DNA is prone to attack by physical and chemical agents generated endogenously and exogenously, producing modified DNA bases (i.e. DNA adducts/lesions), abasic sites, and inter- and intrastrand DNA crosslinks. DNA adducts, if not properly repaired, can lead to blocked replication, misincorporation, and mutation, potentially causing gene deregulation and cancer. Etheno (ε) DNA adducts are exocyclic adducts that, in addition to their use as fluorescent nucleotidfe derivatives,[1] were first recognized as reaction products of DNA with reactive metabolites of the occupational carcinogen vinyl chloride (VC).[2] Endogenous etheno-DNA adducts, arising from lipid peroxidation-derived DNA damage, were also detected in rats[3] and humans[4] without VC exposure. VC is a known carcinogen that induces hepatic angiosarcomas.[5] The major DNA adduct formed by VC, N7-(2-oxoethyl)guanine,[3, 6] is generally not considered to be mutagenic, because in vitro experiments showed that it did not cause detectable miscoding in an assay with modified poly(GC).[7] However, etheno adducts formed by VC (e.g. 1,N6-ethenoadenine, 3,N4-ethenocytosine, N2,3-ethenoguanine (N2,3-εG), and 1,N2-ethenoguanine (1,N2-εG)) have all been shown to be mutagenic in vitro and in bacteria (see N2,3-εG and 1,N2-εG structures in Figure 1a).[8] N2,3-εG is the most abundant endogenous etheno adduct, with levels estimated to be approximately 36 N2,3-εG lesions/cell in livers of untreated rats or humans.[9] A common assumption is that N2,3-εG is highly mutagenic; N2,3-εG is considered to contribute to the carcinogenesis of VC and inflammation-driven malignancies.[10] The dominance of a GC to AT transition in five of six K-ras (oncogene) tumors from VC workers[9] suggests the importance of a G adduct, but the misincorporation characteristics of 1,N2-εG are not consistent with this transition.[8a,f] Little repair of N2,3-εG occurs in VC-exposed rats, since the half-life of this lesion in rat liver and lung (150 days) and in rat kidney (75 days) is quite long.[11] The lability of the glycosidic bond of N2,3-ε-deoxyguanosine (N2,3-ε-dG) makes it difficult to adequately discern its mutagenic potential.[12] Both C and T were incorporated opposite N2,3-εG in a polyribo(G/N2,3-εG) template by avian myeloblastosis virus (AMV) reverse transcriptase.[13] N2,3-ε-Deoxyguanosine triphosphate is inserted opposite T by several polymerases (pols).[8d]
Figure 1.
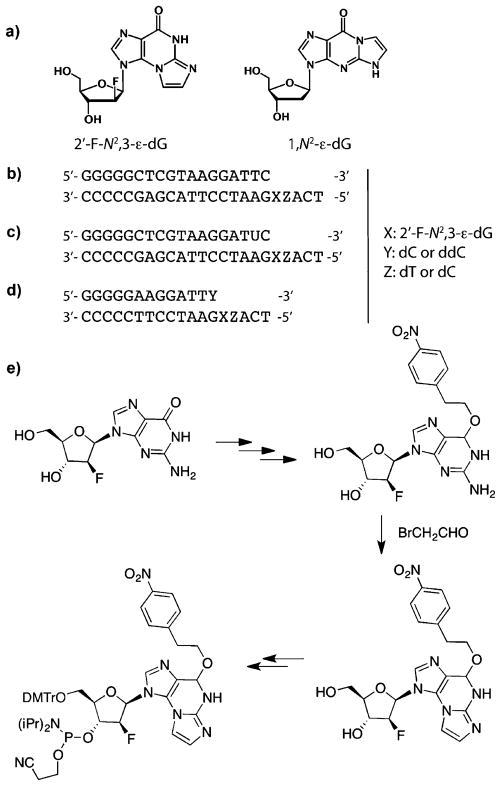
Oligonucleotides used in this study. a) Structural formulas of 2′-F-N2,3-ε-dG and 1,N2-ε-dG. Primer-template DNA sequences used for b) steady-state kinetic analysis, c) primer extension analysis, and d) crystallography. e) Summary of 2′-F-N2,3-ε-2′-deoxyarabinoguanosine phosphoramidite synthesis. The complete procedure is given in Scheme S1 of the Supporting Information. DMTr = dimethoxytrityl.
