Abstract
Purpose
Whether race influences bone loss and fracture risk during androgen deprivation therapy (ADT) for prostate cancer is unknown. Using data from a prospective, clinical trial, we compared bone mineral density (BMD) and fracture between African American and Caucasian men receiving ADT.
Materials and Methods
Subjects (n=516) were in the placebo group of a two-year randomized placebo controlled fracture prevention trial and were African American (n=68) or Caucasian (n=448). We compared baseline characteristics, changes in BMD, and rates of new fractures between races.
Results
Compared to Caucasian men, African American men had higher baseline hip BMD (0.98 ± 0.15 g/m2 versus 0.91 ± 0.15 g/m2; P=0.001) and similar spine BMD (1.09 ± 0.22 versus 1.11 ± 0.22; P=0.51). There was no difference in prevalent vertebral fractures between African American and Caucasian men (7.4% versus 15.0%; P=0.13). Percentage change in hip BMD at two years was similar between African American and Caucasian men (−2.21 ± 0.59% versus −2.54 ± 0.26%; P=0.65). Changes in BMD of the lumbar spine were also similar between African American and Caucasian men (−1.74 ± 0.69 versus −1.30 ± 0.33%; P=0.64). No new vertebral fractures were reported in African American men versus two fractures in Caucasian men.
Conclusions
In a clinical trial, African American men receiving ADT for prostate cancer have higher hip BMD and tended to have fewer prevalent vertebral fractures than Caucasian men. Despite lower baseline risk of osteoporosis and fracture, African American men experience a decline in BMD that is similar to Caucasian men.
Keywords: prostate cancer, androgen deprivation therapy, bone mineral density, fracture, survivorship
INTRODUCTION
Androgen deprivation therapy (ADT) for prostate cancer increases bone turnover, decreases bone mineral density (BMD), and increases rates of clinical fracture 1–7. Treatment-related osteoporosis and fractures adversely impact quality of life and are associated with greater morbidity and mortality1–4.
In the United States, African American men are disproportionately affected by prostate cancer. Compared to Caucasian men, African American men have a 50% greater prostate cancer incidence and two-fold greater prostate cancer mortality rate. Because of this, understanding the effects of ADT on BMD and the risk of fracture in the African American population is of great clinical interest. Osteoporosis and fracture develop in men receiving androgen deprivation therapy due to the effects of the absence of testosterone and estrogen on bone. Previous work investigating BMD in the general population has demonstrated that African American men have significantly higher BMD at all skeletal sites than Caucasian men 8,9. African American men in the general population also have fewer prevalent fractures than Caucasian men10. It is not known whether the effects of ADT on BMD and fractures vary by race.
In a recently reported randomized placebo-controlled trial of men receiving ADT for prostate cancer, toremifene significantly increased BMD and decreased new vertebral fractures11. We used data from the placebo group to compare the effects of ADT on BMD and fracture rates between African American and Caucasian men.
MATERIALS AND METHODS
Study Design
Subjects in these analyses (n=516) were in the intent-to-treat population of the placebo control group of a recently reported two-year randomized controlled trial of toremifene to prevent fractures in men receiving ADT for prostate cancer (clinicaltrials.gov identifier: NCT00129142), resided in United States, and reported their race as either African American (n=68) or Caucasian (n=448)11. The study was sponsored by GTx, Inc. and was designed by GTx, Inc. and the principal investigator (MRS). INC Research (Raleigh, NC) provided data management, Synarc (San Francisco, CA) provided central radiology services, and CRL Medinet (Lenexa, KS) provided central laboratory services.
Subjects
The study included men aged ≥50 years who were receiving ADT for histologically documented prostate cancer and were at an increased risk for fracture based on (1) age ≥70 years, or (2) osteopenia at the femoral neck or L1 to L4 lumbar spine 11. All subjects were members of the placebo arm of the trial from the United States and who identified themselves as either African American or Caucasian. They had baseline serum prostate-specific antigen (PSA) ≤4 ng/mL; adequate bone marrow, liver, and renal function; Zubrod performance status 0 or 1; and received either continuous ADT for ≥6 months or intermittent ADT for ≥12 months before randomization (all subjects received continuous ADT for 24 months following randomization).
