SUMMARY
Fixed orthodontic appliances make it difficult to maintain the oral hygiene, resulting in plaque accumulation. Retention of bacterial plaque, represents a risk for white spot lesions and development of periodontal disease.
Aim
Purpose of this study was to determine in vivo the retention of plaque on three different elastic ligatures, in comparison with stainless steel ligature, to determine a possible association between type of ligatures and accumulation of microorganisms.
Material and Methods
three elastic ligation systems were analyzed for plaque retention: ring-shape, clear, latex ligatures (Leone® Spa), ring-shape, grey, polyurethane ligatures (Micerium® Spa) and grey, polyurethane, Slide low-friction ligatures (Leone® Spa), compared with stainless steel ligatures (Leone® Spa) used as control. Forthy orthodontic patients undergoing fixed orthodontic therapy were selected.
A sample for each type of ligature were applied inside the oral cavity of each subject. Samples were kept in the oral cavity for 28 days, ligating 0.16 X 0.22 stainless steel archwire to stainless steel orthodontic premolars brackets. The presence of bacterical slime was quantified by spectrophotometric method (crystal violet-Bouin’s fixative) and morphological observations was evaluated by Scanning Electron Microscopy (SEM).
Results
From analysis of bacterical slime emerges that all the elastics showed a low plaque retention, especially if compared to the group of steinless steel ligatures, that presented a greater plaque adhesion, statistically significant compared to the Slide group (r<0.0002) and the two elastic groups (r<0.0001). This study reported no significant difference between the Slide ligatures and the traditional elastic ligatures as regards the retention of plaque. SEM images showed presence of cocci, rods and few filamentous organisms and an interbacterial matrix in all observed samples.
Conclusion
Elastomeric ligatures showed a significant lower susceptibility to plaque adhesion, in comparison to the stainless steel of the metallic ligatures. No statistically significant difference was observed among the elastic devices.
Keywords: orthodontic ligature, plaque adhesion, SEM
Introduction
The fixed orthodontic appliance is able to alter the coronal anatomy of the tooth, increasing the number of retentive surfaces and determining a difficulty in controlling the formation and adhesion of plaque (1). Accumulation and retention of bacterial plaque represent a risk for white spot lesion development during orthodontic treatment and development or worsening of periodontal pathologies (2, 3, 4). The labial enamel of teeth ligated with an elastomeric ring exhibits a significantly higher number of micro-organisms in the plaque than that of incisors ligated with stainless steel wire (5). SEM examination of brackets, excess composite, and buccal enamel reveals that mature plaque is present on excess composite at 2 and 3 weeks after bonding, when plaque on the gingival enamel surface is still at an early stage of maturation. The method of ligation does not appear to influence the bacterial morphotypes on composite and enamel surface (6, 7).
Some studies showed that Streptococcus sanguis (which causes gingivitis, papillary hypertrophies and formation of pseudo pockets), Treponema denticola, Actinobacillus actinomycetencomitans, Fusobacterium nucleatum, Streptococcus anginosus and Eubacterium nodatum adhere selectively to the surface of stainless steel orthodontic brackets, while on ceramic brackets there is the proliferation of Streptococcus mutans and Eikenella corrodens, Campylobacter and Selenomonas to a lower extent (8, 9, 10, 11). In proportion to the number of orthodontic bands applied, there is also an increase of lactobacilli, staphylococci, and moving bacterial rods which are all the expression of the aggressiveness of subgingival bacterial plaque (12, 13, 14).
In general, an ideal habitat is created in the oral cavity for the growth and development of Gram negative anaerobic micro-organisms, which are the main cause of periodontitis (2, 15, 16, 17).
Therefore, it is obvious that the chemical and physical characteristics of the various materials and morphology of the numerous orthodontic appliances determine the electiveness of the bacterial species in term of quality and quantity (18, 19).
