Summary
Objectives
the aims of the present study were to compare the haemodynamic effects of oxytocin and carbetocin and to assess the efficacy of these two drugs in terms of blood loss and the additional uterotonic needed in caesarean section at high risk of primary post-partum haemorrhage.
Methods
women in the carbetocin group (group A) received a bolus of 100 μg IV; women in the control group (group B) received 20 IU of oxytocin in 1000 ml of 0,9% Na-Cl solution IV (150 mL/hour). The main parameter evaluated was the haemodynamic effects of drugs and the need for additional uterotonic agents. In addition we compared the drop in haemoglobin level, the uterine tone, the uterine fundal state and the diuresis.
Results
regarding the haemodynamic effects, both drugs have a hypotensive effect, but we found a greater reduction in blood pressure within the oxytocin group. Significantly more women needed additional uterotonic agents in the oxytocin group (23,5% vs 0%, p<0.01), though there was no significant difference in estimated blood loss and in the drop haemoglobin level (p>0.05). There was a significant difference in the diuresis, higher in carbetocin group (1300 ml ± 450 ml vs 1100 ml ± 250 ml, p=0.01).
Conclusions
a single injection of carbetocin appears to be more effective than a continuous infusion of oxytocin to prevent the PPH, with a similar haemodynamic profile and minor antidiuretic effect.
Keywords: post-partum haemorrhage, uterotonic drugs, carbetocin, oxytocin
Introduction
Prevention of post-partum haemorrhage (PPH) is a major issue due to its impact on maternal morbidity and mortality. The primary PPH is defined as blood loss more than 500 mL after vaginal delivery and more than 1000 mL after caesarean section, that occurs in the first 24 hours after delivery. Almost 500.000 women die for this potentially preventable cause each year, and up to an estimated quarter of these deaths uses to occur as a consequence of haemorrhage at time of delivery (1). Non fatal PPH can result in further interventions, severe anaemia, need of blood transfusion, Sheehan’s syndrome (pituitary infarction), coagulopathy and organ damage due to hypotension and shock. PPH diagnosis is based on International Classification of Disease (ICD) codes recorded in Perinatal Database (ICD-9 and ICD-10). Subtypes of PPH identified with ICD-9 and ICD-10 diagnostic codes included: 1) PPH due to retained placenta, 2) PPH due to uterine atony (occurring within 24 hours following delivery), 3) delayed and secondary PPH (occurring after the first 24 hours following delivery) and 4) PPH due to a coagulation defects (2). The study conducted by the International PPH Collaborative Group reports an increasing trend in coded PPH between 1991 and 2006 not only in low income countries, but also in Canada, New South Wales and the USA, as a possible result of increased maternal age at childbirth, increased rate of caesarean delivery, increased rate of induction of labor and higher number of multiple pregnancies (2).
The first cause of haemorrhage at the time of delivery is uterine atony, therefore there is general agreement that active management of the third stage of labour rather than expectant management is recommended (2–4). The third stage of labor is defined as the period that follows delivery and finishes with the delivery of placenta.
The practical guidelines on PPH of the Society of Obstetricians and Gynaecologists of Canada (SOGC) (5) suggest that the active management of the third stage of labour reduces the risk of PPH compared with the expectant management and should be offered and recommended to all women. The administration of uterotonic drugs widely prevents the PPH, significantly decreases the incidence of PPH and therefore it is the main point of active management. Oxytocin (10 IU), administered intra-muscularly, is the preferred medication for the prevention of PPH in low-risk vaginal and caesarean deliveries. Care providers should administer this medication after delivery of the anterior shoulder. Intravenous infusion of oxytocin (20 to 40 IU in 1000 mL, 150 mL/hour) is an acceptable alternative for the active management. Ergonovine can be used but it may be considered a second choice to oxytocin due to the greater risk of maternal adverse effects. Carbetocin, given 100 μg as an IV bolus over 1 minute, instead of continuous oxytocin infusion, can be administered in elective caesarean section for the prevention of PPH, in the attempt to decrease the need for therapeutic uterotonics.
Although the oxytocin is the most widely accepted uterotonic agent, however other drugs are available, but which agent is ideal for prophylactic use is far to be clearly stated (5).
