Abstract
Objective
Evidence-based treatment guidelines have been unable to provide evidence-based guidance on the effects of acupuncture for irritable bowel syndrome (IBS) because the only previous systematic review included only small, heterogeneous and methodologically unsound trials. We conducted a new systematic review and meta-analysis of randomized controlled trials (RCTs) to estimate the effects of acupuncture for treating IBS.
Methods
MEDLINE, the Cochrane Central Register of Controlled Trials, EMBASE, Cumulative Index to Nursing and Allied Health, and the Chinese databases Sino-Med, CNKI, and VIP were searched through November 2011. Eligible RCTs compared acupuncture with sham acupuncture, other active treatments, or no (specific) treatment, and evaluated acupuncture as an adjuvant to another treatment. Our outcomes were overall IBS symptom severity and health-related quality of life. Dichotomous data were pooled to provide a relative risk (RR) of substantial improvement after treatment, and continuous data were pooled to provide a standardized mean difference (SMD) in post-treatment scores between groups.
Results
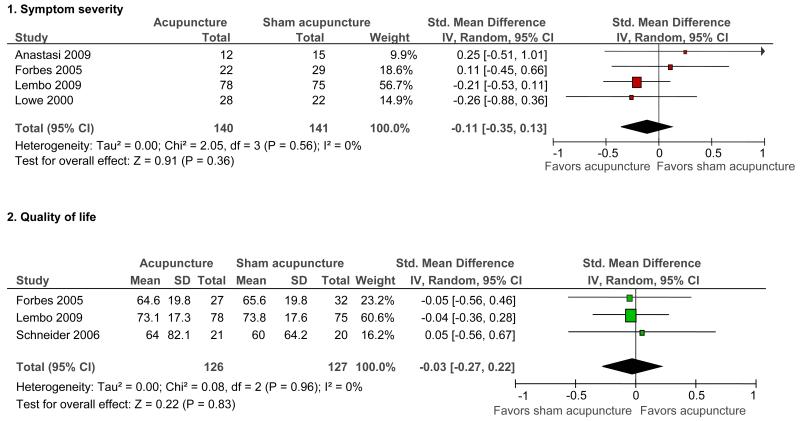
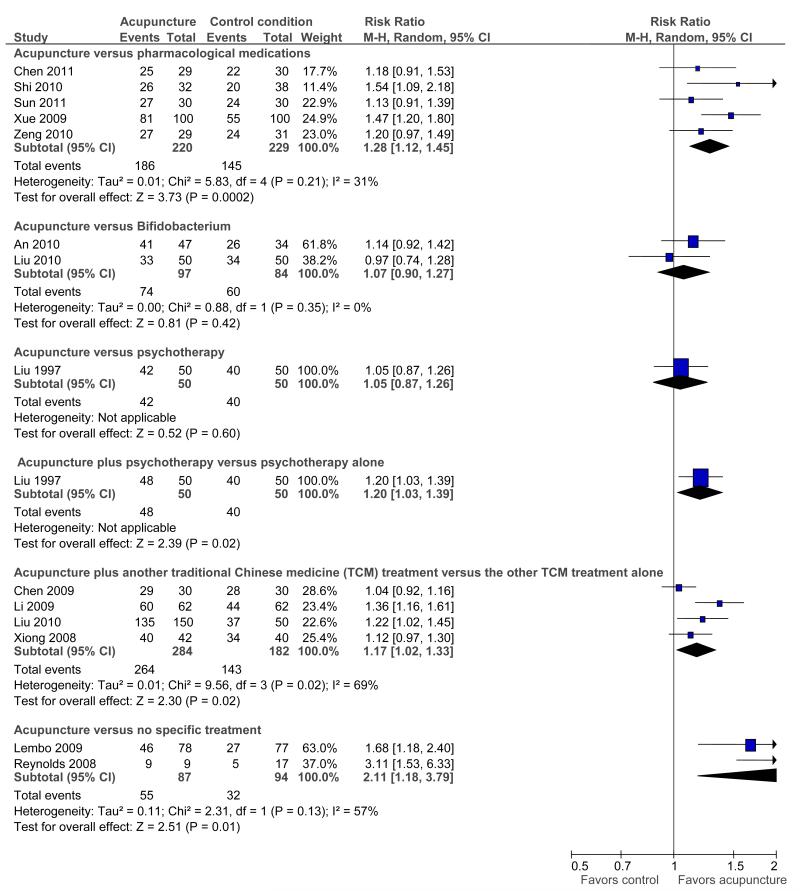
Seventeen RCTs (N=1806) were included. We found no evidence of an improvement with acupuncture relative to sham acupuncture on symptom severity (SMD = −0.11, 95% confidence interval: −0.35 to 0.13; 4 RCTs) or quality of life (SMD = −0.03, −0.27 to 0.22; 3 RCTs). Because of the homogeneity of the results of the sham-controlled trials, results were unaffected by restriction to the 4 sham-controlled RCTs that used adequate randomization, blinding, and had few withdrawals/drop-outs. Among RCTs that did not use a placebo control, acupuncture was more effective than pharmacological therapy (RR of symptom improvement=1.28, 1.12 to 1.45; 5 RCTs) and no (specific) treatment (RR = 2.11, 1.18 to 3.79; 2 RCTs). There was no difference between acupuncture and Bifidobacterium (RR = 1.07, 0.90 to 1.27; 2 RCTs) or between acupuncture and psychotherapy (RR=1.05, 0.87 to 1.26; 1 RCT). Acupuncture as an adjuvant to another Chinese medicine treatment was statistically significantly better than the other treatment alone, in trials with a high risk of bias (RR = 1.17, 1.02 to 1.33; 4 RCTs).
Conclusions
Sham-controlled RCTs have found no benefits of acupuncture relative to a credible sham acupuncture control on IBS symptom severity or IBS-related quality of life. In comparative effectiveness Chinese trials, patients reported greater benefits from acupuncture than from pharmacological therapies. Future trials may help clarify whether or not these reportedly greater benefits of acupuncture relative to pharmacological therapies are due entirely to patients’ preferences for acupuncture or patients’ greater expectations of improvement on acupuncture relative to drugs.
INTRODUCTION
Irritable bowel syndrome (IBS) is a chronic, relapsing gastrointestinal condition characterized by altered bowel habits and abdominal pain and discomfort (1). A systematic review (2) has estimated that 10-15% of adults in North America have IBS, as diagnosed by either the Rome (3) or Manning (4) objective diagnostic criteria. IBS is associated with significant reductions in both health-related quality of life (5) and work productivity (1,6) and increased consumption of medical resources. Indeed, people with IBS consume over 50% more health care resources than age-matched controls without IBS (7,8). The combined direct and indirect costs associated with IBS patients in the United States in 2004 were estimated at over $1 billion (9).
Effective treatments for IBS are needed to relieve symptoms, improve quality of life, and to reduce healthcare utilization. In 2009, the American College of Gastroenterology Task Force conducted a series of systematic reviews to evaluate the efficacy of both pharmacological and non-pharmacological therapies for treating IBS (1). In terms of pharmacological treatments, the Task Force found “poor quality of evidence” for certain antispasmodics and “moderate quality of evidence” for tricyclic antidepressants, selective serotonin reuptake inhibitors, non-absorbable antibiotics (for diarrhea-predominant IBS), and C-2 chloride channel activators (for constipation-predominant IBS). The Task Force found “good quality of evidence” for 5HT3 antagonists and 5HT4 agonists, but noted that these agents carry a possible risk of ischemic colitis and cardiovascular events, respectively, which may limit their utility. A subsequent systematic review showed that the benefits of these 5HT3 antagonists and 5HT4 agonists relative to placebo are “modest” (10). In terms of non-pharmacological therapies, the Task Force found “poor quality of evidence” for psyllium fiber and peppermint oil. The Task Force also noted that preliminary evidence suggested that some probiotics may be effective in reducing IBS symptoms (1). A subsequent systematic review (11) concluded that the specific probiotic B. infantis 35624 has shown repeated efficacy in well-designed randomized controlled trials (RCTs), and can be considered an effective treatment for IBS.
The Task Force was unable to make any recommendations either for or against acupuncture for treating IBS because the only available systematic review available at the time was a Cochrane review (12) which was inconclusive because it included only small, heterogeneous, and methodologically unsound trials. Given the safety of acupuncture (13-15) and the limited availability of other safe and effective treatments for IBS, the question of whether acupuncture is effective for treating IBS is highly relevant. Recently, several RCTs have been published which provide greater evidence to estimate the effects of acupuncture for treating IBS. We have therefore updated our previous Cochrane systematic review and meta-analysis of acupuncture for IBS (12) to assess whether the pooled effects of currently available trials show any benefit of acupuncture in improving symptoms or health-related quality of life in patients with IBS.
METHODS
Search strategy
We searched MEDLINE, the Cochrane Central Register of Controlled Trials (CENTRAL), EMBASE, the Cumulative Index to Nursing and Allied Health, and the Chinese databases Sino-Med (previously called the Chinese Biomedical Database), CNKI, and VIP (through November 2011). We considered all RCTs included in, or ongoing, at the time of the previous version of this review (12). To further avoid the risk of missing eligible trials (16), we also scanned bibliographies of included articles and systematic reviews for further references.
