Abstract
Bioremediation of sediments contaminated with commercial PCBs is potentially achievable by the sequential activity of anaerobic halorespiration to convert higher chlorinated congeners to less chlorinated congeners that are susceptible to aerobic respiratory degradation. The efficacy of bioaugmentation with anaerobic halorespiring “Dehalobium chlorocoercia” DF1 and aerobic Burkholderia xenovorans LB400 added concurrently with GAC as a delivery system was determined in 2-liter laboratory mesocosms containing weathered Aroclor-contaminated sediment from Baltimore Harbor, MD. The greatest effect was seen in the mesocosm bioaugmented with both DF1 and LB400 together, which resulted in an 80% decrease by mass of PCBs, from 8 mg/kg to less than 2 mg/kg after 120 days. There was no significant increase in lesser-chlorinated congeners, indicating that both anaerobic dechlorination by DF1 and aerobic degradation by LB400 occurred. In contrast, non-bioaugmented controls containing filtered culture supernatant showed only 25% decrease in total levels of PCBs after 365 days, which was likely due to biostimulation of the indigenous population by the medium. Direct colony counts and molecular analysis targeting a putative reductive dehalogenase gene of D. chlorocoercia, or the bphA gene of LB400 showed the presence of viable DF1 and LB400 in bioaugmented mesocosms after 365 days, indicating that both non-indigenous strains were sustainable within the indigenous microbial community. These results suggest that an in situ treatment employing the simultaneous application of anaerobic and aerobic microorganisms could be an effective, environmentally sustainable strategy to reduce PCBs levels in contaminated sediment.
INTRODUCTION
Polychlorinated biphenyls (PCBs), manufactured commercially since 1929 as thermally and chemically highly stable, flame- and oxidation-resistant chemicals with excellent dielectric properties, were widely used in transformers, capacitors, printing inks, paints, pesticides and road dust suppression agents. Although their manufacture was banned in the U.S. in 1979 as a result of the Toxic Substances Control Act and subsequently banned worldwide in 2001 by Stockholm Convention on Persistent Organic Pollutants, PCBs persist in the environment where they bioaccumulate in the food chain and act as potential neurotoxins 1, endocrine disruptors 2, and carcinogens 3.
The most common method for treatment of PCB impacted sediments typically utilizes dredging and disposal in landfills which is costly, disruptive to the environment and increases the risk of PCB release into the water column 4. Capping with passive materials such as sand has been tested as an in situ approach for treating PCB impacted sediments, but the vulnerability of the cap to both abiotic and biotic disruption does not completely eliminate the risk of later exposure 5. Recently, the addition of activated carbon to contaminated sediment was shown to be effective in sequestering (by hydrophobic interactions) PCBs from aquatic organisms 6. In these studies, the application of a thin layer of activated carbon to the biologically active surface layer of PCB-impacted sediment resulted in the decrease of bioavailability of PCBs to benthic organisms minimizing the risk of exposure to the food chain 7. Payne et al 8 demonstrated recently that granulated activated carbon did not inhibit microbial dehalogenation of PCBs in sediments when used also as a carrier for dispersing PCB halorespiring microorganisms in PCB impacted sediment mesocosms. The advantage of this “biocatalytic” form of granulated activated carbon is that it both sequesters PCBs bioavailable to benthic organisms and actively remediates them by microbial transformation.
Bioaugmentation has the potential to degrade organohalide contaminants in situ by accelerating the natural biotransformation process. Bioaugmentation with anaerobic halorespiring microorganisms, such as strains of Dehalococcoides mccartyi, has been used successfully for in situ degradation of toxic chlorinated ethenes to non-toxic ethene in contaminated groundwater 9. In contrast to this one-step anaerobic process, degradation of highly chlorinated PCB congeners commonly associated with Aroclor mixtures has been reported thus far to require sequential anaerobic dechlorination of the biphenyl followed by aerobic cleavage and degradation of the remaining partially chlorinated ring structures. Anaerobic halorespiring microorganisms reductively dechlorinate congeners generally with six or more chlorines to less chlorinated congeners that are then vulnerable to aromatic ring cleavage and complete degradation by a consortium of aerobic microorganisms. PCB halorespiring isolates and phylotypes within the halorespiring Chloroflexi have been shown to dechlorinate commercial PCB mixtures in the lab, but this activity is limited to more heavily chlorinated congeners and typically stalls when congeners no longer possess flanked chlorine atoms 10–14. Burkholderia xenovorans LB400, originally isolated from a PCB contaminated landfill in New York, will co-metabolically cleave the aromatic ring of congeners with five or fewer chlorines by dioxygenase attack at either the 2,3 or 3,4 positions 15–17. Bench studies show that resting cells of LB400 degrade PCB congeners with at least one unsubstituted 2,3 or 3,4 position, or congeners containing vicinal unchlorinated carbons (e.g. 2,4,5-2,4,5), when the congeners are present at a concentration of 1 mg/L 15.
As early as 1995 it was recognized that the anaerobic dechlorination of more highly chlorinated congeners followed by the aerobic degradation of those dechlorination products was likely occurring in the environment 18, and enhancing this natural process was suggested to be a potential treatment strategy for PCB impacted sediment. Earlier reports demonstrated that sequentially treating PCB impacted sediment in an anaerobic PCB halorespiring enrichment followed by transfer in an aerobic culture containing LB400 effectively degraded weathered Aroclors by as much as 70% 19–20. However, both studies were conducted in closed microcosms and do not represent in situ conditions.
Previously Payne et al. 8 showed that bioaugmentation of sediment mesocosms simulating in situ conditions with a halorespiring bacterium, “Dehalobium chlorocoercia” DF1, had a stimulatory effect on the dechlorination of weathered Aroclor 1260 (1.3 mg/kg dry wt.), resulting in the loss of approximately 56% by mass of the penta- and higher chlorinated PCBs. Furthermore, the addition of granular activated carbon (GAC), which is currently being field tested as a strong sorbent to reduce PCB bioavailability, had a slight stimulatory effect on dechlorination of PCBs by DF18. In the current study we test the efficacy of bioaugmentation in a single-step process by bioaugmenting mesocosms containing weathered Aroclor 1260 contaminated sediment with anaerobic halorespiring and aerobic degrading bacteria, delivered concurrently to the sediments on GAC. We report the effect of concurrent dechlorination and degradation of weathered PCBs, and we document the fate of the inoculated microorganisms over the course of 365 days.
MATERIALS AND METHODS
Media and growth conditions
“Dehalobium chlorocoercia” DF1 was grown anaerobically in estuarine mineral medium (ECl) with sodium formate (10 mM), PCB 61 (2,3,4,5-PCB; 173 μM), Desulfovibrio sp. extract (1% v/v) as a growth factor and titanium(III) nitrilotriacetate (0.5 mM) as a chemical reductant 8. DF1 was routinely grown in 50 ml of medium sealed under N2-CO2 (4:1) in 160-ml serum bottles with 20-mm Teflon-coated butyl stoppers (West Pharmaceutical, Inc.). Cultures were incubated statically at 30 °C in the dark. Growth was monitored by gas chromatographic analysis of PCB 61 dechlorination to PCB 23 (2,3,5-PCB) and by real-time quantitative PCR (qPCR) of 16S rRNA gene copies (described below).
