Abstract
We investigated the interaction between the corticostriatal glutamatergic afferents and dopamine D1-like and D2-like receptors in the dorsomedial striatum (dm-STR) in attention and executive response control in the five-choice serial reaction time (5-CSRT) task. The competitive NMDA receptor antagonist 3-(R)-2-carboxypiperazin-4-propyl-1-phosphonic acid (CPP) injected in the mPFC impaired accuracy and increased premature and perseverative responding, raising GLU, DA, and GABA release in the dm-STR. The D1-like antagonist SCH23390 injected in the dm-STR reversed the CPP-induced accuracy deficit but did not affect the increase in perseverative responding. In contrast, the D2-like antagonist haloperidol injected in the dm-STR reduced the CPP-induced increase in perseverative responding but not the accuracy deficit. The different roles of dorsal striatal D1-like and D2-like receptor were further supported by the finding that activation of D1-like receptor in the dm-STR by SKF38393 impaired accuracy but not perseverative responding while the D2-like agonist quinpirole injected in the dm-STR increased perseverative responding but did not affect accuracy. These findings suggest that integration of cortical information by D1-like receptors in the dm-STR is a key mechanism of the input selection process of attention while the integration of corticostriatal signals by D2-like receptors preserves the ability to switch from one act/response to the next in a complex motor sequence, thus providing for behavioral flexibility.
Keywords: attention, behavioral flexibility, GLU, DA and GABA release, dorsal striatum, prefrontal cortex
INTRODUCTION
Dopamine (DA) and glutamate (GLU) interact in the regulatory circuits encompassing the prefrontal cortex (PFC) and basal ganglia, and a dysfunctional relationship in their transmission in these circuits may underlie the pervasive executive deficits observed in neuropsychological disorders such as schizophrenia and Parkinson's disease (Owen et al, 1992; Robbins, 1990).
Neuroimaging studies in healthy humans show that selective and divided attention governs the activity in the PFC and cingulate cortex, and in some nuclei of the basal ganglia such as the caudate and globus pallidum (Corbetta et al, 1991); the activity in the striatopallidum plays a role in the top–down influence of the PFC during attention shifts (van Schouwenburg et al, 2010). Glutamate N-methyl-𝒟-aspartate (NMDA) receptor hypofunction in the PFC causes cognitive dysfunctions including executive and attentional deficits in man and experimental animals (Higgins et al, 2003; Javitt and Zukin, 1991; Krystal et al, 1994; Mirjana et al, 2004; Moghaddam and Adams, 1998). NMDA receptor blockade increases frontal cortex metabolism and resting cerebral blood flow in humans (Breier et al, 1997; Vollenweider et al, 1997) and the firing of pyramidal neurons (Jackson et al, 2004) and GLU and DA release in the medial PFC (mPFC) in rats (Ceglia et al, 2004; Moghaddam et al, 1997). Therefore, increased activation of cortical inputs to the striatum may contribute to cognitive deficits. However, there is little direct evidence of increased neurotransmission in the medial region of the dorsal striatum (dm-STR) due to blockade of NMDA receptors in the mPFC.
DA inputs to the dorsal striatum (arising mainly in the substantia nigra) (Anden et al, 1966) commonly converge with corticostriatal glutamate inputs onto the same postsynaptic spines of medium spiny (MS) projection neurons (Hersch et al, 1995). DA effects on striatal outputs are mediated by D1-like and D2-like DA receptors (Missale et al, 1998), which are expressed in disjointed subsets of MS projection neurons; D1-like receptors are preferentially found on those projecting to the substantia nigra pars reticulata, while D2-like receptors are located on neurons that innervate the external globus pallidum (Smith et al, 1998), but also on corticostriatal GLU-ergic terminals (Wang and Pickel, 2002). This distribution of DA receptors provides different modulation of the GLU-ergic cortical information flow and forms the basis for many aspects of basal ganglia functions and dysfunctions.
Interaction between these DA-ergic and GLU-ergic inputs in the dorsal striatum appears vital for the control of motor functions and response control in a reaction time task (Amalric et al, 1994; Chase and Oh, 2000; Di Chiara et al, 1994). Glutamatergic transmission in the striatum is facilitated in D2-deficient mice (Cepeda et al, 2001) while selective over-expression of D2 receptors in the striatum leads to impairments in behavioral flexibility (Kellendonk et al, 2006). Activation of D1 receptors in the striatum potentiates NMDA receptor function (Cepeda et al, 1993) and simultaneous activation of glutamate NMDA and D1-like receptors in the dm-STR seems essential for the control of attention (Agnoli and Carli, 2011).
The main aim of this study was to investigate how D1-like and D2-like receptors in the dorsal striatum contribute to deficits in impulse control, attention and behavioral flexibility induced by blocking NMDA receptors in the mPFC, which alters corticostriatal GLU neurotransmission. We used the five-choice serial reaction time (5-CSRT) task, which entails selective attention and tight organization of a complex response sequence for optimal performance (Carli et al, 1983; Robbins, 2002) and engages fronto-striatal-thalamic circuitry (Christakou et al, 2001; Chudasama et al, 2003; Chudasama and Muir, 2001; Rogers et al, 2001). The dorsal striatum is a functionally heterogeneous brain structure and the medial region appears to contribute to goal-directed actions while its lateral region supports instrumental stimulus-response habits (Balleine and O'Doherty, 2010). Excitotoxic lesion studies in rats show that the neural circuit centered on mPFC and dm-STR is involved in selective attention (Christakou et al, 2001; Rogers et al, 2001) while lesions of the dorsolateral striatum retard learning and cause omissions in the 5-CSRT task (Rogers et al, 2001). Thus, we injected the competitive NMDA receptor antagonist (R)-2-carboxypiperazin-4-propyl-1-phosphonic acid (CPP) into the mPFC in rats concurrently injected in the dm-STR with antagonists for the D1-like and D2-like DA receptors such as SCH23390 or haloperidol, respectively.
