Abstract
In mammalian cells, hypoxia, or inadequate oxygen availability, regulates the expression of a specific set of microRNA, which we have previously termed “hypoxamirs”. Over the past five years, the appreciation of the importance of hypoxamirs in regulating the cellular adaptation to hypoxia has grown dramatically. At a cellular level, each hypoxamir can simultaneously regulate expression of multiple (>100) target genes in order to control fundamental biological processes, including survival, proliferation, angiogenesis, migration, and metabolism, among others. A maladaptive imbalance of these hypoxic phenotypes often drives many ischemic cardiovascular diseases, such as pulmonary hypertension—an enigmatic vascular disorder characterized by pronounced and severe panvasculopathy secondary to diverse upstream etiologies, notably including hypoxia. Yet, despite this pathogenic relationship between hypoxic cell phenotypes and disease, the mechanistic roles of hypoxamirs in modulating pulmonary hypertension remain largely unrecognized. Some advances have been made regarding the contributions of specific hypoxamirs in the development and progression of pulmonary hypertension. New methods are also being developed to more comprehensively study their roles in this complex disease. As a result, a more sophisticated understanding of their pervasive roles in pathogenesis could set the stage for unique diagnostic and therapeutic strategies in pulmonary hypertension.
Key Words: Hypoxia, vascular disease, microRNA, hypoxamirs, pulmonary hypertension
Introduction
Pulmonary hypertension (PH) is a serious and sometimes fatal pulmonary vascular condition that affects a growing number of people worldwide. Clinically, pre-capillary PH (pulmonary arterial hypertension, PAH) is characterized by increased pulmonary arterial pressures and pulmonary vascular resistance, leading to right ventricular failure, volume overload, and sometimes death. At the histological level, PH is characterized by a panvasculopathy, involving the dysregulation of various vascular components (e.g., endothelial, smooth muscle, fibroblast), inflammatory, and perhaps other cell types driven by multiple complex and overlapping molecular pathways active in the pulmonary vasculature [as reviewed in (1)]. Destruction of the arterial lumen is observed in severe forms of PH, resulting in pathognomonic complex vascular lesions termed plexogenic lesions. This vascular pathology can be triggered by seemingly disparate genetic and environmental stimuli, including hypoxia. At the transcriptional level, a majority of the hypoxic adaptations in the pulmonary vasculature are regulated by master transcription factors hypoxia-inducible factor 1-alpha (HIF-1 alpha) and hypoxia-inducible factor 2-alpha (HIF-2 alpha). At the onset of hypoxia, increased HIF levels initiate transcription of more than 100 genes, that affect and regulate a multitude of pulmonary vascular functions such as reactive oxygen species generation/oxidative stress, angiogenesis, vascular cell migration, metabolism, proliferation, and survival (2). Acutely, such adaptations are beneficial in preserving cellular function. However, chronic hypoxic induction of these molecular mechanisms is detrimental, leading to pulmonary arterial remodeling and increasing pulmonary arterial pressures. While the association of chronic hypoxia and PH has been the subject of substantial molecular study in the past decades, the molecular and gene mechanisms that connect hypoxia to downstream manifestations of PH remain enigmatic.
Recent advances in the study of microRNA (miRNA) indicate that these molecules may have substantial and important roles in regulating the physiological and pathophysiological adaptations to hypoxia. Current data estimate that over 1,400 distinct miRNA are predicted to be encoded by the human genome (3). Of these, the expression of a specific and still growing list of miRNA, so-called “hypoxamirs” (2), are known to be dynamically regulated by hypoxia (4). Following transcription and processing in the nucleus and cytoplasm, mature miRNA exist as small (19-23 nucleotides in length) non-coding RNA species (Figure 1). Upon recognition and binding with the RNA induced silencing complex of proteins (RISC), these mature forms then down-regulate expression of specific target messenger RNA (mRNA), via Watson-Crick nucleotide binding to a “seed sequence” typically located at the 3' untranslated region (3' UTR) of mRNA. This miRNA-mRNA interaction results in the down-regulation of the target gene transcript, via either translational repression or transcript degradation. As many as 50-60% of all mammalian messenger RNA (mRNA) transcripts are likely to be subject to miRNA targeting (8,9), showcasing their vast range as ubiquitous modulators of gene expression. Because of the necessity of conserved binding for such target recognition, a number of computational algorithms have been developed [i.e., Targetscan 5 (Conserved) (10) and DIANA (11), among others] that predict mRNA transcripts recognized by specific miRNA. Although all limited by both false positive and false negative predictive rates, these algorithms nonetheless have become powerful bioinformatic tools in rapidly identifying the biological roles of miRNA based on their virtual predicted targets.
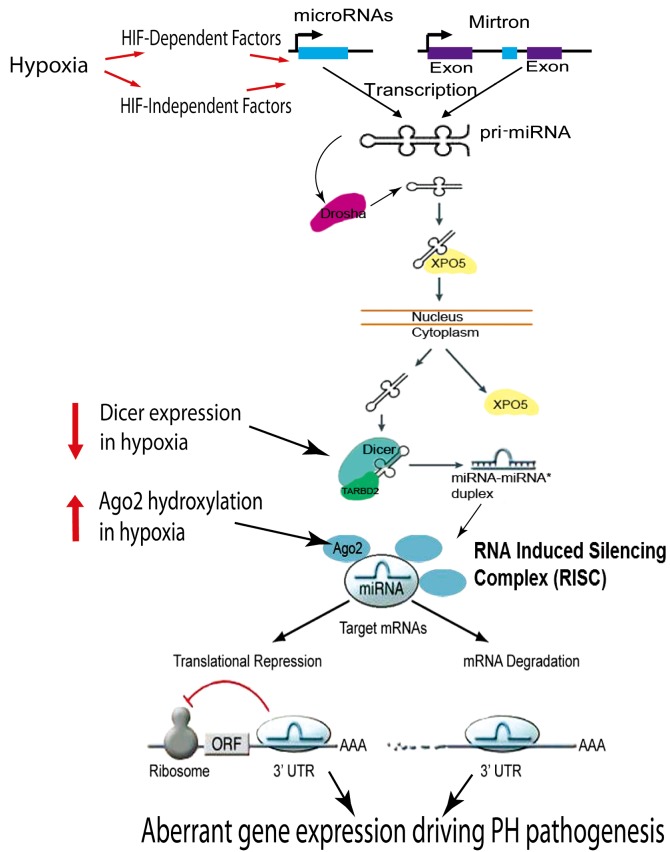
Figure 1.
