Abstract
Vascular endothelium is vulnerable to the attack of glucose-derived oxoaldehydes (glyoxal and methylglyoxal) during diabetes, through the formation of advanced glycation end products (AGEs). Although aminoguanidine (AG) has been shown to protect against the AGE-induced adverse effects, its protection against the glyoxal-induced alterations in vascular endothelial cells (ECs) such as cytotoxicity, barrier dysfunction, and inhibition of angiogenesis has not been reported and we investigated this in the bovine pulmonary artery ECs (BPAECs). The results showed that glyoxal (1–10 mM) significantly induced cytotoxicity and mitochondrial dysfunction in a dose- and time-dependent (4–12 h) fashion in ECs. Glyoxal was also observed to significantly inhibit EC proliferation. The study also revealed that glyoxal induced EC barrier dysfunction (loss of trans-endothelial electrical resistance), actin cytoskeletal rearrangement, and tight junction alterations in BPAECs. Furthermore, the results revealed that glyoxal significantly inhibited in vitro angiogenesis on the Matrigel. For the first time, this study demonstrated that AG significantly protected against the glyoxal-induced cytotoxicity, barrier dysfunction, cytoskeletal rearrangement, and inhibition of angiogenesis in BPAECs. Therefore, AG appears as a promising protective agent in the treatment of AGE-induced vascular endothelial alterations and dysfunction during diabetes, presumably by blocking the reactivity of the sugar-derived dicarbonyls such as glyoxal and preventing the formation of AGEs.
Keywords: Glyoxal, Vascular endothelial cell, Barrier dysfunction, Cytoskeletal rearrangement, Cytotoxicity, Advanced glycation end products, Diabetes
Introduction
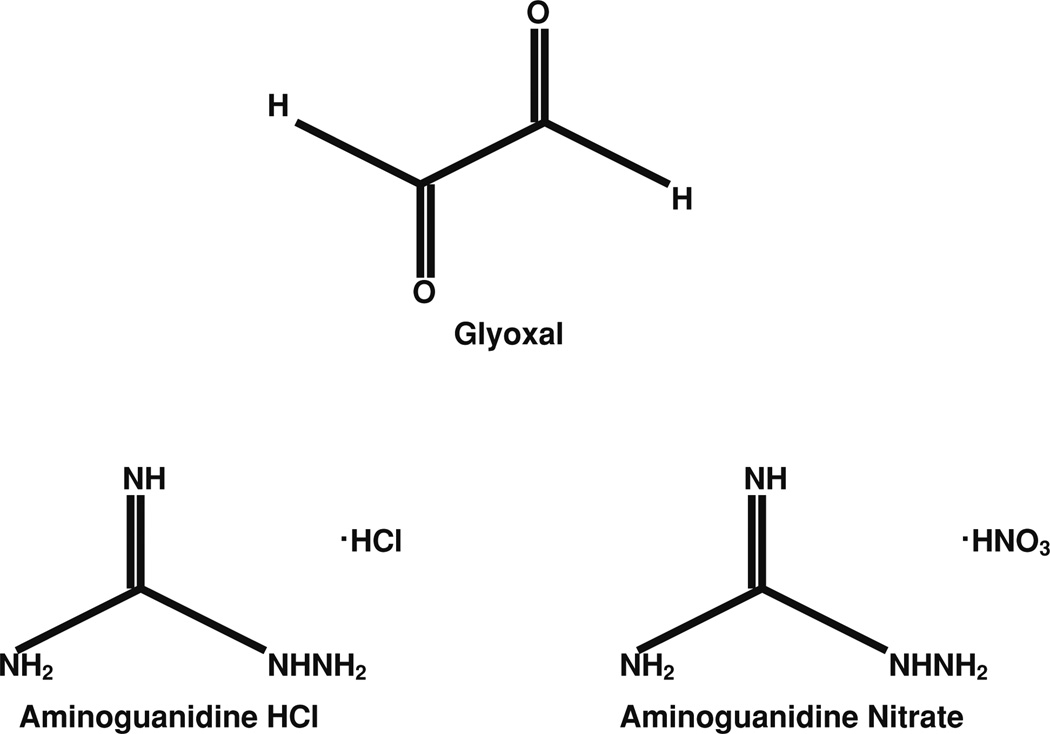
Diabetes causes elevated blood glucose levels known as hyperglycemia [1, 2] due to the inability of insulin production or abnormal resistance to insulin action. Sugar aldehydes (glycoaldehydes), which arise from oxidative stress during hyperglycemia, react with amino groups of proteins and form cross links between proteins, known as the advanced glycation end products (AGEs) [3, 4]. The two most prominent reactive sugar aldehydes encountered during diabetes are glyoxal (Fig. 1) and methylglyoxal [5]. The AGE precursors and AGEs themselves induce cellular damage and have been strongly linked to the development of complications during diabetes such as retinopathy, neuropathy, atherosclerosis, heart disease, and kidney failure [3, 6, 7]. Endothelium plays a pivotal role in the structure and function of the blood vessel and is involved in the vital functions including clotting, angiogenesis, inflammation, and regulation of blood pressure through vasoconstriction and dilation. Vascular endothelium is crucial as the selective barrier against circulating macromolecules and leukocytes between the blood and interstitium [8]. Experimental evidence supports that AGEs formed from glyoxal and methylglyoxal contribute to complications during diabetes and other diseases [7]. This is due to the affinity of glycoaldehydes and AGEs to bind to cellular macromolecules, most notably proteins, thus leading to the alterations in structure and function of the cell [9]. Recently, more attention has been paid to the compounds that are pharmacologically able to prevent the adverse effects of AGEs. They include aminoguanidine (AG), metformin, pioglitazone, angiotensin receptor blockers, angiotensin-converting enzyme inhibitors, pentoxyfylline, metal ion chelators (desferrioxamine and penicillamine), antioxidants (vitamin C or E), amino group capping agents (aspirin), enzymes that cause deglycation of Amadori products (amadoriases), compounds that mostly break α-dicarbonyl cross links (phenacylthiazolium bromide and its stable derivative ALT-711, Alagebrium), and derivatives of aryl ureido and aryl carboxaminido phenoxy isobutyric acids [9]. One of the most notable agents used to prevent the formation of AGEs is AG. Because of its ability to react with the dicarbonyl compounds, such as glyoxal and methylglyoxal, AG (Fig. 1) is an effective blocker of AGEs [10]. AGEs present in circulation can impair the functions of the endothelium by causing cytotoxicity and cell death and lead to atherosclerosis [11, 12]. AG has been shown to scavenge glyoxal and therefore protects against AGEs [10]. However, detailed studies on the glycoaldehyde-induced adverse actions such as anti-angiogenesis, cytoskeletal alterations, and barrier dysfunction in vascular endothelial cells (ECs), at least in vitro, are lacking. In addition, pharmacological protection, offered specifically by AG against such glycoaldehyde-induced EC alterations, has not been reported.
Fig. 1.
Chemical structures of glyoxal and aminoguanidine
Therefore, we hypothesize that the sugar-derived aldehyde, glyoxal, formed during diabetes causes endothelial dysfunction through cytotoxicity, barrier dysfunction, cytoskeletal alterations, and also causes inhibition of angiogenesis. Secondly, as AG is known to offer protection against AGE-mediated nephropathy during diabetes, we envision that AG protects against the glyoxal-induced cytotoxicity, cytoskeletal alterations, and inhibition of angiogenesis in vascular ECs [1, 10, 13, 14]. In order to test our hypothesis, in the current study, we utilized our well-established bovine pulmonary artery EC (BPAEC) system and investigated the glyoxal-induced cytotoxicity, cytoskeletal rearrangements, and barrier dysfunction. We also utilized the widely used EC Matrigel tube formation assay and investigated the effects of glyoxal on the in vitro angiogenesis. In addition, we also investigated the protective effects of AG on the glyoxal-induced EC dysfunctions and inhibition of angiogenesis.
The results of the current study established that the sugar-derived aldehyde and AGE precursor, glyoxal, formed during diabetes caused cytotoxicity, cell morphology alterations, cytoskeletal reorganization, barrier dysfunction, and inhibition of angiogenesis in vascular ECs in vitro, which were all effectively attenuated by the AG treatment.
Materials and methods
Materials
Bovine pulmonary artery endothelial cells (BPAECs) (passage 2) were obtained from Cell Applications Inc. (San Diego, CA). Fetal bovine serum (FBS), trypsin, minimum essential medium (MEM), and non-essential amino acids were obtained from Gibco Invitrogen Corp. (Grand Island, NY). Glyoxal, AG HCl, AG nitrate, N-acetyl-l-cysteine (NAC), meso-2,3-dimercaptosuccinic acid (DMSA), pyridoxamine dihydrochloride, l-carnosine, d-penicillamine, cysteamine, t-octylphenoxypolyethoxyethanol (Triton X-100), bovine serum albumin (BSA), 36.5% formaldehyde solution, 3-[4,5-dimethylthiazol-2-yl]-2, 5-diphenyl tetrazolium bromide (MTT) assay kit, and lactate dehydrogenase cytotoxicity assay (LDH) kit were obtained from Sigma Chemical Co. (St. Louis, MO). [3H]-thymidine was obtained from American Radiolabeled Chemicals, Inc. (St. Louis, MO). ECIS electrode arrays were obtained from Applied Biophysics (Troy, NY). AlexaFluor 568, 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI), and rhodamine–phalloidin were purchased from Molecular Probes Invitrogen Co. (Carlsbad, CA). Mouse anti-ZO-1 antibody was obtained from Zymed Laboratories (San Francisco, CA). Polyoxyethylene sorbitan monolaurate (Tween-20) and 10× Tris-buffered saline (TBS) were obtained from Bio-Rad Laboratories (Hercules, CA).
Cell culture
Bovine pulmonary artery endothelial cells were cultured in MEM supplemented with 10% FBS, non-essential amino acids, antibiotics, and growth factor as described previously [15, 16]. Cells in culture were maintained at 37°C in a humidified environment of 5% CO2–95% air and grown to contact-inhibited monolayers with typical cobblestone morphology. When confluence was reached, cells were trypsinized and sub-cultured in T-75-cm2 flasks or 17.5 or 35-mm tissue culture dishes. Confluent cells showed cobblestone morphology under light microscope and stained positive for Factor VIII. All experiments were conducted between 5 and 20 passages (75–90% confluence).
Cell treatment
Glyoxal was prepared by dissolving in PBS at 70°C and then diluted to desired concentrations in MEM. Other pharmacological agents were prepared directly in MEM to desired concentrations. Cells were then treated with MEM or solutions prepared in MEM as desired.
Cell morphology assay
Morphological changes in BPAECs grown in 35-mm dishes up to 90% confluence, following their exposure to different concentrations of glyoxal (0–10 mM) in the absence or presence of chosen pharmacological agents (0–10 mM) in MEM for 0–24 h at 37°C in a humidified environment of 5% CO2–95% air, were examined as an index of cytotoxicity. Images of cell morphology were digitally captured with the Nikon Eclipse TE2000-S at 20× magnification.
LDH assay of cytotoxicity
Bovine pulmonary artery endothelial cells were grown up to 90% confluence in 17.5-mm dishes (24-well culture plate) pretreated with MEM alone or MEM-containing AG (0–10 mM) for 1 h following which the cells were exposed to MEM-containing glyoxal (0–20 mM) for 4–24 h. At the end of incubation, supernatant was removed, the experiment was terminated with 1 N HCl, and LDH release was determined spectrophotometrically according to the manufacturer’s recommendations (Sigma Chemical Co., St. Louis, MO).
MTT assay of cytotoxicity
Bovine pulmonary artery endothelial cells were grown up to 90% confluence in 17.5-mm dishes pretreated with MEM alone or MEM-containing AG (0–10 mM) for 1 h following which the cells were exposed to MEM-containing glyoxal (0–10 mM) for 4–24 h. At the end of incubation, MTT solution (10% of culture volume) was added and incubated for 3–4 h, the solution was removed, and MTT solvent was added in an amount equal to original culture volume. Absorbance of the reduced MTT was measured according to the manufacturer’s recommendations (Sigma Chemical Co., St. Louis, MO).
[3H]-thymidine incorporation assay for cell proliferation
Bovine pulmonary artery endothelial cells were grown up to 70% confluence in 35-mm dishes. Complete medium was removed from the culture dishes and the treatment solutions were added to the dishes. The treatment medium was then removed and 1 ml of [3H]-thymidine (1 µCi/ml) in MEM was added to each well and incubated for 24 h. After incubations, [3H]-thymidine was removed and cells were washed twice with ice-cold PBS. Cells were then washed twice with 5% trichloroacetic acid (TCA) in distilled water. Washings of 5% TCA were then removed and the cells were treated with 10.25 M NaOH (500 µl) for 30 min to solubilize the cells. The solubilized cell solution (400 µl) was transferred to the scintillation vials followed by the addition of 10 ml of scintillation cocktail, and then the [3H] radioactivity was counted in the Packard Tri-Carb 2900TR Liquid Scintillation Counter.
Fluorescence microscopy of actin stress fibers
Formation of actin stress fibers as an index of endothelial cytoskeletal reorganization was analyzed by fluorescence microscopy. BPAECs grown on sterile glass coverslips (90% confluence) were treated with different concentrations of glyoxal, AG, or glyoxal + AG following which they were rinsed twice with PBS and then fixed with 3.7% formaldehyde in PBS for 10 min at room temperature. The cells were then permeabilized with 0.5% Triton X-100 prepared in TBS containing 0.01% Tween-20 (TBST) for 2 min. The cells were then washed four times with PBS and treated with 1% BSA in TBST for 1 h. Actin stress fibers were visualized by staining the cells with rhodamine–phalloidin (1:50 dilution) in 1% BSA in TBST for 1 h. The cells were then rinsed four times with PBS to remove excess stain, stained with 1% DAPI in PBS for 5 min to visualize the nuclei following which they were washed four times with PBS, mounted, and then examined under Zeiss LSM 510 Confocal/Multiphoton Microscope at 543-nm excitation and 565-nm emission under 63× magnification. The images were captured digitally.
