Abstract
The objective of this study was to evaluate the long-term efficacy of venlafaxine extended release (ER) ≤225 mg/ day in patients with recurrent major depressive disorder (MDD). In this double-blind trial, outpatients with recurrent MDD (N=1096) were randomized to 10 weeks of acute-phase treatment with venlafaxine ER (75–300 mg/ day) or fluoxetine (20–60 mg/day) followed by a 6-month continuation phase and two consecutive 12-month maintenance phases. At the start of each maintenance period, venlafaxine ER responders were randomized to double-blind venlafaxine ER or placebo. In this analysis, data from responders to acute and continuation treatment were analyzed during the combined maintenance phases while receiving venlafaxine ER ≤225 mg/day. Failure to maintain response was defined as an increase in maintenance dose to 300 mg/day or recurrence. Differences were calculated using Kaplan–Meier methods and compared using log-rank tests. Continuation-phase responders (n =114) receiving venlafaxine ER ≤225 mg/ day comprised the analysis population (venlafaxine ER: n= 55; placebo: n= 59). The estimated probability for remaining well across 24 months of maintenance treatment was 67% for venlafaxine ER and 41% for placebo (P = 0.007). Venlafaxine ER effectively maintained response at doses ≤225 mg/day for up to 2.5 years in patients with recurrent MDD. The findings are consistent with those of the full data set.
Keywords: dosing, long-term efficacy, recurrence prevention, selective serotonin reuptake inhibitor/serotonin-norepinephrine reuptake inhibitor
Introduction
Depression is a chronic, recurrent, and disabling condition that is prevalent worldwide. In a pan-European population, the 6-month prevalence of depression (major depression, minor depression, and depressive symptoms) was 17% (Tylee et al., 1999). Depression exacts a significant toll on functional status and quality of life for many patients (Trompenaars et al., 2006), and the societal impact is profound. A recent economic analysis estimated that the total annual cost of depression in Europe was €118 billion in 2004, with direct costs amounting to approximately €42 billion (Sobocki et al., 2006).
The episodic nature of this disorder suggests that a substantial portion of these costs may be related to recurrence. Naturalistic studies have shown that 40% of patients with major depressive disorder (MDD) will have a recurrence within 1 year, and 85% will have a recurrence within 15 years (Mueller et al., 1999). Thus, prophylaxis is an important goal of treatment and constitutes one method to reduce the suffering, disability, and costs associated with recurrent depressive illness.
Surprisingly, few randomized trials have evaluated the efficacy of selective serotonin reuptake inhibitors and serotonin-norepinephrine reuptake inhibitors in long-term prevention of recurrence. Venlafaxine extended release (ER), the first drug classified as a serotonin-norepinephrine reuptake inhibitor, was shown in a randomized, placebo-controlled trial to be effective for recurrent MDD over 2.5 years at doses ranging from 75 to 300 mg/day (Keller et al., 2007a; Montgomery et al., 2004; Shelton et al., 2005). In many countries, however, the approved dosage of venlafaxine ER is ≤225 mg/day, which creates uncertainty about the generalizability of the findings from the whole data set to those settings where venlafaxine ER is not approved up to 300 mg/day. The objective of this analysis was to evaluate the long-term probability of remaining well on venlafaxine ER ≤225 mg/day.
Methods
Study design
This analysis is based on data from the Prevention of Recurrent Episodes of Depression with Venlafaxine ER for Two Years (PREVENT) trial, a randomized, multi-center, multiphase study that has been presented in detail elsewhere (Keller et al., 2007b; Kocsis et al., 2007; Kornstein, 2006). Patients who were included in the primary study were at least 18 years of age and had at least two earlier episodes of major depression in the previous 5 years, as defined using Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) (American Psychiatric Association, 1994) criteria, with earlier episodes being at least 3 months before the start of the current episode. Participants had 17-item Hamilton Rating Scale for Depression (HAM-D17) scores of at least 20 at screening and at least 18 at acute-phase randomization. Depressive symptoms were required to be present for at least 1 month before acute-phase randomization. Patients were excluded if they had a history of treatment-resistant episodes (i.e., had failed≥3 earlier adequate trials of at least two classes of antidepressant medication, or electroconvulsive therapy, or two adequate trials of psychotherapy in the past 3 years); if the index episode was nonresponsive to fluoxetine, venlafaxine, or venlafaxine ER; or if they had a lifetime history of psychotic disorder, bipolar disorder, or obsessive-compulsive disorder, or a primary diagnosis of generalized anxiety disorder, social phobia, or post-traumatic stress disorder in the 6 months before screening. For the purposes of this report, data from the subset of patients who were treatment-responsive [i.e., achieved and maintained response (HAM-D17 <12 and ≥50% decrease from baseline) or remission (HAM-D17≤7)] on venlafaxine ER ≤225 mg/day during the acute and continuation phases were analyzed.
