Abstract
The H-NOX (Heme-Nitric oxide/OXygen binding) family of diatomic gas sensing hemoproteins has attracted great interest. Soluble guanylate cyclase (sGC), the well-characterized eukaryotic nitric oxide (NO) sensor is an H-NOX family member. When NO binds sGC at the ferrous histidine-ligated protoporphyrin-IX, the proximal histidine ligand dissociates, resulting in a 5-coordinate (5c) complex; formation of this 5c complex is viewed as necessary for activation of sGC. Characterization of other H-NOX family members has revealed that while most also bind NO in a 5c complex, some bind NO in a 6-coordinate (6c) complex or as a 5c/6c mixture. To gain insight into the heme pocket structural differences between 5c and 6c Fe(II)–NO H-NOX complexes, we investigated the Extended X-ray Absorption Fine Structure (EXAFS) of the Fe(II)–unligated and Fe(II)–NO complexes of H-NOX domains from three species, Thermoanaerobacter tengcongensis, Shewanella woodyi, and Pseudoalteromonas atlantica. Although the Fe(II)–NO complex of TtH-NOX is formally 6c, we found the Fe-NHis bond is substantially lengthened. Furthermore, although NO binds to SwH-NOX and PaH-NOX as a 5c complex, consistent with histidine dissociation, the EXAFS data do not exclude a very weakly associated histidine. Regardless of coordination number, upon NO-binding, the Fe–Nporphyrin bond lengths in all three H-NOXs contract by ~0.07 Å. This study reveals that the overall heme structure of 5c and 6c Fe(II)–NO H-NOX complexes are substantially similar, suggesting that formal histidine dissociation may not be required to trigger NO/H-NOX signal transduction. The study has refined our understanding of the molecular mechanisms underlying NO/H-NOX signaling.
Introduction
Diatomic gas molecules such as nitric oxide (NO) regulate important biological signal transduction pathways through selective binding with cognate sensor proteins.1-9 The H-NOX (Heme-Nitric oxide/OXygen binding) family of heme-based sensor proteins has been shown to play essential roles in gas molecule sensing in a variety of biological systems.3, 4, 10-13 The H-NOX domain can be defined as a ~200 amino acid protein sequence that binds a histidine-ligated ferrous protoporphyrin IX in a mixed α/β fold.14-16 This fold is exclusive to the H-NOX family.
The best-studied H-NOX family member is soluble guanylate cyclase (sGC), the only confirmed NO sensor in mammals. Upon binding of NO to the H-NOX domain of sGC, the enzymatic activity of sGC is enhanced by over 200-fold, leading to a significant increase in the production of cyclic guanosine monophosphate (cGMP). Downstream, cGMP regulates important physiological processes including neurotransmission, smooth muscle relaxation, and inhibition of platelet aggregation.1, 2, 17, 18 In bacteria, H-NOX proteins have been demonstrated to regulate enzymatic activities including histidine autophosphorylation and cyclic-di-GMP synthesis and hydrolysis in response to NO binding.3, 4, 12, 13 While the downstream physiological consequences of these NO-mediated changes in enzymatic activity are still being elucidated, regulation of biofilm formation has been demonstrated.5, 12, 13
In 2004, the crystal structure of the H-NOX domain from Thermoanaerobacter tengcongensis (Tt H-NOX) was solved to a resolution of 1.77 Å (Figure 1).16 This structure, as well as subsequent H-NOX structures,15, 19-22 has been widely used to model the sGC heme domain as well as other H-NOX family members, yielding many valuable insights into the H-NOX signaling mechanism.21, 23-31 Currently the prevalent hypothesis for NO/H-NOX signaling is that NO binding to H-NOX generates conformational changes to the heme group, most notably dissociation of the proximal histidine ligand.22, 30-34 Subsequently, these changes in heme structure result in a shift of the N-terminal helices on the surface of H-NOX.19-22 Presumably this change in the structure of the H-NOX domain triggers downstream signaling events through changes in domain-domain interactions, either within the protein itself (such as in sGC) or between separate proteins (such as interactions of H-NOX with effector enzymes like histidine kinases and di-guanylate cyclases). The molecular details of this mechanism remain to be fully elucidated, however. In particular, there is still significant disagreement over even the most fundamental components, such as whether NO binds to the proximal or distal face of the heme in its active signaling form.35-39
Figure 1.
Crystal structure of Tt H-NOX and homology models for Sw and Pa H-NOX (structure for Tt H-NOX was generated using PDB 1U55; homology models were generated using HHPRED40). The structures for Tt, Sw, and Pa H-NOX are colored in cyan, green and purple, respectively. The heme cofactor is colored in red and shown in its position in Tt H-NOX.
A problem that significantly impedes the resolution of the mechanism of H-NOX signaling is the lack of structural information for the Fe(II)–unligated and Fe(II)–NO complexes, which represent, respectively, the physiological resting state and active state of these NO sensors. To date, there has been only one instance of successful crystallization of both the Fe(II)–unligated and Fe(II)–NO H-NOX complexes, which was reported for Nostoc sp H-NOX (Ns H-NOX).20 Ns H-NOX binds NO as a mixture of 5-coordinate (5c, with dissociation of the proximal His ligand) and 6-coordinate (6c, with retention of the Fe–NHis bond) complexes, with 6c-NO as the predominant species. As sGC and most other characterized H-NOX domains bind NO in a 5c complex,4, 14, 26, 33 and dissociation of His is thought to be important to trigger signal transduction in the H-NOX family, it is unclear how much relevant structural information can be extracted from this structure. Therefore, additional structural characterization of the heme pocket of the H-NOX Fe(II)–unligated and Fe(II)–NO complexes is highly desirable.
In this work, we use iron K-edge X-ray absorption spectroscopy (XAS) to obtain structural details of the heme pocket of H-NOX. XAS is a powerful technique for examining Fe-heme local structure and electrostatics in hemoproteins, providing information on Fe oxidation state, Fe spin state, the number and type of ligands bound to Fe, and Fe-ligand bond lengths.41, 42 In our previous work, we used iron K-edge XAS to examine the flow of electron density between the iron center and surrounding ligands and X-ray Absorption Near-Edge Structure (XANES) to predict iron-heme displacement upon ligand binding to Tt H-NOX.30 Here we use Extended X-ray Absorption Fine Structure (EXAFS) analysis to obtain precise structures of the H-NOX heme iron for several different H-NOX domains.
Specifically, we investigate the Fe(II)–unligated and Fe(II)–NO complexes for H-NOX domains from three different species, Thermoanaerobacter tengcongensis (Tt), Shewanella woodyi (Sw), and Pseudoalteromonas atlantica (Pa). Our data challenge the requirement of histidine dissociation upon NO binding and support a model in which NO is bound to the distal side of the heme. Furthermore, the results suggest that contraction of the Fe–Nporphyrin bonds, as well as Fe–NHis bond lengthening (but not dissociation), are key structural changes that initiate signal transduction in the H-NOX family.
