Abstract
The process of neurogenesis continues throughout life, with thousands of new neurons generated every day in the mammalian brain. Impairment of hippocampal neurogenesis has been suggested to be involved in neurodegenerative conditions including the cognitive decline associated with aging, Alzheimer's disease, Parkinson's disease, and ionizing radiation. These neurodegenerative conditions are all characterized by proinflammatory changes and increased numbers of activated microglia. Activated microglia produce a variety of pro-inflammatory factors, including IL-6, TNF-α, reactive oxygen species, and nitric oxide, all of which are antineurogenic. These same factors have also been shown to suppress mitochondrial function, but the role of mitochondria in neurogenesis remains barely investigated. This brief review summarizes the findings of several studies that support a role for mitochondrial impairment as part of the mechanism of the reduction of neurogenesis associated with inflammation.
Keywords: doublecortin, inflammation, microglia, lipopolysaccharide
Introduction
Neurogenesis is impaired in several inflammation-associated conditions including cranial irradiation, Alzheimer's disease (AD) and Parkinson's disease (PD), and several age associated brain pathologies that include cognitive decline as a component (Imamura et al. 2003; McGeer et al. 1988; Mizumatsu et al. 2003; Monje et al. 2003; Sparkman and Johnson 2008). While the connection between inflammation and reduced neurogenesis has been indicated in several studies (Ben-Hur et al. 2003; Ekdahl et al. 2003; Liu et al. 2005b; Monje et al. 2003), the mechanisms of the neurogenesis impairment remain poorly understood. Mitochondria are one of the primary targets of inflammatory injury (Halliwell 2006; Hunter et al. 2007; Samavati et al. 2008; Xie et al. 2004). The results of recent studies suggest that mitochondrial function might play an important role in both developmental and adult neurogenesis (Baxter et al. 2009; Calingasan et al. 2008; Kirby et al. 2009; Voloboueva et al.). In this review we highlight the mitochondrial mechanisms involved in inflammation-induced neurogenesis impairment.
Neurogenesis in Neurological Diseases and Disorders
Although the process of neurogenesis almost completely ceases in mammals after development, the generation of new neurons occurs throughout life in two brain regions (Zhao et al. 2008). Thousands of new neurons are born every day in the subgranular zone (SGZ) of the dentate gyrus (DG) and in the sub-ventricular zone (SVZ) (Aimone et al. 2010; Cameron and McKay 2001; Gage 2000). Immature neurons migrate from the SVZ to the olfactory bulb and give rise to several local interneuron populations. In the DG region of hippocampus new neurons are generated from local neuronal progenitor cells (NPC) and eventually develop into excitatory granule cells, the principal projection neurons of DG. New neurons are functionally integrated into existing neuronal circuitry (van Praag et al. 2002; Zhao et al. 2006). A positive correlation has been observed between hippocampal neurogenesis and memory formation and mood regulation in experimental animals (Kempermann et al. 1997; Santarelli et al. 2003; Shors et al. 2001). A recent study suggests that hippocampal neurogenesis might be important in the consolidation stage of memory formation (Kitamura et al. 2009). Changes in adult neurogenesis have been described both in the brains of patients and in animal models of various neurological diseases and disorders (Baker et al. 2004; Leker et al. 2007; Li et al. 2008; Raber et al. 2004b; Zhao et al. 2008).
Brain irradiation, such as that used in the treatment of head and neck tumors, is associated with cognitive impairment and this might involve effects of irradiation on the precursor cells of the hippocampal SGZ (Monje and Palmer 2003; Raber et al. 2004b; Rola et al. 2004). Significant loss of NPC cells occurs within a few hours after relatively low radiation doses (Mizumatsu et al. 2003). In addition to the acute apoptosis of precursor cells, there are long-term radiation-associated effects on these cells. The surviving NPC demonstrate reduced ability to differentiate into mature neurons in a dose-dependent fashion (Mizumatsu et al. 2003; Raber et al. 2004b). The reduction in precursor proliferation is still observed several months after irradiation and is associated with hippocampal-dependent cognitive dysfunction (Raber et al. 2004a; Rola et al. 2004). Experimental stroke in animals promotes increased neurogenesis in both adult neurogenic regions, the SVZ and the SGZ (Arvidsson et al. 2002; Liu et al. 1998; Parent et al. 2002; Zhang et al. 2001). The injured brain releases a variety of diffusible mitogens, like glutamate, erythropoietin, epidermal and vascular endothelial growth factors (EGF and VEGF), that have been implicated in enhancement of proliferation of progenitor cells (Kernie and Parent 2010; Wiltrout et al. 2007). More importantly, the neuroblasts from the SVZ migrate towards the injured regions of the striatum and cortex, and express DARPP-32, a marker of neostriatal spiny neurons upon differentiation (Arvidsson et al. 2002; Parent et al. 2002). However, despite the increased proliferation of the progenitor cells, the majority of newly generated neurons fail to survive, with the number of surviving post-ischemic striatal neurons comprising only ∼0.2% of the number of striatal neurons lost during injury (Arvidsson et al. 2002). It was proposed that inflammatory changes accompanying the ischemic damage contribute to high rates of apoptotic death of stroke-generated neuroblasts observed within the first several weeks after ischemic injury (Kokaia et al. 2006). Studies suggest that interventions promoting increased rates of post-stroke neurogenesis can lead to better functional recovery after stroke (Androutsellis-Theotokis et al. 2006; Leker et al. 2007; Schabitz et al. 2007).
It has been suggested that neurological dysfunction associated with AD could be partly due to impaired NPC formation in the hippocampal SGZ (Zhao et al. 2008). Animal models of AD have provided equivocal data, demonstrating both increased and decreased hippocampal neurogenesis (Biscaro et al. 2009; Gan et al. 2008). The disease severity, use of different animal models, and lineage-specific markers are apparently important factors in the reported discrepancy, but a recent study suggested impaired neurogenesis as an early critical event in the course of Alzheimer's disease (Demars et al. 2010). Deficient maturation of new hippocampal neurons has been reported in AD patients (Jin et al. 2004; Li et al. 2008). Thus strategies promoting survival and maturation of hippocampal NPC might be beneficial in the treatment of AD patients.
In the case of PD, a reduction of neurogenesis has been shown in animal models, as a consequence of dopamine depletion from the neighboring substantial nigra (Baker et al. 2004; Hoglinger et al. 2004; O'Keeffe et al. 2009). It has been shown that dopamine induces proliferation of precursor cells in the subventricular zone through EGF release, and that dopamine depletion leads to decrease in proliferation concomitant with reduction of EGF levels (O'Keeffe et al. 2009). The increased survival of progenitor cells might hold beneficial potential due to the proximity of both principal neurogenic regions to the basal ganglia, where the majority of the pathology is located.
