Abstract
This study investigated the advantages and challenges of using Medication Electronic Monitoring System (MEMS) technology to examine adherence among pediatric kidney transplant patients. Twenty-nine patients participated in the study, with a mean age of 14.03 yr (SD = 3.34, range 8–19 yr). Patients were given a MEMS bottle and cap to be used with their primary immunosuppressant medication over a three-month period. Issues related to study eligibility, recruitment, and participant maintenance were recorded. Patients completed the Debriefing Form regarding their experiences with the MEMS. Many younger patients were on liquid medications affecting the feasibility of this technology across ages. Acceptance of this technology proved difficult, as many patients either declined upfront or dropped out because they did not want to use the MEMS. Of the final sample, 41% found transferring medication into the MEMS bottle difficult and 27.2% reported that the MEMS was a burden and/or difficult to transport. Another 22% of the patients reported that using the MEMS changed their routine, and 10.2% worried about missing their medications. Pediatric transplant centers should be cautious about solely relying on MEMS to examine adherence until more research is conducted on the feasibility, acceptance, and utility of this technology.
Keywords: kidney transplantation, adherence, Medication Electronic Monitoring System, electronic monitoring
Non-adherence among pediatric transplant recipients is a widespread concern with significant implications for the patient’s health and long-term survival. Non-compliance with immunosuppressives has been cited as a major cause of rejection episodes and graft loss, and has been identified as the third leading cause of death in this population (1–3). Despite the importance of adherence, non-adherence is a common occurrence among pediatric transplant groups that has been difficult to ascertain (4).
Attempts to accurately measure adherence have led to the utilization of a variety of methods including patient and parent reports, pill counts, blood serum levels, pharmacy records, and most recently electronic monitoring (4–6). Each type of adherence assessment has limitations that preclude firm conclusions about the validity and reliability of the adherence information garnered (6, 7). Electronic monitoring has been described in the literature as ‘a gold standard’ of adherence assessment (4, 5) because of its superior validity and reliability relative to the other assessment methods. Its use has become more prominent because it is meant to track adherence ‘as it occurs’; however, it remains an ‘indirect method’ as ingestion of the medication cannot be confirmed by viewing the electronic data provided (4–6, 8).
The Medication Event Monitoring System (MEMS) SmartCap or TrackCap (AARDEX Ltd, Union City, CA, USA) is one of these electronic tracking methods. MEMS caps contain an embedded computer chip, which digitally records when pill bottles are opened to dispense medication. The caps are designed to present an in-depth view of medication adherence by providing information on when caps are opened. By tracking this information it is expected that clinicians and/or researchers can track daily adherence to dosages, inter-dose intervals, and drug holidays. The aim is to allow the clinician and/or researcher to have detailed information regarding how the patient dispenses his medication and to determine whether the patient/participant is taking the right number of dosages, as well as properly spacing the dosages to maintain appropriate therapeutic coverage (8).
Unfortunately, clinicians and researchers alike have found some hurdles in the implementation of the technology, and the true objectivity of these electronic monitoring methods have come into question. In their initial implementation, the MEMS caps were widely used with adult chronic illness groups including organ transplant, human immunodeficiency virus (HIV)-positive, and hypertensive populations (9–12). These studies found that the MEMS cap was successful in linking non-adherence as assessed by this electronic technology with medical management and risk (9, 10). However, studies also found challenges. Reports by Bova et al. and Wendel et al. (10, 11) on the adherence of HIV-positive adults found inconsistencies in the use of the MEMS caps by the sample (e.g., multiple dosing during a single opening of the MEMS bottle, missed dosing, and/or MEMS opening without taking the medication). Despite these challenges, the reported usefulness of the MEMS caps in linking measured adherence with medical management has led to its application with pediatric populations.