Results and Discussion
Electronic spectroscopy suggests that H-NOX domains can bind NO in either a 5c or 6c complex
UV/visible spectra of hemoproteins are indicative of the Fe oxidation state as well as the bound ligand. The Fe(II)–unligated and Fe(II)–NO bound complexes for Tt H-NOX, Sw H-NOX, and Pa H-NOX are shown in Figure 2 and the peak absorbance maxima are compared with sGC in Table 1. The spectra for Tt H-NOX14 and Sw H-NOX13 have previously been published; they are included here for comparison. The Fe(II)–unligated state of all three H-NOX proteins has a Soret band maximum at ~430 nm and a merged α/β band at ~560 nm, indicative of a 5-coordinate, high-spin heme complex, similar to sGC and other characterized H-NOX domains (Table 1).14, 26,33 The Soret band maximum at 419 nm and split α/β bands that results from addition of NO to the Tt H-NOX Fe(II)–unligated complex is consistent with a 6c Fe(II)–NO complex with a bound histidine ligand, as previously reported.14 However, the Fe(II)–NO complexes for Sw H-NOX and Pa H-NOX display Soret bands at ~399 nm and split α/β bands at ~570 nm and 543 nm respectively, which are similar to sGC (Table 1). Thus, the electronic spectra of Sw H-NOX and Pa H-NOX are consistent with formation of 5c complexes with the Fe–NHis bonds broken. sGC (bovine) shares 40% homology (24% identity) and 39% homology (22% identity), respectively, with Sw H-NOX and Pa H-NOX. Thus, these two H-NOX proteins are good models of the sGC heme domain; characterization of their interactions with NO should provide insight into the NO/H-NOX activation mechanism, including the H-NOX family member sGC.
Figure 2.
UV/visible spectra of Tt (A), Sw (B) and Pa (C) H-NOX in Fe(II)–unligated (solid) and Fe(II)–NO (dash) states.
Table 1.
UV/visible spectra peak positions and iron edge/pre-edge energies for Tt, Sw and Pa H-NOX in Fe(II)–unligated and Fe(II)–NO states.
| Soreta | β a | α a | Eedgeb | Epre-edgeb | |
|---|---|---|---|---|---|
| Fe(II)-unligated | |||||
| T. tengcongensis | 429 | 560 | 7124.1 | 7112.3 | |
| S. woodyi | 430 | 564 | 7123.4 | 7112.2 | |
| P. atlantica | 431 | 561 | 7123.5 | 7112.6 | |
| sGC | 431 | 555 | cND | cND | |
| Fe(II)-NO | |||||
| T. tengcongensis | 419 | 547 | 577 | 7125.5 | 7113.8 |
| S. woodyi | 399 | 543 | 572 | 7123.7 | 7113.4 |
| P. atlantica | 398 | 543 | 569 | 7124.0 | 7115.4 |
| sGC | 398 | 537 | 572 | cND | cND |
units: nm for UV/visible spectra.
units: eV for X-ray edge/pre-edge energies.
Not determined.
XANES analysis of H-NOX ferrous complexes suggests electronic structure differences in the Fe(II)-NO complexes
X-ray absorption near-edge spectroscopy (XANES) provides qualitative insights into the oxidation state and electronic symmetry of the absorbing atom, as well as the identity of ligating atoms.43, 44 For example, the energy of the iron K-edge in iron-containing compounds indicates the energy required to excite the core shell (1s) electron of the Fe atom. Thus higher edge energies correspond to a greater energy requirement for electron excitation and a more positive effective nuclear charge at the iron center. We have previously determined the Fe K-edge positions for Tt H-NOX, with values for the Fe(II)–unligated and Fe(II)–NO complexes of 7124.1 eV and 7125.5 eV, respectively (Table 1).30 The 1.4 eV increase in edge energy upon NO binding suggests extensive electron density flow from Fe to its surrounding ligands (including NO), generating a more positive heme iron center. Surprisingly, the K-edge energies for Sw and Pa H-NOX Fe(II)–NO complexes are more comparable to those of their Fe(II)–unligated counterparts, increasing by only 0.3 – 0.5 eV (Table 1). The small difference in iron K-edge energies caused by NO association does not exclude the possibility of a change in the electronic environment of Fe upon NO binding, however. The edge energy indicates the net electron density on heme iron; it does not reveal the detailed iron ligand field. The small change in K-edge energy upon NO ligation in Sw and Pa H-NOX can be attributed to the presence of only 5 iron ligands in the Fe(II)–NO complexes of Sw and Pa H-NOX, in contrast to a coordination number of 6 in Tt H-NOX. In Sw and Pa H-NOX, NO binding to the heme iron promotes elongation or dissociation of the proximal histidine ligand, formally maintaining a 5c environment; i.e., the effect of NO association on iron electron density is roughly cancelled out by histidine dissociation, resulting in similar K-edge values. Therefore, the similar edge values for the Fe(II)–unligated and Fe(II)–NO complexes of Sw and Pa H-NOX are consistent with two distinct 5c ligand fields at the heme center.
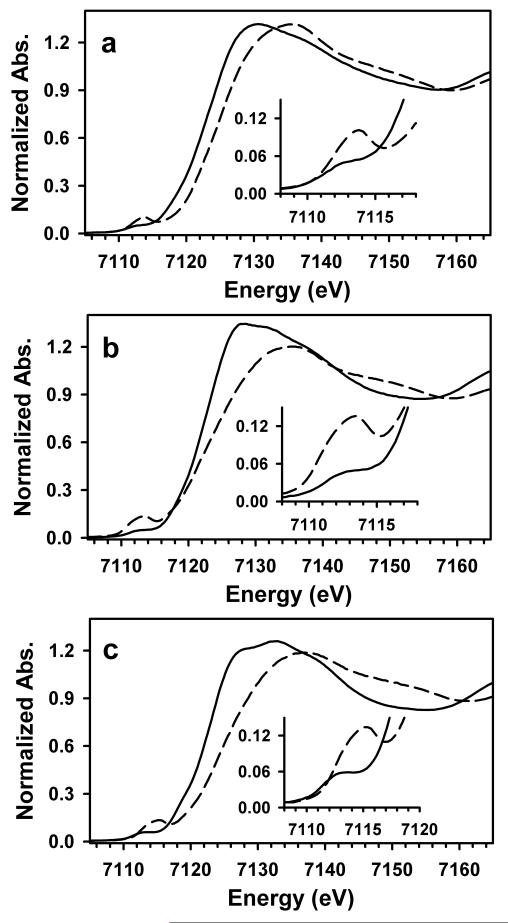
The shape of the rising edge also provides insights into the electronic environment of the heme iron center. The XANES spectra of both the Fe(II)–unligated and NO-bound states of Tt H-NOX, shown in Figure 3a, exhibit rising white-line absorptions of similar intensity and steepness, whereas NO binding to Sw and Pa H-NOX elicits a broadening and decrease in the edge intensity relative to their respective unligated states (Figure 3b,c). In general, the shape and intensity of the K-edge white line is related to both coordination number and structural disorder at the absorbing atom, with higher coordination numbers and more ordered first coordination spheres yielding more intense white-line absorptions.45, 46 The observed edge shape change upon NO binding to Fe(II)–unligated Sw or Pa H-NOX likely reflects increased disorder and/or an increase in the distribution of bond lengths for the heme iron site. We favor this explanation over one involving a decrease in coordination number, presumably from 6c in the Fe(II)–unligated state to 5c in the NO-bound form, because our UV/visible observations suggest both Fe(II)–unligated and NO-bound Sw and Pa H-NOX are 5c.
Figure 3.
XANES spectra of the Fe(II)–unligated (solid lines) and NO-bound (dashed lines) states of H-NOX from Tt (a), Sw (b), and Pa (c). The insets depict an expansion of the pre-edge transition for each sample.
For Tt H-NOX, the similar white-line absorption for both the Fe(II)–unligated and NO-bound forms probably results from the increased coordination number of the Fe(II)–NO form (from 5c in the unligated state to 6c in the NO-bound state, consistent with the UV/visible spectra) being balanced by an increase in the distribution of bond lengths for the iron center. Indeed, comparison of all three of the Fe(II)–unligated H-NOX complexes reveals edge absorptions of nearly identical sharpness and intensity, while the NO-bound form of Tt H-NOX exhibits a clearly steeper edge than those of NO-bound Sw and Pa H-NOX (Figure S1, Electronic Supplementary Information). This distinction likely reflects a somewhat different iron electronic environment in the NO complex of Tt H-NOX.
