Abstract
Objective
To assess clinical and neurocognitive function in children who have undergone liver transplantation for classical maple syrup urine disease (MSUD).
Study design
A total of 35 patients with classical MSUD (age 9.9 ± 7.9 years) underwent liver transplantation between 2004 and 2009. Six patients donated their liver to recipients without MSUD (“domino” transplant). We analyzed clinical outcomes for our cohort and 17 additional cases from the national United Network for Organ Sharing registry; 33 patients completed IQ and adaptive testing before transplantation, and 14 completed testing 1 year later.
Results
Patient and graft survival were 100% at 4.5 ± 2.2 years of follow-up. Liver function was normal in all patients. Branched-chain amino acid levels were corrected within hours after surgery and remained stable, with leucine tolerance increasing more than 10-fold. All domino transplant recipients were alive and well with normal branched-chain amino acid homeostasis at the time of this report. Patient and graft survival for all 54 patients with MSUD undergoing liver transplantation in the United States during this period were 98%and 96%, respectively. One-third of our patients were mentally impaired (IQ ≤ 70) before transplantation, with no statistically significant change 1 year later.
Conclusion
Liver transplantation is an effective long-term treatment for classical MSUD and may arrest brain damage, but will not reverse it.
Despite progress in nutritional and medical management, classical maple syrup urine disease (MSUD) poses a risk of serious neurologic disability and untimely death.1,2 Acute metabolic intoxication causes cerebral edema that can culminate in brain herniation and cardiorespiratory arrest.3,4 Chronic disturbances of branched-chain amino acid (BCAA) and ketoacid homeostasis alter cerebral amino acid uptake and neurotransmitter metabolism and can result in chronic cognitive impairment and mental illness.2,5 The liver expresses 9%–13% of the body’s total branched-chain ketoacid dehydrogenase complex (BCKDH) activity.6 In 2006, we presented evidence that liver transplantation controlled BCAA metabolism in 11 children with MSUD.7 Here we extend our observations to 37 patients followed for a mean of 4.5 years and also review 17 additional cases from the United Network for Organ Sharing (UNOS) registry.
Methods
Between May 2004 and December 2009, 35 patients with classical MSUD (22 males, 13 females) underwent transplantation with deceased-donor livers under an elective protocol at Children’s Hospital of Pittsburgh of the University of Pittsburgh Medical Center.7 Two additional patients who underwent transplantation 13.2 and 5.8 years ago at other centers were also followed at Children’s Hospital of Pittsburgh. IRB approval was obtained for this report. Mean age at transplantation was 9.9 ± 7.9 years (range, 1.7–32.1 years). Immunosuppression was achieved with methylprednisolone (2 mg/kg) premedication, perioperative rabbit antithymocyte globulin (5 mg /kg) intravenous induction, and long-term tacrolimus monotherapy. All patients who underwent transplantation were afebrile, metabolically stable, and selected according to the UNOS match run list. Six patients consented to donate their explanted liver to consenting recipients without MSUD (“domino” transplantation). To manage any metabolic complications, plasma amino acid monitoring was available around the clock, and MSUD hyperalimentation solution could be prepared on demand.7
BCAA homeostasis, weight-adjusted leucine tolerance, and metabolic control during illness were used to assess the efficacy of transplantation. Plasma BCAA levels for 3 MSUD groups (pretransplantation, 1–11 months posttransplantation, and ≥1 year posttransplantation) were compared with a pediatric reference population of 51 children without disorders of amino acid or organic acid metabolism. Groups were studied using ANOVA and the Tukey posttest for pairwise comparisons (with P < .05 indicating significance). Our outcome data were supplemented with information on 17 additional UNOS-registered patients with MSUD who underwent liver transplantation at other US centers during the same time period.
Before transplantation, 33 subjects completed IQ testing using the Routing Test of the Stanford-Binet Intelligence Scales or the Wechsler Abbreviated Scale of Intelligence. Thirty-one subjects were tested for adaptive skills using the Vineland Adaptive Behavior Scale II or Adaptive Behavior Assessment System Second Edition. Associations among pretransplantation test scores and various clinical variables were explored using Spearman correlations (rs). Ten males and 4 females (mean age, 11.5 ± 7.1 years; range, 2–22 years) also completed cognitive and adaptive testing 1 year after liver transplantation, and results were analyzed qualitatively in a separate report.8 For the present work, we analyzed pretransplantation and posttransplantation scores using the paired t-test.9,10
Results
Patient and graft survival were 100% with satisfactory liver function (mean bilirubin level, 0.6 ± 0.5 mg/dL; mean γ-glutamyl transpeptidase level, 31.8 ± 60.4 IU/L) in all 37 patients under our care at a mean posttransplantation follow-up period of 4.5 ± 2.2 years. The longest follow-up period was 13.2 years. All 6 domino transplantation recipients were alive and well, with normal liver function and BCAA homeostasis on unrestricted protein intake, at the time of this report.