Burkholderia xenovorans LB400 was grown aerobically in M9 minimal medium 21 with solid biphenyl crystals (5 mM; solubility in water, 2.89×10−2 mM) as the carbon source and electron donor as described previously 22. LB400 was grown in 100 ml of medium in 500 ml Erlenmeyer flasks loosely sealed with aluminum foil. Cultures were incubated at 30 °C with shaking at 100 rpm. Growth was monitored by measuring optical density at 600nm with a Spectronic 21 (Bausch and Lomb).
Mesocosm experiments
Mesocosms were prepared in glass 2 liter thin-layer chromatography developing tanks (Fisher Scientific) as described previously 8. PCB impacted sediments were collected on March 29, 2010 from the Northwest Branch of Baltimore Harbor (BH) with a petite Ponar grab sampler at 39°16.8_N, 76°36.2_W and stored in the dark under nitrogen at 4 °C prior to use. Total organic carbon in sediment was determined by the loss-on-ignition method by measuring the loss of mass from dried sediment after heating at 400°C for 8 hours 23. Sediment samples were pooled and homogenized in an anaerobic glove bag and two liters were added to each mesocosm tank with 2 cm of indigenous water above the sediment surface. A glass plate covered each mesocosm with a 1 cm gap on one end for air exchange. Water lost due to evaporation was periodically replenished with deionized water to maintain the original osmolarity of the harbor water.
LB400 was prepared as bioaugmentation inoculum in one 100ml culture until an O.D.600 of 1.0 was reached (ca. 4×108 cells/ml). The culture was transferred into a 250 ml Oak Ridge bottle and centrifuged at 22,000 × g for 10 min. The cell pellet was suspended in 100 ml of sterile M9 medium and added to mesocosms as described below. DF1 was prepared as bioaugmentation inoculum in ten 50 ml cultures grown until 50% of PCB 61 was dechlorinated. The cultures were transferred into 250 ml Oak Ridge bottles in an anaerobic glove box, sealed under nitrogen-carbon dioxide (4:1) and centrifuged at 22,000 × g for 30 min. Cell pellets were pooled anaerobically in 50 ml of ECl medium to a final concentration of approximately 5×107 16S rRNA gene copies/ml. Filtered culture supernatant was prepared by passing pooled LB400 and DF1 culture supernatants through a 0.22 micron filter (Millipore) to remove residual cells. DF1 was undetectable in the supernatant based on qPCR (described below).
Granulated activated carbon (GAC) was used as a medium to inoculate mesocosms with either LB400 alone or with both LB400 and DF1 concurrently. In addition, lactate and reduced zero valent iron were tested as electron donors for anaerobic halorespiration in combination with the LB400 or the LB400 and DF1 inocula for a total of nine different experimental mesocosm treatments, including filtered controls. For each of the nine mesocosm treatments, 25 g GAC (CAS# 7440-44-0, type TOG-NDS 80×325, Calgon Carbon Corp.) was prepared as the delivery system for both microorganisms in an anaerobic glove box as follows: (1) GAC with 20 ml of filtered growth medium; (2) GAC with filtered growth medium and sodium lactate (5 mM final concentration); (3) GAC with filtered growth medium and reduced zero valent iron (10 mg/g sediment wet wt.; Fisher Scientific); (4) 10 ml of LB400 (ca. 2 × 106/g sediment wet wt.) adsorbed to 25 g GAC for one hour in an anaerobic glove box; (5) LB400 and sodium lactate adsorbed to GAC for one hour; (6) LB400 adsorbed to GAC and zero valent iron; (7) LB400 and DF1 (ca. 5 × 105/g sediment wet wt.) adsorbed to GAC; (8) LB400, DF1 and sodium lactate adsorbed to GAC; or (9) LB400 and DF1 adsorbed to GAC and reduced zero valent iron. Each of the nine mesocosms was homogenized after addition of its corresponding amendment by stirring with a Teflon spoon, then removed from the anaerobic glove box and incubated at 23 °C in the dark. Mesocosms were sampled by taking triplicate 1 cm cores six cm deep using a random sampling grid. Each core was homogenized prior to analysis for PCBs and DNA as described below.
DNA extraction and enumeration of PCB halorespiring bacteria and LB400 by quantitative PCR
DNA was extracted by adding 0.25 g sediment (wet wt.) from each sample core to a PowerBead microcentrifuge tube (Power Soil DNA Isolation Kit, MOBIO Laboratories, Inc.) as previously described 8. Extracted DNA samples had an A260/280 ratio of ≥ 1.6 and an A260/230 ratio of ≥ 2.0. All DNA samples were diluted to 2 ng/μl in TE buffer.
Enumeration of putative halorespiring Chloroflexi in each subcore was performed by real-time quantitative PCR (qPCR) using iQ SYBR Green Supermix (Bio-Rad Laboratories) and primers specific for the 16S rRNA gene of a deep branching, putative dechlorinating clade within the Chloroflexi (348F/884R, Table S1) 24 as described previously. Alternatively, primers SKFPat9F and SKFPat9R, targeting a putative reductive dehalogenase of DF1 were designed to specifically quantify DF1 in the presence of any indigenous halorespiring bacteria (Table S1). qPCR with SKFPat9F/SKFPat9R was performed using the following program: initial denaturation at 95 °C for 5 min; followed by 35 cycles of 95 °C for 45 sec, 55 °C for 25 sec, and 72 °C for 25 sec. The detection limits of all qPCR assays was 3 × 102 gene copies/g sediment (wet wt.).
Enumeration of LB400 in each subcore was performed by qPCR using iQ SYBR Green Supermix and primers specific for the upstream region of the LB400 bphA gene operon (CIOP0/CIOP1, Table S1) 25. qPCR with CIOP0/CIOP1 was performed using the following program: initial denaturation at 95 °C for 5 min; followed by 35 cycles of 95 °C for 45 sec, 58 °C for 30 sec, and 72 °C for 30 sec.
Community analysis of PCB dechlorinating bacteria by denaturing HPLC
Denaturing HPLC (DHPLC) analyses were performed using a WAVE 3500 HT system (Transgenomic, Inc.) as described previously 26 equipped with a fluorescence detector (excitation 490 nm, emission 520 nm). The primer set 348F/884R was used to monitor putative dechlorinating bacteria within the Chloroflexi 24 and the DF1 specific primer pair SKFPat9F/SKFPat9R was used to monitor DF1. The consensus primer set bphAf668-3/bphAr1153-2 was used to monitor putative biphenyl degrading bacteria and the specific LB400 bphA primer pair CIOP0/CIOP1 was used to monitor LB400. DHPLC fractions corresponding to DF1 and LB400 were sequenced as described previously 26.