Aging promotes a progressive and marked decline in the levels of endogenous neurogenesis (Enwere et al. 2004; Kuhn et al. 1996; Seki and Arai 1995). Impairment of hippocampal neurogenesis has been suggested to be linked to cognitive decline associated with aging (Drapeau and Nora Abrous 2008; Galvan and Jin 2007; Zitnik and Martin 2002). Understanding the age-related changes in neurogenesis could lead to novel treatment strategies that could modulate disease-related processes and induce repair of aged brain.
Neurogenesis and Inflammation
Inflammation plays an important role in the pathogenesis of a variety of neurological disease states that demonstrate altered adult neurogenesis. Pro-inflammatory changes and increased numbers of activated microglia were reported in irradiated animals (Mizumatsu et al. 2003; Monje et al. 2002; Rola et al. 2004). Moreover, the numbers of activated microglia showed a direct correlation with the impairment of neurogenesis (Mizumatsu et al. 2003). It was shown that treatment with the anti-inflammatory agent indomethacin partially reversed irradiation-associated decreases in neurogenesis (Monje et al. 2003). Cerebral ischemia induces acute and prolonged inflammatory processes. Brain ischemic injury is characterized by rapid activation of resident microglia, production of proinflammatory mediators, followed by infiltration of neutrophils, macrophages, and other inflammatory cells (Davies et al. 1999; Hallenbeck 2002; Morioka et al. 1993; Wang et al. 2007). Anti-inflammatory treatment with indomethacin increased the levels of neurogenesis after focal cerebral ischemia (Hoehn et al. 2005). It has been shown that brain inflammation, activated microglia and increased levels of pro-inflammatory cytokines are pathological hallmarks of AD, PD and other neurodegenerative diseases (Imamura et al. 2003; McGeer et al. 1988; McGeer et al. 1987; Rogers et al. 1988). Aging is also characterized by neuroinflammation and increased levels of microglial activation (Sparkman and Johnson 2008).
Microglial inhibition of neurogenesis is mediated by activated, but not resting, microglia (Monje et al. 2003). The first two studies to identify activated microglia as the cell type responsible for suppression of hippocampal neurogenesis were published by Ekdahl and colleagues and Monje and colleagues in 2003 (Ekdahl et al. 2003; Monje et al. 2003). Activated microglia produce a large number of pro-inflammatory factors (Gebicke-Haerter 2001; Hanisch 2002; Pocock and Liddle 2001), and also reactive oxygen species (ROS) and nitric oxide (Rock et al. 2004). Several proinflammatory cytokines produced by reactive microglia, including IL-1β, IL-6, TNF-α, and interferon-γ, are antineurogenic (Ben-Hur et al. 2003; Ekdahl et al. 2003; Liu et al. 2005b; Monje et al. 2003). Increased levels of nitric oxide have been shown to suppress neurogenesis under normal and injury conditions (Moreno-Lopez et al. 2004; Torroglosa et al. 2007). Treatment with the antioxidant α-lipoic acid partially reversed the radiation-induced reduction of doublecortin (Dcx)-positive cells in hippocampus in vivo (Fike et al. 2007).
Inflammation and Mitochondria
Due to the clear importance of gaining a detailed understanding of the mechanisms involved in the inflammation-associated modulation of neurogenesis, it is currently the subject of intense investigation. Several recent studies indicate involvement of mitochondrial function in the processes of NPC survival and differentiation (Baxter et al. 2009; Calingasan et al. 2008; Kirby et al. 2009; Papa et al. 2004). Cellular mitochondria are a major target of inflammation-associated injury. Several interrelated factors can contribute to the impairment of mitochondria associated with inflammation. Tumor necrosis factor-α (TNF-α), a pleiotropic pro-inflammatory cytokine, has been shown to induce mitochondrial damage through suppression of mitochondrial complexes I and IV and pyruvate dehydrogenase activities (Samavati et al. 2008; Stadler et al. 1992; Zell et al. 1997). Exposure to increased ROS levels leads to impairment of mitochondrial oxidative phosphorylation through oxidation of mitochondrial lipids, sulfhydryl groups and iron sulfur complexes of mitochondrial respiratory enzymes (Halliwell 2006; Wagner et al. 1990).
IL-6, a pro-inflammatory cytokine that is extensively produced by activated glia, was recently shown to stimulate increased ROS production in brain (Behrens et al. 2008), thus contributing to other ROS-inducing mechanisms activated during inflammation. Nitric oxide levels are markedly increased in brain during inflammation (Brown 2007). Nitric oxide is a potent inhibitor or mitochondrial cytochrome c oxidase (complex IV) (Brown 1995; Giuffre et al. 1996). It has been shown both in vitro and in vivo that LPS-induced inflammation promotes strong microglial activation and induces mitochondrial dysfunction both in vitro and in vivo (Hunter et al. 2007; Xie et al. 2004) . Brain inflammation also promotes activation of astrocytes, a major glial cell type involved in response to stress and injury as well as normal physiology (Whitney et al. 2009). Reactive astrocytes forming a glial scar can impede neuronal migration and axonal regrowth following brain injury (Anderson et al. 2003). Reactive astrocytes also decrease rates of neurogenesis and increase glial differentiation of neural progenitor cells (Go et al. 2009). Brain injury promotes mitochondrial dysfunction and mitochondrial ROS production in astrocytes (Bambrick et al. 2004; Schipper et al. 2009; Voloboueva et al. 2007). Attenuation of mitochondrial ROS has been shown to decrease the degree of astrocyte activation in vitro (Gonzalez et al. 2007). This suggests that downregulation of mitochondrial ROS production can attenuate astrocyte activation, thus reducing the negative effect of astrocytic activation on neurogenesis following brain injury.
Effect of Mitochondrial Impairment on Neuronal Progenitor Cell Differentiation and Viability
Differentiation of progenitor cells into neurons requires energy in the form of ATP produced by mitochondria for growth of neuronal processes, cytoskeletal remodeling, and organelle transport (Bernstein and Bamburg 2003). Early studies have shown that developing neurons exhibit a marked increase in mitochondrial proteins during early neuronal differentiation (Cordeau-Lossouarn et al. 1991; Vayssiere et al. 1992). It has been recently demonstrated that the transcription factor NeuroD6, which is important for initiation and execution of neuronal differentiation, also induces an increase in total mitochondrial mass (Baxter et al. 2009). Despite these findings few studies have investigated the importance of mitochondrial function in neuronal differentiation and survival under normal and pathological conditions. Studies of mitochondrial biogenesis at the early stages of neurogenesis have suffered from the lack of a good cellular paradigm or accessible animal model. Knockout of transcriptional enzymes important in mitochondrial biogenesis, such as mitochondrial-associated polymerases γPolgG and Tfam, causes early embryonic lethality, at E8.5 (Hance et al. 2005) and E10.5 (Larsson et al. 1998), respectively.