Carbetocin is a long-acting synthetic oxytocin analogue, 1-deamino-1-monocarbo-(2-O-Methyltyrosine)-oxytocin, firstly described in 1987. It has a half-life of 40 minutes (around 4–10 times longer than oxytocin) and uterine contractions occur in less than two minutes after intravenous administration of optimal dosage of 100 μg (6). A single dose of carbetocin has been hypothysed to act as a 16 hours intravenous oxytocin infusion regarding the increase in uterine tone and the reduction of the risk of PPH in elective caesarean section (7).
Several data of literature (8–10) suggest that prophylactic administration of carbetocin may be a good alternative to oxytocin to prevent post-partum haemorrhage, but which uterotonic agent is ideal for prophylactic use is being debated. Nonetheless, primary prevention of a post-partum haemorrhage begins with the assessment of identifiable risk factors.
The aims of the present study were:
- to compare the haemodynamic effects of oxytocin and carbetocin (effects on blood pressure and diuresis)
- to assess the efficacy of these two drugs in terms of intra-operative blood loss and the additional uterotonic needed in caesarean section at high risk of post-partum haemorrhage.
Methods
This is a prospective, case-control study conducted from July 2011 and October 2011 within the Obstetric and Gynaecology tertiary care unit of Fatebenefratelli Isola Tiberina, Rome.
One hundred two women undergoing elective caesarean section were consecutively enrolled, with risk factors for primary post-partum haemorrhage such as: multiple pregnancy, two or more previous caesarean section, presence of uterine fibroids, previous myomectomy, presence of placenta previa, past history of PPH, fetal macrosomia and fetal malformations associated with polyhydramnios.
A written informed consent was asked from eligible women on admission.
The exclusion criteria included the presence of hypertension, preeclampsia, cardiac, renal or liver diseases, epilepsy and general anaesthesia, as well as women with history of hypersensitivity to carbetocin according to the Br National Formulary (8).
Firstly we recruited fifty-one women who received carbetocin (case group A), then we enrolled fifty-one women who received oxytocin (control group B).
Women in the carbetocin group (group A) received a bolus of 100 μg IV at delivery of the anterior shoulder; women in the control group (group B) received 20 IU of oxytocin in 1000 ml of 0,9% NaCl solution IV (150 mL/hour) at delivery of the anterior shoulder.
The primary outcome of this study was the evaluation of the early haemodynamic effects of carbetocin and oxytocin, in terms of impact on the blood pressure (BP) suddenly after the injection. All patients underwent the same combined spinal-epidural (CSE) anaesthesia.
After the application of this anaesthesia, subjects were positioned in recumbent position and for continuous blood pressure measurement a limb cuff (Drager Infinity Delta®, Drager Medical Australia pty ltd) was applied. To evaluate the haemodynamic effects between carbetocin and oxytocin we considered the drop in a blood pressure comparing the BP after combined spinal-epidural (CSE) procedure, 1 minute, 3 minutes and 5 minutes after drug administration, at time of uterine repair and at term of caesarean procedure, on left recumbent position. We recorded the occurrence of nausea, vomiting, flushing, haedache, dyspnea and tachycardia.
The latter important outcome of this study was the need for additional uterotonic agents and the evaluation of the drop in haemoglobin level by comparing the haemoglobin concentration on admission with the measure at 2 hours and 24 hours after delivery. Also the blood loss is checked immediately after caesarean, defining as haemorrhage a blood loss in excess of 1000 ml or more (5). Blood loss was estimated by the surgeon in the usual way (visual estimation, number of used swabs and amount of aspirated blood) (8).
Blood pressure (in mmHg), uterine tone (standardized as Very good, Good, Sufficient, Atony), uterine position (with respect to the umbilical point, UP) were monitored 2 hours, 12 hours and 24 hours after delivery by the same midwife.
All patients had the Foley catheter and urobag in situ for 24 hours after caesarean section and the amount of urine was monitored 2 hours and 12 hours after delivery by the midwife. This study had no external funding source. No author had any potential relationships that may pose conflict of interest.
Statistical analysis
For a power analysis of 90%, the study needed of 50 patients in each group.
Data were expressed as means ± SD or median, as appropriated. Data were tested for normal distribution. Statistical analysis was performed by using Mann Withney non parametric test or χ2 test and Fisher’s exact test for categorical data. P value of less then 0,05 (P<0,05) was considered as statistically significant.
The STATA software version 12.0 was used for the statistical calculations.