PCB extraction and analysis
Sediment samples were extracted using an Accelerated Solvent Extractor (Dionex) following EPA method 3545 as described previously 8. Briefly, approximately 5 grams wet weight sediment was dried in a desiccator with pelletized diatomaceous earth (Dionex) and the dried sediment (1 g) was extracted in an 11 ml stainless steel extraction cell containing 0.6 g Cu and 2.4 g fluorosil on the bottom of the cell and anhydrous Na2SO4 in the void volume. PCB 166 (10 μl stock of 400 μg/L hexane) was added as a surrogate to correct for extraction efficiency. Each sample was extracted with 20 ml of pesticide grade hexane (Acros Organics) at 100 °C and purged with 1 MPa nitrogen. The extract was evaporated to a final volume of 1 ml at 30 °C under nitrogen flow and PCB 30 and PCB 204 (400 μg/L each in 10 μl acetone) were added as internal standards.
PCB congeners were analyzed using a Hewlett-Packard 6890 series II gas chromatograph (GC) with a DB-1 capillary column (60 m by 0.25 mm by 0.25 μm; JW Scientific) and a 63Ni electron capture detector by a modified method of EPA 8082 as previously described 8. Briefly, PCB congeners in a mixture containing Aroclors 1232, 1248, 1262 and fifty-five additional congeners that were potential dechlorination products were quantified with a 10-point calibration curve using PCBs 30 and 204 as internal standards. Using this protocol 173 congeners were resolved in 130 individual peaks. Quantification of co-eluting congeners and total PCB concentrations were calculated as described elsewhere 8.
RESULTS
Characterization of Baltimore Harbor sediment
Sediment from Baltimore Harbor (BH) was black in color and a plume of hydrocarbons was observed on the water surface after the sampler was deployed, which is consistent with relatively high amounts of PAHs reported near this site as recently as 1997 27. Total organic carbon of the pooled and mixed sediment was 18.25% ± 0.11% by dry weight (n = 3) and total PCB concentration was 8.0 ± 2.3 mg/kg (n = 27) with a mean of 3.27 ± 0.47 chlorines per biphenyl. PCB analysis indicated that di-, tri-, and tetra-chlorobiphenyl homologs were dominant, accounting for 2.0 ± 0.7, 2.3 ± 1.2 and 2.2 ± 0.9 mg/kg or about 25% of the total amount of PCBs. Pentachlorobiphenyls accounted for approximately 1 mg/kg; and hexa- and higher chlorinated biphenyl homologs accounted for less than 1 mg/kg of the total PCB content. The total PCB concentration in sediment used in this study was greater than that detected in a sample collected near the same site in 2009 (1.3 mg/kg) and contained a greater fraction of less chlorinated (di- through penta-chlorobiphenyls) than the prior study 8. The observations possibly indicate spatial variation, an influx of sediments impacted with a less chlorinated Aroclor, or extensive weathering of Aroclor 1260, which has been historically detected in BH sediments 28.
Effect of bioaugmentation on reductive dechlorination and degradation of PCBs
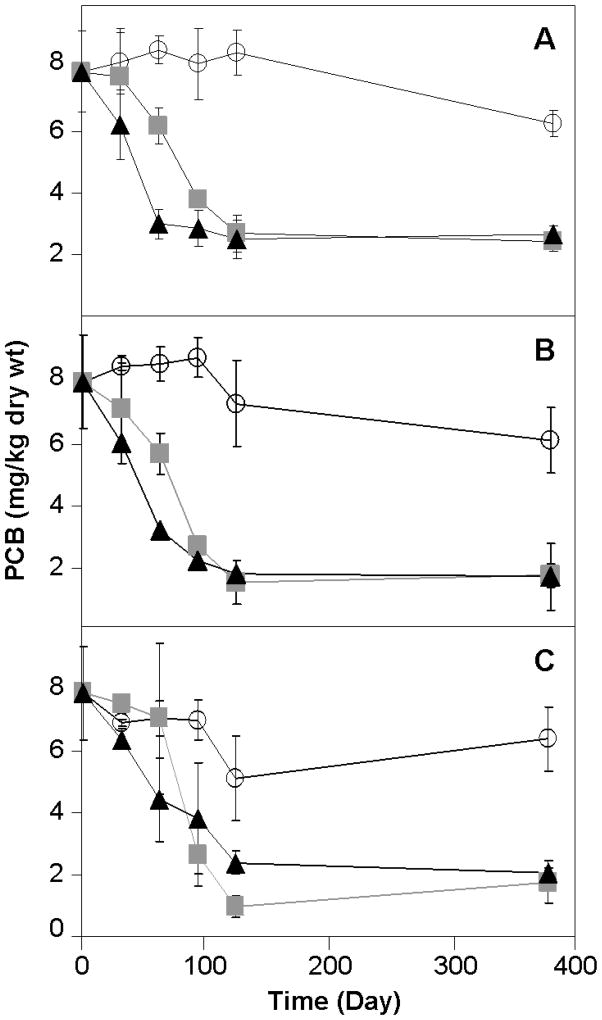
BH sediment mesocosms were bioaugmented with the aerobic PCB degrading LB400 at a final concentration of approximately 2×106 cells/g wet sediment both alone and with the simultaneous addition of the PCB halorespiring DF1 at a final concentration of 2×105 cells/g wet sediment. In mesocosms bioaugmented with GAC plus LB400 or GAC plus LB400 and DF1 together, there was a 25%–75% total loss by mass in total PCBs after 60 days (Figure 1), and the rate plateaued after the first 120 days. Overall, over 80% of the total mass of PCBs was degraded in mesocosms bioaugmented with either LB400 alone or LB400 and DF1 after 360 days. The addition of electron donors sodium lactate or zero valent iron as biostimulants did not have a significant effect on PCB dechlorination or degradation compared with cells alone (Figure 1). Loss of about 25% of the total mass of PCBs occurred in non-bioaugmented negative controls augmented with GAC and filtered culture supernatant after 360 days. This could be due to biostimulation of native microorganisms with factors in filtered spent medium or abiotic factors such as volatilization. However, the apparent loss of PCBs in uninoculated controls was not detected until day 90 of the experiment and was much slower than that seen in bioaugmented mesocosms (Figure 1). There was a decrease of di- through nona-chlorobiphenyls, which was attributed to aerobic degradation by LB400 simultaneously with reductive dechlorination by DF1 without exogenous electron donor (Figure 2).
Figure 1.
Total PCB change over 365 days in mesocosms. Average and standard deviation at time 0 (n = 27) and all other times (n = 3) are shown. A, no added electron donor; B, lactate added as electron donor; C, zero valent iron added as electron donor. Open circles (○), filtered growth media control; Grey squares (
 ), LB400; Black triangles (▲), LB400 and DF1. Error bars are calculated from replicate sediment subsamples.
), LB400; Black triangles (▲), LB400 and DF1. Error bars are calculated from replicate sediment subsamples.
Figure 2.
