Abstract
Purpose of Review
This review addresses our current understanding of the pathogenesis of HIV associated lipohypertrophy, and describes an evidence-based approach to treatment.
Recent Findings
Although the pathogenesis of HIV associated lipohypertrophy remains elusive, recent clinical and laboratory investigations in fatty acid metabolism and growth hormone dynamics have furthered our understanding of the condition. These findings have also paved the way for new therapeutic interventions, of which tesamorelin, an analogue of growth hormone-releasing hormone, has gained recognition as a promising treatment strategy against visceral fat accumulation. Recent randomized placebo-controlled trials of tesamorelin demonstrated significant reductions in visceral adipose tissue, improvement in lipid parameters, and no adverse effects on glucose tolerance. Optimal therapeutic dosing and treatment duration, though, are not yet known. Whether treatment with GHRH-analogues will translate into improved long-term metabolic and cardiovascular outcomes also remains to be seen.
Summary
Although the pathogenesis of HIV lipohypertrophy remains unclear, several theories and observations have led to the development of treatment strategies to counter fat accumulation and its accompanying metabolic complications. Based on clinical trials, analogues of the GH/GHRH axis appear to be most effective in reducing visceral adipose tissue.
Keywords: lipohypertrophy, visceral adipose tissue, HIV, lipodystrophy, tesamorelin
Introduction
With the advent of potent antiretroviral therapy for HIV infection in the mid-1990s, descriptions of morphological changes and metabolic disturbances in treated patients began to emerge. Initially the observed lipoatrophy and central fat accumulation were referred to collectively as “HIV-associated lipodystrophy”. In recent years, there has been increasing recognition that fat loss and fat gain likely represent distinct entities with unique pathogenic mechanisms.
Lipoatrophy is characterized by generalized loss of subcutaneous fat, most evident in the face, arms, legs, and/or buttocks without substantial loss of lean tissue mass. Lipohypertrophy is characterized by excess fat deposition in abdominal visceral adipose tissue (VAT), as well as in the dorsocervical region (i.e. buffalo hump), trunk and/or breasts. There may be co-existing fat deposition in the liver, muscle [1], myocardium, and epicardium [2].
The clinical implications of HIV-associated fat redistribution are both psychosocial and medical. Patients with abdominal obesity report poorer self-image [3], and may be less inclined to initiate or maintain adherence to antiretroviral treatment. The accumulation of VAT is of particular concern from a medical standpoint due to the often concomitant presence of insulin resistance and dyslipidemia [4,5], a clustering of abnormalities akin to the metabolic syndrome. In the general population, the metabolic syndrome significantly increases the risk of incident cardiovascular disease and mortality [6–8], with waist circumference being increasingly recognized as an independent risk factor for myocardial infarction [9]. In a recent report, coronary artery calcium scores were strongly associated with lipohypertrophy, providing evidence for the link between VAT and CVD risk in HIV-infected individuals [•10]. From both psychosocial and medical perspectives then, there has been an urgency to develop treatment strategies to counter the morphologic and metabolic complications of fat accumulation. This review will focus on proposed pathogenesis mechanisms and current treatment options for HIV-associated lipohypertrophy.
Pathogenesis
Epidemiological studies suggest that host factors (e.g. female sex and increasing age), markers of HIV infection itself (e.g. HIV viral load and nadir CD4 count), duration and possibly type of antiretroviral therapy are associated with lipohypertrophy [11]. While protease inhibitors (PIs) as a class have been most cited in the development of lipohypertrophy [11], trunk fat and waist circumference tend to increase after initiation of ART regardless of the type of regimen [12,13]. Based on these epidemiological observations, investigators have proposed a number of theories to explain the pathogenesis of lipohypertrophy, or, more generally, altered fat distribution (lipodystrophy) in HIV-infected patients.
Mitochondrial dysfunction
Mitochondrial dysfunction induced by NRTIs, specifically the thymidine analogues stavudine and zidovudine, has been implicated in the pathogenesis of lipoatrophy [14–16]. Some patients could have preserved visceral fat in the setting of subcutaneous fat loss if there is relative resistance of visceral fat to mitochondrial toxicity. In one study, patients were found to have increased expression of adipogenic transcription factors in abdominal subcutaneous adipose tissue (SAT) after two months of ARV initiation but decreased expression in thigh SAT suggesting that ARVs may have tissue-specific effects leading to lipoatrophy, lipohypertrophy, or a combination of both [17]. The technical challenge of acquiring visceral fat tissue from HIV-infected subjects for laboratory investigation has limited investigation in this area.
Impaired fatty acid metabolism
One model of HIV-associated fat redistribution cites dysregulation of fatty acid metabolism in the adipocyte as the underlying defect in promoting lipodystrophy. This model is based on the finding that patients with HIV lipodystrophy have significantly increased rates of basal lipolysis in the fasted state, increased intra-adipocyte re-esterification and net increase in free fatty acid release into the plasma pool without a proportionate increase in fatty acid oxidation [18]. Furthermore, a study involving ingestion of labeled triglyceride demonstrated that clearance from the plasma chylomicron pool was markedly reduced in patients with HIV-associated fat redistribution [19], implying that there is also a profound defect in fat storage.