Study selection
We included randomized controlled trials, published in any language, as either full articles or abstracts. Because recent research indicates that a large proportion of Chinese-language RCT reports are of studies that are not truly randomized (17), an author interviewed the investigators of Chinese-language RCTs by telephone to determine whether they had used randomization. The interviews were conducted using questions adapted from the survey developed by Wu et al to verify the authenticity of “claimed” randomized trials (17). The same questions were asked of authors of English-language RCTs that did not include details about randomization methods in their published reports. Trials that were found to assign patients by alternation, rotation, or hospital record number were automatically excluded. Trials that used a random method of assignment, but with flaws or suspected flaws in the random assignment process were included, but with their limitations described.
We included trials evaluating traditional Chinese Medicine (TCM) acupuncture for the treatment of adults diagnosed with IBS. TCM acupuncture involves inserting needles into traditional meridian points, usually with the intention of influencing energy flow in the meridian. Needles may also be inserted at additional tender points and electrical stimulation of the needles may be used. Since TCM acupuncture is often accompanied by moxibustion, we included trials using moxibustion as a co-intervention with acupuncture. We excluded trials of dry needling/trigger point therapy, a therapy which is based on principles of Western anatomy and physiology and rejects TCM concepts of energy and meridians. We also excluded RCTs of laser acupuncture, non-invasive electrostimulation (i.e., using electrodes on the skin rather than needles to stimulate acupuncture points (18)), and acupressure, to restrict our focus to the effects of traditional needle acupuncture. Finally, we excluded trials of micropuncture, a non-traditional acupuncture practice which is based on the principle that the ear (or nose, eye, etc.) is a microsystem of the entire body, and in which needles are only inserted on that microsystem.
We included trials comparing acupuncture to sham (placebo) acupuncture, other active non-TCM treatments, and no (specific) treatment, or evaluated acupuncture as an adjuvant to another treatment. We excluded RCTs in which one form of acupuncture was compared with another form of acupuncture or a different type of TCM (e.g., Chinese herbal medicine). Adjunctive treatments, either Western or TCM, were allowed as long as they had been given to both the acupuncture and control groups. Our primary outcomes were overall IBS symptom severity and IBS health-related quality of life. Studies that did not report at least one of these outcomes were excluded.
All records identified by searching were independently screened by at least one reviewer. The full text of potentially relevant reports was obtained and independently reviewed by two authors for eligibility. Disagreements between reviewers were resolved by discussion.
Outcome measures and data extraction
Two recent evaluations of symptom and quality of life measures in IBS concluded that the IBS Adequate Relief question (IBS-AR) (19) and the IBS Symptom Severity Scale (IBS-SSS) (20) possessed responsiveness, face and construct validity and were two of the most appropriate IBS symptom outcome measures, while the IBS Quality of Life measure (IBS-QoL) (21) was the most extensively validated quality of life scale (22,23). For overall symptom severity, we therefore gave preference to the IBS-AR for dichotomous outcomes and to the IBS-SSS for continuous outcomes, while for quality of life outcomes we gave preference to the IBS-QoL. In cases where dichotomous outcomes such as improvement in IBS symptoms were presented in the form of multiple strata, such that we had the option of choosing cutpoints for the dichotomous outcome, we followed the model of Ford et al. and created a dichotomous measure in which all positive outcomes were combined into a single positive category (i.e., improvement) and the remaining strata constituted the negative category (i.e., no improvement) (10,24). When investigators selected a cutpoint on a continuous scale to dichotomize between improvement and no improvement, we used the same cutpoint to define the dichotomous outcome (10).
We extracted outcome data for both short and long-term follow-up points. Short-term follow-up was defined as three months or less after randomization, and long-term follow-up was defined as closest to six months but more than three months after randomization. When we observed multiple short-term follow-up points, we chose to extract the data closest to eight weeks after randomization, which coincided with end of treatment. In cases where participants were lost to follow-up, and the RCT investigators conducted intention-to-treat (ITT) analyses using imputed values for the participants’ missing data, we used these ITT results for our meta-analyses in preference to the available case analyses, if the ITT method for imputing data was described and if it was an appropriate method that would not bias the effect size calculation. If the method that the RCT investigators used for imputing missing data in their ITT analysis was not clearly described or not appropriate, we used the available case data instead (if available) for the primary analysis and the ITT data for a sensitivity analysis. If either only the ITT data or only the available case data were reported, then those available data were used. (We did not impute missing data ourselves for the meta-analyses.) The potential impact of missing data (including missing data that were imputed using a method that was not clearly described) were considered in interpreting the results of the review, taking into account the degree of missing trial data across the treatment arms and the size of the effect estimate of the individual trial and the pooled effect estimate.
All study characteristics and outcome data were independently extracted by two authors, and disagreements were resolved by discussion. When reported data were incomplete or ambiguous, we requested additional information or clarification from the corresponding authors.
Assessment of acupuncture adequacy
Two acupuncturists (LL, XS) who have a combined acupuncture clinical experience of nearly fifty years in treating IBS, and who have previously worked on RCTs of acupuncture, assessed the adequacy of the acupuncture administered in the trials. Six aspects of the acupuncture intervention were assessed for adequacy: choice of acupuncture points; total number of sessions; treatment duration; treatment frequency; needling technique; and acupuncturist’s experience (25-27). The likelihood of the sham intervention to have physiological activity was also assessed, using an open-ended question. The acupuncturist assessors were provided with only the part of the publications that described the acupuncture and sham procedures, so that their assessments could not be influenced by the results of the trials. To test the success of blinding the assessors to the study publication and results, we asked the assessors to guess the identity of each study being assessed. The acupuncturists assessed adequacy independently and achieved consensus by discussion.
Study risk of bias
For each included study, we assessed risk of bias using the Cochrane Collaboration’s risk of bias tool (28), which is comprised of 6 domains that may increase the risk of over- or underestimating an intervention effect. We also evaluated 2 other risk of bias-related factors: baseline comparability and use of an intention to treat analysis.
In judging adequacy of blinding, which is one of the Cochrane risk of bias domains, we assigned sham-controlled trials a judgment of “Unclear” unless we felt certain that the sham control was sufficiently credible in fully blinding participants to the treatment being evaluated (26,27). We considered sham-controlled trials to have a low risk of bias for blinding if the trial either 1) evaluated the credibility of the sham and found the sham to be indistinguishable from true acupuncture or 2) used a penetrating needle or a previously validated sham needle (i.e., Streitberger needle (29)).
Two review authors independently judged whether each risk of bias criterion was adequately met. Any disagreement was resolved by discussion.
Data synthesis and statistical analysis
We only pooled data from trials that used similar control interventions (sham acupuncture, no treatment, or another active treatment), outcome measures (overall IBS symptom severity, IBS-related quality of life), and timing of outcome assessment (short-term, long-term). For pooled data, summary test statistics were calculated using the RevMan software version 5.1 (30) random effects model, to account for expected heterogeneity. We evaluated heterogeneity using the I2 statistic (31), which indicates the proportion of variability across trials not explained by chance alone (32).
For the acupuncture versus sham comparison, data for the symptom severity outcome were presented in some studies as dichotomous data (e.g., adequate symptom relief) and in other studies as continuous data (e.g., symptom severity as measured by the IBS-SSS). We re-expressed odds ratios as standardized mean differences (SMDs), thereby allowing dichotomous and continuous data to be pooled together for this comparison/outcome (32), using the generic inverse variance method in RevMan. For the acupuncture versus sham comparison, all 3 studies that included the quality of life outcome reported continuous data (and 2 out of 3 did not also report dichotomous data), so we pooled these studies using the SMD. For all other comparisons/outcomes, all studies reported dichotomous outcome data (and some did not also report continuous data), so we pooled these studies as relative risks. For the Cochrane version of this review, all continuous and dichotomous data reported for all studies are presented in forest plots. There were no important differences between continuous and dichotomous results for any comparison/outcome.
RESULTS
Results of the search
Our searches identified 1421 citations of which 71 references, corresponding to 65 individual studies, were evaluated in full. Of these 65 studies, 48 were excluded, leaving 17 eligible randomized controlled trials (33-49), including a total of 1806 patients (see Supplementary Figure 1 online). Three of the studies included in our 2006 Cochrane review (50-52) were excluded from this update because either an adequate randomization process was not used (50,51) or the procedure could not be recalled by the author (52).
Table 1 includes a description of trial characteristics and acupuncture and control interventions. The Cochrane version of this review includes an additional figure for the flow of studies through the selection process, as well as the following additional tables: summary of findings table; list of studies that we excluded as well as the reasons for exclusion; full details of the characteristics of the included trials; full details of the risk of bias assessments for each trial; and assessments of adequacy of acupuncture and sham protocols.
Table 1.