The pre-edge features, which are associated with a formally forbidden 1s-to-3d transition that gains intensity by mixing with the 4p orbitals of the iron center,44 support our iron K-edge observations. The pre-edge peak for Tt H-NOX upshifts by ~1.5 eV (from 7112.3 eV to 7113.8 eV) and increases significantly in intensity upon NO binding. This is consistent with a substantial loss of centrosymmetry at the iron center as well as an increase in effective nuclear charge. This observation is in agreement with previous XANES observations on the NO-bound forms of both heme and non-heme iron proteins.47-49
Both Sw and Pa H-NOX exhibit similar changes in their pre-edge peak properties. The Fe(II)–NO complex of Pa H-NOX exhibits an unusually high pre-edge energy of 7115.4 eV; we do not have a good explanation at present for this surprising result. The intensities of the pre-edge peaks for the NO complexes of both Sw and Pa H-NOX are greater than that of Tt H-NOX (Figure S1, Electronic Supplementary Information; note that any pre-edge intensity differences for the unligated states are quite subtle). We can infer that the iron site in the Tt H-NOX Fe(II)–NO complex has less electronic distortion than in either Sw or Pa H-NOX, which is again consistent with a 6c site in the former and 5c sites in the latter two species. In general, our XANES data are consistent with UV/visible data indicating that Sw H-NOX and Pa H-NOX form 5-coordinate Fe(II)–NO complexes, while the NO-bound state of Tt H-NOX is better described as 6-coordinate.
Finally, the edge energy for the Tt H-NOX Fe(II)–unligated state is ~0.6 eV greater than those of Sw and Pa H-NOX (Table 1). This suggests that there are large electrostatic differences in the heme pocket in the respective H-NOX domains, with a more positive heme iron in Tt H-NOX. We reason that this difference is correlated with their distinct functions, as Tt H-NOX is predicted to be an O2 sensor while Sw and Pa H-NOX are expected to be NO sensors. It has been suggested that the severe heme distortion in wildtype Tt H-NOX results in a lowered electron density at the heme iron,50 so a higher edge energy could correlate with a larger heme distortion as well.
EXAFS analysis of 5c and 6c H-NOX Fe(II)–NO complexes reveals only subtle differences in a weak Fe–NHis bond
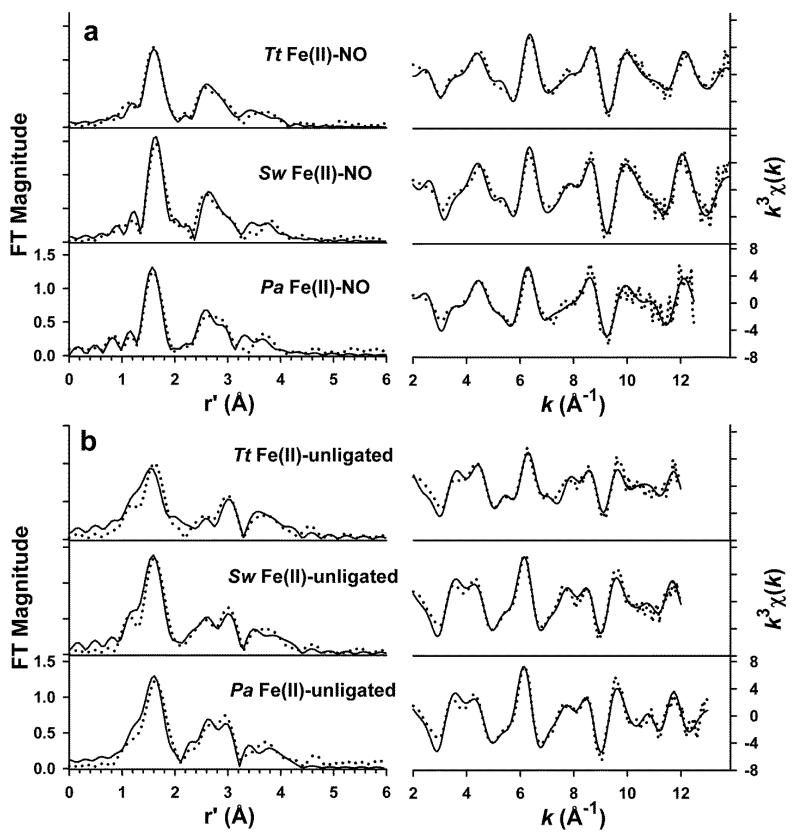
EXAFS is a powerful technique that has been extensively used to examine the coordination chemistry of metal centers in metalloproteins, including heme-based sensor proteins.43, 51, 52 Here we use it to identify the specific molecular details of heme iron conformational changes induced by NO binding to the three different H-NOX domains. The unfiltered k3χ(k) EXAFS spectra and corresponding Fourier transforms (FT) for both the Fe(II)–unligated and NO-bound states of Tt H-NOX, Sw H-NOX and Pa H-NOX are shown in Figure 4, along with representative best fits.
Figure 4.
Representative best fits to the Fourier transforms of the Fe K-edge EXAFS data (k3χ(k), left) and unfiltered EXAFS spectra (k3χ(k), right) for the NO-bound (a) and Fe(II)–unligated (b) states of H-NOX from Tt, Sw, and Pa, as indicated by the labels. Experimental data is shown with dotted lines and fits are shown with solid lines. The spectra in each panel are shown with identical vertical scaling. Fit parameters are given in Table 2 (fit 4 for NO-bound, fit 8 for Fe(II)–unligated) for Tt H-NOX and Table 3 for both NO-bound and Fe(II)–unligated Sw H-NOX and Pa H-NOX.
The EXAFS features of the NO-bound state of all three H-NOX domains are similar to one another, aside from some modest differences in the amplitude of the EXAFS oscillations. The Fourier transforms contain an intense, sharp feature at a phase-uncorrected distance of r’ ~ 1.6 Å, corresponding to first-shell ligands, and a set of peaks at r’ = 2.5 – 4.0 Å that can be assigned to single- and multiple-scattering paths from the pyrrole moieties of the heme group.
While the k3χ(k) EXAFS spectra of the Fe(II)–unligated complexes differ significantly from their Fe(II)–NO congeners, the Fourier transforms are more similar. The most notable difference in the FT is a loss of intensity and slight broadening in the feature at r’ ~ 1.6 Å, which is most evident for the Fe(II)–unligated state of Tt H-NOX (both in relation to the NO-bound form and to the other Fe(II)–unligated spectra).
We carried out a detailed EXAFS analysis of the Fe(II)–NO complex of Tt H-NOX (summarized in Table 2). The EXAFS spectrum of NO-ligated Tt H-NOX can be simulated with a set of 4 Fe–Np scatterers at 1.99 Å associated with the equatorial N donors of the heme, along with a set of single and multiple scattering paths associated with the Cα, Cβ, and Ch atoms assignable to the pyrrole moieties and meso carbons of the heme. Inclusion of a single Fe–N/O scatterer at 1.75 Å attributable to bound NO53 results in a significant improvement in fit quality (Table 2, fit 2). We note that the Debye-Waller factor (σ2) for this shell is fairly large, which may reflect either sub-stoichiometric binding of NO or perhaps multiple modes of NO binding. It was not possible to identify unique multiple-scattering paths involving the Fe–NO unit that would enable determination of the Fe–NO bond angle, presumably due to the intensity of the long-range scattering paths from the heme group. The requirement for a short Fe–N/O scatterer that would significantly distort the centrosymmetry of the iron site agrees nicely with the observed increase in the intensity of the pre-edge feature upon NO binding.
Table 2.