To examine further whether D1-like and D2-like receptors control different aspects of executive attentional processes, we tested the effects of the D1-like agonist SKF38393 and the D2-like agonist quinpirole injected into the dm-STR on rats' performance in the 5-CSRT task. To test directly how blockade of NMDA receptors in the mPFC affected neurotransmission in the dm-STR, we checked how CPP infused into the mPFC affected GLU, DA and GABA levels in the dm-STR and mPFC, concomitantly using dual probe intracerebral microdialysis in awake, freely moving rats.
MATERIALS AND METHODS
Animals. Male hooded Lister rats (Charles River, Italy) were used. The rats weighed between 280–320 g at the start of the experiments, and were housed in pairs until surgery, under temperature-controlled conditions (21 °C) with a day/night cycle (light on 0700 to 1900 hours). Rats used in behavioral studies were kept at about 90% of their free-feeding weight by limiting their access to food to 15 g of food pellets for rats (Altromin, Italy) at the end of each day's testing. Water was available ad libitum. Rats used in microdialysis experiments had free access to food and water.
Procedures involving animals and their care were conducted in conformity with the institutional guidelines that are in compliance with the national (D.L. n. 116, G.U., suppl., 40, 18 Febbraio 1992, Circolare No. 8, G.U., 14 luglio 1994) and international laws and policies (EEC Council Directive 86/609, OJ L 358,1, Dec. 12, 1987; Guide for the Care and Use of Laboratory Animals, US National Research Council, 1996).
Behavioral Studies
The 5-CSRT task. The apparatus consisted of four five-hole operant conditioning chambers (Campden Ins. UK) controlled on-line by Whisker software (Cambridge University Technical Services, UK). The apparatus and all the details of training procedures have been described previously (Carli et al, 1983).
Briefly, rats were trained to wait for a fixed time (5 s) before a brief visual stimulus (0.5 s) was presented in one of the five holes. While the light was on, and for a short period afterwards (limited hold), response in the hole that was illuminated (correct response) resulted in the delivery of a food pellet (45 mg; Sandown Scientific, UK). Responses in the holes that had not been illuminated (incorrect responses) or failure to respond within the limited hold (omissions) caused the house-light to be turned off for a short period (2 s). Responses made before presentation of the visual stimulus during the 5-s waiting period were labeled premature and caused 2 s of lights-off. After a premature response, the trial was restarted so only one premature response per trial was possible. These responses were not counted in the total trials completed. Repeated responses in any hole after a correct or incorrect response but before collecting the food pellet were labeled as perseverative. Perseverative responses after correct or incorrect response were not collected separately and they caused a 2 s of lights-off.
Each daily session consisted of 100 trials or 30 min of testing, whichever was completed first. Each rat had only one session per day on the 5-CSRT task. After about 60 sessions, all rats trained on the 5-CSRT task (n=44) reached a stable performance with a mean of 80% correct responses and no more than 20% omissions at 0.5 s of stimulus duration. Afterwards they underwent stereotaxic surgery for cannulae implantation in the mPFC and/or dm-STR. After surgery, rats had 3–5 days without training followed by about seven training sessions to re-establish pre-surgery levels of baseline performance.
Surgery
Rats were anesthetized with 1 ml/kg ketamine (80 mg/ml) plus xylazine (10 mg/ml) intraperitoneally (i.p.). To reduce tracheobronchial secretions, rats were injected subcutaneously with atropine (1 mg/kg) and then secured in a stereotaxic frame (Kopf Ins., USA) with the incisor bar set at −3.3 mm relative to the interaural line. Bilateral 23-gauge, stainless-steel guide cannulae (Cooper's Needles, UK) were implanted in the dm-STR and in the mPFC using standard stereotaxic techniques. The cannulae were secured to the skull using bone screws and dental cement. The cannulae for dm-STR were at following coordinates: anterior–posterior (AP): +0.7 mm, lateral (L): ±2.2 mm from midline, and dorsal–ventral (DV): −2.5 mm from dura. For mPFC the coordinates were: AP: +3.7 mm, L: ±0.7 mm from midline and DV: −1.6 mm from dura (Paxinos and Watson, 1998). Thirty-gauge stainless-steel stylets flush with the end of the guide cannulae were inserted in the guide cannulae. After surgery, rats were housed singly.
Drugs and experimental design
CPP (Tocris, UK), SCH23390 (RBI, USA), SKF38393 (Sigma, Italy), and quinpirole (Sigma, Italy) were dissolved in phosphate buffer saline (PBS); haloperidol (Lusofarmaco, Italy) was dissolved in vehicle (PBS containing 10–20 μl of 90% lactic acid; the pH of the solution was adjusted to 6 with 1 M NaOH).
On testing days, 1 μl per hemisphere of vehicle, CPP, various doses of haloperidol, SCH23390, SKF38393 and quinpirole were delivered at a rate of 0.5 μl/min through a 30-gauge stainless-steel cannula connected by PE10 tubing to a 10-μl Hamilton syringe mounted in a CMA/400 syringe pump (CMA Microdialysis, Sweden). The injection cannulae needle ends were 2 mm below the guide cannulae. After the end of infusion, the injection cannulae were left in place for 1 min to allow for diffusion.
