Abstract
Oropharyngeal candidiasis is a frequent cause of morbidity in patients with defects in cell-mediated immunity or saliva production. Animal models of this infection are important for studying disease pathogenesis and evaluating vaccines and antifungal therapies. Here we describe a simple murine model of oropharyngeal candidiasis. Mice are rendered susceptible to oral infection by injection with cortisone acetate and then inoculated by placing a swab saturated with Candida albicans sublingually. This process results in a reproducible level of infection, the histopathology of which mimics that of pseudomembranous oropharyngeal candidiasis in patients. Using this model, data are obtained after 5–9 days of work.
INTRODUCTION
Fungi of the genus Candida grow on mucosal surfaces as part of the normal human flora. When either local or systemic antifungal defense mechanisms are impaired, these organisms can overgrow and cause oropharyngeal candidiasis, leading to significant morbidity in patients with HIV/AIDS 1, Sjögren’s syndrome 2, diabetes mellitus 3, and head and neck cancers 4. Although the incidence of oropharyngeal candidiasis has declined in HIV-infected patients treated with highly active antiretroviral therapy 5,6, it continues to be a significant problem in AIDS patients worldwide 7,8. Candida albicans causes at least 80% of cases of oropharyngeal candidiasis in patients with HIV/AIDS 1,9. It is also the most common cause of oropharyngeal candidiasis in patients with Sjogren’s syndrome, diabetes mellitus, and cancer of the head and neck 2–4. Azole antifungal drugs are currently the mainstay of therapy 10. However, resistance to these agents can develop in immunocompromised patients who receive prolonged therapy 1,9. Because of the high prevalence of oropharyngeal candidiasis, there is intense interest in developing new approaches to prevent and treat this disease. While in vitro models mimic some aspects of oropharyngeal candidiasis, experimental animal models are necessary to fully understand the pathogenesis of this disease and to evaluate new treatment and vaccine strategies.
Although rats have been used to study oropharyngeal candidiasis, mice are frequently preferable because of their relative ease of use, lower cost, and the greater availability of gene deletion strains. Murine models of oropharyngeal candidiasis have been used for investigating the immunology of this infection 11–14, candidal virulence factors 15–23, and the efficacy of vaccines and antifungal agents 24–26. Multiple experimental models of oropharyngeal candidiasis have been developed (reviewed in 27). The key difference among these models is the type of immunosuppression that is used to induce susceptibility to oropharyngeal candidiasis.
Immunocompetent mice are generally not colonized with C. albicans. Furthermore, oral inoculation of immunocompetent mice with most strains of C. albicans, such as the sequenced strain SC5314, typically induces only transient oropharyngeal colonization without the development of disease. However, it has been reported that a strain of C. albicans that was isolated from a patient with oropharyngeal candidiasis could colonize the oropharynx of estrogen treated mice that did not received additional immunosuppression 26. Nevertheless, the oral fungal burden of these mice was relatively low and the oral isolate did not induce the typical pseudomembranous oropharyngeal candidiasis that is seen in patients.
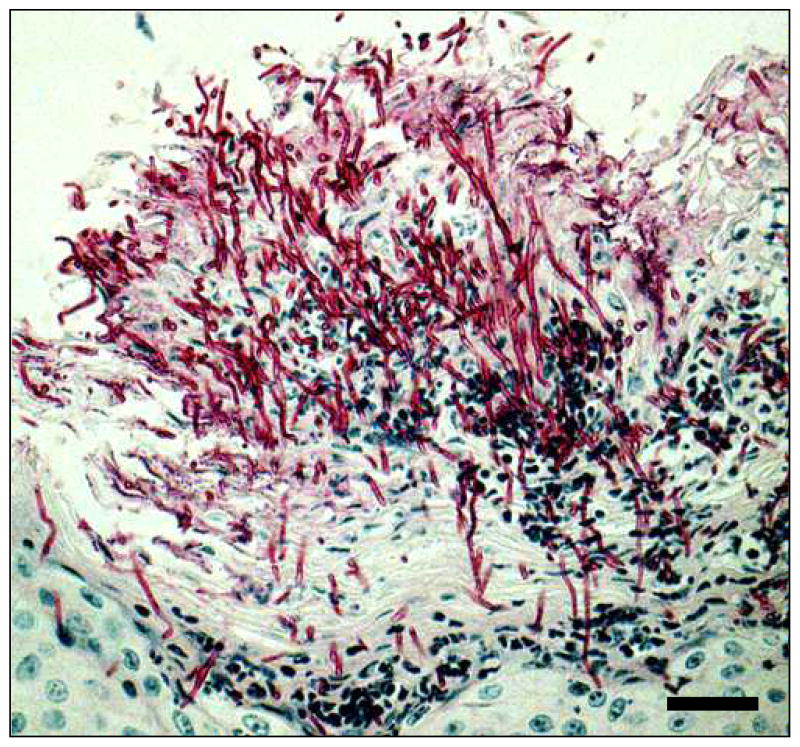
The simplest approach to induce susceptibility to oropharyngeal candidiasis is to treat mice with corticosteroids 19,23,25. The advantages of this approach are that it can be used with multiple different strains of mice, immunosuppression is transient so special housing for the mice is not required, and the histopathology of the lesions mimics those of patients with pseudomembranous oropharyngeal candidiasis (Fig. 1). Also, the use of corticosteroids is medically relevant because patients who use inhaled corticosteroids for asthma are at increased risk of developing oropharyngeal candidiasis 28.
