Summary
Introduction
placental mesenchymal dysplasia (PMD) is a rare placental anomaly characterized by placentomegaly and grape-like vesicles which resemble molar pregnancy.
Case
we report the case of 33-year-old woman (1-gravid) who visited our clinic at 11 weeks of gestation due to a suspected molar pregnancy. Ultrasound examination showed an enlarged placenta with multiple vesicular lesions. Maternal human chorionic gonadotropin level was normal and chorionic villus sampling showed a normal male karyotype (46 XY). The fetus exhibited no specific anomalies and fetal growth was normal during pregnancy with no signs of fetal suffering. At 31 weeks, the pregnancy ended owing to intrauterine fetal death (IUFD). The patient delivered a normal-sized male fetus (1800 g) with no definite anomalies. A pathological examination led to a diagnosis of placental mesenchymal dysplasia.
Conclusion
in the presence of placental ultrasound anomalies with no other sign of fetal suffering, the pregnancy should be considered at risk and, therefore, should be monitored carefully including the option of hospitalization.
Keywords: placental dysplasia, intrauterine death, molar pregnancy
Introduction
Placental mesenchymal dysplasia (PMD) is a rare placental anomaly characterized by placentomegaly and grape like vesicles which resemble molar pregnancy. The incidence of PMD is reported to be 0.02% (1). Distinguishing PMD from its mimics, especially molar pregnancy, is important in order to prevent the unnecessary termination of pregnancy. Unlike molar pregnancies, PMD usually involves a normal fetus. However, PMD has a high incidence of fetal growth restriction (FGR) and intrauterine fetal death (IUFD), and it has been associated with Beckwith-Wiedemann syndrome (macrosomia, visceromegaly, macroglossia, and omphalocele) (2). Pathologically, in PMD, placentas are usually large in size and show edema of stem villi with intact terminal villi and many kinds of vascular anomalies, such as cirsoid chorionic vessels, thrombosis, thickening of vessel wall, vascular stenosis, villous chorangiosis, chorioangioma and fetal thrombotic vasculopathy. There have also been reports of umbilical cords, including tortuous, marked twisted cords, and excessively long cords (3, 4). Absence of trophoblastic proliferation in PMD placentas is the main histological difference from partial moles. We report a case of PMD with intrauterine sudden death of a normal-sized fetus.
Case presentation
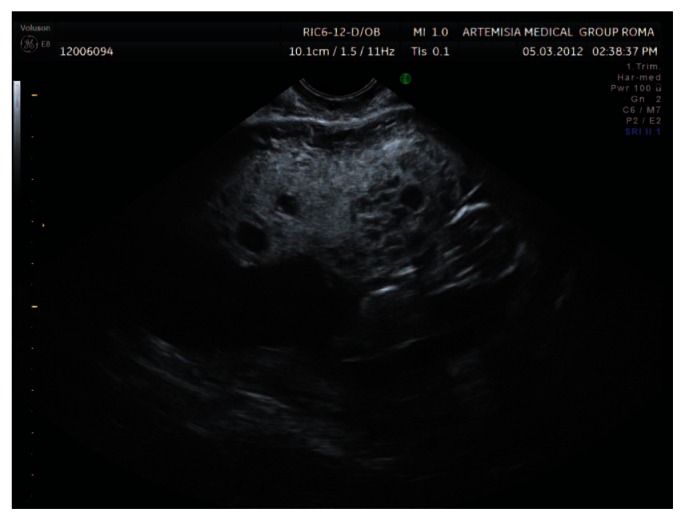
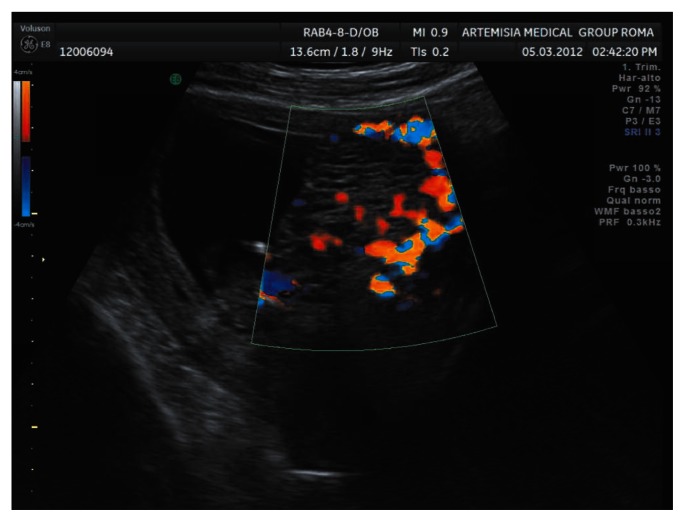
A 33-year old woman with a completely negative anamnesis at her first pregnancy was referred to our centre at 11 weeks of gestation on account of a suspected molar pregnancy. Our scan showed a normal size fetus (crown-rump length 45 mm) with no detectable anomalies at that gestation time and an enlarged placenta with multiple vesicular lesions (Figs. 1, 2). The Doppler study excluded abnormal trophoblastic vascularization.