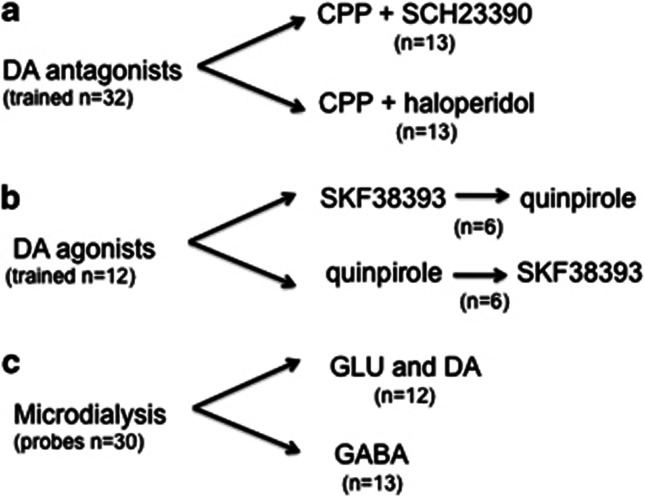
The flowchart in Figure 1 shows the number and distribution of rats in various experiments and the final group sizes of rats whose data were included in the statistical analysis. Rats with cannulae in the dm-STR (n=12) received injections of PBS (1 μl), SKF38393 (100 and 1000 ng/μl), or quinpirole (10 and 100 ng/μl) into the dm-STR and 5 min later their performance was assessed in the 5-CSRT task. SKF38393 and quinpirole were tested in a batch of 12 rats. Rats were divided in two groups of six each. One group started with SKF38393, the other with quinpirole. After completion of the drug dose-response the treatments were reversed. Thus, the effects SKF38393 and quinpirole were tested in 12 rats. The doses were selected based on our preliminary data and studies by Pezze et al (2007) and Haluk and Floresco (2009).
Figure 1.
Flowchart showing the number and distribution of rats in various experiments and the final group sizes of rats whose data were included in the statistical analysis. (a) Thirty-two rats were trained in the 5-CSRT task. The data of six rats with cannulae in both mPFC and dm-STR were excluded, two because of obstruction of the cannula, two because of wrong placement and two because after CPP injection they made >80% omissions. Data from 13 rats receiving CPP+SCH23390 and 13 receiving CPP+haloperidol were included in the statistical analysis. (b) Twelve rats were trained in the 5-CSRT task, in two groups of six. One group started with SKF38393, the other with quinpirole. After completion of the drug-dose response in these rats the treatments were reversed. The final data set for SKF38393 and quinpirole had 12 rats. (c) Thirty rats were implanted with dual probes, one in the mPFC, the other in the ipsilateral dm-STR. Five rats were excluded because of clogging or misplacement of the probes. GLU and DA were measured in the microdialysis samples of the same rat. The final group size was 12 rats. The final group size for GABA measurements was 13 rats.
We tested the effects of combined administration of SCH23390 or haloperidol plus CPP in two different batches of 16 rats each. One batch of rats had combinations of 1 μl vehicle (PBS) or SCH23390 (10 and 50 ng/μl) plus CPP (50 ng/μl) while the other had vehicle (1 μl) or haloperidol (10 and 30 ng/μl) plus CPP (50 ng/μl). SCH23390 and haloperidol were injected into the dm-STR 10 min before the injection of CPP or vehicle into the mPFC. Ten minutes after CPP injection, rats were put in the boxes and their performance assessed. The dose of 50 ng/side of CPP was selected as it lowers accuracy and increases premature and perseverative responses (Carli et al, 2006; Mirjana et al, 2004). The SCH23390 and haloperidol doses were selected on the basis of our pilot experiments and previous studies (Agnoli and Carli, 2011).
Before the start of the experiments, rats were naive to any drug treatment. The various treatments were administered according to a Latin-square design. At least 2 days were left between test days and rats were always tested on these ‘free' days to re-establish the baseline and check for the lasting effects of drugs.
Statistical analysis
The variables selected for analysis were (a) accuracy (%) of visual discrimination (total correct responses/total correct+total incorrect responses × 100); (b) percentage of omissions (total omissions/total correct+total incorrect+total omissions × 100); (c) percentage of premature responses in the holes during the ITI (number of premature responses/total correct+total incorrect responses × 100); (d) the number of perseverative responses in the holes after a correct or an incorrect response; (e) mean correct response latency; and (f) mean latency to collect the earned food pellet (both measured to the nearest 0.001 s).
One-way within-subjects ANOVA was used for the statistical analysis of SKF38393 and quinpirole data. Post hoc comparison of treatment means was done by Dunnett's t test. We used two-way within-subjects ANOVA with factors drug (haloperidol or SCH23390) and CPP to analyze the effects of CPP in combination with haloperidol (H) or SCH23390 (SCH). When two-way ANOVA indicated a significant drug (H or SCH) × CPP interaction (the significance level for F-values was set at P<0.05) or significant main effects of drugs (H or SCH) and CPP, Tukey's test was used to compare the treatments mean values. All statistical tests were done with SAS 9.1 run on PC.