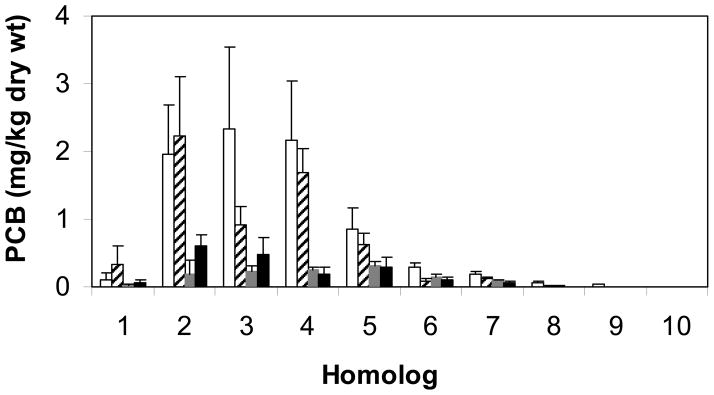
PCB homolog distribution in mesocosms at day 0 (□, n = 9) and day 365 in mesocosms augmented with filtered growth media (
 , n = 3), LB400 (
, n = 3), LB400 (
 , n = 3), and LB400 plus DF1 (■, n = 3). Error bars are calculated from replicate sediment subsamples.
, n = 3), and LB400 plus DF1 (■, n = 3). Error bars are calculated from replicate sediment subsamples.
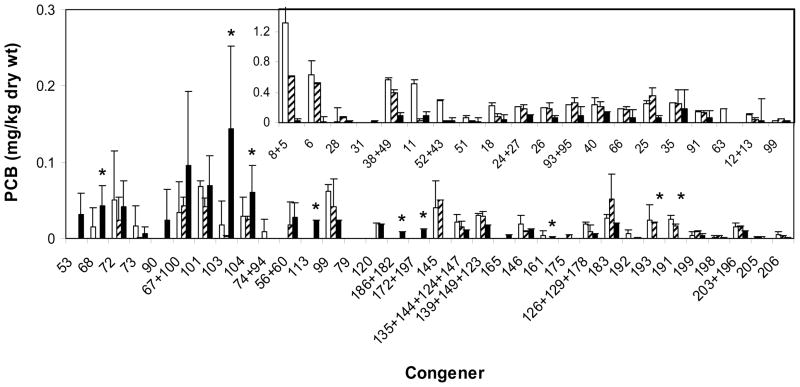
Analysis of single congeners showing the most significant change indicated that dechlorination by DF1 and degradation by LB400 occurred in the mesocosms bioaugmented with both organisms (Figure 3 and Table S2). Disappearance of less chlorinated congeners such as 8/5, 6, etc., occurred rapidly within the first 60 days of bioaugmentation and then stopped, which was likely due to the degradation by LB400 (Figure 1 and data not shown). This activity accounted for more than 50% of the total loss of PCBs observed by mass (Figure 1). However, patterns consistent with the dechlorination of congeners by DF1 were also observed. For example, the disappearance of some highly chlorinated congeners such as 203/196, 145, 193, and 139/149/123 coincided with the appearance of both intermediate and final dechlorination products such as 120, 113, 53, 90 and others (Figure 3 and Table S2). This was observed to as greater extent in the mesocosm inoculated with DF1 than in the mesocosm without the PCB halorespiring microorganism (Figure 3) indicating DF1 was actively dechlorinating highly chlorinated congeners. The results indicate that both aerobic degradation and anaerobic dechlorination occurred within the course of this study when LB400 and DF1 were added together.
Figure 3.
Profile showing the most significant changeS in PCB congener distribution between at day 0 (□) and day 365 in the untreated mesocosm (
 ) and the mesocosm bioaugmented with LB400 plus DF1 (■). Inset; congeners inferred to be degraded by LB400 (no products). An asterisk (*) indicates potential substrates or products of DF1 dechlorination that are significantly different between mesocosm amended with LB400 and LB400 plus DF1 (P < 0.05). Error bars are calculated from replicate sediment subsamples.
) and the mesocosm bioaugmented with LB400 plus DF1 (■). Inset; congeners inferred to be degraded by LB400 (no products). An asterisk (*) indicates potential substrates or products of DF1 dechlorination that are significantly different between mesocosm amended with LB400 and LB400 plus DF1 (P < 0.05). Error bars are calculated from replicate sediment subsamples.
The observation of simultaneous degradation and dechlorination was supported by the analysis of the total Cl per biphenyl ratio in mesocosms augmented with LB400 compared to mesocosms augmented with both LB400 and DF1 (Figure S1). There appears to be a large increase in Cl per biphenyl at day 120 when LB400 (4.3 ± 0.6) or LB400 together with DF1 (3.9 ± 0.2) are present as compared to spent media controls (3.0 ± 0.2), which is consistent with the large decrease in the absolute amounts of lower chlorinated congeners due to aerobic degradation (Figure 3). Between day 120 and day 365, the Cl per biphenyl ratio in mesocosms with both LB400 and DF1 was lower than in mesocosms with LB400 alone (3.1 ± 0.1 compared to 3.60 ± 0.3), which would be expected if dechlorination of higher chlorinated congeners by DF1 occurred.
Sustainability of non-indigenous microorganisms after bioaugmentation
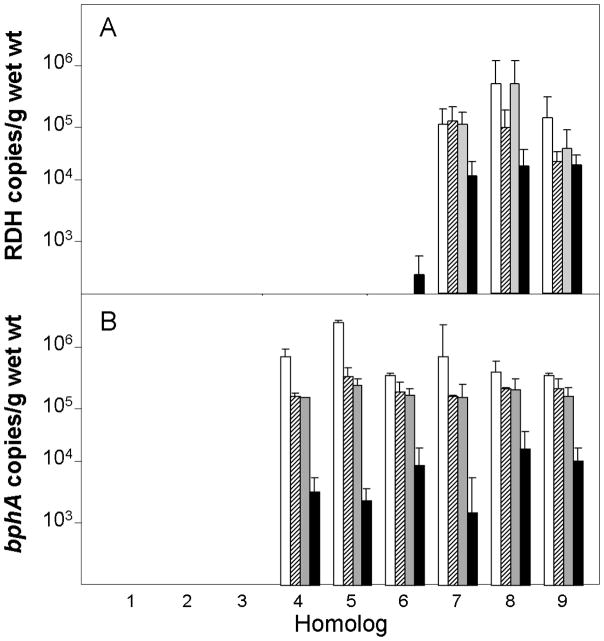
The estimated number of indigenous putative dechlorinating Chloroflexi increased from 3.9 ± 1.0 × 106 to 1.0 ± 0.3 × 107/g sediment based on 16S gene copies, which was greater than the number of DF1 added to bioaugmented mesocosms (data not shown). The number of total indigenous putative dechlorinating Chloroflexi in mesocosms was approximately ten-fold higher than reported previously in BH sediments (ca. 5×106 compared to 5×105/g). The observed difference in total putative dechlorinating Chloroflexi could be due to differences in the spatial distribution of cells or changes in the indigenous community due to the higher PCB concentration observed in the current study (8 vs 1.3 mg/kg). qPCR using DF1 specific primers confirmed that the number of added DF1 cells added to sediment mesocosms was approximately 2×105 gene copies/g sediment (Figure 4A). The number of DF1 gene copies/g in non-bioaugmented Treatments 1 to 5 was below the detection limit of 102 gene copies/g sediment and any remaining cells would be at least 4 orders of magnitude less than bioaugmented treatments. The copies detected in non-biaugmented Treatment 6 might be due to trace amounts of DF1 DNA carried over in the filtered culture supernatant added to the mesocosm. DF1 was maintained through the course of the experiment although its numbers decreased about 10-fold between day 60 and day 365 (Figure 4A). The addition of lactate or zero valent iron as electron donor had no apparent effect on the numbers putative dechlorinating Chloroflexi or DF1 (Figure 4A). The presence of DF1 among indigenous putative halorespiring Chloroflexi in bioaugmented mesocosms was confirmed by PCR followed by DHPLC analysis and sequencing. (Figure 5A). The peak corresponding to the DF1 16s rRNA gene was detected at day 0 and was prominent after 120 days in bioaugmented mesocosms, but not observed in controls (Figure 5A.). The putative DF1 fraction was collected, sequenced, and confirmed to be DF1 (0 or 1 mismatch per 500 bps). In addition, DHPLC analysis was performed on sediment from mesocosms augmented with GAC and filtered culture supernatant only (negative control) or GAC plus the LB400 and DF1 inocula using primers specific for a DF1 reductive dehalogenase (SKFPat9F/R). The corresponding gene fragment was detected at 0, 120, and at 365 days only in the mesocosm augmented with DF1 inoculum, but was below the detection limit in uninoculated controls (Figure 5A).