To explain the development of lipohypertrophy, Sekhar et al. hypothesize that a defect in peripheral adipocytes results in a greater availability of fatty acids in the circulation. The available fatty acids are then selectively deposited in visceral adipose tissue owing to the higher rate of lipid turnover and uptake in visceral adipocytes [20]. The putative cause of this dysregulation is not apparent but could be related to the effects of HIV itself via the HIV-1 accessory protein Vpr [21] or the effects of specific antiretrovirals.
Adiponectin
Adiponectin is an adipocyte-derived hormone that functions as an insulin sensitizer by reducing triglyceride levels and inhibiting gluconeogenesis in the liver. Adiponectin deficiency has been implicated in obesity, insulin resistance and type 2 diabetes in the general population [22,23], and has also been found to correlate inversely with VAT in patients with HIV-associated fat redistribution [24]. Since adiponectin expression is higher in SAT than VAT in obese humans [25], the adiponectin deficiency observed in HIV lipodystrophy may result from the accumulation of VAT in conjunction with the loss of SAT.
Leptin
Leptin is a hormone involved in central regulation of energy homeostasis and insulin resistance. In human obesity, leptin levels are elevated, likely representing a state of leptin resistance [26]. In HIV-infected individuals, leptin levels seem to correlate with body fat phenotype, with the lowest levels seen in patients with lipoatrophy, and the highest levels in patients with lipohypertrophy [27]. Elevated leptin levels can be attributed to increased secretion by adipocytes and/or resistance at the leptin receptor level in those with lipohypertrophy. Whether these alterations in adiponectin and leptin are a cause or consequence of fat redistribution is unclear, but therapeutic interventions to correct their levels are being explored.
Cortisol
Owing to phenotypic similarities with Cushing’s syndrome, the role of cortisol in the pathogenesis of lipohypertrophy has been investigated. Though circulating levels of cortisol are not elevated [28], investigators have found higher ratios of urinary cortisol: cortisone metabolites and higher mRNA levels of 11β-hydroxy-steroid dehydrogenase type 1 (11β-HSD1) in the SAT of patients with HIV-associated lipodystrophy [29]. 11β-HSD1 is an enzyme that catalyzes the conversion of inactive cortisone to cortisol, and its overexpression in transgenic mice leads to visceral obesity, diabetes and dyslipidemia [30]. These data suggest that alterations in intra-abdominal glucocorticoid signaling could account for the Cushingoid characteristics of patients with HIV-associated lipohypertrophy.
Growth Hormone
In obese subjects, the amount of GH secreted per burst is decreased [31], and the response to GHRH and arginine stimulation testing is significantly blunted [32]. Subsequent studies in patients with HIV-associated lipodystrophy have shown similar alterations in GH dynamics. In comparison with age and body mass index-matched HIV-negative men and HIV-infected men without lipodystrophy, pulsatility of GH is maintained in overnight frequent sampling, but mean concentrations of GH are reduced by approximately 50% [33]. Affected patients also show suboptimal GH response to standard GHRH and arginine stimulation testing [34]. Increased somatostatin tone, decreased ghrelin concentrations and direct suppression of GH by elevated free fatty acids may all contribute to impaired GH secretion in HIV-infected men with lipodystrophy [35].
Immune Reconstitution
Finally, immune reconstitution from effective antiretroviral therapy and residual inflammation and immune activation in the setting of suppressed HIV replication could contribute to lipohypertrophy. While there is no direct evidence to support this theory, there is precedent in the oncologic literature where adult survivors of childhood cancers and adults who have undergone bone marrow or stem cell transplantation appear to have a higher prevalence of obesity and metabolic syndrome than the general population [36,37].
Treatment
Because of the potential metabolic and cardiovascular risk posed by fat accumulation, there has been significant interest and research on the treatment of HIV lipohypertrophy, especially in the reduction of VAT (Table).
TABLE.