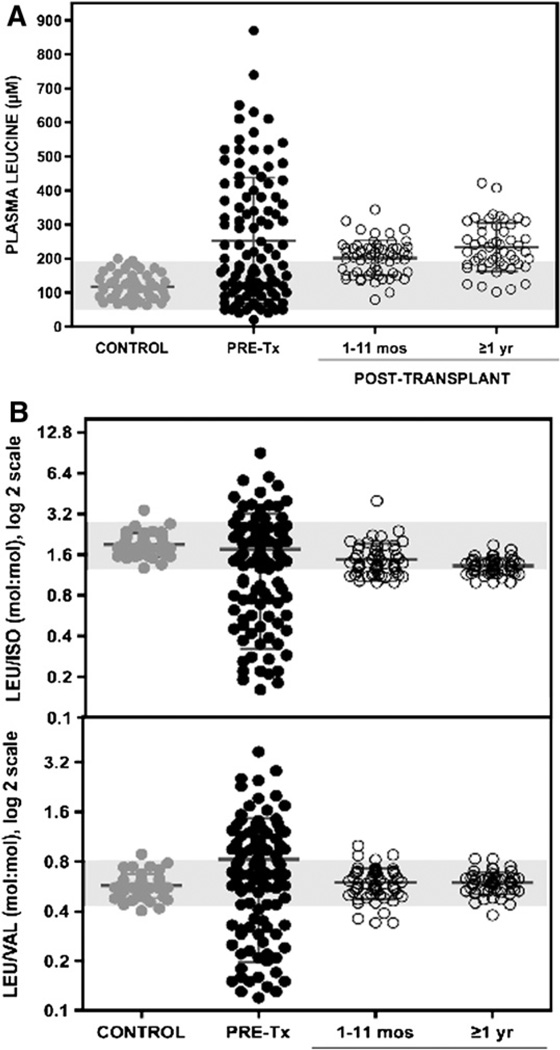
In all of the patients with MSUD who underwent liver transplantation, BCAA metabolism was stable soon after surgery and remained so as leucine tolerance increased from 10–25 mg/kg/day to >150 mg/kg/day (natural protein intake >1.5 g/kg/day). Compared with control subjects (mean leucine level, 119 ± 38 µM), patients with MSUD had 2-fold higher mean plasma leucine values before transplantation (253 ± 185 µM), 1–11 months after transplantation (202 ± 51 µM), and ≥1 year after transplantation (233 ± 71 µM). Although leucine levels were similar before and after transplantation, posttransplantation values were much less variable (F test; P < .0001) and remained tightly regulated relative to isoleucine and valine levels (Figure 1).
Figure 1.
>A, Pooled plasma leucine values in control subjects (gray circles) and patients with MSUD before (black circles) and 1–11 months and ≥1 year after liver transplantation (open circles). Shaded area represents mean ± 2 SD leucine values in 51 normal children. A single exceptional value of 2170 µM (not shown) was seen in a child who developed severe dehydration at 55 months posttransplantation. B, Plasma molar ratios of leucine to isoleucine (upper panel) and leucine to valine (lower panel) showing intact regulation of BCKDH activity after transplant (open circles). Shaded areas represent mean ± 2 SD molar ratios in normal children.
Enzyme activity of the transplanted liver prevented BCAA elevations during illness, with one important exception. One child developed transient leucinosis at 55 months posttransplantation during an episode of gastroenteritis and severe dehydration (leucine, 2170 µM; isoleucine, 1009 µM; valine, 1483 µM; reference ranges, leucine, 119 ± 38 µM; isoleucine, 81 ± 29 µM, valine, 275 ± 57 µM). With routine intravenous hydration therapy, his BCAA levels normalized within a few days (leucine, 110 µM; isoleucine, 70 µM; valine, 219 µM), and no specific metabolic treatment was required.
The Table (available at www.jpeds.com) lists major medical and surgical complications in our cohort. The median length of hospital stay after liver transplantation was 17 days (range, 8–39 days). The most common perioperative problems were organ rejection within 90 days (40%), delayed wound closure (27%), and ventral hernia repair (11%). Two patients underwent successful hepatic arterial revascularization for arterial thrombosis, and 3 patients underwent arterial revisions for ultrasound findings of arterial stenosis. There were no biliary complications. Most patients (78%) are currently receiving low-dose tacrolimus monotherapy (mean, 0.09 mg/kg/day); 9 patients also are receiving low-dose prednisone (mean, 0.17 mg/kg/day). Renal function and glucose homeostasis remained normal after surgery, and only 1 patient developed hypertension. As expected, asymptomatic viremia was common, but cytomegalovirus (n = 1) and Epstein-Barr virus (n = 2) disease were rare. The first patient, who underwent transplantation elsewhere, developed Epstein-Barr virus–induced intestinal posttransplantation lymphoproliferative disease before being transferred to our center. Transient withdrawal of immune suppression resolved lymphomatous changes in her gastrointestinal tract and did not compromise her graft. She did not experience recurrence during 12 years of follow-up.
Table.
Perioperative and postoperative complications in 37 patients
| Number | Percent | |
|---|---|---|
| Postsurgical interventions | ||
| Delayed wound closure | 10 | 27 |
| Ventral hernia repair | 4 | 11 |
| Gastrocutaneous fistula closure* | 2 | 5 |
| Exploratory laparotomy: | ||
| Hepatic artery thrombosis with successful revision | 2 | 5 |
| Hepatic artery revision or graft revision | 3 | 8 |
| Intra-abdominal bleeding | 1 | 3 |
| Partial small bowel obstruction | 1 | 3 |
| Pleurocentesis | 2 | 5 |
| Chest tube drainage | 2 | 5 |
| Bronchoscopy | 1 | 3 |
| Medical complications | ||
| Acute rejection† | 15 | 40 |
| Epstein-Barr virus disease | 2 | 5 |
| Cytomegalovirus disease | 1 | 3 |
| Posttransplantation lymphoproliferative disease‡ | 1 | 3 |
At previous gastrostomy tube sites.