Subjects were excluded who received treatment with a bisphosphonate, Selective Estrogen Receptor Modulator (SERM), parathyroid hormone, teriparatide, finasteride, dutasteride, PC-SPES, saw palmetto, calcitonin, or oral glucocorticoids within 45 days of randomization11. Additional exclusion criteria included prior toremifene treatment; <8 evaluable vertebrae from T4 to L4, or ≥4 prevalent vertebral fractures; weight >300 lbs (due to weight limits for densitometry equipment); or history of thromboembolic disease, chronic hepatitis, or cirrhosis. The institutional review board for each participating institution approved the study, and all subjects provided written informed consent.
Endpoints
The primary endpoint was incidence of new vertebral fractures. Secondary endpoints included the incidence of fragility fractures and changes in BMD at the spine and hip.
Study Procedures
Anteroposterior and lateral radiographs of thoracic and lumbar spine were obtained at baseline, 12 months, and 24 months or at early termination. Vertebral fractures were assessed by an expert reader at the central imaging laboratory who evaluated vertebrae from T4 to L4 using the Genant semiquantitative scoring method 12. Prevalent vertebral fractures were defined as fractures that were present at the screening visit, and new vertebral fractures were defined as fractures in a vertebra without a prevalent fracture 11. Fragility fractures were defined as fractures resulting from trauma equivalent to a fall from less than standing height and were confirmed by expert review of radiographs. The sum of all fractures is defined as fragility fractures plus new vertebral fractures. Pathologic fractures, non-fragility fractures (ie, trauma from a fall greater than standing height), or fractures of the skull, face, or fingers were excluded from the overall fracture analysis.
Dual energy x-ray absorptiometry (DXA) of the spine, hip, and femoral neck was performed at baseline, month 12, and month 24 or at early termination using either a Hologic (Bedford, MA) or Lunar (GE Healthcare, Waukesha, WI) densitometer. Osteopenia was defined as Hologic L1 to L4 <0.926 g/cm2 and femoral neck <0.717 g/cm2, Lunar L1 to L4 <1.050 g/cm2 and femoral neck <0.849 g/cm2. These values were calculated using a T score of <−1 as the definition of osteopenia and then converting the T score into BMD (g/cm2) using the respective machine manufacturer specifications 11. Any subject with ≥7% decrease in BMD of either the hip or spine prior to the end of the trial was withdrawn from the study for safety purposes. Serum levels of bone turnover markers, and testosterone were measured at baseline, month 12, and month 24 and were analyzed at a central laboratory.
Statistical Analyses
Demographic and presenting features at baseline that were recorded as continuous variables: age, duration of ADT, total testosterone, serum PSA, BMD in the spine and hip, T-scores in the spine and hip, BMI, and bone turnover markers were compared between the races using a T-test. The means and one standard deviation are reported. Proportions of subjects with orchiectomies, prevalent fractures at baseline and who were at least osteopenic were compared between the races using the chi-square test. The proportion of subjects who developed new morphometric vertebral fractures and any fracture were compared between the races using Fisher’s exact test. Percentage change in BMD from baseline to months 12 and 24 were compared between the races with a T-test. The 12 and 24 month comparisons to baseline values include only subjects with DXA results at the 12 and 24 month assessments, respectively. Mean change and one standard error of the mean are reported. P values are two-sided and are not adjusted for multiple comparisons. All analyses were performed using SAS/STAT software, version 9.113 .
RESULTS
Baseline Characteristics
These analyses included subjects in the placebo control group who resided in United States and reported their race as either African American (n=68) or Caucasian (n=448). Baseline characteristics of the participants are summarized in Table 1. Mean age of the African American subjects was significantly lower than that of Caucasian subjects (75.0 years ± 8.4 years versus 76.9 years ± 7.1 years; P=0.045). Duration of ADT, prevalence of bilateral orchiectomies, total testosterone, and serum PSA were similar between the groups.
Table 1.