Summary of interventions for HIV-associated visceral adiposity
| Intervention | Selected Studies | N | Dose | VAT outcome |
|---|---|---|---|---|
|
| ||||
| Diet and exercise | Thoni 2002 [38] | 17 | Individualized aerobic training program x 4 months | Reduction in VAT by 12% |
| Dolan 2006 [39] | 40 | Home exercise training program x 4 months | Decrease in waist circumference, but no change in VAT | |
|
| ||||
| Metformin | Hadigan 2000 [40] | 26 | Metformin 500mg bid vs. placebo x 3 months | Reduction in VAT by 6.3% |
| Driscoll 2004 [41] | 37 | Metformin 500mg bid (increased to 850mg bid) +/− exercise training x 3 months | Combination of metformin and exercise training decreased VAT by 8.5% | |
| Kohli 2007 [42] | 48 | Metformin 500mg bid (increased to 1500mg bid) vs. placebo x 6 months | No significant decrease in VAT compared to placebo after adjusting for age, height, baseline VAT and insulin AUC | |
|
| ||||
| Leptin | Lee 2006 [43] | 7 | Recombinant human leptin at 0.02mg/kg bid x 2 months | Decrease in truncal fat by 14.6%, but no significant decrease in VAT |
| Mulligan 2009 [44] | 8 | Recombinant human leptin at 0.01 mg/kg bid x 3 months, then 0.03 mg/kg bid x 3 months | Average reduction in VAT by 32% after 6 months | |
|
| ||||
| Recombinant human growth hormone | Grunfeld 2007 [45] | 325 | rhGH 4mg daily vs. placebo x 12 weeks induction | Reduction in VAT by 20.3% |
| Luzi 2005 [46] | 30 | rhGH 0.028 IU/kg/day vs. placebo x 6 months | Reduction in truncal fat but no specific data on VAT | |
| Hansen 2010 [47] | 46 | rhGH 0.7mg/day vs. placebo x 10 months | Decrease in VAT by 11% | |
| Lo 2008 [48] | 56 | rhGH titrated to upper quartile of normal IGF-1 range (average dose 0.33mg/day) x 18 months | Decrease in VAT by 8.5% | |
|
| ||||
| Tesamorelin (growth hormone-releasing factor) | Falutz 2007 [49] | 412 | Tesamorelin 2mg daily vs. placebo x 26 weeks | Reduction in VAT by 15.2% |
| Falutz 2010 [50] | 404 | Tesamorelin 2mg daily vs. placebo x 6 months (efficacy phase) | Reduction in VAT by 10.9% during efficacy phase | |
| 265 | Re-randomization to tesamorelin 2mg daily vs. placebo x another 6 months (safety extension phase) | Overall reduction in VAT by 17.5% in those treated with tesamorelin x 12 months. Return to baseline VAT in group switching from tesamorelin to placebo. |
||
Switching antiretrovirals
Unlike lipoatrophy, there is no clear association between fat accumulation and specific antiretrovirals. While PIs have been implicated historically, switching PIs out of cART regimens have yielded inconsistent results in reducing adipose tissue [51]. In one small study, switching from lopinavir/ritonavir to atazanavir/ritonavir, a PI that does not affect insulin stimulated glucose uptake in vitro [52–54], resulted in increased muscle glucose uptake, decreased visceral adiposity and improved lipid parameters [55]. Due to inconsistency of results, however, we do not recommend switching antiretrovirals to combat lipohypertrophy. It is not known if choice of contemporary cART regimen affects the likelihood of developing lipohypertrophy or whether earlier initiation of HIV therapy could reduce the incidence of lipohypertrophy.
Diet and Exercise
Lifestyle changes are likely to provide some benefit in treating HIV-associated lipohypertrophy and associated metabolic abnormalities, though data are limited. A small study involving 17 HIV-infected patients with lipodystrophy who underwent a supervised aerobic training program demonstrated a mean decrease of 12.8% in total adipose tissue and 12% in visceral adipose tissue at 4 months [38]. Another study of 40 HIV-infected women who underwent 16-week exercise training program demonstrated improvements in muscle strength, cardiorespiratory fitness, endurance, and a reduction in waist circumference, but ultimately no change in VAT [39]. Since diet and exercise are typically well-tolerated and confer other health benefits, they are recommended in the initial treatment approach to lipohypertrophy.
Metformin
A number of clinical trials have explored the use of metformin in the treatment of lipohypertrophy with most showing modest reductions in waist circumference but inconsistent effects on VAT [40–42, 56]. Metformin use has led to proportionate reductions of SAT and VAT, and documented loss of limb fat in one study [42]. Consequently, metformin should be avoided in patients with concurrent lipoatrophy.
Thiazolidinediones (TZDs)
Several studies have investigated the use of TZDs for HIV-associated lipoatrophy with mixed results and no clear benefit in a recent meta-analysis [57]. Neither rosiglitazone nor pioglitazone significantly reduces VAT in this population. In light of recent data from a meta-analysis suggesting that rosiglitazone may increase cardiovascular risk [58], there does not appear to be a role for TZDs in the treatment of HIV-associated lipohypertrophy.
Testosterone
In a study of 88 HIV-infected men with abdominal obesity randomized to receive testosterone gel or placebo, the testosterone group experienced reductions in total body subcutaneous fat, but did not show any statistically significant reduction in visceral abdominal fat [59].
Leptin
One small study looking at leptin replacement in HIV-infected patients with lipoatrophy found a decrease in truncal fat by 14.6% though no significant change in VAT [43]. In a more recent study of 8 patients with lipoatrophy, hypoleptinemia and insulin resistance, recombinant human leptin decreased visceral fat by 30% with no exacerbation of lipoatrophy [44]. Although these patients were selected for the presence of lipoatrophy, average values for VAT at baseline were comparable to those seen in other studies targeting patients with central fat accumulation. While these positive findings are worthy of further investigation, we are not aware of any ongoing trials of leptin replacement that will definitively assess its role as a potential treatment for lipohypertrophy.
Recombinant human growth hormone
Based on anecdotal observations of improvements of lipohypertrophy in patients receiving recombinant human growth hormone (rhGH) for AIDS-related wasting and the subsequent observation of a relative GH deficiency in HIV-infected patients with fat redistribution, the GH axis has become a target of pharmacologic strategies to reduce visceral fat. In the largest randomized controlled trial, supraphysiologic doses of recombinant human growth hormone (rhGH) at 4mg daily reduced visceral abdominal fat by approximately 20% and improved lipid profiles [45]. However, this relatively high dose of rhGH worsened insulin sensitivity and caused significant side effects of arthralgias, peripheral edema, and carpal tunnel syndrome. Studies of lower rhGH doses [46,47], including physiologic dosing to achieve IGF-1 levels in the upper range of normal [48], have been less robust in reducing VAT but are better tolerated and have modest if any effects on insulin sensitivity. Unfortunately the effects of rhGH wane after treatment discontinuation, with body composition returning to baseline at 12 weeks in several studies. Drug development of rhGH for lipohypertrophy is no longer being pursued.