The primary study had four phases. In the acute phase, patients were randomized to double-blind treatment with venlafaxine ER or fluoxetine (ratio 3 : 1) for 10 weeks. Treatment-responsive patients who completed the acute phase were eligible for the continuation phase, during which they remained on the same study drug for an additional 6 months. Treatment-responsive patients who completed the continuation phase were then eligible to enter two successive, 1-year maintenance phases. As this report focused on the maintenance phases and only patients from the venlafaxine ER arm who entered the maintenance phase were eligible for randomization to the placebo arm, results of the fluoxetine arm are not presented herein.
At the start of the first maintenance phase, patients who were responsive to venlafaxine ER throughout the continuation phase were randomly assigned to double-blind treatment with venlafaxine ER or placebo for 1 year. At the end of that year, those who had not suffered a recurrence and were still taking double-blind study medication were eligible to enter the second maintenance phase. At the start of this second maintenance phase, patients who had been in the venlafaxine ER group during the first phase were randomized again to either continue active therapy or receive placebo; those receiving double-blind placebo continued on the same study medication for another year.
Flexible dosing was used throughout all phases of the trial. Therefore, the dosage of study medication could be increased at the discretion of the prescribing physician and did not require that the patient experience a recurrence.
Efficacy evaluation and event definitions
The primary end point for this analysis was time to failure to maintain response. In the primary analysis, failure to maintain response was defined as either an increase in maintenance dose to venlafaxine ER 300 mg/day (or placebo equivalent) or recurrence (total HAM-D17 >12 and a HAM-D17 reduction of ≤50% from acute-phase baseline at two consecutive visits or at the last valid visit prior to patient’s early discontinuation). To capture the data for those individuals who met criteria for recurrence at a single visit (but did not discontinue), a second analysis was performed using broader criteria for recurrence. This analysis included the primary analysis population and patients who had a total HAM-D17 greater than 12 and a HAM-D17 reduction of ≤50% from acute-phase baseline at only one visit and were determined to have had a recurrence by a panel of experts on review of blinded data.
Two additional analyses were performed to assess the components of the primary outcome separately: (i) time to a dose increase above 225 mg/day in the maintenance phases and (ii) time to recurrence (i.e., total HAM-D17 >12 and a HAM-D17 reduction of ≤50% from acute-phase baseline at two consecutive visits) in the maintenance phases.
Statistical analysis
Kaplan–Meier methods were used to estimate the probabilities of remaining well. Kaplan–Meier estimates of the probability of no dose increase above 225mg/day and of no recurrence were also calculated individually. Comparisons between the venlafaxine ER and placebo groups were made using log-rank tests.