Selected EXAFS fits to the NO-bound and Fe(II)–unligated states of Tt H-NOX.a
| Tt H-NOX Fe(II)–NO | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| fit 1 | fit 2 | fit 3 | fit 4 | ||||||||
| shell | r | σ 2 | shell | r | σ 2 | shell | r | σ 2 | shell | r | σ 2 |
|
|
|
|
|
||||||||
| 1 Fe-NO | 1.75 | 5.1 | 1 Fe-NO | 1.73 | 3.0 | 1 Fe-NO | 1.74 | 5.4 | |||
| 4 Fe-Np | 1.99 | 3.9 | 4 Fe-Np | 1.97 | 3.3 | 4 Fe-Np + 1 Fe- NHis |
1.97 | 5.1 | 4 Fe-Np | 1.97 | 3.4 |
| 1 Fe-NHis | 2.32 | 6.5 | |||||||||
| 8 Fe••Cα | 3.01 | 3.3 | 8 Fe••Cα | 2.99 | 3.6 | 8 Fe••Cα | 2.99 | 3.6 | 8 Fe••Cα | 2.99 | 3.5 |
| 16 Fe••Cα/Np | 3.18 | 6.8 | 16 Fe••Cα/Np | 3.16 | 7.2 | 16 Fe••Cα/Np | 3.16 | 7.2 | 16 Fe••Cα/Np | 3.16 | 7.1 |
| 4 Fe••Ch | 3.10 | 37.5 | 4 Fe••Ch | 3.34 | 4.9 | 4 Fe••Ch | 3.34 | 4.9 | 4 Fe••Ch | 3.35 | 4.5 |
| 8 Fe••Cβ | 4.36 | 6.3 | 8 Fe••Cβ | 4.31 | 5.5 | 8 Fe••Cβ | 4.31 | 5.4 | 8 Fe••Cβ | 4.31 | 5.5 |
| 16 Fe••Cβ/Np | 4.39 | 12.6 | 16 Fe••Cβ/Np | 4.34 | 11.0 | 16 Fe••Cβ/Np | 4.33 | 10.8 | 16 Fe••Cβ/Np | 4.33 | 11.0 |
|
|
|
|
|
||||||||
| F | 0.369 | F | 0.286 | F | 0.315 | F | 0.270 | ||||
| ΔE0 | 8.40 | ΔE0 | 3.76 | ΔE0 | 3.43 | ΔE0 | 3.48 | ||||
|
|
|
|
|
||||||||
|
| |||||||||||
| Tt H-NOX Fe(II)–unligated | |||||||||||
|
| |||||||||||
| fit 5 | fit 6 | fit 7 | fit 8 | ||||||||
| shell | r | σ 2 | shell | r | σ 2 | shell | r | σ 2 | shell | r | σ 2 |
|
|
|
|
|
||||||||
| 4 Fe-Np | 2.02 | 7.2 | 4 Fe-Np + 1 Fe-NHis |
2.02 | 10.0 | 4 Fe-Np | 2.02 | 7.2 | 4 Fe-Np + 1 Fe-NHis |
2.03 | 10.3 |
| 1 Fe-NHis/O | 2.69 | 2.6 | 1 Fe-O | 2.38 | 3.2 | ||||||
| 8 Fe••Cα | 3.11 | 13.4 | 8 Fe••Cα | 3.12 | 15.9 | 8 Fe••Cα | 3.09 | 15.0 | 8 Fe••Cα | 3.12 | 14.2 |
| 4 Fe••Ch | 3.40 | −0.3 | 4 Fe••Ch | 3.40 | −0.4 | 4 Fe••Ch | 3.40 | −0.5 | 4 Fe••Ch | 3.40 | −0.8 |
| 8 Fe••Cβ | 4.39 | 3.7 | 8 Fe••Cβ | 4.39 | 4.0 | 8 Fe••Cβ | 4.39 | 3.8 | 8 Fe••Cβ | 4.39 | 3.3 |
| 16 Fe••Cβ/Np | 4.42 | 7.5 | 16 Fe••Cβ/Np | 4.42 | 8.0 | 16 Fe••Cβ/Np | 4.42 | 7.7 | 16 Fe••Cβ/Np | 4.42 | 6.7 |
|
|
|
|
|
||||||||
| F | 0.381 | F | 0.436 | F | 0.362 | F | 0.411 | ||||
| ΔE0 | 4.95 | ΔE0 | 4.94 | ΔE0 | 4.98 | ΔE0 | 4.98 | ||||
|
|
|
|
|
||||||||
r is in units of Å; σ2 is in units of 10−3 Å2; ΔE0 is in units of eV. All fits are to unfiltered data. F represents a goodness-of-fit parameter. Fourier transform ranges: k = 2.0 – 13.85 Å−1 (resolution = 0.134 Å) for Tt H-NOX Fe(II)–NO; k = 2.0 – 12.0 Å−1 (resolution = 0.157 Å) for Tt H-NOX Fe(II)–unligated. Values of r for the Fe••Cα single scattering and Fe••Cα/Np multiple scattering paths were constrained to a constant difference from one another, while σ2 for the multiple scattering path was constrained to be twice that of the single scattering path. A similar constraint was placed on the Cβ and Cβ/Np combination. Inclusion of the Fe••Cα/Np multiple scattering paths was not required for fits of Fe(II)-unligated data.
Additional attempts were made to fit the inner shell to a six-coordinate Fe(II) site by inclusion of an axially bound Fe–NHis scatterer. A fit with a single shell of 5 (Fe–Np + NHis) scatterers (Table 2, fit 3) resulted in a significant decrease in fit quality and a larger σ2 value for the principal Fe–N shell, suggesting that the Fe-NHis bond, if present, has a bond length very different from those of the equatorial heme nitrogens. Indeed, a weak and fairly disordered Fe–NHis bond of 2.32 Å can be resolved (Table 2, fit 4), providing a modest improvement in fit quality.
The inclusion of a 6th bond, the Fe–NHis bond, is consistent with the electronic spectrum of the Fe(II)–NO complex of Tt H-NOX, thus we include it in our fit, but it is significant that the inclusion of a long Fe–NHis bond only modestly improves the fit relative to a 5c complex. We always obtain electronic spectra of each sample before and after XAS measurements to monitor any change in sample quality due to X-ray exposure, and we therefore know that this sample is NO-bound both before and after EXAFS data collection. This, as well as the fact that there are significant differences in EXAFS spectra of the Fe(II)–unligated and Fe(II)–NO complexes leads us to conclude that the apparent 5c nature of the Fe(II)–NO EXAFS is not due to NO dissociation during data collection. Instead, we conclude that the Fe-NHis bond is formally present, but severely weakened by the NO trans effect.
Similar analyses of the EXAFS were obtained for the NO complexes of Sw and Pa H-NOX (Table 3, Tables S1-S2 in Electronic Supplementary Information). EXAFS data for both species was adequately simulated by a short Fe–N scatterer at 1.72–1.75 Å from bound NO, 4 Fe–Np scatterers at ca. 1.99 Å and a set of single and multiple scattering pathways associated with the heme macrocycle (the Fe••Ch component for the heme meso carbons was not required to fit Pa H-NOX Fe(II)–NO data). In addition, the magnitude of the Debye-Waller factors for the first-shell paths was, in general, identical to or smaller than those obtained for Tt H-NOX Fe(II)–NO, suggesting that the different shape of the XANES spectra for NO-bound Sw and Pa H-NOX reflects an altered iron site geometry compared to that of the Tt H-NOX Fe(II)–NO complex, rather than a greater level of disorder in the Fe site.
Table 3.