A corrected mutation frequency of 13% was calculated for N2,3-εG in an indirect assay, resulting in G to A transitions in Escherichia coli.[8b] Recently, theoretical calculations were used to predict the preferred base-pairing partner of N2,3-εG in the order G > T > A > C.[14] These results may partially explain the miscoding potential of N2,3-εG; however, kinetic and mechanistic details of the interaction of N2,3-ε-dG with replication enzymes are still missing.
2′-Fluoro substitution in nucleosides slows cleavage of the N-glycosidic bond, presumably by destabilization of the transition state and an oxocarbenium ion intermediate.[15] Recently 2′-fluoroarabinose was used to stabilize the glycosidic bond of an established, labile DNA adduct, N7-methylguanine.[16] We hypothesized that such a strategy could be utilized to retard the glycosidic cleavage of N2,3-ε-dG. Here we report a synthetic strategy for the site-specific incorporation of 2′-F-N2,3-ε-2′-deoxyarabinoguanosine (2′-F-N2,3-εdG) into oligonucleotides (Figure 1e and Scheme S1 of Supporting Information). This strategy, based on the use of fluorine as a non-classical isostere (one atom substituting for another) of hydrogen, greatly increased the stability of the glycosidic bond and allowed detailed biochemical and structural studies to be performed. Kinetic and mechanistic details of the replication of N2,3-εG by five representative DNA polymerases were investigated. Three crystallographic structures of Sulfolobus solfataricus P2 DNA polymerase IV (Dpo4) with DNA reveal, for the first time, base-pairing characteristics of N2,3-εG:C and N2,3-εG:T, the two major base pairs identified in single-base insertion and primer-extension assays.
A protected phosphoramidite reagent of 2′-F-N2,3-ε-dG was synthesized from the 2′-fluoro-2′-deoxyarabinoguanine derivative and is described in Figure 1e and the Supporting Information. Protection of the O6 atom is necessary to drive the reaction with bromoacetaldehyde to form N2,3-εG instead of 1,N2-εG.[1b] Two 23-mer oligomers (Figure 1b, c) containing N2,3-εG were utilized in biochemical assays, and two 18-mer oligomers (Figure 1d) were designed based on the existing Dpo4 crystal structures[17] for use in crystallographic studies. The synthetic oligomers were characterized by MALDI-TOF (Figures S9–S14 of Supporting Information), and the presence of N2,3-εG was confirmed by enzymatic digestion (Figures S15, S16 of Supporting Information).
The t1/2 for glycosidic cleavage of 2′-F-N2,3-ε-dG at pH 7.0 and 37°C was 23 ± 4 days in a single-stranded oligonucleotide and 33 ± 6 days in a duplex (Figure S1 of Supporting Information), which is comparable to the t1/2 (around 600 h) reported for sequestering N2,3-εG in a poly(GC/N2,3-ε-dGC) template.[12] The stability of 2′-F-N2,3-ε-dG permitted careful biochemical assays and crystallographic studies.
The miscoding potential of 2′-F-N2,3-ε-dG was examined with steady-state kinetic assays using a survey of prokaryotic and eukaryotic DNA polymerases with different functions, including the replicative bacteriophage pol T7 DNA exonuclease− (pol T7−), the moderately replicative E. coli pol I Klenow fragment exonuclease− (both 5′ to 3′ exo and 3′ to 5′ exo deficient, KF−), and the translesion pols Dpo4, human pol κ, and yeast pol η. A preference for inserting the correct base, C, opposite N2,3-εG was detected with four of the five polymerases(i.e. f < 1; Table 1). Misincorporation of a T residue was seen for all DNA polymerases, with frequencies ranging from 0.22 to 1.0 (Table 1 and Table S1 and S2 of Supporting Information), and some misincorporation of an A residue was also seen for pol T7−. Translesion pols are considered important for processing damaged DNA, although some of them also promote the generation of mutations, in certain cases. As expected, these pols (Dpo4, pol κ, and pol η) showed lower miscoding tendency than the more replicative pols (KF− and pol T7−), indicating poor discrimination of the incoming dNTP with replicative pols when N2,3-εG is present. Catalytic efficiencies (kcat/KM,dNTP) of C residue insertion opposite N2,3-εG (N2,3-εdG:C) showed at least tenfold attenuation compared to insertion opposite an unmodified-G residue (dG:C), with the most significant decrease (over 200-fold) seen for pol T7−. Only small changes in catalytic efficiency were seen for C residue insertion opposite 2′-F-2′-deoxyarabinoguanosine (2′-F-dG), ensuring that the 2′-fluoro modification does not markedly perturb polymerase catalysis.
