Abstract
Purpose
Androgen deprivation therapy (ADT) is associated with increased fracture risk. In a recent Phase III trial, toremifene significantly decreased vertebral fractures in men receiving ADT. Similar to other selective estrogen receptor modulators, toremifene was associated with an increase in venous thromboembolic events (VTEs), with the greatest risk in men aged ≥80 years. This post hoc analysis evaluated the efficacy and safety of toremifene in men aged <80 years.
Materials and Methods
This analysis included 847 subjects aged <80 years; 430 received toremifene 80 mg by mouth daily and 417 received placebo for up to 24 months. The primary endpoint was new vertebral fractures. Secondary endpoints included fragility fractures, bone mineral density (BMD), and safety.
Results
Compared with placebo, toremifene decreased the relative risk of new vertebral fractures by 79.5% (95% CI, 29.8%–94.0%; P<0.005). New vertebral fracture incidence was 1.0% with toremifene and 4.8% with placebo (absolute risk reduction, 3.8%). Compared with placebo, toremifene significantly reduced the incidence of a nontraumatic fracture or >7% bone loss by 24 months (P<0.0001). Toremifene also significantly increased BMD at all measured sites (P<0.001 for all comparisons). The incidence of VTEs was similar in the toremifene and placebo groups (2.1% vs 1.0%, respectively; P=0.26). Rates of other adverse events were also similar between groups.
Conclusions
Toremifene significantly decreased new vertebral fractures in men aged <80 years receiving ADT for prostate cancer. The risk of VTEs was lower than in the overall study population, suggesting an improved benefit–risk profile in younger men.
Keywords: androgen deprivation therapy, osteoporosis, prostate cancer, selective estrogen receptor modulators, toremifene
Introduction
Androgen deprivation therapy (ADT) is the primary treatment for men with metastatic or recurrent prostate cancer.1–3 Although ADT improves survival in certain clinical settings,4 numerous adverse effects have been associated with ADT. Adverse effects attributed to the loss of androgens include sarcopenia (or reduced lean body mass), obesity, anemia, hair loss, erectile dysfunction, and insulin resistance.1,5,6 In addition, because estrogen in men is primarily derived from the aromatization of testosterone, ADT results in low levels of estrogen (>70% reduction) and subsequent estrogen deficiency–related effects.6 These include gynecomastia, hot flashes, mental and emotional symptoms, and unfavorable changes in lipid profile.3,6,7 Studies also indicate an increased risk of incident diabetes and cardiovascular morbidity with both short-term (≤6 mo) and long-term ADT.7–9 Because estrogen plays a central role in male bone resorption and formation10 and maintenance of bone mass,11 ADT may also result in adverse skeletal effects. Specifically, ADT has been shown to increase bone turnover, decrease bone mineral density (BMD), and increase clinical fractures.1,7,12,13 These effects may have serious implications for patient quality of life and long-term survival as well as healthcare costs.
Toremifene is a second-generation selective estrogen receptor modulator (SERM) that exhibits beneficial estrogen-like agonist activity in bone as well as other favorable tissue-specific agonist and antagonist effects. In a Phase III trial of men on ADT, toremifene 80 mg significantly reduced the incidence of new vertebral fractures by 50%; significantly increased BMD at the lumbar spine, hip, and femoral neck; and significantly decreased markers of bone turnover.14 In addition, toremifene had beneficial effects on serum lipids14 and in men with severe hot flashes.15 Although toremifene was well tolerated overall, an increase in the rate of venous thromboembolic events (VTEs) was observed,14 similar to other SERMs.16,17 VTEs were seen primarily in men with known risk factors, including age ≥80 years.
We conducted a post hoc analysis of the Phase III study to assess the efficacy and safety of toremifene 80 mg in men aged <80 years. The study results indicate an improved benefit–risk profile in younger men.