Results
Patients
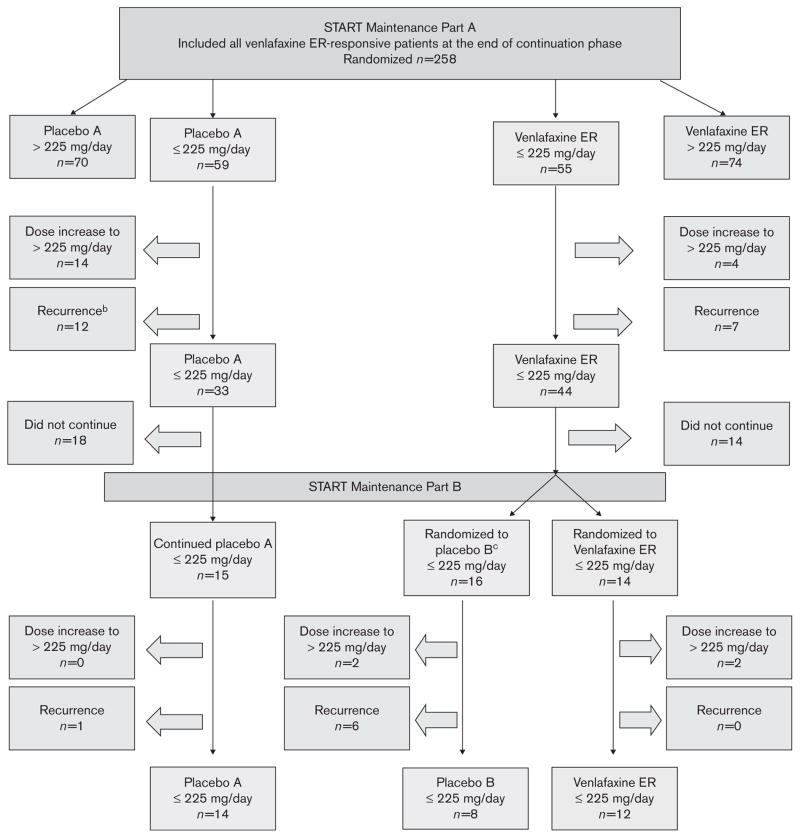
In the primary study, 1096 patients were randomized using a flexible dose design, with 821 receiving venlafaxine ER 75–300 mg/day. Of these patients, 114 were enrolled in the first maintenance phase and did not take any doses of venlafaxine more than 225 mg in the acute and continuation phases, making them eligible for this analysis. At the start of the first maintenance phase, 55 individuals were randomized to the venlafaxine ER group and 59 to the placebo group (Figure 1). For the primary analysis, 69 patients either experienced an event or discontinued treatment during maintenance phase A, and were not counted in the second maintenance phase. Thirty of the 55 patients from the venlafaxine ER group from the first maintenance phase entered the second maintenance phase (14 randomized to venlafaxine ER and 16 to placebo). Fifteen of the 59 patients from the placebo group from the first maintenance phase entered the second maintenance phase.
Fig. 1.
Disposition of patients during combined maintenance phases for primary analysis. aIf a patient experienced both dose increase to >225 mg/d and recurrence, the first event was summarized in this figure. bRecurrence was defined as total HAM-D17 >12 and a HAM-D17 reduction of ≤50% from acute-phase baseline at two consecutive visits or at the last valid visit prior to discontinuation. cPatients who were treated with venlafaxine ER in maintenance A and did not have an event and were then randomized to placebo in maintenance B were censored at the end of maintenance A. ER, extended release.
The demographic and baseline characteristics of the analysis treatment groups were similar (Table 1). Compared with those in the placebo group, those randomized to receive venlafaxine ER were slightly younger (mean age 41.0 years vs. 43.1 years) and more likely to be female (73% female vs. 63% female). Mean HAM-D17 scores were also similar at acute-phase baseline (21.5 vs. 22.2) and at the first maintenance-phase baseline (3.2 vs. 4.5). Moreover, the analysis population was similar to the overall study population for age, sex distribution, and baseline HAM-D17 scores.
Table 1.
Demographic and baseline characteristics of the analysis population
| Characteristic | Venlafaxine ER (n= 55) | Placebo (n=59) |
|---|---|---|
| Mean age, years | 41.0 | 43.1 |
| Sex, N (%) | ||
| Female | 40 (73) | 37 (63) |
| Male | 15 (27) | 22 (37) |
| Mean HAM-D17 score | ||
| Acute-phase baseline | 21.5 | 22.2 |
| Maintenance A baseline | 3.2 | 4.5 |
ER, extended release; HAM-D17, 17-item Hamilton Rating Scale for Depression.