Figure 1.
Histopathology of a typical lesion in the mouse model of oropharyngeal candidiasis after 5 days of infection. The tissue section was stained with periodic acid-Schiff. Scale bar indicates 20 μm.
Another approach is to use mutant strains of mice with defects in cell-mediated immunity. Mouse strains that are susceptible to oropharyngeal candidiasis include those with defects in T cells alone, such as athymic (nu/nu) mice 29, and those with combined natural killer and T cell defects, such as beige-athymic (bg/bg-nu/nu) mice 30 and Tgε26 mice 22. Another mouse strain that is highly susceptible to oropharyngeal candidiasis is the CD4C/HIV(MutA) transgenic strain that expresses human immunodeficiency virus type 1 in immune cells 31. The immune defects in this mouse strain mimics those found in AIDS patients. Finally, it has recently been discovered that the Th17 immune response is critical for host defense against oropharyngeal candidiasis 11. Th17 deficient mice that lack either IL-23p19 or the IL-17 receptor are susceptible to oropharyngeal candidiasis without additional immunosuppression 11. While the use of these mutant strains obviates the need for administration of corticosteroids, these mice are considerably more expensive than most other mouse strains, frequently have limited availability, and may require special housing.
Experimental design
Immunosuppression
The mice are rendered susceptible to oropharyngeal candidiasis by subcutaneous administration of cortisone acetate, which is administered every other day, starting at day -1 relative to infection. Oropharyngeal candidiasis persists as long as the cortisone acetate is administered and resolves after it is stopped. The extent of disease can be adjusted by changing the dose of cortisone acetate. We have not observed disseminated infection in mice that have received cortisone acetate at doses of up to 200 mg kg−1.
Inoculation
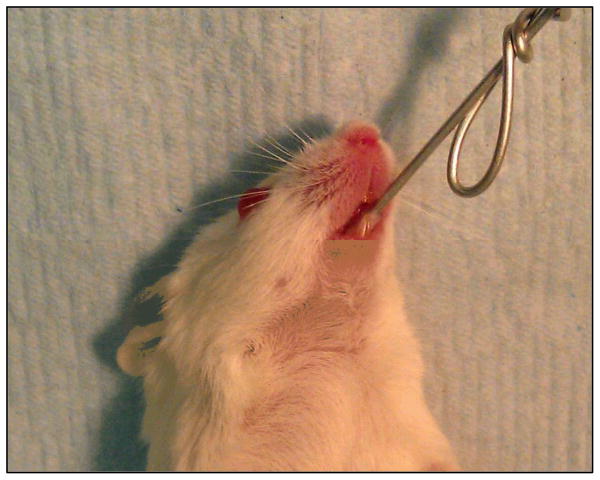
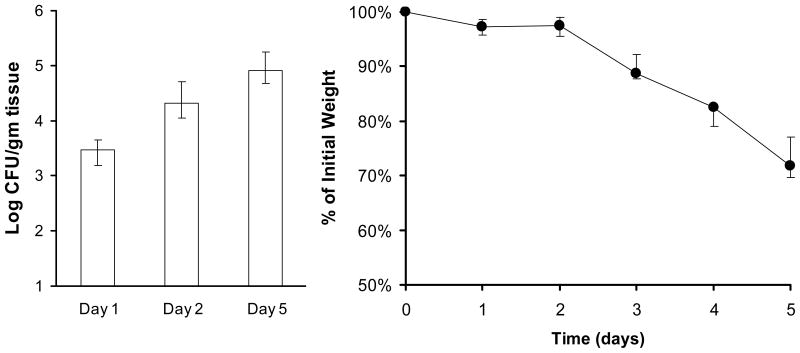
The mice are inoculated by placing a calcium alginate swab saturated with C. albicans blastospores sublingually for 75 min (Fig. 2). The concentration of organisms in the suspension is not critical because the course of the infection appears to go through a bottleneck in which only a low number of organisms are present in the oropharynx on day 1 post-inoculation. The relatively few organisms present in the oropharynx on day 1 subsequently proliferate to high numbers and induce disease over the ensuing days (Fig. 3).