Materials and Methods
Study Design
The Phase III clinical trial was a 2-year, randomized, double-blind, placebo-controlled study of toremifene in men receiving ADT for prostate cancer (ClinicalTrials.gov identifier, NCT00129142). The study was conducted at 144 clinical sites in the United States and Mexico. Subjects received oral toremifene 80 mg daily or matching placebo for up to 24 months. Full details of the study design, subject inclusion/exclusion criteria, and analytical procedures have been previously reported.14 In this post hoc analysis, the efficacy and safety of toremifene for fracture risk reduction were evaluated in a subgroup of men aged <80 years.
Subjects
Briefly, the study enrolled men aged ≥50 years with a serum prostate-specific antigen level ≤4 ng/mL who were at increased risk for fracture based on (1) lumbar spine or femoral neck BMD at or below previously described thresholds or (2) age ≥70 years.14 Eligible subjects had received continuous ADT for ≥6 months or intermittent ADT for ≥12 months before enrollment. Following randomization, subjects were required to receive continuous ADT for the duration of the study. Subjects who received prior treatment with toremifene, received treatment with a SERM or bisphosphonates within 45 days of randomization, or had a history of venous thromboembolic disease were excluded. Of note, 2 subjects in the toremifene treatment group who experienced a VTE during the study were later found to have a prior history of venous thromboembolic disease. All subjects provided written informed consent, and the study protocol was approved by the institutional review board for each participating institution.
Endpoints
The primary study endpoint was new vertebral fractures. Secondary efficacy endpoints included fragility fractures; bone loss >7% by study end; change from baseline in BMD of the lumbar spine, total hip, and femoral neck; and change from baseline in biochemical markers of bone turnover (bone-specific alkaline phosphatase, C-telopeptide, and osteocalcin). The safety and tolerability of treatment with toremifene were also assessed.
Study Procedures
Vertebral fractures at baseline and new morphometric vertebral fractures (T4 to L4) at months 12 and 24 were assessed by an expert reader at a central radiographic imaging laboratory (Synarc Inc., Portland, OR).14 Nontraumatic fractures, defined as those resulting from trauma equivalent to a fall from less than standing height, were also confirmed by expert review of radiographs. BMD was assessed by dual energy x-ray absorptiometry (DEXA) at baseline and months 12 and 24 or at early termination. Any subject with a >7% decrease in BMD of the hip or spine, as confirmed by a follow-up DEXA scan, was withdrawn from the study. Serum levels of bone turnover markers were measured at baseline and months 12 and 24 and were analyzed at a central laboratory. Adverse events (AEs) were reported from the first administration of the study medication through the treatment period and until 30 days after discontinuation. The study protocol did not specify a particular assessment for VTEs; however, those that were clinically detected during the study were confirmed via imaging. Additionally, because hot flashes were monitored on an ongoing basis as a secondary endpoint of the study, they were not recorded as an AE unless recorded by the investigator as atypical or unusually severe.
Statistical Analyses
Safety analyses included the intent-to-treat (ITT) population, defined as all randomized subjects who received ≥1 dose of study medication. Efficacy analyses included the modified ITT (MITT) population, defined as ITT subjects who had ≥1 on-study radiograph. Differences in the distribution of AEs were assessed using the Fisher exact test. The incidence of new vertebral fractures and nontraumatic fractures (or >7% bone loss) at month 24 was compared between treatment arms using the Cochran-Mantel-Haenszel test stratified by country of enrollment.18 The least squares mean (SE) difference between the toremifene and placebo groups in percentage change from baseline to month 24 for BMD and bone turnover markers was calculated using a generalized linear mixed model with country as a covariate. All tests were 2-sided with a significance level of P≤0.05. There were no adjustments for multiple testing.