Best EXAFS fits to the NO-bound and Fe(II)–unligated states of H-NOX from Sw and Pa.a
| Sw H-NOX and Pa H-NOX Fe(II)–unligated and Fe(II)–NO complexes | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
||||||||
| Sw Fe(II)–NO | Pa Fe(II)–NO | Sw Fe(II)–unligated | Pa Fe(II)–unligated | ||||||||
| shell | r | σ 2 | shell | r | σ 2 | shell | r | σ 2 | shell | r | σ 2 |
|
|
|
|
|
||||||||
| 1 Fe-NO | 1.75 | 1.8 | 1 Fe-NO | 1.72 | 5.3 | ||||||
| 4 Fe-Np | 1.99 | 2.0 | 4 Fe-Np | 1.98 | 4.0 | 4 Fe-Np + 1 Fe-NHis |
2.06 | 5.7 | 4 Fe-Np + 1 Fe-NHis |
2.06 | 5.2 |
| 8 Fe••Cα | 3.02 | 3.0 | 8 Fe••Cα | 3.03 | 2.8 | 8 Fe••Cα | 3.11 | 6.6 | 8 Fe••Cα | 3.10 | 3.1 |
| 16 Fe••Cα/Np | 3.19 | 6.1 | 16 Fe••Cα/Np | 3.20 | 5.6 | ||||||
| 4 Fe••Ch | 3.38 | 3.5 | 4 Fe••Ch | 3.41 | 0.55 | 4 Fe••Ch | 3.40 | 2.37 | |||
| 8 Fe••Cβ | 4.36 | 4.8 | 8 Fe••Cβ | 4.37 | 7.1 | 8 Fe••Cβ | 4.42 | 3.8 | 8 Fe••Cβ | 4.42 | 3.9 |
| 16 Fe••Cβ/Np | 4.39 | 9.6 | 16 Fe••Cβ/Np | 4.39 | 14.2 | 16 Fe••Cβ/Np | 4.45 | 7.6 | 16 Fe••Cβ/Np | 4.45 | 7.7 |
|
|
|
|
|
||||||||
| F | 0.342 | F | 0.406 | F | 0.301 | F | 0.306 | ||||
| ΔE0 | 7.65 | ΔE0 | 6.21 | ΔE0 | 3.88 | ΔE0 | 3.31 | ||||
|
|
|
|
|
||||||||
r is in units of Å; σ2 is in units of 10−3 Å2; ΔE0 is in units of eV. All fits are to unfiltered data. F represents a goodness-of-fit parameter. Fourier transform ranges: k = 2.0 – 12.0 Å−1 (resolution = 0.157 Å) for Sw H-NOX Fe(II)–unligated; k = 2.0 – 13.0 Å−1 (resolution = 0.142 Å) for Pa H-NOX Fe(II)–unligated; k = 2.0 – 13.85 Å−1 (resolution = 0.134 Å) for Sw H-NOX Fe(II)–NO; k = 2.0 – 12.5 Å−1 (resolution = 0.150 Å) for Pa H-NOX Fe(II)–NO. Values of r for the Fe••Cα single scattering and Fe••Cα/Np multiple scattering paths were constrained to a constant difference from one another, while σ2 for the multiple scattering path was constrained to be twice that of the single scattering path. A similar constraint was placed on the Cβ and Cβ/Np combination. Inclusion of the Fe••Cα/Np multiple scattering paths was not required for fits of Fe(II)–unligated data.
Interestingly, it was possible to include a long Fe–N (presumably representative of an Fe–NHis bond) scatterer at ca. 2.30 – 2.35 Å and slightly improve fit quality over the five-coordinate fits (Tables S1 & S2, Electronic Supplementary Information). However, we note that for both Sw and Pa H-NOX, the σ2 values for this Fe–NHis shell were larger than that obtained for Tt H-NOX. We can therefore conclude from this analysis that, even if the axial His in NO-ligated Sw and Pa H-NOX is bound to the heme Fe, the binding of this residue is probably weaker than in Tt H-NOX. In view of our combined UV/visible and XANES spectra, as well as EXAFS analysis, we conclude that the Fe–NHis bond is formally broken in the Fe(II)–NO complexes of Sw and Pa H-NOX. However, we cannot ignore that there is still a very weak interaction between heme iron and the dissociated histidine.
EXAFS analysis of Fe(II)-unligated H-NOX provides support for 5c heme iron centers, with evidence for weakly-bound solvent in the hydrophilic Tt H-NOX active site
We next turned to the analysis of EXAFS data obtained for the Fe(II)–unligated forms of Tt, Sw and Pa H-NOX, the results of which are shown in Figure 4 and summarized in Tables 2 and 3, as well as Tables S3 and S4 in the Electronic Supplementary Information. Similar to our observations with the NO-ligated Fe(II) state, the k3χ(k) EXAFS data obtained for Fe(II)–unligated Tt H-NOX is well simulated by a set of single- and multiple-scattering pathways associated with the 4 pyrrole moieties of the heme group, with a disordered set of 4 Fe–Np scatterers at approximately 2.02 Å (Table 2). In contrast to our analysis of the NO-bound form, it is not necessary to include multiple-scattering from a three-body path involving the iron, Cα (alpha carbons of each pyrrole), and Npyrrole (Fe–Cα/Np), and indeed fit quality improves markedly when this path is removed (data not shown). The observed 0.06 Å increase in the average Fe–Npyrrole bond length relative to the NO-ligated form is consistent with a high-spin Fe(II) center in unligated Tt H-NOX.
Addition of a fifth scatterer to the Fe(II)–unligated Tt H-NOX first shell, corresponding to the putative Fe–NHis interaction, did not produce a change in the average Fe–N bond length of 2.02 Å. However, it did lead to a noticeable decrease in fit quality as judged by the magnitude of F, along with an increase in σ2 that presumably reflects a greater distribution of bond lengths (Table 2, fit 6). Attempts to model the Fe–NHis scatterer with a different bond length than the principal Fe–Np shell were unsuccessful, affording an unrealistically long Fe–N distance of 2.69 Å. Indeed, computational studies predicted an Fe–NHis bond length of 2.05 Å for unligated Fe(II) Tt H-NOX, which is very similar to the average 2.02 Å bond length obtained in our analysis.
Studies of structurally well-defined model complexes have shown that the best-fit coordination number can be underestimated in systems with significant disorder in the first coordination sphere.45, 55, 56 We therefore favor the 5-coordinate model of fit 6 over the 4-coordinate sites of fits 5 and 7 (Table 2). Finally, there is evidence for a very long Fe–O/N interaction at ca. 2.38 Å (Table 2, fit 8) that could conceivably be assigned to a weakly bound solvent ligand. Inclusion of this sixth ligand affords a modest improvement in the quality of fit relative to a 5-coordinate fit model.
The complete EXAFS analyses for the unligated Fe(II) forms of Sw and Pa H-NOX are broadly similar to that of Tt H-NOX, in that the EXAFS can be well simulated with a set of 4 – 5 Fe–N/O scatterers at ~2.06 Å, as well as single- and multiple-scattering paths associated with the heme pyrroles. One notable difference is that it is not possible to obtain chemically reasonable bond lengths for a putative solvent ligand that would complete a potential highly distorted 6-coordinate Fe(II) site. In both cases, the Fe–O distance refined to a value of 2.6 – 2.7 Å, clearly too long to be associated with any significant bonding interaction (see Tables S3-S4, Electronic Supplementary Information).
This difference relative to Tt H-NOX can be attributed to variations in the distal pocket environments of Tt, Sw and Pa H-NOX. As shown in Figure 5, Tt H-NOX has a relatively hydrophilic distal pocket with a hydrogen bonding (H-bonding) network involving Trp9, Asn74 and Tyr140 that is known to stabilize iron ligands.23 This same network could conceivably stabilize a solvent water molecule as well. In contrast, Sw and Pa H-NOX are predicted by homology modeling to have very hydrophobic distal pockets and to lack the H-bonding network seen in Tt H-NOX (Sw H-NOX has Leu/Leu/Phe and Pa H-NOX has Leu/Met/Phe in the positions homologous to Tt H-NOX Trp9/Asn74/Tyr140). Therefore, it is not surprising that there is no significant Fe-solvent interaction at the distal pocket of Sw and Pa H-NOX, probably because the pocket is too hydrophobic to facilitate the presence of water.