Figure 2.
Image of an anesthetized mouse with a calcium alginate swab placed sublingually.
Figure 3.
Time course of oropharyngeal candidiasis. (A) Oral fungal burden of mice with oropharyngeal candidiasis. (B) Effects of infection on body weight. Results are the median ± interquartile range of 8–9 mice per time point.
In order to obtain a reproducibly high oral fungal burden, it is necessary to suspend the C. albicans cells in Hanks balanced salt solution (HBSS) and maintain the suspension at 30°C while it is being used to saturate the calcium alginate swabs. If the swabs are saturated with organisms that are suspended in a glucose-free medium such as phosphate buffered saline or if the suspension is kept on ice, then the swabs need to kept sublingually for at least 90 min to consistently induce infection.
Anesthesia
The most difficult step in the inoculation procedure is keeping the mice adequately sedated while avoiding anesthesia-related mortality. Mice that are sedated for a prolonged period of time will develop hypothermia if they are kept at room temperature. Thus, it is important to maintain the animals on a heating pad until they recover from anesthesia. We use a mixture of ketamine and xylazine to induce sedation and then administer repeated doses of ketamine to maintain sedation for the required time. Even with inbred mouse strains there is significant mouse-to-mouse variability in the response to ketamine. Thus, each mouse needs to monitored individually to determine when an additional dose of ketamine needs to be administered. Signs that a mouse is waking up and needs an additional dose of ketamine include movement of the extremities and blinking of the eyes. We usually administer ketamine at 110mg kg−1 as the first and second maintenance doses (if needed) and then decrease this dose by 50% after 55 to 65 min. We do not give any additional ketamine after 65 min, but instead gently restrain any awakening mice for the remaining 10 min.
MATERIALS
REAGENTS
BALB/c mouse (18–25 g) (Taconic Farms, cat. no. Balb-M) !CAUTION Experiments must comply with national and institutional regulations concerning the use of animals for research purposes.
Yeast extract, Bacto (Fisher Scientific, cat. no. DF0127-17-9)
Peptone, Fine powder (Fisher Scientific, cat. no. DF0118-17-0)
Dextrose (D-Glucose) Anhydrous (Fisher Scientific, cat. no. D16)
Cortisone acetate (Sigma-Aldrich, cat. no. C3130)
Tween 80 (Sigma-Aldrich, cat. no. P8074)
Ketamine (Western Medical Supplies, cat. no. 4165)
Xylazine (Western Medical Supplies, cat. no. 5533)
1X PBS, without calcium and magnesium (Fisher Scientific, cat. no. 21-031-CV)
1X HBSS, with calcium and magnesium (Fisher Scientific, cat. no. 21-023-CV)
Sodium chloride (Sigma-Aldrich, cat. no. S271)
Sabouraud dextrose agar (Fisher Scientific, cat. no. B11584)
Chloramphenicol (MP Biomedicals, cat. no. 02190321)
Ethanol (Fisher Scientific, cat. no. 04-355-451)
EQUIPMENT
Shaking incubator
Centrifuge
Heated water bath with temperature control
Isothermal Pads (Braintree Scientific, cat. no. 39DP)
Dry bath
Lamp (Lamps Plus, cat. no. 15358)
Stainless steel tray (13.5 × 9.75 in) (Harvard Apparatus, cat. no. 610213)
1 ml safety syringe with a 5/8 inch, 25 G needle (Fisher Scientific, cat. no. 05-561-29)
1 ml safety syringe with a ½ inch, 29 G needle (Fisher Scientific, cat. no. 05-561-25)
Hemacytometer (Fisher Scientific, cat. no.0267110)
Sterile calcium alginate tipped applicator (Fisher Scientific, cat. no. 22-029-500)
Polypropylene culture test tube, 17 × 100 mm (Fisher Scientific, cat. no. 14-956-1J)
Timer
50 ml conical centrifuge tubes (VWR, cat. no. 89039-656)
Glass borosilicate culture tubes (Fisher Scientific, cat. no. 14-961-32)
Balance
Petri dishes (Fisher Scientific, cat. no. 08-757-13)
Dissecting scissor (Fisher Scientific, cat. no. 08940)
Brown-Adson thumb forcep (Fisher Scientific, cat. no. 3120024)
Pro250 homogenizer (Pro Scientific, cat. no. 01-01250)
10mm × 105mm generator (Pro Scientific, cat. no. 02-10105)
REAGENT SETUP
YPD Broth
Dissolve 5g yeast extract, 10g peptone, and 10g dextrose in 500ml of distilled water. Mix thoroughly and autoclave. Store at room temperature.