Results
Baseline Characteristics
A total of 1284 subjects were randomized to treatment and included in the ITT population for safety analyses. This post hoc analysis included 847 subjects aged <80 years; 430 received toremifene 80 mg by mouth daily and 417 received placebo (Table 1). Mean treatment compliance was 95.7% for patients receiving toremifene and 96.5% for patients receiving placebo. Six patients in the placebo group received bisphosphonates while on study drug, 5 of whom were discontinued from study. The remaining patient received bisphosphonates for 15 days preceding the final study visit. Baseline characteristics were similar between the treatment groups. Most subjects (~55%) were categorized as osteopenic at baseline. The mean duration of ADT was approximately 4 years, similar to that of the overall patient population.14
Table 1.
Baseline Demographic and Clinical Characteristics (ITT Population)
| Characteristic | Aged <80 y n=847
|
|
|---|---|---|
| Toremifene n=430 | Placebo n=417 | |
| Mean (SD) age, y | 72.2 (5.4) | 72.1 (5.5) |
| Race, n (%)a | ||
| White | 296 (68.8) | 281 (67.4) |
| Hispanic | 82 (19.1) | 85 (20.4) |
| Black | 45 (10.5) | 44 (10.6) |
| Asian | 5 (1.2) | 3 (0.7) |
| Other | 2 (0.5) | 4 (1.0) |
| Country of residence, n (%) | ||
| United States | 354 (82.3) | 336 (80.6) |
| Mexico | 76 (17.7) | 81 (19.4) |
| Mean (SD) BMI, kg/m2 | 28.8 (4.4) | 28.5 (4.3) |
| Mean (SD) ADT duration, y | 3.9 (3.3) | 4.0 (3.8) |
| Prevalent vertebral fracture, n (%) | 54 (13.0) | 50 (12.5) |
| Mean (SD) BMD T-score | ||
| Lumbar spine | −0.52 (1.85) | −0.68 (1.71) |
| Total hip | −0.86 (1.14) | −0.93 (1.08) |
| Femoral neck | −1.36 (1.02) | −1.46 (1.02) |
| Trochanter | −0.71 (1.26) | −0.76 (1.24) |
| Mean (SD) BMD, g/cm2 | ||
| Lumbar spine | 1.08 (0.23) | 1.06 (0.22) |
| Total hip | 0.94 (0.16) | 0.93 (0.15) |
| Femoral neck | 0.80 (0.16) | 0.79 (0.15) |
| Trochanter | 0.75 (0.16) | 0.74 (0.16) |
| BMD category,b n (%) | ||
| Osteopenia | 233 (54.7) | 238 (57.9) |
| Normal | 102 (23.9) | 73 (17.8) |
| Osteoporosis | 91 (21.4) | 100 (24.3) |
| Mean (SD) serum PSA, ng/mL | 0.4 (0.9) | 0.4 (0.8) |
| Mean (SD) serum bone-specific alkaline phosphatase, U/L | 29.9 (12.3) | 30.2 (12.8) |
| Mean (SD) serum osteocalcin, ng/mL | 10.8 (5.1) | 10.7 (4.9) |
| Mean (SD) serum C-telopeptide, ng/mL | 0.6 (0.3) | 0.6 (0.3) |
| ADT treatment, n (%) | ||
| LHRHa | 397 (92.3) | 382 (91.6) |
| Orchiectomy | 33 (7.7) | 35 (8.4) |
ADT=androgen deprivation therapy; BMD=bone mineral density; BMI=body mass index; ITT=intent-to-treat; LHRHa=luteinizing hormone releasing hormone agonist; PSA=prostate-specific antigen.
Percentages may not total 100% because of rounding.
Not all subjects had a baseline BMD measurement.
Fractures
Among MITT subjects aged <80 years (n=622), the 2-year incidence of new vertebral fractures was 1.0% in the toremifene group versus 4.8% in the placebo group (P<0.005), corresponding with a relative risk reduction of 79.5% (95% CI, 29.8%–94.0%). Compared with placebo, toremifene significantly reduced the incidence of nontraumatic fractures or >7% bone loss at month 24 (placebo, 21.6% vs toremifene, 9.5%; P<0.0001; Table 2). This was a relative risk reduction of 56.2% (95% CI, 34.4%–70.8%).