Characteristics of randomized controlled trials of acupuncture in irritable bowel syndrome (IBS)
| Study | N a | Country | Diagnosti c criteria used for IBS |
Criteria for improvement in overall IBS symptoms |
Criteria for improvement in IBS-related quality of life |
Time point for outcome assess- ment b |
Acupuncture treatment | Control treatment(s) |
|---|---|---|---|---|---|---|---|---|
| An 2010 (33) |
81 c | China | Rome II | Categoricald | -e | 4 wks EoT) |
Fixed formula with moxibustion at 2 fixed points; 24 sessions over 4 wks |
Combined Bifidobacterium, lactobacillus and Enterococcus faecium capsules (2 pills, 3×/d) |
| Anastasi 1999 (34) |
29 | USA | Rome II/III | CGI | - | 4 wks (EoT) |
Flexible formula with moxibustion at all points; 8 sessions over 4 wks |
Sham acupuncture -- superficial needling 2-3 cm from true acupoints |
| Chen 2009 (36) |
60c | China | Rome II | Categorical | FDG QoL scale |
4 wks (EoT) |
Flexible formula with moxibustion at 4 fixed pts; 12 sessions over 4 wks + Chinese herbal formula |
Chinese herbal formula alone (3 pills, 3×/d) |
| Chen 2011 (35) |
60 | China | Rome III – IBS-D |
Categorical | - | 3 wks (EoT) |
Flexible formula with moxibustion at 3-5 pts; 15 sessions over 3 wks |
Montmorillonite (1 bag, 2×/day) + loperamide (4mg, 3×/day) + pinaverium bromide (50mg, 3×/d)f |
| Forbes 2005 (37) |
59 | UK | Rome I and Manning |
Global symptom score based on patient diary g |
EuroQol | 13 wks (EoT) |
Individualized; 10 sessions over 10 wks |
Sham acupuncture -- penetrating needles at non- acupoints |
| Lembo 2009 (38) h |
230 | USA | Rome II | IBS-AR and IBS-SSS |
IBS-QoL | 3 wks (EoT) |
Flexible formula; 6 sessions over 3 wks |
|
| Li 2009 (39) |
186 | China | Rome III | Categorical | - | 10 wks (EoT) |
Flexible formula with moxibustion at all points; 60 sessions over 10 wks + Tuina spinal massage |
Tuina spinal massage alone (60 sessions over 10 wks) |
| Liu 1997 (40) |
150 | China | Not specified |
Categorical | - | 3-21 wks (EoT) |
Flexible formula with moxibustion at 1 acupoint; 10-60 sessions over 20- 120 days + psychotherapy |
|
| Liu 2010 (41) |
300 | China | Rome III – IBS-D |
Categorical | - | 4 wks (EoT) |
Fixed formula EA; 28 sessions over 4 wks + Chinese herbal formula |
|
| Lowe 2000 (42) |
50 | Canada | Rome (version not stated) |
Dichotomous measure of symptom relief |
IBS-36 | 4 wks (Eot) |
Fixed formula; 8 sessions over 4 wks | Sham acupuncture -- tapping blunt needle on the skin and then taping the needle in place |
| Reynolds 2008 (43) |
30 | UK | Rome II | IBS-SSS | - | 3 mos (EoT) |
Flexible formula; 8 sessions over 3 mos |
Usual care |
| Schneider 2006 (44) |
43 | Germ any |
Rome II | Not measured | FDDQL | 5 wks (EoT) |
Fixed formula; 10 sessions over 5 wks |
Sham acupuncture -- validated Streitberger placebo needles placed 2 cm from true acupoints |
| Shi 2010 (45) |
70 | China | Rome III – IBS-D |
Categorical | - | 5 wks (1 wk after (EoT)i |
Flexible formula EA; 28 sessions over 4 wks |
Pinaverium bromide (50mg, 3×/d) |
| Sun 2011 (46) |
63 | China | Rome III – IBS-D |
Categorical | - | 4 wks (EoT) |
Fixed formula; 20 sessions over 4 wks |
Pinaverium bromide (50mg, 3×/d) |
| Xiong 2008 (47) |
120 | China | Rome II | Categorical | - | 4 wks (EoT) |
Fixed formula with moxibustion at 2 pts; 28 sessions over 4 wks + Chinese herbal formula |
Chinese herbal formula alone |
| Xue 2009 (48) |
210 | China | Rome II | Categorical | - | 3-7 weeks (EoT) |
Fixed formula with moxibustion at 1 point; 20-40 sessions over 3-7 wks |
Sulfasalazine (10 mg, 1×/d) |
| Zeng 2010 (49) |
65 | China | Rome III – IBS-D |
Categorical | - | 30 d (EoT) | Flexible formula with moxibustion at 6 points; 30 sessions over 30 d |
Trimebutine maleate (100mg, 3×/d) |
IBS, irritable bowel syndrome; EoT, end of treatment; wks, weeks; d, day; CGI, Clinical Global Impression Scale (73); acupoints, acupuncture points; FDG QoL, Functional Digestive Diseases Quality of Life Scale, an IBS quality of life questionnaire which had been used in previous Chinese studies but that has not been validated; IBS-D, study restricted eligibility to IBS-D (IBS with diarrhea) subtype patients; EuroQol, EuroQol Group’s rating scale (74); IBS-AR, IBS-Adequate Relief question (19); IBS-SSS, IBS Severity Scoring System (20); IBS-QoL, IBS Quality of Life Measure (22); EA, electroacupuncture; IBS-36 (75); FDDQL, Functional Digestive Diseases Quality of Life Questionnaire (76).
Number randomized.
The time point listed is the number of weeks after randomization.
For these 2 trials, the author did not record nor recall the numbers randomized, nor the numbers of dropouts, and the numbers analyzed are reported here.
For all 11 trials conducted in China, a symptom scale was used to assess the severity of the patients’ overall IBS-related symptoms (e.g., abdominal pain, defecation difficulties, diarrhea) both at baseline and after treatment. For 8 of these trials (33,35,36,41,45-47,49), a percentage improvement from baseline scores was then calculated (i.e., (baseline symptom score – symptom score after treatment)/ baseline symptom score), and this percentage change from baseline was then grouped into 2 (45), 3 (33,49), or 4 (35,36,41,46,47) categories, which were then converted into 2 categories for the meta-analysis, as described in the Methods section. For the other 3 trials (39,40,48), it was not clear how the symptom scale scores were converted into the categorical data. For these 11 trials, the criteria for improvement is listed as “Categorical” in this table.
A dash (-) indicates that the outcome not measured.
The Montmorillonite was given to all patients and the loperamide and pinaverium bromide was added if the diarrhea did not stop.
This symptom diary is based on the Bristol scale (77).
In this trial, patients were randomized to five arms. The first arm was a wait-list control. Participants in the remaining 4 arms were randomized to sham or true acupuncture, with or without an augmented practitioner-patient interaction. There was no main effect of practitioner-patient interaction; therefore we combined the two acupuncture groups (augmented and limited encounter) and the two sham acupuncture groups (augmented and limited encounter) in order to compare the effects of acupuncture and sham acupuncture.
This trial also included an EoT measurement point, for which the results were very similar to the 1 wk post EoT measurement point.
Acupuncture adequacy
All trials included in this review were judged adequate on “Choice of acupoints”, except for the Lowe trial (42), which did not report the acupoints. The acupuncture frequency was judged adequate in all trials except for the Forbes and Reynolds trials (37,43). The acupuncture adequacy assessors were successfully blinded to the study publications and were unable to guess the identity or results of any of the studies they assessed.
Risk of bias in included studies
All sham-controlled trials reported adequate methods for sequence generation and allocation concealment. In 4 out of 5 of the sham-controlled trials (34,37,38,44) (i.e., all except for the Lowe trial (42)), we judged that the shams were likely to be indistinguishable from true acupuncture and that incomplete outcome data was adequately addressed. The Lowe trial was reported only as an abstract, and the completeness of outcome data ascertainment could not be assessed. In the one sham-controlled trial (37) that had a moderate total number of withdrawals (i.e., 8/59), the drop-outs were approximately evenly distributed across treatment groups, the withdrawals were unlikely to be related to knowledge of treatment assignment or effects of the treatment, and the degree of missing data would be unlikely to affect the estimate of the treatment effect in this individual trial or in the meta-analytic estimates.
In the trials comparing acupuncture with another active treatment (33,35,40,41,45,46,48,49), no (specific) treatment (38,43), or evaluating acupuncture as an adjuvant to another treatment received by all trial participants (36,39-41,47), blinding of participants was not possible, and this likely represents the major risk of bias in these trials. In these comparative effectiveness trials, there were also risks of bias associated with the randomization procedure and the follow-up of patients (see Risk of Bias table).
Acupuncture versus sham acupuncture
Five trials (34,37,38,42,44) compared the effects of acupuncture and sham acupuncture. The 5 individual sham-controlled RCTs, and also the pooled analysis, found no statistically significant differences between acupuncture and sham acupuncture, on the outcomes of symptom severity or quality of life. (One trial (44) did not measure the outcome of symptom severity and 2 trials (34,42) did not report quality of life.) For both outcomes, the results of all sham-controlled trials were homogeneous (I2=0%) (see Figure 1). Only the Schneider et al trial (44) included a long-term follow-up time point, and this trial did not find a difference between acupuncture and sham in the quality of life outcome at six months (SMD 0.07, 95% CI −0.54 to 0.69).