Baseline Characteristics by Race
| Race
|
|||
|---|---|---|---|
| White (n=448) | Black (n=68) | p-value | |
|
| |||
| Age (y) | 76.93 ± 7.07 | 75.03 ± 8.37 | 0.045 |
|
| |||
| BMI (kg/m2) | 28.37 ± 4.21 | 28.16 ± 4.07 | 0.70 |
|
| |||
| Orchiectomy at baseline, number (%) | 14 (3.1) | 1 (1.4) | 0.45 |
|
| |||
| Serum PSA, ng/mL | 0.42 ± 0.78 | 0.45 ± 0.92 | 0.76 |
|
| |||
| Prior Years on ADT | 4.34 ± 3.74 | 3.96 ± 2.79 | 0.42 |
|
| |||
| Serum testosterone, ng/dL | 25.61 ± 31.9 | 22.45 ± 5.97 | 0.43 |
|
| |||
| BMD T score | |||
| Lumbar spine | −0.38 ± 1.7 | −0.33 ± 1.9 | 0.85 |
| Total hip | −1.09 ± 1.10 | −0.54 ± 1.1 | 0.001 |
|
| |||
| BMD (g/m2) | |||
| Lumbar spine | 1.11 ± 0.22 | 1.09 ± 0.22 | 0.51 |
| Total hip | 0.91 ± 0.15 | 0.98 ± 0.15 | 0.001 |
|
| |||
| Low BMD any site, number (%) | 356 (79.5) | 44 (64.7) | 0.007 |
|
| |||
| Prevalent vertebral fractures, number (%) | 67 (15) | 5 (7.4) | 0.13 |
|
| |||
| Bone Specific Alk Phos, U/L | 28.28 ± 10.99 | 32.78 ± 10.98 | 0.002 |
|
| |||
| Osteocalcin, ng/mL | 11.29 ± 4.98 | 13.09 ± 5.62 | 0.008 |
|
| |||
| CTX (C-Telopeptide), ng/mL | 0.57 ± 0.25 | 0.70 ± 0.31 | <0.001 |
Baseline BMD, Prevalent Vertebral Fractures, and Bone Turnover
Compared to Caucasian men, African American men had higher markers of bone turnover including significantly higher serum levels of bone specific alkaline phosphatase, osteocalcin, and c-telopeptide (CTX) (P<0.01 for each marker) (Table 1). Further, at baseline, African American men had a higher mean BMD of the total hip (0.98 ± 0.15 g/m2 and 0.91 ± 0.15 g/m2 in African American and Caucasian men, respectively; P<0.001) and higher mean total hip T score (−0.54 ± 1.10 and −1.09 ± 1.10 in African American and Caucasian men, respectively; P<0.001). Mean lumbar spine BMD and lumbar spine T scores were similar between the groups. Significantly fewer African American men had low BMD at any skeletal site, with 67.7% of African American men versus 81.9% of Caucasian men with low BMD at any skeletal site (P<0.01). However, when comparing African American men to Caucasian men, there was no statistically significant difference in prevalent vertebral fractures (7.4% of African American men versus 15.0% of Caucasian men; P=0.13).
