Abstract
Objective
To evaluate whether transfusion of cell saver salvaged, stored at the bedside for up to 24 hours, would decrease the number of post-operative allogeneic RBC transfusions and donor exposures, and possibly improve clinical outcomes.
Design
Prospective, randomized, controlled, clinical trial.
Setting
Pediatric cardiac intensive care unit.
Patients
Infants <20kg (n = 106) presenting for cardiac surgery with cardiopulmonary bypass.
Interventions
Subjects were randomized to a cell saver transfusion group where cell saver blood was available for transfusion up to 24 hours post-collection, or to a control group. Cell saver subjects received cell saver blood for volume replacement and/or RBC transfusions. Control subjects received crystalloid or albumin for volume replacement and RBCs for anemia. Blood product transfusions, donor exposures, and clinical outcomes were compared between groups.
Measurements and Main Results
Children randomized to the cell saver group had significantly fewer RBC transfusions (cell saver: 0.19 ± 0.44 v. control: 0.75 ± 1.2; p = 0.003) and coagulant product transfusions in the first 48 hours post-op (cell saver: 0.09 ± 0.45 v. control: 0.62 ± 1.4; p = 0.013), and significantly fewer donor exposures (cell saver: 0.60 ± 1.4 v. control: 2.3 ± 4.8; p =0.019). This difference persisted over the first week post-op, but did not reach statistical significance (cell saver: 0.64 ± 1.24 v. control: 1.1 ± 1.4; p =0.07). There were no significant clinical outcome differences.
Conclusion
Cell saver blood can be safely stored at the bedside for immediate transfusion for 24 hours post-collection. Administration of cell saver blood significantly reduces the number of RBC and coagulant product transfusions and donor exposures in the immediate post-operative period. Reduction of blood product transfusions has the potential to reduce transfusion-associated complications and decrease post-operative morbidity. Larger studies are needed to determine whether this transfusion strategy will improve clinical outcomes.
Keywords: transfusion, pediatric cardiac surgery, congenital heart disease, red blood cells, cardiopulmonary bypass
INTRODUCTION
Neonates and infants undergoing cardiac surgery with cardiopulmonary bypass (CPB) typically require large numbers of blood product transfusions (1,2). Increasing recognition of the morbidity and mortality associated with red blood cell (RBC) and blood component transfusions in both adult (3–5) and pediatric cardiac surgery (6–8) patients has increased the interest in restriction, modification, and conservation of RBC transfusions. Use of cell saver devices as a blood conservation technique is commonly employed in adult cardiac and non-cardiac surgery without increased complication rates (9). However, technical limitations prevented cell saver use in pediatrics, and only recent advancements have made cell savers feasible for the small volume requirements of neonates and infants (10).
Observational studies suggest that cell saver systems safely decrease the volume of RBC transfusions in children (11) and small infants (12) undergoing surgical procedures, and those having cardiac surgery with CPB (13). Despite this, there is only a single prospective non-randomized cohort study describing experience with postoperative transfusion of intra-operative cell salvage using a dedicated pediatric system (14), and that work was limited by availability of cell saver salvaged blood for only six hours following its collection.
We hypothesized that transfusion of cell saver salvaged blood, for up to 24 hours post collection, can be performed safely, and will reduce the number of post-operative allogeneic RBC transfusions and donor exposures. Reduction of the number of RBC transfusions will limit the known risks of RBC transfusions, will reduce transfusion-associated complications, and may improve clinical outcomes.
The objective of this study is to determine the impact of intra-operative cell saver blood collection, as a blood conservation technique, on post-operative allogeneic blood product administration. The primary aim of the study is to compare the number of allogeneic blood product transfusions in the first 48 hours post-op between patients randomized to receive cell saver blood v. current standard of care for RBC transfusions and volume replacement. Secondary aims are to compare donor exposures, clinical outcome measures and complication rates between patients randomized to receive cell saver blood v. current standard of care.
MATERIALS AND METHODS
Subjects
Children ≤ 20 kg presenting to the University of Rochester Medical Center (URMC) for cardiac surgical repair/palliation with CPB were eligible. Exclusion criteria were weight > 21 kg, if their parent/guardian did not speak English, or if consent could not be obtained. The protocol was approved by the URMC Research Subjects Review Board and was registered with ClinicalTrials.gov (NCT01211366).
Subjects were enrolled at their pre-anesthesia visit, with properly witnessed and documented informed consent. Once enrolled, subjects were divided into groups according to their weight (≤10 kg or ≥ 11 kg) and risk adjusted congenital heart surgery (RACHS-1) score (1–3 “less severe” or 4–6 “more severe”) (15). Block randomization was used to randomize subjects to the cell saver or control transfusion strategy.
Transfusion and Volume Replacement Strategy
The transfusion and volume replacement strategy was initiated following completion of CPB in the operating room and maintained for 24 hours following either collection of cell saver salvaged blood or discontinuation of CPB.
Control Group
Subjects received crystalloid, colloid, or allogeneic RBC transfusions for hemodynamic compromise (tachycardia, hypotension, poor pulses/perfusion, rising lactate, low central venous pressure (CVP), poor urine output) with/without anemia as per our current standard of care.
Volume replacement
Our current standard is for patients with “adequate” hemoglobin levels (not solely defined by a Hb “trigger” but a composite clinical impression) in the setting of hemodynamic instability to receive volume replacement in the form of crystalloid (NS or LR) or colloid (5% albumin).
Blood component transfusions
For patients with evidence of hemodynamic instability and/or poor oxygenation and anemia, allogeneic RBCs (10–15cc/kg) are transfused. Our pediatric cardiac intensive care unit (PCICU) standard of care is to carefully consider each individual patient’s clinical condition in the decision to transfuse RBCs. We do not have a specific transfusion protocol, but take into account the presence or absence of cyanosis, cardiac function, single or bi-ventricular physiology, and the particular cardiac procedure, to ensure that all transfusions are clinically based. Generally, our cyanotic children with single ventricle physiology are transfused for Hb < 9.0 – 10 g/dl and children following bi-ventricular repairs for Hb < 7.0 – 8 g/dl, unless there are signs of clinical compromise (poor hemodynamics or significant oxygen requirement) or ongoing bleeding. This allows the PCICU attending to use their best clinical judgment, based on each subjects’ condition, as to type of volume replacement – saline, albumin, or RBCs. Coagulant products are transfused according to factor or platelet deficiency with ongoing bleeding.