Antibody therapy for steroid-resistant rejection in 3/15 (8% of all patients).
Intestinal posttransplantation lymphoproliferative disease developed in 1 patient who underwent transplantation at another center; it resolved with transient withdrawal of immunosuppression, and the patient has been disease-free for 12 years.
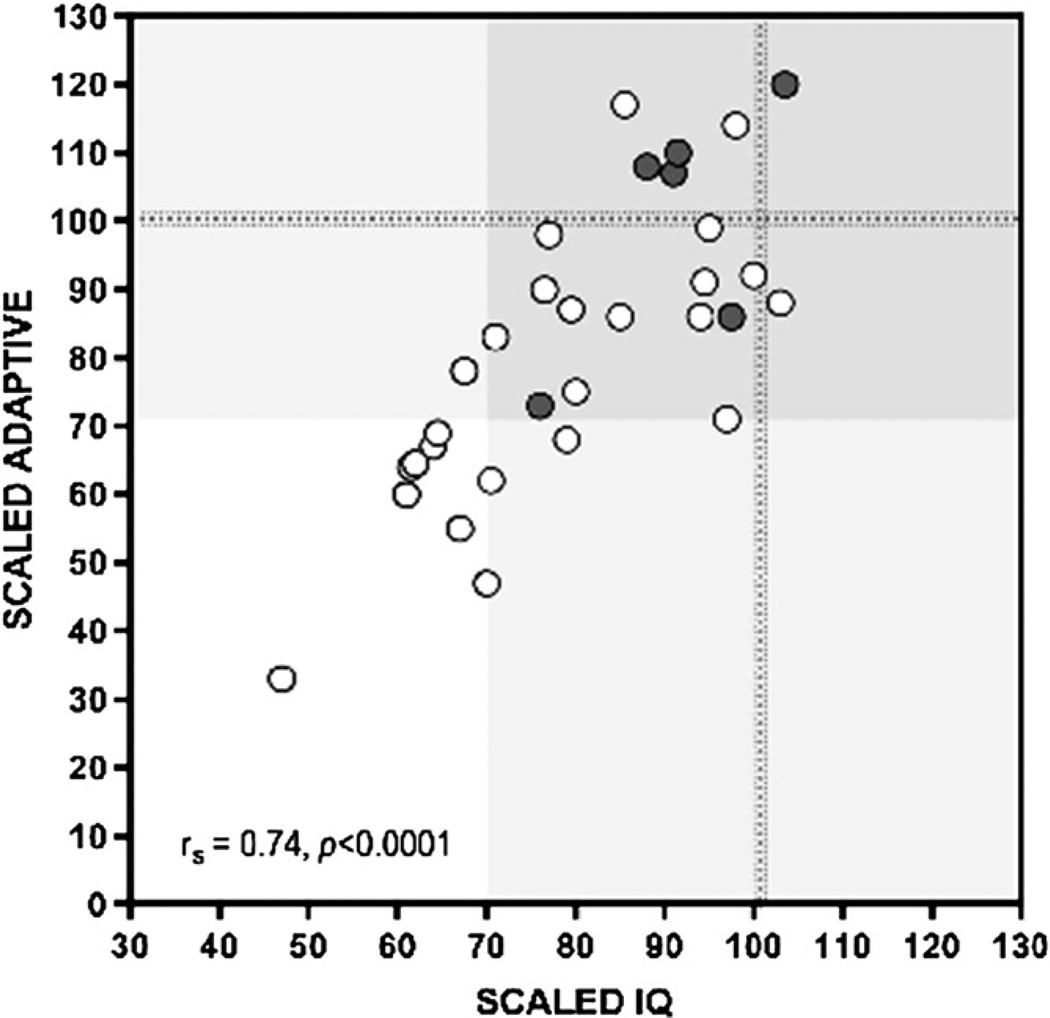
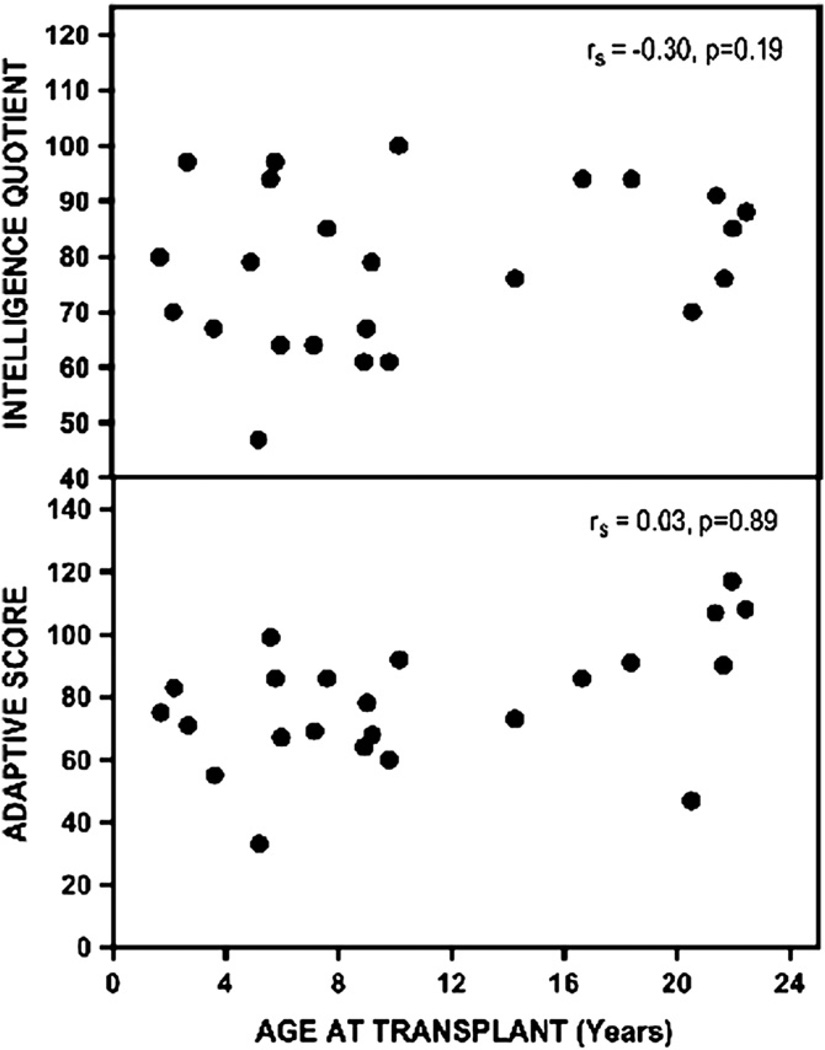
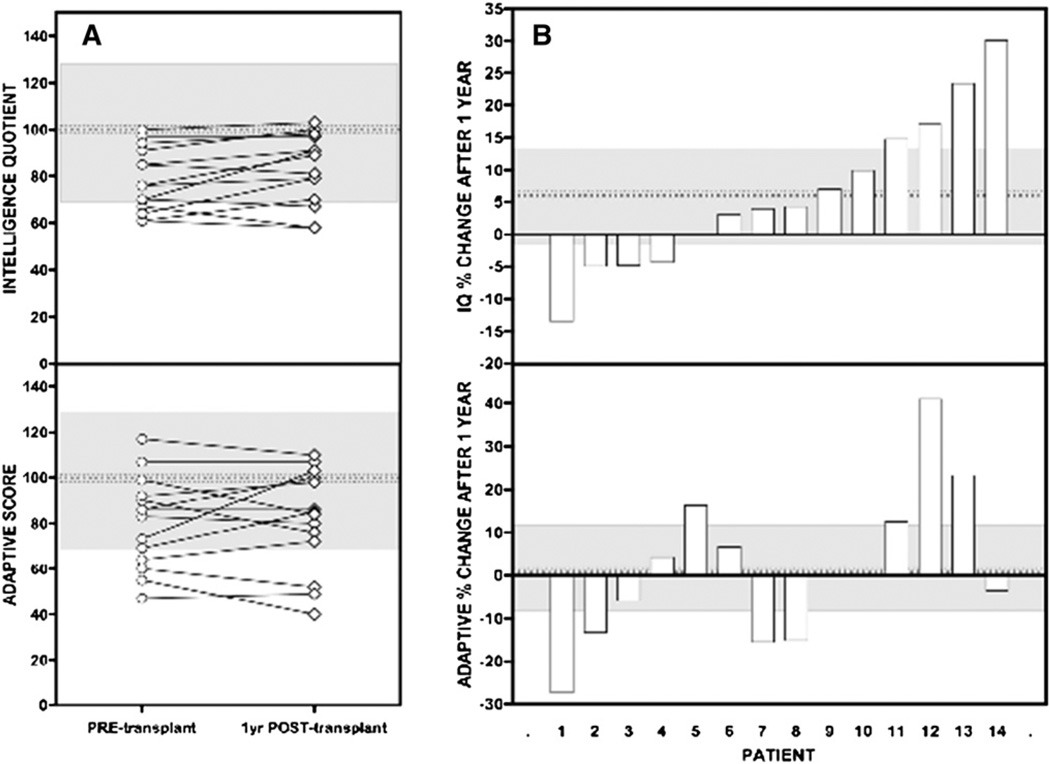
Mean scaled IQ and adaptive scores for 33 pretransplantation patients were 81 ± 15 (range, 47–103) and 82 ± 21 (range, 33–120), respectively (normal scores, 100 ± 15). Eleven patients (33%) had an IQ score in the deficient range (≤70), and only 3 patients were of average or better intelligence (≥100). Twelve patients had an adaptive score <70; only 8 scored average or higher. Scaled IQ was correlated to adaptive function (rs = 0.74; P < .0001) (Figure 2), but there were no significant correlations between IQ or adaptive test scores and age at diagnosis, number of preceding metabolic crises, number of hospitalizations, or age at transplantation (Figure 3). In the subgroup of patients who completed testing 1 year after transplantation,8 scaled IQ and adaptive scores increased by an average of 6.2% (95% CI, −1 to 12.6%; P = .059) and 1.6% (95% CI, −9.3 to 10.7%; P = .779), respectively. These changes were not statistically significant, but the sample size had limited power to detect real differences of this magnitude (Figure 4; available at www.jpeds.com).
Figure 2.