Figure 1.
Aspect of the placenta at 11 weeks of gestation showing multiple vesicular lesions without abnormal vascularization.
Figure 2.
Aspect of the placenta at 11 weeks of gestation showing multiple vesicular lesions without abnormal vascularization.
The analysis of the maternal hCG levels showed a normal value (122.000 mUI/ml) for gestational age. However, maternal alpha-fetoprotein (AFP) was found to be above average (196 ng/ml).
On the same day, we performed a chorionic villus sampling that showed a normal male karyotype (46 XY).
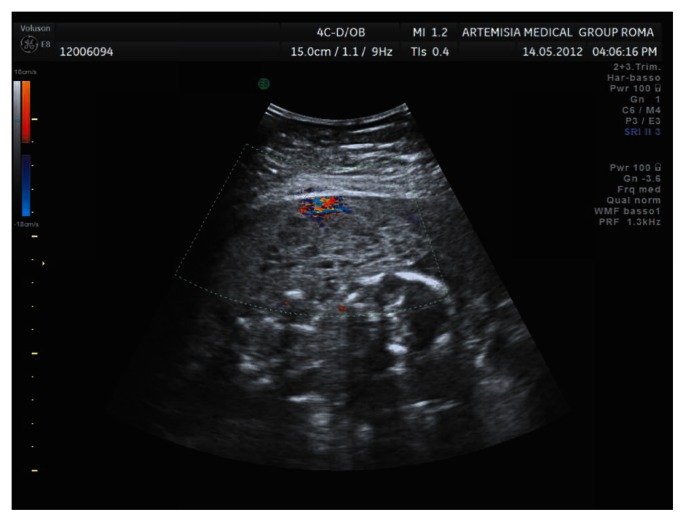
We planned monthly ultrasound scans to monitor the placenta lesions, fetal anatomy and growth. No fetal abnormalities or worsening of placental lesions were observed throughout the pregnancy (Fig. 3). A normal Doppler velocimetry of the uterine arteries was recorded at the time of the anomaly scan.
Figure 3.
Aspect of placenta at 22 weeks of gestation.
At 28 weeks’ gestation, the placental tissue was found to have the same characteristics and the growth of the fetus was regular (estimated fetal weight 1240 g +/− 10%) with a normal flow pattern in the umbilical and cerebral arteries. At 31 weeks, the patient went to the hospital because of a reduction in fetal movement. An intrauterine fetal demise was diagnosed.
After a labor induction, the patient delivered a 1800 g male fetus (50° percentile) with no definite anomalies. The placenta and the umbilical cord were larger than normal. The post mortem examination indicated a normal fetus.
A macroscopic examination showed that the placenta weight was 800 g. with a diameter of between 20 to 25 cm. Average thickness was 3.5 cm. The chorionic vessels were significantly dilated up to a diameter of 3 cm. The cord (40 cm) had a velamentous insertion and was found to be coiled.
The microscopic examination showed focally dilated and cystic villi, some of which were winding, thrombosed and immersed in blood. There was no trophoblastic proliferation.
Macroscopic and microscopic morphology confirmed a diagnosis of “placental mesenchymal dysplasia”.
Discussion
The main differential diagnoses of PMD are partial hydatidiform mole, dichorionic twins of a normal fetus and complete mole, and confined placental mosaicism. In fact, the sonographic features (thickened placenta with hypoechoic spaces) of PMD are very similar to those of partial moles. The other differential diagnoses of these ultrasonographic findings include chorioangiomas and subchorionic hematomas. However, these findings are not as common as in PMD.
Absence of trophoblastic proliferation in PMD placentas is the main histological difference from partial moles. For this reason, in PMD, levels of maternal serum hCG are normal or slightly increased throughout gestation (4). Moreover, the enlargement in the surface transfer area as a result of the increased placental volume and number of vessels within the stem villi is thought to lead to an increased transfer of AFP into the maternal circulation (5).