Results
All relevant maternal subject characteristics in both study groups were comparable, except for the significantly increased use of anticoagulant drugs during pregnancy in carbetocin group (19,6% vs 2%, p<0.05), as summarized in Table 1.
Table 1.
Characteristics of the study population.
| Carbetocin (A) (n=51) | Oxytocin (B) (n=51) | P value | |
|---|---|---|---|
| Maternal Age (mean, SD) | 37,1±5,7 | 36,1±4,1 | 0.33 |
| Gestational age at delivery (median-range) | 38(37–39) | 37(36–38) | 0.01 |
| Weight increase in pregnancy (mean, SD) | 12,2±4,4 | 13,5± 4,2 | 0.15 |
| Previous Abdominal surgery (n,%) | 32 (62,7%) | 22 (43,1%) | 0.05 |
| Use of anticoagulant in pregnancy (n,%) | 10 (19,6%) | 1 (2%) | 0.01 |
The main gestational age at caesarean was 38 weeks in the carbetocin group and 37 in the control group.
Population profile and indication to elective caesarean section were similar for each group, however in the group B the main indication has been the twin pregnancy (21.6% vs 41,2%, p=0.05) (Tab. 2).
Table 2.
Indications to Caesarean Section.
| Carbetocin (A) (%, n) n=51 | Oxytocin (B) (%, n) n=51 | |
|---|---|---|
| Two or more CS | 33,3% (17) | 23,5% (12) |
| Fetal Macrosomia | 11,8% (6) | 2% (1) |
| Placenta praevia | 11,8% (6) | 11,8% (6) |
| Uterine fibroids | 3,9% (2) | 0 |
| History of PPH | 0 | 0 |
| Twin pregnancy | 21,6% (11) | 41,2% (22) |
| Fetal polyhydramnios | 0 | 5,9% (3) |
| Previous myomectomy | 17,6% (9) | 15,7% (8) |
Regarding the haemodynamic effects of carbetocin and oxytocin, both drugs have a hypotensive effect.
Particularly, systolic blood pressure was lower in the oxytocin group at the 5th minute after administration, at uterine closure time, at 12 hours postoperatively, as stated in Table 3.
Table 3.
Systolic and diastolic blood pressure during and after caesarean section.
| Carbetocin (A) | Oxytocin (B) | P value | ||||
|---|---|---|---|---|---|---|
| Systolic | (median, Interquartile range) | |||||
| Skin Incision | 114 | (108–127) | 116 | (102–125) | 0.533 | |
| 1′ after uterotonic administration | 118 | (110–126) | 120 | (113–125) | 0.904 | |
| 3′ after uterotonic administration | 117 | (110–126) | 110 | (106–122) | 0.06 | |
| 5′ after uterotonic administration | 117 | (106 –128) | 106 | (103–116) | <0.01* | |
| Uterine repair | 116 | (107–125) | 111 | (105–125) | <0.01* | |
| Suturing the skin | 114 | (109–126) | 109 | (104–114) | 0.0986 | |
| Post-operative | ||||||
| 2 h after CS | 110 | (100–120) | 105 | (95–110) | 0.0714 | |
| 12 h after CS | 110 | (100–120) | 100 | (100–115) | 0.02* | |
| 24 h after CS | 110 | (100–120) | 110 | (100–110) | 0.0941 | |
| Diastolic | (median, Interquartile range) | |||||
| Skin Incision | 68 | (60–73) | 70 | (60–75) | 0.4208 | |
| 1′ after uterotonic administration | 65 | (58–72) | 70 | (63–75) | 0.1129 | |
| 3′ after uterotonic administration | 68 | (61–74) | 62 | (59–70) | <0.02* | |
| 5′ after uterotonic administration | 64 | (56–70) | 59 | (55–65) | 0.02* | |
| Uterine repair | 66 | (60–71) | 62 | (59–74) | 0.3411 | |
| Suturing the skin | 63 | (55–70) | 59 | (54–64) | 0.0714 | |
| Post-operative | ||||||
| 2 h after CS | 70 | (60–80) | 65 | (60–70) | 0.03* | |
| 12 h after CS | 70 | (60–70) | 60 | (60–70) | 0.04* | |
| 24 h after CS | 70 | (60–70) | 60 | (60–70) | <0.01* | |
statistically significant
In the meanwhile diastolic blood pressures were pairwise lower in the control group with respect to the carbetocin group, at the 3rd, 5th minutes after administration, and both 12 and 24 hours postoperatively, testifying a general greater reduction in blood pressure within the oxytocin group (Tab. 3).