Figure 4.
qPCR analysis using RDH primers (A) for DF-1 and bphA1 primers for LB400 (B) at days 0 (□), 60(
 ), 120 (
), 120 (
 ), and 365 (■). Treatments are: (1) filtered growth media; (2) filtered growth media and sodium lactate; (3) filtered growth media and reduced zero valent iron; (4) LB400; (5) LB400 and sodium lactate; (6) LB400 and zero valent iron; (7) LB400 and DF1; (8) LB400, DF1 and sodium lactate; and (9) LB400, DF1 and reduced zero valent iron. Error bars are calculated from triplicate sediment subsamples.
), and 365 (■). Treatments are: (1) filtered growth media; (2) filtered growth media and sodium lactate; (3) filtered growth media and reduced zero valent iron; (4) LB400; (5) LB400 and sodium lactate; (6) LB400 and zero valent iron; (7) LB400 and DF1; (8) LB400, DF1 and sodium lactate; and (9) LB400, DF1 and reduced zero valent iron. Error bars are calculated from triplicate sediment subsamples.
Figure 5.
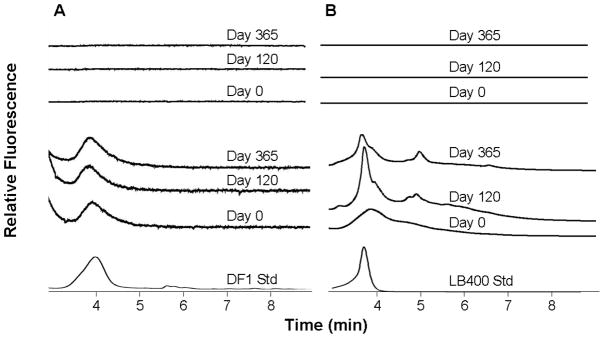
A: DHPLC analysis of mesocosms using DF1 RDH specific primers SKFPat9F/R; and B: DHPLC analysis of mesocosms using LB400 specific primers CIOP0/1. Top A and B chromatograms show mesocosm without bioaugmentation at days 0, 120, and 365. Center A and B chromatograms show mesocosm bioaugmented with LB400 and DF1 at days 0, 120, and 365. The lower chromatograms show show retention times of DF1 reductive dehalogenase and LB400 bphA standards.
LB400 was added to BH mesocosms to a final concentration of approximately 2 × 106 cells/g sediment. Initial qPCR and DHPLC analysis using degenerate universal primers targeting the consensus bphA gene (bphAf668-3/bphAr1153-2) did not detect a change in the number of bphA genes above background level after bioaugmentation of mesocosms with LB400; therefore, a primer pair specific for LB400 bphA (CIOP0/CIOP1) was used to monitor LB400 by qPCR and DHPLC. 25.
LB400-specific bphA genes were detected only in mesocosms bioaugmented with LB400 (Figure 4B). Interestingly, there was approximately a 10-fold decrease in numbers after the first 60 days, which coincided with a decrease in the rate of PCB degradation in mesocosms. The numbers of LB400 enumerated by qPCR decreased a total of 100- to 1000- fold (from 106 initially to 103–104) over the course of 365 days. Approximately 7 × 103 viable LB400 cells were recovered by diluting and plating sediment samples on mineral medium with biphenyl as the sole carbon source after 365 days, confirming that most cells remained viable. DHPLC analysis was performed on sediment from mesocosms augmented with GAC and spent media (negative control) only or GAC plus the LB400 and DF1 inocula using primers specific for the upstream region of the bphA operon of LB400. The gene fragment corresponding to the upstream region of bphA eluted at 4 min with a shoulder at 5 min and was detected only at 0, 120, and at 365 days in the mesocosm augmented with LB400 plus DF1 inocula, but not the uninoculated control (Figure 5B). The addition of lactate or zero valent iron had no detectable effect on the numbers of LB400.
DISCUSSION
In a prior study that focused on reductive dechlorination with anaerobic halorespiring bacteria, 56% mass decrease in penta- and greater weathered PCB congeners was reported within 120 days after bioaugmentation with DF1 in open sediment mesocosms 8. Since many of the products of reductive dechlorination are susceptible to ring-cleavage by aerobic biphenyl degraders the current study examined the effect of bioaugmentation with both halorespiring and aerobic degrading bacteria. Prior reports by others demonstrated that sequential anaerobic and aerobic treatments in laboratory cultures were successful in reducing the total concentration of PCBs in soils and sediments. In contrast to those studies the current study: 1) examined the effect of concurrent bioaugmentation with a cultured anaerobic halorespirer rather than an enrichment culture; 2) employed static sediment mesocosms open to the air to simultaneously create both aerobic and anaerobic zones rather than closed, agitated culture vessels that were sequentially anaerobic and aerobic; 3) employed only indigenous water rather than culture medium; and 4) did not utilize high concentrations of aromatic biostimulants such as biphenyl, brominated biphenyls or chlorobenzoates.