PBS 0.05% Tween 80
Prepare a solution of PBS containing 0.05% (v/v) Tween 80. Filter sterilize and store at room temperature.
Anesthetics
Combined ketamine and xylazine in sterile PBS so that the final concentration of ketamine is 10mg ml−1 and xylazine is 1mg ml−1. For initiation of anesthesia, administer 0.1 ml of this mixture per 10 g of body wt intraperitoneally. Dilute additional ketamine in sterile PBS so that 110 mg kg−1 can be administered in a total volume of 0.2 ml (e.g., 11 mg ml−1 for a 20 g mouse) The anesthetics are not stable for a long period of time and should be prepared the day of infection.
0.85% Saline
Dissolve 8.5g of sodium chloride in 1L of distilled water. Aliquot 5ml of the solution into 50ml conical tubes and 9ml into glass borosilicate culture tubes. Autoclave the tubes and store at 4°C until needed.
Sabouraud dextrose agar
Dissolve 65g of Sabouraud dextrose agar in 1L of distilled water. Autoclave. Then add chloramphenicol at 75mg L−1. Store at room temperature until needed.
PROCEDURE
-
1|
Three days before infection Inoculate a colony of the C. albicans strain into 10ml of YPD broth and incubate in a 30°C shaker. The next day, transfer 100μl of the overnight culture to 10ml fresh YPD broth and incubate in a 30°C shaker overnight. Repeat this step one more time.
-
2|
The day before infection Weigh the animals. Use their average weight to calculate the dose of cortisone acetate, which should be administered at a concentration of 225mg kg−1 in a total volume of 0.2ml. Suspend the cortisone acetate in sterile PBS containing 0.05% Tween 80 (v/v). The cortisone acetate will not dissolve. To make a fine suspension, either vortex the mixture vigorously or sonicate it for 1 minute (at setting 3 on a Branson Sonicator). Store aliquots of the suspension at −80°C.
-
3|
nject the animal with 0.2ml of cortisone acetate subcutaneously in the dorsum of the neck on days -1, 1, and 3 relative to infection. Use a 1ml syringe with a 5/8 inch 25 G needle.
▲ CRITICAL STEP Because the cortisone acetate forms a suspension and does not dissolve, vortex it frequently between injections. Also, because there is some lot-to-lot variation in the potency of cortisone acetate, use the same lot for a given series of experiments.
-
4|
lace the isothermal pads in a 60°C water bath overnight.
-
5|
The day of infection Reduce the water bath temperature to 37°C. Let the isothermal pads sit for 1 hour at 37°C prior to use to avoid overheating the mice.
-
6|
dd 1ml of the YPD overnight culture to 9ml of sterile PBS.
-
7|
entrifuge at 1000 g for 5 minutes.
-
8|
ecant the supernatant. Resuspend pellet in 10ml of sterile PBS.
-
9|
epeat step 7.
-
10|
Decant the supernatant. Resuspend pellet in 10ml of sterile HBSS. Vortex vigorously.
-
11|
Dilute an aliquot of the fungal suspension 1:100 in sterile PBS and count the number of organisms with a hemacytometer.
-
12|
Make up a suspension of 1×106 organisms ml−1 in 5ml of sterile HBSS in the 17×100mm size polypropylene tube.
-
13|
Warm C. albicans suspension to 30°C in a dry block and place the calcium alginate swabs in the suspension approximately 5 min before they are used to inoculate the mice.