There was no significant difference in the amount of estimated blood loss and in the incidence of primary post-partum haemorrhage (>1000 ml) in both groups as shown in Table 4.
Table 4.
Maternal blood loss.
| Carbetocin (A) | Oxytocin (B) | P value | |||
|---|---|---|---|---|---|
| During caesarean section | n° | % | n° | % | |
| < 500 ml | 39 | 76.5 | 35 | 68.6 | 0.18 |
| 500–1000 ml | 12 | 23.5 | 12 | 29.4 | |
| > 1000 ml | 0 | 0.0 | 4 | 7.8 | |
| 2h after caesarean section | |||||
| < 500 ml | 50 | 98.0 | 50 | 98.0 | 1.00 |
| 500–1000 ml | 1 | 2.0 | 1 | 2.0 | |
| 1000 ml | 0 | 0 | 0 | 0 | |
| 12h after caesarean section | |||||
| < 500 ml | 51 | 100.0 | 51 | 100.0 | |
| 500–1000 ml | 0 | 0 | 0 | 0 | 1.00 |
| >1000 ml | 0 | 0 | 0 | 0 | |
| 24h after caesarean section | |||||
| <500 ml | 51 | 100.0 | 51 | 100.0 | |
| 500–1000 ml | 0 | 0 | 0 | 0 | 1.00 |
| >1000 ml | 0 | 0 | 0 | 0 | |
Similarly, in both study groups haemoglobin levels before and after 2 hours and 24 hours a part from delivery were similar, confirming no significant difference in the level of blood loss (Tab. 5), although we found a tendentially lower Hb decrease at 12 hours from delivery in the carbetocin group (−0.7 g/dl vs −1.1 g/dl, p 0.074).
Table 5.
Haemoglobin, hematocrit and platelets levels before and after caesarean section.
| Carbetocin (A) | Oxytocin (B) | P value | |||
|---|---|---|---|---|---|
| Pre-Operative Hb (median interquartile ranges) | |||||
| Hb (g/dl) | 11.4 | (10.7 – 12.1) | 11.8 | (10.9 – 12.3) | 0.33 |
| Ht (%) | 34.6 | (32 – 36.2) | 34 | (30.9 – 36.8) | 0.80 |
| PLT | 222 | (180 – 266) | 206 | (162 – 236) | 0.09 |
| Post-Operative Hb Drop (median interquartile ranges) | |||||
| Hb 2 h after CS (g/dl) | −0.6 | (−1.1 ; −0.1) | −0.7 | (−1.3 ; −0.3) | 0.463 |
| Hb 12 h after CS (g/dl) | −0.7 | (−1.3 ; 0.0) | −1.1 | (−1.9 ; −0.2) | 0.074 |
| Ht 2 h after CS (%) | −1.4 | (−2.8 ; −0.1) | −1.8 | (−4.0 ; 0.4) | 0.25 |
| Ht 12 h after CS (%) | −1.4 | (−3.2 ; 0.2) | −1.9 | (−5.3 ; 0.2) | 0.20 |
| PLT 2 h after CS | −20 | (−52 ; 0) | −7 | (−28 ; 1 ) | 0.171 |
| PLT 12h after CS | −18 | (−46 ; 0) | −12 | (−29 ; 8) | 0.194 |
However, as regarding the secondary outcome, none from the carbetocin group versus 23,5% from the control group, needed additional oxytocics (oxytocin, methylergometrine, sulprostone). Therefore, significantly more women required additional uterotonic agents in the oxytocin group (p<0.01).