Cell Saver Group
Volume replacement
Subjects received cell saver blood for volume replacement if there was evidence of hemodynamic compromise (as described above) without anemia and their Hb was ≤ 13 g/dL (to avoid causing polycythemia). If they had evidence of hemodynamic compromise and their Hb was > 13 g/dL they were given crystalloid or albumin instead of cell saver blood.
Blood component transfusions
Cell saver blood was transfused for the same indications as described above for allogeneic RBCs in control group subjects. The patient’s clinical condition, and not a specific Hb threshold or protocol, was used in the decision to transfuse with cell saver blood.
Cell saver was available for 24 hours post its collection and then discarded. After this time period and for the remainder of their hospital admission cell saver subjects were managed according to the current standard of care as described for the control group above. If all of the cell saver blood was utilized before the 24 hour period elapsed, children were subsequently managed according to the current standard of care as described for the control group and received either allogeneic RBCs or crystalloid/colloid. Coagulant products are transfused according to factor or platelet deficiency with ongoing bleeding.
With the exception of the initial volume/Hb replacement strategy, all participating subjects received the current standard of care for their medical and surgical management.
Surgical Management
Operative and perioperative management including the initiation of anesthesia, CPB technique and surgical repair/palliation procedures were performed per current standard of care at URMC with the sole exception being the collection of cell saver blood at the end of CPB for subjects assigned to that group. The cardiac surgeon was blinded to study assignment to prevent any potential bias of the surgical and/or CPB management. Obvious differences in packaging and labeling of blood products prevented blinding of the perfusionists, anesthesiologists and PCICU personnel.
Vasoactive medications (epinephrine, dopamine, or milrinone drips) were used to maintain hemodynamics during transition after CPB at the discretion of the cardiac surgical team. All patients remained intubated and sedated for transfer to the pediatric cardiac intensive care unit (PCICU). Surgical, CPB and transfusion details were collected, as well as any intraoperative complications (i.e., arrhythmias, acidosis, bleeding or poor ventricular function).
CPB procedures for all subjects
Two bypass circuits were used in this study. All cases used the Terumo System 1 Heart Lung Machine (Terumo Corporation, Tokyo, Japan).
-
For subject’s < 11kg: an Fx05 oxygenator with hardshell reservoir and integrated arterial line filter (Terumo Corporation, Tokyo, Japan) was used. The circuit A/V loop consisted of 3/16” x ¼ “ tubing (Medtronic Inc., Minnesota, USA), and a roller pump with ¼” raceway used in the arterial position. Only the oxygenator is coated with Terumo’s X-coating. Conventional ultrafiltration (CUF) was used on all cases, and modified ultrafiltration (MUF) on none.
The bypass prime consisted of: 200mg/kg mannitol, 1000units heparin, 25mg/kg cephazolin (up to 1gm), methylprednisolone 30mg/kg (only in subjects <6 months of age or in cases requiring deep hypothermic circulatory arrest), aminocaproic acid 250mg, 10–15 mEq 8.4% sodium bicarbonate, 25% albumin, and red blood cells (RBCs) as needed to achieve an “acceptable” hematocrit (HCT) on CPB (as determined by CT surgeon and perfusionists per current standard of care). Circuits primed with RBCs used 25 mL of 25% Albumin, and asanguinous primes used 50 mL of 25% Albumin.
For subjects from 11–20kg: the Terumo RX-15 oxygenator (Terumo Corporation, Tokyo, Japan) with hard-shell reservoir was utilized with a stand-alone arterial line filter, the Pediatric Affinity (Medtronic, Inc., Minnesota, USA). The circuit A-V loop consisted of ¼” x 3/8” tubing, and a roller pump with 3/8” raceway in the arterial position. Only the oxygenator is coated with Terumo’s X-coating. Again, CUF was used on all subjects, and MUF on none. The bypass circuit for these subjects contained the same kg dosages of mannitol, cephazolin, methylprednisolone, aminocaproic acid, and sodium bicarbonate as described above including 2000 units of heparin, 50 mL 25% albumin, and RBCs. Again, RBCs were added if needed to obtain an “acceptable” on-CPB HCT.
Following the termination of CPB and sequestration of the volume in the venous line, the residual pump volume was hemoconcentrated to the lowest possible level. For infants, some of this remaining pump volume was transferred into syringes for re-infusion by the Anesthesia team during the remainder of the intra-operative period. For older subjects, some of this volume was transferred into a 600 mL collection bag for re-infusion by the Anesthesia team as needed.
Cell-saver blood collection
Any blood not re-infused by the anesthesiologist was returned to the perfusionist at the end of the case. This “pump salvage” was then infused into the Fresinius Continuous AutoTransfusion System® (CATS) (Terumo Corporation, Tokyo, Japan). The residual volume in the bypass circuit was also chased into the CATS system, and the total volume processed and washed and transferred into a collection bag labeled with a unique label for patient identification stating “autologous use only”. The cell saver salvaged red cells were transported to the PCICU in a designated “cell saver” blood temperature controlled and monitored cooler. Aliquots for transfusion were drawn off this bag aseptically using a needleless adapter by PCICU nursing staff.
Cell saver coolers were managed at the subject’s bedside maintained between 1 and 6°, in keeping with New York State blood bank regulation 58-2.25, allowing blood to be stored up to 24 hours from collection (16). Blood Bank staff performed quality assurance checks every 4 hours to ensure proper temperature regulation. Cell saver blood not utilized during the intervention period was discarded (after 24 hours from collection).
Post-operative Management
Subjects were managed postoperatively per the current standard of care aside from their transfusion strategy. At the URMC, a PCICU attending is “in house” 24 hr/day and therefore weaning from mechanical ventilation and/or inotropes occurs around the clock. Subjects were weaned from mechanical ventilation and extubated as their cardiopulmonary status allowed. Crystalloid or 5% albumin were infused in 5–20cc/kg boluses for clinical findings of hypovolemia and poor cardiac output (i.e., tachycardia, poor pulses and perfusion, low urine output, or hypotension) as needed to maintain hemodynamic stability. Vasoactive medications were adjusted to maintain hemodynamics and end-organ perfusion. Subjects received anticoagulation (i.e., aspirin, enoxaparin, warfarin) per the current PCICU Anticoagulant strategy as appropriate for their type of cardiac defect, surgical palliation/repair and post-operative medical management.
URMC Blood Bank Procedures
Transfusion of RBC, platelet, thawed whole plasma and cryoprecipitate products were based on a standard PCICU protocol which was adhered to throughout the trial. The RBC transfusion protocol takes into account the cardiac defect and surgical procedure; the overall hemodynamics of the subject (as assessed by blood pressure, central venous pressure (CVP), oxygenation status, distal perfusion/temperature, urine output, serum lactate; for pro-coagulant product transfusions key information includes the presence/absence of active bleeding or mediastinal tube (MT) output, in addition to the Hb, platelet count and tests of hemostasis. RBCs were transfused in a volume of 10–15cc/kg at a rate at the discretion of the attending physician.