Table 1.
Steady-state kinetic analysis of polymerase-catalyzed single-base insertion opposite X in a template sequence of 3′-CCCCCGAG-CATTCCTAAGXTACT-5′.[a]
| Polymerase/base pairing | kcat [min−1] | KM,dNTP [μm] | kcat/KM,dNTP [min−1 μm−1] | f[b] |
|---|---|---|---|---|
| Dpo4 | ||||
| 2′-F-N2,3-εdG:T | 0.52 ± 0.03 | 96 ± 16 | 0.0054 | 0.22 |
| 2′-F-N2,3-εdG:C | 0.37 ± 0.04 | 15 ± 5 | 0.025 | |
| 2′-F-dG:C | 0.63 ± 0.08 | 1.0 ± 0.02 | 0.63 | |
| dG:C | 1.41 ± 0.03 | 7.7 ± 1.0 | 0.18 | |
| Human pol k | ||||
| 2′-F-N2,3-εdG:T | 0.90 ± 0.04 | 111 ± 14 | 0.0082 | 0.37 |
| 2′-F-N2,3-εdG:C | 1.6 ± 0.1 | 73 ± 13 | 0.022 | |
| 2′-F-dG:C | 1.9 ± 0.1 | 2.8 ± 0.3 | 0.68 | |
| dG:C | 1.8 ±0.1 | 20 ± 1 | 0.090 | |
| Yeast pol η | ||||
| 2′-F-N2,3-εdG:T | 0.38 ± 0.015 | 3300 ± 480 | 0.00012 | 0.29 |
| 2′-F-N2,3-εdG:C | 0.38 ± 0.05 | 931 ± 210 | 0.00041 | |
| 2′-F-dG:C | 0.53 ± 0.02 | 26 ± 6 | 0.020 | |
| dG:C | 0.26 ± 0.02 | 45 ± 8 | 0.0058 | |
| Pol T7− | ||||
| 2′-F-N2,3-εdG:T | 0.29 ± 0.03 | 120 ± 20 | 0.0024 | 0.57 |
| 2′-F-N2,3-εdG:A | 0.74 ± 0.06 | 1000 ± 130 | 0.00074 | 0.17 |
| 2′-F-N2,3-εdG:C | 0.27 ± 0.02 | 62 ± 9 | 0.0044 | |
| 2′-F-dG:C | 0.44 ± 0.03 | 12 ± 2 | 0.037 | |
| dG:C | 1.1 ± 0.04 | 1.1 ± 0.2 | 1.0 | |
| KF− | ||||
| 2′-F-N2,3-εdG:T | 3.4 ± 0.2 | 14 ± 3 | 0.24 | 1.0 |
| 2′-F-N2,3-εdG:C | 5.4 ± 0.4 | 24 ± 5 | 0.23 | |
| 2′-F-dG:C | 4.3 ± 0.4 | 1.5 ± 0.6 | 2.9 | |
| dG:C | 3.6 ± 0.4 | 1.5 ± 0.6 | 2.4 | |
X is 2′-F-N2,3-ε-2′-deoxyarabinoguanosine (2′-F-N2,3-ε-dG), 2′-fluoro-2′-deoxyarabinoguanosine (2′-F-dG), or 2′-deoxyguanosine (dG) (complete data are given in Tables S1 and S2 of Supporting Information).
f(misinsertion frequency) = (kcat/KM,dNTP)incorrect/(kcat/KM,dNTP)correct.