Recombinant human growth hormone releasing hormone
Tesamorelin, an analogue of growth hormone-releasing hormone, augments endogeneous GH pulsatility with preservation of the negative feedback inhibition by IGF-1 (Figure). In an initial phase III trial, 412 ARV-treated patients with excess abdominal fat were randomized to tesamorelin at 2mg daily vs. placebo for 26 weeks. At the end of the study, visceral fat decreased by 15% in the treatment group with only a marginal reduction in abdominal SAT and limb fat. Triglyceride levels also declined on tesamorelin, and glucose metabolism was not adversely affected [49]. A second large Phase III study randomized 404 patients to tesamorelin or placebo during a 6 month efficacy phase. In a second 6 month extension phase, 265 patients were re-randomized to continue on tesamorelin or switch to placebo. Patients who received tesamorelin for the entire study period of 12 months demonstrated an overall 18% reduction of VAT. Those who switched from tesamorelin to placebo, however, experienced a reaccumulation of VAT to baseline levels [••50], indicating that treatment effects are not sustained after discontinuation of the medication.
Figure.
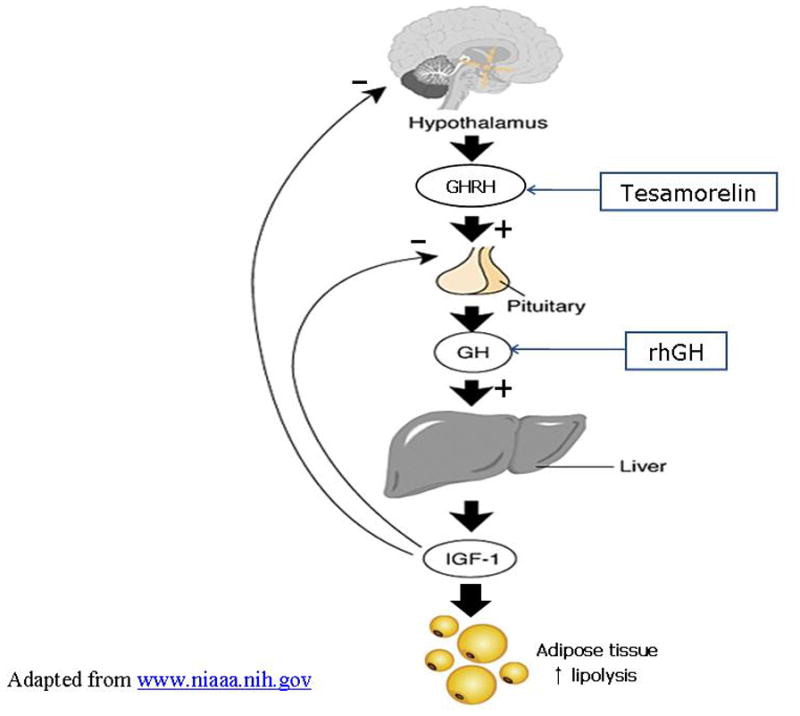
Sites of pharmacologic intervention for HIV lipohypertrophy in the GH/GHRH axis. Tesamorelin preserves the negative feedback of IGF-1 on the pituitary.
Potential limitations of GH or GHRH therapies include cost and uncertainty regarding optimal dosing and duration of treatment. Long-term safety has also not been established. In the first Falutz study, 49% of the patients who received the drug developed IgG antibodies against tesamorelin, and a hypersensitivity reaction was seen in 6 patients, all of whom tested positive for IgG antibodies against tesamorelin [49]. The consequences of antibody development against tesamorelin are unknown, and it is unclear if antibody production is responsible for the predisposition to atopy observed in the tesamorelin group. Other theoretical concerns about the long-term administration of GH or GHRH analogues include increased risk of pituitary neoplasms or other cancers through excessive IGF-1 stimulation.
In June 2010, an FDA advisory committee voted unanimously for the approval of tesamorelin for the treatment of excess abdominal fat in HIV lipodystrophy, and an official decision from the FDA is expected in the latter half of 2010.
IGF-1/IGFBP-3
In patients with pituitary GH deficiency, treatment with recombinant IGF-1 has been shown to increase lean muscle and reduce body fat [60]. In a recent pilot study 13 HIV-infected men with excess central fat and insulin resistance were treated with IGF-1 complexed to its major binding protein, IGF-binding protein-3 (IGFBP-3) to prolong its half-life. After 3 months of treatment, glucose tolerance and peripheral insulin sensitivity improved but visceral adiposity remained unchanged [61].
Surgical interventions
Surgical procedures for fat deposition in the head and neck using liposuction techniques may help some patients. A small case series reported efficacy for dorsocervical fat pad liposuction, though 3 of 10 patients developed a partial late recurrence at 1 year [62].