In 33 pretransplantation patients, there was a strong correlation between IQ (x-axis) and adaptive function (y-axis). Only 3 patients had IQ scores at or above the mean (100; dotted line). One-third had IQ scores in the deficient range (≤70). Shaded areas represent the mean ±2 SD in normal subjects. Gray circles represent Old Order Mennonite subjects and indicate that different cultural background did not bias toward lower test scores. Open circles indicate non-Old Order Mennonite patients.
Figure 3.
There was no correlation between age at transplantation and IQ (upper panel) or adaptive scores (lower panel). No correlations were found between test scores and age at diagnosis, number of hospitalizations, number of metabolic crises, or age at transplantation.
Figure 4.
A, Change in test scores for a subgroup of 14 patients. Pretransplantation IQ (78 ± 14) and adaptive (81 ± 20) scores were no different at 1 year after transplantation (posttransplantation IQ, 83 ± 15; adaptive, 82 ± 22). Gray shaded areas show the normal mean (dotted line) ± 2 SD. B, Mean percentage change (dotted line) and 95% CI (gray shaded area). IQ increased by an average of 6.2% (95% CI, −1.0% to 12.6%; range, −13% to 30%) at 1 year after transplantation, but the difference was not statistically significant (P = .059).
Four children sustained serious brain injury before transplantation. All 4 of these children had spastic diplegia, in 1 case requiring prolonged posttransplantation rehabilitation and multilevel corrective orthopedic surgery. One child was physically and neurologically healthy until age 6, when she developed severe cerebral edema during a metabolic crisis. This child sustained bilateral uncal herniation that occluded both posterior cerebral arteries, causing massive ischemic injury to thalami, sensorimotor areas, and visual cortices. She is now confined to a wheelchair, mentally retarded (IQ 47; adaptive 43), and blind. Transplantation has not allowed any patients to stop treatment for inattention, hyperactivity, or mental illness; 4 posttransplantation patients (11%) are receiving psychostimulant medication, and 6 (16%) are being treated for anxiety, panic, or depression.
Adding 17 cases from the UNOS registry, a total of 54 liver transplantations for MSUD were performed in the United States between January 2004 and February 2010. In this group, patient and graft survival were 98% and 96%, respectively; 1 child died in the immediate postoperative period due to vascular complications in the graft, and 1 other child required retransplantation. Fifty-three patients received a liver from a deceased donor (51 whole livers, 2 split), and the child who died after transplantation received a partial liver from a living donor. Among 17 patients who underwent transplantation at other centers, half were under age 5, one-quarter were aged 6–10, and the remainder were aged 11–34.
Discussion
The largest relative activity of BCKDH is in muscle (54%–66%), with equal contributions by liver and kidney (9%–13% each) and a considerable amount in brain (9%–20%).6 This has several important ramifications. First, it suggests that kidney transplantation might be equally effective in controlling BCAA metabolism; we prefer liver based on superior long-term posttransplantation outcomes and its central role in adaptive intermediary metabolism. Second, the high BCKDH activity of muscle “protects” recipients without MSUD from BCKDH deficiency in domino grafts. Finally, BCKDH deficiency within the brain might cause neurologic sequelae independent of peripheral disturbances of BCAA and branched-chain ketoacid metabolism. A longitudinal study of patients who underwent transplantation early in life is a crucial way to explore this possibility.
Restoring 9%–13% of the body’s BCKDH enzyme activity is sufficient to control BCAA metabolism under most circumstances.6 Unlike an infused enzyme, BCKDH activity in a liver graft can adapt to variable metabolic demands through changes in enzyme expression or phosphorylation.11–13 Although BCAA levels in patients who have undergone transplantation remain approximately 2-fold greater than normal, they are tightly regulated in the face of catabolic illness and unrestricted protein intake (Figure 1).
The protection afforded by liver transplantation has limits. Our experience with 1 patient demonstrates that catabolic stress and dehydration can temporarily overwhelm the capacity of the grafted liver to oxidize BCAAs. Severe dehydration may transiently reduce hepatic blood flow such that total body enzyme activity is insufficient to keep pace with a large catabolic efflux of BCAAs from muscle tissue. This observation reveals quantitative relationships among in vivo BCKDH activity, endogenous protein catabolic rate, and the demand for BCAA oxidation. There are 4 important clinical implications: (1) Clinicians should continue to monitor amino acids in posttransplantation patients, particularly those who develop serious catabolic illness or unexplained encephalopathy3; (2) Visceral ischemia, hepatic vascular compromise, dehydration, or anything that restricts hepatic blood flow can compromise BCAA clearance by the graft3; (3) Centers that perform transplantation in patients with MSUD should be prepared to contend with metabolic crisis if the procedure fails. At minimum, this requires on-demand amino acid monitoring, extemporaneous MSUD hyperalimentation solution, and a capable inpatient metabolic service4; and (4) Despite encouraging results in an experimental murine model of intermediate MSUD,13 hepatocyte stem cell transplantation or hepatic gene therapy might not work for classical MSUD.11–13 Such procedures restore enzyme activity to <30% of host hepatocytes,11,14,15 a quantity representing <5% of whole-body BCKDH activity.6 This might improve leucine tolerance but is unlikely to protect children during catabolic crises. Similar logic applies to the use of living related donors who may harbor a mutant BCKDH subunit allele. We favor deceased donor whole-organ transplantation to maximize BCKDH activity delivered to the patient.