During the orthodontic therapy with fixed appliances, the risk of developing periodontal inflammatory lesions and demineralisations of the enamel, commonly called white spots, increases almost exponentially because the main components of this device, brackets, bands, ligatures and orthodontic wires, are able to:
reduce the physiological mechanisms of self-cleansing operated by the tongue, cheeks, and saliva;
increase the retention of bacterial plaque due to the increase of the number of retentive surfaces for the sub-layers;
cause compression effects damaging oral mucosas;
change the bacterial population from a qualitative and quantitative point of view (19, 20, 21, 22, 23, 24, 25).
It has been observed that after removing the fixed appliance, this risk drops to average levels with the reduction of plaque index, gingival inflammation and initial demineralization lesions (23, 26). These clinical findings are confirmed by micro-biological exams on Streptococcus mutans levels identified prior, during and after the orthodontic treatment (23, 27). The collection of plaque samples, the measurement of colony forming units (CFU) and the comparison with untreated patients, showed an increase of the micro-organism during the active phase of the therapy, a return to pre-therapy levels during the retention phase and constant, long term maintenance (28, 29).
Among the components of the fixed orthodontic appliance, the ligatures are the auxiliary components that allow the proper mechanical connection between the orthodontic arch and the bracket slot, in different therapeutic phase and technique (30).
Stainless steel ligatures and circular elastomeric ligatures (latex or polyurethane) of new generation are generally considered as traditional ligature systems. Slide ligatures are of a new generation; they are made of a harder polyurethane mix compared to traditional elastics and are produced with a new shape that creates passive, low friction contact with the orthodontic bracket (31).
Many studies were carried out concerning the quantitative and qualitative aspect of bacterial adhesion on orthodontic ligature (9, 32).
Forsberg et al., focused on the association between Streptococcus mutans and Lattobacillus colonization and type of ligatures, whether elastomeric or metallic (5, 23). The elastic ligatures accumulate a greater quantity of bacterial plaque and after the application of a fixed appliance, also the number of colonies in the saliva increases. However this increase does not depend on the type of ligature used (5, 23).
There are no significant associations between the gingival index, plaque index and pocket depth in relation to the used ligatures, except for the bleeding index, which is greater in patients with elastic ligatures (33, 34).
The purpose of this research was to carry out a qualitative analysis of the residual plaque on the surface of 4 different orthodontic ligatures, after 4 weeks of permanence in the oral cavity. In addition, a quantity evaluation of the microbic population was carried out through spectrophotometric test.
Materials and methods
Experimental protocol
Volunteers were recruited from patients about to start their orthodontic treatment with maxillary and mandibular fixed appliance. Four type of ligatures have been analysed: ring-shape, clear, latex ligatures (Leone® Spa, Sesto Fiorentino, FI, Italy), ring-shape grey, polyurethane ligatures (Micerium® Spa, Avegno, Ge, Italy), grey, polyurethane Slide low-friction ligatures (Leone® Spa, Sesto Fiorentino, FI, Italy) and stainless steel ligatures (Leone® Spa, Sesto Fiorentino, FI, Italy).
The study sample consisted of 20 male and 20 female subjects (aged 13–17 years). Patients taking antibiotics or having systemic disease were excluded. Informed consent was obtained from parents for all the subjects. Patients were supplied with standardized toothpaste and asked to refrain from any other hygiene products for the duration of the trial.
The specimens tied a stainless steel 0.16 X 0.22 archwire to stainless steel twin premolars brackets (Leone® Spa, Sesto Fiorentino, FI, Italy). Ligatures were placed according to the following scheme: upper second premolars with Slide ligatures, upper first premolars with grey, ring-shape ligatures, lower second premolars with steinless steel ligatures, and lower first premolars with clear, ring-shape ligatures.
After 28 days ligatures were aseptically removed by the same operator that tied them in, transferred in separate test tubes containing a saline solution and cooled (NaCl 0.9%; 4°C). Twenty samples of each type of ligature were collected and taken to the laboratory within 10 minutes.
