Abstract
Background
Bone metastases are a major cause of morbidity and mortality in men with prostate cancer. Preclinical studies suggest that osteoclast inhibition may prevent bone metastases. This phase 3 study evaluated denosumab, a fully human anti-RANKL monoclonal antibody, to prevent bone metastasis or death from any cause in men with non-metastatic castration-resistant prostate cancer (CRPC).
Methods
Men with non-metastatic CRPC at high risk for bone metastasis (PSA ≥8.0 ng/mL and/or PSA doubling time ≤10.0 months) were enrolled in 319 centers from 30 countries. Patients were randomised 1:1 in blinded fashion using an interactive voice response system to receive monthly subcutaneous denosumab 120 mg or placebo. The primary endpoint was bone metastasis-free survival, a composite endpoint determined by time to first occurrence of bone metastasis (symptomatic or asymptomatic) or death.
Results
1432 patients were randomised, 716 to receive denosumab and 716 to receive placebo. Denosumab significantly increased bone metastasis-free survival by a median of 4.2 months over placebo (hazard ratio 0.85 [0.73–0.98]; P=0.028). Denosumab also significantly delayed time to first bone metastasis (hazard ratio 0.84 [0.71–0.98]; P=0.032). Overall survival was similar between groups (hazard ratio 1.01 [0.85–1.20]; P=0.91). Rates of adverse events (AEs) and serious AEs were generally similar between groups, except for osteonecrosis of jaw (ONJ) and hypocalcemia. Yearly cumulative incidence of ONJ for denosumab was: 1%, 3%, 4% in years 1, 2, 3, respectively; overall, less than 5% (n=33). Hypocalcemia occurred in under 2% (n=12) of denosumab and under 1% (n=2) of placebo patients. The blinded treatment phase has been completed.
Conclusion
In men with CRPC, denosumab significantly prolonged bone metastasis-free survival and delayed time to bone metastasis. This is the first large randomised study to demonstrate that targeting the bone microenvironment prevents bone metastasis in men with prostate cancer.
Keywords: urology/prostate disease, denosumab, prostate cancer, prevention, bone metastasis, survival, hormone refractory, castration-resistant
INTRODUCTION
Bone metastases are a major cause of morbidity and mortality in men with prostate cancer.1, 2 Nearly all men with fatal prostate cancer develop bone metastases, and for most of these men, bone is the dominant or only site of metastases.3–5 Bone metastases pose a substantial health and economic burden as they are associated with skeletal-related events (SREs) including pathologic fractures, spinal cord compression, pain, and need for radiation therapy or surgery to bone.6–8 Prevention of bone metastasis represents an important unmet medical need.
Reciprocal interactions between tumor cells and bone appear to explain the bone-dominant pattern of metastases in prostate cancer.9–11 In the bone microenvironment, growth factors secreted by tumor cells induce stromal cells and osteoblasts to express RANKL, an essential mediator of osteoclast formation, function, and survival.12–14 Activation of osteoclasts by RANKL results in increased bone turnover and release of growth factors from bone matrix that may promote establishment of prostate cancer in the skeleton.15 In preclinical models of prostate cancer, osteoclast inhibition prevents bone metastasis.16, 17 RANK expression on prostate cancer cells may also enhance metastatic behavior of tumor cells, with RANKL serving as a potential homing signal to bone marrow.18
Androgen deprivation therapy (ADT) through bilateral orchiectomy or treatment with gonadotropin-releasing hormone (GnRH) agonists or antagonist is standard first-line therapy for metastatic prostate cancer.19, 20 ADT is also frequently used to treat men with non-metastatic prostate cancer.21 Although initial ADT is uniformly effective, nearly all men eventually experience disease progression, despite castrate levels of testosterone, known as castration-resistant prostate cancer (CRPC). 22 In men with progressive non-metastatic CRPC, higher baseline PSA and shorter PSA doubling time are consistently associated with time to first bone metastasis and death.23, 24
Denosumab is a fully human monoclonal antibody that specifically binds and inactivates RANKL. Based on superiority to zoledronic acid in breast and prostate cancer,25, 26 denosumab was approved in the United States for the prevention of skeletal-related events in patients with solid tumors and bone metastases.27 In this randomised, double-blind, placebo-controlled, phase 3 study, we evaluated the effects of denosumab on bone metastasis–free survival in men with CRPC, no evidence of bone metastases, and a high-risk for progression based on elevated PSA and/or short PSA doubling time.
METHODS
Patients
Eligible patients were men ≥18 years of age with histologically confirmed prostate cancer, Eastern Cooperative Oncology Group performance status ≤1, and adequate organ function. Patients had to have received a bilateral orchiectomy or continuous ADT with a GnRH agonist or antagonist for at least 6 months when entering the study.
Patients had to have a total serum testosterone <50 ng/dL (1.72 nM/L), and were to be castration-resistant with three consecutive increasing PSA tests separated by ≥2 weeks and the last two PSAs ≥1.0 ng/mL. High risk for bone metastasis was also required, characterized by PSA ≥8.0 ng/mL within three months prior to randomization and/or PSA doubling time ≤10 months. Baseline renal function was not an eligibility criterion.
Key exclusion criteria included prior or current evidence of radiographically detectable bone metastasis, evidence of metastasis to other organs (except lymph nodes), history or evidence of osteomyelitis or osteonecrosis of jaw (ONJ), prior secondary malignancy within ≤5 years, prior administration of denosumab, intravenous bisphosphonate administration, and use of oral bisphosphonates ≥3 years continuously (<3 years acceptable with washout of ≥1 year before randomization). Anti-neoplastic therapies and concomitant treatments deemed necessary were allowed both prior to enrollment and on study.
All patients had a radioisotope bone scan during screening with subsequent imaging by CT, MRI, or plain radiograph if needed to exclude bone metastases. All screening images were evaluated by a central imaging reader (CoreLab Partners, NJ) in a blinded fashion, with double-reader confirmation and third-reader adjudication in case of disagreement. Patients with imaging results that were either equivocal or consistent with bone metastases at screening were excluded.
The study was approved by the Institutional Review Board or Ethics Committee for each site. Patients provided written informed consent before any study-specific procedure.