Figure 1.
Acupuncture versus sham acupuncture: Symptom severity and quality of life
Acupuncture versus other active treatments
The five trials (35,45,46,48,49) that compared acupuncture versus pharmacological therapies for IBS found that participants receiving acupuncture reported a greater improvement than participants receiving pharmacological therapies (see Figure 2).
Figure 2.
Acupuncture versus another active treatment, as adjuvant to another active treatment, or compared to no specific treatment: Symptom severity
Participants receiving acupuncture were not more likely to have responded to treatment than those treated with psychotherapy (40) or those treated with bifidobacterium (33,41).
Acupuncture as an adjuvant to other active treatments
Five trials (36,39-41,47) compared the combination of adjuvant acupuncture plus another IBS treatment received by all trial participants to the other IBS treatment alone. Pooled results showed that participants receiving adjuvant acupuncture were more likely to have reported improvement than those treated with another Chinese medicine treatment alone (although there was substantial heterogeneity of results and high risks of bias in these trials) (36,39,41,47), or those treated with psychotherapy alone (40).
Acupuncture versus no specific treatment
Two trials (38,43) compared the effects of acupuncture to no specific treatment. In both trials, all participants were allowed to continue receiving standard medical care for IBS, including prescribed medications, but control group participants were not assigned to any additional IBS treatment. Both of these trials showed a statistically significant benefit of acupuncture for improving IBS symptom severity, although there was substantial heterogeneity of results between the 2 trials (I2 = 57%).
Subgroup and sensitivity analyses
For the sham-controlled trials, subgroup analyses on risk of bias or treatment adequacy-related variables would be uninformative because all sham-controlled trials had similar results and no combination of these trials resulted in a pooled statistically significant benefit, for either the symptom severity or quality of life outcome. For trials comparing acupuncture versus pharmacological therapies, restriction to the 4 trials that compared acupuncture versus evidence-based (53) antispasmodic pharmacological therapies (35,45,46,49) had similar results (RR 1.21, 95% CI 1.07 to 1.37, 249 participants, I2=0). For the other comparisons, there were too few trials to attempt subgroup analyses (32).
For the Forbes et al trial (37), which reported both intent-to-treat (ITT) and available case data for the symptom severity outcome, a sensitivity analysis using ITT values instead of the available case values did not result in important differences in the SMDs for this trial.
Safety of acupuncture
Nine trials included descriptions of adverse events associated with acupuncture (33,34,36-38,41,43,45,46). For 8 of these 9 trials (33,34,36-38,41,43,46), no serious adverse events were reported, while the Shi et al trial (45) reported that 1 participant in the electro-acupuncture group withdrew because of syncope.
DISCUSSION
Summary of main results
Five sham-controlled RCTs have tested the effects of acupuncture for IBS, and 4 of these trials used adequate randomization, blinding, and had few withdrawals/drop-outs. None of these sham-controlled RCTs individually found a statistically significant benefit of acupuncture relative to sham acupuncture on the outcomes of symptom severity or quality of life. Similarly, pooling the data from these sham-controlled trials did not result in statistically significant benefits of acupuncture on either outcome. At the same time, 5 Chinese-language comparative effectiveness trials found that patients receiving acupuncture reported greater improvements in IBS symptoms compared to patients receiving pharmacological therapies for IBS.
How should physicians, researchers, and policy-makers interpret these seemingly contradictory trial findings in guiding treatment decisions and future trial design? First, both the comparative effectiveness trials and the sham-controlled trials have important limitations that complicate their interpretations. Namely, in the trials comparing acupuncture versus pharmacological therapy, in which the patients are not “blinded” to the treatment received, expectation effects (i.e., defined as “the impact of expectations on subjective outcomes” (54)), may differ between acupuncture and drugs (27,55,56). Such differences in expectations of benefits between acupuncture and drugs may contribute to different magnitudes of a placebo effect (i.e., a patient’s improvement in symptoms due to an inert treatment, or an inert component of a treatment). Because of the possibility of differential expectations of a benefit from acupuncture versus drugs in these trials (27,55,57), it cannot be determined whether any of the reported benefits of acupuncture are due to a larger biological effect of acupuncture needling relative to drugs, or rather due entirely to the impact of the study participants’ greater expectations of a benefit of acupuncture, on the subjective outcomes that they reported.
A limitation of the sham-controlled trial design is that the high placebo effects of sham acupuncture may preclude the detection of any small, true biological benefits of true acupuncture relative to sham, when patient-reported subjective outcome measures are used. Two “methodological” trials have evaluated the placebo effects of sham acupuncture, on both subjective and objective outcomes (56,58). One such methodological trial (56), designed to compare placebo effects of placebo pills and sham acupuncture, found that, relative to placebo pills, sham acupuncture was more credible as an authentic treatment and resulted in higher subjective patient reports of improvement. This trial also found that the placebo effect was confined to self-reported, subjective outcomes (e.g., pain) and that there was no placebo effect (i.e., no improvement from baseline) for either the placebo acupuncture or placebo pill on the objective outcome that they measured (i.e., grip strength). Another recent methodological trial (58) compared albuterol (i.e., a proven asthma drug) versus sham acupuncture for asthma patients, and found that while only the albuterol had a biological effect on the objective outcome of airway flow, both the sham acupuncture and albuterol groups had dramatic and comparable improvements from baseline on the subjective outcome of patient self-reports of improvement, such that the albuterol showed no benefit relative to the sham acupuncture on self-reported improvement.
These methodological trials suggest that relying exclusively on subjective patient reports, such as those used as outcomes in IBS trials, may result in a failure to detect small biological effects of an active treatment (i.e., true acupuncture) relative to a highly credible, but physiologically inert, sham acupuncture control. Thus, while the high placebo effects among IBS patients (59) make it difficult to show that any pharmacological treatment is superior to an inert placebo pill, demonstrating such an effect may be even more difficult when the placebo control is sham acupuncture.
Overall completeness and applicability of evidence
How externally valid are the results of this review? Namely, do the types of interventions investigated in these studies represent current best practice of acupuncture for IBS? Assessing adequacy of the acupuncture treatment procedure is important because, for instance, basing conclusions about acupuncture efficacy on a suboptimal procedure is “analogous to a pharmaceutical trial formulating conclusions about the efficacy of a drug based on an inadequate dose” (60). For the sham-controlled trials, it might be argued that a possible reason for the lack of benefit might be explained by the fact that there were too few treatment sessions, an inadequate treatment frequency, or an insufficient duration of treatment. All sham-controlled trials were judged by our acupuncture adequacy assessors to have used an adequate number of sessions and duration of treatment. Only the Forbes sham-controlled trial (37) was judged to use an inadequate treatment frequency because this trial involved only 1 acupuncture session per week (for 13 weeks), which even though judged inadequate, probably still well reflects clinical practice. The other sham-controlled trials all used 2 sessions per week, which was judged by the acupuncture adequacy assessors as an adequate treatment frequency, so it seems unlikely that an inadequate frequency of treatment explains the lack of benefit. Although the acupuncture assessors judged the treatment frequency of the sham-controlled trials to be largely adequate, the Chinese language comparative effectiveness trials used a much greater treatment frequency, with daily acupuncture treatments used in 9 out of 11 of these comparative effectiveness trials, and all 5 of these trials that compared acupuncture versus drugs. The higher acupuncture treatment frequency in the Chinese comparative effectiveness trials, relative to the sham-controlled trials, might also help explain the different benefits of acupuncture relative to the controls in these 2 subsets of trials.
Quality of the evidence
Four out of the 5 sham-controlled trials in this review (34,37,38,44) did not have limitations related to a risk of bias criterion. Only the trial by Lowe (42) used a sham control that might not have been sufficiently credible to blind participants to whether they were receiving a true or sham treatment; however, any unblinding to the treatment received in this trial would likely only overestimate the effect of acupuncture (28).
It might be argued that one potential methodological limitation is that 2 of the 5 sham-controlled RCTs (34,37) used a sham control that involved skin penetrating needles inserted at non-acupuncture points that the acupuncture assessors in our review judged to have potential weak physiological activity that might influence the outcome, and which might therefore have biased these 2 RCTs to the null. However, we would not expect this to explain the lack of benefit of acupuncture relative to sham, both because these 2 shams were judged to have potential for only weak physiological activity and also because the other 3 sham-controlled RCTs used shams that were judged unlikely to have physiological effects, and these 3 RCTs also found no benefit of acupuncture relative to sham.