Table 2.
Incidence of Nontraumatic Fractures or >7% Bone Loss at 24 Months (MITT Population)
| Aged <80 y n=622 | |
|---|---|
| Placebo group, n | 315 |
| Fractures, n (%) | 68 (21.6) |
| Toremifene group, n | 307 |
| Fractures, n (%) | 29 (9.5) |
| Relative risk reduction, % (95% CI) | 56.2 (34.4–70.8) |
| P value | P<0.0001a |
MITT=modified intent-to-treat.
P value was calculated using the Cochran-Mantel-Haenszel statistic, stratified by country of enrollment, for testing the association of fracture with treatment.
Bone Mineral Density
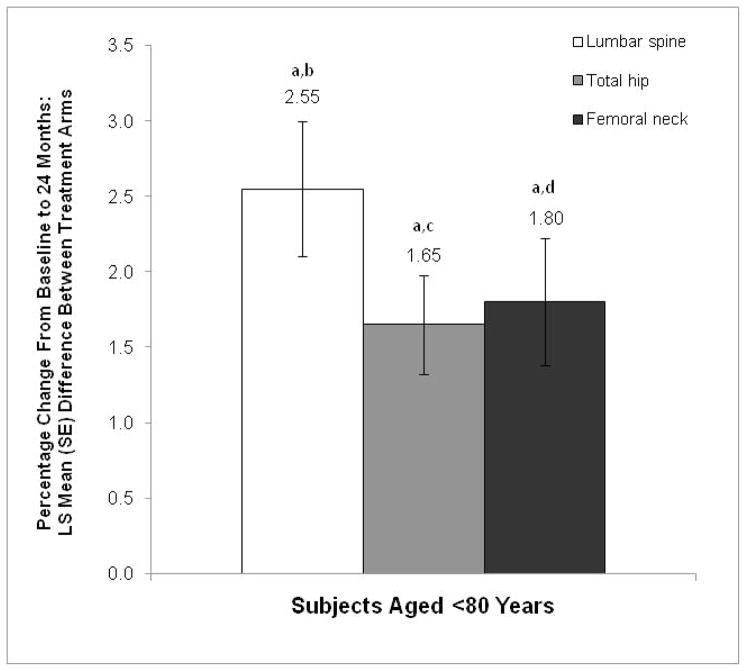
The percentage increase in BMD from baseline to month 24 was significantly greater with toremifene than with placebo at all measured sites among subjects aged <80 years. The least squares mean between-group differences for percentage change in BMD of the lumbar spine, total hip, and femoral neck were 2.55%, 1.65%, and 1.80%, respectively (P<0.001 for all comparisons vs placebo; Figure 1). The mean change from baseline to month 24 is shown in Table 3. The percentage of study discontinuations due to a >7% decrease in BMD of the hip or spine after 2 years was low (~1.1%) and similar between treatment groups (Table 4).
Figure 1.
Percentage change from baseline in BMD at month 24 (MITT population). The LS mean (SE) differences between the toremifene and placebo groups are shown for each site.
BMD=bone mineral density; LS=least squares; MITT=modified intent-to-treat.
aP<0.001 vs placebo.
bMean (SD) change from baseline to month 24: toremifene, 0.01 (0.04); placebo, −0.02 (0.06).
cMean (SD) change from baseline to month 24: toremifene, 0.00 (0.03); placebo, −0.02 (0.03).
dMean (SD) change from baseline to month 24: toremifene, 0.00 (0.03); placebo, −0.02 (0.04).
Table 3.