Determination of plaque adhesion
Specimens were washed 3 times with PBS ( Phosphate Buffered Saline, Sigma-Aldrich, St. Louis, MO, USA) to eliminate bacteria which did not adhere to the material, and then they were incubated for two hours in Bouin fixative (picric acid – acetic acid – methanol) at 25°C.
After being washed with PBS, the samples were incubated in 0.01% crystal violet for 15 minutes at 37°C. Specimens were then treated with 200 ml of 10% Sarkosyl (lauryl sarcosinate sodium). After 5 minutes at 37°C, the supernatant was analysed using a spectrophotometer for micro-plates at 570 nm. The bacterial adhesion on each sample was quantified in relation to the optical density of the control sample (unused ligature).
SEM Morphological analysis
Specimens of each elastic ligature were subject to a preparation phase consisting of fixing, dehydration, and sputtering: the specimens were washed twice with PBS, fixed in a 2.5% glutaraldehyde solution in PBS for 30 minutes and washed twice with PBS. Then specimens were dehydrated with ethanol solutions in PBS at increasing concentrations: twice in a 70% solution 15 minutes, twice in a 90% solution for 15 minutes, and twice in 100% ethanol for 15 minutes.
Finally specimens were treated for 5 minutes with HMDS (Hexamethyl disilazane, Sigma-Aldrich, St. Louis, MO, USA), dried and glued on stubs. Stubs were treated with acetone to eliminate possible impurities and, through a specific device (Quick Drying Silver Paint, Agar Scientific LTD, Stansted, Essex, England), specimens were glued on stubs prior to be subjected to the golden sputtering (SEM Coating System, Bio-Rad, Microscience Division, Hercules, CA, USA), for 4 minutes.
Once cooled down, the specimens were stored vacuum-sealed inside a glass container and analysed within 5 days at SEM (Digital Scanning Microscope, DSM 950, Zeiss).
Results
Table 1 shows the results of the quantitative analysis of plaque that adhered on the 4 materials tested after staying 28 days inside the oral cavity.
Table 1.
Quantitative analysis of plaque: means and standard deviations of Optical Density at 570 nm.
| Method of ligation | Mean (Plaque retention) | SD |
|---|---|---|
| Polyurethane Slide low-friction (GS) | 0.074 | 0.08 |
| Clear, latex, ring-shape ligatures (CR) | 0.048 | 0.10 |
| Grey, polyurethane, ring-shape ligatures (GR) | 0.055 | 0.10 |
| Stainless steel ligatures | 0.183 | 0.07 |
The value is expressed as absorption at 570 nm, which gives a measurement of the slime produced by the bacteria that adhered to the ligatures. From the results it emerges that all the elastics showed a good behaviour, especially if compared to the group of metal ligatures, that presented with a greater plaque adhesion. This difference was statistically significant compared to the Slide group (p<0.0002) and the other two groups of elastic ligatures (p<0.0001).
A small difference was detected between Slide and grey and transparent elastics, but this is probably due to the greater surface of the Slide.
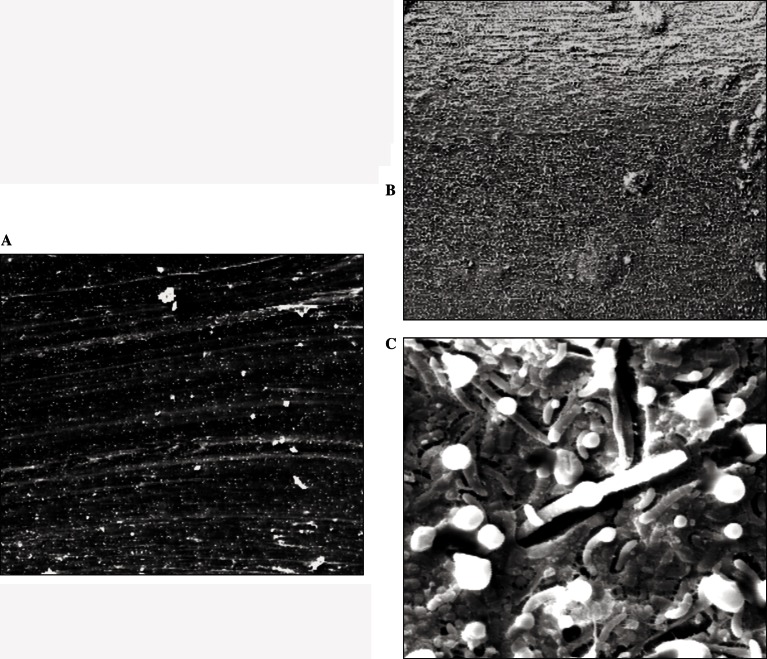
SEM low magnification (X200) images of clear ring-shape ligatures (CR) before and after 4 weeks of permanence in oral cavity are shown in Figure 1A–B. A fine layer of plaque covers the CR ligature. A higher magnification (X5000) image revealed cocci and rods aggregated in an area with surface irregularities and an extracellular amorphous matrix (Fig. 1 C).