Immunofluorescence confocal microscopy of ZO-1 and AGEs
Formation ZO-1 tight junction protein reorganization and formation of AGEs were analyzed by immunofluorescence confocal microscopy. BPAECs grown on sterile glass coverslips (90% confluence) were treated with different concentrations of glyoxal, AG, or glyoxal + AG, then rinsed three times with PBS, and fixed with 3.7% formaldehyde in PBS for 10 min at room temperature. The cells were then rinsed with three times with PBS and permeabilized with 0.25% Triton X-100 prepared in TBS containing 0.01% Tween-20 (TBST) for 5 min. The cells were then washed three times with TBST and treated with TBST containing 1% BSA blocking buffer for 30 min at room temperature. The cells were then incubated for 1 h at room temperature with the primary antibody [anti-ZO-1 and anti-Amadori antibodies (1:200 dilution)] in 1% BSA solution in TBST. After rinsing three times with TBST, the cells were treated with AlexaFluor 568 (1:200 dilution) in 1% BSA in TBST for 1 h. For the visualization of nuclei, the cells were then stained with 1% DAPI in PBS for 5 min. Finally, the cells were washed three times with TBST, mounted, and examined under Zeiss LSM 510 Confocal/Multiphoton Microscope at 543-nm excitation and 600-nm emission under 63× magnification. The images were captured digitally.
Measurement of transendothelial cell electrical resistance
Bovine pulmonary artery endothelial cells were cultured in complete MEM on gold electrodes (Applied Biophysics Inc., Troy, NY) at 37°C in a humidified atmosphere of 5% CO2–95% air and grown to contact-inhibited monolayer with typical cobblestone morphology. TER of the BPAEC monolayer cultured on the gold electrodes was measured on an electric cell substrate impedance sensing system (ECIS; Applied Biophysics Inc., Troy, NY) following treatment of the cells with MEM containing the chosen concentrations of glyoxal, pharmacological agent (e.g., AG), or glyoxal + pharmacological agent in a humidified atmosphere of 5% CO2–95% air at 37°C. The total endothelial electrical resistance, as measured across the EC monolayer, was determined by the combined resistance between the basal and/or cell matrix adhesion. TER measurements were done in triplicate and expressed as normalized resistance for each of the treatments.
In vitro endothelial cell tube formation assay for angiogenesis
In vitro angiogenesis assay was performed using our established Matrigel tube formation assay [17]. BPAECs were grown up to 80% confluence in complete medium (10% FBS, non-essential amino acids, antibiotics, and growth factor) in two separate flasks. Both flasks of cells were starved in minimal media (MEM) at 37°C for 30 min following which AG in minimal medium was added at a final concentration of 5 mM to one of the flasks and continued to incubate for an additional 45 min. During this time, 120 µl of growth factor-reduced Matrigel (BD Discovery Labware, Bedford, MA) was coated in the wells of a 48-well culture plate and placed at 37°C for 1 h to polymerize. The cells from both flasks were then trypsinized, centrifuged, washed, resuspended in minimal medium, and counted. BPAECs (2 × 104) that were not pretreated with AG HCl were added to 1 ml of rich medium containing glyoxal at a final concentration of 1 mM and then immediately transferred to the Matrigel-coated plate [rich medium + glyoxal (1 mM)]. BPAECs (2 × 104) that were pre-treated with AG HCl were added to either 1 ml of minimal medium, or 1 ml of rich medium, or to 1 ml of rich medium supplemented with glyoxal (1 mM) or AG (5 mM) + glyoxal (1 mM). The cells were then allowed to incubate for 12 h at 37°C and subsequently analyzed for the formation of tubes connecting one colony to the next. At the termination of the experiment, the medium was removed and the cells were fixed with 4% paraformaldehyde. Four digital images were captured per well at the same locations in each well, and the tubes were counted in a blinded fashion. The data represent n = 6 for each condition. The statistical analysis was performed using an ANOVA with SYSTAT12 software (San Jose, CA).
Statistical analysis of data
Standard deviation (SD) for each data point was calculated from triplicate samples. Data were subjected to one-way analysis of variance, and pair wise multiple comparisons was done by Dunnett’s method with P < 0.05 indicating significance.
Results
Glyoxal inhibits MTT reduction by endothelial cells
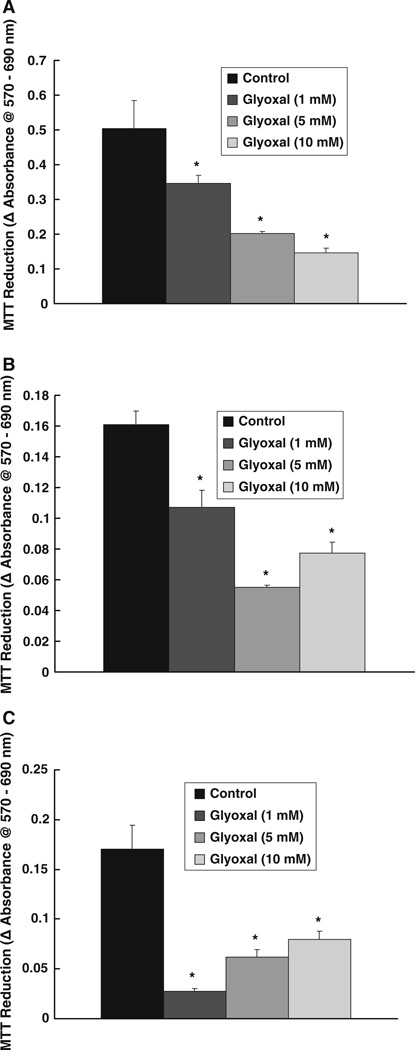
As glycoaldehydes form cross links with cellular macromolecules, we hypothesized that glyoxal would alter the mitochondrial activity and cause cytotoxicity in vascular ECs. Hence, we utilized the MTT assay, which is a widely accepted method to determine the mitochondrial activity of living cells by measuring mitochondrial dehydrogenase activity [18–20]. Glyoxal treatment of BPAECs caused a significant loss of MTT reduction in cells, indicating a decrease in cell growth and cell viability. Glyoxal caused a significant decrease in mitochondrial activity in a dose-dependent manner (1, 5, and 10 mM) in BPAECs as compared to the same in cells treated with vehicle alone at 4, 6, and 12 h of treatment (Fig. 2a–c). With the increase in glyoxal concentration, significantly greater decline in the mitochondrial activity at the shorter time points (4 and 6 h) (Fig. 2a, b) and less dramatic, but a significant decrease of the same at longer treatment time (12 h), were observed (Fig. 2c). These data showed that the glycoaldehyde, glyoxal, caused a striking loss of mitochondrial dehydrogenase activity leading to cytotoxicity in ECs.
Fig. 2.
Glyoxal inhibits MTT reduction by endothelial cells. BPAECs (90% confluent), grown in 17.5-mm dishes (24-well culture plate) were treated with MEM alone or MEM-containing glyoxal (1, 5, and 10 mM) for a 4 h, b 6 h, or c 12 h and incubated at 37°C in a humidified environment of 5% CO2–95% air. At the end of incubation, MTT reduction (as an index of cytotoxicity) was determined spectrophotometrically as described under Materials and methods section. Data represent means ± SD of three independent experiments. * Significantly different at P < 0.05 as compared to the cells treated with MEM alone
Aminoguanidine (AG) protects against glyoxal-induced inhibition of MTT reduction by endothelial cells
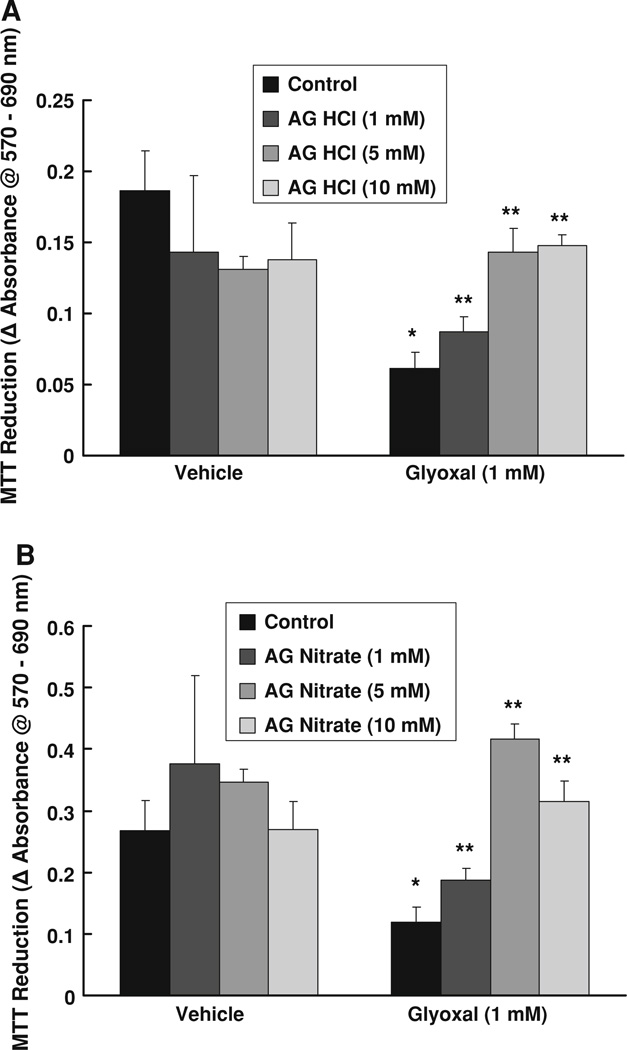
Aminoguanidine has been reported to offer protection against the AGE-induced adverse conditions [21]. Throughout this study, we chose two different widely used salt forms of AG (AG HCl and AG nitrate) to compare the efficacy of both anionic forms of AG in offering protection against the glyoxal-induced adverse cellular effects. Accordingly, here, we investigated whether AG (both AG HCl and AG nitrate) would attenuate glyoxal-induced decrease of mitochondrial activity (MTT reduction) in ECs. BPAECs were pretreated for 1 h with MEM alone or MEM-containing AG HCl or AG nitrate (1, 5, and 10 mM) following which they were cotreated with glyoxal (1 mM) for 6 h. Glyoxal alone caused a significant decrease in the mitochondrial activity, indicating a decrease in cell growth and viability. Both AG HCl and AG nitrate significantly and effectively inhibited the glyoxal-induced suppression of MTT reduction in BPAECs (Fig. 3a, b). These results revealed that AG was effective in offering significant protection against the glyoxal-induced inhibition of mitochondrial function and cytotoxicity in vascular ECs.
Fig. 3.
Aminoguanidine (AG) protects against glyoxal-induced inhibition of MTT reduction by endothelial cells. BPAECs (90% confluent), grown in 17.5-mm dishes (24-well culture plate) were pretreated with MEM alone or MEM-containing a AG HCl or b AG nitrate (1, 5, and 10 mM) for 1 h, following which the cells were treated with MEM alone, MEM-containing AG HCl or AG nitrate (1, 5, 10 mM), MEM-containing glyoxal (1 mM), or MEM-containing AG HCl or AG nitrate (1, 5, and 10 mM) + glyoxal (1 mM) for 6 h and incubated at 37°C in a humidified environment of 5% CO2–95% air. At the end of incubation, MTT reduction (as an index of cytotoxicity) was determined spectrophotometrically as described under Materials and methods section. Data represent means ± SD of three independent experiments. * Significantly different at P < 0.05 as compared to the cells treated with MEM alone. ** Significantly different at P < 0.05 as compared to the control cells treated with MEM-containing glyoxal
Glyoxal induced LDH release by endothelial cells
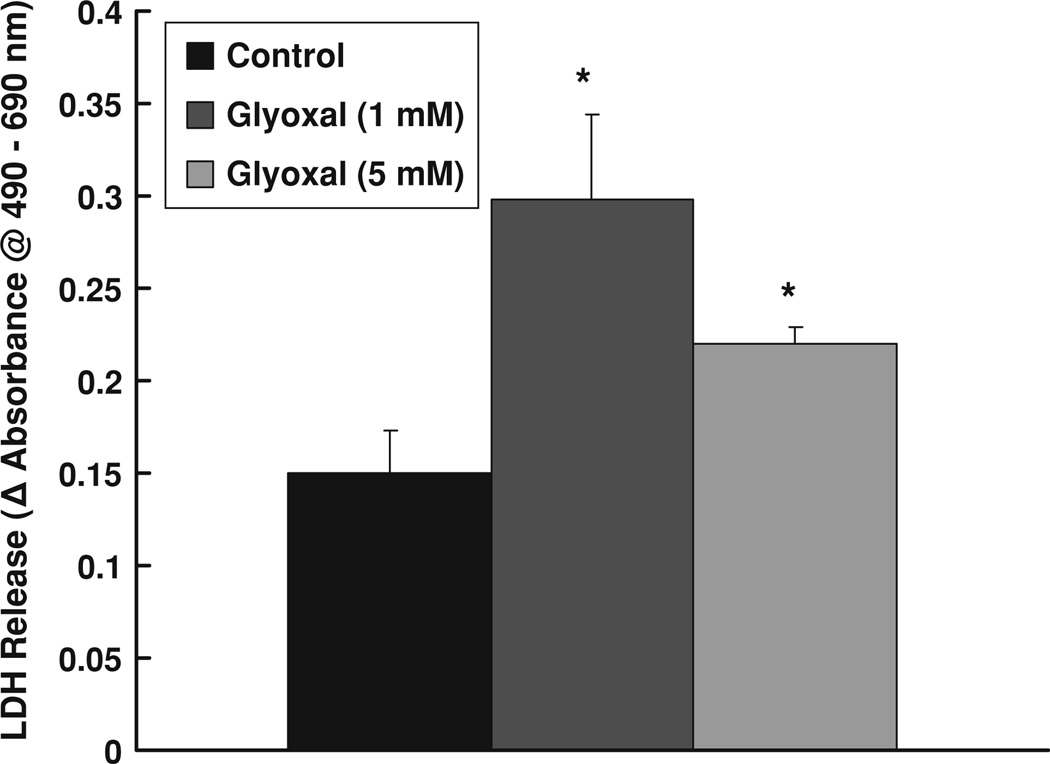
As our above results showed that glyoxal caused significant loss of mitochondrial function, we further investigated the membrane damage and macromolecule leak from the ECs as an index of cytotoxicity. Release (leak) of cytoplasmic LDH is widely utilized to confirm cytotoxicity. Therefore, we investigated the glyoxal-induced LDH release from BPAECs as an index of cytotoxicity. Glyoxal at a dose of 1.0 mM caused a significant increase in LDH release from cells at 12 h of treatment as compared to the same in the control MEM-treated cells under identical conditions (Fig. 4). Upon increasing the concentration of glyoxal up to 5 mM, although resulted in a significant increase in LDH release from BPAECs as compared to the same in the control cells, the same was slightly lower than that was observed in cells treated with 1.0 mM glyoxal (Fig. 4). These results confirmed that glyoxal significantly increased LDH release from BPAECs and hence the cytotoxicity in ECs.