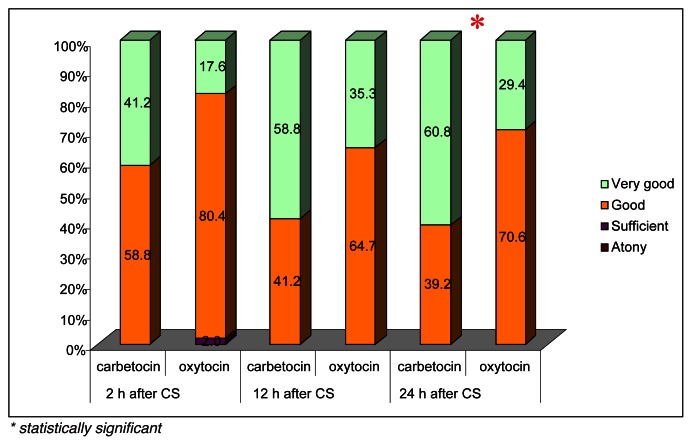
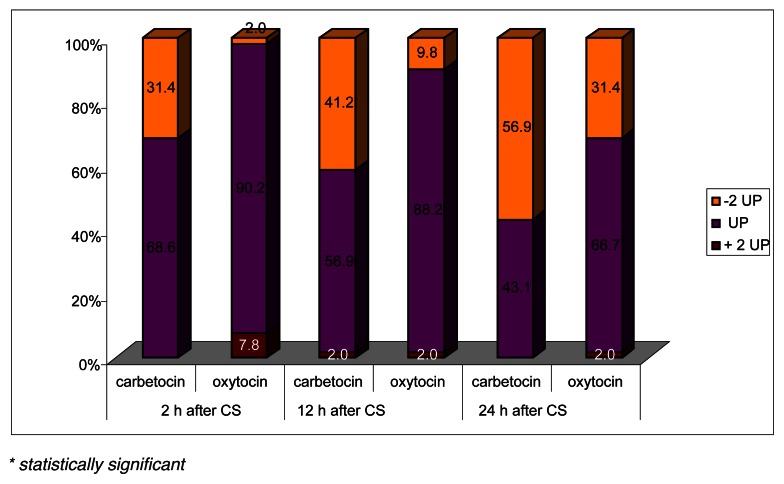
At the same, there was a significant difference in the uterine tone and in the fundal height as shown in Figure 1 and 2. The uterine contractility was better in the carbetocin group at 2, 12 and 24 hours after caesarean section, and the difference was statistically significant at 24 hours (p<0.05). The fundus was significantly below 2 cm from the umbilical point (-2UP) in patients of group A at 2 and 12 hours on the ward (p<0.05) with respect to patients undergoing oxytocin administration.
Figure 1.
Uterine tone.
* statistically significant
Figure 2.
Position of the uterine fundus to the umbilical point (UP).
* statistically significant
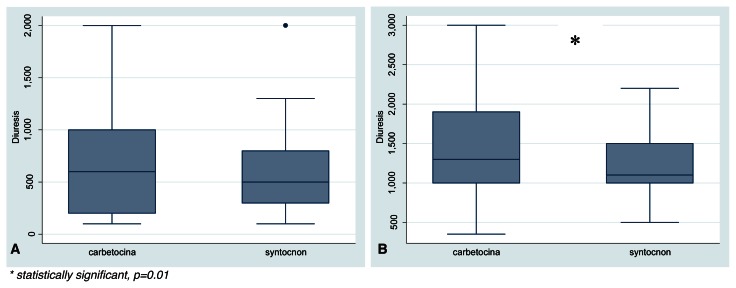
Patients treated with carbetocin were observed to have a higher diuresis than patients belonging to oxytocin group, especially 12 hours after caesarean section (Fig. 3), and the difference was statistically significant (1300 ml ± 450 ml vs 1100 ml ± 250 ml, p=0.01).
Figure 3.
A) Diuresis at 2 and B) 12 hours after caesarean section.
* statistically significant, p=0.01
There wasn’t any recorded important adverse effects in both study groups, instead nausea and vomiting was observed with similar frequency in both study groups.
Discussion
This is one of the few studies, at the best of our knowledge, comparing carbetocin with oxytocin in elective caesarean sections with risk factors for primary post-partum haemorrhage, concerning both the haemodynamic effects of these drugs, and the prevention of PPH.
Nonetheless up to date, which uterotonic agent is suitable for prophylactic use is being debated and literature lacks of clear endpoints on this item.
The primary outcome of the study is the evaluation of immediate haemodynamic effects of carbetocin administration. We know that the haemodynamic effects of an oxytocin bolus consist of systemic vasodilation, with important hypotension, tachycardia and increase of cardiac output, resulting in dose-dependent hypotension and tachycardia (11–13). These haemodynamic side effects, especially in patients with hypovolaemia or cardiac diseases, may lead to myocardial ischemia (14). A recent study of Moertl et al. shows that patients treated with oxytocin has a more pronounced hypotension and haemodynamic rebound than patients treated with carbetocin, with comparable effects on the cardiovascular system (15).