Pediatric researchers in HIV and transplant have branched out and used the MEMS cap to examine adherence in children (2, 4, 13, 14). Although the findings have yielded promising results regarding the ability of the MEMS system to accurately track adherence, they have also pointed toward certain limitations. The literature suggests that younger users might need devices that provide more flexibility (e.g., pharmacy refills) (8). In addition, it is not yet clear how often younger populations engage in the behaviors described of adult patients (e.g., opening and closing the bottle without taking any medication), how caregivers may influence the child’s use of the MEMS, and whether the MEMS technology itself has any impact on adherence behaviors of pediatric patients. The present study sought to investigate the advantages and challenges faced when utilizing MEMS technology, to examine adherence, and to obtain direct feedback from pediatric kidney transplant patients.
Methods
Study design
This is a descriptive study examining the authors’ experience utilizing MEMS cap methodology with pediatric transplant patients. For this study, patients were recruited from a large children’s hospital in the northeastern USA containing a pediatric Kidney Transplant Center, serving infants through young adults aged zero to 25 yr. All recruitment and study procedures were approved by Committees for the Protection of Human Subjects.
A list of patients who had received a kidney transplantation at our center one to four yr prior to recruitment was obtained from a transplant coordinator. Patients were eligible for participation in the study if the patients: (i) had a viable graft; (ii) were taking Prograf or Rapamune (because Prograf and Rapamune are usually kept in a pharmacy bottle transfer to a white non-see-through MEMS cap bottle did not compromise medication use for these patients) as their primary immunosuppressant; (iii) were not on dialysis; and (iv) and patient and their parents were willing to participate in the study for a length of three months. Written informed consent was obtained according to the Institutional Review Board guidelines. Parents and patients older than 14 yr gave informed consent and assent procedures were followed for patients younger than 14 yr. Families were informed that the cap contained a computer chip and that the purpose of the study was to understand medication-taking patterns among transplant patients.
All patients and parents who agreed to participate in the study were asked to use the MEMS bottle and cap to store their immunosuppressant medication over a three-month period. MEMS caps were distributed to participants during a scheduled clinic visit. Families were asked to bring the patient primary immunosuppressant medication with them to their subsequent medical appointment at the time of recruitment and were later called the day prior to their clinic visit to remind them to bring their medication. A nurse working with the research team transferred the medication to the MEMS cap bottle under sterile conditions and labeled the MEMS bottle with necessary medical information. Both patient and parent were instructed in the proper use of the MEMS cap and bottle at the time of recruitment and again during the initial study session when the MEMS was distributed. Training was provided by either a trained research assistant or the first author. The patient and family was then called prior to a scheduled clinic visit three months later and asked to bring back their MEMS cap bottle with them to clinic for pick-up and data upload. If the family was not scheduled to come to clinic within the scheduled time period for the study, a self-addressed stamped envelop was sent to the family with instructions to return the MEMS cap bottle in the mail after the immunosuppressant medication had been returned to their original pharmacy bottle. All participants were aware that the MEMS cap was tracking their adherence.
Researchers kept a record of any issues that arose during participant selection, recruitment, and study maintenance. Patient comments and concerns about using the MEMS were noted throughout the study. In addition, at the end of the study patients and their caregivers were administered the Debriefing Form regarding their experiences in utilizing the MEMS cap. The Debriefing Form was administered by a trained research assistant or the first author either in person or on the telephone and responses were noted on the form by the interviewer.