Fig. 4.
Glyoxal induced LDH release by endothelial cells. BPAECs (90% confluent), 17.5-mm dishes (24-well culture plate) were treated with MEM alone or MEM-containing glyoxal (1 and 5 mM) for 12 h at 37°C in a humidified environment of 5% CO2–95% air. At the end of incubation, release of LDH into the medium (as an index of cytotoxicity) was determined spectrophotometrically as described under Materials and methods section. Data represent means ± SD of three independent experiments. * Significantly different at P < 0.05 as compared to the cells treated with MEM alone
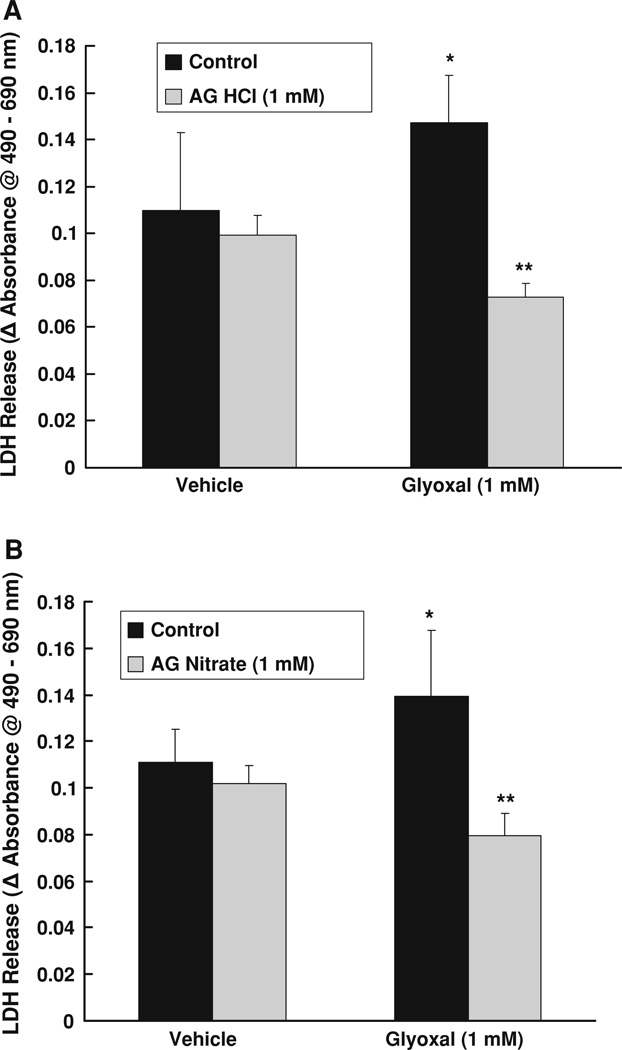
Aminoguanidine (AG) protects against glyoxal-induced LDH release by endothelial cells
In the current study, our earlier experiments revealed that AG effectively attenuated the glyoxal-induced decrease of MTT reduction in BPAECs. Therefore, here, we investigated whether AG would attenuate the glyoxal-induced LDH release (cytotoxicity) in BPAECs. Glyoxal (1.0 mM) caused a significant increase in LDH release after 12 h of exposure of cells as compared to the same in the control cells treated with MEM alone. Treatment of BPAECs with both AG HCl and AG nitrate (1.0 mM) significantly and completely attenuated the glyoxal-induced LDH release from BPAECs (Fig. 5a, b). These results established that AG protected against the glyoxal-induced cytotoxicity as evidenced from the attenuation of the glyoxal-induced LDH release from ECs.
Fig. 5.
Aminoguanidine (AG) protects against glyoxal-induced LDH release by endothelial cells. BPAECs (90% confluent), 17.5-mm dishes (24-well culture plate) were pre-treated with MEM alone or MEM-containing a AG HCl or b AG nitrate (1 mM) for 1 h, following which the cells were treated with MEM alone, MEM-containing AG HCl or AG nitrate (1 mM), MEM-containing glyoxal (1 mM), or MEM-containing AG HCl or AG nitrate (1 mM) + glyoxal (1 mM) for 12 h at 37°C in a humidified environment of 5% CO2–95% air. At the end of incubation, release of LDH into the medium (as an index of cytotoxicity) was determined spectrophotometrically as described under Materials and methods section. Data represent means ± SD of three independent experiments. * Significantly different at P < 0.05 as compared to the cells treated with MEM alone. ** Significantly different at P < 0.05 as compared to the control cells treated with MEM-containing glyoxal
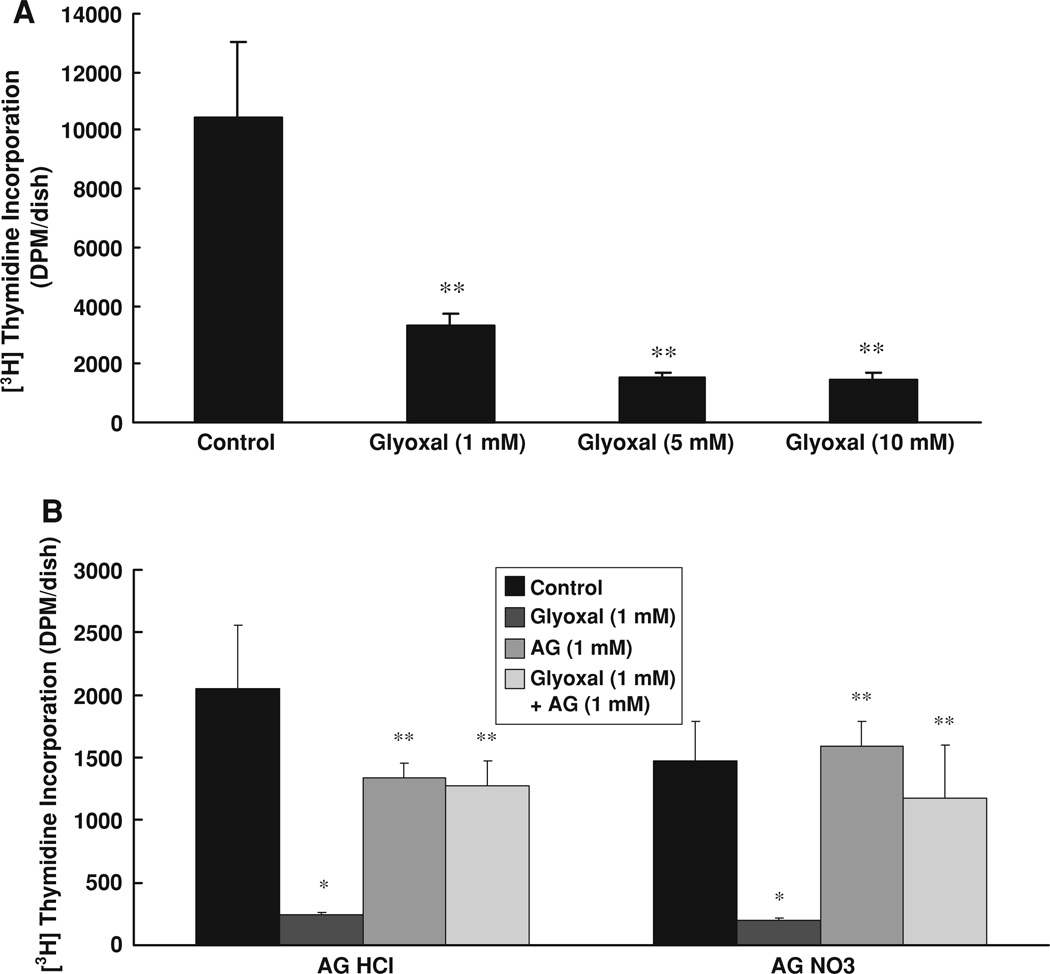
Aminoguanidine (AG) protects against glyoxal-induced inhibition of [3H]-thymidine incorporation by endothelial cells
The results of the above experiments in the present study clearly revealed that glyoxal caused cytotoxicity in BPAECs, which was effectively protected by AG. Therefore, here, we investigated whether glyoxal would cause loss of cellular replication and AG treatment would rescue the ECs from such insult. In order to investigate that, we adopted the [3H]-thymidine incorporation by BPAECs to investigate whether glyoxal would alter their ability to replicate and AG would protect the cells against such adverse effect. The results revealed that BPAECs treated with glyoxal (1, 5, and 10 mM) for 6 h exhibited a significant decline in the cellular DNA synthesis (Fig. 6a) indicating that glyoxal, in a dose-response fashion, drastically inhibited EC replication. However, treatment of BPAECs with both AG HCl and AG nitrate (1.0 mM) significantly restored the ability of cells to replicate under glyoxal (1.0 mM) treatment (Fig. 6b), revealing that AG offered protection against the glyoxal-induced inhibition of cellular DNA synthesis and replication in BPAECs.
Fig. 6.
Aminoguanidine (AG) protects against glyoxal-induced inhibition of [3H]-thymidine incorporation by endothelial cells. BPAECs (90% confluent), grown in 35-mm dishes (6-well culture plate) were treated for 12 h with MEM alone or MEM-containing a glyoxal (1, 5, and 10 mM) or b cells were pre-treated with AG HCl (1 mM) or AG nitrate (1 mM) for 1 h and then treated with MEM alone, MEM-containing AG HCl or AG nitrate (1 mM), MEM-containing glyoxal (1 mM), or MEM-containing AG HCl or AG nitrate (1 mM) + glyoxal (1 mM) for 12 h at 37°C in a humidified environment of 5% CO2–95% air. At the end of incubation, cells were labeled with [3H]-thymidine (1 µCi/ml) in complete EC medium for 24 h. Cell replication at the end of the treatment was assayed by determining [3H]-thymidine incorporation into the cells as outlined in the Materials and methods section. Data represent means ± SD of three independent experiments. * Significantly different at P < 0.05 as compared to the cells treated with MEM alone. ** Significantly different at P < 0.05 as compared to the control cells treated with MEM-containing glyoxal
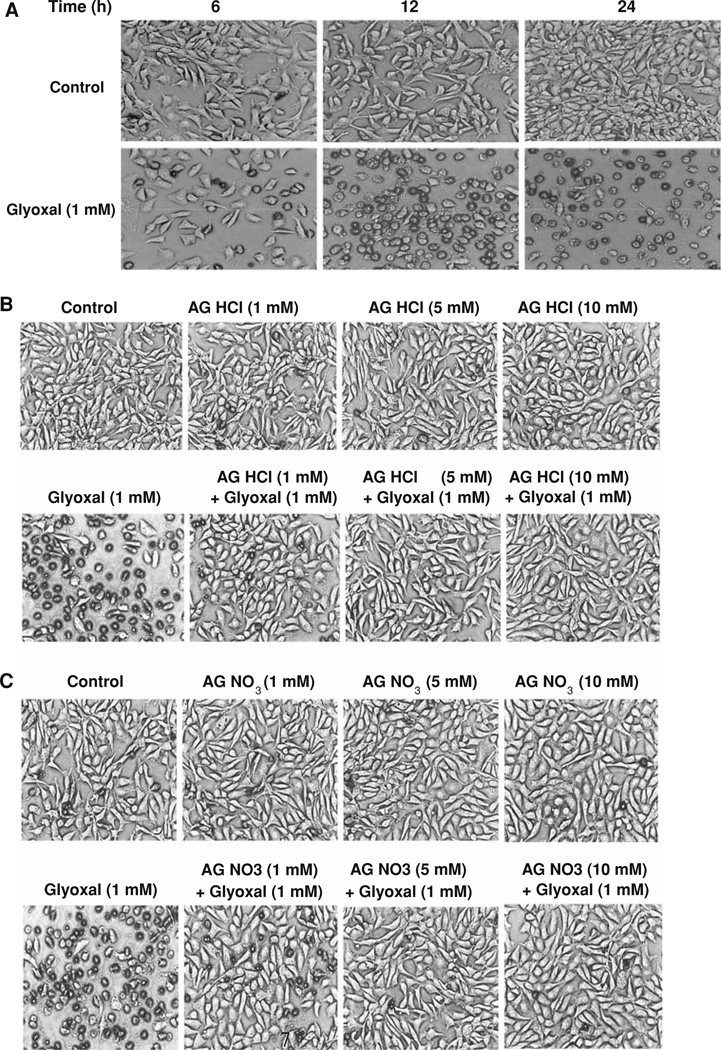
Aminoguanidine (AG) protects against glyoxal-induced alterations in cell morphology
Experiments conducted in the present study so far clearly revealed that glyoxal caused loss of mitochondrial function, cytotoxicity (LDH release), and loss of ability to replicate in ECs and all of these were effectively protected by AG. As the alterations in cell morphology are widely used as index of cytotoxicity, here, we investigated whether glyoxal would alter the cell morphology as an index of cellular structural alterations and cytotoxicity and if AG would protect against such alterations in ECs. Phase contrast microscopic images revealed that BPAECs treated with glyoxal (1.0 mM) exhibited the loss of cell morphology in a time-dependent manner (Fig. 7a). After 6 h of treatment of cells with glyoxal, the loss of cell morphology and inhibition of cell growth were evident, and more striking changes in cell morphology were noticed as the time of treatment with glyoxal progressed up to 12 and 24 h. In addition, treatment of BPAECs with AG HCl and AG nitrate (5 mM) protected against the glyoxal-induced loss of cell morphology (Fig. 7b, c). These results established that glyoxal caused complete loss of cell morphology and AG offered protection against such adverse effect in BPAECs.