Change from Baseline to Month 24 in BMD
| Treatment Location | n | Mean (SD), g/cm2 | Median (Range), g/cm2 |
|---|---|---|---|
| Toremifene (n=430) | |||
| Lumbar spine | 206 | 0.01 (0.04) | 0.01 (−0.17, 0.16) |
| Total hip | 207 | 0.00 (0.03) | 0.00 (−0.11, 0.07) |
| Femoral neck | 207 | 0.00 (0.03) | 0.00 (−0.11, 0.11) |
| Placebo (n=417) | |||
| Lumbar spine | 200 | −0.02 (0.06) | −0.02 (−0.43, 0.21) |
| Total hip | 196 | −0.02 (0.03) | −0.02 (−0.10, 0.11) |
| Femoral neck | 196 | −0.02 (0.04) | −0.01 (−0.13, 0.11) |
BMD=bone mineral density.
Patients with bone mineral density data for both baseline and month 24.
Table 4.
Incidence of AEs (ITT Population)
| AEs, No. (%) of Subjects | Aged <80 y n=847
|
|
|---|---|---|
| Toremifene n=430 | Placebo n=417 | |
| General | ||
| Any AE | 325 (75.6) | 311 (74.6) |
| Serious AEs | 80 (18.6) | 73 (17.5) |
| Deaths due to AEs | 6 (1.4) | 6 (1.4) |
| Discontinuations due to AEs | 65 (15.1) | 45 (10.8) |
| Venous thromboembolic events | ||
| Any event | 9 (2.1) | 4 (1.0) |
| Fatal events | 0 | 0 |
| Events in first year | 6 (1.4) | 2 (0.5) |
| Events in second year | 3 (0.7) | 2 (0.5) |
| Cardiovascular events | ||
| MI | 4 (0.9) | 2 (0.5) |
| Stroke | 2 (0.5) | 3 (0.7) |
| Most common AEsa | ||
| Arthralgia | 32 (7.4) | 49 (11.8)b |
| Back pain | 30 (7.0) | 21 (5.0) |
| Dizziness | 25 (5.8) | 20 (4.8) |
| Pain in extremity | 25 (5.8) | 21 (5.0) |
| Constipation | 23 (5.4) | 17 (4.1) |
| Fatigue | 20 (4.7) | 22 (5.3) |
| Hematuria | 15 (3.5) | 23 (5.5) |
| Hypertension | 13 (3.0) | 21 (5.0) |
| Discontinuation because of bone loss >7% at 24 months | 4 (0.9) | 5 (1.2) |
AE=adverse event; ITT= intent-to-treat; MI=myocardial infarction.
Incidence ≥5% in either treatment group.
P<0.05 vs toremifene (Fisher exact test).
Bone Turnover Markers
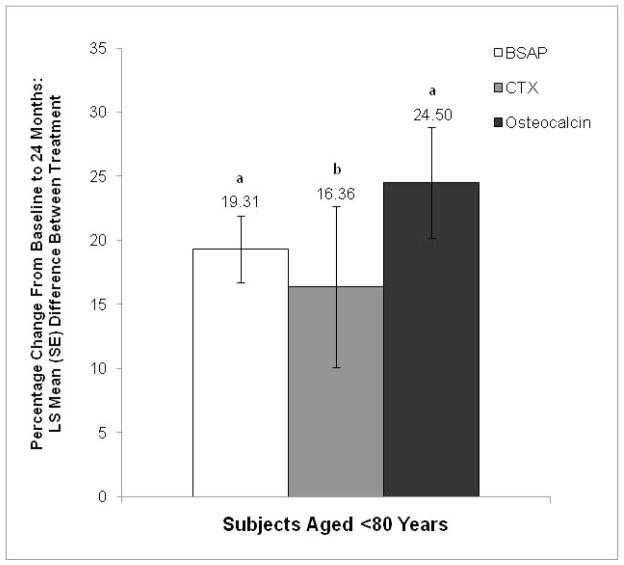
Toremifene significantly decreased biochemical markers of bone turnover from baseline to month 24 compared with placebo among subjects aged <80 years (Figure 2). Significant least squares mean between-group differences were found for bone-specific alkaline phosphatase (19.31%; P<0.001 vs placebo), C-telopeptide (16.36%; P=0.01 vs placebo), and osteocalcin (24.50%; P<0.001 vs placebo).
Figure 2.