Sampling and setting
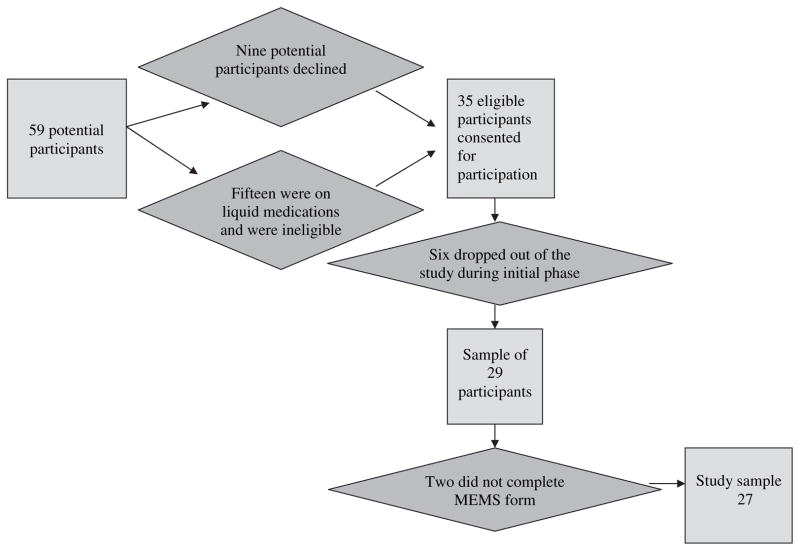
Based on inclusion criteria 59 transplant patients from the pediatric Kidney Transplant Center at the children’s hospital were deemed eligible for study participation. However, not all of them were able to participate. Nine (9) patients declined participation for a variety of reasons, with a primary reason of not wanting to disrupt their medication taking routine by introducing the MEMS cap into it. Most of these patients utilized pillboxes and did not want to alter a proven method of medication management for their families. Fifteen (15) younger patients were on liquid medications and therefore were not able to use the MEMS technology for storing their medication. A total of 35 patients were recruited into the study, but nine (9) participants dropped out of the study after consenting. Of the nine who dropped out after consenting three did so after completing the first phase of the study, and one of these participants completed the MEMS Debriefing Form. The primary reasons for dropping out of the study included not wanting to use the MEMS cap, not having enough time to participate, and health reasons. Figure 1 provides a flow diagram of the selection and recruitment process.
Fig. 1.
Flow diagram of selection and recruitment process.
A final sample of 29 participants met all inclusion criteria, gave informed consent to participate in the study, and completed at least the first phase of the larger study. The sample included 21 males and eight females, with a mean age of 14.03 yr (s.d. = 3.34, range eight to 19 yr). Participants were an average of 35.51 (±10.8) months post-transplant. Patients in this sample missed an average of 23% of their prescribed doses over the three-month duration of the study, with about a third of the patients (n = 10) missing more than 10% of their prescribed doses.
Although the majority of participants (69%) were Caucasian, there was a good representation of patients from minority backgrounds (13.8% African-American, 3.4% Asian, 6.9% Latino, and 6.9% were multi-ethnic). The median annual household income was approximately $61 000–70 000 (range <$12 000 to >$80 000).
Variables and measurement
MEMS
Patients were given a MEMS bottle and cap to be used with their primary immunosuppressant medication. The caps included individual identifying numbers that allowed for easy tracking of individual data. When participants returned the cap, specialized software was used to link the cap to a PC to download data about dosing and adherence for the period of use. The data obtained from the MEMS caps was not only used to assess adherence, but also to assess the ability of the MEMS cap to capture adherence data readily and without complication (e.g., malfunction and data loss) were also noted throughout the study.
Debriefing form
The MEMS Debriefing Form (Table 1) examined participants’ experiences in using the MEMS caps, including transferring of medications to the MEMS bottle, ease of use, differences in routines between utilizing the MEMS cap and their typical medication dispensing system, and perceived impact of the MEMS cap on their adherence. The Debriefing Form also included an open-ended space for participant comments. This measure was developed by the authors incorporating feedback from patients in previous studies and discussions with researchers from other US pediatric hospitals who had utilized the MEMS technology with pediatric transplant patients.
Table 1.
MEMS Debriefing Form

|
Data collection
Participants were recruited over a 10-month period from October 2004 to August 2005 to obtain feedback regarding their experiences with the MEMS technology. Patients recruited for this study were part of a larger study examining the impact of non-adherence after kidney transplantation on resource utilization and medical complications. Data were collected either in a private office during scheduled clinic visits or over the telephone during scheduled telephone conversations arranged with the patient and family. In this study, only the data regarding the experiences of using the MEMS caps are presented.