Fig. 7.
Aminoguanidine (AG) protects against glyoxal-induced alterations in cell morphology. BPAECs (90% confluent), grown in 35-mm dishes (6-well culture plate) were treated with MEM alone or MEM-containing a glyoxal 1 mM for 6, 12, and 24 h. Cells were then examined after pre-treatment with MEM alone or MEM-containing b AG HCl or c AG nitrate (1, 5, and 10 mM) for 1 h then treated with MEM alone, MEM-containing AG HCl or AG nitrate (1, 5, and 10 mM), MEM-containing glyoxal (1 mM), or MEM-containing AG HCl or AG nitrate (1, 5, and 10 mM) + glyoxal (1 mM) for 12 h at 37°C in a humidified environment of 5% CO2–95% air. At the end of the incubation period, cells were examined under light microscope at a magnification of 20× as described under Materials and methods section. Each micrograph is a representative picture obtained from three independent experiments conducted under identical conditions
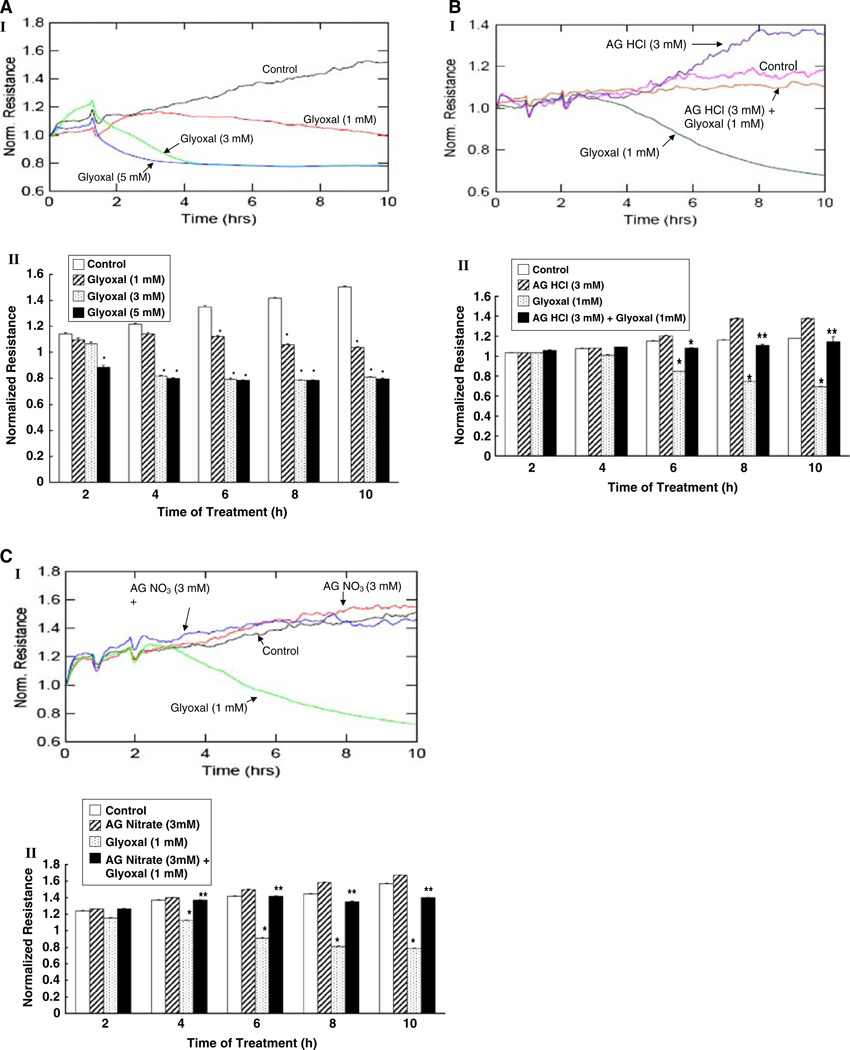
Aminoguanidine (AG) protects against glyoxal-induced endothelial cell barrier dysfunction
Earlier experiments of this study clearly established that glyoxal caused the complete loss of cell morphology which was totally rescued by AG treatment in BPAECs. This led us to hypothesize that the glyoxal-induced loss of cell morphology would lead to or indicative of the glyoxal-induced EC barrier dysfunction. As the EC barrier dysfunction reflects in the decrease in trans-endothelial electrical resistance (TER) [22], here, we determined the glyoxal-induced decrease in TER and the protective effects of AG against such alterations in BPAEC monolayers on gold electrodes with the aid of Electric Cell Substrate Impedance Sensing (ECIS). Glyoxal (1–5 mM) induced a dose- and time-dependent decrease in TER in BPAEC monolayer (Fig. 8a–I). At 2 h of treatment of cells, glyoxal, only at the highest tested dose (5 mM), caused a significant decrease in TER. At 4 h of treatment of BPAEC monolayer, glyoxal at both 3 and 5 mM doses, induced a significant drop in TER as compared to the TER in control MEM-treated EC monolayer. However, beginning with 6 h of treatment of cells with glyoxal (1–5 mM), a significant dose- and time-dependent (up to 10 h) decrease in TER was evident as compared to the same in the control untreated BPAEC monolayer (Fig. 8a–II).
Fig. 8.
Aminoguanidine (AG) protects against glyoxal-induced endothelial cell barrier dysfunction. BPAEC monolayers were cultured in complete MEM on gold electrodes and exposed to a MEM alone or MEM-containing glyoxal (1–5 mM), b MEM alone, MEM-containing AG HCl (3 mM), glyoxal (1 mM), or AG HCl (3 mM) + glyoxal (1 mM), and c MEM alone, MEM-containing AG nitrate (3 mM), glyoxal (1 mM), or AG nitrate (3 mM) + glyoxal (1 mM) for 10 h in a humidified atmosphere of 5% CO2–95% air at 37°C and the TER was measured continuously in an ECIS system as described under Materials and methods section. Inset I is a representative tracing of the normalized resistance values of three independent experiments conducted under identical conditions. Inset II is the graphical representation of the average normalized resistance values obtained from three independent determinations at specified time points. Values are represented as means ± SD from three independent determinations. * P < 0.05 versus MEM-treated control cells. ** P < 0.05 versus pharmacological agent-treated cells
Having observed that glyoxal induced the loss of TER in BPAEC monolayer, we investigated whether certain established anti-AGE protective compounds including d-penicillamine, pyridoxamine, AG HCl, and AG nitrate would offer protection against the glyoxal (1 mM)-induced decline in TER in BPAEC monolayer. d-penicillamine (1 mM) exhibited slight but significant attenuation of the glyoxal-induced decrease in TER and upon increasing the concentration of d-penicillamine from 1 to 5 mM did not appear to enhance the extent of attenuation of the glyoxal-induced decrease in TER in BPAEC monolayer (data not shown).
We also investigated whether pyridoxamine, another pharmacological agent reported to protect against the AGE-induced adverse effects, would attenuate the glyoxal-induced decrease of TER in BPAEC monolayer. Pyridoxamine (3 mM) significantly attenuated the glyoxal (1.0 mM)-induced decline in TER in BPAEC monolayer at 6, 8, and 10 h of treatment (data not shown).
Earlier experiments of the present study revealed that both AG HCl and AG nitrate were effective in protecting against the glyoxal-induced cytotoxicity and cell morphological alterations. Therefore, here, we investigated whether AG in the form of HCl and nitrate would protect against the glyoxal-induced decrease in TER in BPAECs. As shown in Fig. 8b and c, both AG HCl and nitrate (3 mM) significantly and almost completely attenuated the glyoxal-induced loss of TER in BPAEC monolayer. These results established that AG protected against the glyoxal-induced EC barrier dysfunction as determined by the decline in TER in EC monolayer.
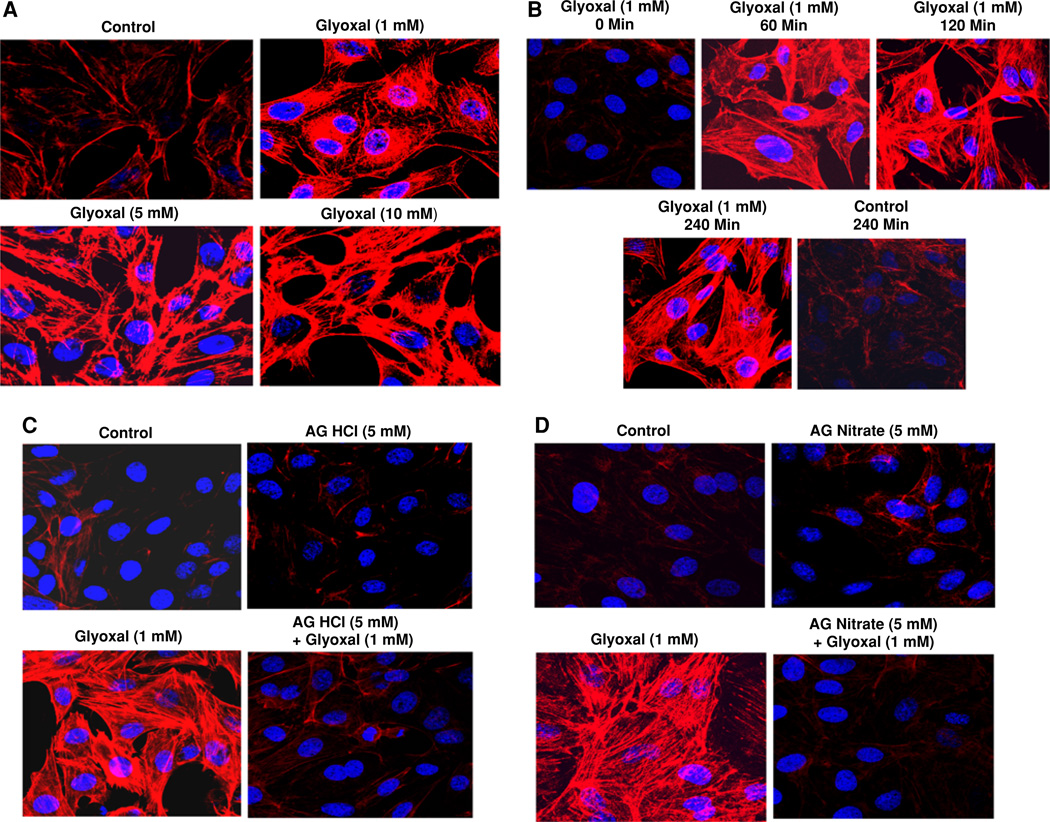
Aminoguanidine (AG) protects against glyoxal-induced actin cytoskeletal reorganization in endothelial cells
Cytoskeleton plays a central role in the regulation of cell size and shape and motility [23]. Actin cytoskeletal reorganization in ECs under stress is known to contribute to the stress-induced loss of TER and barrier dysfunction in vascular EC monolayers [24]. Actin stress fiber formation in ECs during stress is a commonly recognized cytoskeletal response indicating the actin cytoskeletal reorganization [25]. Hence, we examined whether glyoxal would cause the actin cytoskeletal reorganization (stress fiber formation) and if AG would protect against such EC response. Glyoxal induced the formation of actin stress fibers in BPAECs in a dose-dependent (1–10 mM) manner at 4 h of treatment of cells (Fig. 9a). The formation of actin stress fibers was intense with the increase in time of exposure (0–240 min) of BPAECs to glyoxal (1 mM) (Fig. 9b). Furthermore, treatment of cells with both AG HCl and nitrate (5 mM) completely attenuated the glyoxal (1 mM)-induced actin stress fibers formation in BPAECs at 4 h of treatment of cells (Fig. 9c, d). Overall, these results established that glyoxal induced the actin cytoskeletal reorganization and AG protected against such cytoskeletal response in ECs.
Fig. 9.