In 2007, Simon et al2 described the social consequences of brain disease in 22 adult patients with MSUD. Educational and professional development was poor, and few held a steady job. Most could not live independently, maintain a steady relationship, or raise children. Consistent with these observations, we found a large burden of cognitive impairment in pretransplantation patients with MSUD. At least one-third had significant mental deficiency (ie, IQ or adaptive score ≤ 70) that could not be attributed to any single clinical variable (Figures 2 and 3). Our results are similar to those of others, who found mean IQ scores of 78 ± 24 (n = 16; unaffected siblings 92 ± 5) and 74 ± 14 (n = 22; control subjects 107 ± 9) in patients with MSUD.16,17 The neurologic outcome for each individual reflects many interacting factors, including genetic background, quality of longitudinal metabolic control, frequency of amino acid monitoring, prevalence of hyperleucinemia and prolonged BCAA (particularly valine) deficiencies, unfavorable molar ratios of leucine to its amino acid competitors at the blood-brain barrier, and prevention of catastrophic brain injuries during illness.2,3,5 Our correlation data (Figure 3) suggest that these factors exert the strongest influence during the period of rapid brain growth of infancy and early childhood.5
These observations indicate that liver transplantation may arrest the progression of brain damage, and also suggest that chronic cognitive and motor impairments associated with MSUD reflect structural changes to the brain18 rather than reversible neurotransmitter deficiencies.19,20 This is consistent with the previously reported findings of abnormal cell orientation, dendrite morphology, and spine density in the postmortem brain of a child with MSUD.18 Such microscopic damage is not readily apparent on magnetic resonance imaging, and would change slowly, if at all, after transplantation. In some cases, injury to the nervous system is more overt. Before transplantation, approximately one-third of patients suffered brain damage that led to serious mental or physical disability. These findings underscore the fundamental rationale for liver transplantation as a neuroprotective strategy and may justify pursuit of this option at a young age.
Despite the long-term neurologic protection that liver transplantation affords, transplantation is a complicated and potentially dangerous endeavor. With a multidisciplinary approach focused on the volatility and unpredictability of the disease, we achieved 100% patient and graft survival over an extended period. Complications of immune suppression compared favorably with those in other transplantation populations, and rates of hypertension, posttransplantation diabetes, viral illness, renal insufficiency, and vascular and biliary complications were low. However, a single death and an additional retransplantation reported by UNOS over the same time period underscore the risks involved. Transplantations should be performed only at centers in which considerable pediatric transplantation experience is coupled with expert management of MSUD.
Liver transplantation is an effective alternative to dietary treatment in patients with MSUD, but specific indications for surgery will vary from case to case. Under most circumstances, only patients with severe enzyme deficiency (ie, “classical” disease) will be considered for transplantation. Certain mutations are known to abrogate BCKDH function,21 and residual enzyme activity can be studied using in vivo labeled leucine oxidation or ex vivo fibroblast enzyme activity.22,23 However, an operational understanding of residual enzyme activity can be achieved without such methods by examining weight- and age-adjusted leucine tolerance, metabolic responses to illness, and the stability of plasma concentration relationships among BCAAs.5 Residual enzyme activity is only one variable influencing the decision. Other variables to consider, particularly in developing countries, include the availability of medical foods, convenience and speed of amino acid monitoring, and access to emergency metabolic care. The up-front cost of transplantation also will affect the treatment decision in some cases, especially in uninsured patients. Although frequent hospitalization for encephalopathic crises would support transplantation, a history of “good metabolic control” in a patient with a classical phenotype is no guarantee of future stability and should not be considered a contraindication to the procedure. Metabolic crises can arise at any time and without warning; each such episode is a risk for brain swelling and death.
The ability to treat MSUD and other serious metabolic conditions with liver transplantation expands the list of indications for this procedure.24–27 However, deceased donor organs are in short supply, and we acknowledge objections to “elective” transplantation for metabolic disorders when more than 1500 people die each year on the liver waitlist. Although an in-depth analysis of the ethics of transplantation for MSUD is beyond the scope of this article, this published experience addresses several concerns that have been expressed by Ross.28 First, decision making for patients with metabolic disease requires the type of longer-term data on both graft- and nongraft-related outcomes, such as the neurocognitive outcomes considered here. Successful elimination of sustained BCAA dysregulation and preliminary evidence of the stabilization of neurocognitive function are important elements that will aid clinical and community decision making. Although matched controlled subjects not undergoing transplantation were not included in the present analysis, the collective published experience by us and others supports the long-term benefits of transplantation. In addition, the success of domino transplantation reported here and in additional cases29 also may mitigate some of these concerns.
Finally, it is also important to remember that MSUD, even if detected early, is still a lethal condition in many countries. As of 1988, before we established a local metabolic service in Lancaster County, 14 of 36 (39%) Mennonite patients with MSUD died of brain herniation by age 6 years. In 2006, Vietnam began using tandem mass spectrometry to screen newborns for MSUD. Fourteen cases were diagnosed during the first 3 years of the program. Among 12 patients diagnosed as newborns, 11 are dead, and the sole survivor is disabled. Two remaining patients, diagnosed at 6 months and 2.5 years, have brain damage (Vu Chi Dung, personal communication, April 14, 2011). In Vietnam as elsewhere, liver transplantation potentially may be more readily available than medical foods and inpatient services to manage MSUD.
