4.1466-1471.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterfield N, Dowling A, Sharma S, Daborn PJ, Potter U, Ffrench-Constant RH. Oral toxicity of Photorhabdus luminescens W14 toxin complexes in Escherichia coli. Appl. Environ. Microbiol. 2001;67:5017–5024. doi: 10.1128/AEM.67.11.5017-5024.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park Y, Herbert EE, Cowles CE, Cowles KN, Menard ML, Orchard SS, Goodrich-Blair H. Clonal variation in Xenorhabdus nematophila virulence and suppression of Manduca sexta immunity. Cell. Microbiol. 2007;9:645–656. doi: 10.1111/j.1462-5822.2006.00815.x. [DOI] [PubMed] [Google Scholar]
- Park Y, Kim Y, Putnam SM, Stanley DW. The bacterium Xenorhabdus nematophilus depresses nodulation reactions to infection by inhibiting eicosanoid biosynthesis in tobacco hornworms, Manduca sexta. Arch. Insect Biochem. Physiol. 2003;52:71–80. doi: 10.1002/arch.10076. [DOI] [PubMed] [Google Scholar]
- Cowles KN, Cowles CE, Richards GR, Martens EC, Goodrich-Blair H. The global regulator Lrp contributes to mutualism, pathogenesis and phenotypic variation in the bacterium Xenorhabdus nematophila. Cell. Microbiol. 2007;9:1311–1323. doi: 10.1111/j.1462-5822.2006.00873.x. [DOI] [PubMed] [Google Scholar]
- Cowles KN, Goodrich-Blair H. Expression and activity of a Xenorhabdus nematophila haemolysin required for full virulence towards Manduca sexta insects. Cell. Microbiol. 2005;7:209–219. doi: 10.1111/j.1462-5822.2004.00448.x. [DOI] [PubMed] [Google Scholar]
- Goodrich-Blair H, Clarke DJ. Mutualism and pathogenesis in Xenorhabdus and Photorhabdus: two roads to the same destination. Mol. Microbiol. 2007;64:260–268. doi: 10.1111/j.1365-2958.2007.05671.x. [DOI] [PubMed] [Google Scholar]
- Eleftherianos I, Baldwin H, ffrench-Constant RH, Reynolds SE. Developmental modulation of immunity: changes within the feeding period of the fifth larval stage in the defence reactions of Manduca sexta to infection by Photorhabdus. J. Insect Physiol. 2008;54:309–318. doi: 10.1016/j.jinsphys.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Kavanagh K, Reeves EP. Exploiting the potential of insects for in vivo pathogenicity testing of microbial pathogens. FEMS Microbiol. Rev. 2004;28:101–112. doi: 10.1016/j.femsre.2003.09.002. [DOI] [PubMed] [Google Scholar]