MicroRNA biogenesis and mechanisms of action in hypoxia. Hypoxia can regulate the expression of miRNA through HIF-dependent and HIF-independent mechanisms. Hypoxia-responsive miRNA may undergo several nuclear and cytosolic processing steps prior to expression as mature and biologically active species. It has been reported that hypoxia increases Ago2 hydroxylation to increase miRNA expression and activity (5); hypoxia also decreases Dicer expression to depress miRNA expression (6). Mature miRNA are taken up into the RNA induced silencing complex (RISC) in order to recognize complementary sites in the 3'untranslated region (UTR) of target gene messenger RNA (mRNA) via Watson-Crick base pairing. At this point, miRNA negatively regulate gene expression via either translational repression or mRNA degradation. Proven to be critically important in the regulation of numerous other cellular processes and cardiovascular diseases, the functions of hypoxamirs in pulmonary hypertension remain mostly undefined. [Adapted with permission from (3,7)]
Taken together, miRNA are suspected to carry various central regulatory roles in the development and progression of pulmonary hypertension (PH), especially in the context of hypoxic stress. However, the degree to which they are involved is still under active investigation. To date, only a handful of miRNA have been characterized as bona fide modulators in pulmonary vascular disease. This review will focus on the pathogenic dysregulation of hypoxamirs and their established as well as predicted importance in the molecular mechanisms of PH. We will highlight specific studies demonstrating the role of distinct hypoxamirs in multiple signaling pathways integral to PH, such as the tumor growth factor β (TGF-β) bone morphogenetic protein receptor-2 (BMPRII), and Rho-kinase pathways. We will review experimental approaches utilizing both the loss- and gain-of function in order to substantiate actions of these hypoxamirs in the development of PH in vivo. Finally, we will explore the evidence that predicts the importance of other as-of-yet poorly studied hypoxamirs in PH.
Growing list of hypoxamirs
The comprehensive and validated list of hypoxamirs that are either up- or down-regulated by low oxygen exposure in various biological contexts continues to expand yearly (Figure 2). Some bona fide hypoxamirs may carry minimal importance in PH, given specific patterns of expression outside the pulmonary vascular space. Nonetheless, a substantial number of as-of-yet unexplored hypoxamirs likely play central mechanistic roles in the etiology of this disease (4,14,15). A few hypoxamirs, such as miR-210, are known to be directly up-regulated by HIF itself, as a consequence of HIF binding to HIF-response elements (HRE) in their transcriptional promoter sites (2,16). Alternatively, in the case of miR-424, its induction in hypoxia has been reported to induce a program of feedback regulation on HIF activity itself, leading to a pervasive, albeit complex, miR-424-dependent control over other downstream HIF-dependent miRNA (17). Furthermore, the expression of many other hypoxamirs is likely modulated by a dynamic cascade of PH-relevant and hypoxia-associated stimuli that indirectly contribute to miRNA induction or degradation. For example, inflammatory signaling is triggered by hypoxia, which in turn increases the expression of the hypoxamirs miR-146a/b (18) and miR-181b (19). Finally, stemming from the underpinnings of cross-talk between BMP signaling and hypoxia in the pulmonary vasculature (20,21), a multitude of other hypoxamirs may be regulated by virtue of their linked response to bone morphogenetic protein (BMP) stimulation [as previously catalogued by Parikh and colleagues (4)] under low oxygen stress. This area of investigation is particularly exciting, given that genetic haploinsufficiency of the BMP Receptor Type II (BMPR2) is the predominant cause of familial PH in humans (>80% of cases) [as reviewed by (1)]. In that vein, BMPR2 is a direct target of many miRNA including the hypoxamirs miR-21 (4) and miR-17 (22), among others. Furthermore, BMP signaling plays an important role in the post-transcriptional processing and maturation of miR-21, which in turn down-regulates BMPR2 in an autoregulatory feedback loop (23). It remains to be seen how the actions of miR-21 and other hypoxamirs further intersect with BMP signaling; but given the large number of predicted sites for hypoxamir binding throughout the members of the BMP/SMAD signaling pathway (SY Chan, unpublished observations), we would expect a substantial level of cross-regulation among hypoxamirs and BMP pathways.
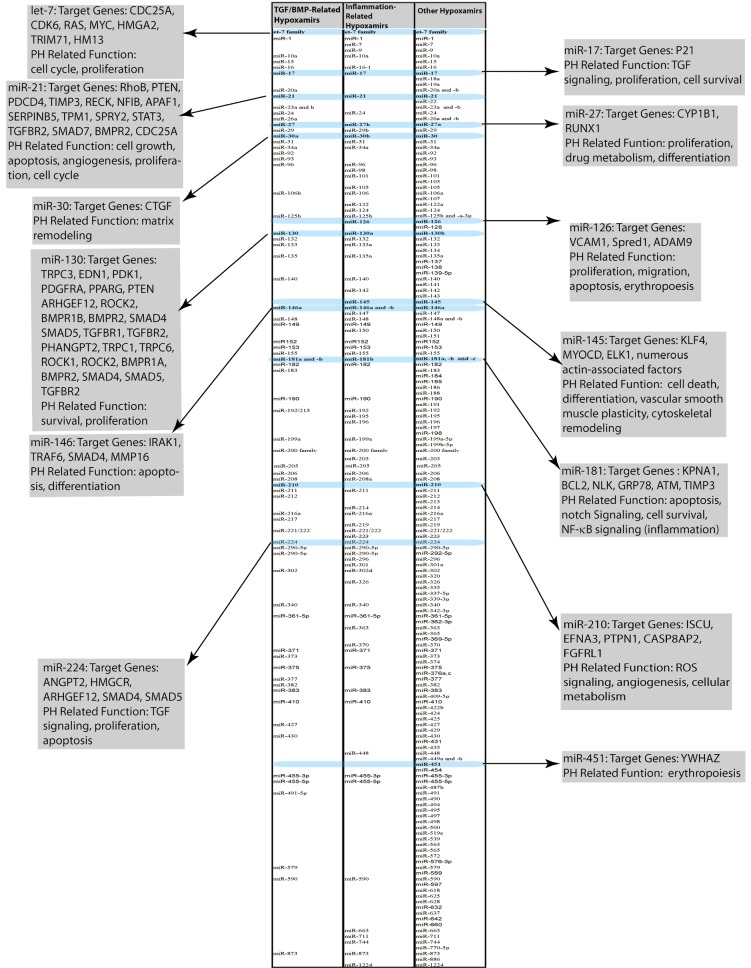
Figure 2.