Conclusions
The pathogenesis of HIV-related lipohypertrophy has been attributed to several complex processes, but ultimately is most likely multi-factorial in nature. Ongoing research may yield important insights into visceral obesity and the metabolic syndrome in the general population. Despite limited understanding of the mechanisms underlying lipohypertrophy, investigators have made advances in treatment strategies for this complication. A reasonable treatment approach to lipohypertrophy is to initiate standard methods of weight reduction through diet and exercise. In patients with concomitant type 2 diabetes, metformin can be considered if there is minimal, if any, lipoatrophy. Of the potential treatments on the horizon, tesamorelin appears to be the most promising though its optimal use requires further study.
Acknowledgments
This work was supported in part by the National Institute of Allergy and Infectious Diseases (K24 AI 78884). The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Footnotes
Disclosure Statement:
MJG has served as an ad hoc consultant to Bristol-Myers Squibb and Pfizer, has received research support from Pfizer, and has received study drug donation from Merck Serono and GlaxoSmithKline.
References
- 1.Torriani M, Hadigan C, Jensen ME, Grinspoon S. Psoas muscle attenuation measurement with computed tomography indicates intramuscular fat accumulation in patients with the HIV-lipodystrophy syndrome. J Appl Physiol. 2003;95:1005–1010. doi: 10.1152/japplphysiol.00366.2003. [DOI] [PubMed] [Google Scholar]
- 2.Lo J, Abbara S, Rocha-Filho JA, et al. Increased epicardial adipose tissue volume in HIV-infected men and relationships to body composition and metabolic parameters. AIDS. 2010;24(13):2127–30. doi: 10.1097/QAD.0b013e32833c055a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blanch J, Rousaud A, Martinez E, et al. Factors associated with severe impact of lipodystrophy on the quality of life of patients infected with HIV-1. Clin Infect Dis. 2004;38:1464–70. doi: 10.1086/383573. [DOI] [PubMed] [Google Scholar]
- 4.Grunfeld C, Rimland D, Gibert CL, et al. Association of Upper Trunk and Visceral Adipose Tissue Volume With Insulin Resistance in Control and HIV-Infected Subjects in the FRAM Study. J Acquir Immune Defic Syndr. 2007;46(3):283–290. doi: 10.1097/qai.0b013e31814b94e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wohl D, Scherzer R, Heymsfield S, et al. The association of regional adipose tissue with lipid and lipoprotein levels in HIV-infected men. J Acquir Immune Defic Syndr. 2008;48(1):44–52. doi: 10.1097/QAI.0b013e31816d9ba1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ford ES. Risks for all-cause mortality, cardiovascular disease, and diabetes associated with the metabolic syndrome: a summary of the evidence. Diabetes Care. 2005;28(7):1769–78. doi: 10.2337/diacare.28.7.1769. [DOI] [PubMed] [Google Scholar]
- 7.Galassi A, Reynolds K, He J. Metabolic syndrome and risk of cardiovascular disease: a meta-analysis. Am J Med. 2006;119(10):812–9. doi: 10.1016/j.amjmed.2006.02.031. [DOI] [PubMed] [Google Scholar]
- 8.Gami AS, Witt BJ, Howard DE, et al. Metabolic syndrome and risk of incident cardiovascular events and death: a systematic review and meta-analysis of longitudinal studies. J Am Coll Cardiol. 2007;49(4):403–14. doi: 10.1016/j.jacc.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 9.Yusuf S, Hawken S, Ounpuu S, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case control study. Lancet. 2004;364:937–952. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 10•.Guaraldi G, Stentarelli C, Zona S, et al. Lipodystrophy and anti-retroviral therapy as predictors of sub-clinical atherosclerosis in human immunodeficiency virus infected subjects. Atherosclerosis. 2010;208(1):222–7. doi: 10.1016/j.atherosclerosis.2009.06.011. An important study providing evidence for the link between HIV associated lipohypertrophy and CVD risk, as suggested by the presence of coronary artery calcium. [DOI] [PubMed] [Google Scholar]
- 11.Lichtenstein KA. Redefining lipodystrophy syndrome: risks and impact on clinical decision making. J Acquir Immune Defic Syndr. 2005;39:395–400. doi: 10.1097/01.qai.0000167478.28051.3a. [DOI] [PubMed] [Google Scholar]
- 12.Dubé MP, Komarow L, Mulligan K, et al. Long-term body fat outcomes in antiretroviral-naive participants randomized to nelfinavir or efavirenz or both plus dual nucleosides. Dual X-ray absorptiometry results from A5005s, a substudy of Adult Clinical Trials Group 384. J Acquir Immune Defic Syndr. 2007;45(5):508–14. doi: 10.1097/QAI.0b013e3181142d26. [DOI] [PubMed] [Google Scholar]