-
14|
Remove the isothermal pads from the water bath.
▲ CRITICAL STEP The isothermal pads are critical for keeping the mice warm and preventing hypothermia while the animals are sedated.
-
15|
Place two isothermal pads within the stainless steel pan and cover with a paper towel.
-
16|
Using a 1ml syringe with ½ inch 29 gauge needle, Inject each mouse intraperitoneally with the ketamine/xylazine mixture, administering 0.1ml per 10gm of body weight. This dosage will induce 20–30 min of anesthesia.
-
17|
After the animal is anesthetized, place a saturated calcium alginate swab sublingually for 75 minutes. Place the animal in the supine position on the isothermal pad (Fig. 2).
?TROUBLESHOOTING
-
18|
Monitor each mouse and administer additional doses of ketamine (without xylazine) as necessary to maintain sedation. Although the duration of anesthesia varies with different strains of mice, most will require two additional doses of ketamine to maintain anesthesia for the full 75 min.
▲ CRITICAL STEP Do not administer additional doses of xylazine to maintain anesthesia because it has a longer half-life than ketamine and there is a risk of overdose if multiple doses of xylazine are given.
?TROUBLESHOOTING
-
19|
Five days post infection Set the water-bath temperature to 50°C. Melt the Sabouraud dextrose agar and let the agar sit at 50°C until needed.
-
20|
Administer the anesthetic mixture as described in step 16. Once the animal is anesthetized, euthanize by cervical dislocation.
● TIMING The mice can be sacrificed as early as 1 day post infection to study the initiation of infection.
-
21|
Using the dissecting scissors and forceps, excise the tongue and attached oral tissues and place on a pre-labeled Petri dish. Keep on ice until the tissue weight is recorded.
-
22|
Note It is frequently useful to examine the extent of infection by histopathologic analysis. To obtain tissue for histopathology, use a scalpel or razor blade to cut the tongue into two pieces with a longitudinal incision in the sagittal plane. Fix one half of the tongue in zinc-buffered formalin for 4 h at room temperature and process the other half for quantitative culture as described below. Store the fixed tissue in 80% ethanol prior to processing for histopathology.
-
23|
Weigh each tissue sample and record weight.
-
24|
Transfer each tissue sample to a 50ml conical tube containing 5ml of 0.85% saline and place the tube on ice.
-
25|
Homogenize the sample for approximately 8–10 sec. with the homogenizer with the speed control set at 3. If the tissue is not completely homogenized, cool the tube on ice and then repeat homogenization for an additional 8–10 sec.
-
26|
Remove the Sabouraud dextrose agar from the water-bath and place the bottle under cold running water. The agar should be cooled to a comfortable temperature when held against the skin.
-
27|
Prepare serial 10-fold dilutions of the homogenate by adding 1 ml of the homogenate to 9 ml of 0.85% sterile saline.
-
28|
Plate undiluted homogenate and each of the serial dilutions in Sabouraud dextrose agar by combining 1ml of the sample with 12 ml of molten Sabouraud dextrose agar in a Petri dish. Gently swirl the dish to mix.
-
29|
Incubate the Petri dishes at 35°C overnight.
-
30|
The next day, count the colony forming units.
?TROUBLESHOOTING
Steps 17 and 18
As emphasized above, maintaining adequate anesthesia while avoiding anesthesia-related mortality is critical. Occasionally a mouse will not be adequately sedated after receiving the ketamine/xylazine mixture. If a mouse remains awake 10 min after the first injection, administer 0.2 ml of the ketamine maintenance solution.
If the initial sedation is successful, but the mice awaken too quickly (within 10 min or less), make sure that the temperature of the procedure room is at least 20°C. Alternatively, place a heat lamp over the mice to provide additional warming.
If the mice either die during anesthesia or remain sedated for a prolonged time after the 75 min inoculation period, make sure that they are being kept at the correct temperature and are neither becoming hypothermic nor hyperthermic. In addition, try reducing the maintenance dose of ketamine, either by increasing the time interval between doses or reducing the dose that is administered.