Compiled list of hypoxamirs related to TGF/BMP signaling, inflammatory stimuli, and hypoxia alone. Examples of hypoxamirs with relevance to PH are highlighted. These examples have either been experimentally established or predicted based on studies performed in other biological contexts. Descriptions include a brief synopsis of miRNA target genes and functions relevant to PH. Lists compiled from (4-6,12,13)
Besides the dysregulation of specific miRNAs directly by HIF or HIF-related pathways, hypoxia may also control miRNA processing and synthesis in general, leading to global alterations in miRNA expression. Wu and colleagues have reported that hypoxia increased the expression of type I collagen prolyl-4-hydroxylase [C-P4H(I)], which led to prolyl-hydroxylation and accumulation of Argonaute2 (Ago2), a critical component of the RISC. Hydroxylation of Ago2 is required for the association of Ago2 with heat shock protein 90 (Hsp90), which is necessary for the loading of microRNAs (miRNAs) into the RISC and translocation to stress granules (SGs). Consequently, in vascular smooth muscle cells, Ago2 hydroxylation was found to induce the endonuclease activity of Ago2 and increase the expression and activity of numerous hypoxamirs. Thus, these results offer one reasonable molecular explanation of the dynamic up-regulation of multiple hypoxamirs in response to low oxygen. On the other hand, more recently Ho and colleagues found that in vascular endothelial cells, hypoxia down-regulated the miRNA processing enzyme Dicer, leading to a subsequent decrease in the levels of various mature miRNA (6). The Dicer-dependent miR-185 was among these down-regulated miRNA and, in turn, was found to directly target HIF-2 and control consequent HIF-dependent adaptive responses. Considering the results of both Wu et al. and Ho et al., it is difficult to discern the utility of hypoxic induction of global miRNA activity via Ago2 hydroxylation while simultaneously reducing global miRNA expression via Dicer down-regulation. It is possible that these seemingly opposing mechanisms may reflect complex differences in hypoxic adaptation that greatly depend upon specific cellular or molecular context. Alternatively, it remains to be seen whether modulation of Ago2 versus Dicer activity may preferentially affect particular subsets of hypoxamirs, thus allowing for an additional level of specificity in miRNA modulation. Nonetheless, both studies emphasize the deeply rooted and complex molecular connections linking hypoxia and global miRNA activity in the hypoxic vasculature.
Some progress has also been made in identifying dynamic alterations in hypoxamir expression specifically in hypoxia-induced PH. In the first comprehensive miRNA expression screen in PH, Caruso et al. performed a miRNA array analysis of whole lung tissues obtained from two independent models of PH, chronic hypoxic PH and monocrotaline PH (12). To capture the dynamic changes in miRNA expression through pathogenesis, lung analyses were performed at serial time points consistent with disease progression. Of the 350 miRNAs studied, only five were consistently altered across both PH models, inferring a shared contribution of these miRNA in multiple types of PH pathogenesis. Down-regulation of let-7f, miR-22, and miR-30c and up-regulation of miR-322 and miR-451 were reported. Conversely, and perhaps reflective of these distinct modes of pathogenesis, some significant changes in miRNA expression profiles were observed following comparison between hypoxia-dependent and monocrotaline-dependent models. For example, miR-21 and let-7a expression were significantly reduced only in the setting of monocrotaline-induced PH. By expression array, other miRNA were found to be dysregulated in hypoxia-treated mice, but these were not further validated by more rigorous additional assays, such as RT-PCR (reverse transcription-polymerase chain reaction). Based on their robust expression levels and consistent dysregulation across multiple time points of disease, miR-322, miR-451, miR-21, miR-22, miR-30c, let-7f, and let-7a were selected for further analysis. Following RT-PCR and in situ analysis of paraffin-embedded lungs derived from idiopathic pulmonary arterial hypertension (IPAH) patients, miR-21 was found to be down-regulated while miR-451 was up-regulated as compared with non-diseased tissue. In a follow-up study, Yang et al. performed similar miRNA screening in the lungs of chronic hypoxic mice, which showed consistent increases in miR-21 miR-451, miR-210, and miR-144 when compared against normoxic mouse lung (13). In total, these findings represent the first and most comprehensive screens of miRNA reported in hypoxia-induced PH. Notably, however, none of these data sets were derived from human disease tissue, and none specifically localized these changes to the dysregulated pulmonary vasculature. In fact, thus far, all hypoxamirs that have been identified as specifically dysregulated and/or mechanistically important in PH have also been described to carry additional functions in other tissue types beyond the pulmonary vasculature. Thus, when considering the challenges of obtaining pulmonary vascular tissue for pathologic or molecular study in human PH patients, there is no facile method for the clinician to readily assess “real-time” alterations in pulmonary vascular hypoxamir expression as disease progresses.
Notably, some of these hypoxamirs in chronic PH have also been found to be dysregulated in acute hypoxic lung disease such as acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) (e.g., miR-146a and miR-155) [as reviewed by (24)]. However, alterations of most of these miRNA have been attributed to increased inflammation (i.e., in neutrophils, monocytes) rather than a direct vascular response to acute hypoxic exposure. Further studies which better compare the differences of hypoxamir expression between acute hypoxia diseases and chronic hypoxia PH are warranted and could offer novel insight into the explanation of PH crisis in acute hypoxia.
MiR-21 in PH
Given the importance of hypoxia in PH and the large and ever expanding list of miRNA regulated by hypoxia, efforts are ongoing to define the mechanistic role of specific hypoxamirs in the control of pulmonary vascular disease. MiR-21 has been among the first hypoxamirs to be studied in this context (Figure 3), as it carries robust pleiotropic activity in various other pathological contexts [as reviewed in (25)] and importantly in cardiovascular diseases such as cardiac fibrosis and heart failure (26), aortic aneurysm progression (27), and peripheral vascular disease (28). Notably, miR-21 is up-regulated in numerous cellular contexts by upstream triggers of PH (4), including hypoxia (16,29) as well as TGF-β/BMP signaling (30), and pro-inflammatory cytokines such as interleukin-6 (IL-6) (31,32). On a molecular level, we have found that constitutive HIF expression increases mature miR-21 expression in pulmonary arterial endothelial cells (4). Although a HIF response element (HRE) has been predicted in the promoter of this miRNA (29), hypoxia-induced up-regulation of miR-21 likely is driven by both HIF-dependent and HIF-independent mechanisms (such as induction of AKT2 activity) (33). Direct gene targets of miR-21 are also numerous, many of which include tumor suppressor genes that, when repressed by miR-21 in transformed cell types, drive proliferation and suppress apoptosis (25). However, while miR-21 carries bona fide oncogenic function in many contexts, emerging data indicate that control of each direct target by miR-21 differs depending on the biological context, and that some of these targets may be less relevant in controlling function in the pulmonary vascular compartment.