Data analysis
Data were analyzed using the Statistical Package for the Social Sciences 12.0 for Windows 2000 (SPSS Inc., Chicago, IL). Twenty-two participants utilized the MEMS cap bottle as asked (i.e., they kept their medications in the bottle for the duration of the study). Two of the participants organized their weekly medications in a pillbox and only ‘opened and closed’ the empty MEMS cap bottle during designated dosing times, two participants did not use the MEMS cap bottle at all, and three participants did not return the bottle as requested at the end of the study. Of this group, 27 participants completed the MEMS Debriefing Form. We were unable to contact the two participants who did not complete the Debriefing Form. These participants were also two of the participants who did not return the MEMS bottle at the end of the study. A summary of responses for each item can be found in Table 2. In the following sections we discuss common themes that emerged from participant responses.
Table 2.
Participant responses to Medication Electronic Monitoring System (MEMS) Debriefing Form
| Question* | Percentage yes† | Percentage no† |
|---|---|---|
| Did you find transferring medications difficult? | 41 | 59 |
| Where you able to use MEMS cap at all? | 100 | 0 |
| Did you find using MEMS cap any different? | 70 | 30 |
| Did using MEMS cap make you remember your medications more? | 19 | 81 |
| Did utilizing MEMS cause any differences in medication taking habits? | 22 | 78 |
| Did utilizing MEMS make it less likely medications would be missed? | 19 | 81 |
| Did MEMS increase the likelihood of missing medications? | 4 | 96 |
Descriptive responses summarized in the text.
Data sample based on n of 27 participants.
Challenges experienced in utilizing the MEMS caps
Difficulty of transferring medication
Eleven participants found transferring medications into the MEMS bottle difficult, describing several challenges. Some reported forgetting to transfer the medication into the MEMS bottle or keeping up with the medications in the bottle. Others had trouble getting used to the bottle, thought the bottle was too ‘top heavy’ and bulky to carry around in a pocket. Some patients complained that putting the medications in the MEMS cap added an extra task to or altered the family’s regular medication regimen routine.
Difference of MEMS cap vs. typical routine
Nineteen participants agreed that using the MEMS cap and bottle had been different than their typical routine, while the other eight participants reported that utilizing the MEMS cap had been similar to their medication taking routine. Seven of the participants reported that the MEMS cap constituted an extra step/burden and/or was difficult to transport making utilization of this technology unappealing. Six participants reported that the MEMS cap changed their usual routine (e.g., using a pillbox), which they did not like, increasing the chance that they would not use it accurately. Lastly, three participants worried about forgetting to take their medications when using the MEMS bottle.
Increased likelihood of missing medication
Despite the drawbacks discussed by the participants, only one of the 27 participants who completed the Debriefing Form reported that the MEMS cap could increase the likelihood of missing medication, as a result of using the MEMS instead of a pillbox.
Positive aspects of utilizing the MEMS caps
Decreased likelihood of missing medication
Participants were asked whether the MEMS cap altered their medication taking habits in a positive manner. Five participants reported that the MEMS cap made it less likely to miss medications. Four participants reported that seeing the bottle prompted them or helped them remember to take their medication, while one participant reported that the bottle’s distinguishable shape and size assisted in adherence efforts.
Because some patients endorsed certain items on the Debriefing Form and yet others provided more that one answer, there was some overlap in both negative and positive responses.
MEMS data reporting
Beyond the effects reported by participants, it is important to note that the MEMS caps were extremely useful in the provision of individual adherence data. The data that were collected and downloaded were in excellent condition providing detailed information regarding daily dosages, inter-dose intervals and overall pattern of adherence for this sample of patients. The technology was physically resistant to damage, withstanding four inadvertent drops and trips through airport metal detectors without any loss of information.
Discussion
Adherence rates for childhood chronic illness approach those of adult populations, and unfortunately pediatric transplant patients make up part of this group (6). Employing a multipronged approach to the assessment of adherence is particularly important if changes in adherence rates are to be achieved (15).