Percentage change from baseline in bone turnover markers at month 24 (MITT population). The LS mean (SE) differences between the toremifene and placebo groups are shown for each marker.
BSAP=bone-specific alkaline phosphatase; CTX=C-telopeptide; LS=least squares; MITT=modified intent-to-treat.
aP<0.001 vs placebo.
bP=0.01 vs placebo.
Adverse Events
The overall rate of AEs was similar in the toremifene and placebo treatment arms (75.6% and 74.6%, respectively; Table 4). The most common AEs (occurring in ≥5% of subjects in either treatment group) were arthralgia, back pain, dizziness, pain in extremity, constipation, fatigue, hematuria, and hypertension. There were no significant differences in the incidence of serious AEs, deaths, or study discontinuations due to AEs between the toremifene and placebo treatment arms (Table 4).
Toremifene was associated with a slight, nonsignificant increase in the rate of VTEs compared with placebo (2.1% vs 1.0%, respectively; P=0.26; Table 4). Specifically, 9 of 430 subjects in the toremifene group had a VTE (6 with deep vein thrombosis [DVT] and 3 with pulmonary embolisms [PEs]) versus 4 of 417 subjects in the placebo group (1 with DVT and 3 with PEs). There were no fatal VTEs and no between-group differences in the incidence of stroke (Table 4).
Rates of myocardial infarction were similar between treatment arms (Table 4). Increases in prostate-specific antigen levels were observed in both the toremifene and placebo groups, with mean (SD) changes from baseline to 24 months of 1.6 (12.4) and 0.4 (1.8) ng/mL, respectively.
Discussion
In a recently reported randomized controlled trial,14 toremifene significantly decreased new vertebral fractures (50% relative risk reduction vs placebo; P=0.05) and increased BMD of the lumbar spine, hip, and femoral neck (P<0.0001 for all comparisons vs placebo), but was associated with a greater incidence of VTEs compared with placebo (2.6% vs 1.1%, respectively). The current post hoc analysis suggests an improved benefit–risk profile for men aged <80 years. Compared with placebo, toremifene significantly decreased new vertebral fractures (79.5% relative risk reduction; P<0.005). Toremifene also significantly reduced the incidence of nontraumatic fragility fractures or bone loss >7% compared with placebo (P<0.0001) and significantly increased BMD at the lumbar spine, total hip, and femoral neck from baseline to month 24, with significant differences from placebo at all measured sites (P<0.001). Concomitant decreases from baseline in bone turnover markers (bone-specific alkaline phosphatase, C-telopeptide, and osteocalcin) were seen in men treated with toremifene. These decreases were substantially greater than with placebo, and significant between-group differences in the percentage change from baseline to month 24 were noted for all markers (P≤0.01). In addition, the rate of VTEs in men treated with toremifene was 2.1%, which was slightly lower than that previously reported for the overall population (2.6%).14
Only one other large, randomized controlled trial has evaluated treatment-related fractures in men with prostate cancer. In a trial of 1468 men receiving ADT for prostate cancer, denosumab significantly increased BMD at all sites (P≤0.001 for all comparisons vs placebo) and reduced new vertebral fractures by 62% (P=0.006 vs placebo) after 3 years.19 In contrast to denosumab, toremifene has potential nonskeletal benefits, including significant improvements in serum lipids14 and reduction in hot flashes15 and breast tenderness.20
Venous thromboembolic events are a recognized adverse effect of SERMs. Even before treatment, patients with prostate cancer may have an elevated risk of VTEs. A significant association between cancer and VTE risk has been demonstrated.21 Numerous clinical and inherited or genetic factors have been implicated in this elevated VTE risk, including obesity, prior VTE, advanced age, and factor V Leiden.22 In addition, the increased risk of VTEs found with the SERM tamoxifen in the treatment of breast cancer has been associated with high body mass index, elevated diastolic blood pressure, the presence of atherosclerosis, and hyperlipidemia.16 Although the Phase III trial of toremifene demonstrated an increased rate of VTEs with toremifene in the overall patient population,14 there was no significant difference in VTE incidence between toremifene and placebo among men aged <80 years in this analysis. Thus, older age appears to be a potentially important risk factor for treatment with toremifene.