Study Design
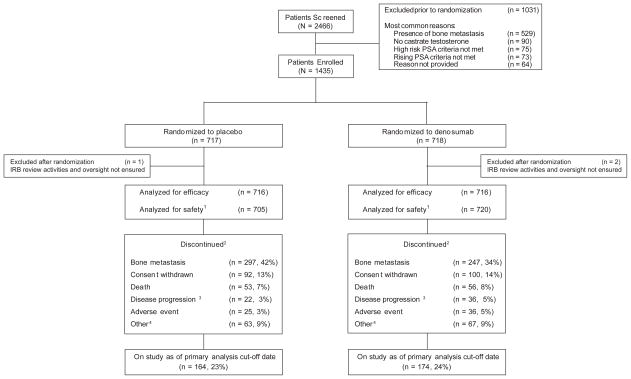
This is a global, multicenter, phase 3, double-blind, randomised, placebo-controlled study in patients with CRPC who were at high risk for developing bone metastases. Patients were enrolled in 319 centers from 30 countries between February 2006 and July 2008. The event-driven date of the primary analysis cut-off was July 2010. Thus, all enrolled patients had a chance to receive investigational product for at least 24 months.
Patients were randomly assigned 1:1 and received subcutaneous (SC) denosumab 120 mg or SC placebo (sterile saline) every 4 weeks until the date when approximately 660 men developed bone metastasis or died (Figure 1). Daily supplementation with calcium ≥500 mg and vitamin D ≥400 IU was strongly recommended, unless hypercalcemia (albumin-adjusted serum calcium ≥2.9 mM/L [11.5 mg/dL] or ionized calcium >1.5 mM/L) developed. Study duration from first patient enrollment to primary analysis cut-off date was approximately 54 months. Patients were discontinued from treatment when bone metastasis occurred so they could receive standard treatment for bone metastasis per investigator discretion. These patients were followed for survival in blinded fashion for up to an additional 3 years after discontinuation of investigational product.
Figure 1.
Patient Enrollment, Randomization, and Disposition
1Patients were analyzed for safety according to their treatment received, regardless of treatment assigned; 4 patients randomized to placebo received denosumab in error.
2Patients who no longer participated in monthly assessments; patients who withdrew consent or were lost to follow up were not followed for survival
3Not in bone.
4Other = administrative decision, noncompliance, lost to follow-up, protocol deviation, ineligibility determined.
Study procedures included medical history and physical examination, vital signs, radioisotope bone scans (every 4 months), radiographic skeletal surveys (yearly), hematology, serum chemistry, denosumab level and antidenosumab antibody assays, urine collection, central laboratory PSA, and testosterone assessments. Investigators were also trained to perform oral examinations (every 6 months). Bone scans were performed every 4 months to detect bone metastases; a confirmed diagnosis of bone metastases required a second imaging modality (CT, MRI, or plain radiograph). All radiographic assessments were also performed by the central reader in a blinded fashion with double-reader confirmation and adjudication by a third-reader in case of disagreement.
Extra-skeletal progression of prostate cancer and concomitant medications were recorded by investigators. Potential cases of ONJ were identified using a predefined list of oral-related Medical Dictionary for Regulatory Activities preferred terms in addition to clinical review, and adjudicated by an independent, blinded panel of experts. A data monitoring committee reviewed safety and efficacy data approximately twice yearly.
Randomisation and Masking
Blinded allocation of patients to treatment occurred via interactive voice response system. An individual independent of the study team prepared the computer-generated randomization schedule; all patients, investigators, and persons involved in study conduct remained blinded throughout the study. Randomization was stratified by PSA criteria (both ≥8.0 ng/mL and doubling time ≤10.0 months versus either one of these criteria) and previous or current chemotherapy for prostate cancer (yes/no). A randomly permuted block design with a block size of four was applied.
Endpoints
The primary efficacy endpoint was bone metastasis-free survival, as determined by time to first occurrence of bone metastasis (symptomatic or asymptomatic) or death from any cause. Secondary endpoints were time to first bone metastasis (symptomatic or asymptomatic, excluding deaths) and overall survival (including deaths on-study and during follow-up). Key exploratory prostate cancer endpoints included overall prostate cancer progression (i.e., centrally confirmed bone metastasis and investigator-determined extra-skeletal prostate cancer progression), prostate cancer progression-free survival (i.e., progression as defined above or death), proportion of patients with symptomatic bone metastasis (bone metastases that were symptomatic at the time of radiologic detection), and change from baseline in PSA level. Changes from baseline in bone turnover markers were also assessed.
Safety was assessed at regular intervals and included adverse events graded by Common Terminology Criteria for Adverse Events (CTCAE) version 3. Safety endpoints included incidence of treatment-emergent adverse events, changes in laboratory values, and incidence of anti-denosumab antibodies.
Statistical Analysis
Based on the assumption that the hazard ratio (HR) of denosumab versus placebo was 0.8, a sample size of 1,400 men was projected to provide sufficient study events (i.e., 660 events) at approximately 80% power and significance level of 0.025 using a 1-sided maximum likelihood test, which is equivalent to 0.05 using a 2-sided test. Only 2-sided p-values will be presented.
In this intention-to-treat analysis, the primary and secondary endpoints were assessed hierarchically. The secondary endpoint of time to first bone metastasis was tested only if the primary endpoint of bone metastasis-free survival was statistically significant in favor of denosumab. If time to first bone metastasis was also statistically significant in favor of denosumab, overall survival was tested.
The Cox proportional hazards model, stratified by the randomization stratification factors, with treatment groups as independent variables was used to compare primary and secondary endpoints between treatment groups for all randomised patients, providing hazard ratio, 95% confidence interval (CI), and two-sided P-value based on the Wald test. Descriptive statistics were used for PSA levels and for change in bone turnover markers from baseline to 2 years.
Safety analyses were performed for all randomised patients who received 1 dose of investigational drug. No formal statistical testing was performed for safety analyses. The cut-off date for the primary analysis was July 2010. The database was locked on December 7, 2010 to allow time for radiologic confirmation of newly detected bone-scan lesions up to July 2010 and for data collection and cleaning. This study is registered with ClinicalTrials.gov [identifier NCT00286091].
Role of the Funding Source
Amgen Inc. provided the study drug and collaborated with investigators on protocol design, data analysis and interpretation, and preparation of this report. All authors had access to pertinent study data; the corresponding author (MS) had full access to the study data for interpretation and drafting of the manuscript. A medical writer provided by Amgen Inc. assisted authors in drafting and finalizing the report. The corresponding author was responsible for the final decision to submit for publication.