These problems led to development of transmitochondrial technology, in which enucleated somatic cells that harbor pathological mtDNA mutations are fused with a cell line with chemically ablated endogenous mtDNA (King and Attardi 1989; Trounce and Wallace 1996). In an early transmitochondrial cybrid model of mtDNA disease affecting complex I, decreased production of both differentiated neurons and glial cells was observed (Wong et al. 2002). A recent in vitro study that also employed a transmitochondrial cybrid model demonstrated that embryonic stem cells containing pathogenic mitochondrial DNA mutations, particularly mutations leading to impaired complex I activity, are compromised in neuronal differentiation (Kirby et al. 2009). Dependence of neuronal differentiation on complex I function was already suggested in an earlier study (Papa et al. 2004). A recent in vivo study has shown that the impairment of mitochondrial α-ketoglutarate-dehydrogenase complex (KGDHC) activity results in decreased neurogenesis in the SGZ of hippocampus (Calingasan et al. 2008). This observation is consistent with another study that demonstrated that thiamine deficiency induces cognitive dysfunction in mice and impairs hippocampal neurogenesis (Zhao et al. 2008). KGDHC is a thiamine phosphate-dependent enzyme, and it is well established that thiamine deficiency leads to impaired KGDHC activity in the brain (Gibson et al. 1984). Overexpression of the mitochondrial protective and anti-apoptotic protein Bcl-xl has been shown to promote neuronal differentiation in vitro (Chang et al. 2007) and improve survival of neuronal precursor cells in the lesioned striatum after focal cerebral ischemia in animals (Doeppner et al. 2009). Mitochondria play a critical role in regulating cellular ROS levels in various neurodegenerative diseases (Keating 2008). Mild alterations in redox state leading to oxidation of the redox sensitive histone deacetylase Sirt1 have been shown to suppress proliferation of neural progenitor cells and direct their differentiation towards the astroglial lineage (Prozorovski et al. 2008).
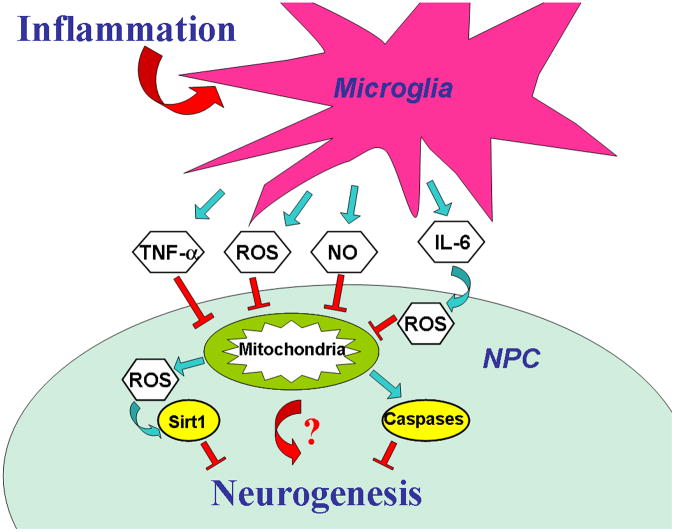
Different cell types have different sensitivities to interventions causing mitochondrial dysfunction. Inhibition of mitochondrial function promotes rapid loss of mitochondrial potential and cell death in neurons (Bolanos and Almeida 2006). On the other hand, mitochondrial inhibition in astrocytes induces strong upregulation of glycolysis without promoting significant changes in cell viability (Almeida et al. 2001). It has also been shown that changes in energetic demands can affect the cell's ability to maintain mitochondrial potential in the face of mitochondrial impairment (Voloboueva et al. 2007). A recent study demonstrated that while various cell lines, like HeLa, XP30RO and GM10115, can tolerate mitochondrial DNA (mtDNA) depletion for extended time periods, neural precursor cells die within a short time after mtDNA depletion (Fike et al. 2009). A summary of a putative mechanism of inflammation-induced neurogenesis impairment is presented in Fig. 4.
Fig. 4.
Putative mechanisms of inflammation-induced impairment of neurogenesis. During inflammation activated microglia produce pro-inflammatory cytokines, ROS and nitric oxide (NO), all of which inhibit mitochondrial function in neuronal progenitor cells (NPC). Mitochondrial damage leads to increased levels of mitochondrial ROS production and may suppress neurogenesis through mechanisms including Sirt1 oxidation. Severe impairment of mitochondrial function leads to cell death by necrosis or by activation of apoptotic signaling and activation of caspases. Other mechanisms connecting NPC mitochondrial function and neurogenesis require further investigation.
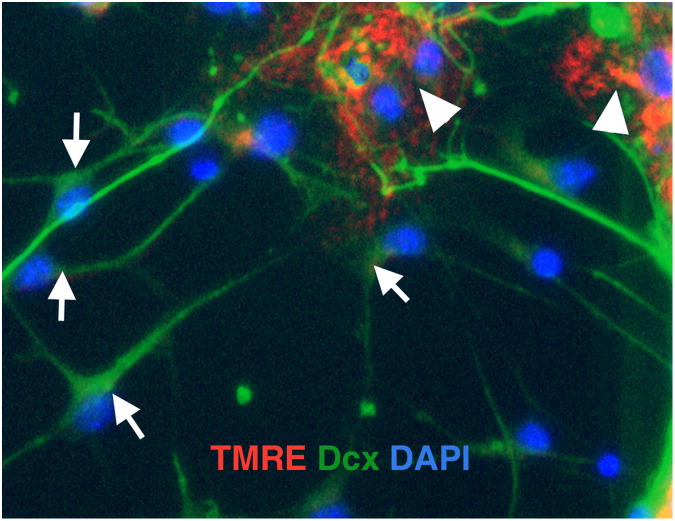
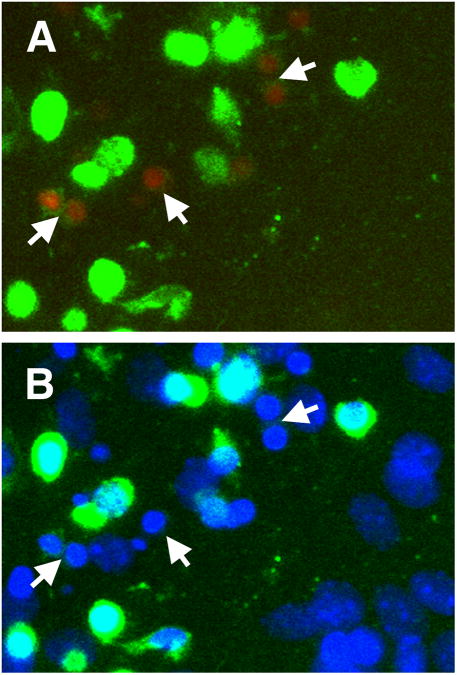
In our studies we observed that mitochondrial inhibition promotes rapid loss of mitochondrial membrane potential in immature Dcx -positive neurons (Fig. 1) associated with induction of apoptotic markers in Dcx+ cells (Fig. 2). We demonstrated that 14-16 h of mitochondrial inhibition with antimycin A promoted a significant drop in the viability of Dcx+ cells, in striking contrast to co-cultured astrocytes and oligodendrocytes, that showed no change in viability. Dcx+ cells that co-expressed MAP2, a marker of more mature neurons, also demonstrated reduced vulnerability to mitochondrial inhibition, compared to less mature neurons expressing only Dcx but not MAP2 (Voloboueva et al. 2010). Moreover, differentiation of NPC cells under conditions of mitochondrial inhibition for 4 days resulted in complete absence of Dcx+ cells, while control cultures demonstrated a significant fraction of differentiated Dcx+ cells (Fig. 3). These findings indicate that immature doublecortin (Dcx)-positive neurons are uniquely sensitive, compared to matured neurons and glia, to conditions impairing mitochondrial metabolism. As discussed above, inflammation promotes release of a variety of pro-inflammatory factors that inhibit mitochondrial function. In line with that, protection of mitochondrial function with a variety of mitochondrial protective compounds has been shown to be protective against inflammation-associated loss of Dcx+ cells in vitro. Also, overexpression of mitochondrial Hsp70 (mtHsp70), a mitochondrial chaperone that has been shown to protect mitochondrial function in several previous studies (Liu et al. 2005a; Voloboueva et al. 2008; Williamson et al. 2008; Xu et al. 2009), led to protection of mtHsp70-overexpressing Dcx+ cells against an in vitro inflammatory injury (Voloboueva et al. 2010).