Similar clinical circumstances exist throughout the world. Our cohort included 2 children from India and Brazil who had no consistent medical care and would have died or been crippled without liver transplantation. Even within the United States, metabolic services for MSUD are inconsistent. Some families travel long distances to see a specialist once or twice yearly and cannot find appropriate care during emergencies. Monitoring practices vary from region to region, and families sometimes wait up to days or weeks for amino acid results. For most patients, fragmented and unreliable subspecialty services disintegrate further as they pass into adulthood. Even under ideal clinical circumstances,5 classical MSUD is a volatile and dangerous condition that hangs like a sword of Damocles over the child and family. Cases of serious brain disease reported in this cohort are a stark reminder of the fear and anxiety that families live with daily; a consistent effect of transplantation is to relieve families of this psychological burden. Indeed, the recommendation for liver transplantation during the multidisciplinary evaluation of the child with significant neurologic injury was based on the overall assessment that her medical care could be facilitated and further neurologic injury potentially abrogated by successful transplantation. Although ongoing study of both long-termtransplantation and nontransplantation outcomes is needed, this report provides important support for liver transplantation for MSUD as a sound ethical and medical practice.
Liver transplantation substitutes one set of problems for another. It sets the risks of surgery and immune suppression against those of acute and chronic brain injury. Parents and young adults who elect transplantation understand the tradeoff and place their priority on neurologic health. Ongoing clinical investigation is needed to determine whether neurologic protection is sustained over the long term and at acceptable costs.
Acknowledgments
We thank the children and families who continue to inspire our work. Many clinicians, nurses, physician assistants, nurse practitioners, and students made important contributions to their care. We especially wish to acknowledge the contributions of Dr Nicholas L. Rider, Donna Robinson, and Christine Hendrickson (Clinic for Special Children); Lynn Seward, Kim Haberman, JoAnne Blice, Tammy Fazzolare PA-C, Lisa Remaley PA-C, and Lorna Cropcho (Children’s Hospital of Pittsburgh of UPMC). Dr. VũChí Dũng, Head of the Department of Medical Genetics, Metabolism, and Endocrinology at the National Hospital of Pediatrics in Hanoi, graciously shared newborn screening and follow-up data.
Funded by charitable contributions from the Old Order Mennonite and Amish communities to the nonprofit Clinic for Special Children and the Hillman Foundation. The authors and affiliated clinicians had sole responsibility for the clinical care described herein, the development of the manuscript, and interpretation of the data and outcomes, with no role from any external funding sponsors.
Glossary
- BCAA
Branched-chain amino acid
- BCKDH
Branched-chain ketoacid dehydrogenase complex
- MSUD
Maple syrup urine disease
- UNOS
United Network for Organ Sharing
Footnotes
The authors declare no conflicts of interest.
References
- 1.Riviello JJ, Jr, Rezvani I, DiGeorge AM, Foley CM. Cerebral edema causing death in children with maple syrup urine disease. J Pediatr. 1991;119:42–45. doi: 10.1016/s0022-3476(05)81036-4. [DOI] [PubMed] [Google Scholar]
- 2.Simon E, Schwarz M, Wendel U. Social outcome in adults with maple syrup urine disease (MSUD) J Inherit Metab Dis. 2007;30:264. doi: 10.1007/s10545-007-0475-4. [DOI] [PubMed] [Google Scholar]
- 3.Hoffmann B, Helbling C, Schadewaldt P, Wendel U. Impact of longitudinal plasma leucine levels on the intellectual outcome in patients with classic MSUD. Pediatr Res. 2006;59:17–20. doi: 10.1203/01.pdr.0000190571.60385.34. [DOI] [PubMed] [Google Scholar]
- 4.Simon E, Wendel U, Schadewaldt P. Maple syrup urine disease: treatment and outcome in patients of Turkish descent in Germany. Turk J Pediatr. 2005;47:8–13. [PubMed] [Google Scholar]
- 5.Strauss KA, Wardley B, Robinson D, Hendrickson C, Rider NL, Puffenberger EG, et al. Classical maple syrup urine disease and brain development: principles of management and formula design. Mol Genet Metab. 2010;99:333–345. doi: 10.1016/j.ymgme.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suryawan A, Hawes JW, Harris RA, Shimomura Y, Jenkins AE, Hutson SM. A molecular model of human branched-chain amino acid metabolism. Am J Clin Nutr. 1998;68:72–81. doi: 10.1093/ajcn/68.1.72. [DOI] [PubMed] [Google Scholar]
- 7.Strauss KA, Mazariegos GV, Sindhi R, Squires R, Finegold DN, Vockley G, et al. Elective liver transplantation for the treatment of classical maple syrup urine disease. Am J Transplant. 2006;6:557–564. doi: 10.1111/j.1600-6143.2005.01209.x. [DOI] [PubMed] [Google Scholar]