RESULTS
Study Population
The study included 1432 randomised patients (716 in the denosumab group and 716 in the placebo group) (Figure 1). A total of 705 events occurred, of which 605 were bone metastases and 100 were deaths. Of the 605 patients with bone metastases, 165 were denoted by investigator as symptomatic and 440 as asymptomatic. Median time on study was 20.2 months with denosumab and 19.0 months with placebo. The most common reasons for study discontinuation were bone metastasis (38%), consent withdrawal (13%), and death (8%). Twenty-four percent of patients continued on study at the time of the primary analysis. Patients were exposed to denosumab for a median (Q1, Q3) of 19 (9, 30) months and to placebo for a median of 18 (9, 30) months.
Baseline demographics and disease characteristics were generally balanced between treatment groups (Table 1). Median age was 74.0 years with denosumab and 74.0 years with placebo. Median time from diagnosis of prostate cancer to study entry was 6.1 years in each group. Median PSA levels and ADT duration were also balanced.
Table 1.
Baseline Demographics and Disease Characteristics
| Characteristic | Placebo N = 716 |
Denosumab N = 716 |
|---|---|---|
| Race, n (%) | ||
| White/Caucasian | 604 (84) | 606 (85) |
| Black | 35 (5) | 41 (6) |
| Hispanic | 37 (5) | 32 (4) |
| Other | 40 (6) | 37 (5) |
| Region, n (%) | ||
| North America | 237 (33) | 228 (32) |
| Europe | 309 (43) | 299 (42) |
| Rest of World | 170 (24) | 189 (26) |
| Median (Q1, Q3) age, years | 74.0 (67.5, 80.0) | 74.0 (67.0, 80.0) |
| ≥ 65 years old, n (%) | 600 (84) | 598 (84) |
| Median (Q1, Q3) time from diagnosis to study entry, years | 6.10 (3.6, 9.5) | 6.10 (3.5, 9.1) |
| Median (Q1, Q3) PSA levels, ng/mL | 12.5 (4.9, 28.5) | 12.2 (4.7, 27.5) |
| PSA dual risk factors1, n (%)*± | 346 (48) | 346 (48) |
| PSA single risk factor2, n (%)*± | 370 (52) | 370 (52) |
| Prior chemotherapy, n (%)*± | 55 (8) | 55 (8) |
| Median (Q1, Q3) duration of prior ADT, months | 47.1 (27.5, 77.5) | 47.2 (27.0, 74.9) |
| Local therapy (prostatectomy and/or radiation), n (%) | 331 (46) | 313 (44) |
| Current lymphatic disease, n (%) | 88 (12) | 93 (13) |
| Gleason score at diagnosis, n (%) | ||
| ≤ 7 | 432 (60) | 404 (56) |
| 8–10 | 214 (30) | 237 (33) |
| Missing | 70 (10) | 75 (11) |
| ECOG status, n (%) | ||
| 0 | 514 (72) | 505 (71) |
| 1 | 199 (28) | 210 (29) |
| 2 | 3 (0.4) | 1 (0.1) |
| Median (Q1, Q3) urinary N-telopeptide corrected for urine creatinine | 25.4 (16.0, 39.2) | 23.2 (15.7, 39.9) |
| Median (Q1, Q3) bone-specific alkaline phosphatase | 13.5 (10.4, 17.7) | 13.1 (10.5, 17.6) |
Per randomization;
Stratification factor
PSA ≥ 8.0 ng/mL within 3 months prior to randomization and PSA doubling time ≤10.0 months
PSA <8.0 ng/mL within 3 months prior to randomization and PSA doubling time ≤10.0 months OR PSA ≥8.0 ng/mL within 3 months prior to randomization and PSA doubling time >10.0 months
PSA: prostate-specific antigen, ECOG: Eastern Cooperative Oncology Group
Use of secondary hormone therapies during the study was reported for 38% (n=275) vs. 42% (n=302) and of chemotherapy/biologics was 28% (n=198) vs. 25% (n=176) for denosumab and placebo, respectively, with no notable differences in individual treatment types.
Efficacy
Bone Metastasis-free Survival
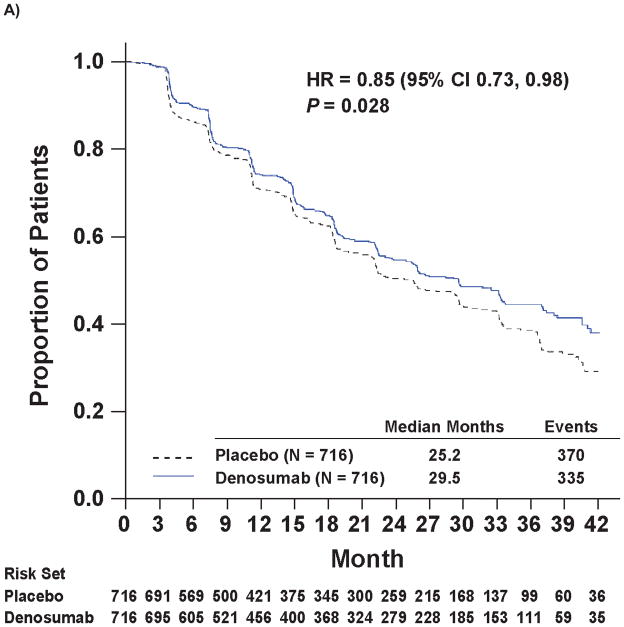
Denosumab therapy prolonged bone metastasis-free survival (time to first occurrence of bone metastasis or death from any cause) compared with placebo by 4.2 months. The median bone metastasis-free survival was 29.5 months with denosumab and 25.2 months with placebo, representing a decrease in risk of 15% (hazard ratio 0.85; 95% CI 0.73, 0.98; P=0.028; Figure 2A).