All blood is pre-storage leukoreduced, irradiated using a CIS-US IBL 437 blood irradiator (delivering 2500 cg), and ABO identical. Red cells with short storage duration (storage days) were utilized as available (usually < 14 days for red cells; platelets were 3–5 days of storage). Platelets were transfused in a volume of 10mL/kg for a platelet count <20K, or <50K + significant bleeding. Platelets and coagulant components (fresh-frozen plasma, cryoprecipitate and/or recombinant factors) were transfused according to PCICU protocol which was adhered to throughout the trial.
Clinical Outcome Measures
Number of RBC and component blood product transfusions, donor exposures, and volume of crystalloid/colloid administered were recorded. Length of mechanical ventilation, vasoactive agents, PCICU and hospital length of stay was followed. Infections (based on clinical and culture data), bleeding complications and thrombosis (based on clinical and radiographic data) were recorded. Mediastinal tube drainage, Hb, platelet and coagulant protein levels were also followed.
wrCRP
For the first three post-operative days (POD) a wide range C-reactive protein (wrCRP) was measured. wrCRP was performed at the URMC inpatient laboratory, analyzed by immunoturbidmetric assay (ADVIA 2400 Chemistry System-Bayer ADVIA kit, Bayer Healthcare, Tarrytown, NY).
Statistical analysis
Sample size calculations were conducted before initiation of the study. The study was designed to have adequate power for the primary hypothesis. Previous data regarding URMC blood utilization for pediatric cardiac patients was available from previous studies. It was calculated that a sample size of 53 subjects per group would provide 80% power to detect a 25% absolute decrease in the number of RBC transfusions, which is considered clinically significant.
Descriptive statistics were used to summarize demographics and outcome measures by treatment group. These analyses include means, standard deviations for continuous variables, or frequencies and proportions for categorical variables. For data that was not normally distributed median values were compared using nonparametric tests. For normally distributed data, t tests were performed to test for significance. SPSS version 17.0 (SPSS, Chicago, IL) was used for all statistical analysis. Analyses were performed according to the intention-to-treat principle and include data from all randomized subjects. A p < 0.05 was considered statistically significant.
RESULTS
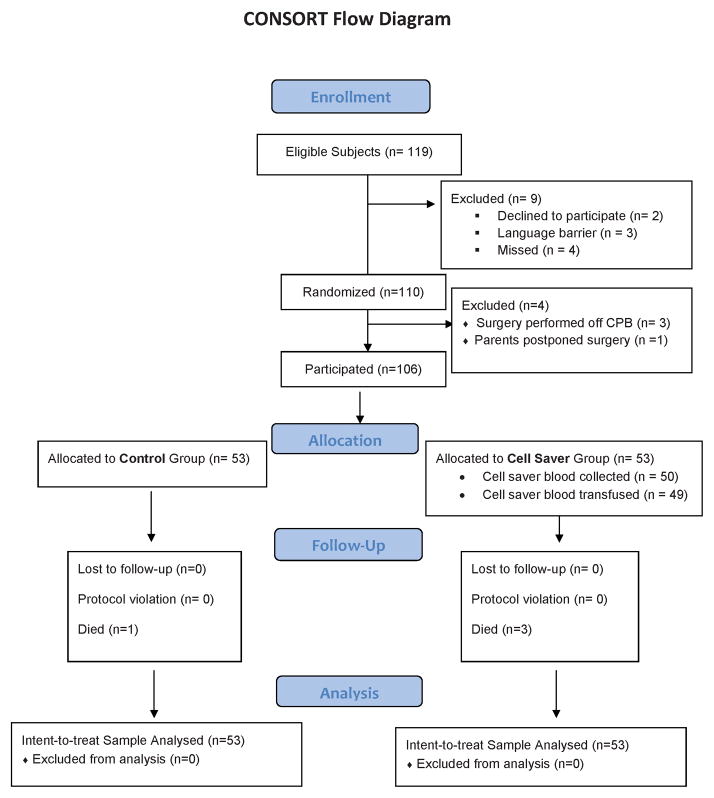
From November 2010 to December 2011 119 children were eligible for study participation. See CONSORT diagram (Figure 1). Families of two subjects refused participation, in four cases families were unavailable before consent could be obtained, and a significant language barrier existed in 3 cases preventing acquisition of informed consent. 110 children were randomized but in 3 cases surgery was able to be performed without CPB and in one case surgery was postponed. 106 (89% of original eligibility; 95% of eligible subjects undergoing CPB) subjects underwent study procedures; 53 per group. No subjects were lost to follow up. No protocol violations occurred. All subjects were analyzed according to intent-to-treat.
Figure 1.
CONSORT diagram.
Subject Characteristics
There were no statistically significant differences in baseline characteristics between each treatment group. There were trends towards smaller, younger and more surgically complex subjects (higher RACHS score and single ventricle physiology) in the cell saver group. A larger number of subjects in the control group received coagulant products in the OR and had delayed sternal closure than subjects in the cell saver group. The CPB duration and surgical management were similar. The Hb and arterial lactate levels were similar on PCICU admission (Table 1). The cardiac morphology and surgical procedures of subjects in each group is presented in Table 2.
Table 1.
Subject Characteristics and surgical details.