Fig. 1.
Treatment with the mitochondrial complex III inhibitor antimycin A (2 μM, 4 h) promotes loss of mitochondrial membrane potential in Dcx+ cells (arrows, green staining), while nearby cells retain mitochondrial potential, as evidenced by red staining with the mitochondrial membrane potential sensitive dye tetramethylrhodamine ethyl ester (TMRE) arrowheads. Cell nuclei are counterstained with DAPI (blue).
Fig. 2.
Dcx+ cells (green) demonstrate signs of apoptotic cell death (red staining with Magic Red Live caspase 3&7 reagent), arrows, after 12 h of treatment with the mitochondrial inhibitor antimycin A (2 μM) (A). Note rapid disappearance of green Dcx staining in apoptotic cells. The bottom panel shows Dcx and nuclear DAPI staining of the same area
Fig. 3.
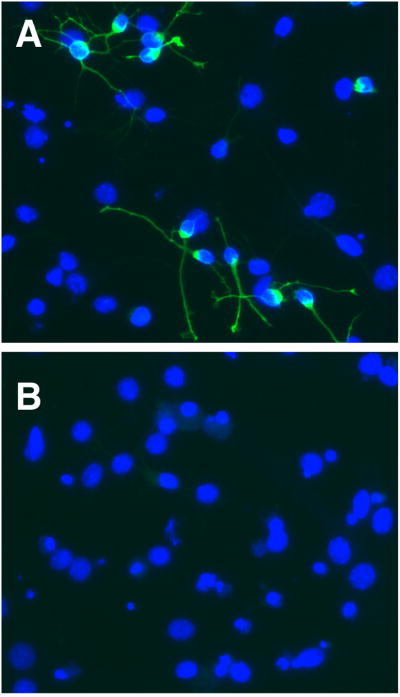
Control cultures of NPC isolated from newborn mouse brains demonstrate about 25% Dcx+ cells (green) after 4 days of differentiation (A). NPC cultures differentiated under the same conditions, but co-treated with mitochondrial inhibitor antimycin A (2 MM) lack cells with Dcx+ staining (B).
Summary
Clarifying the mechanisms underlying the inflammation-associated impairment of neurogenesis may help identify novel therapeutic targets for treatment of a variety of neurodegenerative disorders. The results of several recent studies, both in vitro and in vivo, indicate the involvement of mitochondrial mechanisms in the modulation of neurogenesis, and support the concept that mitochondrial protection could enhance rates of neurogenesis under conditions of inflammation.
Acknowledgments
The work was supported in part by NIH grants GM49831, NS053898 and NS014543 to RGG.
References
- Aimone JB, Deng W, Gage FH. Adult neurogenesis: integrating theories and separating functions. Trends Cogn Sci. 2010;14(7):325–337. doi: 10.1016/j.tics.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida A, Almeida J, Bolanos JP, Moncada S. Different responses of astrocytes and neurons to nitric oxide: the role of glycolytically generated ATP in astrocyte protection. Proc Natl Acad Sci U S A. 2001;98(26):15294–15299. doi: 10.1073/pnas.261560998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson MF, Blomstrand F, Blomstrand C, Eriksson PS, Nilsson M. Astrocytes and stroke: networking for survival? Neurochem Res. 2003;28(2):293–305. doi: 10.1023/a:1022385402197. [DOI] [PubMed] [Google Scholar]
- Androutsellis-Theotokis A, Leker RR, Soldner F, Hoeppner DJ, Ravin R, Poser SW, Rueger MA, Bae SK, Kittappa R, McKay RD. Notch signalling regulates stem cell numbers in vitro and in vivo. Nature. 2006;442(7104):823–826. doi: 10.1038/nature04940. [DOI] [PubMed] [Google Scholar]
- Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8(9):963–970. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- Baker SA, Baker KA, Hagg T. Dopaminergic nigrostriatal projections regulate neural precursor proliferation in the adult mouse subventricular zone. Eur J Neurosci. 2004;20(2):575–579. doi: 10.1111/j.1460-9568.2004.03486.x. [DOI] [PubMed] [Google Scholar]
- Bambrick L, Kristian T, Fiskum G. Astrocyte mitochondrial mechanisms of ischemic brain injury and neuroprotection. Neurochem Res. 2004;29(3):601–608. doi: 10.1023/b:nere.0000014830.06376.e6. [DOI] [PubMed] [Google Scholar]
- Baxter KK, Uittenbogaard M, Yoon J, Chiaramello A. The neurogenic basic helix-loop-helix transcription factor neuroD6 concomitantly increases mitochondrial mass and regulates cytoskeletal organization in the early stages of neuronal differentiation. ASN Neuro. 2009 doi: 10.1042/AN20090036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens MM, Ali SS, Dugan LL. Interleukin-6 Mediates the Increase in NADPH-Oxidase in the Ketamine Model of Schizophrenia. The Journal of Neuroscience. 2008;28(51):13957–13966. doi: 10.1523/JNEUROSCI.4457-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Hur T, Ben-Menachem O, Furer V, Einstein O, Mizrachi-Kol R, Grigoriadis N. Effects of proinflammatory cytokines on the growth, fate, and motility of multipotential neural precursor cells. Mol Cell Neurosci. 2003;24(3):623–631. doi: 10.1016/s1044-7431(03)00218-5. [DOI] [PubMed] [Google Scholar]
- Bernstein BW, Bamburg JR. Actin-ATP hydrolysis is a major energy drain for neurons. J Neurosci. 2003;23(1):1–6. doi: 10.1523/JNEUROSCI.23-01-00002.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biscaro B, Lindvall O, Hock C, Ekdahl CT, Nitsch RM. Abeta immunotherapy protects morphology and survival of adult-born neurons in doubly transgenic APP/PS1 mice. J Neurosci. 2009;29(45):14108–14119. doi: 10.1523/JNEUROSCI.2055-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolanos JP, Almeida A. Modulation of astroglial energy metabolism by nitric oxide. Antioxid Redox Signal. 2006;8(5-6):955–965. doi: 10.1089/ars.2006.8.955. [DOI] [PubMed] [Google Scholar]
- Brown GC. Nitric oxide regulates mitochondrial respiration and cell functions by inhibiting cytochrome oxidase. FEBS Lett. 1995;369(2-3):136–139. doi: 10.1016/0014-5793(95)00763-y. [DOI] [PubMed] [Google Scholar]
- Brown GC. Mechanisms of inflammatory neurodegeneration: iNOS and NADPH oxidase. Biochemical Society transactions. 2007;35(5):1119–1121. doi: 10.1042/BST0351119. [DOI] [PubMed] [Google Scholar]