Aminoguanidine (AG) protects against glyoxal-induced actin cytoskeletal reorganization in endothelial cells. BPAECs (90% confluent), grown in 35-mm dishes (6-well culture plate) on glass coverslips were treated with MEM alone or MEM-containing a glyoxal (1, 5, and 10 mM) for 4 h or MEM-containing b glyoxal (1 mM) for 1, 2, and 4 h. Cells were then examined after pretreatment with MEM alone or MEM-containing c AG HCl or d AG nitrate (5 mM) for 1 h then treated with MEM alone, MEM-containing AG HCl or AG nitrate (5 mM), MEM-containing glyoxal (1 mM), or MEM-containing AG HCl or AG nitrate (5 mM) + glyoxal for 4 h at 37°C in a humidified environment of 5% CO2–95% air. At the end of the incubation period, cells were stained as described under Materials and methods section and examined under confocal fluorescence microscope at a magnification of 63×. Each digitally captured image is a representative picture obtained from three independent experiments conducted under identical conditions
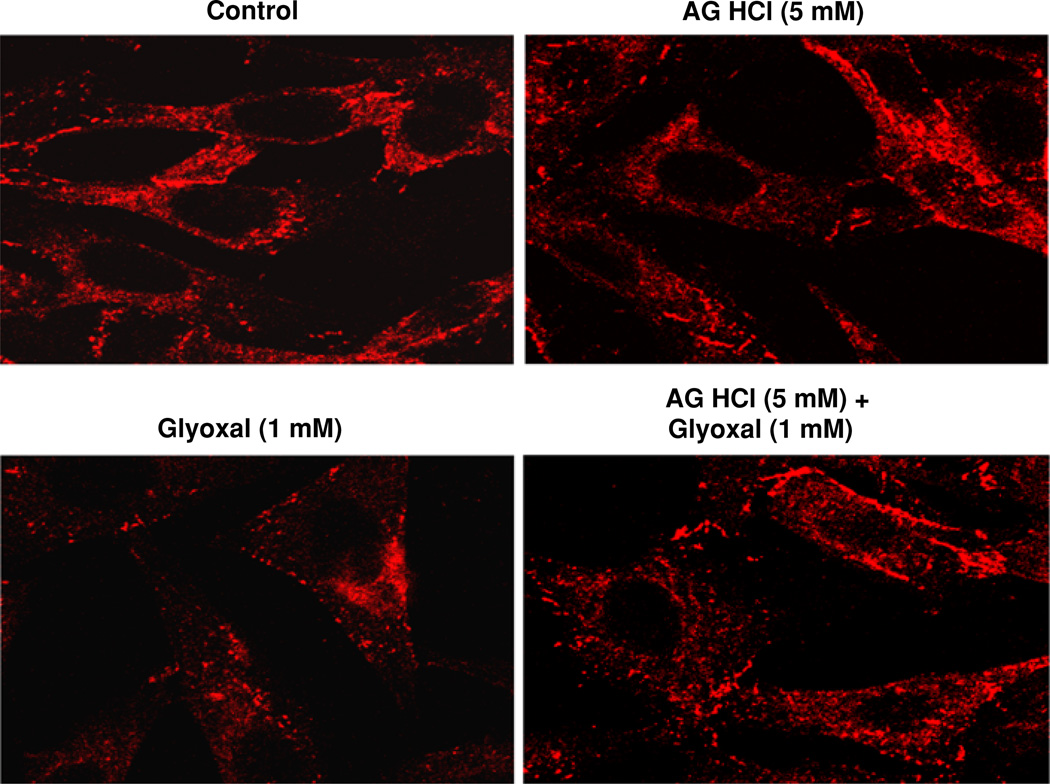
Aminoguanidine (AG) protects against glyoxal-induced tight junction alterations in endothelial cells
Tight junctions are highly crucial for the cell-to-cell contact and maintenance of tight barrier in EC monolayer [26]. One of the important tight junction proteins, ZO-1, is a key player that contributes to the maintenance of cellular tight junctions [27]. Stress is known to alter the EC tight junctions through the alterations in ZO-1 protein [28]. Therefore, here, we investigated whether glyoxal would alter the ZO-1 protein organization and if AG would attenuate such alteration. As shown in Fig. 10, glyoxal (1.0 mM) caused marked disappearance of regular localization of ZO-1 around the cell periphery as seen in the control untreated BPAECs at 4 h of treatment. Also, treatment of cells with AG HCl (5 mM) restored the glyoxal-induced disturbance of ZO-1 in BPAECs at 4 h of exposure. These results demonstrated that glyoxal induced the disappearance of ZO-1 protein causing the alteration in tight junctions ECs, which were protected by AG.
Fig. 10.
Aminoguanidine (AG) protects against glyoxal-induced tight junction alterations in endothelial cells. BPAECs (90% confluent), grown in 35-mm dishes (6-well culture plate) on glass coverslips were pre-treated with MEM alone or MEM-containing AG HCl (5 mM) for 1 h. Cells were then treated with MEM alone, MEM-containing AG HCl (5 mM), MEM-containing glyoxal (1 mM), or MEM-containing AG HCl (5 mM) + glyoxal (1 mM) and incubated at 37°C for 4 h in a humidified environment of 5% CO2–95% air. At the end of the incubation period, cells were stained as described under Materials and methods section and examined under confocal fluorescence microscope at a magnification of 63×. Each digitally captured image is a representative picture obtained from three independent experiments conducted under identical conditions
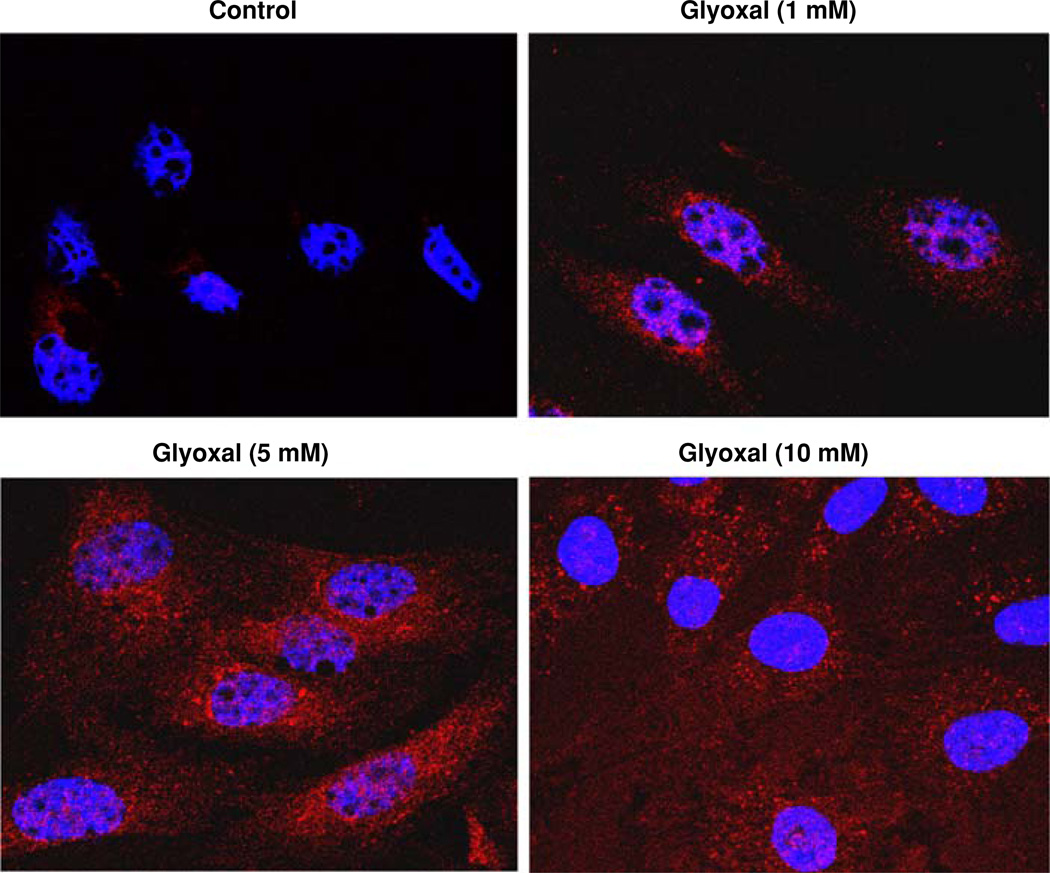
Glyoxal-mediated in vitro formation of AGEs in endothelial cells
Glycoaldehydes such as glyoxal react with different amino acid residues of intact cellular protein targets resulting in the formation of AGEs (including the Amadori Products) which are identified as important players in causing adverse cellular functions [29]. Therefore, we envisioned that treatment of ECs with glyoxal would cause the in situ formation of AGEs and investigated this with the aid of anti-Amadori antibody immunohistochemical staining and confocal microscopy. BPAECs treated with glyoxal (1, 5, and 10 mM) for 4 h exhibited a dose-dependent formation of AGEs (Amadori Products) in cells (Fig. 11). These results clearly revealed the intracellular formation of AGEs in ECs upon treatment with glyoxal.
Fig. 11.
Glyoxal-mediated in vitro formation of AGEs in endothelial cells. BPAECs (90% confluent), grown in 35-mm dishes (6-well culture plate) on glass coverslips were pretreated with MEM alone or MEM-containing glyoxal (1, 5, 10 mM) for 4 h and incubated at 37°C in a humidified environment of 5% CO2–95% air. At the end of the incubation period, cells were stained as described under Materials and methods section and examined under confocal fluorescence microscope at a magnification of 63×. Each digitally captured image is a representative picture obtained from three independent experiments conducted under identical conditions
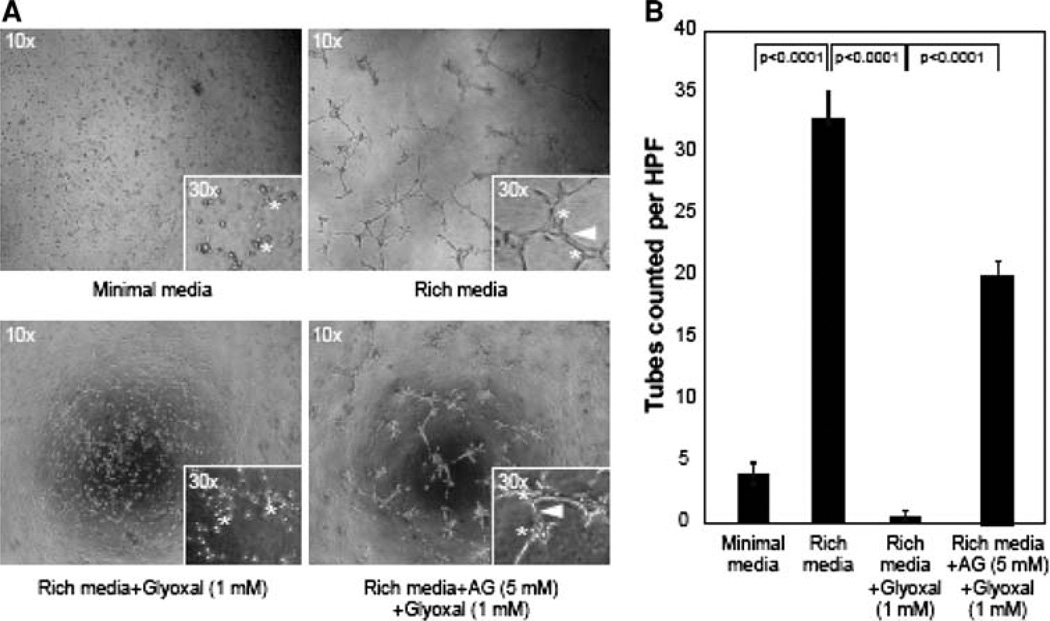
Aminoguanidine (AG) protects against glyoxal-induced inhibition of in vitro angiogenesis in endothelial cells
Endothelial cells play an essential role in the formation and growth of new blood vessels from pre-existing vessels is known as angiogenesis. This process is important during growth and development, as well as wound healing, which is impaired during diabetes. As our earlier experiments of the current study demonstrated that glyoxal caused cytotoxicity, loss of cell morphology, decrease in TER, barrier dysfunction, and cytoskeletal reorganization, we hypothesized that glyoxal would also inhibit the angiogenesis. Therefore, we examined whether glyoxal would inhibit angiogenesis in vitro (tube formation on Matrigel) and AG would protect against such responses. The results showed that glyoxal (1 mM) almost completely inhibited tube formation in BPAECs on Matrigel (Fig. 12). Furthermore, treatment of cells with AG HCl offered significant protection against the glyoxal-induced inhibition of tube formation in BPAECs (Fig. 12). These results established that glyoxal inhibited angiogenesis in vitro in BPAECs and AG effectively rescued the cells from the glyoxal-induced inhibition of angiogenesis.
Fig. 12.
Aminoguanidine (AG) protects against glyoxal-induced inhibition of in vitro angiogenesis in endothelial cells. BPAECs were grown up to 80% confluence in complete medium in two separate flasks. Both flasks of cells were starved in minimal media (MEM) at 37°C for 30 min and following that AG HCl (5 mM) in MEM was added to one of the flask and continued to incubate for an additional 45 min. The cells from both flasks were then added to the Matrigel-**coated plate [Rich media + Glyoxal (1 mM)] as described in the Materials and methods section. a BPAECs that were pre-treated with AG HCl were added to either 1 ml of minimal media (Minimal media), 1 ml of rich media (Rich media), or to 1 ml of rich media supplemented with Glyoxal (1 mM) [Rich media + AG (5 mM) + Glyoxal (1 mM)]. The cells were allowed to incubate for 12 h at 37°C and subsequently analyzed for the development of tubes connecting one colony to the next. The media was removed and the cells were fixed with 4% paraformaldehyde. b Four digital images were captured per well at the same locations in each well and the tubes were counted in a blinded fashion. The data represent n = 6 for each condition. The statistical analysis was performed using an ANOVA with SYSTAT12 software (San Jose, CA)
Discussion
The current study revealed that the glucose-derived oxoaldehyde (carbonyl), glyoxal, caused cytotoxicity, alterations in cell morphology, mitochondrial dysfunction, cytoskeletal rearrangement, barrier dysfunction, inhibition of DNA synthesis and cell replication, and inhibition of angiogenesis in vascular ECs. The findings of this study also demonstrated that the known blocker of AGEs [10], AG, attenuated the adverse effects caused by glyoxal, a known AGE precursor in ECs [30].