The quality of the evidence is also limited by the fact that all sham-controlled trials except the Lembo trial (38) had small sample sizes and were each underpowered to detect a small benefit of the acupuncture protocol evaluated. Although these trials may have been adequately powered to detect a moderate to large benefit of acupuncture relative to sham, an effect size of this magnitude may have been unreasonable to expect, considering that even specific 5HT4 agonists (i.e., tegaserod) and 5HT3 antagonists (i.e., alosetron and cilansetron), which are the only treatments with “good quality of evidence” for treating IBS (1) have only a modest efficacy. Although a meta-analysis of the 5 sham-controlled trials increases the statistical power to detect an effect, a limitation of pooling trials with different acupuncture protocols is that we cannot rule out the possibility that larger trials or meta-analyses focusing on one of these protocols might show a benefit of treatment. In addition, although the meta-analysis point estimates suggest no effects, the meta-analysis confidence intervals include the possibility that there could be small benefits which could be important to patients. A final limitation of the sham-controlled trial evidence base, related to the small sample sizes, and also the heterogeneity of participants, is that these trials did not restrict eligibility to specific subtypes of IBS patients, and the proportions of patients with different IBS subtypes differed across trials. An individual patient data (IPD) meta-analysis would be necessary to address whether acupuncture has different effects on different subtypes of IBS patients, although the relatively small numbers of patients would be unlikely to provide a confident answer to this question.
In the Chinese language comparative effectiveness trials, in addition to the primary risk of bias associated with the absence of patient blinding, there were also risks of bias associated with the randomization procedure and the follow-up of patients. Notably, for 5 of these trials (36,39-41,48), there were equal sized treatment groups, and the trial investigators could not adequately explain during our telephone surveys how this was achieved. This raises the possibility that the randomization might not have been adequately generated or concealed (61). The notion that randomized trials should have equal numbers in each treatment group has been shown to commonly lead clinical trial investigators to force equality by unscientific means (61). Indeed, previous methodological reviews of this issue have found that over one-half of trials using simple, unrestricted randomization schemes report equal numbers in each group (61,62), and 88% of reported randomized trials have been shown to exclude some randomized participants from their analysis (62). The Chinese trials with high risks of bias associated with the randomization and/or the accounting of randomized patients in the outcomes assessments evaluated acupuncture as an adjuvant to either another Chinese medicine treatment or psychotherapy, or compared acupuncture versus psychotherapy, probiotics, or a drug not indicated or commonly used for IBS (i.e., sulfasalazine (48)). Therefore, the findings from these comparisons should be considered only hypothesis generating, and are not included in our overall conclusions. In contrast, there was an overall low risk of bias in the 4 comparative effectiveness trials that found acupuncture more effective than 2 antispasmodic pharmacological therapies shown to be effective for IBS (53,63) (i.e., pinaverium bromide (35,45,46) and Trimebutine maleate (49)).
Authors’ conclusions
Implications for practice
People with IBS have few treatment options available. Pharmacological therapies have modest benefits (24), can have high costs, and some of the newer drugs have been withdrawn from the market because of side effects (64,65). Safe, non-pharmacological therapies that may allow patients to feel more empowered and more in control of their symptoms should be evaluated for effectiveness. However, evaluating such complex non-pharmacological therapies for IBS (e.g., mindfulness meditation (66), hypnotherapy (67)) poses challenges, particularly in regards to selecting a placebo control or a credible alternative treatment control.
While acupuncture can theoretically be compared with a sham acupuncture “placebo” control, a fundamental challenge has been developing a sham acupuncture control that is sufficiently believable to patients as to be indistinguishable from true acupuncture, and yet at the same time not so similar to true acupuncture that the sham has a therapeutic effect of its own and is therefore not an inert placebo. The sham acupuncture controls used in 4 of the 5 sham-controlled trials in this review appeared to be believable as authentic treatments, but 2 of the 5 sham-controlled trials used sham controls that might have had weak physiological activity, and therefore these shams may not have been completely inert placebos. While none of the sham-controlled trials showed a benefit of acupuncture relative to sham acupuncture, it is still not clear whether these findings are because acupuncture has no true biological effect above and beyond a placebo, or whether instead acupuncture has small biological effects, but the small sample sizes and heterogeneity of participants and interventions in these trials precluded detecting a statistically significant pooled benefit of acupuncture over sham, or whether any biological effects of true acupuncture cannot be detected because they are overridden and obscured by the large placebo effects of the sham control (56,58). Evidence from 4 Chinese language comparative effectiveness trials (35,45,46,49) showed acupuncture to be superior to 2 antispasmodic drugs, both of which have consistently been shown to be effective in high quality trials (53,63), although neither is approved for treatment of IBS in the United States (63). Patient preferences and expectations may partly explain the positive findings of these trials comparing acupuncture to drugs. That is, if the trial participants have pretreatment preferences for acupuncture over drugs, these preferences may have influenced the participants’ later assessments of their subjective states, as reported on the patient-reported outcome measures used (27,55,57,68).
In addition to efficacy, safety and costs are other considerations. Safety is best determined with large prospective surveys of practitioners and 3 such surveys (13-15) show that serious adverse events after acupuncture are rare. There was 1 adverse event associated with acupuncture in the 9 trials that reported this outcome (33,34,36-38,41,43,45,46), although relatively small sample sizes limit the usefulness of this safety data. Finally, patients would also need to consider costs because acupuncture treatment often needs to be paid for out of pocket, at least in part.
Implications for research
Considering that our meta-analysis found no differences between acupuncture and sham, and also considering that there are limited resources available to conduct trials of acupuncture, a non-proprietary therapy, additional sham-controlled trials of acupuncture among IBS patients should not be a high priority in acupuncture research, at least until the large, ongoing sham-controlled trial, which is expected to complete data collection in March 2013, is published (Principal Investigator: Anastasi; n=171). This trial compares a sham control with 2 different acupuncture test treatment groups, one test group using a fixed formula and the other test group using an individualized treatment approach, for patients with diarrhea-predominant IBS. If this trial shows no benefit of acupuncture relative to the sham, then the need for additional sham-controlled trials would seem questionable. However, if this ongoing sham-controlled trial shows a benefit, then future sham-controlled trials building upon the results of this trial (e.g., restriction to diarrhea predominant IBS patients; using the same acupoints as used in this trial) would certainly be warranted. Such future sham-controlled trials should use non-penetrating, but demonstrably credible, shams to control for placebo effects, and ideally these sham needles should be placed far away from the true acupuncture points.
Because of the difficulties of controlling for placebo effects in acupuncture for IBS trials, which typically evaluate strictly subjective, patient-reported outcomes (e.g., symptom severity, quality of life), another approach forward for research is the evaluation of objective or semi-objective outcomes in IBS patients, using pragmatic and cost-effectiveness trials. Indeed, a recently completed trial (n=220) (Principal Investigator: MacPherson), compared the effectiveness and cost-effectiveness of acupuncture plus usual general practitioner (GP) care versus usual GP care alone, on the semi-objective outcomes of medication use, health service use, and days lost from work (69). Although this trial does not include a placebo control, because the outcome measures being assessed in this trial are semi-objective, its results will be less influenced by expectation effects (70-72), than trials that assess only strictly subjective outcomes (i.e., patient reports of symptom improvement). Indeed, the Rome criteria for design of IBS treatment trials note that placebo effects “are especially a problem where end points are subjective.” (23) If this recently completed cost-effectiveness trial shows that acupuncture reduces healthcare utilization, then whether the resulting cost-savings are due to a specific effect of acupuncture needling or non-specific effects (e.g., greater autonomy and empowerment of patients, positive patient-practitioner relationship) seems of secondary importance.
However, it must be borne in mind that the patient population who elected to participate in this acupuncture trial may have stronger a priori beliefs about the benefits of acupuncture, than does the average population of IBS patients. As a result, the non-specific effects experienced by the patients in this unblinded trial may not be generalizable to the average population of IBS patients. However, because this pragmatic trial was designed to test whether acupuncture may be helpful as an additional option to standard GP care alone, its results may be generalizable to the subset of IBS patients in general practice who would elect to receive acupuncture, who may also have a priori expectations for acupuncture to be beneficial. To produce results generalizable to the average population of IBS patients, investigators of future pragmatic trials might minimize the recruitment of participants with a preference for acupuncture by not specifying, in the recruitment of patients, that acupuncture is one of the treatment options being investigated.
Future comparative effectiveness trials would also be helpful to validate and extend the preliminary evidence in this review, which suggests that acupuncture is associated with greater improvements in subjective patient self-assessments than pharmacological therapies. As previously mentioned, a limitation of the acupuncture versus pharmacological therapy trials in this review is that they did not use a design that controlled for the effects of patients’ expectations for improvement, patient preferences, and non-specific therapeutic factors. Indeed, in the Chinese trials included in this review, the patients may well have had pre-treatment preferences for acupuncture, considering that these trials were conducted at hospitals of traditional Chinese medicine. Because acupuncture may elicit a greater expectation effect than pharmacological therapies or other active treatments (27,55,56), particularly among participants who have a preference for acupuncture, investigators conducting future trials that compare acupuncture with other active therapies should consider asking participants about their preferences and expectations (before and after the intervention), and studying the potential effects of pre-treatment preferences on study outcomes. Such trials should also include a credibility questionnaire to establish that the treatments being compared are perceived by the patients as equally credible treatments for IBS symptoms (66). Future comparative effectiveness trials in the West should also consider using a daily frequency of acupuncture, as was used in the Chinese trials in this review. However, even with additional well-designed trials, the truth about the effects of acupuncture for IBS will likely always be difficult to assess because the complexities and potential biases inherent to both the comparative effectiveness and sham acupuncture control designs makes it difficult to evaluate the subjective, patient-reported outcomes typically used in IBS trials.