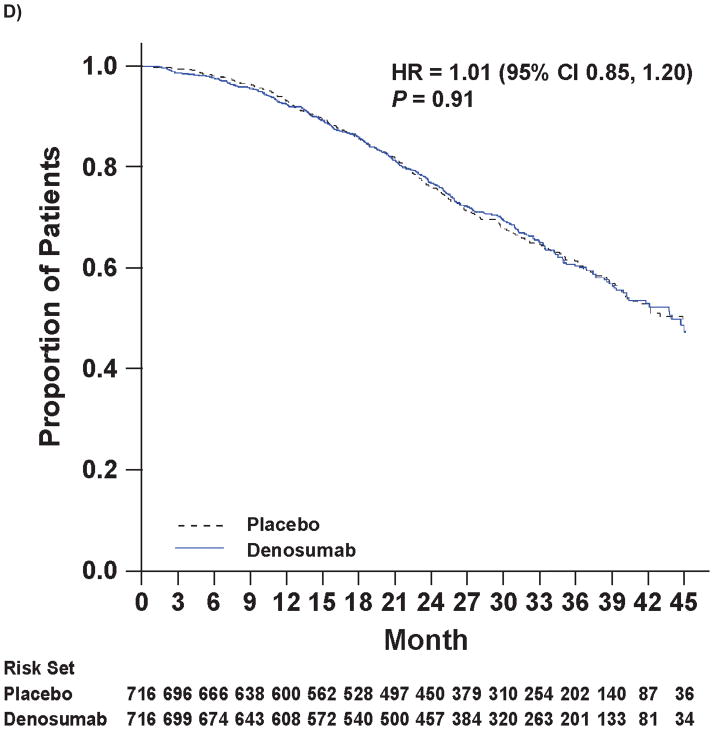
Figure 2.
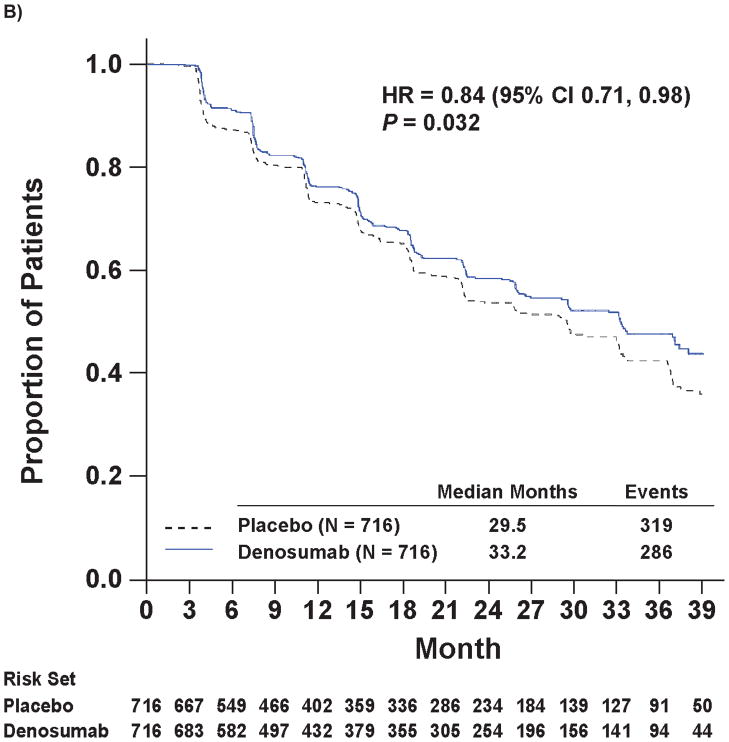
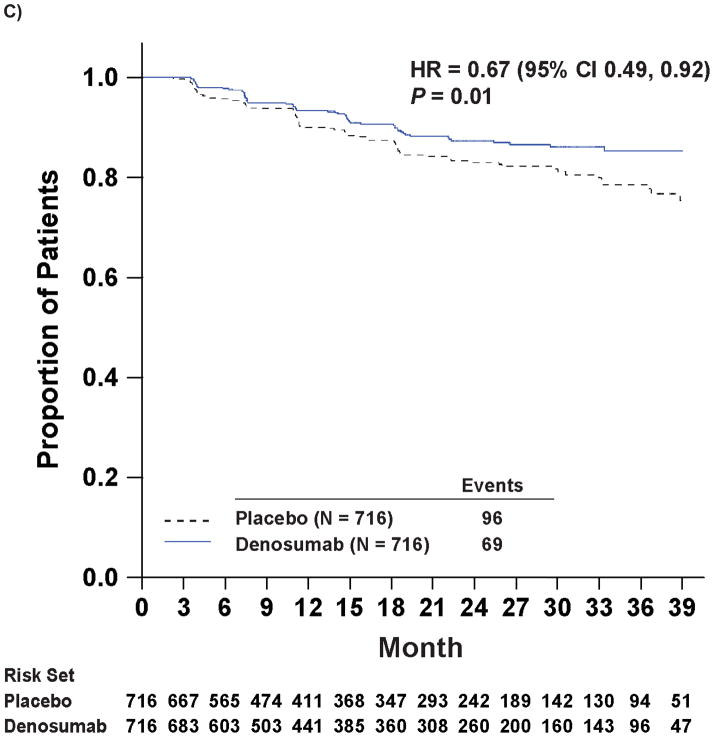
Kaplan-Meier Curves of Key Efficacy Endpoints
Panel A shows the primary endpoint of bone metastasis-free survival. Panels B and C show the time to bone metastases (asymptomatic or symptomatic) and time to symptomatic bone metastasis, respectively. Panel D shows overall survival.
Note: All curves were truncated when the combined risk sets dropped to below 3.5% of the total risk set (i.e., when there were 50 patients or less in the combined risk sets).
Time to Bone Metastasis
Denosumab therapy was also associated with increased time to first bone metastasis (either symptomatic or asymptomatic, excluding deaths) and increased time to symptomatic bone metastasis. Median time to first bone metastasis was 33.2 months with denosumab and 29.5 months with placebo (hazard ratio 0.84; 95% CI: 0.71, 0.98; P=0.032; Figure 2B). Symptomatic bone metastasis were reported in 9.6% (n=69) of denosumab patients and 13.4% (n=96) of placebo patients (P=0.03), and time to symptomatic bone metastasis was increased with denosumab by 33% (hazard ratio 0.67 [0.49, 0.92]; P=0.01; Figure 2C).
Disease Progression, Overall Survival and Progression-Free Survival
The median time to overall prostate cancer progression, an exploratory endpoint defined as confirmed bone metastasis and investigator-determined prostate cancer progression, was 22.3 months with denosumab and 21.9 months with placebo (hazard ratio 0.90 [0.78, 1.03], P=0.13). Overall survival (including deaths on study and during follow-up) was similar for both groups. Median overall survival was 43.9 months with denosumab and 44.8 months with placebo (hazard ratio 1.01; [0.85, 1.20]; P=0.91; Figure 2D). Progression-free survival (an exploratory endpoint defined as confirmed bone metastasis, investigator-determined prostate cancer progression, or death) was 21.7 months (median) with denosumab and 19.3 months with placebo (hazard ratio 0.89 [0.78, 1.02], P=0.09).