This Phase III trial is the first prospective study to evaluate the incidence of new morphometric vertebral fractures as a primary endpoint in the setting of men receiving ADT treatment for prostate cancer. Although this was a large prospective trial, one potential limitation is that the benefit–risk profile was evaluated as a post hoc analysis. A future Phase III trial of toremifene in men aged <80 years is warranted to confirm these findings.
Conclusions
Treatment of ADT-related bone loss in men with prostate cancer remains an unmet medical need. To date, there is minimal clinical evidence of agents that can significantly reduce fracture risk in these patients, and there are no agents approved by the US Food and Drug Administration for fracture prevention in men receiving ADT for prostate cancer.23 Toremifene is a promising agent in development for this indication and has demonstrated significant reductions in fracture risk and bone turnover rate and significant elevations in BMD in a Phase III clinical trial. Data from the current post hoc analysis indicate that men aged <80 years are likely to have the greatest benefit from toremifene therapy, with robust increases in BMD and reductions in bone turnover markers and fracture risk, without a concomitant increase in the risk of VTEs.
Acknowledgments
This study was supported by GTx, Inc. Dr. M.R. Smith is supported by a National Institutes of Health K24 Midcareer Investigator Award (K24CA121990) and research awards from the Prostate Cancer Foundation. Dr. Jamie L. Kistler of Complete Publication Solutions, LLC, provided editorial and medical writing assistance, which was supported by GTx, Inc.
Abbreviations
- ADT
androgen deprivation therapy
- AE
adverse event
- BMD
bone mineral density
- BMI
body mass index
- BSAP
bone-specific alkaline phosphatase
- CTX
C-telopeptide
- DEXA
dual energy x-ray absorptiometry
- DVT
deep vein thrombosis
- ITT
intent-to-treat
- LS
least squares
- MI
myocardial infarction
- MITT
modified intent-to-treat
- PE
pulmonary embolism
- PSA
prostate-specific antigen
- SERM
selective estrogen receptor modulator
- VTE
venous thromboembolic event
Footnotes
Conflict of Interest/Disclosure Information: M.R. Smith has a financial interest and/or other relationship with GTx, Inc. and Amgen, Inc. S.B. Malkowicz has a financial interest and/or other relationship with GTx, Inc., Amgen, Inc., and Centocor/Johnson & Johnson. M.K. Brawer, M.L. Hancock, R.A. Morton, and M.S. Steiner are employees of GTx, Inc.
References
- 1.Saylor PJ, Keating NL, Smith MR. Prostate cancer survivorship: prevention and treatment of the adverse effects of androgen deprivation therapy. J Gen Intern Med. 2009;24 (Suppl 2):S389. doi: 10.1007/s11606-009-0968-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prostate Cancer. V1.2010. Fort Washington, PA: National Comprehensive Cancer Network; 2009. Clinical Practice Guidelines in Oncology™. [Google Scholar]
- 3.Hoimes CJ, Kelly WK. Redefining hormone resistance in prostate cancer. Ther Adv Med Oncol. 2010;2:107. doi: 10.1177/1758834009356433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kumar S, Shelley M, Harrison C, et al. Neo-adjuvant and adjuvant hormone therapy for localised and locally advanced prostate cancer. Cochrane Database Syst Rev. 2006:CD006019. doi: 10.1002/14651858.CD006019.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharifi N, Gulley JL, Dahut WL. Androgen deprivation therapy for prostate cancer. JAMA. 2005;294:238. doi: 10.1001/jama.294.2.238. [DOI] [PubMed] [Google Scholar]
- 6.Kim HS, Freedland SJ. Androgen deprivation therapy in prostate cancer: anticipated side-effects and their management. Curr Opin Support Palliat Care. 2010;4:147. doi: 10.1097/SPC.0b013e32833bd913. [DOI] [PubMed] [Google Scholar]