The development and utilization of electronic monitoring with pediatric populations is a promising and growing field in pediatric transplantation. The ability to obtain a wealth of adherence information objectively and to track deviations in medication taking routines (e.g., dosing schedule, inter-dose intervals, and missed doses) makes the MEMS an attractive addition to other methods of adherence measurement. Studies with pediatric populations have shown the utility of the MEMS; however, they have not yet fully explored the drawbacks of utilizing this technology with younger patients as other studies have shown with adults (9, 10, 15). This exploratory study provided descriptive data regarding the challenges of utilizing this technology with pediatric transplant patients.
In general, the MEMS cap was a useful device that provided an abundance of data regarding medication-taking habits. Patients who spoke positively regarding the MEMS caps reported that the caps provided a visual reminder of the need to take their medication. One patient stated that utilizing the MEMS cap and bottle added an incentive to remaining adherent, while others reported that simply utilizing the bottle helped them remember their medications more consistently. Despite this, there were clear challenges in the use of the MEMS. For example, patients on liquid medications were unable to participate given that the MEMS caps are not appropriate for use with liquid medications. There were also many instances of patients who refused to participate stating that the use of the MEMS cap and bottle would be too much of a burden, a finding that has also been evident in the adult literature and has been rumored to cause a self-selecting bias (15). This ‘self-selecting bias’ presents another challenge as non-adherent patients might refuse participation out of concern that their adherence patterns would be discovered with electronic monitoring, while adherent patients might not want to disrupt a system of medication management (e.g., use of a pillbox) that has proved effective in the past. In this study, the bias often manifested itself in participants who described their distaste in changing their routines in order to incorporate the MEMS (15). Specifically, concerns regarding ease of use of the MEMS were clearly evident, some patients described the bulkiness of the device, and yet others commented on the inconvenience of needing to withdraw medications at specific times of day. The findings are consistent with findings from the adult literature (9, 10, 15) and point toward the limitations of using the MEMS with younger as well as older populations. In addition, a recent study with adult renal transplant patients by De Geest et al. (15) suggests that a three-month period of monitoring with the MEMS caps might not be sufficient to truly capture ‘adherence behavior’; however, our aim in this article was to present patients’ and parents’ opinions regarding their experience with the MEMS caps.
Transplant centers that dedicate time to educating patients about organization of their post-transplant medications in pillboxes need to be flexible and also cautious when relying on MEMS technology to examine patient adherence. In conducting this study we have identified several strategies that may increase the feasibility and acceptability of using MEMS technology with the pediatric transplant population: (1) allowing patients to open and close their MEMS cap bottles when taking the medication out of their pillbox without needing to keep their medications in the MEMS bottle; (2) incorporating the use of the MEMS caps into an established routine of pillbox use such that patients keep the MEMS cap bottle directly behind their weekly pillbox and slide the bottle along the back as they move through the week; (3) keeping a picture of the pill inside the appropriate compartments of the pillbox (e.g., keeping a picture of a Prograf pill in the AM compartment if that is when the medication should be taken) so that when medications are dispensed from the pillbox the picture will serve as a reminder to take the medication in the MEMS bottle; and (4) allowing patients to note in a diary when they remove more than one dose at a time for a special occasion (e.g., baseball game/birthday party/sleep over) so that the data can be adjusted manually in the database. These strategies can help incorporate the MEMS into established medication taking routines and therefore may be perceived as less disruptive by patients leading to increased acceptability and cooperation.
This study provided preliminary descriptive data regarding the limitations of using the MEMS caps with pediatric transplant patients. Future studies should further examine the role that these factors play in adherence within pediatric populations to ensure that the results obtained from the technology are valid and reliable given the reported obstacles. Because patients had clear views regarding the impact of the MEMS caps on their adherence, it is suggested that a debriefing form similar to the one utilized in this study be included in future studies. Such a step would assist researchers in determining the effects of the technology itself on adherence findings.
Acknowledgments
The study was supported by a grant from The Joseph Stokes, Jr Research Institute at The Children’s Hospital of Philadelphia.