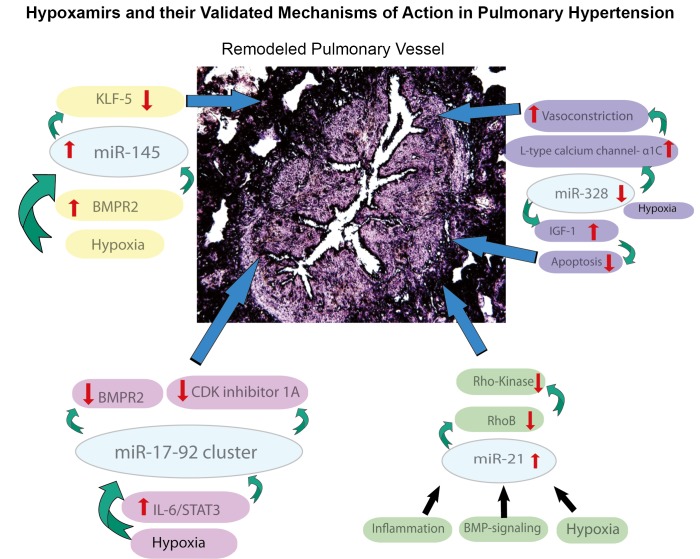
Figure 3.
Validated functions of hypoxamirs in the control of PH
Importantly, expression of miR-21 is dysregulated in the diseased pulmonary vasculature of humans and animal models exposed to hypoxic stress. In recent studies by Parikh and colleagues (4) and Bockmeyer and colleagues (34) in situ analysis of miRNA expression in remodeled pulmonary arteries and plexiform lesions in human PH lungs revealed up-regulation of miR-21 expression as compared with non-diseased vessels. This pattern of up-regulation was also observed in pulmonary hypertensive mice either exposed to chronic hypoxia for three weeks or subjected to constitutive expression of HIF-1 via a conditional genetic deletion of the Von-Hippel Lindau (VHL) protein (4). In contrast, Caruso and colleagues have reported a down-regulation, rather than up-regulation, of miR-21 in lung homogenate in rodent and human PH (12). Differences in the clinical context of disease may partially explain these results. Nonetheless, dynamic alterations of this particular hypoxamir certainly reflect its potentially central role in controlling the progression of PH.
Independent of such expression screening for alterations of miR-21 in diseased pulmonary vasculature, the putative importance of miR-21 was also emphasized by a novel computational gene network-based analysis performed by our group (4). Briefly, there is a growing appreciation that miRNA frequently target multiple related genes in the same functional pathways in order to coordinately regulate a given biological phenotype. By leveraging this idea, we specifically sought to identify miRNA that may robustly regulate disease phenotype by targeting multiple related genes in functionally integrated pathways. To do so, a functional network of PH-associated genes (“the PH-network”) was derived from the scientific literature and mapped using consolidated databases of molecular interactions (35-43). Using the highly sensitive and specific miRNA target prediction algorithm, TargetScan 5 (Conserved) (9), a hypergeometric analysis was then performed to rank miRNA according to the proportion of their predicted targets found within the PH-network. In doing so, 29 miRNA groups were found to have a less than 5% probability that the overlap of their predicted target list with the PH-network occurred by chance and thus, are most likely to coordinate pathogenic effects within the PH-network. MiR-21 was the second most highly ranked miRNA -- a ranking consistent with its known associations with hypoxic stress and the diseased pulmonary vasculature.
Because of its robust ranking, we chose to further investigate miR-21 as a central regulator of PH. To explore the molecular mechanisms controlled by this miRNA that are relevant to PH, the targets of miR-21 encompassed within the PH-network were analyzed for their known regulation of vasoactive phenotypes. The GTPase RhoB, a previously confirmed target of miR-21 in cancer cells (44), emerged as a promising candidate, given its activation of Rho kinase, which itself induces pulmonary vascular remodeling and PH in rodents and humans [as reviewed in (45)]. Importantly, among several candidate PH-modifying miRNA, only miR-21 was predicted to regulate RhoB by our analysis. Thus, we predicted that other disease-relevant miRNA would be less likely to interfere with or compensate for miR-21’s actions on RhoB. Indeed, we found that in cultured human pulmonary arterial endothelial cells (HPAEC) miR-21 down-regulated RhoB expression, leading to subsequent decrease in Rho kinase activity. Consequently, miR-21 induces downstream molecular programs consistent with decreased angiogenesis and vasodilation, which would be expected to protect against the pathogenic mechanisms driving PH (1). In vivo, upon induction of PH in miR-21-null mice, lung vascular RhoB expression and Rho-kinase activity were increased, accompanied by exaggerated PH severity. Notably, since our findings were reported, the function of RhoB as a pathogenic factor in PH was independently confirmed by Wojciak-Stothard and colleagues (46). Therefore, our findings not only demonstrated the importance of miR-21 in the development of PH but also provided the first confirmation that network biology can help to elucidate novel molecular mechanisms in PH.
Although the actions of miR-21 certainly appear central to the progression of PH, it should be noted that reports of its functions in the pulmonary vasculature have not been altogether consistent. A previous report indicated that miR-21 may elicit hyperproliferation in hypoxic cultured pulmonary arterial smooth muscle cells (PASMCs) (47). Furthermore, in vivo use of antisense inhibitors of miR-21 may induce a similar hyperproliferative phenotype, ultimately leading to a decrease of pulmonary vascular remodeling in hypoxic rodents (13). The functional validity of in vivo inhibitors for miR-21, in particular, has been questioned, in light of discrepancies with genetic “knock-out” studies of cardiac disease (48). Nonetheless, given the pleiotropic nature of this molecule, it is possible that subtle differences in disease trigger, or manifestation of PH may dramatically alter miR-21-dependent actions. Future exploration of these roles of miR-21 in different cellular and PH contexts may further emphasize the significance of these differences and thus better reflect its robust and likely very complex control of this disease.