| Control Group N = 53 | Cell Saver Group N = 53 | Significance | |
|---|---|---|---|
| Age (months) | 5 (0.3–72) | 3 (0.1–84) | z = −0.983; p = 0.326 |
| Neonate (≤30 days) | 16 (30.2%) | 44 (83%) | z = −0.816; p = 0.414 |
| Weight (kg) | 5.3 (1.8–18) | 4.5 (2.3–17.2) | z = −1.087; p = 0.277 |
| Male sex | 23 (43.4%) | 25 (35.7%) | z = −0.388; p = 0.698 |
| RACHS score 4–6 | 14 (26.4%) | 22 (31.4%) | z = −1.633; p = 0.102 |
| Cyanotic | 27 (50.9%) | 30 (42.9%) | z = −0.582; p = 0.561 |
| Single Ventricle | 10 (18.9%) | 16 (22.9%) | z = −1.348; p = 0.178 |
| Admission Hb (g/dl) | 12.7 ± 1.8 | 12.8 ± 1.65 | p = 0.764 |
| Admit lactate (mmol/L) | 3.55 ± 2.52 | 3.13 ± 1.51 | p = 0.295 |
| CPB time (min) | 125 ± 59 | 130 ± 60 | p = 0.676 |
| Aortic cross clamp | 46 (87%) | 46 (87%) | p = 1.0 |
| time (min) | 79 ± 54 | 77 ± 50 | p = 0.861 |
| DHCA | 5 (9%) | 12 (23%) | z = −1.43; p = 0.153 |
| time (min) | 27 ± 10 | 33 ± 14 | p = 0.449 |
| Dressing time (min) | 69 ± 39 | 61 ± 21 | p = 0.147 |
| RBC in prime | 35 (66%) | 39 (74%) | z = −0.842; p = 0.400 |
| Coagulant Products | 6 (11.3%) | 2 (3.7%) | z = −1.464; p = 0.143 |
| OR duration (min) | 267 ± 98 | 270 ± 82 | p = 0.860 |
| Delayed sternal closure | 8 (15%) | 4 (8%) | z = −1.585; p = 0.113 |
Data is presented as median (range); mean ± SD or number (%). Student’s t-tests were used for normally distributed data and Wilcoxon Signed Ranks test for data that was not normally distributed. Z test for two proportions was utilized to compare number (%).
RACHS = risk adjusted congenital heart surgery score; Hb = hemoglobin; CPB = cardiopulmonary bypass; DHCA = deep hypothermic cardiac arrest; RBC = red blood cell; OR = operating room.
Table 2.
Surgical palliations and procedures.
| Control Group N = 53 | Cell Saver Group N = 53 | |
|---|---|---|
| Norwood (Sano) or DKS | 1 (1.9%) | 7 (13.2%) |
| BT or Central Shunt | 3 (5.7%) | 1 (1.9%) |
| Arterial Switch | 5 (9.4%) | 5 (9.4%) |
| Systemic to Pulmonary Shunt + PA unifocalization | 1 (1.9%) | 3 (5.7%) |
| IAA or hypoplastic aortic arch +/− coarct repair | 4 (7.5%) | 3 (5.7%) |
| PA band and Aortic arch repair | 0 | 2 (3.8%) |
| Truncus arteriosus repair | 2 (3.8%) | 1 (1.9%) |
| Atrioventricular septal defect repair | 4 (7.5%) | 7 (13.2%) |
| Bidirectional Glenn | 3 (5.7%) | 6 (11.3%) |
| Fontan (extra-cardiac conduit) | 5 (9.4%) | 3 (5.7%) |
| Tetralogy of Fallot +/− coarct repair | 5 (9.4%) | 5 (9.4%) |
| Rastelli | 1 (1.9%) | 0 |
| Ross | 2 (3.8%) | 0 |
| Aortic Root Reconstruction | 1 (1.9%) | 0 |
| Pulmonary valve repair | 2 (3.8%) | 0 |
| Anomalous Coronary Artery repair | 0 | 1 (1.9%) |
| TAPVR repair | 4 (7.5%) | 1 (1.9%) |
| Right ventricle o Pulmonary artery conduit | 1 (1.9%) | 1 (1.9%) |
| ASD +/− VSD repair or AP window | 7 (13.2%) | 5 (9.4%) |
| Aortic Membrane resection | 0 | 1 (1.9%) |
| PAPVR and ASD repair | 2 (3.8%) | 1 (1.9%) |
DKS = Damus-Kaye-Stansel; BT = Blallock-Taussig; PA = pulmonary artery; IAA = interrupted aortic arch; coarct = aortic coarctation; TAPVR = total anomalous pulmonary venous return; ASD = atrial septal defect; VSD = ventricular septal defect; PAPVR = partial anomalous pulmonary venous return; AP = aortopulmonary.
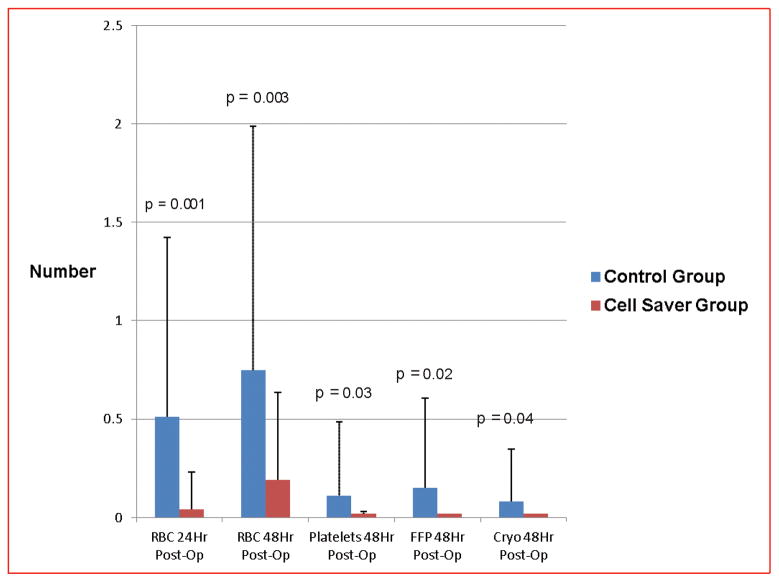
Number of RBC transfusions (Figure 2)
Figure 2.
Number of red blood cell and component transfusions in the first 48 hours post-operation between each group.
The mean number of RBC transfusions in the first 24 hours following discontinuation of CPB was significantly less in subjects randomized to the cell saver group than the control group (cell saver: 0.04 ± 0.19 v. control: 0.51 ± 0.91; p = 0.001). This reduction in RBC transfusions was maintained for the first 48 hours post-op (cell saver: 0.19 ± 0.44 v. control: 0.75 ± 1.2; p = 0.003).
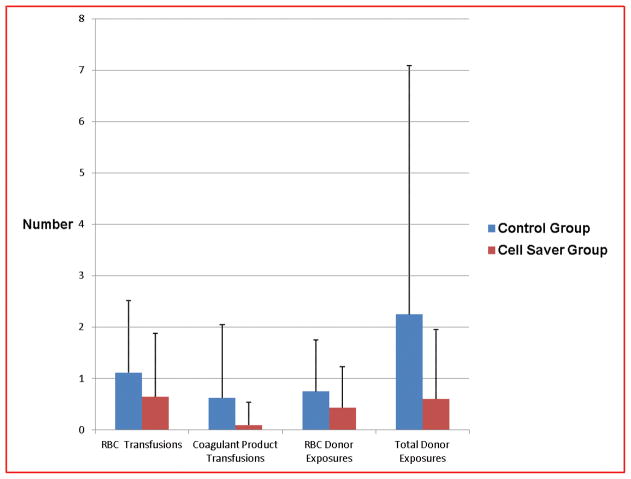
When the first seven days post-op were examined to see if the study intervention transfusion strategy had a longer-term impact, the total number of RBC transfusions remained numerically less in the cell saver group, but did not reach statistical significance (cell saver: 0.64 ± 1.24 v. control: 1.1 ± 1.4; p =0.07) (Figure 3).