References
- 1.Lurie S, Shemesh LS, Sheiner PA, et al. Non-adherence in pediatric liver transplant recipients – An assessment of risk factors and natural history. Pediatr Transplant. 2000;4:200–206. doi: 10.1034/j.1399-3046.2000.00110.x. [DOI] [PubMed] [Google Scholar]
- 2.Shemesh E. Non-adherence to medication following pediatric liver transplantation. Pediatr Transplant. 2004;8:600–605. doi: 10.1111/j.1399-3046.2004.00238.x. [DOI] [PubMed] [Google Scholar]
- 3.Sudan DL, Shaw BW, Jr, Langnas AN. Causes of late mortality in pediatric liver transplant recipients. Ann Surg. 1998;227:289–295. doi: 10.1097/00000658-199802000-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neu AM. Special issues in pediatric kidney transplantation. Adv Chronic Kidney Dis. 2006;13:62–69. doi: 10.1053/j.ackd.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 5.Riekert KA, Rand CS. Electronic monitoring of medication adherence: When is high-tech best? J Clin Psychol Med Settings. 2002;9:25–34. [Google Scholar]
- 6.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487–492. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 7.Zelikovsky N, Walsh A. Medical adherence. In: Kaplan BS, Meyers K, editors. Pediatric Nephrology and Urology: The Requisites in Pediatrics. Vol. 3. Philadelphia, PA: Elsevier Mosby; 2007. pp. 69–75. Ch. 9. [Google Scholar]
- 8.Farley J, Hines S, Musk A, et al. Assessment of adherence to antiviral therapy in HIV-infected children using the Medication Event Monitoring System, pharmacy refill, provider assessment, caregiver self-report, and appointment keeping. J Acquir Immune Defic Syndr. 2003;33:211–218. doi: 10.1097/00126334-200306010-00016. [DOI] [PubMed] [Google Scholar]
- 9.Dobbels F, DeGeest S, van Cleemput J, et al. Effect of late medication non-compliance on outcome after heart transplantation: A 5-year follow-up. J Heart Lung Transplant. 2004;23:1245–1251. doi: 10.1016/j.healun.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 10.Bova CA, Fennie KP, Knafl GJ, et al. Use of electronic monitoring devices to measure antiretroviral adherence: Practical considerations. AIDS Behav. 2005;9:103. doi: 10.1007/s10461-005-1685-0. [DOI] [PubMed] [Google Scholar]
- 11.Wendel CS, Mohler MJ, Kroesen K, et al. Barriers to use of electronic adherence monitoring in an HIV clinic. Ann Pharmacother. 2001;35:1010–1015. doi: 10.1345/aph.10349. [DOI] [PubMed] [Google Scholar]
- 12.Marquez-Contreras E, Marteli-Claros N, Gil-Guillen V, et al. Compliance Group of the Spanish Society of Hypertension (SEE) Efficacy of a home bound blood pressure monitoring programme on therapeutic compliance in hypertension: The EAPACUM-HTA study. J Hypertens. 2006;24:169–175. doi: 10.1097/01.hjh.0000198023.53859.a2. [DOI] [PubMed] [Google Scholar]
- 13.Wiener L, Riekert K, Ryder C, et al. Assessing medication adherence in adolescents with HIV when electronic monitoring is not feasible. AIDS Patient Care STDS. 2004;18:527–538. doi: 10.1089/apc.2004.18.527. [DOI] [PubMed] [Google Scholar]
- 14.Blowey DL, Hebert D, Arbus GS, et al. Compliance with cyclosporine in adolescent renal transplant recipients. Pediatr Nephrol. 1997;11:547–551. doi: 10.1007/s004670050335. [DOI] [PubMed] [Google Scholar]
- 15.De Geest S, Schäfer-Keller P, Denhaerynck K, et al. Supporting medication adherence in renal transplantation (SMART): A pilot RCT to improve adherence to immunosuppressive regimens. Clin Transplant. 2006;20:359–368. doi: 10.1111/j.1399-0012.2006.00493.x. [DOI] [PubMed] [Google Scholar]