- Calingasan NY, Ho DJ, Wille EJ, Campagna MV, Ruan J, Dumont M, Yang L, Shi Q, Gibson GE, Beal MF. Influence of mitochondrial enzyme deficiency on adult neurogenesis in mouse models of neurodegenerative diseases. Neuroscience. 2008;153(4):986–996. doi: 10.1016/j.neuroscience.2008.02.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron HA, McKay RD. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J Comp Neurol. 2001;435(4):406–417. doi: 10.1002/cne.1040. [DOI] [PubMed] [Google Scholar]
- Chang MY, Sun W, Ochiai W, Nakashima K, Kim SY, Park CH, Kang JS, Shim JW, Jo AY, Kang CS, Lee YS, Kim JS, Lee SH. Bcl-XL/Bax proteins direct the fate of embryonic cortical precursor cells. Mol Cell Biol. 2007;27(12):4293–4305. doi: 10.1128/MCB.00031-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordeau-Lossouarn L, Vayssiere JL, Larcher JC, Gros F, Croizat B. Mitochondrial maturation during neuronal differentiation in vivo and in vitro. Biol Cell. 1991;71(1-2):57–65. doi: 10.1016/0248-4900(91)90051-n. [DOI] [PubMed] [Google Scholar]
- Davies CA, Loddick SA, Toulmond S, Stroemer RP, Hunt J, Rothwell NJ. The progression and topographic distribution of interleukin-1beta expression after permanent middle cerebral artery occlusion in the rat. J Cereb Blood Flow Metab. 1999;19(1):87–98. doi: 10.1097/00004647-199901000-00010. [DOI] [PubMed] [Google Scholar]
- Demars M, Hu YS, Gadadhar A, Lazarov O. Impaired neurogenesis is an early event in the etiology of familial Alzheimer's disease in transgenic mice. J Neurosci Res. 2010;88(10):2103–2117. doi: 10.1002/jnr.22387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doeppner TR, Dietz GP, El Aanbouri M, Gerber J, Witte OW, Bahr M, Weise J. TAT-Bcl-x(L) improves survival of neuronal precursor cells in the lesioned striatum after focal cerebral ischemia. Neurobiol Dis. 2009;34(1):87–94. doi: 10.1016/j.nbd.2008.12.013. [DOI] [PubMed] [Google Scholar]
- Drapeau E, Nora Abrous D. Stem Cell Review Series: Role of neurogenesis in age-related memory disorders. Aging Cell. 2008;7(4):569–589. doi: 10.1111/j.1474-9726.2008.00369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekdahl CT, Claasen JH, Bonde S, Kokaia Z, Lindvall O. Inflammation is detrimental for neurogenesis in adult brain. Proc Natl Acad Sci U S A. 2003;100(23):13632–13637. doi: 10.1073/pnas.2234031100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enwere E, Shingo T, Gregg C, Fujikawa H, Ohta S, Weiss S. Aging results in reduced epidermal growth factor receptor signaling, diminished olfactory neurogenesis, and deficits in fine olfactory discrimination. J Neurosci. 2004;24(38):8354–8365. doi: 10.1523/JNEUROSCI.2751-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fike JR, Rola R, Limoli CL. Radiation response of neural precursor cells. Neurosurg Clin N Am. 2007;18(1):115–127. x. doi: 10.1016/j.nec.2006.10.010. [DOI] [PubMed] [Google Scholar]
- Fike JR, Rosi S, Limoli CL. Neural precursor cells and central nervous system radiation sensitivity. Semin Radiat Oncol. 2009;19(2):122–132. doi: 10.1016/j.semradonc.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage FH. Mammalian neural stem cells. Science. 2000;287(5457):1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- Galvan V, Jin K. Neurogenesis in the aging brain. Clin Interv Aging. 2007;2(4):605–610. doi: 10.2147/cia.s1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan L, Qiao S, Lan X, Chi L, Luo C, Lien L, Yan Liu Q, Liu R. Neurogenic responses to amyloid-beta plaques in the brain of Alzheimer's disease-like transgenic (pPDGF-APPSw,Ind) mice. Neurobiol Dis. 2008;29(1):71–80. doi: 10.1016/j.nbd.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebicke-Haerter PJ. Microglia in neurodegeneration: Molecular aspects. Microscopy Research and Technique. 2001;54(1):47–58. doi: 10.1002/jemt.1120. [DOI] [PubMed] [Google Scholar]
- Gibson GE, Ksiezak-Reding H, Sheu KF, Mykytyn V, Blass JP. Correlation of enzymatic, metabolic, and behavioral deficits in thiamin deficiency and its reversal. Neurochem Res. 1984;9(6):803–814. doi: 10.1007/BF00965667. [DOI] [PubMed] [Google Scholar]
- Giuffre A, Sarti P, D'Itri E, Buse G, Soulimane T, Brunori M. On the mechanism of inhibition of cytochrome c oxidase by nitric oxide. J Biol Chem. 1996;271(52):33404–33408. doi: 10.1074/jbc.271.52.33404. [DOI] [PubMed] [Google Scholar]
- Go HS, Shin CY, Lee SH, Jeon SJ, Kim KC, Choi CS, Ko KH. Increased proliferation and gliogenesis of cultured rat neural progenitor cells by lipopolysaccharide-stimulated astrocytes. Neuroimmunomodulation. 2009;16(6):365–376. doi: 10.1159/000228911. [DOI] [PubMed] [Google Scholar]
- Gonzalez A, Pariente JA, Salido GM. Ethanol stimulates ROS generation by mitochondria through Ca2+ mobilization and increases GFAP content in rat hippocampal astrocytes. Brain Res. 2007;1178:28–37. doi: 10.1016/j.brainres.2007.08.040. [DOI] [PubMed] [Google Scholar]
- Hallenbeck JM. The many faces of tumor necrosis factor in stroke. Nat Med. 2002;8(12):1363–1368. doi: 10.1038/nm1202-1363. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Oxidative stress and neurodegeneration: where are we now? J Neurochem. 2006;97(6):1634–1658. doi: 10.1111/j.1471-4159.2006.03907.x. [DOI] [PubMed] [Google Scholar]
- Hance N, Ekstrand MI, Trifunovic A. Mitochondrial DNA polymerase gamma is essential for mammalian embryogenesis. Hum Mol Genet. 2005;14(13):1775–1783. doi: 10.1093/hmg/ddi184. [DOI] [PubMed] [Google Scholar]
- Hanisch UK. Microglia as a source and target of cytokines. Glia. 2002;40(2):140–155. doi: 10.1002/glia.10161. [DOI] [PubMed] [Google Scholar]