Neurochemical Study
Microdialysis procedure
Behaviorally naive rats used in microdialysis experiments were anesthetized i.p. with 3 ml/kg Equithesin (composition in mM: pentobarbital 39, chloral hydrate 256, MgSO4 86, ethanol 10% v/v, propylene glycol 39.6% v/v), and placed on a stereotaxic frame. Two dialysis probes were implanted, one in the mPFC and one in the ipsilateral dm-STR. The anteroposterior and lateral stereotaxic coordinates were the same as for the rats in the behavioral experiments. The dorsoventral coordinate for mPFC was DV −4.8 mm from dura and for dm-STR was DV −6.0 from dura.
Concentric dialysis probes were constructed with Cuprophan membrane (216 μm outer diameter, 3000 Da cutoff, Sorin Biomedica, Mirandola, Italy), essentially as described by Robinson and Whishaw (1988). The membrane exposed to the brain tissue was 3 mm long. About 24 h after surgery, the probes were perfused with artificial cerebrospinal fluid (aCSF) at 1 μl/min with a CMA/100 pump (CMA/Microdialysis, Stockholm, Sweden). At this time, the acute damage due to probe insertion has fully recovered and the neurotransmitter release is largely sensitive to tetrodotoxin and elevated KCl in the perfusion medium (Calcagno and Invernizzi, 2010; Ceglia et al, 2004). Samples of dialysate were collected every 20 min and stored at 4 °C. A stabilization period was allowed before dialysis sampling. When basal extracellular levels of GLU, DA, and GABA in the mPFC and dm-STR remained stable (not differing by >20%) for at least three consecutive samples, CPP 100 μM (dissolved in aCSF) or vehicle (aCSF) was perfused in the mPFC for 60 min. The concentration of 100 μM CPP was selected as it raises reliably GLU levels, to the same extent as a bolus injection of 50 ng/μl CPP in the mPFC (Calcagno et al, 2009).
Chromatographic analysis
Samples of dialysate from mPFC and dm-STR were split into two portions; 5 μl for the GLU and 15 μl for the DA determination. In an additional experiment, dialysate samples (15 μl) were used to assay GABA. GLU and GABA were determined by high-performance liquid chromatography (HPLC) with fluorometric detection after pre-column derivatization with o-phthalaldehyde/β-mercaptoethanol (Sigma-Aldrich, Milan, Italy) reagent according to Donzanti and Yamamoto (1988). DA was determined by HPLC coupled to an electrochemical detector, as described elsewhere (Invernizzi et al, 1992).
Statistical analysis
Time course microdialysis data for GLU, DA, and GABA samples from PFC and dm-STR were analyzed separately by repeated-measure one-way ANOVA with CPP as between-subject factor and time as within-subject factor. The analysis was applied to the part of the curve between 0 and 120 min. The significance level was set at P<0.05.
Histology
After completion of the behavioral and microdialysis experiments, rats were anaesthetized with 3 ml/kg i.p. Equithesin and killed by decapitation. The brains were removed and postfixed in 4% formalin solution, then transferred to 20% sucrose in 0.2 M PBS. Three days later, the brains were frozen in n-pentane and stored at −20 °C. Coronal sections were cut at 30 μm in a Cryo-cut and stained with cresyl violet. Inspection of the stained slices under the light microscope and the trajectory of gliosis produced by the cannulae or microdialysis probe allowed their location and tip to be estimated and mapped on the rat atlas (Paxinos and Watson, 1998).
RESULTS
Histology and Baseline Performance
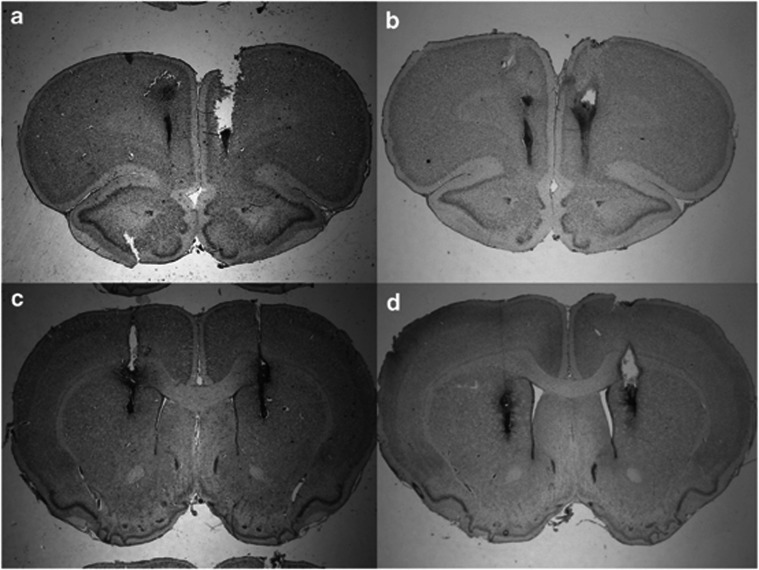
Examination of stained coronal sections of the brains showed that multiple injections into the mPFC and dm-STR caused limited tissue damage. Figure 2 presents photographs of histological sections showing examples of the bilateral placements of injection sites in the mPFC and/or dm-STR. Supplementary Figure 1S shows schematic drawings of the injections tip from rats whose data were included in statistical analysis.
Figure 2.
Representative histological coronal sections of rat brain stained with cresyl violet showing the tracts of bilateral injections in the mPFC (a, b) and in the dm-STR (c, d).