In agreement, we report a good haemodynamic profile in carbetocin group with substantially unmodified levels of systolic and diastolic blood pressure with respect to the beginning of the surgical procedure and moreover we describe lower BP levels in the control group (group B) in almost all the study times after drug administration, even if this scenario seems to be tendential and not always statistically significative. This result suggests that carbetocin seems to have an acceptable haemodynamic safety profile. Currently, pre-eclampsia, eclampsia and general anaesthesia are contraindications to the administration of carbetocin, but this data suggest a new threshold in the attempt to enlarge the therapy indications.
On this item, Su et al. in the Cochrane of 2007 regarding “Oxytocin agonists for preventing postpartum haemorrhage” and in the Cochrane 2012 regarding “Carbetocin for preventing post-partum haemorrhage”, conclude that the use of carbetocin is more effective than oxytocin for preventing PPH in women undergoing caesarean section, but the data and the evidences were still insufficient (4–10, 16).
Regarding the literature about carbetocin, Danzereau et al. (17) firstly described a lower additional uterotonic need for treatment of uterine atony in women who took carbetocin soon after delivery.
Also Borruto et al. (9) described a lower rate of additional oxytocic need in women undergoing carbetocin administration during CS and we do reach the same conclusions as many other researches (7, 8), but it is difficult to compare our study with the mentioned ones cause the strict selection of our study population according to risk factors. For instance Attilakos et al., in their interesting paper, excluded placenta previa and multiple pregnancies from the study or pregnant women being under 37 weeks’ gestation (8).
We find a definitively lack of additional uterotonic need after CS in the carbetocin receiving women at high risk for PPH.
Also, our results suggest the effectiveness of carbetocin compared to oxytocin regarding the uterine contraction and tonicity. Indeed we have shown that the uterine contractility was better in the carbetocin group at 2, 12 and 24 hours after caesarean section, as well as the fundus was significantly below 2 cm from the umbilical point (-2UP) in patients of group A after 2 and 12 hours.
In fact we did not demonstrate any difference in the amount of blood loss after caesarean section and in the drop of haemoglobin level within 2 hours and 24 hours, but we showed in the oxytocin group a significant need (23,5%) of additional uterotonic agents. Previous studies have shown that carbetocin could induce maternal tachycardia and facial flushing (18, 19), but none in our carbetocin subgroup had this adverse events.
Regarding the effects of carbetocin to the diuresis, this recognition goes back to the early 1960s with clinical observations showing that systemic infusion of the high-dosage oxytocin in obstetric patients causes antidiuresis, indicating vasopressin-like effects (20, 21).
In fact these two hormones are synthesized in the hypothalamus of humans and transported through the neurohypophysial tract to the posterior pituitary gland for storage or secretion, and they display close similarities in chemical structure, (differing by only two aminoacids and exhibiting similar intron-exon structures) (22).
In recent years the study of the molecular basis for antidiuretic effect has shown a long- and short-term effects on renal acquaporin (AQP) water channels in the cells of the collecting duct, mediated by V2 receptors. The long-term effect leads to increased expression of AQP, whereas the short-term effects involve trafficking of AQP to the apical membrane of the principal cells (23, 24). Therefore, the oxytocin seems to act as an antidiuretic hormone, mimicking the short-term action of vasopressin on water permeability, albeit with somewhat lower potency (25) and an administration of oxytocin for a longer period may cause antidiuresis, resulting in water intoxication (20, 21). In literature there is only one other study that compares the effects of carbetocin and oxytocin on the diuresis, which shows that the carbetocin has a moderate antidiuretic effect without statistically significant difference in urine output between women treated with carbetocin or oxytocin (26).
In contrast with these results, in our study we report in the oxytocin group (group B) an important diuresis contraction both 2 hours and 12 hours after caesarean section, whereas in carbetocin group (group A) we observe a significant higher diuresis than observed in the oxytocin group, especially 12 hours after caesarean section.
Therefore, despite the carbetocin is a synthetic oxytocin analogue, the small difference in the molecular structure could determine not only the uterotonic stronger action, but also the difference in the biologic function as the absence of antidiuretic effect, and this is an important result in the attempt to define a more comprehensive carbetocin profile.