On Study BMD and Fractures
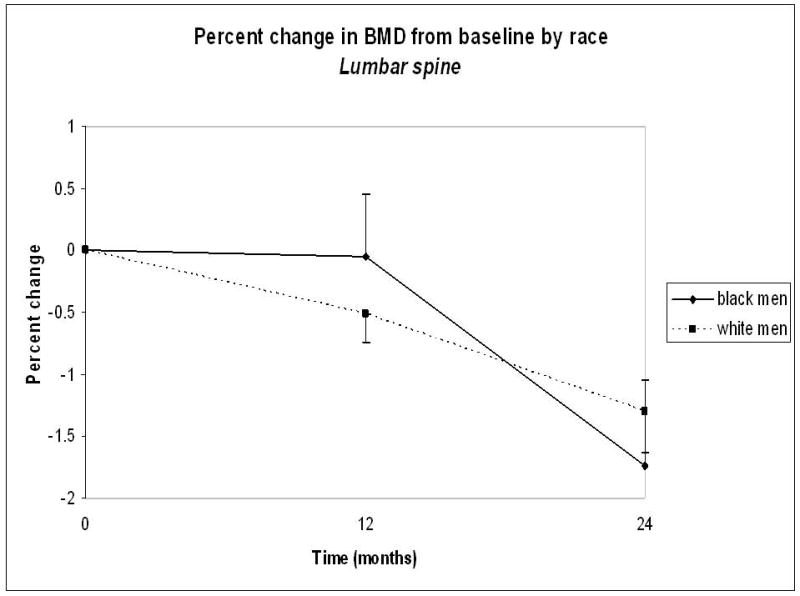
Percentage change in BMD during the study was similar between African American and Caucasian men (Figure 1). From baseline to month 24, mean lumbar spine BMD decreased by 1.74% ± 0.69% in African American men and by 1.30% ± 0.33% in Caucasian men (P=0.64). Similarly, total hip BMD decreased by 2.21% ± 0.59% in African American men and by 2.54% ± 0.26% in Caucasian men (P=0.65). The two-year incidence of any fracture was 9.8% in Caucasian men and 2.9% in African American men (P=0.07). There were no new vertebral fractures in African American men versus 4.5% of Caucasian men who developed new fractures during the study (P=0.09).
Figure 1.

DISCUSSION
Using data from the placebo group of a large prospective randomized clinical trial of men receiving ADT for prostate cancer, we observed that African American men have higher BMD at baseline but similar numbers of prevalent vertebral fractures compared to Caucasian men. Changes in BMD during ongoing ADT were similar for African American and Caucasian men. While African American men had fewer new vertebral fractures than Caucasian men, this observation did not reach statistical significance.
Our observation that African American men had higher baseline BMD than Caucasian men is consistent with prior observations from the general population8, 9, 10,14. Several mechanisms may contribute to greater BMD in African American men. African American men appear to achieve higher peak bone mass in early adulthood than Caucasian men15. Various explanations have been suggested for this difference, including decreased calcium excretion from the kidneys, greater secretion of growth hormone, and higher levels of 17-β estradiol levels in African American men as opposed to Caucasian men16, 17. Skeletal response to vitamin D may also vary between races18.
African American men had fewer but not a statistically significantly different number of prevalent vertebral fractures compared to Caucasian men. The trend that we observed is consistent with studies of fracture rates in African American and Caucasian men in the general population 19. The Baltimore Men’s Osteoporosis Study evaluated prevalent fractures in 542 men (415 Caucasian men and 127 African American men) over the age of 65 years. In that study, fewer African American men suffered vertebral fractures than Caucasian men (0.9% versus 7.3%).
Changes in BMD of the lumbar spine and hip were similar for African American and Caucasian subjects in these analyses. In the general population of older Americans, the decrease in BMD over time also appears to be similar for African American and Caucasian men. MrOS is a large observational study of Caucasian men (n=3869) and African American men (n=138) ≥65 years of age recruited from various sites across the United States 20. In agreement with our findings, annual decreases in BMD of the hip were similar for African American and Caucasian men in the MrOS study. In another observational study of older men, deceases in BMD of the hip were similar between African American and Caucasian men after adjusting for other variables 8.
Caucasian men in our study trended towards having significantly more fractures during ADT and tended to have more new vertebral fractures. This is consistent with the observation that African American men in the general population have a lower rate of fracture than Caucasian men 19. Given evidence demonstrating similar rates of decline in BMD in African American and Caucasian men, we postulate that higher baseline BMD in African American men likely accounts for our observed lower fracture rates. With extended exposure to ADT, we expect African American men to have increasing rates of fracture as their BMD falls.
The large sample size and prospective design are strengths of these analyses. Because there were few fracture events in the study population as a whole, however, the analyses may have been underpowered to detect differences in fracture rates between African American and Caucasian men. The African American men were younger than the Caucasian men in this study and these differences in age may have contributed to the observed racial differences in BMD and fractures.