Efficacy outcomes
During the combined maintenance phases, six patients in the venlafaxine ER ≤225 mg/day group required a dose increase to 300 mg/day (four in the first maintenance phase and two in the second maintenance phase), seven patients met the primary criteria for recurrence (all during the first maintenance phase); three of these 13 patients had a dose increase followed by a recurrence. In the placebo group, 14 patients required a dose increase to 300 mg/day (all during the first maintenance phase); 13 patients met the criteria for recurrence (12 during the first maintenance phase and one in the second maintenance phase); five of these 27 patients had a dose increase followed by a recurrence (Figure 1).
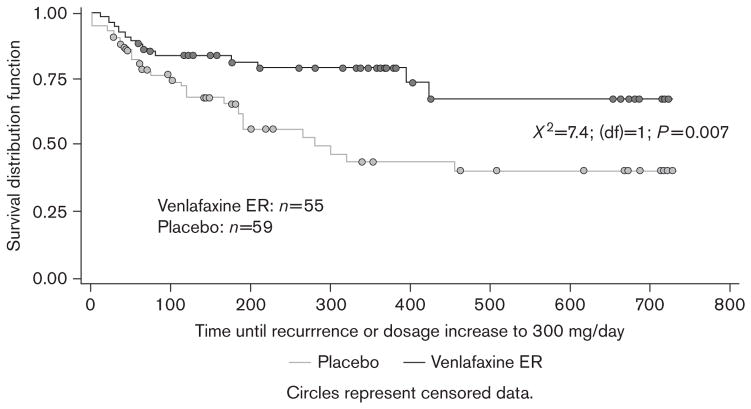
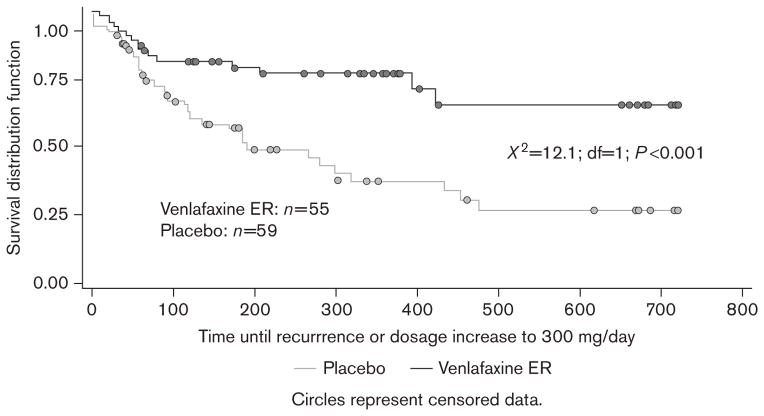
Kaplan–Meier analysis of data across 2 years of double-blind maintenance therapy revealed that the probability of remaining well was significantly greater for patients receiving venlafaxine ER ≤225 mg/day than for those receiving placebo. The Kaplan–Meier probability estimates for remaining well in the primary analysis were 67% for venlafaxine ER ≤225 mg/day and 41% for placebo [log-rank χ2=7.4; degrees of freedom (df)=1; P=0.007] (Figure 2). The number-needed-to-treat value associated with the primary analysis was 4.5. Similar results were obtained when patients meeting criteria for recurrence at a single visit were included. Using this broader definition of failure to maintain response, Kaplan–Meier probability estimates for remaining well were 66% for venlafaxine ER ≤225 mg/day and 27% for placebo (χ2=12.1; df=1; P<0.001) (Figure 3).
Fig. 2.
Primary analysis: Kaplan–Meier curve for probability of maintaining response at ≤225 mg/day of study treatment across the combined 2 years of maintenance therapy. Failure to maintain response was defined as increase in maintenance dose to venlafaxine extended release (ER) 300 mg/day or having total 17-item Hamilton Rating Scale for Depression (HAM-D17) more than 12 and a HAM-D17 reduction of ≤50% from acute-phase baseline at two consecutive visits or at the last valid visit before discontinuation.
Fig. 3.