Prostate Specific Antigen
Median (Q1, Q3) PSA values at baseline were 12.2 (4.7, 27.5) μg/mL for denosumab group and 12.5 (4.9, 28.5) μg/mL for placebo. Median absolute values of PSA over time were similar in denosumab and placebo groups.
Markers of Bone Turnover
Biochemical markers of bone turnover decreased significantly with denosumab treatment compared with placebo (P<0.001 for each comparison). Among patients with bone turnover assessments at baseline and month 22, levels of urinary N-telopeptide corrected for urine creatinine decreased from baseline by a median (Q1, Q3) of 68% (−82%, −48%) for denosumab (n=305) and increased by 1% (−37%, 88%) for placebo (n=284) at month 22. Levels of bone-specific alkaline phosphatase decreased from baseline by 49% (−61%, −35%) and 7% (−25%, 16%) for denosumab (n=345) and placebo (n=340), respectively, at month 22.
Safety
The most common AEs were back pain, constipation, arthralgia, diarrhea, and urinary tract infection, which occurred at similar rates between treatment groups (Table 2). AEs leading to study discontinuation occurred in 11% (n=79) of denosumab- and 10% (n=67) of placebo-treated patients. Rates of serious AEs were approximately 46% with both denosumab (n=329) and placebo (n=323).
Table 2.
Subject Incidence of Adverse Events
| Placebo N = 705 |
Denosumab N = 720 |
|
|---|---|---|
| Any adverse event, n (%) | 655 (93) | 676 (94) |
| Most common adverse events, n (%) | ||
| Back pain | 156 (22) | 168 (23) |
| Constipation | 119 (17) | 127 (18) |
| Arthralgia | 112 (16) | 123 (17) |
| Diarrhea | 102 (14) | 111 (15) |
| Urinary tract infection | 96 (14) | 108 (15) |
| Serious adverse events, n (%) | 323 (46) | 329 (46) |
| Most common serious adverse events, n (%) | ||
| Urinary retention | 31 (4) | 54 (8) |
| Hematuria | 24 (3) | 35 (5) |
| Prostate cancer | 21 (3) | 15 (2) |
| Anemia | 12 (2) | 22 (5) |
| Urinary tract infection | 14 (2) | 15 (2) |
| Grade 3, 4, or 5 adverse events, n (%) | 353 (50) | 381 (53) |
| Adjudicated positive osteonecrosis of the jaw | 0 | 33 (5) |
| Hypocalcemia | 2 (<1) | 12 (2) |
Denosumab therapy was associated with increased incidence of ONJ and hypocalcemia. ONJ occurred in less than 5% (n=33) of men receiving denosumab and no men receiving placebo, at rates of 1% (n=8), 3% (n=21), and 4% (n=30) at end of years 1, 2, and 3, respectively, with denosumab. Oral risk factors occurred in 94% (n=31) of patients with ONJ, including tooth extraction (70%; n=23), poor oral hygiene (55%; n=18), and dental appliance use (48%; n=16). Limited interventions (i.e., curettage and debridement) were required in 64% (n=21) of patients with ONJ and bone resection was performed in 6% (n=2); the remaining 30% (n=10) of patients were managed with oral rinses/antibiotics. As of February 1, 2011, resolution (mucosal healing) occurred in 39% (n=13) of patients with ONJ.
Hypocalcemia was seen more often with denosumab; rates of grade 3 or 4 hypocalcemia (central laboratory) were reported in 1% (n=9) of denosumab and in no placebo patients. Symptomatic hypocalcemia was reported in one patient receiving denosumab; he was not taking calcium and vitamin D at the time of the event.
No difference in creatinine profiles was observed between denosumab and placebo on study. Neutralizing anti-denosumab antibodies were not detected.
DISCUSSION
In this global placebo-controlled randomised trial of men with high-risk CRPC, treatment with denosumab was associated with improved bone metastasis-free survival. Treatment with denosumab was also associated with prolonged time to first bone metastasis, with fewer symptomatic bone metastases.
Several randomised controlled trials have evaluated the effects of other drugs on development of metastases in men with prostate cancer. In a study of 508 men with high-risk hormone-naïve/sensitive nonmetastatic prostate cancer, clodronate failed to improve symptomatic bone metastasis-free survival compared with placebo.28 In a study of 941 men with CRPC and no bone metastases, atrasentan (an endoethelin-1 receptor antagonist) did not significantly improve time to disease progression or time to first bone metastasis compared with placebo.5 A trial of zoledronic acid in men with non-metastatic CRPC was stopped early after the observed event rate was lower than expected; published observations from the placebo group informed the eligibility criteria used to select men at high risk to develop bone metastases in the current study.24
This study has several major strengths. Although there are no evidence-based guidelines or established standards of care for salvage therapy in men with non-metastatic CRPC, other treatments were allowed on study to increase the generalizability of the study results and improve patient and clinician acceptance of the randomised design. Study treatment was discontinued at diagnosis of bone metastases because other therapies are approved to treat men with metastatic CRPC.29 Bone scans were performed every four months to detect bone metastases and were confirmed by a second imaging modality. Readers who were blinded to treatment assignments centrally reviewed all radiographic studies. The primary efficacy analysis of bone metastasis-free survival was based on a large number of informative events.
The study design required that patients discontinue investigational product following development of bone metastasis so they could receive standard treatment for prevention of SREs once bone metastasis had occurred. This limited our ability to evaluate overall survival with denosumab, as approximately 80% of the deaths occurred in patients who had discontinued investigational product. With a median time from bone metastasis to death of 19 months, a treatment effect of denosumab on overall survival would be difficult to observe, particularly in the context of all other prostate cancer therapies used during that period which could influence survival. This same requirement also limited our ability to determine when asymptomatic bone metastases became symptomatic as patients were removed from the study once a bone metastasis was detected and symptoms may not yet have occurred.
Denosumab was associated with increased incidence of ONJ and hypocalcemia. The rate of ONJ in this study was similar when adjusted for exposure to that previously reported for denosumab, at the same dose and schedule, in another large trial in prostate cancer patients with bone metastasis. Median exposure to denosumab in the current study was longer than in the SRE trial.26 In the current study, most cases of ONJ were managed conservatively and over 40% resolved. Grade 3 or 4 hypocalcemia was observed in 1% of patients and only 1 patient developed symptomatic hypocalcemia.