Figure 3.
Number of transfusions and donor exposures post-operative days # 0–7 between each group.
Number of Coagulant Product Transfusions
The mean number of component transfusions (platelets, fresh frozen plasma (FFP), and cryoprecipitate (CRYO)) administered in the first two post-operative days was significantly less in subjects randomized to the cell saver group as compared to controls (Platelets: cell saver: 0 ± 0 v. control: 0.11 ± 0.38; p = 0.03; FFP: cell saver: 0 ± 0 v. control: 0.15 ± 0.46; p = 0.02; CRYO: cell saver: 0 ± 0 v. control: 0.08 ± 0.27; p = 0.04) (Figure 2). This effect persisted over the POD #0–7 (cell saver: 0.09 ± 0.45 v. control: 0.62 ± 1.43; p = 0.012) (Figure 3). Only 2 subjects, both in the control group received any coagulant products after the first 48 hours post-op. One of these subjects received one platelet and one FFP transfusion on POD#3, and the other received one cryoprecipitate transfusion on POD #3.
Donor exposures
The mean number of RBC donor exposures during the first seven post-op days was numerically but not statistically less in the cell saver group when compared to controls (cell saver: 0.43 ± 0.8 v. control: 0.75 ± 1.0; p = 0.07). However the mean number of total donor exposures (RBC and coagulant products) during this period was significantly less in the cell saver group when compared to controls (cell saver: 0.60 ± 1.35 v. control: 2.3 ± 4.8; p =0.019) (Figure 2).
When subgroup analysis was performed with subjects divided according to low or high RACHS scores, and blood transfusions and donor exposures were compared between study intervention groups, the results remained consistent with those for the entire subject population for both time periods. Therefore even the lower risk subjects randomized to the cell saver group received significantly fewer autologous RBC transfusions in the first 48 hours post-op and had significantly fewer donor exposures.
Cell Saver Utilization
Cell saver blood salvage was collected in 50/53 (94%) subjects assigned to that group. In three cell saver subjects there was insufficient volume at the end of the case for collection. The mean volume of cell saver collected in those fifty subjects was 38.6 mL/kg ± 20.5 mL/kg (range: 4 to 107 mL/kg). Cell saver was transfused in the OR in 45/50 (90%) of subjects where it was available. In the five cases where it was not given in the OR, 4 subjects received cell saver salvaged RBCs postoperatively in the PCICU. Therefore cell saver blood was utilized in 49/50 (98%) subjects in which it was collected, or 49/53 (92.4%) of subjects randomized to the cell saver group.
Clinical Outcomes
Overall survival was 102 subjects (96%); the four children who died had single ventricle physiology. Of the three deaths in the cell saver group, the first occurred in a neonate with renal, hepatic and severe right ventricular dysfunction prior to Norwood with Sano modification. She died several hours after surgery before any cell saver blood was transfused. The second death was a neonate with hypoplastic left heart syndrome (HLHS) who was 16 days s/p Norwood with Sano modification following multiple cardiac arrests. The third death was a 6 year old with HLHS s/p BGD with diminished RV function and severe tricuspid valve regurgitation who underwent a fenestrated Fontan and tricuspid valve repair. He died POD#22 from Klebsiella pneumonia with pulmonary hypertension. The control group fatality was a neonate with critical aortic stenosis and mitral valve stenosis who died POD#112 from respiratory failure secondary to pulmonary hypertension and bacterial and viral pneumonia.
No subjects required extracorporeal membrane oxygenation either pre or postoperatively. Only one subject (<1%) in the control group required surgical re-exploration for bleeding post-op. There were no significant differences in post-operative clinical outcome variables between subjects in each treatment group (Table 3). Seventeen (16%) of all subjects were diagnosed with a nosocomial infection confirmed with microbiology culture during hospital admission (bacteremia in 9 cases, pneumonia in 7, necrotizing enterocolitis in 1 and pneumonia and bacteremia in 2 cases). No infections occurred before POD#4. There was no difference in the number of infections between treatment groups (cell saver: 9 v. control group: 8; z = −0.263; p = 0.792). There was no significant difference in POD#1 & 2 wrCRP between treatment groups.
Table 3.
Secondary outcome variables between treatment groups.
| Control Group N = 53 | Cell Saver Group N = 53 | Significance | |
|---|---|---|---|
| Mechanical vent (days) | 3 (0.04–112) | 2 (0.13–37) | z = −1.377; p = 0.168 |
| Inotropic/Pressor (days) | 4 (0–30) | 3 (0.08–38) | z = −0.917; p = 0.359 |
| Crystalloid Post-Op (cc/kg) | 3.57 (0–100) | 0 (0–111.5) | z = −1.157; p = 0.247 |
| Albumin Post-Op (cc/kg) | 0 (0–178) | 0 (0–195) | z = −1.220; p = 0.222) |
| Infection (culture proven) | 8 (15%) | 9 (18.8) | z = −0.263; p = 0.792) |
| Thrombosis | 5 (9.4%) | 2 (2.9%) | z = −1.142; p = 0.253 |
| CVA | 1 (1.9%) | 0 | z = −0.917; p = 0.322 |
| MT drainage (days) | 10 (5–34) | 8 (4–27) | z = −1.276; p = 0.202 |
| PCICU LOS (days) | 5 (2–112) | 5 (2–45) | z = −0.780; p = 0.435 |
| Hospital LOS (days) | 12 (5–112) | 10 (5–67) | z = −1.357; p = 0.175 |
| Survival | 52 (98.1%) | 50 (94%) | z = −1.015; p = 0.310 |
| wrCRP POD#1 (mg/L) | 29.4 (0.6–114) | 53 (4.5–116) | z = −1.731; p = 0.083 |
| wrCRP POD#2 (mg/L) | 44.9 (0.2–203) | 57.3 (5.9–220) | z = −1.202; p = 0.229 |
MT = mediastinal tube; CVA = cerebral vascular accident; PCICU = pediatric cardiac intensive care unit; LOS = length of stay; wrCRP = wide range C-reactive protein; POD = post operative day.