MiR-145 in PH
Recently, miR-145 has also been implicated in the pathogenesis of PH (Figure 3). As a miRNA that is highly expressed in vascular smooth muscle cells, the vascular functions of miR-145 have been studied in substantial detail by multiple independent groups. MiR-145 is encoded and typically expressed along with miR-143. At the molecular level, miR-143 and miR-145 (both members of the miR-143/145 family) are modulated by serum response factor (SRF) and myocardin, both of which are key central factors that regulate smooth muscle cell phenotype. In response to up-regulation, these miRNA directly repress a related group of multiple target genes that influence SRF activity and actin dynamics, notably including KLF5 and thus driving consequent modulation of myocardin itself (49,50). Correspondingly, this miRNA cluster carries robust functions in maintaining smooth muscle differentiation in vascular health as well as cytoskeletal dynamics and phenotypic switching during various types of vascular injury or disease in vivo (49-53). Interestingly, Hergenreider and colleagues have reported that miR-143/145 can be released from vascular endothelial cells in response to KLF2-dependent laminar flow for transport to vascular smooth muscle cells and subsequent phenotypic modulation (54). Recently, it has been demonstrated that hypoxia can dynamically regulate the expression of the miR-143/145 family in the cardiomyocyte (55) and renal tissue (56). Similarly, BMP signaling activates transcription of miR-143/145 in vascular smooth muscle cells (57). Thus, similar to the regulatory programs controlling miR-21, both hypoxia and BMP signaling appear to converge upon the regulation of the miR-143/145 family with obvious direct implications to the known triggers of PH.
Consistent with these observations, Caruso and colleagues have recently reported the direct control of PH by miR-145 (14). Expression of miR-145 was found to be up-regulated in the lungs of pulmonary hypertensive mice exposed to chronic hypoxia and in the lungs of BMPR2-deficient mice. Correspondingly, miR-145 was increased in pulmonary tissue of humans suffering from idiopathic and heritable PH; in primary cultured smooth muscle cells derived from such remodeled vessels; and in primary PASMCs cultured from patients with BMPR2 mutations. Importantly, either genetic deletion of miR-145 or antisense inhibition of miR-145 in vivo retarded PH development in chronically hypoxic mice, indicating the pathogenic endogenous role of this miRNA in promoting disease. Interestingly, antisense inhibition of miR-143 did not affect disease progression, suggesting the unique importance of miR-145 despite sharing similar regulation of expression as well as many similar gene targets. Thus, evidence points toward miR-145 as a putative therapeutic target in the pulmonary vasculature of PH patients. Yet, it still remains unclear which of the many validated and potent gene targets of miR-145 may primarily drive these pathogenic actions in vivo. Given the pleiotropic characteristics of most miRNAs, we would expect that these functional relationships should be evaluated to appreciate all pulmonary and systemic effects of such a therapeutic strategy.
MiR-328 in PH
Recently, Guo and colleagues have found a role for the hypoxamir miR-328 in hypoxia-induced PH (Figure 3) (15). Unlike miR-21 and miR-145, the cardiovascular actions of miR-328 have been less well studied, but it has been recently implicated in adverse atrial electric remodeling and the development of atrial fibrillation through targeting L-type Ca2+ channel genes (58). Guo and colleagues have also reported that miR-328 was substantially down-regulated in pulmonary arteries after hypoxic exposure, resulting in vasoconstriction and remodeling. Conversely, no significant expression change was appreciated in other arterial beds (thoracic, mesenteric, etc.). In vivo, transgenic overexpression of miR-328 led to decreased right ventricular systolic pressure and pulmonary arterial remodeling (as assessed by wall thickness) in both hypoxia and normoxia. At the molecular level, these authors confirmed that miR-328 directly represses L-type calcium channel-α1C expression and that this inhibition reduced the pulmonary arterial vasoconstrictive response. Additionally, miRNA-328 was found to down-regulate an additional target gene, the insulin growth factor 1 receptor, subsequently driving PASMC apoptosis. Thus, miR-328 appears to dually influence cell survival and vasomotor tone in order to protect specifically against PH-relevant phenotypes. Further confirmation of these actions in human PH is pending.
MiR-17-92 in PH
The miR-17-92 cluster is one of the most well-characterized miRNA families controlling cell development, apoptosis, and proliferation in a variety of cellular and disease contexts [as reviewed by (59)]. From a cardiovascular perspective, it directly regulates angiogenic potential in vascular endothelial cells in vivo (60). Hypoxia down-regulates miR-17-92 expression in a p53-dependent fashion (61). Conversely, overexpression of c-myc induces miR-17-92, which can directly target and repress the expression of HIF-1 (62). Thus, the miR-17-92 cluster is intricately linked to hypoxia and HIF-related activity. Similar to both miR-21 and miR-145, this miRNA cluster also regulates BMP signaling. For miR-17 and miR-20a (both members of the miR-17-92 cluster), the BMPR2 transcript is a predicted and validated target in cultured vascular cells (63). A recent study by Pullamsetti and colleagues has shown that inhibition of miR-17 attenuated PH (Figure 3) in both hypoxia-treated mice and monocrotaline-treated rats (22). While alteration of BMPR2 activity may have partially contributed to this phenotype, miR-17-dependent suppression of another target gene, cyclin dependent kinase inhibitor 1A, also appeared to be important in inducing a PH-relevant hyperproliferative phenotype in cultured PASMCs. A similar phenotype was also described by Brock and colleagues through inhibiting miR-20a (64), where rescue of BMPR2 function was associated with the observed reduction of PH severity in hypoxia-treated mice. Taken together, these studies suggest a complex yet active role for the miR-17-92 cluster in the etiology of PH. Especially in this context, it will be important in the future to develop approaches that can better predict and validate the coordinated and overlapping roles of these miRNA and their targets in order to understand their comprehensive, rather than isolated, actions in the pulmonary vasculature.
Predicted roles of other hypoxamirs in PH
Besides miR-21, miR-145, miR-328, and miR-17-92, direct mechanistic evidence for the role of additional hypoxamirs in PH is sparse (Figure 2). Courboulin and colleagues have reported a down-regulation of miR-204 in diseased PASMCs in human and rodent models of PH, leading to alterations in its direct target (domain-containing tyrosine phosphatase 2), SHP2 and consequent alterations in Src, NFATc2 (nuclear factor of activated T-cells, cytoplasmic, calcineurin-dependent 2) and pulmonary vascular metabolism (65). Ultimately, these authors found that such a mechanism influences PH progression in vivo and thus suggests this miRNA as a viable therapeutic target in this disease. Notably, hypoxia induces miR-204 in cardiomyocytes (66), and its regulation by hypoxia in PH could contribute in specific circumstances of disease. However, the reported down-regulation of miR-204 in most forms of PH likely suggests that stimuli other than hypoxia primarily drive the currently described miR-204-dependent disease phenotype.