In 1889, Stephen Paget first proposed the “seed and soil” hypothesis of interactions between tumor cells and the host microenvironment to explain why some cancers preferentially spread to specific anatomic sites.9 An extensive body of contemporary preclinical research suggests a “vicious cycle” of complex bidirectional interactions between prostate cancer cells and the bone microenvironment, and tumor-bone interactions have been advanced as the foremost mechanism for the bone-dominant pattern of metastases in prostate cancer.10 Our finding that denosumab increases bone metastasis-free survival provides the first direct clinical evidence for the important role of the bone microenvironment/RANKL signaling in development of bone metastases in men with prostate cancer.
In summary, denosumab treatment was associated with increased bone metastasis-free survival in men with high-risk CRPC based on prolongation of time to first bone metastasis.
“RESEARCH IN CONTEXT” DISPLAY PANEL.
Systematic Review
We searched the PubMed database up to April 20, 2011 without language restriction for full papers reporting randomised controlled trials with the search terms “prostate cancer” and “bone metastases”. Our search identified three randomised controlled trials to prevent bone metastases in men with prostate cancer. In a study of men with high risk nonmetastatic prostate cancer, clodronate failed to improve symptomatic bone metastasis-free survival compared with placebo.28 In a study of men with castration-resistant prostate cancer and no bone metastases, atrasentan (an endoethelin-1 receptor antagonist) did not significantly improve time to disease progression or time to first bone metastasis compared with placebo.5 A randomised placebo-controlled trial of zoledronic acid in men with castration-resistant nonmetastatic prostate cancer was terminated prematurely after the observed event rate was lower than expected; there was no difference in bone metastasis-free survival between the groups.24 Analyses of the latter study indicated that only higher PSA and shorter PSA doubling time were independently associated with bone metastasis-free survival. Those observations informed the eligibility criteria and event rate assumptions for our study.
Interpretation
The significant improvement in bone-metastasis free survival and time to first bone metastasis observed with denosumab treatment in our study represents the first time any bone-targeted agent has delayed the time to bone metastasis or death in men with prostate cancer. Our findings also provide the first direct clinical evidence for the important role of the bone microenvironment/RANKL signaling in the development of bone metastases in men with prostate cancer.
Acknowledgments
Funding: Amgen Inc.
The authors would like to thank additional study investigators, study coordinators and nurses, and the patients and their families for their contributions to this study. We thank the Data Monitoring Committee for their diligence in monitoring the study. We also thank Vidya Setty, MPH, MBA, supported by Amgen Inc. for medical writing assistance with this manuscript.
APPENDIX
Study Investigators
Argentina: MAB Arnold, P Atchabaian, CR Blajman, EH Bogado, LG Casasola, M Chacon, F Coppola, MA Costa, OH Damia, CA Delfino, L Fein, A Hannois, GD Jarchum, L Koliren, JA Lacava, JDL Laur, GL Lerzo, DE Levy, DE Maldonado, R Martinelli, SV Metrebian, LF Montes De Oca, A Nolazco, E Richardet, JJ Rozanec, JJ Zarba
Australia: M Brown, B Chern, M Frydenberg, R Gardiner, H Gurney, P Mainwaring, G Marx, D Nicol, N Pavlakis, C Shannon, J Shapiro, S Wood, S Wong, C Underhill
Belgium: L Hoekx, W Oosterlinck, T Roumeguère, C Schulman
Brazil: JL Amaro, G Atta, S Azevedo, MZ Baptista, CA Beato, LA Bruno, MH Bustillo, C Cabral, V Da Silva Teixeira, G De Castro Junior, AG Faion, MHH Federico, BL Ferrari, U Ferreira, FA Franke, S Glina, DB Jendiroba, M Liberatti, ACP Martins, CMC Mathias, A Murad, A Notari, C Oliveira, MDC Perez, M Rabinowits, R Ribeiro, LC Rocha, FH Souza, FD Tomasich, M Zereu
Bulgaria: H Markova, M Racheva, S Stratev, A Tomova
Canada: L Aaron, C Andreou, J Barkin, R Buckley, M Carmel, R Casey, M Chetner, G Di Costanzo, B Donnelly, S Flax, B Goldfarb, M Gleave, E Hirshberg, U Jain, K Jansz, T Kinahan, L Klotz, W Leung, M Liquornik, W Love, A Mathur, C Morash, A Nabid, P Ouellette, B Palmer, A Patrick, P Pommerville, R Rendon, R Siemens, A So, G Steinhoff, J Trachtenberg, E Woods, J Zadra
Czech Republic: P Hesoun, J Hyncica, J Jansa, J Kasl, K Odrazka, I Pavlik, J Pernicka, J Schraml, R Skoumal, M Urban, V Vitu, F Zatura
Finland: P Hellstrom, A Rannikko, M Ruutu, J Viitanen
France: G Benoit, P Beuzeboc, I Biliiet, B Chauvet, M Colombel, R Delva, B Duclos, JM Ferrero, A Fléchon, F Goldwasser, G Gravis, I Krakowski, F Lesaunier, C Linassier, S Oudard, X Pivot, F Priou, F Rolland M Vannetzel