- 8.Shellmer DA, DeVito Dabbs A, Dew MA, Noll RB, Feldman H, Strauss KA, et al. Cognitive and adaptive functioning after liver transplantation for maple syrup urine disease: a case series. Pediatr Transplant. 2011;15:58–64. doi: 10.1111/j.1399-3046.2010.01411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Motulsky H. Statistics Guide. 4 ed. San Diego, CA: GraphPad Software, Inc; 2005. [Google Scholar]
- 10.Altman DG. Practical Statistics for Medical Research. New York: Chapman & Hall; 1991. [Google Scholar]
- 11.Ito M, Nagata H, Miyakawa S, Fox IJ. Review of hepatocyte transplantation. J Hepatobiliary Pancreat Surg. 2009;16:97–100. doi: 10.1007/s00534-008-0023-0. [DOI] [PubMed] [Google Scholar]
- 12.Koyata H, Cox RP, Chuang DT. Stable correction of maple syrup urine disease in cells from a Mennonite patient by retroviral-mediated gene transfer. Biochem J. 1993;295:635–639. doi: 10.1042/bj2950635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Skvorak KJ, Hager EJ, Arning E, Bottiglieri T, Paul HS, Strom SC, et al. Hepatocyte transplantation (HTx) corrects selected neurometabolic abnormalities in murine intermediate maple syrup urine disease (iMSUD) Biochim Biophys Acta. 2009;1792:1004–1010. doi: 10.1016/j.bbadis.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Toietta G, Mane VP, Norona WS, Finegold MJ, Ng P, McDonagh AF, et al. Life-long elimination of hyperbilirubinemia in the Gunn rat with a single injection of helper-dependent adenoviral vector. Proc Natl Acad Sci USA. 2005;102:3930–3935. doi: 10.1073/pnas.0500930102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brunetti-Pierri N, Stapleton GE, Law M, Breinholt J, Palmer DJ, Zuo Y, et al. Efficient, long-term hepatic gene transfer using clinically relevant HDAd doses by balloon occlusion catheter delivery in nonhuman primates. Mol Ther. 2009;17:327–333. doi: 10.1038/mt.2008.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaplan P, Mazur A, Field M, Berlin JA, Berry GT, Heidenreich R, et al. Intellectual outcome in children with maple syrup urine disease. J Pediatr. 1991;119:46–50. doi: 10.1016/s0022-3476(05)81037-6. [DOI] [PubMed] [Google Scholar]
- 17.Hilliges C, Awiszus D, Wendel U. Intellectual performance of children with maple syrup urine disease. Eur J Pediatr. 1993;152:144–147. doi: 10.1007/BF02072492. [DOI] [PubMed] [Google Scholar]
- 18.Kamei A, Takashima S, Chan F, Becker LE. Abnormal dendritic development in maple syrup urine disease. Pediatr Neurol. 1992;8:145–147. doi: 10.1016/0887-8994(92)90038-z. [DOI] [PubMed] [Google Scholar]
- 19.Zinnanti WJ, Lazovic J, Griffin K, Skvorak KJ, Paul HS, Homanics GE, et al. Dual mechanism of brain injury and novel treatment strategy in maple syrup urine disease. Brain. 2009;132:903–918. doi: 10.1093/brain/awp024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prensky AL, Moser HW. Brain lipids, proteolipids, and free amino acids in maple syrup urine disease. J Neurochem. 1966;13:863–874. doi: 10.1111/j.1471-4159.1966.tb05882.x. [DOI] [PubMed] [Google Scholar]
- 21.Nellis MM, Kasinski A, Carlson M, Allen R, Schaefer AM, Schwartz EM, et al. Relationship of causative genetic mutations in maple syrup urine disease with their clinical expression. Mol Genet Metab. 2003;80:189–195. doi: 10.1016/s1096-7192(03)00144-6. [DOI] [PubMed] [Google Scholar]
- 22.Elsas LJ, Ellerine NP, Klein PD. Practical methods to estimate whole-body leucine oxidation in maple syrup urine disease. Pediatr Res. 1993;33:445–451. doi: 10.1203/00006450-199305000-00005. [DOI] [PubMed] [Google Scholar]
- 23.Simon E, Flaschker N, Schadewaldt P, Langenbeck U, Wendel U. Variant maple syrup urine disease (MSUD): the entire spectrum. J Inherit Metab Dis. 2006;29:716–724. doi: 10.1007/s10545-006-0276-1. [DOI] [PubMed] [Google Scholar]
- 24.Schauer R, Stangl M, Lang T, Zimmermann A, Chouker A, Gerbes AL, et al. Treatment of Crigler-Najjar type 1 disease: relevance of early liver transplantation. J Pediatr Surg. 2003;38:1227–1231. doi: 10.1016/s0022-3468(03)00273-2. [DOI] [PubMed] [Google Scholar]
- 25.Shneider BL. Pediatric liver transplantation in metabolic disease: clinical decision making. Pediatr Transplant. 2002;6:25–29. doi: 10.1034/j.1399-3046.2002.1p057.x. [DOI] [PubMed] [Google Scholar]
- 26.McDiarmid SV, Millis MJ, Olthoff KM, So SK. Indications for pediatric liver transplantation. Pediatr Transplant. 1998;2:106–116. [PubMed] [Google Scholar]
- 27.Rela M, Muiesan P, Heaton ND, Corbally M, Hajj H, Mowat AP, et al. Orthotopic liver transplantation for hepatic-based metabolic disorders. Transpl Int. 1995;8:41–44. doi: 10.1007/BF00366709. [DOI] [PubMed] [Google Scholar]
- 28.Ross LF. An ethical and policy analysis of elective transplantation for metabolic conditions diagnosed by newborn screening. J Pediatr. 2010;156:139–144. doi: 10.1016/j.jpeds.2009.06.067. [DOI] [PubMed] [Google Scholar]
- 29.Khanna A, Hart M, Nyhan WL, Hassanein T, Panyard-Davis J, Barshop BA. Domino liver transplantation in maple syrup urine disease. Liver Transpl. 2006;12:876–882. doi: 10.1002/lt.20744. [DOI] [PubMed] [Google Scholar]