Figure 1.
SEM micrograph of clear, elastomeric, ring-shape ligatures (CR). Before use (A-×200) and after 4 weeks of permanence in oral cavity at original magnification ×200(B) and ×5000(C).
Grey polyurethane Slide low-friction ligatures (GS) showed at low magnification (X200) irregular and thick deposit of plaque (2B), probably due to the irregular surface of ligature (Fig. 2 A, B). At a higher magnification (X5000) the surface exhibited a prevalence of cocci, rods and some filaments (Fig. 2 C). Bacterial colonization of grey, polyurethane, ring-shape ligatures (GR) is presented in Figure 3 where the surface irregularity of the ligature before use is shown (Fig. 3 A). A fine layer of plaque deposition on GR ligatures is revealed at a low magnification (X200) (Fig. 3 B). Abundant bacteria are present, rods are prevalent, with some cocci, as shown at a higher magnification (X5000) (Fig. 3 C).
Figure 2.
SEM micrograph of grey polyurethane Slide low-friction ligatures (GS). Before use (A-×200) and after 4 weeks of permanence in oral cavity at original magnification ×200(B) and ×5000(C).
Figure 3.
SEM micrograph of grey, polyurethane, ring-shape ligatures (GR). Before use (A-×200) and after 4 weeks of permanence in oral cavity at original magnification ×200(B) and ×5000(C).
Discussion
Adhesion of micro-organisms to the surfaces is the result of specific lectin-similar reactions, electro-static interactions, and Van der Waals forces; the surfaces with higher surface free energy show a favourable effect on bacterial adhesion. The initial attack is an important factor that determines the further colonization. The attack mechanisms and following adhesion can vary significantly from one material to another. There is a close relationship between microbic colonization and surface free energy, hydrophobicity and zeta potential of interacting surfaces (6). The materials from which the three ligatures are made showed extremely low adhesive properties towards plaque, compared to the stainless steel of metallic ligatures. This indicates that the surface of elastomeric ligatures boasts low wettability, thus reducing the adhesion of salivary proteins, the production of polysaccharides and therefore bacterial colonization. Clear and grey elastics showed the same behaviour in terms of bacterial adhesion, but the clear ones presented greater absorption of the crystal violet colorant, indicating that the material could anyhow absorb small molecules of saliva, as bacterial endotoxins, even if it does not absorb bacteria. It has already been proven that some types of elastic ligatures can absorb and release molecules such as fluorine (32, 35).
Elastic ligatures showed a lower slime production if compared to stainless steel ligation, but a supragingival plaque accumulation consisting of cocci and rods was present on all types of ligatures by 4 week. A more mature plaque with an intermicrobial matrix was present on transparent elastomeric round ligatures, that presented a rough and porous surface, even if they did not present greater slime production. The residues on the surface are probably due to the precipitation of a mineralized protein film, therefore a precipitation of calcium with a non specific mechanism. Spirochetes were not displayed in many specimens. Coccoid plaque was predominant on GS ligatures, with little presence of the intercellular matrix. Predominant rods population with a mature intermicrobial matrix was present on GR samples.