DISCUSSION
Adults undergoing cardiac surgical procedures who receive large numbers of blood product transfusions have worse clinical outcomes (3–5,18). Transfusion of salvaged cell saver blood during adult cardiac surgery is commonly performed in order to limit the number of allogeneic blood products administered (19–22). A 2009 meta-analysis examined 31 randomized trials of 2282 patients and found that use of intraoperative cell saver blood reduced the rate of exposure to RBCs and/or coagulant blood products, and decreased the mean volume of blood products transfused per patient (23). Children having cardiac surgical procedures also receive large numbers of blood product transfusions with the associated risks of those transfusions (6–8), but processing systems of salvaged blood for auto-transfusion have been unavailable until recently. Technical advancements now allow for volume-independent collection of salvaged blood (24) that can be utilized for even the smallest infants, but prospective clinical studies exploring the effect of cell saver salvaged blood in pediatric cardiac surgery are lacking.
We present the first prospective randomized clinical trial examining the impact of transfusion of cell saver salvaged blood in pediatric cardiac surgery on clinical outcomes. We found that transfusion of washed intra-operative cell saver blood salvage significantly decreased the need for allogeneic RBCs in the first 48 hours postop. A numeric but not quite statistically significant decrease in RBC transfusions throughout POD # 0–7 was also seen. These results would indicate that transfusion of cell saver blood salvage is a useful blood conservation strategy in this heavily transfused population. Certainly as the number of ICU days increases, the immediate reduction in allogeneic transfusions will have less of an impact on the overall number of transfusions that follow. However, transfusion of autologous cell saver salvage blood instead of additional allogeneic RBCs may reduce the immunogenic, inflammatory and infectious impact on the recipient, and may reduce ensuing post-operative complications.
The reduced number of platelet, FFP and cryoprecipitate transfusions required for patients in the cell saver treatment arm was unexpected but significant. There were a greater number of delayed chest closures in control group subjects which raises the question whether this group had more post-operative bleeding that required coagulant product transfusions. The reason for delayed chest closure was not included in our data collection. At our institution delayed chest closure is routinely performed for hemodynamic instability, inadequate oxygenation or ventilation, and/or pulmonary hypertension, in addition to concern for inadequate hemostasis. We did not include the volume of mediastinal tube (MT) drainage in our data collection which is a limitation of this study. Data regarding MT volume and thickness would have provided more information regarding the indication for coagulant and RBC product transfusions in respect to bleeding. Future studies with more attention to post-operative bleeding are needed to confirm these results. It is possible that the use of autologous RBC transfusion in the form of cell saver may have improved hemostatic function than stored allogeneic RBCs. Conversely, since the study is not blinded, the availability of cell saver red cells may have caused attending staff to be less aggressive in their use of pro-coagulant products. In general, our patients rarely receive platelets, FFP or cryoprecipitate, so the differences seen may not be generalizable to environments where these products are more freely administered.
The only prospective study describing use of cell savers in pediatric open heart surgery was performed by Hanna Golab et al (2008) (14). Despite protocol violations, they found a reduction in allogeneic blood transfusions and no adverse effects in infants who received intra-operative collected cell salvage. The Golab study is important as it provides evidence for the safety of intraoperative cell salvage in pediatric patients. However, the study is limited as it used cell salvage only for up to 6 hours postoperatively. It is well recognized that a predictable and significant decrease in cardiac output occurs 7–10 hours post-CPB which is the time during which volume and RBC resuscitation is most likely to be required (17). Therefore the Golab study did not encompass the most critical period during which cell salvage would be of greatest benefit. Furthermore the Golab study focused on 6hr and 24hr blood transfusions as their outcome. However Golab cites that in many cases allogeneic blood was transfused in the ICU even though there was sufficient amount of cell saver product available. They state this as a major study limitation, and indeed lack of compliance with their study protocol hampers confidence in their results. Additionally, they did not follow subjects for other clinical outcome measures such as duration of mechanical ventilation or ICU length of stay.
The current study was designed to examine the transfusion strategy’s impact on RBC transfusions in the immediate post-operative period (1st 48hrs), but also explored its impact on the first 7 days post-op. In the current study cell saver blood was transfused not only for clinically significant anemia, but also for volume expansion. Transfusion of cell saver blood (in the place of albumin or crystalloid) likely also contributed to our findings, maintaining intravascular volume and optimizing hemodynamics in lieu of additional RBC transfusions.
Although not powered to assess differences in clinical outcomes measures, data regarding the known complications of transfusions were examined for the entire hospitalization. There was a trend toward fewer thrombotic events, fewer inotropic, ventilator and MT days, and hospital length of stay. The heterogenous nature of the study subjects resulted in large ranges of our clinical outcome variables and may have prevented finding a greater between group differences. It is possible that a more homogenous population would demonstrate a greater effect of the cell saver on administration of crystalloid/colloid in the first 48 hours which possibly would lead to less peripheral and pulmonary edema and shorter duration of mechanical ventilation and MT drainage. It is also possible that this study is underpowered to demonstrate an effect on late complications and larger studies powered for clinical outcomes and focused on both short and long term complications are warranted.
Lastly, the Golab study included fresh frozen plasma in their pump prime and a post-operative transfusion trigger of a hematocrit of 30%. At the URMC we do not use FFP in our prime, nor use a transfusion trigger and allow the hematocrit to fall lower than 30% in stable patients before transfusing pRBCs. This difference in transfusion practices likely influenced their results and may have exaggerated the benefit of use of cell salvage in their study. Indeed, at URMC Hb levels are commonly accepted as low as 7.0 g/dL even in these children status post cardiac surgical repairs or palliations. If our transfusion protocol goals involved a higher threshold Hb level, a greater between group difference likely would have been found.
Whether future advances in blood banking will allow for cell saver blood to be maintained for >24 hours post-collection, remains unknown. Certainly if cell saver blood could be transfused over a longer time period following its collection, the number of allogeneic RBC transfusions would only decrease further.
This was a pilot study to assess feasibility of this blood conservation strategy. Therefore it was not powered to assess for clinical outcomes. A larger study with a more homogenous population focusing on clinical outcomes would be of great interest. Certainly the clinical indication for blood cell transfusion (i.e., hemodynamic instability, poor cardiac function, post-operative bleeding), in addition to the transfusion itself, directly impacts clinical outcomes. Future trials designed to independently examine the effect of blood cell transfusions, controlling for severity of illness are needed.
Although not powered to assess clinical outcome variables, the association between greater number of RBC transfusions and worse clinical outcomes is well established. Any transfusion strategy that decreases the number of allogeneic RBC transfusions will decrease transfusion-associated infectious risks, thrombotic complications, transfusion-related immunomodulation and potentially overall mortality.