To gain insight into the capability of reading and extending beyond N2,3-εG by polymerases, Dpo4 was characterized in terms of its ability to catalyze full-length extension reactions. Sequences of the products were determined and relative yields were estimated (summarized in Table 2 and Table S3 of Supporting Information) from LC-MS/MS results (Tables S4–S17 and Figures S2–S7 of Supporting Information). The primer was readily extended by Dpo4, bypassing N2,3-ε-dG, similar to that seen for 2′-F-dG and unmodified G templates. With the T-containing template (3′-εGT-5′; Z = T from Figure 1), Dpo4 produced a higher yield of extension products with C incorporated opposite the lesion (52%, Table 2) compared to T (43%, Table 2). Similar results were seen with the C-containing template (3′-εGC-5′; Z = C from Figure 1) shown in Table S3 of the Supporting Information. Thus, the insertion of T opposite N2,3-εG underscores the mutagenic potential of this lesion. A general trend of T misinsertion observed for the five polymerases studied herein is in concert with reports by Singer et al. for catalysis by AMV reverse transcriptase in a polyribo(GC) template containing N2,3-εG,[13] but the results (pairing with C > T > A) are at considerable variance with model calculations.[14]
Table 2.
Products of the extension of template–primer complexes by Dpo4.[a]
| 3′-CCCCCGAGCATTCCTAAGXTACT 5′-GGGGGCTCGTAAGGATUC |
Yield [%] | Base added | |
|---|---|---|---|
| CCATGA | 45 | C | |
| CCATGAA | 7 | ||
| CTATGA | 35 | T | |
| X: 2′-F-N2,3-ε-dG | CTATGAA | 8 | |
| CAATGA | 4 | A | |
| CGATGA | < 1 | G | |
| CATGA | 1 | deletion | |
| 2′-F-dG | CCATGA | 100 | C |
| dG | CCATGA | 100 | C |
X is 2′-F-N2,3-ε-2′-deoxyarabino-guanosine (2′-F-N2,3-ε-dG), 2′-fluoro-2′-deoxyarabinoguanosine (2′-F-dG), or (unmodified) 2′-deoxyguanosine (dG). Mass spectrometry data used to derive these results are presented in Figures S2–S7 and Tables S4–S16 of Supporting Information.
To understand the base-pairing mechanisms of N2,3-εG with C and T residues (see above), we determined crystal structures of two ternary complexes Dpo4·DNA·dCTP (Dpo4-1, 3′-εGC-5′; Dpo4-2, 3′-εGT-5) at 2.3 Å resolution and a binary complex of Dpo4·DNA (Dpo4-3, with ddT opposite N2,3-εG) at 3.5 Å resolution (Figure 2, refinement statistics summarized in Table S17 of Supporting Information). The active sites of all three structures resemble the reported configuration of the so-called “type I” Dpo4–DNA complex,[17b] where one base pair is accommodated at the active site, and the 5′ base in the template is rotated over 90° away (Figure 2a, c and Figure S8 of Supporting Information). Base pairing of N2,3-εG with dCTP in ternary complexes (templates: 3′-εGC-5′ and 3′-εGT-5′) showed both N2,3-εG and dCTP in an anti conformation. Interestingly, electron density suggested that the G residue 3′ to the lesion is most likely in the syn conformation to form a better stacking interaction with εG. A Watson–Crick-like configuration was seen for N2,3-εG:C base pairing (Figure 2b), whereas N2,3-εG:T mispairing resembles a sheared base pair (Figure 2 d). Interestingly, Singer et al.[13] had suggested “wobble” pairing but of a very different type.
Figure 2.