Supplementary Material
Table 2.
Risk of bias summary*
| Adequate sequence generation |
Allocation concealment |
Patient blinding |
Incomplete outcome data addressed |
Free of selective reporting |
Baseline compara- bility a |
ITT b | |
|---|---|---|---|---|---|---|---|
| An (2010) (33) | Yes | [Yes] | No | [Unclear]C | Yes | Unclear | No |
| Anastasi (1999) (34) | [Yes] | [Yes] | Yes d | Yes | Yes | Yes | No |
| Chen (2009) (36) | [No] e | [No] | No | [Unclear]f | Yes | Yes | No |
| Chen (2011) (35) | Yes | [Yes] | No | [Yes] | Yes | Yes | No |
| Forbes (2005) (37) | Yes | [Yes] | Yes g | Yes | Yes | Yes | Yes |
| Lembo (2009) (38) | Yes | Yes | Yes h | Yes | Yes | Yes | Yes |
| Li (2009) (39) | [No] e | [No] | No | [Unclear]C | Yes | Yes | No |
| Liu (1997) (40) | [Unclear]i | [Unclear] | No | [Unclear]C | Yes | Unclear | No |
| Liu (2010) (41) | [Unclear]i | [No] | No | [Unclear]j | Yes | Unclear | No |
| Lowe (2000) (42) | [Yes] | [Yes] | Uncleark | UnclearC | Yes | Yes | Unclear |
| Reynolds (2008) (43) | [Yes] | [Yes] | No | Yes | No | Yes | Yes |
| Schneider (2006) (44) | [Yes] | Yes | Yes h | Yes | Yes | Yes | No |
| Shi (2010) (45) | [Yes] | [Yes] | No | Yes | Yes | Yes | Yes |
| Sun (2011) (46) | [Yes] | [No] | No | Yes | Yes | Yes | No |
| Xiong (2008) (47) | [Yes] | [No] | No | [Unclear]C | Yes | Unclear | No |
| Xue (2009) (48) | [Unclear]i | [Unclear] | No | Yes | Yes | Yes | No |
| Zeng (2010) (49) | Yes | [Yes] | No | Yes | Yes | Yes | No |
We followed the Cochrane Handbook criteria (28) for making judgments about the risk of bias for each of the 6 domains in the Cochrane Risk of Bias tool. Additional data obtained from RCT authors is enclosed in brackets to allow such data to be differentiated from the data included only in the publications.
For the baseline comparability criterion to score “Yes”, a comparison of the symptom scores between the treatment and control group(s) at baseline needed to be reported.
For the ITT criterion to score “Yes”, an intention-to-treat analysis needed to be reported.
In these trials, the authors did not report the numbers of drop-outs in their publication, and did not have records of the numbers of drop-outs to provide during the telephone interviews.
For the sham control, penetrating needles were superficially inserted at nonpoints which were 2-3 cm away from the true points, and placebo moxibustion was performed above the same sham points without generating a heat sensation.
The authors of these trials confirmed in telephone interviews that a few patients were non-randomly assigned to achieve identical sized treatment groups.
For this trial, the authors endeavored to maintain equal group sizes, by eliminating participants who withdrew during the trial and replacing them with new patients, and the number of such replacements was not recorded by the author.
For the sham control, acupuncture needles were inserted at areas on the body that do not correspond to acupuncture points and are deemed to have no therapeutic value. The points and needling technique were varied somewhat each week, as was also done in the true acupuncture group, who received individualized point selection.
For the sham control in these trials, the Streitberger placebo needle was used, which has been previously validated as a sufficiently credible sham (29). The Streitberger needles were placed close to the genuine acupuncture points in both trials. In both trials, the participants were acupuncture naïve.
This criterion was scored as “Unclear” because the authors of these 3 trials were unable to explain how equal sample sizes were achieved.
For this trial, the authors reported no drop-outs, which would be unusual in a 4 week trial of 300 participants.
The sham control used in this trial was judged to have been potentially detectable as a fake treatment by the trial participants. The sham procedure involved tapping a blunt needle on the skin and then taping the needle in place. Although this procedure was described as “validated”, we are unaware of a validation study for this procedure. Also, in the trial report there was no description of whether or not the patients were required to have never previously used acupuncture, and there were no reported tests for checking the success of the blinding.
Financial support
Brian Berman, Lixing Lao, Eric Manheimer, and L. Susan Wieland were partially funded by Grant Number R24 AT001293 from the National Center for Complementary and Alternative Medicine (NCCAM) of the US National Institutes of Health. Brian Berman and Lixing Lao were also partially funded by NIH Grant Number 1U19 AT003266. The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the NCCAM, or the US National Institutes of Health. Xueyong Shen and Ke Cheng were funded by the National Basic Research Program of China (973 Program, 2009CB522901, 2012CB518502), Key Program of Shanghai and State Administration of TCM of China (S30304), Science Foundation of Shanghai Municipal Commission of Science and Technology (09DZ1976600, 09DZ1974303, 10DZ1975800), and Shanghai Leading Academic Discipline Project (T0302 and B112).
Footnotes
CONFLICT OF INTEREST/STUDY SUPPORT
Guarantor of the article:
Eric Manheimer, MS
Specific author contributions:
Study concept and design: Eric Manheimer, L. Susan Wieland, Brian Berman, Ke Cheng, Lixing Lao, Xueyong Shen
Data extraction: Eric Manheimer, Ke Cheng, Shih Min Li, L. Susan Wieland
Acupuncture adequacy assessments: Lixing Lao, Shen Xueyong
Analysis and interpretation of data: Eric Manheimer
Drafting the manuscript: Eric Manheimer, with L. Susan Wieland contributing to the Methods section
Critically revised manuscript for important intellectual content and provided approval of the final draft submitted: Brian Berman, Ke Cheng, Lixing Lao, Shih Min Li, Eric Manheimer, Xueyong Shen, L. Susan Wieland
Potential competing interests:
None
Contributor Information
Eric Manheimer, Center for Integrative Medicine, University of Maryland School of Medicine, 520 W. Lombard St., East Hall, Baltimore, Maryland, 21201 USA.
L. Susan Wieland, Center for Integrative Medicine, University of Maryland School of Medicine, 520 W. Lombard St., East Hall, Baltimore, Maryland, 21201 USA.
Ke Cheng, College of Acupuncture-Moxibustion and Tuina, Shanghai University of Traditional Chinese Medicine, Shanghai Research Center for Acupuncture and Meridians, Shanghai, China.
Shih Min Li, Departamento de Clinica Medica, Universidade Federal de Santa Catarina, Florianopolis, SC, Brazil.
Xueyong Shen, College of Acupuncture-Moxibustion and Tuina, Shanghai University of Traditional Chinese Medicine, Shanghai Research Center for Acupuncture and Meridians, Shanghai, China.
Brian M. Berman, Center for Integrative Medicine, University of Maryland School of Medicine, 520 W. Lombard St., East Hall, Baltimore, Maryland, 21201 USA.
Lixing Lao, Center for Integrative Medicine, University of Maryland School of Medicine, 520 W. Lombard St., East Hall, Baltimore, Maryland, 21201 USA.