- Hoehn BD, Palmer TD, Steinberg GK. Neurogenesis in rats after focal cerebral ischemia is enhanced by indomethacin. Stroke. 2005;36(12):2718–2724. doi: 10.1161/01.STR.0000190020.30282.cc. [DOI] [PubMed] [Google Scholar]
- Hoglinger GU, Rizk P, Muriel MP, Duyckaerts C, Oertel WH, Caille I, Hirsch EC. Dopamine depletion impairs precursor cell proliferation in Parkinson disease. Nat Neurosci. 2004;7(7):726–735. doi: 10.1038/nn1265. [DOI] [PubMed] [Google Scholar]
- Hunter RL, Dragicevic N, Seifert K, Choi DY, Liu M, Kim HC, Cass WA, Sullivan PG, Bing G. Inflammation induces mitochondrial dysfunction and dopaminergic neurodegeneration in the nigrostriatal system. J Neurochem. 2007;100(5):1375–1386. doi: 10.1111/j.1471-4159.2006.04327.x. [DOI] [PubMed] [Google Scholar]
- Imamura K, Hishikawa N, Sawada M, Nagatsu T, Yoshida M, Hashizume Y. Distribution of major histocompatibility complex class II-positive microglia and cytokine profile of Parkinson's disease brains. Acta Neuropathol. 2003;106(6):518–526. doi: 10.1007/s00401-003-0766-2. [DOI] [PubMed] [Google Scholar]
- Jin K, Peel AL, Mao XO, Xie L, Cottrell BA, Henshall DC, Greenberg DA. Increased hippocampal neurogenesis in Alzheimer's disease. Proc Natl Acad Sci U S A. 2004;101(1):343–347. doi: 10.1073/pnas.2634794100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating DJ. Mitochondrial dysfunction, oxidative stress, regulation of exocytosis and their relevance to neurodegenerative diseases. J Neurochem. 2008;104(2):298–305. doi: 10.1111/j.1471-4159.2007.04997.x. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386(6624):493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- Kernie SG, Parent JM. Forebrain neurogenesis after focal Ischemic and traumatic brain injury. Neurobiol Dis. 2010;37(2):267–274. doi: 10.1016/j.nbd.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King MP, Attardi G. Human cells lacking mtDNA: repopulation with exogenous mitochondria by complementation. Science. 1989;246(4929):500–503. doi: 10.1126/science.2814477. [DOI] [PubMed] [Google Scholar]
- Kirby DM, Rennie KJ, Smulders-Srinivasan TK, Acin-Perez R, Whittington M, Enriquez JA, Trevelyan AJ, Turnbull DM, Lightowlers RN. Transmitochondrial embryonic stem cells containing pathogenic mtDNA mutations are compromised in neuronal differentiation. Cell Prolif. 2009;42(4):413–424. doi: 10.1111/j.1365-2184.2009.00612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura T, Saitoh Y, Takashima N, Murayama A, Niibori Y, Ageta H, Sekiguchi M, Sugiyama H, Inokuchi K. Adult neurogenesis modulates the hippocampus-dependent period of associative fear memory. Cell. 2009;139(4):814–827. doi: 10.1016/j.cell.2009.10.020. [DOI] [PubMed] [Google Scholar]
- Kokaia Z, Thored P, Arvidsson A, Lindvall O. Regulation of stroke-induced neurogenesis in adult brain--recent scientific progress. Cereb Cortex. 2006;16(1):i162–167. doi: 10.1093/cercor/bhj174. [DOI] [PubMed] [Google Scholar]
- Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16(6):2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson NG, Wang J, Wilhelmsson H, Oldfors A, Rustin P, Lewandoski M, Barsh GS, Clayton DA. Mitochondrial transcription factor A is necessary for mtDNA maintenance and embryogenesis in mice. Nat Genet. 1998;18(3):231–236. doi: 10.1038/ng0398-231. [DOI] [PubMed] [Google Scholar]
- Leker RR, Soldner F, Velasco I, Gavin DK, Androutsellis-Theotokis A, McKay RD. Long-lasting regeneration after ischemia in the cerebral cortex. Stroke. 2007;38(1):153–161. doi: 10.1161/01.STR.0000252156.65953.a9. [DOI] [PubMed] [Google Scholar]
- Li B, Yamamori H, Tatebayashi Y, Shafit-Zagardo B, Tanimukai H, Chen S, Iqbal K, Grundke-Iqbal I. Failure of neuronal maturation in Alzheimer disease dentate gyrus. J Neuropathol Exp Neurol. 2008;67(1):78–84. doi: 10.1097/nen.0b013e318160c5db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Solway K, Messing RO, Sharp FR. Increased neurogenesis in the dentategyrus after transient global ischemia in gerbils. J Neurosci. 1998;18(19):7768–7778. doi: 10.1523/JNEUROSCI.18-19-07768.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Liu W, Song XD, Zuo J. Effect of GRP75/mthsp70/PBP74/mortalin overexpression on intracellular ATP level, mitochondrial membrane potential and ROS accumulation following glucose deprivation in PC12 cells. Mol Cell Biochem. 2005a;268(1-2):45–51. doi: 10.1007/s11010-005-2996-1. [DOI] [PubMed] [Google Scholar]
- Liu YP, Lin HI, Tzeng SF. Tumor necrosis factor-alpha and interleukin-18 modulate neuronal cell fate in embryonic neural progenitor culture. Brain Res. 2005b;1054(2):152–158. doi: 10.1016/j.brainres.2005.06.085. [DOI] [PubMed] [Google Scholar]
- McGeer PL, Itagaki S, Boyes BE, McGeer EG. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson's and Alzheimer's disease brains. Neurology. 1988;38(8):1285–1291. doi: 10.1212/wnl.38.8.1285. [DOI] [PubMed] [Google Scholar]
- McGeer PL, Itagaki S, Tago H, McGeer EG. Reactive microglia in patients with senile dementia of the Alzheimer type are positive for the histocompatibility glycoprotein HLA-DR. Neurosci Lett. 1987;79(1-2):195–200. doi: 10.1016/0304-3940(87)90696-3. [DOI] [PubMed] [Google Scholar]
- Mizumatsu S, Monje ML, Morhardt DR, Rola R, Palmer TD, Fike JR. Extreme sensitivity of adult neurogenesis to low doses of X-irradiation. Cancer Res. 2003;63(14):4021–4027. [PubMed] [Google Scholar]