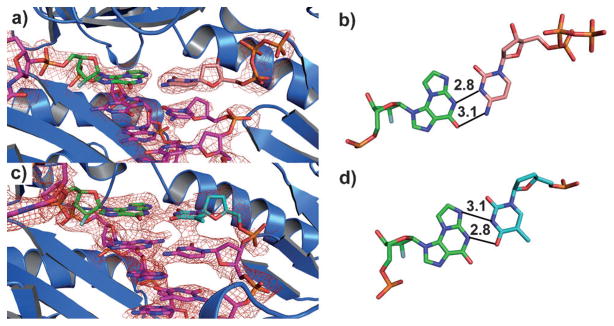
Crystal structures of Dpo4·N2,3-εG-DNA complexes (Z = C in the template). a) Ternary complex of Dpo4 with dCTP and N2,3-εG-containing duplex DNA, (Dpo4-1) and b) the orientation of the bases with proposed hydrogen-bonding mechanism (distances shown in Å). c) Binary complex of Dpo4 with ddT across from N2,3-εG in the DNA duplex (Dpo4-3) and d) the orientation of the bases with proposed hydrogen-bonding mechanism. The quality of the data is demonstrated using non-biased omit electron density maps, displayed as red mesh, at 1.0 σ in (a) and (c). Colors of the atoms: O, red; N, blue; P, orange; F, gray.
Significant differences in the replication patterns and mechanisms exist when comparing current results to our previously reported 1,N2-εG, an isomer of N2,3-εG formed through similar pathways.[6b] Differences in catalytic efficiencies and miscoding frequencies for the two lesions are summarized in Table S18 of the Supporting Information. Overall, 1,N2-εG has a much higher miscoding potential, with potential base pairing with A, T, or G by different pols.[17c,18] Extension beyond 1,N2-εG by Dpo4 yields mainly deletion products (−1 and −2),[17c] whereas these were rare for N2,3-εG (approximately 1%). Crystal structures of Dpo4 with 1,N2-εG resemble “type II” structures,[17b] where the 5′ base in the template is oriented in the active site to pair with the incoming nucleotide, which explains the deletion products observed in primer extension reactions.
In summary, we have successfully used a non-classical isostere approach to stabilize an important, labile DNA lesion, N2,3-εG.[19] Kinetic assays using representative DNA polymerases allow quantitative assessment of the miscoding tendency of this lesion and underscore the diversity of biological effects that can result from isomeric DNA adducts. Structural insights reveal the base-pairing mechanisms of the correct base C and miscoded base T with one of the DNA polymerases (Dpo4). The most common mispairing is consistent with the reported GC to AT transition mutations observed in the second base of codon 13 of the K-ras gene in five out of six human VC-induced angiosarcomas,[9, 20] which are not explained by known C or G adducts (3,N4-ethenocytosine, N7-(2-oxoethyl)G, or 1,N2-εG).[8f, 18, 19, 21] Thus, this adduct (N2,3-εG) may be more relevant to the VC-induced tumors, and its presence in unexposed humans may be an issue in disease, in that the misincorporation patterns (N2,3-εG:T) appear very consistently throughout DNA polymerases (Table 1) and have also been detected with human pol ι.[22] The stability of the 2′-fluoro-modified lesion is adequate for more complex biological studies, for example, cellular site-specific mutagenesis and DNA repair.
Supplementary Material
Footnotes
This work was supported in part by United States Public Health Service grants R01 ES010546, R01 ES010375, P01 ES05355, T32 ES007028, and P30 ES000267. We acknowledge beamline scientists Drs. Zdzislaw Wawrzak and David Smith (LS-CAT, Argonne National Laboratory) for helping collecting crystallographic data and Albena Kozekova for assistance in oligonucleotide purification.
Supporting information for this article (experimental details) is available on the WWW under http://dx.doi.org/10.1002/anie.201109004.