We therefore conclude that a single injection of carbetocin appears to be more effective than a continuous infusion of oxytocin to maintain adequate uterine tone, with a similar safety profile and minor antidiuretic effect, in the third stage and in the first 24 hours after delivery defined “four stage of labor” (9).
Acknowledgments
We would like to thank so much Dr. Federica Rossi for the technical support.
References
- 1.World Health Organisation. The World health report 2005: make every mother and child count. Geneva: 2005. [Google Scholar]
- 2.Knight M, Callaghan WM, Berg C, Alexander S, Bouvier-Colle MH, Ford JB, et al. Trends in postpartum hemorrhage in high resource countries: a review and recommendations from the International Postpartum Hemorrhage Collaborative Group. BMC Pregnancy Childbirth. 2009;9:55. doi: 10.1186/1471-2393-9-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cotter AM, Ness A, Tolosa JE. Prophylactic oxytocin for the third stage of labour. Cochrane Database Syst Rev. 2001;4:CD001808. doi: 10.1002/14651858.CD001808.pub2. [DOI] [PubMed] [Google Scholar]
- 4.Su LL, Chong YS, Samuel M. Oxytocin agonists for preventing postpartum haemorrhage. Cochrane Database Syst Rev. 2007;3:CD005457. doi: 10.1002/14651858.CD005457.pub2. [DOI] [PubMed] [Google Scholar]
- 5.Leduc D, Senikas V, Lalonde AB. Clinical Practice Obstetrics Committee; Society of Obstetricians and Gynaecologists of Canada. Active management of the third stage of labour: prevention and treatment of postpartum hemorrhage. J Obstet Gynaecol Can. 2009;31:980–983. doi: 10.1016/S1701-2163(16)34329-8. [DOI] [PubMed] [Google Scholar]
- 6.Sweeney G, Holbrook AM, Levine M, Yip M, Alfredson K, Cappi S, et al. Pharmacokinetics of carbetocin, a long acting oxytocin analogue, in nonpregnant women. Curr Ther Res. 1990;47:528–540. [Google Scholar]
- 7.Boucher M, Horbay GL, Griffin P, Deschamps Y, Desjardins C, Schulz M, et al. Double-blind randomised comparison of the effect of carbetocin and oxytocin on intraoperative blood loss and uterine tone of patients undergoing caesarean section. J Perinatol. 1998;18:202–207. [PubMed] [Google Scholar]
- 8.Attilakos G, Psaroudakis D, Ash J, Buchanan R, Winter C, Donald F, et al. Carbetocin versus oxytocin for the prevention of postpartum haemorrhage following caesarean section: the results of a double-blind randomised trial. BJOG. 2010;117:929–936. doi: 10.1111/j.1471-0528.2010.02585.x. [DOI] [PubMed] [Google Scholar]
- 9.Borruto F, Treisser A, Comparetto C. Utilization of carbetocin for prevention of postpartum haemorrhage after caesarean section: a randomized clinical trial. Arch Gynecol Obstet. 2009;280:707–712. doi: 10.1007/s00404-009-0973-8. [DOI] [PubMed] [Google Scholar]
- 10.Boucher M, Nimrod CA, Tawagi GF, Meeker TA, Rennicks White RE, Varin J. Comparison of carbetocin and oxyocin for the prevention of postpartum haemorrhage following vaginal delivery: a double-blind randomized trial. J Obstet Gynaecol Can. 2004;26:481–488. doi: 10.1016/s1701-2163(16)30659-4. [DOI] [PubMed] [Google Scholar]
- 11.Pinder AJ, Dresner M, Calow C, Shorten GD, O’Riordan J, Jhonson R. Haemodynamic changes caused by oxytocin during caesarean delivery under spinal anaesthesia. Int J Obstet Anesth. 2002;11:156–159. doi: 10.1054/ijoa.2002.0970. [DOI] [PubMed] [Google Scholar]
- 12.Thomas JS, Koh SH, Cooper GM. Haemodynamic effects of oxytocin given as i.v. bolus or infusion on women undergoing caesarean delivery. Br J Anaesth. 2007;98:116–119. doi: 10.1093/bja/ael302. [DOI] [PubMed] [Google Scholar]