ANTICIPATED RESULTS
After 5 days of infection, the oral fungal burden of mice infected with C. albicans SC5314 is typically 105–106 CFU per gram of tissue and the mice exhibit significant weight loss (Fig. 3), but no mortality. Because of the magnitude of this weight loss, the experiment should be terminated at this time point to prevent unnecessary suffering. Reducing the dose of cortisone acetate may decrease the severity of disease and enable the experiment to be carried out for a longer period of time. This animal model can be used to analyze the virulence of C. albicans mutants 16,17,32, efficacy of antifungal agents 25, and host response to mucosal infection 11. The use of 7 to 8 mice per experimental group usually provides sufficient power to detect a biologically significant 10-fold difference in oral fungal burden.
Acknowledgments
This work was supported by grant R01DE017088 and contract No. N01-AI-30041 from the National Institutes of Health, USA.
References
- 1.Sangeorzan JA, et al. Epidemiology of oral candidiasis in HIV-infected patients: colonization, infection, treatment, and emergence of fluconazole resistance. Am J Med. 1994;97:339–346. doi: 10.1016/0002-9343(94)90300-x. [DOI] [PubMed] [Google Scholar]
- 2.Rhodus NL, Bloomquist C, Liljemark W, Bereuter J. Prevalence, density, and manifestations of oral Candida albicans in patients with Sjogren’s syndrome. J Otolaryngol. 1997;26:300–305. [PubMed] [Google Scholar]
- 3.Willis AM, et al. Oral candidal carriage and infection in insulin-treated diabetic patients. Diabet Med. 1999;16:675–679. doi: 10.1046/j.1464-5491.1999.00134.x. [DOI] [PubMed] [Google Scholar]
- 4.Redding SW, et al. Epidemiology of oropharyngeal Candida colonization and infection in patients receiving radiation for head and neck cancer. J Clin Microbiol. 1999;37:3896–3900. doi: 10.1128/jcm.37.12.3896-3900.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arribas JR, et al. Impact of protease inhibitor therapy on HIV-related oropharyngeal candidiasis. Aids. 2000;14:979–985. doi: 10.1097/00002030-200005260-00009. [DOI] [PubMed] [Google Scholar]
- 6.Martins MD, Lozano-Chiu M, Rex JH. Declining rates of oropharyngeal candidiasis and carriage of Candida albicans associated with trends toward reduced rates of carriage of fluconazole-resistant C. albicans in human immunodeficiency virus-infected patients. Clin Infect Dis. 1998;27:1291–1294. doi: 10.1086/515006. [DOI] [PubMed] [Google Scholar]
- 7.Kerdpon D, et al. Oral manifestations of HIV infection in relation to clinical and CD4 immunological status in northern and southern Thai patients. Oral Dis. 2004;10:138–144. doi: 10.1046/j.1601-0825.2003.00990.x. [DOI] [PubMed] [Google Scholar]
- 8.Chidzonga MM. HIV/AIDS orofacial lesions in 156 Zimbabwean patients at referral oral and maxillofacial surgical clinics. Oral Dis. 2003;9:317–322. doi: 10.1034/j.1601-0825.2003.00962.x. [DOI] [PubMed] [Google Scholar]
- 9.Revankar SG, et al. Clinical evaluation and microbiology of oropharyngeal infection due to fluconazole-resistant Candida in human immunodeficiency virus-infected patients. Clin Infect Dis. 1998;26:960–963. doi: 10.1086/513950. [DOI] [PubMed] [Google Scholar]
- 10.Pappas PG, et al. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;48:503–535. doi: 10.1086/596757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Conti HR, et al. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J Exp Med. 2009;206:299–311. doi: 10.1084/jem.20081463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saunus JM, et al. Early activation of the interleukin-23-17 axis in a murine model of oropharyngeal candidiasis. Mol Oral Microbiol. 2010;25:343–356. doi: 10.1111/j.2041-1014.2010.00570.x. [DOI] [PubMed] [Google Scholar]
- 13.Ho AW, et al. IL-17RC is required for immune signaling via an extended SEF/IL-17R signaling domain in the cytoplasmic tail. J Immunol. 2010;185:1063–1070. doi: 10.4049/jimmunol.0903739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nett JE, Marchillo K, Spiegel CA, Andes DR. Development and validation of an in vivo Candida albicans biofilm denture model. Infect Immun. 2010;78:3650–3659. doi: 10.1128/IAI.00480-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun JN, et al. Host cell invasion and virulence mediated by Candida albicans Ssa1. PLoS Pathog. 2010;6:e1001181. doi: 10.1371/journal.ppat.1001181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chiang LY, et al. Candida albicans protein kinase CK2 governs virulence during oropharyngeal candidiasis. Cell Microbiol. 2007;9:233–245. doi: 10.1111/j.1462-5822.2006.00784.x. [DOI] [PubMed] [Google Scholar]