The increasing incidence of Type 1 and Type 2 diabetes has had an extreme impact on morbidity and mortality around the world. Both types of diabetes lead to hyperglycemia because the body loses the ability to properly regulate blood glucose [31]. Hyperglycemia has been linked to microvascular diseases such as nephropathy, retinopathy, and neuropathy as well as macrovascular diseases such as coronary heart disease, stroke, and peripheral artery disease [32]. The major cause of endothelial dysfunction during diabetes is hyperglycemia [33]. During diabetes, high blood glucose levels lead to the formation of elevated concentrations of α-oxoaldehydes in circulation [34]. These highly reactive carbonyls bind to proteins, nucleic acids, and other molecules in the body, disrupting their structure and function, and leading to a variety of complications including vascular disorders, atherosclerosis, hypertension, and Alzheimer’s disease [9]. The glucose-derived aldehydes eventually undergo a series of chemical rearrangements to form more stable and essentially irreversible AGEs [35]. AGEs have been linked to a vast number of complications during diabetes, one of the major concerns being its role in the pathogenesis of cardiovascular disease. Cross linking by AGEs leads to vascular stiffening and endothelial dysfunction [35, 36]. The mechanism of how these sugar-derived α-oxoaldehydes cause vascular EC dysfunction is not yet thoroughly understood [37]. Therefore, in this study, we focused on the alterations in the cultured vascular ECs, caused primarily by a glucose-derived reactive α-oxoaldehyde, glyoxal, that is formed during hyperglycemia.
Diabetes-associated complications have been shown to be associated with glycation of proteins by glucose [7]. Such glycation of proteins leads to the formation of Schiff’s base and fructosamine adducts through a reaction of glucose with the N-terminal and lysyl side chain amino groups of proteins [7] and has been shown to play a role in the onset of diabetic nephropathy, neuropathy, retinopathy, and vascular diseases [7]. Experimental evidences suggest that α-oxoaldehydes such as glyoxal, methylglyoxal, and 3-deoxyglucosone may also arise from proteins while undergoing glucose-mediated glycation [7].
Glyoxal has been shown to react with arginine to form the imidazolium and with lysine to form N-(carboxymethyl) lysine (CML), an AGE adduct [37]. It has been estimated that nearly 40–50% of the AGEs [Nε-(carboxymethyl) lysine] are formed from a Schiff’s base adduct originating from glyoxal [38]. By nature, glyoxal is a dicarbonyl and it undergoes reaction with two lysine residues in the proteins giving rise to dimers of glyoxal and lysine known as the cross links of protein glycinamide [39, 40]. Glyoxal has been shown to form adducts with the nucleic acids (DNA and RNA) leading to mutations, and α-oxoaldehydes-mediated glycation has been implicated in diabetic vascular damage [37, 41]. Methylglyoxal, another species of oxoaldehyde, is reported to cause protein modifications under diabetes including the alterations of mitochondrial proteins in the diabetic kidney [42, 43]. Glyoxal has been documented to cause cytotoxicity, especially to hepatocytes through GSH depletion, mitochondrial dysfunction, and oxidative stress [37]. These reports reinforce our current findings that the glucose-derived aldehyde, glyoxal, caused cytotoxicity, altered cell morphology, loss of mitochondrial function, and inhibition of DNA and cell replication in BPAECs, probably through different mechanisms such as protein and DNA adduct formation, suppressed NADPH generation, mitochondrial dysfunction, and oxidative stress.
Vasculopathy during diabetes is known to play a causative role in several cardiovascular diseases [44]. AGEs have been linked with the development of diabetic vascular injury including an array of both macrovascular and microvascular complications involving the formation of AGE-mediated cross links in the basement membrane of the extracellular matrix through the receptor for AGE (RAGE) [45, 46]. Overall, diabetic vascular injury due to elevated glucose levels is attributed to the glycative, glyoxidative, carbonyl, and oxidative stresses [45, 47]. Rats receiving methylglyoxal have exhibited diabetes-like microvascular alterations and damage [48]. In patients with Type 2 diabetes, elevated levels of AGEs in serum, in association with endothelial dysfunction, have been observed [49]. Hyperglycemia has been shown to induce the formation of elevated levels of intracellular AGEs in the cultured ECs that is dependent on ROS [50]. AGEs exert their effects on vascular ECs by interacting with the RAGE and a lactoferrin-like polypeptide [51]. AGEs thereby mediate oxidative stress (lipid peroxidation) in the vascular ECs by interacting with the RAGE leading to altered gene expression and vascular abnormalities [52, 53].
The underlying mechanism of diabetes-induced vascular endothelial dysfunction behind the diabetic vasculopathy is not thoroughly understood [54, 55]. Increase in endothelial permeability to macromolecules and cells (hyperpermeability) leading to vascular dysfunction and blood vessel leak is known to be associated with several pathological states including diabetes [56]. RAGE has emerged as an important player in the diabetes-associated microvascular and macrovascular complications [57]. AGE, through interaction with RAGE, has been shown to cause in vitro EC permeability increase and diabetic vascular hyperpermeability wherein oxidant-mediated mechanism plays a key role [56]. The oxoaldehyde, glyoxal, used in the current study apparently formed adducts with macromolecules such as proteins in BPAECs similar to that of AGEs formed during diabetes or hyperglycemia. This is also supported by our current finding in this study that glyoxal treatment of BPAECs led to the intracellular formation of Amadori products as revealed by the confocal immunofluorescence microscopy (Fig. 11). Thus, the observed adverse effects caused by glyoxal in BPAECs in the current study might be due to the reactivity of glyoxal with EC proteins and/or formation of AGE-like adducts.
The sugar-derived carbonyls do contribute to the protein cross linking [58], thus bringing changes in the protein structure and function. AGE-modified human serum albumin (HSA) has been shown to induce actin cytoskeletal reorganization and hyperpermeability in ECs [59, 60]. However, in the current study, we determined that the well-defined glucose-derived α-oxoaldehyde, glyoxal alone, was effective in inducing the cytoskeletal- and permeability-altering effects in ECs. Our current results were similar to those induced by treating ECs with the preformed AGEs or adducts of HSA-glucose oxidation products as shown earlier by Xiao-Hua et al. [59, 60]. Both glucose-derived oxoaldehydes and AGE-modified proteins do cause EC cytoskeletal rearrangement and hyperpermeability, probably sharing the common or different signaling pathways that need to be determined. Albumin-derived AGEs have been shown to disrupt the vascular EC junctions such as the cadherins and catenins which are associated with the increased EC permeability [61]. In the current study, we demonstrated that glyoxal induced the reorganization of the tight junction protein, ZO-1, in BPAECs suggesting that the glucose-derived α-oxoaldehyde was effective in altering EC tight junctions leading to the EC barrier dysfunction and hyperpermeability as revealed by the increase in TER in BPAEC monolayers.
Healthy status of the EC is highly central for angiogenesis and new vessel formation. Our results revealed that glyoxal inhibited in vitro angiogenesis (tube formation) from BPAECs in culture. The observed findings of the current study that glyoxal caused cytotoxicity, altered cell morphology, mitochondrial dysfunction, inhibition of cell replication, and cytoskeletal alterations clearly supported that such alterations probably were not favorable for the glyoxal-treated BPAECs to engage in angiogenesis. Glycated basic fibroblast growth factor (FGF-2), similar to AGEs found in hyperglycemia, has been shown to inhibit mitogenesis and capillary tube formation in the aortic ECs through the alterations of signal transduction pathways involving protein tyrosine kinases (PTyKs), mitogen-activated protein kinases (MAPKs), and tyrosine kinase receptors [62]. Also, oxoaldehydes have been shown to activate specific signaling pathways in vascular ECs involving several key kinases such as PTyKs, p38 MAPK, extracellular signal-regulated kinase, and JNK [63]. Along those lines, the role of PTyK and MAPK signaling pathways could be inferred in the glyoxal-induced inhibition of angiogenesis by BPAECs in the current study.
Glycation during hyperglycemic conditions and oxidative stress-mediated lipid peroxidation contribute to the formation of reactive carbonyl species (RCS) and induction of carbonyl stress [64]. During hyperglycemia, oxidation of glucose and lipid peroxidation have been shown to generate glyoxal that forms DNA adducts, such as the glyoxalated deoxycytidine and deoxyguanosine [41, 65, 66]. Methylglyoxal-modified fibronectin has been demonstrated to alter the retinal capillary cell viability [67]. These studies have established that glyoxals induce adduct formation and damage in cellular DNA and this could be the basis for the glyoxal-induced loss of cell replication and loss of [3H]thymidine incorporation (cell proliferation) in BPAECs as observed in the current study. Glyoxal cytotoxicity to various cellular systems is documented but the underlying molecular mechanisms are yet to be unraveled [37]. Glyoxal cytotoxicity to hepatocytes has been linked with the GSH depletion, oxidative stress, and mitochondrial damage [37]. In another study while delineating the mechanism(s) of cytotoxicity in hepatocytes, glyoxal, up to a concentration of 10 mM has been attributed to oxidative stress caused by glyoxal [3]. Taking these studies into consideration, in the current study, oxidative stress could be considered as a possible player in mediating the cytotoxicity of glyoxal in BPAECs.
Glyoxal, up to 0.4 mM, has been demonstrated to cause apoptosis in the human embryonic lung epithelial cells [68]. In immortalized E1A-NR3 retinal cells, glyoxal, up to 0.8 mM concentration, has been shown to cause cytotoxicity, alterations in cell morphology, mitochondrial damage, and DNA damage in association with the formation of immunocytochemically detectable AGEs [69]. Another study with human keratinocytes has revealed that glyoxal at a dose of 10 mM causes enhanced nuclear proteasome activation due to glyoxidative stress [70]. This specific study also reported that glyoxal, in a dose-dependent manner from 10 to 30 mM, induces significant decline in keratinocyte proliferation with significant formation of CML in the cells. In this report, the authors have justified for using such high concentrations of glyoxal supported by their argument that the high concentration of glyoxal (10 mM) used in their study could be significantly decreased in the cells due to its enzymatic and non-enzymatic degradation prior to targeting the cell nucleus. Concurring with the arguments of Cevantes-Laurean et al. [70], in our current study, we also used high concentrations of glyoxal (1–10 mM) in our BPAEC model and justified the use of those high doses of glyoxal for EC treatment by conceiving that although ECs in the current study were treated with high concentrations of glyoxal (1–10 mM), the levels of available glyoxal at the target sites could have been drastically lowered due to the formation of Schiff’s base adducts, AGEs, and also enzymatic and non-enzymatic degradation. Glyoxal is also known to be metabolically detoxified by an enzymatic mechanism involving glyoxalase which converts glyoxal into glycolic acid [70], which has been shown in several cellular models. However, glyoxal at higher concentrations has been shown to inhibit the glyoxal-metabolizing enzymes [3]. Although high concentrations of glyoxal well above the detected levels of glyoxal in diabetic conditions (~27.2 µg/ml) [3] were used in the current study, this is the first study reporting the glyoxal-induced alterations in vascular ECs leading to cytotoxicity, cytoskeletal alterations, and barrier dysfunction. Nevertheless, the reactivity of the α-oxoaldehydes including glyoxals, the steady-state levels of glyoxals, and the lack of an appropriate analytical technique to determine the levels of free glyoxals in cells and tissues should be considered while focusing on the choice of dose(s) of glyoxal in experimental studies.