The National Comprehensive Cancer Center Network and other published guidelines recommend screening men receiving ADT according to guidelines from the National Osteoporosis Foundation for the general population21–23. These guidelines recommend supplemental vitamin D and calcium daily for all men receiving ADT. They also recommend drug therapy for men with a 10 year probability of hip fracture >3% or a 10 year probability of major osteoporosis-related fracture >20% as estimated using FRAX 21–22. FRAX is a tool developed by the World Health Organization that estimates a patient’s 10 year risk of fracture based on various patient characteristics24. Factors in the risk calculation include race, sex, history of smoking or alcohol use, glucocorticoid use, previous fracture, and bone mineral density at the patient’s femoral neck if that data is available. Although African American men in our study had higher baseline BMD and trended towards having fewer fractures than Caucasian men, they experienced similar high rates of bone loss during ADT suggesting increasing fracture risk over time. Despite higher baseline BMD, African American men in our study were as susceptible to the detrimental effects of ADT on bone as Caucasian men, and should be monitored while receiving ADT to assure treatment for low BMD is initiated when appropriate. We recommend close monitoring of BMD for men of all races during ADT treatment to according to established guidelines.
CONCLUSIONS
Among men receiving ADT for prostate cancer, African American men have higher BMD at the hip but a similar amount of prevalent vertebral fractures compared to Caucasian men. Reduction in BMD during ongoing ADT was similar for African American and Caucasian men. We advocate checking baseline and yearly BMD in all men undergoing treatment with ADT given similar decline in BMD and fracture rates in both races despite African American men starting with a higher baseline BMD.
References
- 1.Maillefert JF, Sibilia J, Michel F, et al. Bone mineral density in men treated with synthetic gonadotropin-releasing hormone agonists for prostatic carcinoma. J Urol. 1999;161(4):1219–1222. [PubMed] [Google Scholar]
- 2.Berruti A, Dogliotti L, Terrone C, et al. Changes in Bone Mineral Density, Lean Body Mass and Fat Content as Measured by Dual Energy X-Ray Absorptiometry in Patients With Prostate Cancer Without Apparent Bone Metastases Given Androgen Deprivation Therapy. J Urol. 2002;167(6):2361–2367. [PubMed] [Google Scholar]
- 3.Daniell HW, Dunn SR, Ferguson DW, et al. Progressive osteoporosis during androgen deprivation therapy for prostate cancer. J Urol. 2000;163(1):181–186. [PubMed] [Google Scholar]
- 4.Diamond T, Campbell J, Bryant C, et al. The effect of combined androgen blockade on bone turnover and bone mineral densities in men treated for prostate carcinoma: longitudinal evaluation and response to intermittent cyclic etidronate therapy. Cancer. 1998;83(8):1561–1566. [PubMed] [Google Scholar]
- 5.Shahinian VB, Kuo Y, Freeman JL, et al. Risk of Fracture after Androgen Deprivation for Prostate Cancer. N Engl J Med. 2005;352(2):154–164. doi: 10.1056/NEJMoa041943. [DOI] [PubMed] [Google Scholar]
- 6.Smith MR, Lee WC, Brandman J, et al. Gonadotropin-releasing hormone agonists and fracture risk: a claims-based cohort study of men with nonmetastatic prostate cancer. J Clin Oncol. 2005;23(31):7897–7903. doi: 10.1200/JCO.2004.00.6908. [DOI] [PubMed] [Google Scholar]
- 7.Smith MR, Boyce SP, Moyneur E, et al. Risk of clinical fractures after gonadotropin-releasing hormone agonist therapy for prostate cancer. J Urol. 2006;175(1):136–139. doi: 10.1016/S0022-5347(05)00033-9. https://phstwlp1.partners.org:2443/login?url=http://ovidsp.ovid.com/ovidweb.cgi?T=JS&NEWS=N&PAGE=fulltext&AN=16406890&D=medl. [DOI] [PubMed] [Google Scholar]