The greatest effect was seen in the bioaugmented mesocosms without electron donor amendments, which resulted in over 80% decrease by mass of PCBs, from 8 mg/kg to less than 2 parts per million after 365 days (Fig. 1). Bioaugmenting with both microorganisms reduced the lag time for PCB degradation compared with LB400m alone, but did not show a significant affect on the final PCB concentration after 365 days (P < .05). In a prior mesocosm study by Payne et al. 8 BH sediments bioaugmented with DF1 showed a 56% decrease in PCB by mass after 120 days. Although there was a stimulation of PCB dechlorination by DF1 in the current study (Figure 3), the average Cl/biphenyl was significantly less (3.27 vs 4.76), which suggests a greater proportion of congeners were likely susceptible to more rapid aerobic degradation. Non-bioaugmented controls unamended or amended with electron donors lactate or zero valent iron showed only 25%, 24%, or 19% decrease, respectively, in total levels of PCBs after 365 days. In addition, congener analysis showed the appearance of congeners such as PCB 53, 56/60, 90 and 113 that were not products of DF1, indicating some stimulation of indigenous PCB halorespiring populations after bioaugmentation; a phenomenon that has been observed before in soils and sediments augmented with DF1 8, 29. Prior studies on sequential anaerobic-aerobic treatments reported up to 70% decrease in PCB concentration in weathered Aroclor 1248 sediment (100 mg/kg) after 19 weeks treatment with a dechlorinating enrichment followed by 19 weeks treatment with LB400 19; 67% decrease in PCB concentration in weathered Aroclor 1260 sediment (59 mg/kg) after 16 weeks treatment with a dechlorinating enrichment followed by 7 days treatment with LB400 20; 57% decrease in PCB concentration in spiked Aroclor 1242 in sediment (70 mg/kg) after 52 weeks treatment with a dechlorinating enrichment followed by 30 days treatment with LB400 30. The current study showed concurrent anaerobic-aerobic treatment achieved degradation levels greater than sequential bioagumentation observed in these prior studies. There are several possible reasons for greater efficacy exhibited by concurrent treatment in the present work. Kuo et al.31 reported that chlorobenzoates generated by the more chlorinated biphenyl ring after hydroxylation act as primers for reductive dechlorination. Sequential anaerobic-aerobic treatment would preclude exposure of actively growing anaerobic PCB dechlorinators to chorobenzoates subsequently generated after transfer into aerobic culture. However, formation of concurrent anaerobic and aerobic microniches on particles such as GAC within close spatial proximity could promote exchange of intermediates such as aerobicaly generated biostimulants effectively simulating the natural environment. Furthermore, sequential anaerobic-aerobic treatments were conducted in flasks on a shaker that would disrupt biofim formation, which could have a critical role in promoting exchange of degradation intermediates between anaerobic and aerobic communities. Lunsdorf et al 32 proposed that clay particles perform an essential role in biofilm formation possibly acting as a nutrient shuttle for mediating transfer of toxic hydrophobic substrates such as PCBs to biodegrading microbes. Concurrent degradation by formation of descrete populations of anaerobes and aerobes in close proximity likely mimics natural attenuation processes by indigenous species in the environment.
Bioaugmentation significantly reduced the lag time for degradation compared with untreated mesocosms. Addition of LB400 with DF1 decreased the lag time prior to initiation of PCB degradation, although it did not affect significantly the rate or final extent of PCB degradation (Fig. 1). After bioaugmentation with both microorganisms there was net aerobic degradation activity detected in the first 120 days, then net reductive dechlorination activity detected between 120 and 365 days (Figure S1) resulting in accumulation of di- and tri-chlorobiphenyls. This observation suggests anaerobic halorespiration occurred throughout the 365 day incubation period, but aerobic degradation subsided to negligible rates after 120 days. A similar threshold has been reported in aerated cultures, which suggests oxygen limitation due to slow oxygen diffusion rates in the static system was not a factor 19–20,30. Interestingly, PCB congener concentrations remained near the half-saturation constant (Ks) for uptake by LB400 in pure culture 33 and should have been catabolized, but such biocatalysts exhibit poor survival and catabolic activity in PCB-impacted sediments since they require a non-chlorinated cometabolic substrate for sustained growth 34. Since viable LB400 was detected after 365 days and it might be possible to stimulate degradation rates by adding a catabolic substrate such as biphenyl or benzoate, which would stimulate growth and cause induction of bph genes 22. However, there was a net accumulation of congeners with 3 to 4 chlorines in the ortho position such as PCB 103, 104, 100 and 68 that are likely resistant to aerobic degradation (Figure 3). These congeners contain chorines in 3 or more ortho positions on the biphenyl rings, which likely prevented access of the 2,3 biphenyl dioxygenase to both rings due to steric hinderance 17. The accumulation of mono- and di-o-chlorobiphenyls and non-o-chlorobiphenyls suggests that catabolic activity of LB400 might also have decreased over time due to inhibitory factors. Dai et al35 showed that o-chlorinated metabolites of aerobic PCB degradation strongly inhibit aromatic ring cleavage by 2,3-dihydroxybiphenyl 1,2-dioxygenase, promote inactivation and interferes with the degradation of other PCB congeners. There have been attempts to enhance degradation activities of aerobic degraders by altering genes, but degradation of congeners with chorines in the 2,6 positions is still inhibited 36. Although anaerobic dechlorination of chlorines in the ortho positions is rare in nature, total degradation could potentially be enhanced by bioaugmenting with ortho-dechlorinating bacteria such as bacterium o-17 that would reduce the amount of ortho chlorines/biphenyl thereby promoting greater aerobic degradation 37.
It is likely that rates decreased due in part to limited bioavailability associated with the partition coefficient of the specific sediment matrix. The biodegradation of hydrophobic organic substances often exhibits a biphasic behavior similar to that observed for desorption consisting of an initial phase of rapid degradation followed by a phase of much slower activity 38. As observed for PAH degradation, the extent of possible PCB degradation will likely be limited to the initial rapidly desorbing fraction of the weathered Aroclors. Increasing the mobility of PCBs with surfactants have been shown to increase the initial rate of degradation, they do not lower threshold biodegradation levels in PCB impacted soils and sediments indicating that they do not have a significant effect on the desorbtion rate and bioavailability of the remianing PCBs 39–41. The effectiveness of bioremediation in reducing the total chemical levels of PCBs will vary with the bioavailable fraction, which is dictated by the binding characteristics of sediment particles in a specific environment 42–43. Although bioremediation is unlikely to eliminate the total detectable PCBs in impacted sediments, microbial bioremediation has the potential to catalyze degradation of the bioavailable fraction, thereby reducing the risk factors associated with PCBs in the environment 44.
The results of this study indicate that PCB degradation after concurrent bioaugmentation with aerobic degrading and anaerobic dechlorinating bacteria is a potentially tractible approach for in-situ treatment of PCB-impacted sediments. In addition, the report shows the importance of using static sediment mesocosms to assess the effectivenes of bioaugentation in conditions that more closely mimic the environment. Although aerobic degradation was only detected in the first 120 days, anaerobic dechlorination continued to day 365 and viable inocula of both the PCB transforming anaerobe and aerobe were detected at the end of the study indicating that they could succesfully compete with the indigenous population. This long-term viability suggests that enhanced dechlorination has the potential to continue beyond 365 days. Even at a reduced rate this enhanced dechlorination could result in a long-term enhancement of dechlorination/degradation after the initial transformation of the most bioavailable congeners. Furthermore, as reported in our previous study with DF1, we show that PCB congeners are bioavaiable to LB400 in the presence of a strong sorbent such as GAC. Both LB400 and DF1 were adsorbed to GAC prior to inoculation into sediments providing an effective solid substrate for dispersing bioamendments for sediments treatment while concurrently reducing the bioavaialiblty of PCB to the higher food chain during the microbial transformation 6. The results suggest that bioaugmentation has potential as a lower cost and environmentally sustainable alternative to dredging for the in situ reduction of PCB levels in impacted sediments. Future studies to assess the effects of bioremediation on the long-term fate of slowly desorbing PCBs combined with bioassays to assess changes in bioavailability would provide a more accurate assessment of the efficacy of bioremediation for reducing risks associated with PCBs to negligible levels in the environment.