Rats' baseline performance in the 5-CSRT task (measured by accuracy of visual discrimination, the number of premature and perseverative responses, and the speed of responding and omissions) after surgery and before the intracerebral drug infusions did not differ between experimental groups. No performance measure after vehicle treatment was significantly different between experimental groups. Rats returned to perform at their baseline level of accuracy (80–90%), making <20% omissions and only a small number of premature and perseverative responses on the between-injections days (data not shown). Thus, the surgery, the various treatments and the multiple injections had no lasting effect on performance.
Effects of D1-like and D2-like Antagonists on CPP-Induced Impairments in Attentional Performance
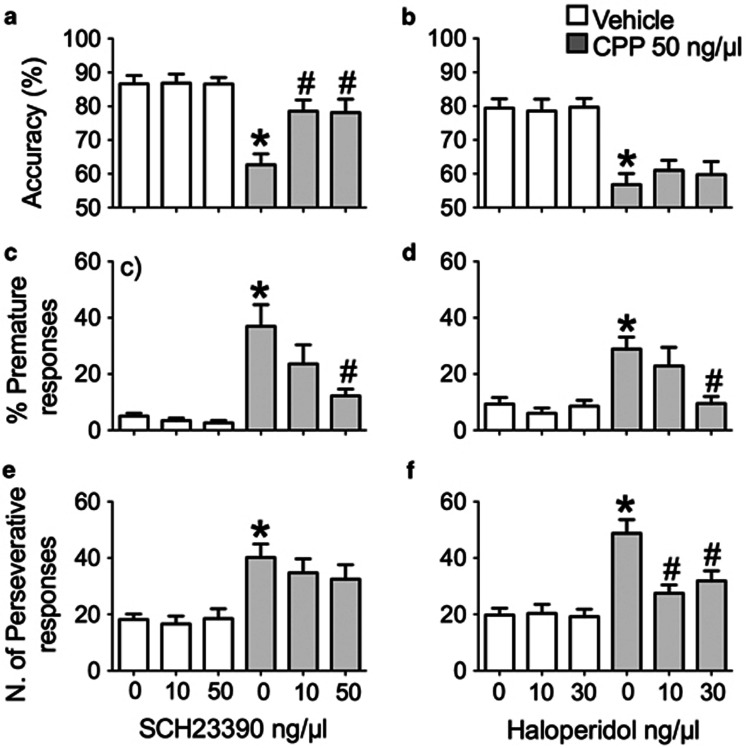
Two groups of rats received injections of either the D1-like antagonist SCH23390 or the D2-like antagonist haloperidol into the dm-STR followed by injections of CPP into the mPFC. The results are illustrated in Figure 3 and Table 1.
Figure 3.
Effects of SCH23390 or haloperidol injected into the dm-STR on CPP-induced deficit in accuracy, measured by the percentage of correct responses (a, b) and loss of response control indicated by the percentage of premature (c, d) and the number of perseverative (e, f) responses. Histograms (mean±SEM of 13 rats) on the left-hand side show the effects of vehicle (1 μl) or SCH23390 (10 and 50 ng/μl) injected into the dm-STR 10 min before an injection of vehicle (1 μl) or CPP (50 ng/μl) into the mPFC. Histograms on the right-hand side (mean±SEM of 13 rats) present the effects of vehicle (1 μl) or haloperidol (10 and 30 ng/μl) injected into the dm-STR 10 min before an injection of vehicle or 50 ng/μl CPP into the mPFC. Rats were tested 10 min after receiving CPP. *P<0.05 vs 0+Vehicle; #P<0.05 vs 0+CPP (Tukey's test).
Table 1. Effects of SCH23390 (a) and Haloperidol (b) Injected in the dm-STR on Omissions and Speed of Responding in the 5-CSRT Task of Rats Given Vehicle or CPP in the mPFC.
| Treatment | Omissions (%) | Correct response latency (s) |
|---|---|---|
| (a) | ||
| V+V | 17.3±1.9 | 0.66±0.027 |
| SCH 10+V | 22.5±3.5 | 0.68±0.030 |
| SCH 50+V | 19.8±5.9 | 0.71±0.032 |
| V+CPP | 32.9±4.0* | 1.07±0.033* |
| SCH 10+CPP | 30.7±5.5 | 1.09±0.090 |
| SCH 50+CPP | 35.7±4.5 | 1.08±0.102 |
| (b) | ||
| V+V | 20.7±2.8 | 0.65±0.026 |
| H 10+V | 20.5±2.9 | 0.68±0.042 |
| H 30+V | 24.1±5.9 | 0.71±0.040 |
| V+CPP | 34.5±2.6* | 0.96 ±0.091* |
| H 10+CPP | 32.7±4.2 | 1.02±0.092 |
| H 30+CPP | 38.4±2.7 | 1.07±0.098 |
The values are expressed as mean±SEM of 13 rats in (a) and 13 rats in (b). Vehicle (V) (1 μl) and SCH23390 (10 and 50 ng/μl) (SCH 10 and SCH 50) or haloperidol (10 and 30 ng/μl) (H 10 and H 30) were injected into the dm-STR 10 min before an injection of vehicle (V) or 50 ng/μl CPP into the mPFC. The combinations of SCH23390 plus CPP (a) and haloperidol plus CPP (b) were administered 72 h apart according to a Latin square design.
*P<0.05 vs V+V (Tukey's test).
Injections of SCH23390 or haloperidol into the dm-STR followed by vehicle injections in the mPFC had no effect on any measure of performance (for all measures, the mean changes were <10% and comparison of vehicle means with those of various doses by Tukey's test showed P>0.05). However, in both experimental groups injection of CPP in the mPFC had profound effects on the 5-CSRT task performance, reducing accuracy, and increasing premature and perseverative responding, omissions and latencies to make a correct response. Within subject two-way ANOVA showed significant main effects of CPP (values not shown) and further comparison of controls (V+Vehicle) and CPP (V+CPP) by Tukey's test showed P<0.05 for all these measures.