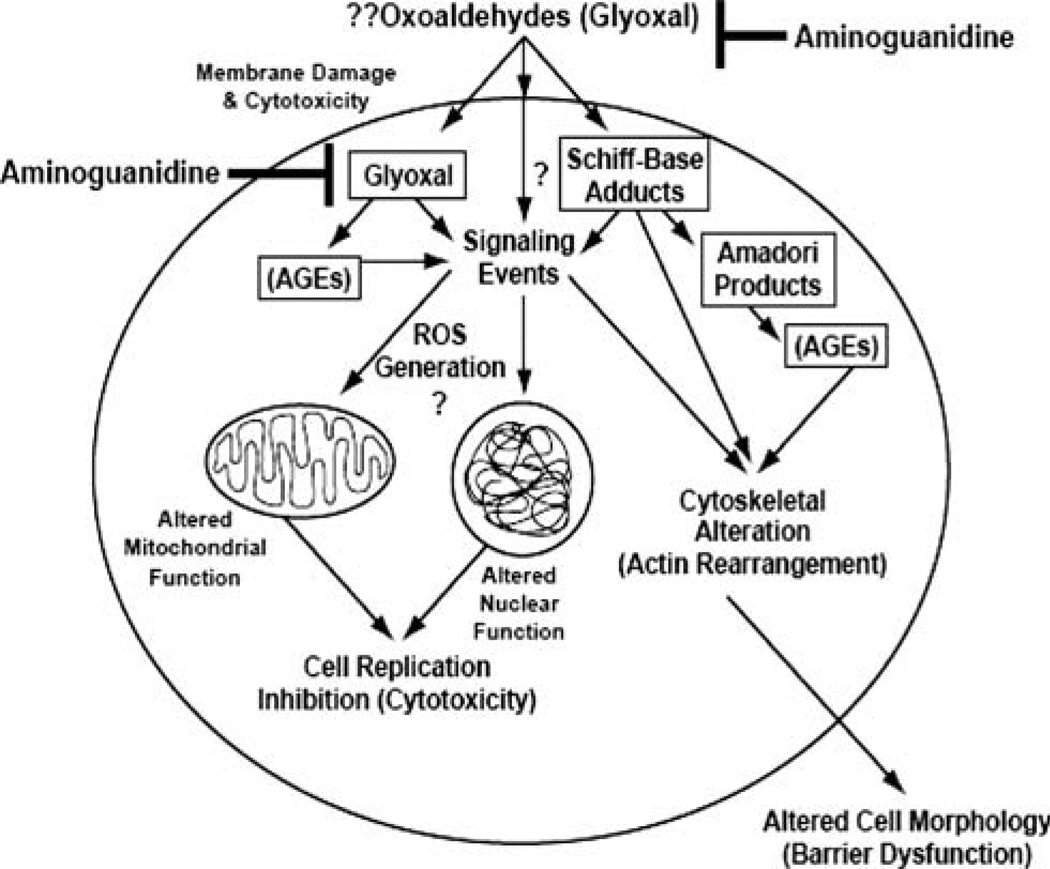
Aminoguanidine is a therapeutic agent which scavenges carbonyl compounds through the reaction with α,α-dicarbonyl precursors, such as glyoxal, to form 3-amino-1,2,4-triazines and prevents the formation of AGEs [10]. In the current study, we did not observe the generation of ROS (as index of oxidative stress) in BPAECs upon treatment with glyoxal. Although the possibility of interference with the antioxidant enzymes such as catalase of BPAECs caused by glyoxal could very well prevail, it is conceivable that the observed protection offered by AG against the glyoxal-induced adverse effects in BPAECs could be due to the complexation (scavenging) of AG with glyoxal rendering the oxoaldehyde inactive. When AG (1 g/l) is administered through drinking water to diabetic rats, diabetes-associated tissue and AGEs as well as albuminuria and mesangial expansion in the kidney are attenuated [10, 71]. While studies have shown the effectiveness of AG in protection against AGEs in the kidneys, reports are lacking on the ability of AG to protect against the glyoxal-induced or AGE-induced vascular EC alterations. Also, we observed that AG HCl and AG nitrate alone at mM doses used in the current study did not cause any adverse cellular effects assuring that AG at pharmacological doses was safe in BPAECs in vitro. However, the current study revealed that AG protected against the glyoxal-induced EC dysfunction and anti-angiogenesis probably through complexing with glyoxal and rendering it inactive or less reactive (Scheme 1). AG, with its ability to protect the integrity and function of the endothelium against the sugar-derived oxoaldehyde and AGEs attack, has the potential for treatment of diabetic vascular endothelial disorders.
Scheme 1.
Proposed mechanism of glyoxal-induced cytotoxicity, cytoskeletal reorganization, barrier dysfunction, and inhibition of angiogenesis in endothelial cells and aminoguanidine protection
Acknowledgements
This work was supported by the National Institutes of Health grant HL 067176-05, EB 004031, DK076566 and Dorothy M. Davis Heart and Lung Research Institute funds. Excellent technical support provided by Ms. Jessica N. Mazerik and Ms. Valorie Ciapala is appreciated.
Contributor Information
Sean M. Sliman, Lipid Signaling and Lipidomics and Vasculotoxicity Laboratory, Division of Pulmonary, Allergy, Critical Care, and Sleep Medicine, Department of Internal Medicine, The Ohio State University College of Medicine, Columbus, OH, USA
Timothy D. Eubank, Lipid Signaling and Lipidomics and Vasculotoxicity Laboratory, Division of Pulmonary, Allergy, Critical Care, and Sleep Medicine, Department of Internal Medicine, The Ohio State University College of Medicine, Columbus, OH, USA
Sainath R. Kotha, Lipid Signaling and Lipidomics and Vasculotoxicity Laboratory, Division of Pulmonary, Allergy, Critical Care, and Sleep Medicine, Department of Internal Medicine, The Ohio State University College of Medicine, Columbus, OH, USA
M. Lakshmi Kuppusamy, Lipid Signaling and Lipidomics and Vasculotoxicity Laboratory, Division of Pulmonary, Allergy, Critical Care, and Sleep Medicine, Department of Internal Medicine, The Ohio State University College of Medicine, Columbus, OH, USA.
Shariq I. Sherwani, Lipid Signaling and Lipidomics and Vasculotoxicity Laboratory, Division of Pulmonary, Allergy, Critical Care, and Sleep Medicine, Department of Internal Medicine, The Ohio State University College of Medicine, Columbus, OH, USA
Elizabeth Susan O’Connor Butler, Lipid Signaling and Lipidomics and Vasculotoxicity Laboratory, Division of Pulmonary, Allergy, Critical Care, and Sleep Medicine, Department of Internal Medicine, The Ohio State University College of Medicine, Columbus, OH, USA.
Periannan Kuppusamy, Lipid Signaling and Lipidomics and Vasculotoxicity Laboratory, Division of Pulmonary, Allergy, Critical Care, and Sleep Medicine, Department of Internal Medicine, The Ohio State University College of Medicine, Columbus, OH, USA.
Sashwati Roy, Lipid Signaling and Lipidomics and Vasculotoxicity Laboratory, Division of Pulmonary, Allergy, Critical Care, and Sleep Medicine, Department of Internal Medicine, The Ohio State University College of Medicine, Columbus, OH, USA.
Clay B. Marsh, Lipid Signaling and Lipidomics and Vasculotoxicity Laboratory, Division of Pulmonary, Allergy, Critical Care, and Sleep Medicine, Department of Internal Medicine, The Ohio State University College of Medicine, Columbus, OH, USA
David M. Stern, College of Medicine, University of Cincinnati, Cincinnati, OH, USA
Narasimham L. Parinandi, Email: narasimham.parinandi@osumc.edu, Lipid Signaling and Lipidomics and Vasculotoxicity Laboratory, Division of Pulmonary, Allergy, Critical Care, and Sleep Medicine, Department of Internal Medicine, The Ohio State University College of Medicine, Columbus, OH, USA; Room 611-A, Division of Pulmonary, Critical Care, and Sleep Medicine, Dorothy M. Davis Heart and Lung Research Institute, The Ohio State University, 473 W. 12th Avenue, Columbus, OH 43210, USA.
References
- 1.Abdel-Rahman E, Bolton WK. Pimagedine: a novel therapy for diabetic nephropathy. Expert Opin Investig Drugs. 2002;11(4):565–574. doi: 10.1517/13543784.11.4.565. [DOI] [PubMed] [Google Scholar]
- 2.Laakso M. Hyperglycemia and cardiovascular disease in type 2 diabetes. Diabetes. 1999;48(5):937–942. doi: 10.2337/diabetes.48.5.937. [DOI] [PubMed] [Google Scholar]
- 3.Shangari N, O’Brien PJ. The cytotoxic mechanism of glyoxal involves oxidative stress. Biochem Pharmacol. 2004;68(7):1433–1442. doi: 10.1016/j.bcp.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 4.Yim MB, Yim HS, Lee C, Kang SO, Chock PB. Protein glycation: creation of catalytic sites for free radical generation. Ann N Y Acad Sci. 2001;928:48–53. [PubMed] [Google Scholar]
- 5.Tan D, Wang Y, Lo CY, Ho CT. Methylglyoxal: its presence and potential scavengers. Asia Pac J Clin Nutr. 2008;17:261–264. [PubMed] [Google Scholar]
- 6.Hartog JW, Voors AA, Bakker SJ, Smit AJ, van Veldhuisen DJ. Advanced glycation end-products (AGEs) and heart failure: pathophysiology and clinical implications. Eur J Heart Fail. 2007;9(12):1146–1155. doi: 10.1016/j.ejheart.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 7.Thornalley PJ, Langborg A, Minhas HS. Formation of glyoxal, methylglyoxal and 3-deoxyglucosone in the glycation of proteins by glucose. Biochem J. 1999;344:109–116. [PMC free article] [PubMed] [Google Scholar]
- 8.Lum H, Roebuck KA. Oxidant stress and endothelial dysfunction. Am J Physiol Cell Physiol. 2001;280(4):C719–C741. doi: 10.1152/ajpcell.2001.280.4.C719. [DOI] [PubMed] [Google Scholar]
- 9.Desai K, Wu L. Methylglyoxal and advanced glycation endproducts: new therapeutic horizons? Recent Pat Cardiovasc Drug Discov. 2007;2(2):89–99. doi: 10.2174/157489007780832498. [DOI] [PubMed] [Google Scholar]
- 10.Thornalley PJ. Use of aminoguanidine (Pimagedine) to prevent the formation of advanced glycation endproducts. Arch Biochem Biophys. 2003;419:31–40. doi: 10.1016/j.abb.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 11.Chang T, Wu L. Methylglyoxal, oxidative stress, and hypertension. Can J Physiol Pharmacol. 2006;84:1229–1238. doi: 10.1139/y06-077. [DOI] [PubMed] [Google Scholar]
- 12.Grossin N, Wautier JL. Protein glycation and endothelium dysfunction. J Soc Biol. 2007;201(2):175–184. doi: 10.1051/jbio:2007022. [DOI] [PubMed] [Google Scholar]
- 13.Bourajjaj M, Stehouwer CDA, van Hinsbergh VWM, Schalkwijk CG. Role of methylglyoxal adducts in the development of vascular complications in diabetes mellitus. Biochem Soc Trans. 2003;31:1400–1402. doi: 10.1042/bst0311400. [DOI] [PubMed] [Google Scholar]
- 14.Ravelojaona V, Peterszegi G, Molinari J, Gesztesi JL, Robert L. Demonstration of the cytotoxic effect of advanced glycation endproducts (AGEs) J Soc Biol. 2007;201(2):185–188. doi: 10.1051/jbio:2007023. [DOI] [PubMed] [Google Scholar]
- 15.Varadharaj S, Steinhour E, Hunter MG, Watkins T, Baran CP, Magalang U, et al. Vitamin C-induced activation of phospholipase D in lung microvascular endothelial cells: regulation by MAP kinases. Cell Signal. 2006;18:1396–1407. doi: 10.1016/j.cellsig.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 16.Hagele TJ, Mazerik JN, Gregory A, Kaufman B, Magalang U, Kuppusamy M, et al. Mercury activates vascular endothelial cell phospholipase D through thiols and oxidative stress. Int J Toxicol. 2007;26:57–69. doi: 10.1080/10915810601120509. [DOI] [PubMed] [Google Scholar]
- 17.Parinandi NL, Sharma A, Eubank TD, Kaufman BF, Kutala VK, Marsh CB, et al. Nitroaspirin (NCX-4016), an NO donor, is antiangiogenic through induction of loss of redox-dependent viability and cytoskeletal reorganization in endothelial cells. Antioxid Redox Signal. 2007;9(11):1837–1849. doi: 10.1089/ars.2007.1603. [DOI] [PubMed] [Google Scholar]
- 18.Green PS, Leeuwenburgh C. Mitochondrial dysfunction is an early indicator of doxorubicin-induced apoptosis. Biochim Biophys Acta. 2002;1(1588):94–101. doi: 10.1016/s0925-4439(02)00144-8. [DOI] [PubMed] [Google Scholar]
- 19.Madesh M, Bhaskar L, Balasubramanian KA. Enterocyte viability and mitochondrial function after graded intestinal ischemia and reperfusion in rats. Mol Cell Biochem. 1997;167(1–2):81–87. doi: 10.1023/a:1006871622049. [DOI] [PubMed] [Google Scholar]
- 20.Yamamoto Y, Kobayashi Y, Devi SR, Rikiishi S, Matsumoto H. Aluminum toxicity is associated with mitochondrial dysfunction and the production of reactive oxygen species in plant cells. Plant Physiol. 2002;128(1):63–72. [PMC free article] [PubMed] [Google Scholar]
- 21.Reddy VP, Beyaz A. Inhibitors of the Maillard reaction and AGE breakers as therapeutics for multiple diseases. Drug Discov Today. 2006;11(13–14):646–654. doi: 10.1016/j.drudis.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 22.Yin F, Watsky MA. LPA and S1P increase corneal epithelial and endothelial cell transcellular resistance. Invest Ophthalmol Vis Sci. 2005;46(6):1927–1933. doi: 10.1167/iovs.04-1256. [DOI] [PubMed] [Google Scholar]
- 23.Bogatcheva NV, Verin AD. The role of cytoskeleton in the regulation of vascular endothelial barrier function. Microvasc Res. 2008;76(3):202–207. doi: 10.1016/j.mvr.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chiang ET, Camp SM, Dudek SM, Brown ME, Usatyuk PV, Zaborina O, et al. Protective effects of high-molecular weight polyethylene glycol (PEG) in human lung endothelial barrier regulation: role of actin cytoskeletal rearrangement. Microvasc Res. 2008 Dec 11; doi: 10.1016/j.mvr.2008.11.007. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vandenbroucke E, Mehta D, Minshall R, Malik AB. Regulation of endothelial junctional permeability. Ann N Y Acad Sci. 2008;1123:134–145. doi: 10.1196/annals.1420.016. [DOI] [PubMed] [Google Scholar]
- 26.Fasano A. Regulation of intercellular tight junctions by zonula occludens toxin and its eukaryotic analogue zonulin. Ann N Y Acad Sci. 2000;915:214–222. doi: 10.1111/j.1749-6632.2000.tb05244.x. [DOI] [PubMed] [Google Scholar]
- 27.Xu Y, Gong B, Yang Y, Awasthi YC, Woods M, Boor PJ. Glutathione-S-transferase protects against oxidative injury of endothelial cell tight junctions. Endothelium. 2007;14(6):333–343. doi: 10.1080/10623320701746263. [DOI] [PubMed] [Google Scholar]