Supplementary Material
Acknowledgments
This work was supported by the National Institute of Environmental Health Science Superfund Research Program (5R01ES-016197-02) and U.S. Department of Defense, Strategic Environmental Research, and Development Program (ER-1502).
Footnotes
SUPPORTING INFORMATION AVAILABLE
Figure showing changed in chlorines per biphenyl over 365 days in mesocosms, table of PCR primers used in the study and table of inferred pathways resulting from PCB dechlorination and degradation mesocosms. This information is available free of charge via the Internet at http://pubs.acs.org/.
REFERECNES CITED
- 1.Jacobson JL, Jacobson SW. Intellectual Impairment In Children Exposed to Polychlorinated Biphenyls In Utero. New England J Med. 1996;335:783–789. doi: 10.1056/NEJM199609123351104. [DOI] [PubMed] [Google Scholar]
- 2.Adami HO, Lipworth L, Titus-Ernstoff L, Hsieh CC, Hanberg A, Ahlborg U, Baron J, Trichopoulos D. Organochlorine compounds and estrogen-related cancers in women. Cancer Causes & Control. 1995;6:551–66. doi: 10.1007/BF00054165. [DOI] [PubMed] [Google Scholar]
- 3.Silberhorn EM, Glauert HP, Robertson LW. Carcinogenicity of polyhalogenated biphenyls: PCBs and PBBs. Crit Rev Toxicol. 1990;20:440–96. doi: 10.3109/10408449009029331. [DOI] [PubMed] [Google Scholar]
- 4.EPA. Final Report. 2005. Contaminated sediment remediation guidance for hazardous waste sites; EPA-540-R-05–012. [Google Scholar]
- 5.Perelo LW. Review: In situ and bioremediation of organic pollutants in aquatic sediments. J Haz Mat. 2010;177:81–89. doi: 10.1016/j.jhazmat.2009.12.090. [DOI] [PubMed] [Google Scholar]
- 6.Ghosh U, Luthy RG, Cornelissen G, Werner D, Manzie CA. In-situ Sorbent Amendments: A New Direction in Contaminated Sediment Management. Environ Sci Technol. 2011;45:1163–1168. doi: 10.1021/es102694h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Millward RN, Bridges TS, Ghosh U, Zimmerman JR, Luthy RG. Addition of activated carbon to sediments to reduce PCB bioaccumulation by a polychaete (Neanthes arenaceodentata) and an amphipod (Leptocheirus plumulosus) Environ Sci Technol. 2005;39:2880–2887. doi: 10.1021/es048768x. [DOI] [PubMed] [Google Scholar]
- 8.Payne RB, Chun C, May HD, Sowers KR. Enhanced reductive dechlorination of polychlorinated biphenyl impacted sediment by bioaugmentation with a dehalorespiring bacterium. Env Sci Technol. 2011;45:8772–8779. doi: 10.1021/es201553c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Major DW, McMaster ML, Cox EE. Field demonstration of successful bioaugmentation to achieve dechlorination of tetrachloroethene to ethene. Environ Sci Technol. 2002;36:5106–5116. doi: 10.1021/es0255711. [DOI] [PubMed] [Google Scholar]
- 10.Bedard DL, Ritalahti KM, Loffler FE. The Dehalococcoides population in sediment-free mixed cultures metabolically dechlorinates the commercial polychlorinated biphenyl mixture Aroclor 1260. Appl Environ Microbiol. 2007;73:2513–21. doi: 10.1128/AEM.02909-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adrian L, Dudkova V, Demnerova K, Bedard DL. “Dehalococcoides” sp. strain CBDB1 extensively dechlorinates the commercial polychlorinated biphenyl mixture Aroclor 1260. Appl Environ Microbiol. 2009;75:4516–4524. doi: 10.1128/AEM.00102-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bedard DL, Bailey JJ, Reiss BL, Jerzak GVS. Development and characterization of stable sediment-free anaerobic bacterial enrichment cultures that dechlorinate Aroclor 1260. Appl Environ Microbiol. 2006;72:2460–2470. doi: 10.1128/AEM.72.4.2460-2470.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fagervold SK, May HD, Sowers KR. Microbial reductive dechlorination of Aroclor 1260 in Baltimore Harbor sediment microcosms is catalyzed by three phylotypes within the phylum Chloroflexi. Appl Environ Microbiol. 2007;73:3009–18. doi: 10.1128/AEM.02958-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cutter LA, Watts JEM, Sowers KR, May HD. Identification of a microorganism that links its growth to the reductive dechlorination of 2,3,5,6-chlorobiphenyl. Environ Microbiol. 2001;3:699–709. doi: 10.1046/j.1462-2920.2001.00246.x. [DOI] [PubMed] [Google Scholar]
- 15.Bopp LH. Degradation of highly chlorinated PCBs by Pseudomonas strain LB400. J Ind Microbiol. 1986;1:23–29. [Google Scholar]
- 16.Furukawa K, Miyazaki T. Cloning of a gene cluster encoding biphenyl and chlorobiphenyl degradation in Pseudomonas pseudoalcaligenes. J Bacteriol. 1986;166:392–8. doi: 10.1128/jb.166.2.392-398.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bedard DL, Unterman R, Bopp LH, Brennan MJ, Haberl ML, Johnson C. Rapid assay for screening and characterizing microorganisms for the ability to degrade polychlorinated biphenyls. Appl Environ Microbiol. 1986;51:761–8. doi: 10.1128/aem.51.4.761-768.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abramowicz DA. Aerobic and anaerobic PCB biodegradation in the environment. Environ Health Perspectives. 1995;103:97–9. doi: 10.1289/ehp.95103s497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Evans BS, Dudley CA, Klasson KT. Sequential anaerobic-aerobic biodegradation of PCBs in soil slurry microcosms. Appl Biochem Biotechnol. 1996;57–58:885–894. doi: 10.1007/BF02941769. [DOI] [PubMed] [Google Scholar]
- 20.Master ER, Lai VW, Kuipers B, Cullen WR, Mohn WW. Sequential anaerobic-aerobic treatment of soil contaminated with weathered Aroclor 1260. Environ Sci Technol. 2002;36:100–3. doi: 10.1021/es001930l. [DOI] [PubMed] [Google Scholar]
- 21.Sambrook J, Fritsch EF, Maniatis T. Molecular cloning- a laboratory manual. Cold Spring Harbour Press; N.Y: 1989. [Google Scholar]