- Monje ML, Mizumatsu S, Fike JR, Palmer TD. Irradiation induces neural precursor-cell dysfunction. Nat Med. 2002;8(9):955–962. doi: 10.1038/nm749. [DOI] [PubMed] [Google Scholar]
- Monje ML, Palmer T. Radiation injury and neurogenesis. Current Opinion in Neurology. 2003;16(2):129–134. doi: 10.1097/01.wco.0000063772.81810.b7. [DOI] [PubMed] [Google Scholar]
- Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302(5651):1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- Moreno-Lopez B, Romero-Grimaldi C, Noval JA, Murillo-Carretero M, Matarredona ER, Estrada C. Nitric oxide is a physiological inhibitor of neurogenesis in the adult mouse subventricular zone and olfactory bulb. J Neurosci. 2004;24(1):85–95. doi: 10.1523/JNEUROSCI.1574-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morioka T, Kalehua AN, Streit WJ. Characterization of microglial reaction after middle cerebral artery occlusion in rat brain. J Comp Neurol. 1993;327(1):123–132. doi: 10.1002/cne.903270110. [DOI] [PubMed] [Google Scholar]
- O'Keeffe GC, Tyers P, Aarsland D, Dalley JW, Barker RA, Caldwell MA. Dopamine-induced proliferation of adult neural precursor cells in the mammalian subventricular zone is mediated through EGF. Proc Natl Acad Sci U S A. 2009;106(21):8754–8759. doi: 10.1073/pnas.0803955106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papa S, Petruzzella V, Scacco S, Vergari R, Panelli D, Tamborra R, Corsi P, Picciariello M, Lambo R, Bertini E, Santorelli FM. Respiratory complex I in brain development and genetic disease. Neurochem Res. 2004;29(3):547–560. doi: 10.1023/b:nere.0000014825.42365.16. [DOI] [PubMed] [Google Scholar]
- Parent JM, Vexler ZS, Gong C, Derugin N, Ferriero DM. Rat forebrain neurogenesis and striatal neuron replacement after focal stroke. Ann Neurol. 2002;52(6):802–813. doi: 10.1002/ana.10393. [DOI] [PubMed] [Google Scholar]
- Pocock JM, Liddle AC. Microglial signalling cascades in neurodegenerative disease. In: Castellano B, Lopez MNS, editors. Progress in Brain Research. Elsevier; 2001. pp. 555–565. [DOI] [PubMed] [Google Scholar]
- Prozorovski T, Schulze-Topphoff U, Glumm R, Baumgart J, Schroter F, Ninnemann O, Siegert E, Bendix I, Brustle O, Nitsch R, Zipp F, Aktas O. Sirt1 contributes critically to the redox-dependent fate of neural progenitors. Nat Cell Biol. 2008;10(4):385–394. doi: 10.1038/ncb1700. [DOI] [PubMed] [Google Scholar]
- Raber J, Fan Y, Matsumori Y, Liu Z, Weinstein PR, Fike JR, Liu J. Irradiation attenuates neurogenesis and exacerbates ischemia-induced deficits. Ann Neurol. 2004a;55(3):381–389. doi: 10.1002/ana.10853. [DOI] [PubMed] [Google Scholar]
- Raber J, Rola R, LeFevour A, Morhardt D, Curley J, Mizumatsu S, VandenBerg SR, Fike JR. Radiation-induced cognitive impairments are associated with changes in indicators of hippocampal neurogenesis. Radiat Res. 2004b;162(1):39–47. doi: 10.1667/rr3206. [DOI] [PubMed] [Google Scholar]
- Rock RB, Gekker G, Hu S, Sheng WS, Cheeran M, Lokensgard JR, Peterson PK. Role of Microglia in Central Nervous System Infections. Clin Microbiol Rev. 2004;17(4):942–964. doi: 10.1128/CMR.17.4.942-964.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J, Luber-Narod J, Styren SD, Civin WH. Expression of immune system-associated antigens by cells of the human central nervous system: relationship to the pathology of Alzheimer's disease. Neurobiol Aging. 1988;9(4):339–349. doi: 10.1016/s0197-4580(88)80079-4. [DOI] [PubMed] [Google Scholar]
- Rola R, Raber J, Rizk A, Otsuka S, VandenBerg SR, Morhardt DR, Fike JR. Radiation-induced impairment of hippocampal neurogenesis is associated with cognitive deficits in young mice. Exp Neurol. 2004;188(2):316–330. doi: 10.1016/j.expneurol.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Samavati L, Lee I, Mathes I, Lottspeich F, Huttemann M. Tumor necrosis factor alpha inhibits oxidative phosphorylation through tyrosine phosphorylation at subunit I of cytochrome c oxidase. J Biol Chem. 2008;283(30):21134–21144. doi: 10.1074/jbc.M801954200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R. Requirement of Hippocampal Neurogenesis for the Behavioral Effects of Antidepressants. Science. 2003;301(5634):805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Schabitz WR, Steigleder T, Cooper-Kuhn CM, Schwab S, Sommer C, Schneider A, Kuhn HG. Intravenous brain-derived neurotrophic factor enhances poststroke sensorimotor recovery and stimulates neurogenesis. Stroke. 2007;38(7):2165–2172. doi: 10.1161/STROKEAHA.106.477331. [DOI] [PubMed] [Google Scholar]
- Schipper HM, Gupta A, Szarek WA. Suppression of glial HO-1 activity as a potential neurotherapeutic intervention in AD. Curr Alzheimer Res. 2009;6(5):424–430. doi: 10.2174/156720509789207985. [DOI] [PubMed] [Google Scholar]
- Seki T, Arai Y. Age-related production of new granule cells in the adult dentate gyrus. Neuroreport. 1995;6(18):2479–2482. doi: 10.1097/00001756-199512150-00010. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Miesegaes G, Beylin A, Zhao M, Rydel T, Gould E. Neurogenesis in the adult is involved in the formation of trace memories. Nature. 2001;410(6826):372–376. doi: 10.1038/35066584. [DOI] [PubMed] [Google Scholar]