In this specific patient population that commonly requires multiple cardiac surgeries and RBC transfusions and/or cardiac transplantation, increased donor exposures makes future transfusions and matching for transplant more difficult. Any strategy that limits donor exposure in this patient population is highly desirable.
A single RBC unit costs the URMC $205 to obtain from a regional blood center. That price is increased with required testing, reagents, and processing, raising the price to $220.00 in variable costs. Consumables for the cell saver system cost $203.00/subject. Reduction of transfusion-related complications and/or decreased duration of mechanical ventilation and PCICU length of stay would make cell saver systems extremely cost effective. As blood is a limited resource, continued blood conservation efforts are highly desirable.
This is a small, single institution study that was not powered to assess for clinical outcome differences. Increased subject numbers would allow for comparison of postoperative complications between the two groups (i.e., thrombosis, infection, duration of mechanical ventilation, vasoactive agents and length of stay) which would be of great interest. Restriction of eligible patients to those ≤10 kg, and/or those with the highest RACHS scores, which are our most heavily transfused subjects, would potentially illustrate a greater impact of the study intervention as well and be most cost effective.
A limitation of this study is the variability of the subject population. Larger studies with a more homogeneous population focusing on neonates and smaller infants powered to test for clinical outcomes would be of great interest. Another limitation is the potential bias inherent to non-blinded studies. Obvious differences in packaging and labeling of blood products prevented blinding of the attending physician. The decision to transfuse RBCs (in the form of salvaged cell saver blood or allogeneic RBCs) or crystalloid/ albumin for volume replacement relied upon the judgment of the attending physician. Since the attending physician was not blinded to the subjects’ treatment group assignment, he/she was likely influenced by having, or not having, salvaged cell saver blood immediately available at the bedside.
The attending may have been more judicious in their volume replacement or more tolerant of a lower hemoglobin level, knowing that a RBC product was immediately available for transfusion if the subject’s clinical condition were to change rapidly. In such a setting, the attending may have been more restrictive in their transfusion and/or volume replacement with the security of having the salvaged blood immediately available. Alternatively, the attending may have been more liberal with their transfusion and/or volume replacement in subjects in the cell saver group simply due to the fact that salvaged cell saver blood was immediately available, did not require the typical steps to prepare and obtain allogeneic RBCs from the blood bank, and since it did not constitute an additional donor exposure. Strict targeting of Hb levels might have made our intervention groups’ transfusion management more uniform, but does not allow for clinical judgment of the physician, which by definition is not in the best interest of the study subjects.
To our knowledge, this is the only prospective randomized trial of clinical outcomes following re-administration of cell saver blood stored for up to 24 hours at the bedside. This study is strengthened by its high enrollment, with excellent capture of eligible subjects. There was an extremely high rate of collection of subjects randomized to the cell saver group, and subject data was analyzed according to intent-to-treat. Also, as there were no protocol violations and no subjects were lost to follow up we believe our results are a true reflection of the impact of the study intervention.
CONCLUSIONS
Cell saver blood can be safely stored at the bedside, with multiple aliquots drawn off for transfusion up to 24 hours post-collection. This strategy is highly desirable as it allows RBCs to be immediately available, without increased donor exposure, to correct for anemia and for volume replacement in a critically ill population. Rate of infections and inflammation were not increased in subjects receiving cell saver blood.
Cell saver use significantly reduced the number of RBC transfusions in the immediate post-operative period. Furthermore, subjects receiving cell saver blood required significantly fewer coagulant product transfusions and donor exposures. There was a trend toward decreased volume of crystalloid infused post-op, decreased thrombosis, and shorter duration of mechanical ventilation and inotropes. Larger studies are needed to confirm these results and to determine whether the decreased number of blood product transfusions and exposures are associated with improved clinical outcomes. Cell savers are cost-effective. Use of cell savers to limit RBC transfusions and donor exposures should be considered in pediatric cardiac surgery with CPB.
Acknowledgments
We thank the children and families who participated in this study. We also thank Regina Cable, PNP, for her assistance with consent procedures. We thank Hongyue Wang PhD for her assistance with sample size calculations and our power analysis. We thank the pediatric cardiac operating room staff, anesthesiologists and perfusionists for assistance with study procedures. We thank the PCICU attendings, fellows and staff, and the Blood Bank/Transfusion Service technical staff for their assistance with study procedures.
Financial support: The Fresinius Continuous AutoTransfusion SystemR (CATS ) was donated for use at the URMC by Terumo Corporation. This did not have any role in the study design, collection, analysis and/or in the decision to submit the manuscript for publication. There are no relationships with industry.
Footnotes
Reprints will not be ordered.
Conflict of interest statement: There is no conflict of interest for any author. No author has any financial or personal relationship with other people or organizations that could inappropriately influence his/her work.