Other Authors have conducted interesting studies to evaluate which type of ligature, whether elastic or metallic, favours the accumulation of plaque, with the purpose of verifying a possible association between the type of ligature and increase of some indexes of periodontal disease. The elastics showed a greater number of micro-organisms compared to metal ligatures, but this difference did not appear to be significant.
All elastic ligatures showed a reduced slime production in comparison with stainless steel ligature in vivo. Clear round elastomeric ligatures showed a more mature plaque with an organized intercellular matrix in comparison with other elastomeric ligatures.
References
- 1.Rosenbloom RG, Tinanoff N. Salivary Streptococcus mutans levels in patients before, during and after orthodontic treatment. Am J Orthod Dentofacial Orthop. 1991 Jul;100(1):35–7. doi: 10.1016/0889-5406(91)70046-Y. [DOI] [PubMed] [Google Scholar]
- 2.Davies TM, Shaw WC, Worthington HV, Addy M, Dummer P, Kingdon A. The effect of orthodontic treatment on plaque and gengivitis. Am J Orthod Dentofacial Orthop. 1991 Feb;99(2):155–61. doi: 10.1016/0889-5406(91)70118-G. [DOI] [PubMed] [Google Scholar]
- 3.Forsberg Cm, Brattstrom V, Malmberg E, Nord CE. Ligature wires and elastomeric rings: two methods of ligating, and their association with microbial colonization of streptococcus mutans and lactobacilli. Eur J Orthod. 1991 Oct;13(5):416–20. doi: 10.1093/ejo/13.5.416. [DOI] [PubMed] [Google Scholar]
- 4.Zimmer BW, Rottwinkel Y. Assessing patient-specific decalcification risk in fixed orthodontic treatment and its impact on prophylactic procedures. Am J Orthod Dentofacial Orthop. 2004 Sep;126(3):318–24. doi: 10.1016/j.ajodo.2003.09.031. [DOI] [PubMed] [Google Scholar]
- 5.Sukontapatipark W, el-Agroudi MA, Selliseth NJ, Thunold K, Selving KA. Bacterial colonization associated with fixed orthodontic appliance. A Scanning electron microscopy study. Eur J Orthod. 2001 Oct;23(5):475–84. doi: 10.1093/ejo/23.5.475. [DOI] [PubMed] [Google Scholar]
- 6.Benson PE, Douglas CW, Martin MV. Fluoridated elastomers: effect on the microbiology of plaque. Am J Orthod Dentofacial Orthop. 2004 Sep;126(3):325–30. doi: 10.1016/j.ajodo.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 7.Eliades T, Eliades G, Brantley WA. Microbial attachment on orthodontic appliances: I. Wettability and early pellicle formation on bracket materials. Am J Orthod Dentofacial Orthop. 1995 Oct;108(4):351–60. doi: 10.1016/s0889-5406(95)70032-3. [DOI] [PubMed] [Google Scholar]
- 8.Fournier A, Payant L, Bouclin R. Adherence of Streptococcus mutans to orthodontic brackets. Am J Orthod Dentofacial Orthop. 1998 Oct;114(4):414–7. doi: 10.1016/s0889-5406(98)70186-6. [DOI] [PubMed] [Google Scholar]
- 9.Knoernschild KL, Rogers HM, Lefebvre CA, Fortson WM, Schuster GS. Exdotoxin affinity for orthodontic brackets. Am J Orthod Dentofacial Orthop. 1999 Jun;115(4):634–9. doi: 10.1016/s0889-5406(99)70288-x. [DOI] [PubMed] [Google Scholar]
- 10.Ogaard B, Rolla G, Arends J, ten Cate JM. Orthodontic appliances and enamel demineralization. Part 2. Prevention and treatment of lesions. Am J Orthod Dentofacial Orthop. 1988 Aug;94(2):123–8. doi: 10.1016/0889-5406(88)90360-5. [DOI] [PubMed] [Google Scholar]