Figure 5.
Model of the distal pocket of Tt (a), Sw (b) and Pa (c) H-NOX. These models are based on the crystal structure of Tt H-NOX (PDB: 1U55) and homology models for Sw and Pa H-NOX, as shown in Figure 1. Tt (a), Sw (b) and Pa (c) H-NOX are colored in cyan, green and purple, respectively. The heme macrocycle in Tt H-NOX is used for all three H-NOXs to demonstrate the relative position of the distal pocket residues. Only in Tt H-NOX is there a hydrophilic distal pocket forming a H-bonding network (shown with yellow dashed lines).
In addition, the NO binding kinetics further confirms a more polar distal pocket in Tt H-NOX. It was determined that the NO dissociation rate constants (koff) are 5.6 × 10−4 s−1, 15.4 × 10−4 s−1 and 8.6 × 10−4 s−1 for Tt26, Sw13 and Pa (unpublished data), respectively. The NO association rate constants (kon) are not known, but are thought to be diffusion limited (≈108 M−1 s−1).32, 34 Therefore, we can infer that NO binds to Tt H-NOX most tightly as a result of having the smallest koff value, which can be attributed to the stabilizing effect of the H-bonding network in the distal pocket of Tt H-NOX (Figure 5a).
Implications for the signal transduction mechanism in the H-NOX family
Our EXAFS analysis leads to the conclusion that the Fe(II)–NO complexes of all three H-NOX domains are similar. Tt, Sw, and Pa H-NOX Fe(II)–NO complexes can all be summarized as having a strong 5c site consisting of porphyrin-derived and NO ligands as well as some contribution towards a 6th ligand from a weak (to extremely weak) Fe–NHis bond. The fact that they can all be described as having a weakly associated His bond leads us to consider that dissociation of the His ligand may not represent the most important structural change upon NO ligation, and thus it may not be the most important factor triggering signal transduction in H-NOX domains.
This is in agreement with the report of the co-existing 5-coordinate and 6-coordinate NO complexes in the Ns H-NOX crystal structure,20 as well as the mixture of 5c/6c structures that has been observed for Fe(II)–NO H-NOX complexes from Nostoc punctiforme and Legionella pneumophila.26 Also consistent with the notion that dissociation of the Fe-His bond may not be imperative for activation of H-NOX signal transduction, weak activation of sGC by CO (forms a 6c complex) as well as full activation of sGC by CO plus an allosteric ligand (still 6c) has been reported.54 Thus, the formal coordination number of the heme iron would not seem to be critical for triggering signal transduction in the H-NOX family.
Furthermore, if our model is correct, it seems unlikely that NO is bound in the proximal pocket in the active, signaling state of sGC and other H-NOX proteins, as has been proposed in other studies.35-39 To be certain, our EXAFS data does not rule out the possibility that the short Fe–N/O scatterer, assigned to NO, is bound in the proximal pocket. However, the presence of a weak 6th bond, assigned here to Fe–NHis, suggests that the proximal coordination site is open (or partially occupied with His). An alternate explanation is that there is a weak 6th bond in the distal pocket, with NO bound in the proximal pocket. A solvent molecule is perhaps the most obvious candidate, although weak association with distal residues such as Tyr140 in Tt H-NOX or Met74 in Pa H-NOX is also a possibility (Figure 5; there is no obvious potential ligand in Sw H-NOX). These distal pocket residues are all >3 Å from the heme iron based on crystal structures of Tt and Ns H-NOX, but it is conceivable that if NO were to bind to the proximal side of the heme, there would be a change in these distal pocket distances. Nonetheless, the most likely scenario is that the weak 6th ligand is, indeed, from the proximal pocket His in all three H-NOXs. Thus, the model most consistent with all the available data20, 26 is that in sGC and other H-NOX domains, NO binds in the distal pocket.
Finally, in our previous XANES analysis of Tt H-NOX, we observed that NO binding leads to only a subtle change in the displacement of the iron atom out of the mean heme plane.30 This agrees with the contraction in the average Fe–Np bond lengths upon NO binding (1.97 Å for – NO complex, versus 2.03 Å for –unligated complex). The combined effect of NO binding and Fe–NHis bond weakening would be expected to drive the iron atom towards the distal side. However, the contraction of the Fe–Np bonds shrinks the heme cavity, and therefore partially prevents the heme iron atom from moving more completely into the distal pocket. This likely enables H-NOX proteins to provide mechanistic control over the extent of heme distortion31 and heme-induced protein conformational change,19-22 thus regulating signal transduction.
Conclusion
The heme domain of sGC and other H-NOX domains constitute an important family of hemoproteins that sense diatomic molecules such as NO, and in response, regulate biological signal transduction pathways. The mechanism of activation of these domains by diatomic gas binding is still unclear, however. To explore the detailed molecular basis of NO/H-NOX signaling pathways, we used EXAFS to investigate the Fe(II)–unligated and Fe(II)–NO complexes of three different H-NOX proteins, Tt H-NOX, Sw H-NOX, and Pa H-NOX. We found that NO forms a similar complex with all three H-NOXs. This complex is best described as a strong 5c ligand set, consisting of 4 Fe–N bonds with the heme and 1 Fe–N bond to NO, as well as a weak 6th ligand, most likely from an incompletely dissociated proximal Fe-His bond. According to our EXAFS analysis, this 6th Fe-His bond in Tt H-NOX is slightly stronger than the corresponding 6th ligand in Sw and Pa H-NOX. This, coupled with UV/visible and XANES data that support a 6th ligand to Tt H-NOX but not to Sw or Pa H-NOX lead us to conclude that Tt H-NOX forms a 6c complex with NO while Sw and Pa H-NOX bind NO in a 5-coordinate manner, with the proximal histidine dissociated, as has been reported for sGC.
Nonetheless, based on these data, we propose that there is no significant difference in the structures of the 5c and 6c NO complexes in H-NOX domains. Instead, they are only distinguished by slight separation along a continuum from a strong Fe–NHis bond to no Fe–NHis bond. This discovery suggests that histidine dissociation may not be required to trigger NO/H-NOX signal transduction, as has been widely hypothesized. Instead we propose that Fe–NHis lengthening together with contraction of the Fe-heme bonds are the relevant structural changes upon NO ligation that promote signal transduction. These data also suggest a mechanism for NO-ligation mediated control of heme flattening, which has been shown to be involved in NO/H-NOX signal transduction.31
Experimental
Protein cloning, expression, and purification
Tt H-NOX30, 57 and Sw H-NOX13 were expressed and purified as previously described. PCR was used to amplify Patl_1532 from Pseudoalteromonas atlantica genomic DNA (ATCC) using Expand polymerase (Roche). Upstream and downstream primers contained NdeI and XhoI restriction sites, respectively. The amplified PCR product was cloned into pET-20b (Novagen) and sequenced. Cell culture procedures for Pa H-NOX were carried out as previously described for other H-NOX proteins.13, 26, 30, 57 Purification of the Pa H-NOX protein took advantage of the C-terminal His tag and was carried out as previously described for other H-NOX proteins.13, 26,6,30, 57 All proteins were >95% pure as estimated by SDS-PAGE.
Sample preparation
Preparation of Fe(II)-unligated and Fe(II)-NO complexes was carried out as previously described.26, 57
Electronic spectroscopy
All UV/visible spectra were recorded on a Cary 100 Bio spectrophotometer equipped with a constant temperature bath set to 20 °C.