- 8.Tracy JK, Meyer WA, Flores RH, et al. Racial differences in rate of decline in bone mass in older men: the Baltimore men’s osteoporosis study. J Bone Miner Res. 2005;20(7):1228–1234. doi: 10.1359/JBMR.050310. [DOI] [PubMed] [Google Scholar]
- 9.George A, Tracy JK, Meyer WA, et al. Racial differences in bone mineral density in older men. J Bone Miner Res. 2003;18(12):2238–2244. doi: 10.1359/jbmr.2003.18.12.2238. [DOI] [PubMed] [Google Scholar]
- 10.Nelson DA, Jacobsen G, Barondess DA, et al. Ethnic differences in regional bone density, hip axis length, and lifestyle variables among healthy black and white men. J Bone Miner Res. 1995;10(5):782–787. doi: 10.1002/jbmr.5650100515. [DOI] [PubMed] [Google Scholar]
- 11.Smith MR, Morton RA, Barnette KG, et al. Toremifene to Reduce Fracture Risk in Men Receiving Androgen Deprivation Therapy for Prostate Cancer. J Urol. 2010;184(4):1316–1321. doi: 10.1016/j.juro.2010.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Genant HK. Assessment of vertebral fractures in osteoporosis research. J Rheumatol. 1997;24(6):1212–1214. [PubMed] [Google Scholar]
- 13.SAS Institute Inc. User’s Guide. Cary, NC: SAS Institute Inc; 2004. SAS/STAT® 9.1. [Google Scholar]
- 14.Nam HS, Shin MH, Zmuda JM, et al. Race/ethnic differences in bone mineral densities in older men. Osteoporos Int. 2010 doi: 10.1007/s00198-010-1188-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bell NH, Gordon L, Stevens J, et al. Demonstration that bone mineral density of the lumbar spine, trochanter, and femoral neck is higher in black than in white young men. Calcif Tissue Int. 1995;56(1):11–13. doi: 10.1007/BF00298737. [DOI] [PubMed] [Google Scholar]
- 16.Bell NH, Yergey AL, Vieira NE, et al. Demonstration of a difference in urinary calcium, not calcium absorption, in Black and White adolescents. J Bone Miner Res. 1993;8:1111–1115. doi: 10.1002/jbmr.5650080912. [DOI] [PubMed] [Google Scholar]
- 17.Wright NM, Renault J, Willi S, et al. Greater secretion of growth hormone in Black than White men: Possible factor in greater bone mineral density – a clinical research center study. J Clin Endocrinol Metab. 1995;80:2291–2297. doi: 10.1210/jcem.80.8.7543111. [DOI] [PubMed] [Google Scholar]
- 18.Gutierrez OM, Farwell WR, Kermah D, et al. Racial differences in the relationship between vitamin D, bone mineral density, and parathyroid hormone in the National Health and Nutrition Examination Survey. Osteoporos Int. 2010 doi: 10.1007/s00198-010-1383-2. online publication 17 September 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tracy JK, Meyer WA, Grigoryan M, et al. Racial differences in the prevalence of vertebral fractures in older men: the Baltimore Men’s Osteoporosis Study. Osteoporos Int. 2006;17(1):99–104. doi: 10.1007/s00198-005-1919-z. [DOI] [PubMed] [Google Scholar]
- 20.Sheu Y, Cauley JA, Wheeler VW, et al. Age-related decline in bone density among ethnically diverse older men. Osteoporos Int. 2010 doi: 10.1007/s00198-010-1330-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Watts NB, Lewiecki EM, Miller PD, et al. National Osteoporosis Foundation. Clinician’s Guide to Prevention and Treatment of Osteoporosis and the World Health Organization Fracture Risk Assessment Tool (FRAX): what they mean to the bone densitometrist and bone technologist. J Clin Densitom. 2008;11(4):473–477. doi: 10.1016/j.jocd.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 22.Mohler J, et al. National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology Prostate Cancer V.3.2010. [DOI] [PubMed] [Google Scholar]
- 23.Saylor PJ, Keating NL, Smith MR. Prostate Cancer Survivorship: Prevention and Treatment of the Adverse Effects of Androgen Deprivation Therapy. J Gen Intern Med. 2009;24(Suppl 2):389–94. doi: 10.1007/s11606-009-0968-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.FRAX: WHO risk assessment tool. [accessed November 18, 2010];Fracture risk information. Available from URL: http://www.shef.ac.uk/FRAX/