- 11.Paolantonio M, di Girolamo G, Pedrazzoli V, di Murro C, Picciani C, Catamo G, Cattabriga M, Piccolomini R. Occurrence of Actinobacillus actinomycetemcomitans in patients wearing orthodontic appliances. A cross-sectional study. J Clin Periodontol. 1996 Feb;23(2):112–8. doi: 10.1111/j.1600-051x.1996.tb00543.x. [DOI] [PubMed] [Google Scholar]
- 12.Atack NE, Sandy JR, Addy M. Periodontal and microbiological changes associated with the placement of orthodontic appliances. A review. J Periodontol. 1996 Feb;67(2):78–85. doi: 10.1902/jop.1996.67.2.78. [DOI] [PubMed] [Google Scholar]
- 13.Ristic M, Vlahovic Svabic M, Sasic M, Zelic O. Effects of fixed orthodontic appliances on subgingival microflora. Int J Dent Hyg. 2008 May;6(2):129–36. doi: 10.1111/j.1601-5037.2008.00283.x. [DOI] [PubMed] [Google Scholar]
- 14.Sharma NC, Lyle DM, Qaqish JG, Galustians J, Schuller R. Effect of a dental water jet with orthodontic tip on plaque and bleeding in adolescent patients with fixed orthodontic appliances. Am J Orthod Dentofacial Orthop. 2008 Apr;133(4):565–71. doi: 10.1016/j.ajodo.2007.12.008. quiz 628.e1–2. [DOI] [PubMed] [Google Scholar]
- 15.Benoist HM, Ngom PI, Seck-Diallo A, Diallo PD. Treatment of localized gingival hipertrophy induced by orthodontic appliances using modified Widman flap. A case report. Odontostomatol Trop. 2007 Sep;30(119):5–9. [PubMed] [Google Scholar]
- 16.Petti S, Barbato E, Simonetti D’Arca A. Effect of orthodontic therapy with fixed and removable appliances on oral microbiota: a six-month longitudinal study. New Microbiol. 1997 Jan;20(1):55–62. [PubMed] [Google Scholar]
- 17.Ristic M, Vlahovic Svabic M, Sasic M, Zelic O. Clinical and microbiological effects of fixed orthodontic appliances on periodontal tissues in adolescents. Orthod Craniofac Res. 2007 Nov;10(4):187–95. doi: 10.1111/j.1601-6343.2007.00396.x. [DOI] [PubMed] [Google Scholar]
- 18.Gusberti FA. Clinical and microbiological periodontal aspects in orthodontic treatments. Schweiz Monatsschr Zahnmed. 1984 May;94(5):462–8. [PubMed] [Google Scholar]
- 19.Kiekens RM, Kuijpers-Jagtman AM. Iatrogenic effects of orthodontic therapy. Ned Tijdschr Tandheelkd. 2000 Apr;107(4):173–7. [PubMed] [Google Scholar]
- 20.De Franco DJ, Spiller RE, Jr, von Fraunhofer JA. Frictional resistances using Teflon-coated ligatures with various bracket-archwire combinations. Angle Orthod. 1995;65(1):63–72. doi: 10.1043/0003-3219(1995)065<0063:FRUTLW>2.0.CO;2. discussion 73–4. [DOI] [PubMed] [Google Scholar]
- 21.Gomes SC, Varela CC, da Veiga SL, Rösing CK, Oppermann RV. Periodontal conditions in subjects following orthodontic therapy. A preliminary study. Eur J Orthod. 2007 Oct;29(5):477–81. doi: 10.1093/ejo/cjm050. [DOI] [PubMed] [Google Scholar]
- 22.Lo BA, Di Marco R, Milazzo I, Nicolosi D, Calì G, Rossetti B, Blandino G. Microbiological and clinical periodontal effects of fixed orthodontic appliances in pediatric patients. New Microbiol. 2008 Apr;31(2):299–302. [PubMed] [Google Scholar]