Germany: D Hempel, MS Michel, J Roigas, M Siegsmund, A Stenzl, M Wirth
Hungary: G Body, JM Feher, G Papp, F Torzsok
India: J Desai, P Jayaprakash, P Julka, B Kashyapi, A Mohan, N Mohanty, PVLN Murthy, V Rajagopala, S Raval, TC Sadasukhi, S Sharma, S Shroff, SL Tolani, H Tongaonkar
Ireland: S McDermott
Italy: F Boccardo, L Dogliotti, N Gebbia, G Vespasiani
Lativa: A Brize, E Vjaters
Lithunia: A Ulys
Mexico: AG Ambriz, IL Caballero, DC Dominguez, J Espinosa de los Monteros, CP Gahbler, JJ Gastelum, M Gomez-Roman, LG Guerra, GG Meza, JAF Munguia, AH Porras, SG Perez, JMU Pérez-Toriz, JA Robles-Avina, RDG Samano, JGL Quintanilla
Netherlands: J.L.H.R. Bosch, J.L. Bruins, K.P.J. Delaere, R.F. Kropman, P. Verhagen H.J.E.J. Vrijhof or E.L. Koldewijn
New Zealand: P Gilling, F Kueppers, P Meffan, I Mundy
Poland: K Bar, T Dmowski, P Jarzemski, P Milecki, M Rurarz, J Sokolowski, R Szczesniewski, K Szkarlat, R Szwedowski, P Trypens, M Wyczolkowski, H Zielinski
Portugal: M Apolinario, JL Carneiro de Moura, H Correia, F Gomes, A Mota, JFS Pinto, N Sousa
Russia: O Karyakin, L Roman, I Rusakov, A Zhivov
Serbia: V Colovic, M Lazic, S Micic
Slovakia: V Balaz, R Barilla, M Brezovsky, F Goncalves, J Kliment, J Marencak, L Valansky
South Africa: J Bahlmann, T Botha, S Cornish, J Enslin, A Pontin, R Rencken, E Strasheim, J Van Wyk
Spain: A Alcaraz, AR Antolín, J Carballido, VM Carrero, J Castiñeiras, E Solsona
Switzerland: U Huber
Ukraine: G Bondar, I Bondarenko, L Chybisov, Y Hotko, F Kostev, I Kostynskyy, V Lesovoy, V Paramonov, Y Shparyk, O Volodimir Lyulko
United Kingdom: D Dearnaley, N James, P Hoskin, R McMenemin
United States: P Acevedo, G Adams, F Ahmann, E Aly, S Amar, P Arnold, S Axelrad, J Bailen, K Baker, L Baumann, D Beccia, K Belkoff, M Berger, M Bhandari, M Bidair, D Bilhartz, K Bloom, F Cabanillas, J Camps, S Chang, V Charu, R Clark, E Cobos, J Cochran, S Coffield, G Colombo, R Concepcion, J Corman, B Cowan, R D’Anna, G Daniels, H Deeths, M Efros, H Epstein, H Fisher, J Forrest, S Freedman, W Friedel, D Friedland, J Gandhi, A Geringer, R Given, D Gleason, S Goel, E Goldfischer, H Goldstein, R Greengold, D Guth, S Hall, W Harper, L Hart, S Hopkins, W Hrushesky, H Hudson, R Israeli, P Iyer, R Kahnoski, J Kaminetsky, G Karlin, M Keaton, L Keeler, D Keiller, M Kelley, S Koukol, R Lance, H Lanctin, S Lacy, D Lipsitz, A Lipton, J Lyne, J McGettigan, M Madorsky, R Mazo, J McMurray, B Mehlhaff, E Meiri, D Mobley, W Mobley, W Moseley, R Moss, J Mullen, M Murdock, S Nash, C Orth, R Perez-Marrero, A Perzin, D Petrylak, T Phillis, W Pittman, D Reed, P Rosen, S Rosenburg, D Sahasrabudhe, W Schiff, P Sethi, J Sharkey, A Sidhom, J Sotolongo, F Snoy, JW Stallings, C Steidle, B Stein, N Stein, D Sussman, S Tagawa, C Teigland, G Thomas, E Torgerson, M Toth, R Tutrone, P Van Veldhuizen, B Wachs, W Waterfield, S Weiss, WG Wells, K Westenfelder, R Wurzel, J Young, J Zachary, N Zinner
Footnotes
Trial Registration: This study is registered with ClinicalTrials.gov with the identifier NCT00286091.
Prior Presentation: These data were previously presented at the 2011 Annual Congress of the American Urological Association (AUA), Washington DC, USA, May 17, 2011.
AUTHOR CONTRIBUTIONS
All authors had access to pertinent study data, contributed to data analysis and interpretation, and reviewed and commented on multiple drafts of the manuscript. The corresponding author (MS) had full access to the study data for interpretation and drafting of the manuscript, and was responsible for the final decision to submit for publication.
CONFLICTS OF INTEREST
M Smith, R Coleman, K Fizazi, and F Saad have been consultants for Amgen and Novartis; and L Karsh, B Tombal, H Van Poppel, J Chin, J Morote, K Miller, P Sieber, TL Tammela, and N Shore have been consultants for Amgen.
K Fizazi, F Saad, R Coleman, and B Tombal have participated in speakers’ bureaus for Amgen and Novartis. N Shore, K Miller, P Sieber, T Borkowski, and J Morote have participated in speakers’ bureaus for Amgen. K Fizazi has received travel funds from Amgen and Novartis; and B Egerdie, L Karsh, B Tombal, J Chin, K Miller, T Borkowski, and N Shore have received travel funds from Amgen. M Smith, F Saad, L Karsh, TL Tammela, and N Shore have received research funding from Amgen; and R Coleman has received research funding from Novartis. R Coleman has received honoraria from Amgen and Novartis; and M Smith, L Karsh, and N Shore have received honoraria from Amgen. R Coleman has provided expert testimony for Novartis. F Gomez Veiga and R Damião have no conflicts of interest to disclose. Z Ye, A Kupic, R Dansey, and C Goessl are employees of Amgen and have received stock/stock options from Amgen.