Coined “the master hypoxamir” (67), miR-210 is a prime example of miRNA with pleiotropic vascular functions with presumed, but with as-of-yet unproven, roles in PH. Because of its direct HIF dependency, miR-210 has been implicated as a contributor to a number of hypoxic and ischemic related diseases in vivo including ischemic heart disease (68), as well as in an array of cancers (69). MiR-210 has also been found to be up-regulated in lung tissue of chronically hypoxic mice suffering from pulmonary hypertension (13). The actions of miR-210 in PH could be linked to down-regulation of the iron sulfur complex assembly proteins 1/2 (ISCU1/2) (70) and additional mitochondrial electron transport proteins (71,72) which repress mitochondrial metabolism in favor of glycolysis for energy production. This adaptation is essential for cell survival during acute hypoxic stress, but carries pathogenic consequences in chronic hypoxic and ischemic vascular diseases [as reviewed in (73)]. Alternatively, by repressing ephrin-A3, the actions of miR-210 have been linked to angiogenesis which could also affect the development of PH (74). Thus, it follows that up-regulation of miR-210 in the hypoxic pulmonary vasculature may represent a sentinel pathogenic event that triggers down-regulation of a cadre of PH-relevant targets and resultant pathogenic vascular dysregulation. Yet, the activity of miR-210 in PH is unknown and awaits validation.
Stemming from studies of miRNA actions in other biological settings, we can make predictions regarding the importance of other hypoxamirs in PH (Figure 2). Yet as the number of hypoxamirs relevant to PH grows, we will likely need better tools to appropriately understand how these miRNA act in a coordinate fashion to influence hypoxic adaptation and disease manifestations. Specifically, we envision that improvements in both computational modeling and in vivo manipulation of multiple miRNA will be necessary to adequately study hypoxamir biology on a systems-wide level.
Conclusions
Over the past five years, our understanding regarding the critical mechanisms by which hypoxamirs influence hypoxic adaptation in both healthy and diseased tissue has greatly matured. While the importance of only a few hypoxamirs has been confirmed specifically in pulmonary vascular function, many more are expected to carry unique and essential roles in the pathogenesis of PH and thus may serve as robust therapeutic targets. Of note, miRNA-based therapy in vivo continues to be the subject of intensive investigation in both the academic and commericial biotechnology sectors, with the most promising results using chemically modified oligonucleotide antisense inhibitors (e.g., antagomirs or anti-miRs). While the delivery of such inhibitors to the pulmonary vasculature in vivo has yet to be adequately explored, potential challenges of their use are the off-target effects that can result from the high concentrations necessary for vascular delivery as well as inability to specifically target the pulmonary vasculature without affecting other tissue beds. Nonetheless, by combining the powerful predictive methods of network biology with traditional yet continually advancing laboratory techniques in the study of PH, we expect that a more complete hypoxamir “interactome” in this disease will emerge and provide fertile grounds for the study of miRNA as new therapeutic targets in PH. Yet, challenges will remain both in deciphering the mechanistic interconnections linking networks of hypoxamirs and their targets and in identifying those pathways that are the most essential in the control of PH in vivo.
Acknowledgements
This work was supported in part by the NIH, the Pulmonary Hypertension Association, Gilead Sciences, and the Lerner, Harris, and Watkins funds (S.Y.C.).
We thank Ms. Erica Kates and Ms. Stephanie Tribuna for expert administrative assistance.
Disclosure: The authors declare no conflict of interest.
References
- 1.Chan SY, Loscalzo J. Pathogenic mechanisms of pulmonary arterial hypertension. J Mol Cell Cardiol 2008;44:14-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chan SY, Loscalzo J. MicroRNA-210: a unique and pleiotropic hypoxamir. Cell Cycle 2010;9:1072-83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Esteller M.Non-coding RNAs in human disease. Nat Rev Genet 2011;12:861-74 [DOI] [PubMed] [Google Scholar]
- 4.Parikh VN, Jin RC, Rabello S, et al. MicroRNA-21 integrates pathogenic signaling to control pulmonary hypertension: results of a network bioinformatics approach. Circulation 2012;125:1520-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu C, So J, Davis-Dusenbery BN, et al. Hypoxia potentiates microRNA-mediated gene silencing through posttranslational modification of Argonaute2. Mol Cell Biol 2011;31:4760-74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ho JJ, Metcalf JL, Yan MS, et al. Functional importance of Dicer in the adaptive cellular response to hypoxia. J Biol Chem 2012;287:29003-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Rooij E, Olson EN. MicroRNAs: powerful new regulators of heart disease and provocative therapeutic targets. J Clin Invest 2007;117:2369-76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked byadenosines, indicates that thousands of human genes are microRNA targets. Cell 2005;120:15-20 [DOI] [PubMed] [Google Scholar]
- 9.Friedman RC, Farh KK, Burge CB, et al. Most mammalian mRNAs are conservedtargets of microRNAs. Genome Res 2009;19:92-105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grimson A, Farh KK, Johnston WK, et al. MicroRNA targeting specificity in mammals: determinants beyond seed pairing. Mol Cell 2007;27:91-105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kiriakidou M, Nelson PT, Kouranov A, et al. A combined computational-experimental approach predicts human microRNA targets. Genes Dev 2004;18:1165-78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caruso P, MacLean MR, Khanin R, et al. Dynamic changes in lung microRNA profiles during the development of pulmonary hypertension due to chronic hypoxia and monocrotaline. Arterioscler Thromb Vasc Biol 2010;30:716-23 [DOI] [PubMed] [Google Scholar]