- Sparkman NL, Johnson RW. Neuroinflammation associated with aging sensitizes the brain to the effects of infection or stress. Neuroimmunomodulation. 2008;15(4-6):323–330. doi: 10.1159/000156474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadler J, Bentz BG, Harbrecht BG, Silvio MD, Curran RD, Billiar TR, Hoffman RA, Simmons RL. Tumor Necrosis Factor Alpha Inhibits Hepatocyte Mitochondrial Respiration. Annals of Surgery. 1992;216(5):539–546. doi: 10.1097/00000658-199211000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torroglosa A, Murillo-Carretero M, Romero-Grimaldi C, Matarredona ER, Campos-Caro A, Estrada C. Nitric oxide decreases subventricular zone stem cell proliferation by inhibition of epidermal growth factor receptor and phosphoinositide-3-kinase/Akt pathway. Stem Cells. 2007;25(1):88–97. doi: 10.1634/stemcells.2006-0131. [DOI] [PubMed] [Google Scholar]
- Trounce I, Wallace DC. Production of transmitochondrial mouse cell lines by cybrid rescue of rhodamine-6G pre-treated L-cells. Somat Cell Mol Genet. 1996;22(1):81–85. doi: 10.1007/BF02374379. [DOI] [PubMed] [Google Scholar]
- van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 2002;415(6875):1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vayssiere JL, Cordeau-Lossouarn L, Larcher JC, Basseville M, Gros F, Croizat B. Participation of the mitochondrial genome in the differentiation of neuroblastoma cells. In Vitro Cell Dev Biol. 1992;28A(11-12):763–772. doi: 10.1007/BF02631065. [DOI] [PubMed] [Google Scholar]
- Voloboueva LA, Duan M, Ouyang Y, Emery JF, Stoy C, Giffard RG. Overexpression of mitochondrial Hsp70/Hsp75 protects astrocytes against ischemic injury in vitro. J Cereb Blood Flow Metab. 2008;28(5):1009–1016. doi: 10.1038/sj.jcbfm.9600600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voloboueva LA, Lee SW, Emery JF, Palmer TD, Giffard RG. Mitochondrial protection attenuates inflammation-induced impairment of neurogenesis in vitro and in vivo. J Neurosci. 2010;30(37):12242–12251. doi: 10.1523/JNEUROSCI.1752-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voloboueva LA, Suh SW, Swanson RA, Giffard RG. Inhibition of mitochondrial function in astrocytes: implications for neuroprotection. J Neurochem. 2007;102(4):1383–1394. doi: 10.1111/j.1471-4159.2007.4634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner KR, Kleinholz M, Myers RE. Delayed decreases in specific brain mitochondrial electron transfer complex activities and cytochrome concentrations following anoxia/ischemia. J Neurol Sci. 1990;100(1-2):142–151. doi: 10.1016/0022-510x(90)90025-i. [DOI] [PubMed] [Google Scholar]
- Wang Q, Tang XN, Yenari MA. The inflammatory response in stroke. J Neuroimmunol. 2007;184(1-2):53–68. doi: 10.1016/j.jneuroim.2006.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney NP, Eidem TM, Peng H, Huang Y, Zheng JC. Inflammation mediates varying effects in neurogenesis: relevance to the pathogenesis of brain injury and neurodegenerative disorders. J Neurochem. 2009;108(6):1343–1359. doi: 10.1111/j.1471-4159.2009.05886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson CL, Dabkowski ER, Dillmann WH, Hollander JM. Mitochondria protection from hypoxia/reoxygenation injury with mitochondria heat shock protein 70 overexpression. Am J Physiol Heart Circ Physiol. 2008;294(1):H249–256. doi: 10.1152/ajpheart.00775.2007. [DOI] [PubMed] [Google Scholar]
- Wiltrout C, Lang B, Yan Y, Dempsey RJ, Vemuganti R. Repairing brain after stroke: a review on post-ischemic neurogenesis. Neurochem Int. 2007;50(7-8):1028–1041. doi: 10.1016/j.neuint.2007.04.011. [DOI] [PubMed] [Google Scholar]
- Wong A, Cavelier L, Collins-Schramm HE, Seldin MF, McGrogan M, Savontaus ML, Cortopassi GA. Differentiation-specific effects of LHON mutations introduced into neuronal NT2 cells. Hum Mol Genet. 2002;11(4):431–438. doi: 10.1093/hmg/11.4.431. [DOI] [PubMed] [Google Scholar]
- Xie Z, Smith CJ, Van Eldik LJ. Activated glia induce neuron death via MAP kinase signaling pathways involving JNK and p38. Glia. 2004;45(2):170–179. doi: 10.1002/glia.10314. [DOI] [PubMed] [Google Scholar]
- Xu L, Voloboueva LA, Ouyang Y, Emery JF, Giffard RG. Overexpression of mitochondrial Hsp70/Hsp75 in rat brain protects mitochondria, reduces oxidative stress, and protects from focal ischemia. J Cereb Blood Flow Metab. 2009;29(2):365–374. doi: 10.1038/jcbfm.2008.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zell R, Geck P, Werdan K, Boekstegers P. Tnf-α and IL-1α inhibit both pyruvate dehydrogenase activity and mitochondrial function in cardiomyocytes: Evidence for primary impairment of mitochondrial function. Molecular and Cellular Biochemistry. 1997;177(1):61–67. doi: 10.1023/a:1006896832582. [DOI] [PubMed] [Google Scholar]
- Zhang RL, Zhang ZG, Zhang L, Chopp M. Proliferation and differentiation of progenitor cells in the cortex and the subventricular zone in the adult rat after focal cerebral ischemia. Neuroscience. 2001;105(1):33–41. doi: 10.1016/s0306-4522(01)00117-8. [DOI] [PubMed] [Google Scholar]
- Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132(4):645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- Zhao C, Teng EM, Summers RG, Ming GI, Gage FH. Distinct Morphological Stages of Dentate Granule Neuron Maturation in the Adult Mouse Hippocampus. The Journal of Neuroscience. 2006;26(1):3–11. doi: 10.1523/JNEUROSCI.3648-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zitnik G, Martin GM. Age-related decline in neurogenesis: Old cells or old environment? Journal of Neuroscience Research. 2002;70(3):258–263. doi: 10.1002/jnr.10384. [DOI] [PubMed] [Google Scholar]