- 17.Park H, et al. Role of the fungal Ras-protein kinase A pathway in governing epithelial cell interactions during oropharyngeal candidiasis. Cell Microbiol. 2005;7:499–510. doi: 10.1111/j.1462-5822.2004.00476.x. [DOI] [PubMed] [Google Scholar]
- 18.Kamai Y, Kubota M, Hosokawa T, Fukuoka T, Filler SG. Contribution of Candida albicans ALS1 to the pathogenesis of experimental oropharyngeal candidiasis. Infect Immun. 2002;70:5256–5258. doi: 10.1128/IAI.70.9.5256-5258.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dwivedi P, et al. Role of Bcr1-activated genes Hwp1 and Hyr1 in Candida albicans oral mucosal biofilms and neutrophil evasion. PLoS ONE. 2011;6:e16218. doi: 10.1371/journal.pone.0016218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hao B, et al. Candida albicans RFX2 encodes a DNA binding protein involved in DNA damage responses, morphogenesis, and virulence. Eukaryot Cell. 2009;8:627–639. doi: 10.1128/EC.00246-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marquis M, et al. CD8+ T cells but not polymorphonuclear leukocytes are required to limit chronic oral carriage of Candida albicans in transgenic mice expressing human immunodeficiency virus type 1. Infect Immun. 2006;74:2382–2391. doi: 10.1128/IAI.74.4.2382-2391.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sundstrom P, Balish E, Allen CM. Essential role of the Candida albicans transglutaminase substrate, hyphal wall protein 1, in lethal oroesophageal candidiasis in immunodeficient mice. J Infect Dis. 2002;185:521–530. doi: 10.1086/338836. [DOI] [PubMed] [Google Scholar]
- 23.Wolf JM, Johnson DJ, Chmielewski D, Davis DA. The Candida albicans ESCRT pathway makes Rim101-dependent and -independent contributions to pathogenesis. Eukaryot Cell. 2010;9:1203–1215. doi: 10.1128/EC.00056-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spellberg BJ, et al. Efficacy of the anti-Candida rAls3p-N or rAls1p-N vaccines against disseminated and mucosal candidiasis. J Infect Dis. 2006;194:256–260. doi: 10.1086/504691. [DOI] [PubMed] [Google Scholar]
- 25.Kamai Y, Kubota M, Hosokawa T, Fukuoka T, Filler SG. New model of oropharyngeal candidiasis in mice. Antimicrob Agents Chemother. 2001;45:3195–3197. doi: 10.1128/AAC.45.11.3195-3197.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rahman D, Mistry M, Thavaraj S, Challacombe SJ, Naglik JR. Murine model of concurrent oral and vaginal Candida albicans colonization to study epithelial host-pathogen interactions. Microbes Infect. 2007;9:615–622. doi: 10.1016/j.micinf.2007.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Naglik JR, Fidel PL, Jr, Odds FC. Animal models of mucosal Candida infection. FEMS Microbiol Lett. 2008;283:129–139. doi: 10.1111/j.1574-6968.2008.01160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rachelefsky GS, Liao Y, Faruqi R. Impact of inhaled corticosteroid-induced oropharyngeal adverse events: results from a meta-analysis. Ann Allergy Asthma Immunol. 2007;98:225–238. doi: 10.1016/S1081-1206(10)60711-9. [DOI] [PubMed] [Google Scholar]
- 29.Farah CS, et al. Primary role for CD4(+) T lymphocytes in recovery from oropharyngeal candidiasis. Infect Immun. 2002;70:724–731. doi: 10.1128/iai.70.2.724-731.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cantorna MT, Balish E. Mucosal and systemic candidiasis in congenitally immunodeficient mice. Infect Immun. 1990;58:1093–1100. doi: 10.1128/iai.58.4.1093-1100.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Repentigny L, et al. Mucosal candidiasis in transgenic mice expressing human immunodeficiency virus type 1. J Infect Dis. 2002;185:1103–1114. doi: 10.1086/340036. [DOI] [PubMed] [Google Scholar]
- 32.Cheng S, et al. The role of Candida albicans NOT5 in virulence depends upon diverse host factors in vivo. Infect Immun. 2005;73:7190–7197. doi: 10.1128/IAI.73.11.7190-7197.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]