- 13.Yang S, Banerjee S, Freitas AD, et al. miR-21 regulates chronic hypoxia-induced pulmonary vascular remodeling. Am J Physiol Lung Cell Mol Physiol 2012;302:L521-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caruso P, Dempsie Y, Stevens HC, et al. A Role for miR-145 in Pulmonary Arterial Hypertension: Evidence From Mouse Models and Patient Samples. Circ Res 2012;111:290-300 [DOI] [PubMed] [Google Scholar]
- 15.Guo L, Qiu Z, Wei L, et al. The microRNA-328 regulates hypoxic pulmonary hypertension by targeting at insulin growth factor 1 receptor and L-type calcium channel-α1C. Hypertension 2012;59:1006-13 [DOI] [PubMed] [Google Scholar]
- 16.Kulshreshtha R, Ferracin M, Wojcik SE, et al. A microRNA signature of hypoxia. Mol Cell Biol 2007;27:1859-67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghosh G, Subramanian IV, Adhikari N, et al. Hypoxia-induced microRNA-424expression in human endothelial cells regulates HIF-α isoforms and promotesangiogenesis. J Clin Invest 2010;120:4141-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taganov KD, Boldin MP, Chang KJ, et al. NF-kappaB-dependent induction ofmicroRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci U S A 2006;103:12481-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun X, Icli B, Wara AK, et al. MicroRNA-181b regulates NF-κB-mediated vascular inflammation. J Clin Invest 2012;122:1973-90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu X, Chang MS, Mitsialis SA, et al. Hypoxia regulates bone morphogenetic protein signaling through C-terminal-binding protein 1. Circ Res 2006;99:240-7 [DOI] [PubMed] [Google Scholar]
- 21.Hagen M, Fagan K, Steudel W, et al. Interaction of interleukin-6 and the BMP pathway in pulmonary smooth muscle. Am J Physiol Lung Cell Mol Physiol 2007;292:L1473-9 [DOI] [PubMed] [Google Scholar]
- 22.Pullamsetti SS, Doebele C, Fischer A, et al. Inhibition of microRNA-17 improves lung and heart function in experimental pulmonary hypertension. Am J Respir Crit Care Med 2012;185:409-19 [DOI] [PubMed] [Google Scholar]
- 23.Parikh VN, Chan SY. Inflammatory mechanisms in pulmonary hypertension. In: Wang YX. eds. Recent Advances in Pulmonary Vascular Biology. In press. Kerala, India: Research Signpost, 2012. [Google Scholar]
- 24.Zhou T, Garcia JG, Zhang W. Integrating microRNAs into a system biologyapproach to acute lung injury. Transl Res 2011;157:180-90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krichevsky AM, Gabriely G. miR-21: a small multi-faceted RNA. J Cell Mol Med 2009;13:39-53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thum T, Gross C, Fiedler J, et al. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature 2008;456:980-4 [DOI] [PubMed] [Google Scholar]
- 27.Maegdefessel L, Azuma J, Toh R, et al. MicroRNA-21 blocks abdominal aortic aneurysm development and nicotine-augmented expansion. Sci Transl Med 2012;4:122ra22. [DOI] [PMC free article] [PubMed]
- 28.Ji R, Cheng Y, Yue J, et al. MicroRNA expression signature and antisense-mediated depletion reveal an essential role of MicroRNA in vascular neointimal lesion formation. Circ Res 2007;100:1579-88 [DOI] [PubMed] [Google Scholar]
- 29.Gorospe M, Tominaga K, Wu X, et al. Post-Transcriptional Control of the Hypoxic Response by RNA-Binding Proteins and MicroRNAs. Front Mol Neurosci 2011;4:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Davis BN, Hilyard AC, Lagna G, et al. SMAD proteins control DROSHA-mediated microRNA maturation. Nature 2008;454:56-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Löffler D, Brocke-Heidrich K, Pfeifer G, et al. Interleukin-6 dependent survival of multiple myeloma cells involves the Stat3-mediated induction of microRNA-21 through a highly conserved enhancer. Blood 2007;110:1330-3 [DOI] [PubMed] [Google Scholar]
- 32.Iliopoulos D, Jaeger SA, Hirsch HA, et al. STAT3 activation of miR-21 and miR-181b-1 via PTEN and CYLD are part of the epigenetic switch linking inflammation to cancer. Mol Cell 2010;39:493-506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Polytarchou C, Iliopoulos D, Hatziapostolou M, et al. Akt2 regulates all Akt isoforms and promotes resistance to hypoxia through induction of miR-21 upon oxygen deprivation. Cancer Res 2011;71:4720-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bockmeyer CL, Maegel L, Janciauskiene S, et al. Plexiform vasculopathy of severe pulmonary arterial hypertension and microRNA expression. J Heart Lung Transplant 2012;31:764-72 [DOI] [PubMed] [Google Scholar]
- 35.Rual JF, Venkatesan K, Hao T, et al. Towards a proteome-scale map of the human protein-protein interaction network. Nature 2005;437:1173-8 [DOI] [PubMed] [Google Scholar]
- 36.Stelzl U, Worm U, Lalowski M, et al. A human protein-protein interaction network: a resource for annotating the proteome. Cell 2005;122:957-68 [DOI] [PubMed] [Google Scholar]
- 37.Cusick ME, Yu H, Smolyar A, et al. Literature-curated protein interaction datasets. Nat Methods 2009;6:39-46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aranda B, Achuthan P, Alam-Faruque Y, et al. The IntAct molecular interaction database in 2010. Nucleic Acids Res 2010;38:D525-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ceol A, Chatr Aryamontri A, Licata L, et al. MINT, the molecular interaction database: 2009 update. Nucleic Acids Res 2010;38:D532-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wingender E, Chen X, Fricke E, et al. The TRANSFAC system on gene expression regulation. Nucleic Acids Res 2001;29:281-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Diella F, Gould CM, Chica C, et al. Phospho.ELM: a database of phosphorylation sites--update 2008. Nucleic Acids Res 2008;36:D240-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hornbeck PV, Chabra I, Kornhauser JM, et al. PhosphoSite: A bioinformatics resource dedicated to physiological protein phosphorylation. Proteomics 2004;4:1551-61 [DOI] [PubMed] [Google Scholar]
- 43.Hong KH, Lee YJ, Lee E, et al. Genetic ablation of the BMPR2 gene in pulmonary endothelium is sufficient to predispose to pulmonary arterial hypertension. Circulation 2008;118:722-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Connolly EC, Van Doorslaer K, Rogler LE, et al. Overexpression of miR-21promotes an in vitro metastatic phenotype by targeting the tumor suppressor RHOB. Mol Cancer Res 2010;8:691-700 [DOI] [PubMed] [Google Scholar]