Injections of D1-like and D2-like antagonists into the dm-STR had different effects on CPP-induced performance changes. The accuracy deficit was abolished by SCH23390 (Figure 3a) but not by haloperidol (Figure 3b). Statistical analysis by within-subject two-way ANOVA of accuracy data showed a significant interaction between SCH23390 and CPP (F(2,60)=6.4, P=0.0031) but not haloperidol and CPP (F2,60=0.4, P=0.6). Planned comparisons of mean accuracy indicated that both 10 and 50 ng/μl SCH23390 increased the accuracy of CPP-injected rats (SCH 10+CPP or SCH 50+CPP vs V+CPP, Tukey's test P<0.05).
Both SCH23390 (Figure 3c) and haloperidol (Figure 3d) dose dependently reduced the CPP-induced increase in premature responses. Statistical analysis of these responses showed a significant interaction for SCH23390 × CPP (F(2,60)=3.7, P=0.029) and haloperidol × CPP (F2,60=4.0, P=0.02). Further comparison of the means showed that the CPP-induced increase (V+CPP vs V+Vehicle, P<0.05) was reduced by 50 ng/μl SCH23390 (SCH 50+CPP vs V+CPP, P<0.05) but not by 10 ng/μl (SCH 10+CPP vs V+CPP, P>0.05). Similarly, 30 ng/μl haloperidol (V+CPP vs H30+CPP, P<0.05) but not 10 ng/μl (V+CPP vs H10+CPP, P>0.05) reduced the CPP-induced increase in premature responding.
The CPP-induced increase in perseverative responding was reversed by haloperidol (Figure 3f) but not SCH23390 (Figure 3e), with a significant interaction for haloperidol × CPP (F2,60=4.5, P=0.01) but no interaction for SCH23390 × CPP (F(2,60)=0.9, P=0.4). Post hoc comparisons of treatment means showed that the CPP-induced increase in perseverative responding was reduced by both doses of haloperidol (H10+CPP and H30+CPP both vs V+CPP, P<0.05).
As shown in Table 1, CPP increased the percentage of omissions and correct response latencies equally in both groups of rats and these effects were not affected by any dose of SCH23390 (interaction SCH23390 × CPP: omissions, F(2,60)=0.2, P=0.8; correct response latency, F(2,60)=0.9, P=0.4) (see Table 1a) or haloperidol (interaction haloperidol × CPP: omissions, F2,60=1.7, P=0.3; correct response latency, F2,60=0.3, P=0.7) (see Table 1b). Incorrect response latencies and latencies to collect the food reward were not affected by CPP, SCH23390, haloperidol, or their combinations (data not shown).
Activation of D1-like or D2-like Receptors in the dm-STR and Rats Performance in the 5-CSRT Task
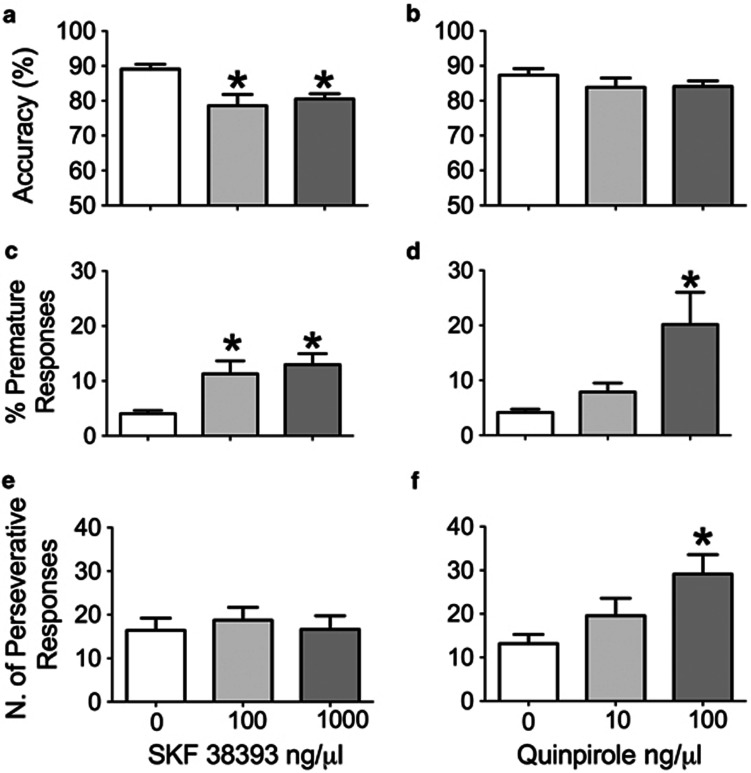
The D1-like agonist SKF38393 and the D2-like agonist quinpirole had different patterns of effects on performance in the 5-CSRT task. SKF38393 injected into the dm-STR reduced accuracy (Figure 4a) and increased premature responses (Figure 4c) but had no effect on perseverative responding (Figure 4e). In contrast, quinpirole had no effect on accuracy (Figure 4b) but increased premature (Figure 4d) and perseverative responses (Figure 4f).
Figure 4.