- 13.Shikuma CM, Yang Y, Glesby MJ, et al. Metabolic effects of protease inhibitor-sparing antiretroviral regimens given as initial treatment of HIV-1 Infection (AIDS Clinical Trials Group Study A5095) J Acquir Immune Defic Syndr. 2007;44(5):540–50. doi: 10.1097/QAI.0b013e318031d5a0. [DOI] [PubMed] [Google Scholar]
- 14.Brinkman K, Smeitink JA, Romijn JA, Reiss P. Mitochondrial toxicity induced by nucleoside-analogue reverse-transcriptase inhibitors is a key factor in the pathogenesis of antiretroviral-therapy-related lipodystrophy. Lancet. 1999;354(9184):1112–5. doi: 10.1016/S0140-6736(99)06102-4. [DOI] [PubMed] [Google Scholar]
- 15.Shikuma CM, Hu N, Milne C. Mitochondrial DNA decrease in subcutaneous adipose tissue of HIV-infected individuals with peripheral lipoatrophy. AIDS. 2001;15(14):1801–9. doi: 10.1097/00002030-200109280-00009. [DOI] [PubMed] [Google Scholar]
- 16.Kakuda T, Brundage R, Anderson P, et al. Nucleoside reverse transcriptase inhibitor-induced mitochondrial toxicity: an etiology for lipodystrophy. AIDS. 1999;13:2311. doi: 10.1097/00002030-199911120-00019. [DOI] [PubMed] [Google Scholar]
- 17.Kratz M, Purnell JQ, Breen PA, et al. Reduced Adipogenic Gene Expression in Thigh Adipose Tissue Precedes Human Immunodeficiency Virus-Associated Lipoatrophy. J Clin Endocrinol Metab. 2008;93(3):959–966. doi: 10.1210/jc.2007-0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sekhar RV, Jahoor F, White AC, Pownall HJ, Visnegarwala F, Rodriguez-Barradas MC, Sharma M, Reeds PJ, Balasubramanyam A. Metabolic basis of HIV-lipodystrophy syndrome. Am J Physiol Endocrinol Metab. 2002;283(2):E332–7. doi: 10.1152/ajpendo.00058.2002. [DOI] [PubMed] [Google Scholar]
- 19.Sekhar RV, Jahoor F, Pownall HJ, et al. Severely dysregulated disposal of postprandial triacylglycerols exacerbates hypertriacylglycerolemia in HIV lipodystrophy syndrome. Am J Clin Nutr. 2005;81(6):1405–10. doi: 10.1093/ajcn/81.6.1405. [DOI] [PubMed] [Google Scholar]
- 20.Van Harmelen V, Lonnqvist F, Thorne A, et al. Noradrenaline-induced lipolysis in isolated mesenteric, omental and subcutaneous adipocytes from obese subjects. Int J Obesity Rel Metab Dis. 1997;21:972–979. doi: 10.1038/sj.ijo.0800504. [DOI] [PubMed] [Google Scholar]
- 21.Kino T, Gragerov A, Kopp JB, et al. The HIV-1 virion-associated protein vpr is a coactivator of the human glucocorticoid receptor. J Exp Med. 1993;189:51–62. doi: 10.1084/jem.189.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arita Y, Kihara S, Ouchi N, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257(1):79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- 23.Weyer C, Funahashi T, Tanaka S, et al. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab. 2001;86(5):1930–5. doi: 10.1210/jcem.86.5.7463. [DOI] [PubMed] [Google Scholar]
- 24.Addy C, Gavrila A, Tsiodras S, et al. Hypoadiponectinemia is associated with insulin resistance, hypertriglyceridemia, and fat redistribution in human immunodeficiency virus-infected patients treated with highly active antiretroviral therapy. J Clin Endocrinol Metab. 2003;88(2):627–636. doi: 10.1210/jc.2002-020795. [DOI] [PubMed] [Google Scholar]
- 25.Fain JN, Madan JK, Hiler ML, et al. Comparison of the Release of Adipokines by Adipose Tissue, Adipose Tissue Matrix, and Adipocytes from Visceral and Subcutaneous Abdominal Adipose Tissues of Obese Humans. Endocrinology. 2004;145(5):2273– 2282. doi: 10.1210/en.2003-1336. [DOI] [PubMed] [Google Scholar]
- 26.Mantzoros CS. The role of leptin in human obesity and disease: a review of current evidence. Ann Intern Med. 1999;130:671–80. doi: 10.7326/0003-4819-130-8-199904200-00014. [DOI] [PubMed] [Google Scholar]
- 27.Nagy GS, Tsiodras S, Martin LD, et al. Human immunodeficiency virus type 1-related lipoatrophy and lipohypertrophy are associated with serum concentrations of leptin. Clin Infect Dis. 2003;36:795–802. doi: 10.1086/367859. [DOI] [PubMed] [Google Scholar]
- 28.Miller KK, Daly PA, Sentochnik D, et al. Pseudo-Cushing’s syndrome in human immunodeficiency virus infected patients. Clin Infect Dis. 1998;27:68–72. doi: 10.1086/514638. [DOI] [PubMed] [Google Scholar]
- 29.Sutinen J, Kannisto K, Korsheninnikova E, et al. In the lipodystrophy associated with highly active antiretroviral therapy, pseudo-Cushing’s syndrome is associated with increased regeneration of cortisol by 11beta-hydroxysteroid dehydrogenase type 1 in adipose tissue. Diabetologia. 2004;47(10):1668–71. doi: 10.1007/s00125-004-1508-2. [DOI] [PubMed] [Google Scholar]