- 23.Magno AFF, Enoki C, Ito IY, Matsumoto MAN, Faria G, Nelson-Filho P. In-vivo evaluation of the contamination of Super Slick elastomeric rings by Streptococcus mutans in orthodontic patients. Am J Orthod Dentofacial Orthop. 2008;133:S104–9. doi: 10.1016/j.ajodo.2006.04.054. [DOI] [PubMed] [Google Scholar]
- 24.Ogaard B, Rolla G, Arends J. Orthodontic appliances and enamel demineralization. Part 1. Lesion development. Am J Orthod Dentofacial Orthop. 1988 Jul;94(1):68–73. doi: 10.1016/0889-5406(88)90453-2. [DOI] [PubMed] [Google Scholar]
- 25.Van Gastel J, Quirynem M, Teughels W, Pauwels M, Coucke W, Carels C. Microbial Adhesion on different bracket types in vitro. Angle Orthod. 2009;79:915–921. doi: 10.2319/092908-507.1. [DOI] [PubMed] [Google Scholar]
- 26.Sallum EJ, Nouer DF, Klein MI, Gonçalves RB, Machion L, Wilson Sallum A, Sallum EA. Clinical and microbiologic changes after removal of orthodontic appliances. Am J Orthod Dentofacial Orthop. 2004 Sep;126(3):363–6. doi: 10.1016/j.ajodo.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 27.Read-Ward GE, Jones SP, Davies EH. A comparison of self-ligating and conventional orthodontic bracket system. Br J Orthod. 1997 Nov;24(4):390–17. doi: 10.1093/ortho/24.4.309. [DOI] [PubMed] [Google Scholar]
- 28.Brêtas SM, Macari S, Elias AM, Ito IY, Matsumoto MA. Effect of 0.4% stannous fluoride gel on Streptococci mutans in relation to elastomeric rings and steel ligatures in orthodontic patients. Am J Orthod Dentofacial Orthop. 2005 Apr;127(4):428–33. doi: 10.1016/j.ajodo.2003.12.024. [DOI] [PubMed] [Google Scholar]
- 29.Pan YC, Zhang D, Fu MK. Changes of Streptococcus mutans concentration of plaque during fixed appliance treatment. Zhonghua Kou Qiang Yi Xue Za Zhi. 2007 Jan;42(1):41–2. [PubMed] [Google Scholar]
- 30.Tuncer AV, Baylas H. Examination of the effects of various orthodontic appliances on periodontal tissues. Turk Ortodonti Derg. 1990 Apr;3(1):13–8. [PubMed] [Google Scholar]
- 31.De Souza Ra, de Araujo Magnani MBB, Nouer DF, da Silva CO, Klein MI, Sallum EA, Goncalves RB. Periodontal and microbiologic evaluation of 2 methods of archwire ligation: Ligature wires and elastomeric rings. Am J Orthod Dentofacial Orthop. 2008;134:506–12. doi: 10.1016/j.ajodo.2006.09.067. [DOI] [PubMed] [Google Scholar]
- 32.Doherty UB, Benson PE, Higham SM. Fluoride-releasing elastomeric ligatures assessed with the in situ caries model. Eur J Orthod. 2002;24:371–378. doi: 10.1093/ejo/24.4.371. [DOI] [PubMed] [Google Scholar]
- 33.Rimondini L, Cerroni L, Carrassi A, Torricelli P.Bacterial colonization of zirconia ceramic surfaces: an in vitro and in vivo study Int J Oral Maxillofac Implants 2002November–Dec176793–8. [PubMed] [Google Scholar]
- 34.Turkkahraman H, Sayin MO, Bozkurt FY, Yetkin Z, Kaya S, Onal S. Archwire ligation techniques microbial colonization, and periodontal status in orthodontically treated patients. Angle Orthod. 2005 Mar;75(2):231–6. doi: 10.1043/0003-3219(2005)075<0227:ALTMCA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 35.Casaglia A, Dominici F, Pachì F, Turlà R, Cerroni L. Morphological observations and Fractological considerations on orthodontics miniscews. Minerva Stomatol. 2010 Sep;59(9):465–76. [PubMed] [Google Scholar]