Effects of SKF38393 and quinpirole on accuracy, measured by the percentage of correct responses (a, b) and response control indicated by the percentage of premature (c, d) and the number of perseverative (e, f) responses. Histograms present mean values±SEM of 12 rats. Vehicle (1 μl), SKF 38393 (100 and 1000 ng/μl), or quinpirole (10 and 100 ng/μl) were injected into the dm-STR and their performance was assessed 5 min later. *P<0.05 vs Vehicle (0) (Dunnett's t-test).
Repeated-measure one-way ANOVA of accuracy data indicated a significant effect of SKF38393 (F(2,22)=6.03, P=0.008) (Figure 4a) but not quinpirole (F(2,22)=1.30,P=0.29) (Figure 4b). Thus, comparisons of mean accuracy showed that rats injected with either 100 or 1000 ng/μl SKF38393 had lower accuracy than vehicle-injected rats (P<0.05).
Statistical analysis of premature responses showed significant effects of SKF38393 (F(2,22)=4.98, P=0.02) (Figure 4c) and quinpirole (F(2,22)=4.8, P=0.02) (Figure 4d). Compared with vehicle, premature responses rose to the same level after both doses of SKF38393 (both P<0.05) whereas premature responses after quinpirole increased only after the highest dose, 100 ng/μl (P<0.05 compared with vehicle). Perseverative responses were increased after quinpirole (F(2,22)=4.62, P=0.02) but not SKF38393 (F(2,22)=0.42, P=0.65). Compared with vehicle, 100 (P<0.05) but not 10 ng/μl (P>0.05) quinpirole increased perseverative responses (Figure 4f).
Table 2 shows that the D1 receptor agonist SKF38393 reduced omissions (F(2,22)=3.29, P=0.05) and shortened correct response latency (F(2,22)=6.64, P=0.005). Both doses of SKF38393 significantly reduced the correct response latency (comparison with vehicle, P<0.05) although neither dose had any significant effect on omissions (P>0.05). Quinpirole had no effect on omissions (F(2,22)=2.89, P=0.08) or correct response latency (F(2,22)=0.21, P=0.81).
Table 2. Effects of SKF38393 (a) and Quinpirole (b) Injected in the dm-STR on Omissions and Speed of Responding in the 5-CSRT Task.
| Treatment (ng/μl) | Omissions (%) | Correct response latency (s) |
|---|---|---|
| (a) | ||
| Vehicle | 21.3±1.3 | 0.69±0.036 |
| SKF 100 | 15.6±2.5 | 0.62±0.031* |
| SKF 1000 | 17.2±2.9 | 0.60±0.028* |
| (b) | ||
| Vehicle | 18.9±2.2 | 0.64±0.040 |
| QUIN 10 | 25.0±2.9 | 0.67±0.044 |
| QUIN 100 | 22.0±2.4 | 0.67±0.034 |
The values are expressed as mean±SEM of 12 rats. Vehicle (1 μl) and SKF38393 (100 and 1000 ng/μl) (SKF 100 and SKF 1000) or quinpirole (10 and 100 ng/μl) (QUIN 10 and QUIN 100) were injected into the dm-STR 5 min before the test session. The doses of SKF38393 (a), quinpirole (b) and vehicle were administered 72 h apart according to a Latin square design.
*P<0.05 vs Veh+Veh (Tukey's test).
Blockade of NMDA Receptors in the mPFC on Extracellular GLU, DA and GABA in the mPFC and dm-STR
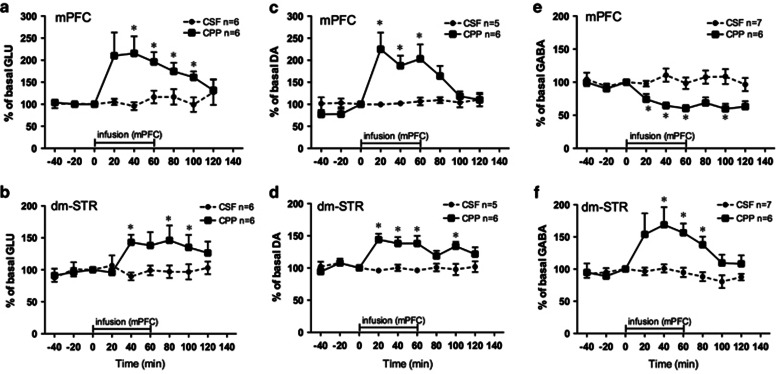
To determine whether blockade of NMDA receptors in the mPFC altered neurotransmission in the dm-STR, we measured the time course of effects of intra-PFC CPP infusion on extracellular GLU, DA, and GABA by dual probe microdialysis in the same rat. Figure 5 shows the effects in the mPFC (a, c, e) and dm-STR (b, d, f). Figure 6 presents photographs of representative histological sections showing microdialysis probes placements in the mPFC and dm-STR.
Figure 5.
Effects of CPP infused into the mPFC on glutamate (GLU), dopamine (DA), and GABA levels in the mPFC (a, c, e) and dm-STR (b, d, f) measured by dual probe microdialysis. Each rat had two probes, one in the left or right mPFC the other in the ipsilateral dm-STR. Once extracellular levels of GLU, DA, and GABA in the mPFC and dm-STR were stable, perfusion medium (aCSF) in the mPFC was replaced with a medium containing 100 μM CPP for 60 min. Then, it was switched back to the aCSF for another 60 min. Probes in the dm-STR were perfused with aCSF for the whole experiment (2 h). The horizontal bar indicates the duration of CPP infusion in the mPFC. Samples from the probes in mPFC and dm-STR were collected every 20 min. Data are percentages of basal values. (a, b) CSF (n=6) CPP (n=6); (b, d) CSF (n=5), CPP (n=6); (e, f) CSF (n=7) CPP (n=6). *P<0.05 vs aCSF (Student's t-test).