- 30.Masuzaki H, Paterson J, Shinyama H, et al. A transgenic model of visceral obesity and the metabolic syndrome. Science. 2001;294:2166–2170. doi: 10.1126/science.1066285. [DOI] [PubMed] [Google Scholar]
- 31.Veldhuis JD, Liem AY, South S, et al. Differential impact of age, sex-steroid hormones, and obesity on basal versus pulsatile growth hormone secretion in men as assessed in an ultrasensitive chemiluminescence assay. J Clin Endocrinol Metab. 1995;80:3209–3222. doi: 10.1210/jcem.80.11.7593428. [DOI] [PubMed] [Google Scholar]
- 32.Ghigo E, Procopio M, Boffano GM, et al. Arginine potentiates but does not restore the blunted growth hormone response to growth hormone-releasing hormone in obesity. Metabolism. 1992;41:560–563. doi: 10.1016/0026-0495(92)90220-5. [DOI] [PubMed] [Google Scholar]
- 33.Rietschel P, Hadigan C, Corcoran C, et al. Assessment of Growth Hormone Dynamics in Human Immunodeficiency Virus- Related Lipodystrophy. J Clin Endocrinol Metab. 2001;86(2):504–10. doi: 10.1210/jcem.86.2.7175. [DOI] [PubMed] [Google Scholar]
- 34.Koutkia P, Canavan B, Breu J, Grinspoon S. Growth hormone (GH) responses to GH-releasing hormone-arginine testing in human immunodeficiency virus lipodystrophy. J Clin Endocrinol Metab. 2005;90:32–8. doi: 10.1210/jc.2004-1342. [DOI] [PubMed] [Google Scholar]
- 35.Koutkia P, Meininger G, Canavan B, et al. Metabolic regulation of growth hormone by free fatty acids, somatostatin, and ghrelin in HIV-lipodystrophy. Am J Physiol Endocrinol Metab. 2004;286(2):E296–E303. doi: 10.1152/ajpendo.00335.2003. [DOI] [PubMed] [Google Scholar]
- 36.Taskinen M, Saarinen-Pihkala UM, Hovi L, Lipsanen-Nyman M. Impaired glucose tolerance and dyslipidaemia as late effects after bone-marrow transplantation in childhood. Lancet. 2000;356(9234):993–7. doi: 10.1016/S0140-6736(00)02717-3. [DOI] [PubMed] [Google Scholar]
- 37.Annaloro C, Usardi P, Airaghi L, et al. Prevalence of metabolic syndrome in long-term survivors of hematopoietic stem cell transplantation. Bone Marrow Transplant. 2008;41(9):797–804. doi: 10.1038/sj.bmt.1705972. [DOI] [PubMed] [Google Scholar]
- 38.Thoni GJ, Fedou C, Brun JF, et al. Reduction of fat accumulation and lipid disorders by individualized light aerobic training in human immunodeficiency virus infected patients with lipodystrophy and/or dyslipidemia. Diabetes Metab. 2002;28(5):397–404. [PubMed] [Google Scholar]
- 39.Dolan SE, Frontera W, Librizzi J, et al. Effects of a supervised home-based aerobic and progressive resistance training regimen in women infected with human immunodeficiency virus: a randomized trial. Arch Intern Med. 2006;166(11):1225–31. doi: 10.1001/archinte.166.11.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hadigan C, Corcoran C, Basgoz N, et al. Metformin in the treatment of HIV lipodystrophy syndrome: a randomized controlled trial. JAMA. 2000;284(4):472–7. doi: 10.1001/jama.284.4.472. [DOI] [PubMed] [Google Scholar]
- 41.Driscoll SD, Meininger GE, Lareau MT, et al. Effects of exercise training and metformin on body composition and cardiovascular indices in HIV-infected patients. AIDS. 2004;18(3):465–73. doi: 10.1097/00002030-200402200-00013. [DOI] [PubMed] [Google Scholar]
- 42.Kohli R, Shevitz A, Gorbach S, Wanke C. A randomized placebo-controlled trial of metformin for the treatment of HIV lipodystrophy. HIV Med. 2007;8(7):420–6. doi: 10.1111/j.1468-1293.2007.00488.x. [DOI] [PubMed] [Google Scholar]
- 43.Lee JH, Chan JL, Sourlas E, et al. Recombinant Methionyl Human Leptin Therapy in Replacement Doses Improves Insulin Resistance and Metabolic Profile in Patients with Lipoatrophy and Metabolic Syndrome Induced by the Highly Active Antiretroviral Therapy. J Clin Endocrinol Metab. 2006;91(7):2605–11. doi: 10.1210/jc.2005-1545. [DOI] [PubMed] [Google Scholar]
- 44.Mulligan K, Khatami H, Schwarz JM, et al. The effects of recombinant human leptin on visceral fat, dyslipidemia, and insulin resistance in patients with HIV-associated lipoatrophy and hypoleptinemia. J Clin Endocrinol Metab. 2009;94:1137–1144. doi: 10.1210/jc.2008-1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grunfeld C, Thompson M, Brown SJ, et al. Recombinant human growth hormone to treat HIV-associated adipose redistribution syndrome: 12 week induction and 24-week maintenance therapy. J Acquir Immune Defic Syndr. 2007;45(3):286–97. doi: 10.1097/QAI.0b013e3180691145. [DOI] [PubMed] [Google Scholar]
- 46.Luzi L, Meneghini E, Oggionni S, et al. GH treatment reduces truncal adiposity in HIV-infected patients with lipodystrophy: a randomized placebo-controlled study. Eur J Endocrinol. 2005;153:781–789. doi: 10.1530/eje.1.02039. [DOI] [PubMed] [Google Scholar]