X-ray absorption spectroscopy
H-NOX protein samples were exchanged into XAS sample buffer (50 mM HEPES, 50 mM NaCl, 20% glycerol, pH 7.5) and concentrated to 1-2 mM using spin columns from Millipore (Microcon Ultracel YM-3, 3000 molecular weight cut off (MWCO) and 0.5 mL maximum volume). The concentrated protein samples were transferred to copper sample holders (10 mm wide × 5 mm high × 0.5 mm thick) with Kapton windows, flash frozen in liquid nitrogen, and stored at −80 °C before XAS experiments. Flash freezing is not expected to significantly alter the structure of the H-NOX protein or Fe-heme complexes.
XAS data collection was carried out at the National Synchrotron Light Source (NSLS) at Brookhaven National Laboratory (BNL) on beamline X3B. A sagittally focused Si (111) double crystal monochromator was used for energy selection, with a downstream nickel-coated mirror providing vertical focusing and rejection of higher harmonics. Frozen samples were cooled to 20-40 K during data collection with a closed-cycle liquid helium Displex cryostat. Iron Kα fluorescence was detected with Canberra 13 or 31 element solid-state germanium detectors. An iron foil was used as an internal reference to account for any energy shifts in the monochromator, with the reference energy set to 7112.0 eV. Data were collected in 10 eV steps in the pre-edge region (6912 – 7092 eV), 0.3 eV steps in the edge region (7092 – 7162 eV) and steps of 0.05k above the edge (7162 – 7859 eV). 6-9 scans for each complex were taken and averaged for data analysis. In order to minimize radiation damage to the samples, we made multiple samples for the same complex and collected at most 2 scans per X-ray exposed spot; samples were routinely monitored for evidence of photo-reduction, as indicated by red-shifts in the edge energies and/or a loss of pre-edge peak intensity. XAS data were aligned, background-removed, normalized, and merged using Athena.58 The Fe K-edge energies were determined from the first derivative of the average XAS spectra after smoothing (in some cases the zero-crossing of the smoothed second derivative was used for verification). The derivative spectra were smoothed using Origin 7.0.
EXAFS analysis were carried out using EXAFSPAK.59 Theoretical phase and amplitude parameters were calculated using FEFF 8.4060 for simple models of the active sites of H-NOX that were based on the coordinates of the O2-bound state of Tt HNOX.16 These parameters were used by the opt program of the EXAFSPAK package. Structural models were refined iteratively during the course of the analysis to reflect the EXAFS-derived metrical parameters. For most refinements, the coordination number of a given shell was a fixed parameter, and was varied iteratively while bond lengths (r) and Debye-Waller factors (σ2) were allowed to freely float. The amplitude reduction factor S0 was fixed at 0.9, while the edge shift parameter ΔE0 was allowed to float as a single value for all shells in fits of a given sample. Therefore, in any given fit, the number of floating parameters was typically equal to (2 × num shells) + 1. Two additional constraints were applied to fits where multiple-scattering from the heme group was simulated to reduce the number of free parameters and provide a geometrical restraint. Specifically, the values of r for the Fe••Cα (or Fe••Cα) single scattering paths and the Fe••Cα/Np (or Fe••Cα/Np) multiple scattering paths were constrained to a constant difference from one another, while σ2 for the multiple scattering path was constrained to be twice that of the single scattering path. E0, the point at which k = 0 Å−1, was taken as the value used during normalization of the data in Athena, and was typically between 7123 – 7126 eV for datasets examined here. The goodness of fit F was defined simply as [Σk6(χexptl-χcalc)2 / Σk6χexptl2]½.
Supplementary Material
Acknowledgements
This work was financially supported by the National Science Foundation (grant CHE-0910771 to E.M.B.). XAS experiments were carried out at beamline X3B of the National Synchrotron Light Source (NSLS) at Brookhaven National Laboratory. Beamline X3B is operated by the Case Center for Synchrotron Biosciences, supported by NIH NIBIB grant P30-EB-009998. NSLS is supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences, under Contract No. DE-AC02-98CH10886. We also wish to thank Taemee Pak for cloning Pa H-NOX.
Footnotes
Electronic supplementary information (ESI) available:
Biobiographic references
- 1.Ignarro LJ. Biochem. Pharmacol. 1991;41:485–490. doi: 10.1016/0006-2952(91)90618-f. [DOI] [PubMed] [Google Scholar]
- 2.Waldman SA, Murad F. Pharmacol. Rev. 1987;39:163–196. [PubMed] [Google Scholar]
- 3.Wang Y, Dufour YS, Carlson HK, Donohue TJ, Marletta MA, Ruby EG. Proc. Natl. Acad. Sci. U.S.A. 2010;107:8375–8380. doi: 10.1073/pnas.1003571107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Price MS, Chao LY, Marletta MA. Biochemistry. 2007;46:13677–13683. doi: 10.1021/bi7019035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barraud N, Schleheck D, Klebensberger J, Webb JS, Hassett DJ, Rice SA, Kjelleberg S. J. Bacteriol. 2009;191:7333–7342. doi: 10.1128/JB.00975-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gong W, Hao B, Chan MK. Biochemistry. 2000;39:3955–3962. doi: 10.1021/bi992346w. [DOI] [PubMed] [Google Scholar]
- 7.Gong W, Hao B, Mansy SS, Gonzalez G, Gilles-Gonzalez MA, Chan MK. Proc. Natl. Acad. Sci. U.S.A. 1998;95:15177–15182. doi: 10.1073/pnas.95.26.15177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hao B, Isaza C, Arndt J, Soltis M, Chan MK. Biochemistry. 2002;41:12952–12958. doi: 10.1021/bi020144l. [DOI] [PubMed] [Google Scholar]
- 9.Lanzilotta WN, Schuller DJ, Thorsteinsson MV, Kerby RL, Roberts GP, Poulos TL. Nat. Struct. Biol. 2000;7:876–880. doi: 10.1038/82820. [DOI] [PubMed] [Google Scholar]
- 10.Gray JM, Karow DS, Lu H, Chang AJ, Chang JS, Ellis RE, Marletta MA, Bargmann CI. Nature. 2004;430:317–322. doi: 10.1038/nature02714. [DOI] [PubMed] [Google Scholar]
- 11.Karow DS, Pan D, Davis JH, Behrends S, Mathies RA, Marletta MA. Biochemistry. 2005;44:16266–16274. doi: 10.1021/bi051601b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carlson HK, Vance RE, Marletta MA. Mol. Microbiol. 2010;77:930–942. doi: 10.1111/j.1365-2958.2010.07259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu N, Xu Y, Hossain S, Huang N, Coursolle D, Gralnick J, Boon EM. Manuscript in revision. 2012. [DOI] [PubMed] [Google Scholar]
- 14.Karow DS, Pan D, Tran R, Pellicena P, Presley A, Mathies RA, Marletta MA. Biochemistry. 2004;43:10203–10211. doi: 10.1021/bi049374l. [DOI] [PubMed] [Google Scholar]