In the present case, the placenta was enlarged and with multiple vesicular lesions from 11 weeks.
Villocentes is showed a normal male karyotype, 46 XY, the maternal hCG level was normal and the serum AFP level was higher than normal throughout gestation.
These biochemical, cytogenetic and morphological characteristics of the placenta suggested PMD.
During the third trimester, the chorionic plate vessels in a PMD placenta are dilated and tortuous (5). However, in cases that terminate before 20 weeks of gestation, the chorionic plate vessels are not dilated, and the normal and abnormal areas are not clearly linear, suggesting that the vascular malformations develop progressively (6). As the pregnancy advances, tangled congested vessels grossly resembling gray-white or dark-red wormlike structures may be identified within the parenchyma and are often most prominent in the sub chorionic plate region near the fetal surface (7). The causes of intrauterine fetal death (IUFD) currently remain unclear and may be heterogeneous. Thrombosis of chorionic vessels and umbilical cord anomalies are thought to be likely causes of IUFD in PMD cases, and Truc et al. (3) reported that IUFD may be explained by a potentially chronic hypoxia which is a consequence of obstructive fetal vascular thrombosis and a decrease in maternal-fetal gas exchange as a result of an insufficient amount of normal chorionic villi. In our case, histological examination revealed that the dilated cirsoid chorionic vessels were fragile and that part of the vessel wall had ruptured, resulting in a hemorrhage and the formation of a hematoma. Although fetal thrombotic vasculopathy was found in a part of the affected lesion, this hemorrhage was thought to have provoked sudden death of the fetus at late gestational age (31 weeks) because no significant chronic hypoxic anomaly of the fetus, including growth restriction, was observed.
Conclusions
PMD should be included in a differential sonographic diagnosis of cystic lesions of the placenta, especially when a phenotypically normal-appearing fetus has been identified. When the prenatal characteristics of PMD are detected (such as an elevated AFP and normal hCG level in the mother, a normal karyotype revealed by villocentesis and dilated subchorionic vessels revealed by ultrasonography during the third trimester), labour at an optimal time before term could be considered, since sudden IUFD might occur. An accurate monitoring, including the hospitalization of the patient when necessary, could save the life of the fetus.
References
- 1.Lokan J, Chan YF, Agnesta F. Placental mesenchymal dysplasia. Pathology. 2002;34:375–378. doi: 10.1080/003130202760120571. [DOI] [PubMed] [Google Scholar]
- 2.Paradinas FJ, Sebire NJ, Fisher RA, Rees HC, Foskett M, Seckl MJ, Newlands ES. Pseudo-partial moles: placental stem vessel hydrops and the association with Beckwith-Wiedemann syndrome and complete moles. Histopathology. 2001 Nov;39(5):447–454. doi: 10.1046/j.1365-2559.2001.01256.x. [DOI] [PubMed] [Google Scholar]
- 3.Truc P, Julie S, Carla S. Placental Mesenchymal Dysplasia is associated with high rates of intrauterine growth restriction and fetal demise. A report of 11 new cases and a review of the literature. Am J Clin Pathol. 2006;126:67–78. doi: 10.1309/RV45-HRD5-3YQ2-YFTP. [DOI] [PubMed] [Google Scholar]
- 4.Parveen Z, Tongson-Ignacio JE, Fraser CR, Killeen JL, Thompson KS. Placental mesenchymal dysplasia. Pathol Lab Med. 2007;131:131–137. doi: 10.5858/2007-131-131-PMD. [DOI] [PubMed] [Google Scholar]
- 5.Natori M, Tanaka M, Ishimoto H, Gohda N, Kiyokawa K, Yamauchi J, Miyazaki T, Kobayashi T, Nozawa S, Takagi T. Relation of gestational age, maternal body weight and age or serum alpha-fetoprotein and human chorionic gonadotropin at second-trimester. Nippon Sanka Fujinka Gakkai Zasshi. 1994;46:562–566. [PubMed] [Google Scholar]
- 6.Moscoso G, Jauniaux E, Hustin J. Placental vascular anomaly with diffuse mesenchymal stem villous hyperplasia. A new clinico-pathological entity? Pathol Res Pract. 1991;187:324328. doi: 10.1016/s0344-0338(11)80791-0. [DOI] [PubMed] [Google Scholar]
- 7.Jauniaux E, Nicolaides KH, Hustin J. Perinatal features associated with placental mesenchymal dysplasia. Placenta. 1997;18:701–706. doi: 10.1016/s0143-4004(97)90012-6. [DOI] [PubMed] [Google Scholar]