- 13.Butwick AJ, Coleman L, Cohen SE, Riley ET, Carvalh B. Minimum effective bolus dose of oxytocin during elective caesarean delivery. Br J Anaesth. 2010;104:338–343. doi: 10.1093/bja/aeq004. [DOI] [PubMed] [Google Scholar]
- 14.Svanstrom MC, Biber B, Hanes M, Johansson G, Naslund U, Balfors EM. Signs of myocardial ischemia after injection of oxytocin and methylergometrine during caesarean section. Br J Anaesth. 2008;100:683–689. doi: 10.1093/bja/aen071. [DOI] [PubMed] [Google Scholar]
- 15.Moertl MG, Friedrich S, Krashl J, Wadsack C, Lang U, Schlembach D. Haemodynamic effects of carbetocin and oxytcin given as intravenous bolus on women undergoing caesarean delivery: a randomised trial. B J Obstet and Gynaecol. 2011;118:1349–1356. doi: 10.1111/j.1471-0528.2011.03022.x. [DOI] [PubMed] [Google Scholar]
- 16.Su LL, Ching YS, Samuel M. Carbetocin for preventing post-partum haemorrhage. Cochrane Database Syst Rev. 2012;15:CD005457. doi: 10.1002/14651858.CD005457.pub3. [DOI] [PubMed] [Google Scholar]
- 17.Dansereau J, Joshi AK, Helewa ME, Doran TA, Lange IR, Luther ER, et al. Double-blind comparison of carbetocin versus oxytocin in prevention of uterine atony after caesarean section. Am J Obstet Gynecol. 1999 Mar;180( 3Pt 1):670–676. doi: 10.1016/s0002-9378(99)70271-1. [DOI] [PubMed] [Google Scholar]
- 18.Sweeney G, Holbrook AM, Levine M, Yip M, Alfredson K, Cappi S. Pharmacokinetics of carbetocin, a long acting oxytocin analogue, in nonpregnant women. Curr Ther Res. 1990;47:528–540. [Google Scholar]
- 19.van Dongen PWJ, Vebruggen MM, de Groot ANJA, van Roosmalen J, Sporken JMJ, Shulz M. Ascending dose tolerance study of intramuscular carbetocin administration after normal vaginal birth. Eur J Obstet Gynecol Reprod Biol. 1998;77:181–187. doi: 10.1016/s0301-2115(97)00260-1. [DOI] [PubMed] [Google Scholar]
- 20.Pittman JG. Water intoxication due oxytocin. N Engl J Med. 1963;268:481–482. doi: 10.1056/NEJM196302282680908. [DOI] [PubMed] [Google Scholar]
- 21.Potter RR. Water retention due to oxytocin. Obstet Gynecol. 1964;23:699–702. [PubMed] [Google Scholar]
- 22.Sauville E, Carney D, Battey J. The human vasopressin gene is linked to the oxytocin gene and is selectively expressed in a cultured lung cancer cell line. J Biol Chem. 1985;260:10236–10241. [PubMed] [Google Scholar]
- 23.Li C, Wang W, Summer SN, Westfall TD, Brooks DP, Schirier RW. Molecular mechanism of antidiuretic effect of oxytocin. J Am Soc Nephrol. 2008;19:225–232. doi: 10.1681/ASN.2007010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nielsen S, Frokiaer J, Merples D, Kwon TH, Agre P, Knepper MA. Acquaporins in the kidney: from molecules to medicine. Physiol Rev. 2002;82:205–244. doi: 10.1152/physrev.00024.2001. [DOI] [PubMed] [Google Scholar]
- 25.Chou CL, Di Giovanni SR, Meja R, Nielsen S, Knepper MA. Oxytocin is a antidiuretic hormone. I. Concentration dependence of action. Am J Physiol. 1995;269:70–77. doi: 10.1152/ajprenal.1995.269.1.F70. [DOI] [PubMed] [Google Scholar]
- 26.De Bonis M, Torricelli M, Leoni L, Berti P, Ciani V, Puzzutiello R, et al. Carbetocin versus oxytocin after caesarean section: similar efficacy but reduced pain perception in women with high risk of postpartum haemorrhage. The J of Maternal-Fetal and Neonatal Med. 2012;25:732–735. doi: 10.3109/14767058.2011.587920. [DOI] [PubMed] [Google Scholar]