References
- 1.Willems A, Harrington K, Lacroix J, et al. Comparison of two red-cell transfusion strategies after pediatric cardiac surgery: a subgroup analysis. Crit Care Med. 2010;38:649–56. doi: 10.1097/CCM.0b013e3181bc816c. [DOI] [PubMed] [Google Scholar]
- 2.Szekely A, Cserep Z, Sapi E, et al. Risks and predictors of blood transfusion in pediatric patients undergoing open heart operations. Ann Thorac Surg. 2009;87:187–97. doi: 10.1016/j.athoracsur.2008.09.079. [DOI] [PubMed] [Google Scholar]
- 3.Murphy GJ, Reeves BC, Rogers CA, Rizvi SIA, Culliford L, Angelini GD. Increased Mortality, Postoperative Morbidity, and Cost After Red Blood Cell Transfusion in Patients Having Cardiac Surgery. Circulation. 2007;116:2544–52. doi: 10.1161/CIRCULATIONAHA.107.698977. [DOI] [PubMed] [Google Scholar]
- 4.Koch CG, Li L, Duncan AI, Mihaljevic T, Cosgrove DM, Loop FD, Starr NJ, Blackstone EH. Morbidity and mortality risk associated with red blood cell and blood-component transfusion in isolated coronary artery bypass grafting. Crit Care Med. 2006;34:1608–16. doi: 10.1097/01.CCM.0000217920.48559.D8. [DOI] [PubMed] [Google Scholar]
- 5.Engoren MC, Habib RH, Zacharias A, et al. Effect of blood transfusion on long-term survival after cardiac operation. Ann Thorac Surg. 2002;74:1180–86. doi: 10.1016/s0003-4975(02)03766-9. [DOI] [PubMed] [Google Scholar]
- 6.Kneyber MC, Hersi MI, Twisk JW, Markhorst DG, Plotz FB. Red blood cell transfusion in critically ill children is independently associated with increased mortality. Intensive Care Med. 2007;33:1414–55. doi: 10.1007/s00134-007-0741-9. [DOI] [PubMed] [Google Scholar]
- 7.Kipps AK, Wypij D, Thiagarajan RR, Bacha EA, Newburger JW. Blood transfusion is associated with prolonged duration of mechanical ventilation in infants undergoing reparative cardiac surgery. Pediatr Crit Care Med. 2011;12(1):52–6. doi: 10.1097/PCC.0b013e3181e30d43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salvin JW, Scheurer MA, Laussen PC, Wypij D, Polito A, Bacha EA, Pigula FA, McGowan FX, Costello JM, Thiagarajan RR. Blood Transfusion After Pediatric Cardiac Surgery is Associated with Prolonged Hospital Stay. Ann Thorac Surg. 2011;91:204–11. doi: 10.1016/j.athoracsur.2010.07.037. [DOI] [PubMed] [Google Scholar]
- 9.Wang G, Bainbridge D, Martin J, Cheng D. The Efficacy of an Intraoperative Cell Saver During Cardiac Surgery: A Meta-Analysis of Randomized Trials. Anesth Analg. 2009;109(2):320–30. doi: 10.1213/ane.0b013e3181aa084c. [DOI] [PubMed] [Google Scholar]
- 10.Booke M, Hagemann O, Van Aken H, Erren M, Wullenweber J, Bone HG. Intraoperative autotransfusion in small children: An in vitro investigation to study its feasibility. Anesth Analg. 1999;88:763–5. doi: 10.1097/00000539-199904000-00015. [DOI] [PubMed] [Google Scholar]
- 11.Dahmani S, Orliaguet GA, Meyer PG, Blanot S, Renier D, Carli PA. Perioperative blood salvage during surgical correction of craniosynostosis in infants. Br J Anesth. 2000;85:550–5. doi: 10.1093/bja/85.4.550. [DOI] [PubMed] [Google Scholar]
- 12.Orliaguet GA, Bruyere M, Meyer PG, Blanot S, Renier D, Carli PA. Comparison of perioperative blood salvage and postoperative reinfusion of drained blood during surgical correction of craniosynostosis in infants. Paediatric Anaesthesia. 2003;13(9):797–804. doi: 10.1046/j.1460-9592.2003.01155.x. [DOI] [PubMed] [Google Scholar]
- 13.Liu J, Ji B, Feng Z, Zhao J, Li C, Li B, Long C. The effect of preprocessing stored red blood cells on neonates undergoing corrective cardiac surgery. ASAIO J. 2007;53:680–3. doi: 10.1097/MAT.0b013e31815a5edb. [DOI] [PubMed] [Google Scholar]
- 14.Golab HD, Scohy TV, de Jong PL, Takkenberg JJM, Bogers AJJC. Intraoperative cell salvage in infants undergoing elective cardiac surgery: a prospective trial. European J Cardio Thorac Surg. 2008;34:354–9. doi: 10.1016/j.ejcts.2008.04.047. [DOI] [PubMed] [Google Scholar]
- 15.Jenkins KJ, Gauvreau K, Newburger JW, Spray TL, Moller JH, Iezzoni LI. Consensus-based method for risk adjustment for surgery for congenital heart disease. J Thorac Cardiovac Surg. 2002;123(1):110–8. doi: 10.1067/mtc.2002.119064. [DOI] [PubMed] [Google Scholar]
- 16.Standards for Blood Banks and Transfusion Services. 26. American Association of Blood Banking (AABB) Press; Bethesda, MD: 2009. [Google Scholar]
- 17.Castaneda AR, Jonas RA, Mayer JE Jr, Hanley FL, editors. Cardiac Surgery of the Neonate and Infant. W.B. Saunders Co: Philadelphia PA; 1994. Chapter 5. Perioperative Care: Management of the infant and neonate with congenital heart disease; p. 75. [Google Scholar]
- 18.Scott BH, Seifert FC, Grimson R. Blood transfusion is associated iwth increased resource utilisaion, morbidity and mortality in cardiac surgery. Ann Card Anaesth. 2008;11:15–19. doi: 10.4103/0971-9784.38444. [DOI] [PubMed] [Google Scholar]
- 19.Dalrymple-Hay MJ, Dawkins S, Pack L, Deakiin CD, Sheppard S, Ohri SK, Haw MP, Livesey SA, Monro JL. Autotransfuion decreases blood usage following cardiac surgery-a prospective randomized trial. Cardiovasc Surg. 2001;9:184–7. doi: 10.1016/s0967-2109(00)00100-9. [DOI] [PubMed] [Google Scholar]
- 20.Laub GW, Dharan M, Riebman JB, Chen C, Moore R, Bailey BM, Fernandez J, Adkins MS, Anderson W, McGrath LB. The impact of intraoperative autotransfusion on cardiac surgery. A prospective randomized double-blind study. Chest. 1993;104:686–9. doi: 10.1378/chest.104.3.686. [DOI] [PubMed] [Google Scholar]
- 21.Murphy GJ, Allen SM, Unsworth-White J, Lewis CT, Dalrymple-Hay MJ. Safety and efficacy of perioperative cell salvage and autotransfusion after coronary artery bypass grafting: a randomized trial. Ann Thorac Surg. 2004;77:1553–9. doi: 10.1016/j.athoracsur.2003.10.045. [DOI] [PubMed] [Google Scholar]
- 22.Murphy GJ, Rogers CS, Lansdowne WB, Channon I, Alwair H, Cohen A, Caputo M, Angelini GD. Safety, efficacy, and cost of intraoperative cell salvage and autotransfusion after off-pump coronary artery bypass surgery: a randomized trial. J Thorac Cardiovasc Surg. 2005;130:20–8. doi: 10.1016/j.jtcvs.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 23.Wang G, Bainbridge D, Martin J, Cheng D. The Efficacy of an Intraoperative cell saver during cardiac surgery: a meta-analysis of randomized trials. Anesth Analg. 2009;109:320–30. doi: 10.1213/ane.0b013e3181aa084c. [DOI] [PubMed] [Google Scholar]
- 24.Booke M, Hagemann O, Van Aken H, Erren M, Wullenweber J, Bone HG. Intraoperative autotransfusion in small children: An in vitro investigation to study its feasibility. Anesth Analg. 1999:763–5. doi: 10.1097/00000539-199904000-00015. [DOI] [PubMed] [Google Scholar]