- 45.Connolly MJ, Aaronson PI. Key role of the RhoA/Rho kinase system in pulmonary hypertension. Pulm Pharmacol Ther 2011;24:1-14 [DOI] [PubMed] [Google Scholar]
- 46.Wojciak-Stothard B, Zhao L, Oliver E, et al. Role of RhoB in theregulation of pulmonary endothelial and smooth muscle cell responses to hypoxia. Circ Res 2012;110:1423-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sarkar J, Gou D, Turaka P, et al. MicroRNA-21 plays a role in hypoxia-mediated pulmonary artery smooth muscle cell proliferation and migration. Am J Physiol Lung Cell Mol Physiol 2010;299:L861-71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morrisey EE. The magic and mystery of miR-21. J Clin Invest 2010;120:3817-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cheng Y, Liu X, Yang J, et al. MicroRNA-145, a novel smooth muscle cell phenotypic marker and modulator, controls vascular neointimal lesion formation. Circ Res 2009;105:158-66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Elia L, Quintavalle M, Zhang J, et al. The knockout of miR-143 and -145 alters smooth muscle cell maintenance and vascular homeostasis in mice: correlates with human disease. Cell Death Differ 2009;16:1590-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xin M, Small EM, Sutherland LB, et al. MicroRNAs miR-143 and miR-145 modulate cytoskeletal dynamics and responsiveness of smooth muscle cells to injury. Genes Dev 2009;23:2166-78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cordes KR, Sheehy NT, White MP, et al. miR-145 and miR-143 regulate smooth muscle cell fate and plasticity. Nature 2009;460:705-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Albinsson S, Suarez Y, Skoura A, et al. MicroRNAs are necessary for vascular smooth muscle growth, differentiation, and function. Arterioscler Thromb Vasc Biol 2010;30:1118-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hergenreider E, Heydt S, Tréguer K, et al. Atheroprotective communication between endothelial cells and smooth muscle cells through miRNAs. Nat Cell Biol 2012;14:249-56 [DOI] [PubMed] [Google Scholar]
- 55.Mayorga ME, Penn MS. miR-145 is differentially regulated by TGF-β1 and andischaemia and targets Disabled-2 expression and wnt/β-catenin activity. J Cell Mol Med 2012;16:1106-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bussolati B, Moggio A, Collino F, et al. Hypoxia modulates the undifferentiated phenotype of human renal inner medullary CD133+ progenitors through Oct4/miR-145 balance. Am J Physiol Renal Physiol 2012;302:F116-28 [DOI] [PubMed] [Google Scholar]
- 57.Davis-Dusenbery BN, Chan MC, Reno KE, et al. Down-regulation of Kruppel-like factor-4 (KLF4) by microRNA-143/145 is critical for modulation of vascular smooth muscle cell phenotype by transforming growth factor-beta and bone morphogenetic protein 4. J Biol Chem 2011;286:28097-110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lu Y, Zhang Y, Wang N, et al. MicroRNA-328 contributes to adverse electrical remodeling in atrial fibrillation. Circulation 2010;122:2378-87 [DOI] [PubMed] [Google Scholar]
- 59.Mendell JT. miRiad roles for the miR-17-92 cluster in development and disease. Cell 2008;133:217-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bonauer A, Carmona G, Iwasaki M, et al. MicroRNA-92a controls angiogenesis and functional recovery of ischemic tissues in mice. Science 2009;324:1710-3 [DOI] [PubMed] [Google Scholar]
- 61.Yan HL, Xue G, Mei Q, et al. Repression of the miR-17-92 cluster by p53 has an important function in hypoxia-induced apoptosis. EMBO J 2009;28:2719-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Taguchi A, Yanagisawa K, Tanaka M, et al. Identification of hypoxia-inducible factor-1 alpha as a novel target for miR-17-92 microRNA cluster. Cancer Res 2008;68:5540-5 [DOI] [PubMed] [Google Scholar]
- 63.Brock M, Trenkmann M, Gay RE, et al. Interleukin-6 modulates the expression of the bone morphogenic protein receptor type II through a novel STAT3-microRNA cluster 17/92 pathway. Circ Res 2009;104:1184-91 [DOI] [PubMed] [Google Scholar]
- 64.Brock M, Samillan VJ, Trenkmann M, et al. AntagomiR directed against miR-20a restores functional BMPR2 signalling and prevents vascular remodelling in hypoxia-induced pulmonary hypertension. Eur Heart J. 2012 doi: 10.1093/eurheartj/ehs060. [Epub ahead ofprint] [DOI] [PubMed] [Google Scholar]
- 65.Courboulin A, Paulin R, Giguère NJ, et al. Role for miR-204 in human pulmonary arterial hypertension. J Exp Med 2011;208:535-48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jian X, Xiao-Yan Z, Bin H, et al. MiR-204 regulate cardiomyocyte autophagy induced by hypoxia-reoxygenation through LC3-II. Int J Cardiol 2011;148:110-2 [DOI] [PubMed] [Google Scholar]
- 67.Chan YC, Banerjee J, Choi SY, et al. miR-210: the master hypoxamir. Microcirculation 2012;19:215-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hu S, Huang M, Li Z, et al. MicroRNA-210 as a novel therapy for treatment of ischemic heart disease. Circulation 2010;122:S124-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Huang X, Ding L, Bennewith KL, et al. Hypoxia-inducible mir-210 regulates normoxic gene expression involved in tumor initiation. Mol Cell 2009;35:856-67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chan SY, Zhang YY, Hemann C, et al. MicroRNA-210 controls mitochondrial metabolism during hypoxia by repressing the iron-sulfur cluster assembly proteins ISCU1/2. Cell Metab 2009;10:273-84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen Z, Li Y, Zhang H, et al. Hypoxia-regulated microRNA-210 modulates mitochondrial function and decreases ISCU and COX10 expression. Oncogene 2010;29:4362-8 [DOI] [PubMed] [Google Scholar]
- 72.Puisségur MP, Mazure NM, Bertero T, et al. miR-210 is overexpressed inlate stages of lung cancer and mediates mitochondrial alterations associated with modulation of HIF-1 activity. Cell Death Differ 2011;18:465-78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tuder RM, Davis LA, Graham BB. Targeting energetic metabolism: a new frontier in the pathogenesis and treatment of pulmonary hypertension. Am J Respir Crit Care Med 2012;185:260-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fasanaro P, D’Alessandra Y, Di Stefano V, et al. hypoxia and inhibits the receptor tyrosine kinase ligand Ephrin-A3. J Biol Chem 2008;283:15878-83 [DOI] [PMC free article] [PubMed] [Google Scholar]