Secondary analysis: Kaplan–Meier curve for probability of maintaining response at ≤225 mg/day of study treatment across the combined 2 years of maintenance therapy. Failure to maintain response was defined as increase in maintenance dose to venlafaxine extended release (ER) 300 mg/day or having total 17-item Hamilton Rating Scale for Depression (HAM-D17) more than 12 and a HAM-D17 reduction of ≤50% from acute-phase baseline at one visit and a recurrence as determined by a panel of experts on review of blinded data.
Patients receiving venlafaxine ER ≤225 mg/day also did better on each of the individual components of the primary endpoint. The probability of not increasing the dose to 300 mg/day was significantly greater for the venlafaxine ER group (76% for venlafaxine ER ≤225 mg and 57% for placebo; χ2=5.9; df=1; P=0.015) (Figure 4). Although the estimated probability of not having a recurrence was greater in the venlafaxine ER group, the difference did not reach the level of statistical significance (76% for venlafaxine ER and 58% for placebo; χ2=2.8; df=1; P=0.092) (Figure 5).
Fig. 4.
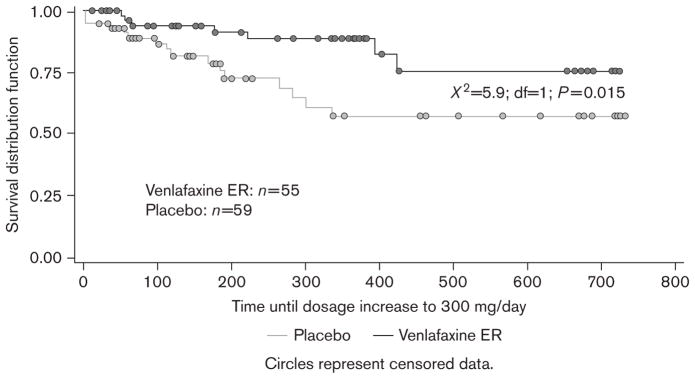
Kaplan–Meier curve for probability of no dose increase above 225 mg/day of study treatment across the combined 2 years of maintenance therapy. ER, extended release.
Fig. 5.
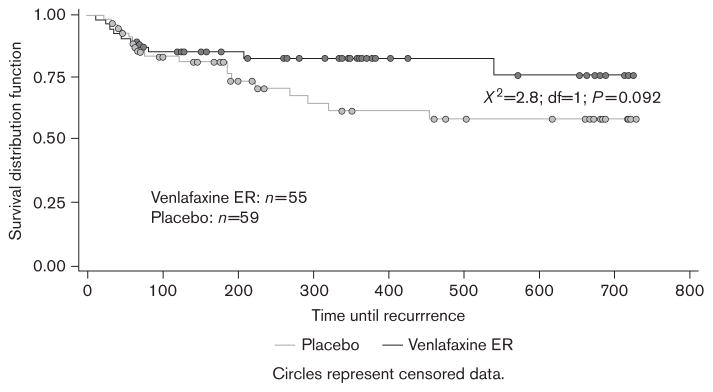
Kaplan–Meier curve for probability of no recurrence across the combined 2 years of maintenance therapy. Recurrence was defined as having total 17-item Hamilton Rating Scale for Depression (HAM-D17) more than 12 and a HAM-D17 reduction of ≤50% from acute-phase baseline at two consecutive visits or at the last valid visit before patient’s early discontinuation.
Average daily dose
The average daily dose of venlafaxine ER (or placebo equivalent) during maintenance treatment (from the start of the first maintenance phase until the time of recurrence, dose increase, randomization to placebo at the start of the second maintenance phase, or discontinuation from the study) was determined for each patient. The mean daily dose of venlafaxine ER (or placebo equivalent) was 159 mg (SD=57) in the venlafaxine ER group and 172 mg (SD=46) in the placebo group.