REFERENCES
- 1.Brandt LJ, Chey WD, Foxx-Orenstein AE, et al. An evidence-based position statement on the management of irritable bowel syndrome. Am J Gastroenterol. 2009;104(Suppl 1):S1–35. doi: 10.1038/ajg.2008.122. [DOI] [PubMed] [Google Scholar]
- 2.Saito YA, Schoenfeld P, Locke GR., 3rd. The epidemiology of irritable bowel syndrome in North America: a systematic review. Am J Gastroenterol. 2002;97:1910–5. doi: 10.1111/j.1572-0241.2002.05913.x. [DOI] [PubMed] [Google Scholar]
- 3.Vanner SJ, Depew WT, Paterson WG, et al. Predictive value of the Rome criteria for diagnosing the irritable bowel syndrome. Am J Gastroenterol. 1999;94:2912–7. doi: 10.1111/j.1572-0241.1999.01437.x. [DOI] [PubMed] [Google Scholar]
- 4.Saito YA, Locke GR, Talley NJ, et al. A comparison of the Rome and Manning criteria for case identification in epidemiological investigations of irritable bowel syndrome. Am J Gastroenterol. 2000;95:2816–24. doi: 10.1111/j.1572-0241.2000.03192.x. [DOI] [PubMed] [Google Scholar]
- 5.El-Serag HB, Olden K, Bjorkman D. Health-related quality of life among persons with irritable bowel syndrome: a systematic review. Aliment Pharmacol Ther. 2002;16:1171–85. doi: 10.1046/j.1365-2036.2002.01290.x. [DOI] [PubMed] [Google Scholar]
- 6.Maxion-Bergemann S, Thielecke F, Abel F, et al. Costs of irritable bowel syndrome in the UK and US. Pharmacoeconomics. 2006;24:21–37. doi: 10.2165/00019053-200624010-00002. [DOI] [PubMed] [Google Scholar]
- 7.Longstreth GF, Wilson A, Knight K, et al. Irritable bowel syndrome, health care use, and costs: a U.S. managed care perspective. Am J Gastroenterol. 2003;98:600–7. doi: 10.1111/j.1572-0241.2003.07296.x. [DOI] [PubMed] [Google Scholar]
- 8.Talley NJ, Gabriel SE, Harmsen WS, et al. Medical costs in community subjects with irritable bowel syndrome. Gastroenterology. 1995;109:1736–41. doi: 10.1016/0016-5085(95)90738-6. [DOI] [PubMed] [Google Scholar]
- 9.Everhart JE. The burden of digestive diseases in the United States. US Department of Health and Human Services, Public Health Service, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. US Government Printing Office; Washington, DC: 2008. p. 142. NIH Publication No. 09-6443. [Google Scholar]
- 10.Ford AC, Talley NJ, Schoenfeld PS, et al. Efficacy of antidepressants and psychological therapies in irritable bowel syndrome: systematic review and meta-analysis. Gut. 2009;58:367–78. doi: 10.1136/gut.2008.163162. [DOI] [PubMed] [Google Scholar]
- 11.Brenner DM, Moeller MJ, Chey WD, et al. The utility of probiotics in the treatment of irritable bowel syndrome: a systematic review. Am J Gastroenterol. 2009;104:1033–49. doi: 10.1038/ajg.2009.25. quiz 1050. [DOI] [PubMed] [Google Scholar]
- 12.Lim B, Manheimer E, Lao L, et al. Acupuncture for treatment of irritable bowel syndrome. Cochrane Database Syst Rev. 2006;(4) doi: 10.1002/14651858.CD005111.pub2. Art. No.: CD005111. DOI: 10.1002/14651858.CD005111.pub2. [DOI] [PubMed] [Google Scholar]
- 13.MacPherson H, Thomas K, Walters S, et al. The York acupuncture safety study: prospective survey of 34 000 treatments by traditional acupuncturists. BMJ. 2001;323:486–7. doi: 10.1136/bmj.323.7311.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Melchart D, Weidenhammer W, Streng A, et al. Prospective investigation of adverse effects of acupuncture in 97 733 patients. Arch Intern Med. 2004;164:104–5. doi: 10.1001/archinte.164.1.104. [DOI] [PubMed] [Google Scholar]
- 15.White A, Hayhoe S, Hart A, et al. Adverse events following acupuncture: prospective survey of 32 000 consultations with doctors and physiotherapists. BMJ. 2001;323:485–6. doi: 10.1136/bmj.323.7311.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ford AC, Guyatt GH, Talley NJ, et al. Errors in the conduct of systematic reviews of pharmacological interventions for irritable bowel syndrome. Am J Gastroenterol. 2010;105:280–8. doi: 10.1038/ajg.2009.658. [DOI] [PubMed] [Google Scholar]
- 17.Wu T, Li Y, Bian Z, et al. Randomized trials published in some Chinese journals: how many are randomized? Trials. 2009;10:46. doi: 10.1186/1745-6215-10-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ezzo JM, Richardson MA, Vickers A, et al. Acupuncture-point stimulation for chemotherapy-induced nausea or vomiting. Cochrane Database Syst Rev. 2006;(2) doi: 10.1002/14651858.CD002285.pub2. Art. No.: CD002285. DOI: 10.1002/14651858.CD002285.pub2. [DOI] [PubMed] [Google Scholar]
- 19.Mangel AW, Hahn BA, Heath AT, et al. Adequate relief as an endpoint in clinical trials in irritable bowel syndrome. J Int Med Res. 1998;26:76–81. doi: 10.1177/030006059802600203. [DOI] [PubMed] [Google Scholar]
- 20.Francis CY, Morris J, Whorwell PJ. The irritable bowel severity scoring system: a simple method of monitoring irritable bowel syndrome and its progress. Aliment Pharmacol Ther. 1997;11:395–402. doi: 10.1046/j.1365-2036.1997.142318000.x. [DOI] [PubMed] [Google Scholar]
- 21.Patrick DL, Drossman DA, Frederick IO, et al. Quality of life in persons with irritable bowel syndrome: development and validation of a new measure. Dig Dis Sci. 1998;43:400–11. doi: 10.1023/a:1018831127942. [DOI] [PubMed] [Google Scholar]
- 22.Bijkerk CJ, de Wit NJ, Muris JW, et al. Outcome measures in irritable bowel syndrome: comparison of psychometric and methodological characteristics. Am J Gastroenterol. 2003;98:122–7. doi: 10.1111/j.1572-0241.2003.07158.x. [DOI] [PubMed] [Google Scholar]
- 23.Irvine EJ, Whitehead WE, Chey WD, et al. Design of treatment trials for functional gastrointestinal disorders. Gastroenterology. 2006;130:1538–51. doi: 10.1053/j.gastro.2005.11.058. [DOI] [PubMed] [Google Scholar]
- 24.Ford AC, Brandt LJ, Young C, et al. Efficacy of 5-HT3 antagonists and 5-HT4 agonists in irritable bowel syndrome: systematic review and meta-analysis. Am J Gastroenterol. 2009;104:1831–43. doi: 10.1038/ajg.2009.223. quiz 1844. [DOI] [PubMed] [Google Scholar]
- 25.Furlan AD, van Tulder MW, Cherkin DC, et al. Acupuncture and dry-needling for low back pain. Cochrane Database Syst Rev. 2005;(1) doi: 10.1002/14651858.CD001351.pub2. Art. No.: CD001351. DOI: 10.1002/14651858.CD001351.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manheimer E, Cheng K, Linde K, et al. Acupuncture for peripheral joint osteoarthritis. Cochrane Database Syst Rev. 2010;(1) doi: 10.1002/14651858.CD001977.pub2. Art. No.: CD001977. DOI:10.1002/14651858.CD001977.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manheimer E, Linde K, Lao L, et al. Meta-analysis: acupuncture for osteoarthritis of the knee. Annals of Internal Medicine. 2007;146:868–77. doi: 10.7326/0003-4819-146-12-200706190-00008. [DOI] [PubMed] [Google Scholar]
- 28.Higgins JPT, Altman DG, Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration; [accessed 5 October 2011]. 2011. Chapter 8: Assessing risk of bias in included studies. Version 5.1.0 [updated March 2011] Available from www.cochrane-handbook.org. [Google Scholar]
- 29.Streitberger K, Kleinhenz, et al. Introducing a Placebo Needle into Acupuncture Research. Lancet. 1998;352:364–5. doi: 10.1016/S0140-6736(97)10471-8. [DOI] [PubMed] [Google Scholar]
- 30.Review Manager (RevMan) [Computer program] Version 5.1. The Nordic Cochrane Centre, The Cochrane Collaboration; Copenhagen: 2011. [Google Scholar]
- 31.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deeks JJ, Higgins JPT, Altman DG, Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011] The Cochrane Collaboration; [accessed 5 October 2011]. 2011. Chapter 9: Analysing data and undertaking meta-analyses. Available from www.cochrane-handbook.org. [Google Scholar]
- 33.An G, Li N, Zhai G, et al. [Evaluation of the therapeutic effect of acupuncture and moxibustion on irritable bowel syndrome] Shanghai Journal of Acupuncture and Moxibustion. 2010;29:354–6. [Google Scholar]
- 34.Anastasi JK, McMahon DJ, Kim GH. Symptom management for irritable bowel syndrome: a pilot randomized controlled trial of acupuncture/moxibustion. Gastroenterol Nurs. 2009;32:243–55. doi: 10.1097/SGA.0b013e3181b2c920. [DOI] [PubMed] [Google Scholar]
- 35.Chen Q. [Clinical study on irritable bowel syndrome of diarrhea type with Chang-three-needle technique] Guangzhou University of Traditional Chinese Medicine Master’s Thesis; Guangzhou: 2011. [Google Scholar]
- 36.Chen Y, Lai X. [Clinical observation on combined warming needle and patented Chinese medicine for irritable bowel syndrome] Journal of Acupuncture and Tuina Science. 2009:274–7. [Google Scholar]
- 37.Forbes A, Jackson S, Walter C, et al. Acupuncture for irritable bowel syndrome: a blinded placebo-controlled trial. World J Gastroenterol. 2005;11:4040–4. doi: 10.3748/wjg.v11.i26.4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lembo AJ, Conboy L, Kelley JM, et al. A treatment trial of acupuncture in IBS patients. Am J Gastroenterol. 2009;104:1489–97. doi: 10.1038/ajg.2009.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li W. [Treatment of warming needle moxibustion combined with spinal manipulation on irritable bowel syndrome] China Practical Medicine. 2009;4:212–3. [Google Scholar]