Contributor Information
Dr. Linlin Zhao, Department of Biochemistry and Center in Molecular Toxicology, Vanderbilt University School of Medicine, Nashville, TN 37232-0146 (USA)
Dr. Plamen P. Christov, Department of Chemistry and Center in Molecular Toxicology, Vanderbilt University School of Medicine, Nashville, TN 37235-1822 (USA)
Dr. Ivan D. Kozekov, Department of Chemistry and Center in Molecular Toxicology, Vanderbilt University School of Medicine, Nashville, TN 37235-1822 (USA)
Dr. Matthew G. Pence, Department of Biochemistry and Center in Molecular Toxicology, Vanderbilt University School of Medicine, Nashville, TN 37232-0146 (USA)
Dr. Pradeep S. Pallan, Department of Biochemistry and Center in Molecular Toxicology, Vanderbilt University School of Medicine, Nashville, TN 37232-0146 (USA)
Prof. Dr. Carmelo J. Rizzo, Departments of Chemistry and Biochemistry and Center in Molecular Toxicology, Vanderbilt University, Nashville, TN 37235-1822 (USA)
Prof. Dr. Martin Egli, Department of Biochemistry and Center in Molecular Toxicology, Vanderbilt University School of Medicine, Nashville, TN 37232-0146 (USA)
Prof. Dr. F. Peter Guengerich, Email: f.guengerich@vanderbilt.edu, Department of Biochemistry and Center in Molecular Toxicology, Vanderbilt University School of Medicine, Nashville, TN 37232-0146 (USA)
References
- 1.a) Secrist JA, Weber G, Leonard NJ, Barrio JR. Biochemistry. 1972;11:3499–3506. doi: 10.1021/bi00769a001. [DOI] [PubMed] [Google Scholar]; b) Sattsangi PD, Leonard NJ, Frihart CR. J Org Chem. 1977;42:3292–3296. doi: 10.1021/jo00440a020. [DOI] [PubMed] [Google Scholar]; c) Leonard NJ. Chemtracts: Biochem Mol Biol. 1993;4:251– 284. [Google Scholar]
- 2.a) Barbin A, Brésil H, Croisy A, Jacquignon P, Malaveille C, Montesano R, Bartsch H. Biochem Biophys Res Commun. 1975;67:596–603. doi: 10.1016/0006-291x(75)90854-2. [DOI] [PubMed] [Google Scholar]; b) Laib RJ, Bolt HM. Toxicology. 1977;8:185– 195. doi: 10.1016/0300-483x(77)90007-5. [DOI] [PubMed] [Google Scholar]
- 3.Fedtke N, Boucheron JA, Walker VE, Swenberg JA. Carcinogenesis. 1990;11:1287– 1292. doi: 10.1093/carcin/11.8.1287. [DOI] [PubMed] [Google Scholar]
- 4.Gonzalez-Reche LM, Koch HM, Weiss T, Muller J, Drexler H, Angerer J. Toxicol Lett. 2002;134:71– 77. doi: 10.1016/s0378-4274(02)00165-0. [DOI] [PubMed] [Google Scholar]
- 5.Int Agency Res Cancer Monogr Eval Carcinog Risks Hum. 1987;7(Suppl):373–376. [Google Scholar]
- 6.a) Swenberg JA, Fedtke N, Ciroussel F, Barbin A, Bartsch H. Carcinogenesis. 1992;13:727–729. doi: 10.1093/carcin/13.4.727. [DOI] [PubMed] [Google Scholar]; b) Müller M, Belas FJ, Blair IA, Guengerich FP. Chem Res Toxicol. 1997;10:242– 247. doi: 10.1021/tx960124i. [DOI] [PubMed] [Google Scholar]
- 7.Barbin A, Laib RJ, Bartsch H. Cancer Res. 1985;45:2440–2444. [PubMed] [Google Scholar]
- 8.a) Basu AK, Wood ML, Niedernhofer LJ, Ramos LA, Essigmann JM. Biochemistry. 1993;32:12793–12801. doi: 10.1021/bi00210a031. [DOI] [PubMed] [Google Scholar]; b) Cheng KC, Preston BD, Cahill DS, Dosanjh MK, Singer B, Loeb LA. Proc Natl Acad Sci USA. 1991;88:9974–9978. doi: 10.1073/pnas.88.22.9974. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Shibutani S, Suzuki N, Matsumoto Y, Grollman AP. Biochemistry. 1996;35:14992–14998. doi: 10.1021/bi961446o. [DOI] [PubMed] [Google Scholar]; d) Singer B, Kusmierek JT, Folkman W, Chavez F, Dosanjh MK. Carcinogenesis. 1991;12:745–747. doi: 10.1093/carcin/12.4.745. [DOI] [PubMed] [Google Scholar]; e) Zhang WF, Johnson F, Grollman AP, Shibutani S. Chem Res Toxicol. 1995;8:157–163. doi: 10.1021/tx00043a021. [DOI] [PubMed] [Google Scholar]; f) Akasaka S, Guengerich FP. Chem Res Toxicol. 1999;12:501– 507. doi: 10.1021/tx980259j. [DOI] [PubMed] [Google Scholar]