- 28.Harhaj NS, Antonetti DA. Regulation of tight junctions and loss of barrier function in pathophysiology. Int J Biochem Cell Biol. 2004;36(7):1206–1237. doi: 10.1016/j.biocel.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 29.Thornalley PJ. Protein and nucleotide damage by glyoxal and methylglyoxal in physiological systems—role in ageing and disease. Drug Metabol Drug Interact. 2008;23(1–2):125–150. doi: 10.1515/dmdi.2008.23.1-2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Asami J, Odani H, Ishii A, Oide K, Sudo T, Nakamura A, et al. Suppression of AGE precursor formation following unilateral ureteral obstruction in mouse kidneys by transgenic expression of alpha-dicarbonyl/l-xylulose reductase. Biosci Biotechnol Biochem. 2006;70(12):2899–2905. doi: 10.1271/bbb.60311. [DOI] [PubMed] [Google Scholar]
- 31.Triggle CR. The early effects of elevated glucose on endothelial function as a target in the treatment of type 2 diabetes. Timely Top Med Cardiovasc Dis. 2008;4:12. E3. [PubMed] [Google Scholar]
- 32.Meeuwisse-Pasterkamp SH, van der Klauw MM, Wolffenbuttel BH. Type 2 diabetes mellitus: prevention of macrovascular complications. Expert Rev Cardiovasc Ther. 2008;6(3):323–341. doi: 10.1586/14779072.6.3.323. [DOI] [PubMed] [Google Scholar]
- 33.Hadi HA, Suwaidi JA. Endothelial dysfunction in diabetes mellitus. Vasc Health Risk Manag. 2007;3(6):853–876. [PMC free article] [PubMed] [Google Scholar]
- 34.Vasdev S, Gill V, Singai P. Role of advanced glycation end products in hypertension and atherosclerosis: therapeutic implications. Cell Biochem Biophys. 2007;49(1):48–63. doi: 10.1007/s12013-007-0039-0. [DOI] [PubMed] [Google Scholar]
- 35.Esper RJ, Vilarino JO, Machado RA, Paragano A. Endothelial dysfunction in normal and abnormal glucose metabolism. Adv Cardiol. 2008;45:17–43. doi: 10.1159/000115120. [DOI] [PubMed] [Google Scholar]
- 36.Smit AJ, Hartog JW, Voors AA, van Veldhuisen DJ. Advanced glycation endproducts in chronic heart failure. Ann N Y Acad Sci. 2008;1126:225–230. doi: 10.1196/annals.1433.038. [DOI] [PubMed] [Google Scholar]
- 37.Shangari N, Bruce WR, Poon R, O’Brien PJ. Toxicity of glyoxals-role of oxidative stress, metabolic detoxification and thiamine deficiency. Biochem Soc Trans. 2003;31:1390–1393. doi: 10.1042/bst0311390. [DOI] [PubMed] [Google Scholar]
- 38.Ruggiero-Lopez D, Lecomte M, Moinet G, Patereau G, Lagarde M, Wiernsperger N. Reaction of metformin with dicarbonyl compounds. Possible implications in the inhibition of advanced glycation end product formation. Biochem Pharmacol. 1999;58(11):1765–1773. doi: 10.1016/s0006-2952(99)00263-4. [DOI] [PubMed] [Google Scholar]
- 39.Wells-Knetch KJ, Zyzak DV, Litchfield JE, Thorpe SR, Baynes JW. Mechanism of autoxidative glycosylation: Identification of glyoxal and arabinose as intermediates in the autoxidative modification of proteins by glucose. Biochemistry. 1995;34:3702–3709. doi: 10.1021/bi00011a027. [DOI] [PubMed] [Google Scholar]
- 40.Lee J, Zen Q, Ozaki H, Wang L, Hand AR, Hla T, et al. Dual roles of tight junction-associated protein, zonula occludens-1, in sphingosine 1-phosphate-mediated endothelial chemotaxis and barrier integrity. J Biol Chem. 2006;281(39):29190–29200. doi: 10.1074/jbc.M604310200. [DOI] [PubMed] [Google Scholar]
- 41.Thornalley PJ. Endogenous alpha-oxoaldehydes and formation of protein and nucleotide advanced glycation end products in tissue damage. Novartis Found Symp. 2007;285:229–243. doi: 10.1002/9780470511848.ch17. [DOI] [PubMed] [Google Scholar]
- 42.Schoneich C. Protein modification in aging: an update. Exp Gerontol. 2006;41:807–812. doi: 10.1016/j.exger.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 43.Ahmed N. Advanced glycation endproducts—role in pathology of diabetic complications. Diabetes Res Clin Pract. 2005;67:3–21. doi: 10.1016/j.diabres.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 44.Wautier JL, Wautier MP. Blood cells and vascular cell interactions in diabetes. Clin Hemorheol Microcirc. 2001;25(2):49–53. [PubMed] [Google Scholar]
- 45.Jakus V, Rietbrock N. Advanced glycation end-products and the progress of diabetic vascular complications. Physiol Res. 2004;53:131–142. [PubMed] [Google Scholar]
- 46.Goldin A, Beckman JA, Schmidt AM, Creager MA. Advanced glycation end products sparking the development of diabetic vascular injury. Circulation. 2006;114:597–605. doi: 10.1161/CIRCULATIONAHA.106.621854. [DOI] [PubMed] [Google Scholar]
- 47.Wu L. Is methylglyoxal a causative factor for hypertension development? Can J Physiol Pharmacol. 2006;84:129–139. doi: 10.1139/Y05-137. [DOI] [PubMed] [Google Scholar]
- 48.Berlanga J, Cibrian D, Guillen I, Freyre F, Alba JS, Lopez-Saura P, et al. Methylglyoxal administration induces diabetes-like microvascular changes and perturbs the healing process of cutaneous wounds. Clin Sci. 2005;109:83–95. doi: 10.1042/CS20050026. [DOI] [PubMed] [Google Scholar]
- 49.Tan KC, Chow WS, Ai VH, Metz C, Bucala R, Lam KS. Advanced glycation end products and endothelial dysfunction in type 2 diabetes. Diabetes Care. 2002;25(6):1055–1059. doi: 10.2337/diacare.25.6.1055. [DOI] [PubMed] [Google Scholar]
- 50.Giardino I, Edelstein D, Brownlee M. BCL-2 expression or antioxidants prevent hyperglycemia-induced formation of intracellular advanced glycation endproducts in bovine endothelial cells. J Clin Invest. 1996;97(6):1422–1428. doi: 10.1172/JCI118563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schmidt AM, Mora R, Cao R, Yan S-D, Brett J, Ramakrishnan R, et al. The endothelial cell binding site for advanced glycation end products consists of a complex: an integral membrane protein and a lactoferrin-like polypeptide. J Biol Chem. 1994;269(13):9882–9888. [PubMed] [Google Scholar]
- 52.Yan SD, Schmidt AM, Anderson GM, Zhang J, Brett J, Zou YS, et al. Enhanced cellular oxidant stress by the interaction of advanced glycation end products with their receptors/binding proteins. J Biol Chem. 1994;269(13):9889–9897. [PubMed] [Google Scholar]
- 53.Wautier JL, Wautier MP, Schmidt AM, Anderson GM, Hori O, Zoukourian C, et al. Advanced glycation end products (AGEs) on the surface of diabetic erythrocytes bind to the vessel wall via a specific receptor inducing oxidant stress in the vasculature: a link between surface-associated AGEs and diabetic complications. Proc Natl Acad Sci USA. 1994;91:7742–7746. doi: 10.1073/pnas.91.16.7742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Duckworth WC. Hyperglycemia and cardiovascular disease. Curr Atheroscler Rep. 2001;5:383–391. doi: 10.1007/s11883-001-0076-x. [DOI] [PubMed] [Google Scholar]
- 55.Guerci B, Kearney-Schwartz A, Bohme P, Zannad F, Drouin P. Endothelial dysfunction and type 2 diabetes. Part 1: physiology and methods for exploring the endothelial function. Diabetes Metab. 2001;4(1):425–434. [PubMed] [Google Scholar]
- 56.Wautier JL, Zoukourian C, Chappey O, Wautier MP, Guillausseau PJ, Cao R, et al. Receptor-mediated endothelial cell dysfunction in diabetic vasculopathy. J Clin Invest. 1996;97(1):238–243. doi: 10.1172/JCI118397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nishizawa Y, Koyama H. Endogenous secretory receptor for advanced glycation end-products and cardiovascular disease in end-stage renal disease. J Ren Nutr. 2008;18(1):76–82. doi: 10.1053/j.jrn.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 58.Glomb MA, Monnier VM. Mechanism of protein modification by glyoxal and glycoaldehyde, reactive intermediates of the Maillard reaction. J Biol Chem. 1995;279(17):10017–10026. doi: 10.1074/jbc.270.17.10017. [DOI] [PubMed] [Google Scholar]
- 59.Guo XH, Huang QB, Chen B, Wang SY, Hou FF, Fu N. Mechanism of advanced glycation end products-induced hyperpermeability in endothelial cells. Acta Physiologica Sinica. 2005;57(2):205–210. [PubMed] [Google Scholar]
- 60.Guo XH, Huang QB, Chen B, Wang SY, Li Q, Zhu YJ, et al. Advanced glycation end products induce actin rearrangement and subsequent hyperpermeability of endothelial cells. APMIS. 2006;113:874–883. doi: 10.1111/j.1600-0463.2006.apm_372.x. [DOI] [PubMed] [Google Scholar]
- 61.Otero K, Martinez F, Beltran A, Gonzalez D, Herrera B, Quintero G, et al. Albumin-derived advanced glycation end-products trigger the disruption of the vascular endothelial cadherin complex in cultured human and murine endothelial cells. Biochem J. 2001;359:567–574. doi: 10.1042/0264-6021:3590567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Duraisamy Y, Slevin M, Smith N, Bailey J, Zweit J, Smith C, et al. Effect of glycation on basic fibroblast growth factor induced angiogenesis and activation of associated signal transduction pathways in vascular endothelial cells: possible relevance to wound healing in diabetes. Angiogenesis. 2001;4:277–288. doi: 10.1023/a:1016068917266. [DOI] [PubMed] [Google Scholar]
- 63.Akhand AA, Hossain K, Mitsui H, Kato M, Miyata T, Inagi R, et al. Glyoxal and methylglyoxal trigger distinct signals for map family kinases and caspase activation in human endothelial cells. Free Radic Biol Med. 2001;31(1):20–30. doi: 10.1016/s0891-5849(01)00550-0. [DOI] [PubMed] [Google Scholar]
- 64.De Bont R, van Larebeke N. Endogenous DNA damage in humans: a review of quantitative data. Mutagenesis. 2004;19(3):169–185. doi: 10.1093/mutage/geh025. [DOI] [PubMed] [Google Scholar]
- 65.Mistry N, Podmore I, Cooke M, Butler P, Griffiths H, Herbert K, et al. Novel monoclonal antibody recognition of oxidative DNA damage adduct, deoxycytidine-glyoxal. Lab Invest. 2003;83(2):241–250. doi: 10.1097/01.lab.0000053915.88556.ed. [DOI] [PubMed] [Google Scholar]
- 66.Roberts MJ, Wondrak GT, Laurean DC, Jacobson MK, Jacobson EL. DNA damage by carbonyl stress in human skin cells. Mutat Res. 2003;522(1–2):45–56. doi: 10.1016/s0027-5107(02)00232-4. [DOI] [PubMed] [Google Scholar]
- 67.Nagaraj RH, Oya-Ito T, Bhat M, Liu B. Dicarbonyl stress and apoptosis of vascular cells: prevention by alphaB-crystallin. Ann N Y Acad Sci. 2005;1043:158–165. doi: 10.1196/annals.1333.020. [DOI] [PubMed] [Google Scholar]
- 68.Kasper M, Roehlecke C, Witt M, Fehrenbach H, Hofer A, Miyata T, et al. Induction of apoptosis by glyoxal in human embryonic lung epithelial cell line L132. Am J Respir Cell Mol Biol. 2000;23(4):485–491. doi: 10.1165/ajrcmb.23.4.4117. [DOI] [PubMed] [Google Scholar]
- 69.Reber F, Kasper M, Siegner A, Kniep E, Seigel G, Funk RH. Alteration of the intracellular pH and apoptosis induction in a retinal cell line by the AGE-inducing agent glyoxal. Graefes Arch Clin Exp Ophthalmol. 2002;240(12):1022–1032. doi: 10.1007/s00417-002-0588-2. [DOI] [PubMed] [Google Scholar]
- 70.Cervantes-Laurean D, Roberts MJ, Jacobson EL, Jacobson MK. Nuclear proteasome activation and degradation of carboxymethylated histones in human keratinocytes following glyoxal treatment. Free Radic Biol Med. 2005;38:786–795. doi: 10.1016/j.freeradbiomed.2004.11.030. [DOI] [PubMed] [Google Scholar]
- 71.Peyroux J, Sternberg M. Advanced glycation endproducts (AGEs): pharmacological inhibition in diabetes. Pathol Biol. 2006;54:405–419. doi: 10.1016/j.patbio.2006.07.006. [DOI] [PubMed] [Google Scholar]