Discussion
PREVENT was a large, multisite, randomized trial evaluating the long-term safety and efficacy of venlafaxine ER in patients receiving a range of doses from 75 to 300 mg/day. The study included two placebo-controlled, 1-year maintenance phases. A post-hoc analysis of data from the PREVENT trial was conducted to estimate the probability of remaining well at a dose of venlafaxine ER ≤225 mg/day over 2.5 years of follow-up. The results of our analysis confirmed that the probability of remaining well was significantly greater for patients who received venlafaxine ER ≤225 mg/day compared with those who received placebo. This difference was significant either when the primary definition of failure to maintain response was used (i.e., dose increase to 300 mg/day or recurrence as defined by total HAM-D17 >12 and a HAM-D17 reduction of ≤50% from acute-phase baseline at two consecutive visits) or when the definition was broadened to include those who had a dose increase to 300 mg/day or met recurrence criteria at a single visit. Patients who switched from active drug to placebo during the maintenance phase were also significantly more likely to have a ‘dose increase’ than patients who continued to take active venlafaxine ER. Although the estimated probability of no recurrence (i.e., based on HAM-D17 score) was greater for the venlafaxine ER group than the placebo group, the difference was not significant. This absence of statistical significance may be a consequence of the small sample size.
The results of a recent meta-analysis of publicly available data from 35 clinical trials of four new-generation antidepressants, including venlafaxine, that was conducted by Kirsch et al. (Kirsch et al., 2008), suggests antidepressants are not effective to a clinically relevant degree in patients with baseline HAM-D17 scores less than 28. Although direct comparisons between the current findings and these previous results are made difficult by variations in methodology (Kirsch and colleagues analyzed short-term treatment effects), we feel that a clinically meaningful long-term effect was observed in this sample of patients with a mean baseline HAM-D17 score of approximately 22. The current results, which are also fully consistent with the findings of an earlier meta-analysis conducted by Geddes et al. (Geddes et al., 2003), demonstrate that patients with recurrent MDD who respond to antidepressant treatment obtain substantial benefit from ongoing preventive therapy. In particular, the magnitude of the effect of ongoing venlafaxine ER therapy in this study, as quantified by a number needed to treat of less than 5, represents a large and clinically meaningful benefit.
The PREVENT study design used a flexible dose approach that precludes a formal analysis of dose–response relationships to determine whether the use of 300 mg/day dosing distorted results. It may be that pharmacotherapists tended toward dose escalation, because the goal of treatment was to achieve response/ remission in the earlier phases to ensure entry into the maintenance phase of treatment. This possibility complicates the evaluation of efficacy because our analysis only applies to the subgroup of randomized patients who were treated in a portion of the full dose range. Assessing recurrence in the population of patients who received a dose ≤225 mg/day in either the active treatment or placebo groups introduces a systematic bias if the study population who had their doses increased to 300 mg/day were inherently different from those who did not require a dose adjustment. Moreover, randomization in the maintenance phase cannot mitigate this bias. Therefore, several perspectives were used in the present analyses in addition to the most direct approach of simply reporting the recurrence rates to help us best evaluate efficacy in this dosage range.
The power of this post-hoc analysis is limited because the number of patients who were at risk of not remaining well became progressively smaller over time, in large part because of sequential randomization steps. As a result, the number of patients included in this subanalysis who actually completed the second maintenance phase was quite small. The significant treatment effect presented here is, however, consistent with the likelihood of remaining recurrence-free presented in the primary analysis of the PREVENTstudy data [i.e., year 1: venlafaxine ER: 77%; placebo: 58%; P=0.005 (Kocsis et al., 2007); year 2: venlafaxine ER: 92%; placebo: 55%; P<0.001 (Keller et al., 2007a); combined maintenance phase: venlafaxine ER: 71%; placebo: 53%; P=0.005 (Keller et al., 2007a)].
In their totality, the current findings indicate that among patients with recurrent MDD who are responsive to acute-phase and continuation-phase treatment with doses of venlafaxine ER ≤225 mg/day, the likelihood of remaining well is significantly greater among those who receive up to 2 years of maintenance treatment with these doses compared with those who are switched to placebo. Moreover, their risk of recurrence is similar to that of individuals treated with a higher dose of venlafaxine ER.