- 47.Hansen BR, Haugaard SB, Jensen FK, et al. Long-term high-physiological-dose growth hormone reduces intra-abdominal fat in HIV-infected patients with a neutral effect on glucose metabolism. HIV Med. 2010;11(4):266–75. doi: 10.1111/j.1468-1293.2009.00775.x. [DOI] [PubMed] [Google Scholar]
- 48.Lo J, You SM, Canavan B, et al. Low-dose physiological growth hormone in patients with HIV and abdominal fat accumulation: a randomized controlled trial. JAMA. 2008;300(5):509–19. doi: 10.1001/jama.300.5.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Falutz J, Allas S, Blot K, et al. Metabolic effects of a growth hormone-releasing factor in patients with HIV. N Engl J Med. 2007;357:2359–2370. doi: 10.1056/NEJMoa072375. [DOI] [PubMed] [Google Scholar]
- 50••.Falutz J, Potvin D, Mamputu JC, et al. Effects of tesamorelin, a growth hormone-releasing factor, in HIV-infected patients with abdominal fat accumulation: a randomized placebo-controlled trial with a safety extension. J Acquir Immune Defic Syndr. 2010;53(3):311–22. doi: 10.1097/QAI.0b013e3181cbdaff. Second large phase III trial showing that tesamorelin is an effective and well-tolerated treatment intervention to reduce VAT in patients with HIV associated lipohypertrophy. [DOI] [PubMed] [Google Scholar]
- 51.Drechsler H, Powderly WG. Switching effective antiretroviral therapy: a review. Clin Infect Dis. 2002;35(10):1219–30. doi: 10.1086/343050. [DOI] [PubMed] [Google Scholar]
- 52.Parker RA, Flint OP, Mulvey R, et al. Endoplasmic reticulum stress links dyslipidemia to inhibition of proteasome activity and glucose transport by HIV protease inhibitors. Mol Pharmacol. 2005;67:1909–1919. doi: 10.1124/mol.104.010165. [DOI] [PubMed] [Google Scholar]
- 53.Yan Q, Hruz PW. Direct comparison of the acute in vivo effects of HIV protease inhibitors on peripheral glucose disposal. J Acquir Immune Defic Syndr. 2005;40:398–403. doi: 10.1097/01.qai.0000176654.97392.c7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim RJ, Wilson CG, Wabitsch M, et al. HIV protease inhibitor-specific alterations in human adipocyte differentiation and metabolism. Obesity. 2006;14:994–1002. doi: 10.1038/oby.2006.114. [DOI] [PubMed] [Google Scholar]
- 55.Stanley TL, Joy T, Hadigan CM, et al. Effects of switching from lopinavir/ritonavir to atazanavir/ritonavir on muscle glucose uptake and visceral fat in HIV-infected patients. AIDS. 2009;23(11):1349–57. doi: 10.1097/QAD.0b013e32832ba904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mulligan K, Yang Y, Wininger DA, et al. Effects of metformin and rosiglitazone in HIV-infected patients with hyperinsulinemia and elevated waist/hip ratio. AIDS. 2007;21(1):47–57. doi: 10.1097/QAD.0b013e328011220e. [DOI] [PubMed] [Google Scholar]
- 57.Sheth SH, Larson RJ. The efficacy and safety of insulin-sensitizing drugs in HIV-associated lipodystrophy syndrome: a meta-analysis of randomized trials. BMC Infect Dis. 2010;10:183. doi: 10.1186/1471-2334-10-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356(24):2457–71. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- 59.Bhasin S, Parker Ra, Sattler F, et al. Effects of Testosterone Supplementation on Whole Body and Regional Fat Mass and Distribution in Human Immunodeficiency Virus-Infected Men with Abdominal Obesity. J Clin Endocrinol Metab. 2007;92(3):1049–1057. doi: 10.1210/jc.2006-2060. [DOI] [PubMed] [Google Scholar]
- 60.Mauras N, Martinez V, Rini A, Guevara-Aguirre J. Recombinant human insulin-like growth factor I has significant anabolic effects in adults with growth hormone receptor deficiency: studies on protein, glucose, and lipid metabolism. J Clin Endocrinol Metab. 2000;85:3036–3042. doi: 10.1210/jcem.85.9.6772. [DOI] [PubMed] [Google Scholar]
- 61.Rao MN, Mulligan K, Tai V, et al. Effects of Insulin-Like Growth Factor (IGF)-I/IGF-Binding Protein-3 Treatment on Glucose Metabolism and Fat Distribution in Human Immunodeficiency Virus-Infected Patients with Abdominal Obesity and Insulin Resistance. J Clin Endocrinol Metab. 2010 Jul 7; doi: 10.1210/jc.2009-2502. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hultman CS, McPhail LE, Donaldson JH, Wohl DA. Surgical Management of HIV-Associated Lipodystrophy: Role of Ultrasonic-Assisted Liposuction and Suction-Assisted Lipectomy in the Treatment of Lipohypertrophy. Annals of Plastic Surgery. 2007;58 (3):255–263. doi: 10.1097/01.sap.0000248128.33465.83. [DOI] [PubMed] [Google Scholar]