- 15.Nioche P, Berka V, Vipond J, Minton N, Tsai A-L, Raman CS. Science. 2004;306:1550–1553. doi: 10.1126/science.1103596. [DOI] [PubMed] [Google Scholar]
- 16.Pellicena P, Karow DS, Boon EM, Marletta MA, Kuriyan J. Proc. Natl. Acad. Sci. U.S.A. 2004;101:12854–12859. doi: 10.1073/pnas.0405188101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Denninger JW, Marletta MA. Biochim. Biophys. Acta. 1999;1411:334–350. doi: 10.1016/s0005-2728(99)00024-9. [DOI] [PubMed] [Google Scholar]
- 18.Schmidt H, Walter U. Cell. 1994;78:919–925. doi: 10.1016/0092-8674(94)90267-4. [DOI] [PubMed] [Google Scholar]
- 19.Erbil WK, Price MS, Wemmer DE, Marletta MA. Proc. Natl. Acad. Sci. U.S.A. 2009;106:19753–19760. doi: 10.1073/pnas.0911645106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma X, Sayed N, Beuve A, van den Akker F. EMBO J. 2007;26:578–588. doi: 10.1038/sj.emboj.7601521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Olea C, Boon EM, Pellicena P, Kuriyan J, Marletta MA. ACS Chem. Biol. 2008;3:703–710. doi: 10.1021/cb800185h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olea C, Herzik MA, Kuriyan J, Marletta MA. Protein Sci. 2010;19:881–887. doi: 10.1002/pro.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boon EM, Huang SH, Marletta MA. Nat. Chem. Biol. 2005;1:53–59. doi: 10.1038/nchembio704. [DOI] [PubMed] [Google Scholar]
- 24.Boon EM, Marletta MA. Curr. Opin. Chem. Biol. 2005;9:441–446. doi: 10.1016/j.cbpa.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 25.Boon EM, Marletta MA. J. Inorg. Biochem. 2005;99:892–902. doi: 10.1016/j.jinorgbio.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 26.Boon EM, Davis JH, Tran R, Karow DS, Huang SH, Pan D, Miazgowicz MM, Mathies RA, Marletta MA. J. Biol. Chem. 2006;281:21892–21902. doi: 10.1074/jbc.M600557200. [DOI] [PubMed] [Google Scholar]
- 27.Capece L, Estrin DA, Marti MA. Biochemistry. 2008;47:9416–9427. doi: 10.1021/bi800682k. [DOI] [PubMed] [Google Scholar]
- 28.Tran R, Boon EM, Marletta MA, Mathies RA. Biochemistry. 2009;48:8568–8577. doi: 10.1021/bi900563g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weinert EE, Plate L, Whited CA, Olea C, Marletta MA. Angew. Chem. Int. Ed. 2010;49:720–723. doi: 10.1002/anie.200904799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dai Z, Boon EM. J. Inorg. Biochem. 2011;105:784–792. doi: 10.1016/j.jinorgbio.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muralidharan S, Boon EM. J. Am. Chem. Soc. 2012 doi: 10.1021/ja211576b. doi 10.1021/ja211576b. [DOI] [PubMed] [Google Scholar]
- 32.Zhao Y, Brandish PE, Ballou DP, Marletta MA. Proc. Natl. Acad. Sci. U.S.A. 1999;96:14753–14758. doi: 10.1073/pnas.96.26.14753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stone JR, Marletta MA. Biochemistry. 1994;33:5636–5640. doi: 10.1021/bi00184a036. [DOI] [PubMed] [Google Scholar]
- 34.Stone JR, Marletta MA. Biochemistry. 1996;35:1093–1099. doi: 10.1021/bi9519718. [DOI] [PubMed] [Google Scholar]
- 35.Lawson DM, Stevenson CEM, Andrew CR, Eady RR. EMBO J. 2000;19:5661–5671. doi: 10.1093/emboj/19.21.5661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsai A-L, Berka V, Sharina I, Martin E. J. Biol. Chem. 2011 doi: 10.1074/jbc.M111.290304. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsai A-L, Berka V, Martin E, Olson JS. Biochemistry. 2011;51:172–186. doi: 10.1021/bi2015629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marti MA, Capece L, Crespo A, Doctorovich F, Estrin DA. J. Am. Chem. Soc. 2005;127:7721–7728. doi: 10.1021/ja042870c. [DOI] [PubMed] [Google Scholar]
- 39.Pixton DA, Petersen CA, Franke A, van Eldik R, Garton EM, Andrew CR. J. Am. Chem. Soc. 2009;131:4846–4853. doi: 10.1021/ja809587q. [DOI] [PubMed] [Google Scholar]
- 40.Söding J, Biegert A, Lupas AN. Nucleic Acids Res. 2005;33:W244–W248. doi: 10.1093/nar/gki408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chance B, Powers L, Ching Y, Poulos T, Schonbaum GR, Yamazaki I, Paul KG. Arch. Biochem. Biophys. 1984;235:596–611. doi: 10.1016/0003-9861(84)90234-0. [DOI] [PubMed] [Google Scholar]
- 42.Chance M, Parkhurst L, Powers L, Chance B. J. Biol. Chem. 1986;261:5689–5692. [PubMed] [Google Scholar]
- 43.Scott RA. In: Physical Methods in Bioinorganic Chemistry. Spectroscopy and Magnetism. Que L Jr., editor. University Science Books; Sausalito, CA: 2000. pp. 465–527. [Google Scholar]
- 44.Westre TE, Kennepohl P, DeWitt JG, Hedman B, Hodgson KO, Solomon EI. J. Am. Chem. Soc. 1997;119:6297–6314. [Google Scholar]
- 45.Stemmler TL, Sossong TM, Goldstein JI, Ash DE, Elgren TE, Kurtz DM, Penner-Hahn JE. Biochemistry. 1997;36:9847–9858. doi: 10.1021/bi9702795. [DOI] [PubMed] [Google Scholar]
- 46.Jacquamet L, Aberdam D, Adrait A, Hazemann J-L, Latour J-M, Michaud-Soret I. Biochemistry. 1998;37:2564–2571. doi: 10.1021/bi9721344. [DOI] [PubMed] [Google Scholar]
- 47.Aitken JB, Thomas SE, Stocker R, Thomas SR, Takikawa O, Armstrong RS, Lay PA. Biochemistry. 2004;43:4892–4898. doi: 10.1021/bi049645s. [DOI] [PubMed] [Google Scholar]
- 48.Rich AM, Armstrong RS, Ellis PJ, Lay PA. J. Am. Chem. Soc. 1998;120:10827–10836. [Google Scholar]
- 49.Shu L, Chiou Y-M, Orville AM, Miller MA, Lipscomb JD, Que L. Biochemistry. 1995;34:6649–6659. doi: 10.1021/bi00020a010. [DOI] [PubMed] [Google Scholar]
- 50.Olea C, Kuriyan J, Marletta MA. J. Am. Chem. Soc. 2010;132:12794–12795. doi: 10.1021/ja106252b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miyatake H, Mukai M, Adachi S.-i., Nakamura H, Tamura K, Iizuka T, Shiro Y, Strange RW, Hasnain SS. J. Biol. Chem. 1999;274:23176–23184. doi: 10.1074/jbc.274.33.23176. [DOI] [PubMed] [Google Scholar]
- 52.Nakajima H, Honma Y, Tawara T, Kato T, Park S-Y, Miyatake H, Shiro Y, Aono S. J. Biol. Chem. 2001;276:7055–7061. doi: 10.1074/jbc.M003972200. [DOI] [PubMed] [Google Scholar]
- 53.McCleverty JA. Chem. Rev. 2004;104:403–418. doi: 10.1021/cr020623q. [DOI] [PubMed] [Google Scholar]
- 54.Ibrahim M, Derbyshire ER, Marletta MA, Spiro TG. Biochemistry. 2010;49:3815–3823. doi: 10.1021/bi902214j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Riggs-Gelasco PJ, Stemmler TL, Penner-Hahn JE. Coord. Chem. Rev. 1995;144:245–286. [Google Scholar]
- 56.Riggs-Gelasco P. Ph.D. dissertation. University of Michigan; 1995. [Google Scholar]
- 57.Dai Z, Boon EM. J. Am. Chem. Soc. 2010;132:11496–11503. doi: 10.1021/ja101674z. [DOI] [PubMed] [Google Scholar]
- 58.Ravel B, Newville M. J. Synchrot. Radiat. 2005;12:537–541. doi: 10.1107/S0909049505012719. [DOI] [PubMed] [Google Scholar]
- 59.George GN. Stanford Synchrotron Radiation Lightsource. SLAC National Accelerator Laboratory; Stanford, CA: 2000. EXAFSPAK. [Google Scholar]
- 60.Ankudinov AL, Ravel B, Rehr JJ, Conradson SD. Phys. Rev. B. 1998;58:7565. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.