- 22.Denef VJ, Patrauchan MA, Florizone C, Park J, Tsoi TV, Verstraete W, Tiedje JM, Eltis LD. Growth substrate- and phase-specific expression of biphenyl, benzoate, and C1 metabolic pathways in Burkholderia xenovorans LB400. J Bacteriol. 2005;187:7996–8005. doi: 10.1128/JB.187.23.7996-8005.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Konare H, Yost RS, Doumbia M, McCarty GW, Jarju A, Kablan R. Loss on ignition: Measuring soil organic carbon in soils of the Sahel, West Africa. African J Agr Res. 2010;5:3088–3095. [Google Scholar]
- 24.Fagervold SK, Watts JEM, May HD, Sowers KR. Sequential reductive dechlorination of meta-chlorinated polychlorinated biphenyl congeners in sediment microcosms by two different Chloroflexi phylotypes. Appl Environ Microbiol. 2005;71:8085–8090. doi: 10.1128/AEM.71.12.8085-8090.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beltrametti F, Reniero D, Backhaus S, Hofer B. Analysis of transcription of the bph locus of Burkholderia sp. strain LB400 and evidence that the ORF0 gene product acts as a regulator of the bphA1 promoter. Microbio. 2001;147:2169–2182. doi: 10.1099/00221287-147-8-2169. [DOI] [PubMed] [Google Scholar]
- 26.Kjellerup BV, Sun X, Ghosh U, May HD, Sowers KR. Site-specific microbial communities in three PCB-impacted sediments are associated with different in situ dechlorinating activities. Environ Microbiol. 2008;10:1296–1309. doi: 10.1111/j.1462-2920.2007.01543.x. [DOI] [PubMed] [Google Scholar]
- 27.Baker J, Mason R, Cornwell J, Ashley J, Halka J, Hill J. UMCES[CBL] 97–142. Maryland Department of the Environment; Aug, 1997. Spatial mapping of sedimentary contaminants in the Baltimore Harbor/Patapsco River/Back River system. [Google Scholar]
- 28.Morgan WP, Sommer SE. Polychlorinated biphenyls in Baltimore Harbor sediments. Bull Environ Cont Toxicol. 1979;22:413–419. doi: 10.1007/BF02026964. [DOI] [PubMed] [Google Scholar]
- 29.May HD, Miller GS, Kjellerup BV, Sowers KR. Dehalorespiration with polychlorinated biphenyls by an anaerobic ultramicrobacterium. Appl Environ Microbiol. 2008;74:2089–2094. doi: 10.1128/AEM.01450-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodrigues JLM, Kachel CA, Aiello MR, Quensen JF, Maltseva OV, Tsoi TV, Tiedje JM. Degradation of Aroclor 1242 dechlorination products in sediments by Burkholderia xenovorans LB400(ohb) and Rhodococcus sp. Strain RHA1(fcb) Appl Environ Microbiol. 2006;72:2476–2482. doi: 10.1128/AEM.72.4.2476-2482.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuo CE, Liu SM, Liu C. Biodegradation of coplanar polychlorinated biphenyls by anaerobic microorganisms from estuarine sediments. Chemosphere. 1999;39:1445–1458. doi: 10.1016/s0045-6535(99)00046-6. [DOI] [PubMed] [Google Scholar]
- 32.Lunsdorf H, Erb RW, Abraham WR, Timmis KN. “Clay Hutches”: a novel interaction between bacteria and clay minerals. Environ Microbiol. 2000;2:161–168. doi: 10.1046/j.1462-2920.2000.00086.x. [DOI] [PubMed] [Google Scholar]
- 33.Rein A, Fernqvist MM, Mayer P, Trapp S, Bittens M, Karlson UG. Degradation of PCB congeners by bacterial strains. Appl Microbiol Biotechnol. 2007;77:469–481. doi: 10.1007/s00253-007-1175-6. [DOI] [PubMed] [Google Scholar]
- 34.Potrawfke T, Lohnert TH, Timmis KN, Wittich RM. Mineralization of low-chlorinated biphenyls by Burkholderia sp. strain LB400 and by a two member consortium upon directed interspecies transfer of chlorocatechol pathway genes. Appl Microbiol Biotechnol. 1998;50:440–446. [Google Scholar]
- 35.Dai S, Vaillancouirt FH, Maaroufi H, FDrouin NM, Neau DB, Snieckus V, olin JT, Eltis LD. Identification and analysis of a bottleneck in PCB degradation. Nature Structural Biology. 2002;9:934–939. doi: 10.1038/nsb866. [DOI] [PubMed] [Google Scholar]
- 36.Hrywna Y, Tsoi TV, Maltseva OV, Quensen JF, Tiedje JM. Construction and characterization of two recombinant bacteria that grow on ortho- and para-substituted chlorobiphenyls. Appl Environ Microbiol. 1999;65:2163–2169. doi: 10.1128/aem.65.5.2163-2169.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fagervold SK, Watts JEM, May HD, Sowers KR. Effects of bioaugmentation on indigenous PCB dechlorinating activity in sediment microcosms. Wat Res. 2011;45:3899–3907. doi: 10.1016/j.watres.2011.04.048. [DOI] [PubMed] [Google Scholar]
- 38.Cornelissen G, Hassell KA, Van Noort PC, Kraaij R, van Ekeren PJ, Dijkema C, de Jager PA, Govers HAJ. Slow desorption of PCBs and chlorobenzenes from soils and sediments: relations with sorbent and sorbate characteristics. Environ Poll. 1998;108:69–80. doi: 10.1016/s0269-7491(99)00203-1. [DOI] [PubMed] [Google Scholar]
- 39.Jafvert CT, Vanhoof PL, Chu W. The phase distribution of polychlorobiphenyl congeners in surfactant-amended sediment slurries. Wat Res. 1995;29:2387–2397. [Google Scholar]
- 40.Cho YC, Oostrofsky EB, Rhee GY. Effects of a rhamnolipid biosurfactant on the reductive dechlorination of polychlorinated biphenyls by St. Lawrence River (North America) microorganisms. Environ Toxicol Chem. 2004;23:1425–1430. doi: 10.1897/03-473. [DOI] [PubMed] [Google Scholar]
- 41.Kim J, Frohnhoefer RC, Cho YC, Cho DW, Rhee GY. Reductive dechlorination of low concentration polychlorinated biphenyls as affected by a rhamnolipid biosurfactant. J Microbiol Biotechnol. 2008;18:1564–1571. [PubMed] [Google Scholar]
- 42.Talley JW, Ghosh U, Tucker SG, Furey JS, Luthy RG. Particle-Scale Understanding of the Bioavailability of PAHs in Sediment. Env Sci Technol. 2002;36:477–483. doi: 10.1021/es010897f. [DOI] [PubMed] [Google Scholar]
- 43.Luthy RG, Aiken GR, Brusseau ML, Cunningham SD, Gschwend PM, Pignatello JJ, Reinhard M, Traina S, WJWJ, Westall JC. Sequestration of hydrophobic organic contaminants by geosorbents. Environ Sci Technol. 1997;31:3341–3347. [Google Scholar]
- 44.Alexander M. Aging, bioavailability, and overestimation of risk from environmental pollutants. Env Sci Technol. 2000;34:4259–4265. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.