Acknowledgments
This clinical trial and analysis were sponsored by Wyeth Research, Collegeville, Pennsylvania, USA. The authors thank Ann Sherwood, PhD, Lorraine M. Sweeney, and Jennifer B. Hutcheson of ADVOGENT for their writing and editorial assistance.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- Geddes JR, Carney SM, Davies C, Furukawa TA, Kupfer DJ, Frank E, et al. Relapse prevention with antidepressant drug treatment in depressive disorders: a systematic review. Lancet. 2003;361:653–661. doi: 10.1016/S0140-6736(03)12599-8. [DOI] [PubMed] [Google Scholar]
- Keller M, Trivedi MH, Thase ME, Shelton RC, Kornstein SG, Nemeroff CB, et al. The Prevention of Recurrent Episodes of Depression with Venlafaxine for Two Years (PREVENT) study: outcomes from the two-year and combined maintenance phases. J Clin Psychiatry. 2007a;68:1246–1256. doi: 10.4088/jcp.v68n0812. [DOI] [PubMed] [Google Scholar]
- Keller MB, Trivedi MH, Thase ME, Shelton RC, Kornstein SG, Nemeroff CB, et al. The Prevention of Recurrent Episodes of Depression with Venlafaxine for Two Years (PREVENT) study: outcomes from the acute and continuation phases. Biol Psychiatry. 2007b;62:1371–1379. doi: 10.1016/j.biopsych.2007.04.040. [DOI] [PubMed] [Google Scholar]
- Kirsch I, Deacon BJ, Huedo-Medina TB, Scoboria A, Moore TJ, Johnson BT. Initial severity and antidepressant benefits: a meta-analysis of data submitted to the Food and Drug Administration. PLoS Med. 2008;5:e40260–e40268. doi: 10.1371/journal.pmed.0050045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocsis JH, Thase ME, Trivedi MH, Shelton RC, Kornstein SG, Nemeroff CB, et al. Prevention of recurrent episodes of depression with venlafaxine ER in a 1-year maintenance phase from the PREVENT Study. J Clin Psychiatry. 2007;68:1014–1023. doi: 10.4088/jcp.v68n0706. [DOI] [PubMed] [Google Scholar]
- Kornstein SG. Beyond remission: rationale and design of the Prevention of Recurrent Episodes of Depression with Venlafaxine for Two Years (PREVENT) Study. CNS Spectr. 2006;11:28–34. doi: 10.1017/s1092852900015236. [DOI] [PubMed] [Google Scholar]
- Montgomery SA, Entsuah R, Hackett D, Kunz NR, Rudolph RL. Venlafaxine versus placebo in the preventive treatment of recurrent major depression. J Clin Psychiatry. 2004;65:328–336. doi: 10.4088/jcp.v65n0307. [DOI] [PubMed] [Google Scholar]
- Mueller TI, Leon AC, Keller MB, Solomon DA, Endicott J, Coryell W, et al. Recurrence after recovery from major depressive disorder during 15 years of observational follow-up. Am J Psychiatry. 1999;156:1000–1006. doi: 10.1176/ajp.156.7.1000. [DOI] [PubMed] [Google Scholar]
- Shelton C, Entsuah R, Padmanabhan SK, Vinall PE. Venlafaxine XR demonstrates higher rates of sustained remission compared to fluoxetine, paroxetine or placebo. Int Clin Psychopharmacol. 2005;20:233–238. doi: 10.1097/00004850-200507000-00007. [DOI] [PubMed] [Google Scholar]
- Sobocki P, Jonsson B, Angst J, Rehnberg C. Cost of depression in Europe. J Ment Health Policy Econ. 2006;9:87–98. [PubMed] [Google Scholar]
- Trompenaars FJ, Masthoff ED, Van Heck GL, Hodiamont PP, De Vries J. Relationship between mood related disorders and quality of life in a population of Dutch adult psychiatric outpatients. Depress Anxiety. 2006;23:353–363. doi: 10.1002/da.20180. [DOI] [PubMed] [Google Scholar]
- Tylee A, Gastpar M, Lepine JP, Mendlewicz J. DEPRES II (Depression Research in European Society II): a patient survey of the symptoms, disability and current management of depression in the community. DEPRES Steering Committee. Int Clin Psychopharmacol. 1999;14:139–151. doi: 10.1097/00004850-199905002-00001. [DOI] [PubMed] [Google Scholar]