- 9.Swenberg JA, Lu K, Moeller BC, Gao LN, Upton PB, Nakamura J, Starr TB. Toxicol Sci. 2011;120:S130–S145. doi: 10.1093/toxsci/kfq371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nair U, Bartsch H, Nair J. Free Radical Biol Med. 2007;43:1109– 1120. doi: 10.1016/j.freeradbiomed.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 11.Mutlu E, Collins LB, Stout MD, Upton PB, Daye LR, Winsett D, Hatch G, Evansky P, Swenberg JA. Chem Res Toxicol. 2010;23:1485– 1491. doi: 10.1021/tx1001767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuśmierek JT, Folkman W, Singer B. Chem Res Toxicol. 1989;2:230– 233. doi: 10.1021/tx00010a003. [DOI] [PubMed] [Google Scholar]
- 13.Singer B, Spengler SJ, Chavez F, Kuśmierek JT. Carcinogenesis. 1987;8:745– 747. doi: 10.1093/carcin/8.5.745. [DOI] [PubMed] [Google Scholar]
- 14.Srinivasadesikan V, Sahu PK, Lee SL. J Phys Chem B. 2011;115:10537– 10546. doi: 10.1021/jp202738v. [DOI] [PubMed] [Google Scholar]
- 15.a) Marquez VE, Tseng CKH, Mitsuya H, Aoki S, Kelley JA, Ford H, Roth JS, Broder S, Johns DG, Driscoll JS. J Med Chem. 1990;33:978–985. doi: 10.1021/jm00165a015. [DOI] [PubMed] [Google Scholar]; b) Schaerer OD, Verdine GL. J Am Chem Soc. 1995;117:10781–10782. [Google Scholar]; c) Sinnott ML. Chem Rev. 1990;90:1171– 1202. [Google Scholar]
- 16.Lee S, Bowman BR, Ueno Y, Wang S, Verdine GL. J Am Chem Soc. 2008;130:11570– 11571. doi: 10.1021/ja8025328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.a) Ling H, Boudsocq F, Plosky BS, Woodgate R, Yang W. Nature. 2003;424:1083–1087. doi: 10.1038/nature01919. [DOI] [PubMed] [Google Scholar]; b) Ling H, Boudsocq F, Woodgate R, Yang W. Cell. 2001;107:91–102. doi: 10.1016/s0092-8674(01)00515-3. [DOI] [PubMed] [Google Scholar]; c) Zang H, Goodenough AK, Choi JY, Irimia A, Loukachevitch LV, Kozekov ID, Angel KC, Rizzo CJ, Egli M, Guengerich FP. J Biol Chem. 2005;280:29750– 29764. doi: 10.1074/jbc.M504756200. [DOI] [PubMed] [Google Scholar]
- 18.a) Choi JY, Zang H, Angel KC, Kozekov ID, Goodenough AK, Rizzo CJ, Guengerich FP. Chem Res Toxicol. 2006;19:879–886. doi: 10.1021/tx060051v. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Langouët S, Müller M, Guengerich FP. Biochemistry. 1997;36:6069– 6079. doi: 10.1021/bi962526v. [DOI] [PubMed] [Google Scholar]
- 19.For examples of the use of modifications to stabilize DNA adducts see: Takeshita M, Chang CN, Johnson F, Will S, Grollman AP. J Biol Chem. 1987;262:10171–10179.Haraguchi K, Greenberg MM. J Am Chem Soc. 2001;123:8636–8637. doi: 10.1021/ja0160952.Mueller H, Hopfinger M, Carell T. Chem Bio Chem. 2008;9:1617– 1622. doi: 10.1002/cbic.200700690.
- 20.Marion MJ, De Vivo I, Smith S, Luo JC, Brandt-Rauf PW. Int Arch Occup Environ Health. 1996;68:394– 398. doi: 10.1007/BF00377858. [DOI] [PubMed] [Google Scholar]
- 21.Moriya M, Zhang W, Johnson F, Grollman AP. Proc Natl Acad Sci USA. 1994;91:11899– 11903. doi: 10.1073/pnas.91.25.11899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pence MG, Guengerich FP. 2012 unpublished results. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.