References
- 1.Norgaard M, Jensen AO, Jacobsen JB, Cetin K, Fryzek JP, Sorensen HT. Skeletal related events, bone metastasis and survival of prostate cancer: a population based cohort study in Denmark (1999 to 2007) J Urol. 2010 Jul;184(1):162–7. doi: 10.1016/j.juro.2010.03.034. [DOI] [PubMed] [Google Scholar]
- 2.Sathiakumar N, Delzell E, Morrisey MA, Falkson C, Yong M, Chia V, et al. Prostate Cancer and Prostatic Disease. 2011. Mortality following bone metastasis and skeletal-related events among men with prostate cancer: a population-based analysis of U.S. Medicare beneficiaries, 1999–2006. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 3.Coleman RE. Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat Rev. 2001;27(3):165–76. doi: 10.1053/ctrv.2000.0210. [DOI] [PubMed] [Google Scholar]
- 4.Gralow JR, Biermann JS, Farooki A, Fornier MN, Gagel RF, Kumar RN, et al. NCCN Task Force Report: Bone Health in Cancer Care. J Natl Compr Canc Netw. 2009 Jun;7(Suppl 3):S1–S32. doi: 10.6004/jnccn.2009.0076. quiz S3–S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nelson JB, Love W, Chin JL, Saad F, Schulman CC, Sleep DJ, et al. Phase 3, randomized, controlled trial of atrasentan in patients with nonmetastatic, hormone-refractory prostate cancer. Cancer. 2008 Nov 1;113(9):2478–87. doi: 10.1002/cncr.23864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coleman RE. Skeletal complications of malignancy. Cancer. 1997;80(8 Suppl):1588–94. doi: 10.1002/(sici)1097-0142(19971015)80:8+<1588::aid-cncr9>3.3.co;2-z. [DOI] [PubMed] [Google Scholar]
- 7.Coleman RE, Smith P, Rubens RD. Clinical course and prognostic factors following bone recurrence from breast cancer. Br J Cancer. 1998;77(2):336–40. doi: 10.1038/bjc.1998.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reed SD, Radeva JI, Glendenning GA, Saad F, Schulman KA. Cost-effectiveness of zoledronic acid for the prevention of skeletal complications in patients with prostate cancer. J Urol. 2004;171(4):1537–42. doi: 10.1097/01.ju.0000116777.94426.60. [DOI] [PubMed] [Google Scholar]
- 9.Talmadge JE, Fidler IJ. AACR centennial series: the biology of cancer metastasis: historical perspective. Cancer Res. 2010 Jul 15;70(14):5649–69. doi: 10.1158/0008-5472.CAN-10-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guise T. Examining the metastatic niche: targeting the microenvironment. Semin Oncol. 2010 Oct;37( Suppl 2):S2–14. doi: 10.1053/j.seminoncol.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 11.Roodman GD. Mechanisms of bone metastasis. N Engl J Med. 2004 Apr 15;350(16):1655–64. doi: 10.1056/NEJMra030831. [DOI] [PubMed] [Google Scholar]
- 12.Fuller K, Wong B, Fox S, Choi Y, Chambers TJ. TRANCE is necessary and sufficient for osteoblast-mediated activation of bone resorption in osteoclasts. J Exp Med. 1998;188(5):997–1001. doi: 10.1084/jem.188.5.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lacey DL, Tan HL, Lu J, Kaufman S, Van G, Qiu W, et al. Osteoprotegerin ligand modulates murine osteoclast survival in vitro and in vivo. Am J Pathol. 2000;157(2):435–48. doi: 10.1016/S0002-9440(10)64556-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93(2):165–76. doi: 10.1016/s0092-8674(00)81569-x. [DOI] [PubMed] [Google Scholar]
- 15.Dougall WC, Chaisson M. The RANK/RANKL/OPG triad in cancer-induced bone diseases. Cancer Metastasis Rev. 2006 Dec;25(4):541–9. doi: 10.1007/s10555-006-9021-3. [DOI] [PubMed] [Google Scholar]
- 16.Guise TA, Mohammad KS, Clines G, Stebbins EG, Wong DH, Higgins LS, et al. Basic mechanisms responsible for osteolytic and osteoblastic bone metastases. Clin Cancer Res. 2006 Oct 15;12(20 Pt 2):6213s–6s. doi: 10.1158/1078-0432.CCR-06-1007. [DOI] [PubMed] [Google Scholar]
- 17.Zhang J, Dai J, Qi Y, Lin DL, Smith P, Strayhorn C, et al. Osteoprotegerin inhibits prostate cancer-induced osteoclastogenesis and prevents prostate tumor growth in the bone. J Clin Invest. 2001 May;107(10):1235–44. doi: 10.1172/JCI11685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones DH, Nakashima T, Sanchez OH, Kozieradzki I, Komarova SV, Sarosi I, et al. Regulation of cancer cell migration and bone metastasis by RANKL. Nature. 2006 Mar 30;440(7084):692–6. doi: 10.1038/nature04524. [DOI] [PubMed] [Google Scholar]
- 19.Heidenreich A, Pfister D, Ohlmann CH, Engelmann UH. Androgen deprivation for advanced prostate cancer. Urologe A. 2008 Mar;47(3):270–83. doi: 10.1007/s00120-008-1636-2. [DOI] [PubMed] [Google Scholar]
- 20.Loblaw DA, Virgo KS, Nam R, Somerfield MR, Ben-Josef E, Mendelson DS, et al. Initial hormonal management of androgen-sensitive metastatic, recurrent, or progressive prostate cancer: 2006 update of an American Society of Clinical Oncology practice guideline. J Clin Oncol. 2007 Apr 20;25(12):1596–605. doi: 10.1200/JCO.2006.10.1949. [DOI] [PubMed] [Google Scholar]
- 21.Sharifi N, Gulley JL, Dahut WL. Androgen deprivation therapy for prostate cancer. JAMA. 2005 Jul 13;294(2):238–44. doi: 10.1001/jama.294.2.238. [DOI] [PubMed] [Google Scholar]
- 22.Scher HI, Halabi S, Tannock I, Morris M, Sternberg CN, Carducci MA, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008 Mar 1;26(7):1148–59. doi: 10.1200/JCO.2007.12.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith MR, Cook R, Lee KA, Nelson JB. Disease and host characteristics as predictors of time to first bone metastasis and death in men with progressive castration-resistant nonmetastatic prostate cancer. Cancer. 2010 doi: 10.1002/cncr.25762. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith MR, Kabbinavar F, Saad F, Hussain A, Gittelman MC, Bilhartz DL, et al. Natural history of rising serum prostate-specific antigen in men with castrate nonmetastatic prostate cancer. J Clin Oncol. 2005;23(13):2918–25. doi: 10.1200/JCO.2005.01.529. [DOI] [PubMed] [Google Scholar]
- 25.Stopeck AT, Lipton A, Body JJ, Steger GG, Tonkin K, de Boer RH, et al. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, double-blind study. J Clin Oncol. 2010 Dec 10;28(35):5132–9. doi: 10.1200/JCO.2010.29.7101. [DOI] [PubMed] [Google Scholar]
- 26.Fizazi K, Carducci M, Smith M, Damião R, Brown J, Karsh L, et al. A randomised, double-blind study of denosumab versus zoledronic acid in the treatment of bone tetastases in men with castration-resistant prostate cancer. Lancet. 2011;377:813–22. doi: 10.1016/S0140-6736(10)62344-6. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.XGEVA™. [Package Insert] Thousand Oaks, CA: Amgen Inc; 2010. [Google Scholar]
- 28.Mason MD, Sydes MR, Glaholm J, Langley RE, Huddart RA, Sokal M, et al. Oral sodium clodronate for nonmetastatic prostate cancer--results of a randomized double-blind placebo-controlled trial: Medical Research Council PR04 (ISRCTN61384873) J Natl Cancer Inst. 2007 May 16;99(10):765–76. doi: 10.1093/jnci/djk178. [DOI] [PubMed] [Google Scholar]
- 29.Mohler J, Bahnson RR, Boston B, Busby JE, D’Amico A, Eastham JA, et al. NCCN clinical practice guidelines in oncology: prostate cancer. J Natl Compr Canc Netw. 2010 Feb;8(2):162–200. doi: 10.6004/jnccn.2010.0012. [DOI] [PubMed] [Google Scholar]