- 7.Isbarn H, Boccon-Gibod L, Carroll PR, et al. Androgen deprivation therapy for the treatment of prostate cancer: Consider both benefits and risks. Eur Urol. 2009;55:62. doi: 10.1016/j.eururo.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith MR. Androgen deprivation therapy and risk for diabetes and cardiovascular disease in prostate cancer survivors. Curr Urol Rep. 2008;9:197. doi: 10.1007/s11934-008-0035-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keating NL, O’Malley AJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol. 2006;24:4448. doi: 10.1200/JCO.2006.06.2497. [DOI] [PubMed] [Google Scholar]
- 10.Falahati-Nini A, Riggs BL, Atkinson EJ, et al. Relative contributions of testosterone and estrogen in regulating bone resorption and formation in normal elderly men. J Clin Invest. 2000;106:1553. doi: 10.1172/JCI10942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Slemenda CW, Longcope C, Zhou L, et al. Sex steroids and bone mass in older men. Positive associations with serum estrogens and negative associations with androgens. J Clin Invest. 1997;100:1755. doi: 10.1172/JCI119701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith MR. Androgen deprivation therapy for prostate cancer: new concepts and concerns. Curr Opin Endocrinol Diabetes Obes. 2007;14:247. doi: 10.1097/MED.0b013e32814db88c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shahinian VB, Kuo YF, Freeman JL, et al. Risk of fracture after androgen deprivation for prostate cancer. N Engl J Med. 2005;352:154. doi: 10.1056/NEJMoa041943. [DOI] [PubMed] [Google Scholar]
- 14.Smith MR, Morton RA, Barnette G, et al. Toremifene to reduce fracture risk in men receiving androgen deprivation therapy for prostate cancer. J Urol. 2010;184:1316. doi: 10.1016/j.juro.2010.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gingrich J, Aliotta P, Morton RA. Phase III trial in men on ADT demonstrates a reduction in hot flashes in men on toremifene 80 mg compared to placebo. Can Urol Assoc J. 2009;3:S165. [Google Scholar]
- 16.Decensi A, Maisonneuve P, Rotmensz N, et al. Effect of tamoxifen on venous thromboembolic events in a breast cancer prevention trial. Circulation. 2005;111:650. doi: 10.1161/01.CIR.0000154545.84124.AC. [DOI] [PubMed] [Google Scholar]
- 17.Ettinger B, Black DM, Mitlak BH, et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. JAMA. 1999;282:637. doi: 10.1001/jama.282.7.637. [DOI] [PubMed] [Google Scholar]
- 18.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719. [PubMed] [Google Scholar]
- 19.Smith MR, Egerdie B, Hernandez Toriz N, et al. Denosumab in men receiving androgen-deprivation therapy for prostate cancer. N Engl J Med. 2009;361:745. doi: 10.1056/NEJMoa0809003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fisher H, Kaufman R, Morton RA. Toremifene 80 mg phase 3 trial in men on ADT demonstrates improvement in gynecomastia compared to placebo. Can Urol Assoc J. 2009;3:S164. [Google Scholar]
- 21.Noble S, Pasi J. Epidemiology and pathophysiology of cancer-associated thrombosis. Br J Cancer. 2010;102 (Suppl 1):S2. doi: 10.1038/sj.bjc.6605599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Caprini JA, Arcelus JI, Reyna JJ. Effective risk stratification of surgical and nonsurgical patients for venous thromboembolic disease. Semin Hematol. 2001;38:12. doi: 10.1016/s0037-1963(01)90094-0. [DOI] [PubMed] [Google Scholar]
- 23.Lee RJ, Saylor PJ, Smith MR. Treatment and prevention of bone complications from prostate cancer. Bone. 2011;48:88. doi: 10.1016/j.bone.2010.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]