- 40.Liu GZ. [Treatment of acupuncture and moxibustion plus psychotherapy for irritable bowel syndrome] Chinese Acupuncture and Moxibustion. 1997;10:611–2. [Google Scholar]
- 41.Liu Q, Wang Z, Zhang W, et al. [Clinical observation on Geshanxiaoyao Decoction combining acupuncture effect on the life quality of patients with irritable bowel syndrome] Journal of Zhejiang University of Traditional Chinese Medicine. 2010;34:510–3. [Google Scholar]
- 42.Lowe C, Depew W, Vanner S. A placebo-controlled, double-blind trial of acupuncture in the treatment of irritable bowel syndrome (IBS) Gastroenterology. 2000;118:A3168. [Google Scholar]
- 43.Reynolds JA, Bland JM, MacPherson H. Acupuncture for irritable bowel syndrome an exploratory randomised controlled trial. Acupunct Med. 2008;26:8–16. doi: 10.1136/aim.26.1.8. [DOI] [PubMed] [Google Scholar]
- 44.Schneider A, Enck P, Streitberger K, et al. Acupuncture treatment in irritable bowel syndrome. Gut. 2006;55:649–54. doi: 10.1136/gut.2005.074518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shi X, Luo J, Tan T. [Clinical observation of electroacupuncture on diarrhea-predominant irritable bowel syndrome] Journal of New Chinese Medicine. 2010;42:72–4. [Google Scholar]
- 46.Sun JH, Wu XL, Xia C, et al. Clinical evaluation of Soothing Gan and invigorating Pi acupuncture treatment on diarrhea-predominant irritable bowel syndrome. Chin J Integr Med. 2011;17:780–5. doi: 10.1007/s11655-011-0875-z. [DOI] [PubMed] [Google Scholar]
- 47.Xiong X, Lin Y. [Acupuncture plus traditional Chinese herb medicine in treating 42 patients with diarrhea-predominant IBS] Journal of Fujian University of TCM. 2008;18:38–40. [Google Scholar]
- 48.Xue Y, Tian X. [The clinical research of the relationship between radicals and irritable bowel syndrome and the effect of acupuncture] Chinese Archives of Traditional Chinese Medicine. 2009;27:111–2. [Google Scholar]
- 49.Zeng Y, Bao Y, Chu J, et al. [Effect of moxibustion for diarrhea-dominant irritable bowel syndrome patients in Dog Days] Chinese Archives of Traditional Chinese Medicine. 2010;28:1774–6. [Google Scholar]
- 50.Fireman Z, Segal A, Kopelman Y, et al. Acupuncture treatment for irritable bowel syndrome. A double-blind controlled study. Digestion. 2001;64:100–3. doi: 10.1159/000048847. [DOI] [PubMed] [Google Scholar]
- 51.Liao YC. [Acupuncture treatment for 132 cases of irritable bowel syndrome] Clinical Journal of Acupuncture and Moxibustion. 2000;16:13–4. [Google Scholar]
- 52.Liu M. [Clinical report of ear acupuncture treatment for functional gastric disorder] Shanghai Journal of Acupuncture and Moxibustion. 1995;14:247–8. [Google Scholar]
- 53.Ruepert L, Quartero AO, de Wit NJ, et al. Bulking agents, antispasmodics and antidepressants for the treatment of irritable bowel syndrome. Cochrane Database Syst Rev. 2011;(8) doi: 10.1002/14651858.CD003460.pub3. Art. No.: CD003460. DOI: 10.1002/14651858.CD003460.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Flum DR. Interpreting surgical trials with subjective outcomes: avoiding UnSPORTsmanlike conduct. JAMA. 2006;296:2483–5. doi: 10.1001/jama.296.20.2483. [DOI] [PubMed] [Google Scholar]
- 55.O’Connell NE, Wand BM, Goldacre B. Interpretive bias in acupuncture research?: A case study. Eval Health Prof. 2009;32:393–409. doi: 10.1177/0163278709353394. [DOI] [PubMed] [Google Scholar]
- 56.Kaptchuk TJ, Stason WB, Davis RB, et al. Sham device v inert pill: randomised controlled trial of two placebo treatments. BMJ. 2006;332:391–7. doi: 10.1136/bmj.38726.603310.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Linde K, Witt CM, Streng A, et al. The impact of patient expectations on outcomes in four randomized controlled trials of acupuncture in patients with chronic pain. Pain. 2007;128:264–71. doi: 10.1016/j.pain.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 58.Wechsler ME, Kelley JM, Boyd IO, et al. Active albuterol or placebo, sham acupuncture, or no intervention in asthma. N Engl J Med. 2011;365:119–26. doi: 10.1056/NEJMoa1103319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Spiller RC. Problems and challenges in the design of irritable bowel syndrome clinical trials: experience from published trials. Am J Med. 1999;107:91S–97S. doi: 10.1016/s0002-9343(99)00086-8. [DOI] [PubMed] [Google Scholar]
- 60.Ezzo J, Lao L, Berman B. Assessing clinical efficacy of acupuncture: what has been learned from systematic reviews of acupuncture? In: Stux G, Hammerschlag R, editors. Clinical acupuncture: scientific basis. Springer; New York: 2001. pp. 113–130. [Google Scholar]
- 61.Schulz KF, Grimes DA. Unequal group sizes in randomised trials: guarding against guessing. Lancet. 2002;359:966–70. doi: 10.1016/S0140-6736(02)08029-7. [DOI] [PubMed] [Google Scholar]
- 62.Adetugbo K, Williams H. How well are randomized controlled trials reported in the dermatology literature? Arch Dermatol. 2000;136:381–5. doi: 10.1001/archderm.136.3.381. [DOI] [PubMed] [Google Scholar]
- 63.Jailwala J, Imperiale TF, Kroenke K. Pharmacologic treatment of the irritable bowel syndrome: a systematic review of randomized, controlled trials. Ann Intern Med. 2000;133:136–47. doi: 10.7326/0003-4819-133-2-200007180-00013. [DOI] [PubMed] [Google Scholar]
- 64.Pasricha PJ. Desperately seeking serotonin… A commentary on the withdrawal of tegaserod and the state of drug development for functional and motility disorders. Gastroenterology. 2007;132:2287–90. doi: 10.1053/j.gastro.2007.04.057. [DOI] [PubMed] [Google Scholar]
- 65.Thompson CA. Alosetron withdrawn from market. Am J Health Syst Pharm. 2001;58:13. doi: 10.1093/ajhp/58.1.13a. [DOI] [PubMed] [Google Scholar]
- 66.Gaylord SA, Palsson OS, Garland EL, et al. Mindfulness training reduces the severity of irritable bowel syndrome in women: results of a randomized controlled trial. Am J Gastroenterol. 2011;106:1678–88. doi: 10.1038/ajg.2011.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lindfors P, Unge P, Arvidsson P, et al. Effects of gut-directed hypnotherapy on IBS in different clinical settings-results from two randomized, controlled trials. Am J Gastroenterol. 2011 Oct 4; doi: 10.1038/ajg.2011.340. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 68.Kalauokalani D, Cherkin DC, Sherman KJ, et al. Lessons from a trial of acupuncture and massage for low back pain: patient expectations and treatment effects. Spine (Phila Pa 1976) 2001;26:1418–24. doi: 10.1097/00007632-200107010-00005. [DOI] [PubMed] [Google Scholar]
- 69.MacPherson H, Bland M, Bloor K, et al. Acupuncture for irritable bowel syndrome: a protocol for a pragmatic randomised controlled trial. BMC Gastroenterol. 2010;10:63. doi: 10.1186/1471-230X-10-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hrobjartsson A, Gotzsche PC. Placebo interventions for all clinical conditions. Cochrane Database Syst Rev. 2004;(3) doi: 10.1002/14651858.CD003974.pub2. Art. No.: CD003974. DOI:10.1002/14651858.CD003974.pub2. [DOI] [PubMed] [Google Scholar]
- 71.Wood L, Egger M, Gluud LL, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta-epidemiological study. BMJ. 2008;336:601–5. doi: 10.1136/bmj.39465.451748.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Manheimer E. Selecting a control for in vitro fertilization and acupuncture randomized controlled trials (RCTs): how sham controls may unnecessarily complicate the RCT evidence base. Fertil Steril. 2011;95:2456–61. doi: 10.1016/j.fertnstert.2011.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Whitehead WE, Corazziari E, Prizont R, et al. Definition of a responder in clinical trials for functional gastrointestinal disorders: Report on a symposium. In: Drossman DA, editor. ROME II: The functional gastrointestinal disorders. 2nd ed. Degnon Associates; McLean, VA: 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Johnson JA, Coons SJ. Comparison of the EQ-5D and SF-12 in an adult US sample. Qual Life Res. 1998;7:155–66. doi: 10.1023/a:1008809610703. [DOI] [PubMed] [Google Scholar]
- 75.Groll D, Vanner SJ, Depew WT, et al. The IBS-36: a new quality of life measure for irritable bowel syndrome. Am J Gastroenterol. 2002;97:962–71. doi: 10.1111/j.1572-0241.2002.05616.x. [DOI] [PubMed] [Google Scholar]
- 76.Chassany O, Marquis P, Scherrer B, et al. Validation of a specific quality of life questionnaire for functional digestive disorders. Gut. 1999;44:527–33. doi: 10.1136/gut.44.4.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Heaton KW, Ghosh S, Braddon FE. How bad are the symptoms and bowel dysfunction of patients with the irritable bowel syndrome? A prospective, controlled study with emphasis on stool form. Gut. 1991;32:73–9. doi: 10.1136/gut.32.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.