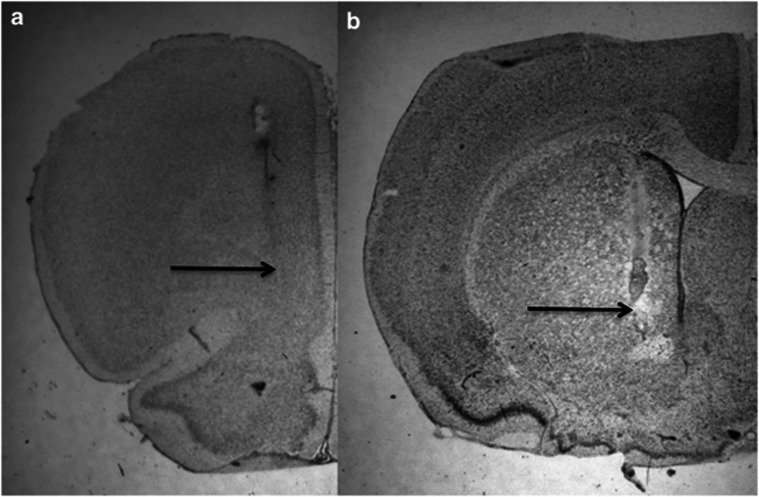
Figure 6.
Representative histological coronal sections of the rat brain showing the tracts of the microdialysis probe in the mPFC (a) and in the ipsilateral dm-STR (b). Arrows indicate the tip of the probes.
Once extracellular levels of GLU, DA, and GABA in the mPFC and dm-STR were stable, animals were infused with aCSF or 100 μM CPP in the mPFC for 60 min and the levels of GLU, DA, and GABA monitored for another 60 min (basal levels of GLU, DA, and GABA are reported in Supplementary Materials).
Glutamate
CPP infusion in the mPFC increased GLU in the PFC and in the dm-STR. Extracellular GLU levels in the mPFC (Figure 5a) rose by about 210% at 20–40 min and gradually returned to baseline after switching off CPP infusion. Statistical analysis of mPFC data showed a significant main effect of CPP (F(1,10)=13.9, P=0.004), no significant effect of time (F(6,60)=2.1, P=0.06) but a marginally significant interaction CPP × time (F(6,60)=2.3, P=0.05). In the dm-STR (Figure 5b), the increase in GLU levels was slower, reaching a plateau (146%) about 60–100 min after the start of infusion but then rapidly returning to baseline. dm-STR data showed a significant main effect of CPP (F(1,10)=11.2, P=0.007) but no effects of time (F(6,60)=2.2, P=0.06) or interaction CPP × time (F(6,60)=1.9, P=0.10).
Dopamine
Figure 5c shows that DA levels in the PFC reached about 220% 20–60 min after the start of CPP infusion. When infusion was switched off, DA started to decline towards the baseline. The increase in DA levels in the dm-STR (Figure 5d) was less pronounced than in the mPFC, reaching a plateau (about 140%) between 20 and 60 min, and persisting for 40 min after switching off CPP infusion. Statistical analysis of data for mPFC and dm-STR showed significant effects of CPP (mPFC, F(1,9)=15,2 P=0.004; dm-STR, F(1,9)=38.3, P=0.0002) and CPP × time (mPFC, F(6,54)=2.5, P=0.03; dm-STR, F(6,54)=3.1, P=0.01); the effects of time was significant for mPFC (F(6,54)=2.3, P=0.04) but not dm-STR (F(6,54)=2.1, P=0.06).
GABA
CPP lowered extracellular GABA in the mPFC (Figure 5e). From 20 min after the start of CPP infusion, extracellular GABA levels dropped reaching a maximum decrease of about 40% at 60 min. GABA did not return to baseline after CPP infusion was switched off. Statistical analysis indicated a significant effect of CPP (F(1,66)=15.8, P=0.002), time (F(6,66)=2.4, P=0.04) but not CPP × time (F(6,84)=2.1, P=0.06). In the dm-STR (Figure 5f), extracellular GABA rose to about 150–170% between 20 and 60 min after intra-mPFC infusion of CPP. When CPP was switched off, GABA gradually returned towards the baseline. Statistical analysis showed a significant main effect of CPP (F(1,66)=8.4, P=0.01) and time (F6,66)=3.2, P=0.007) and a significant interaction CPP × time (F(6,66)=3.0, P=0.01).
DISCUSSION
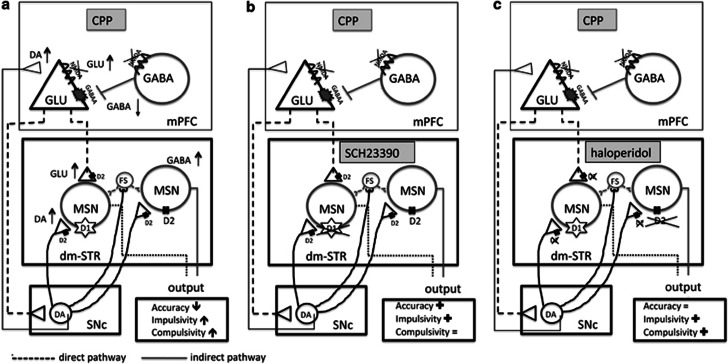
The main findings of this study are that injection of a competitive NMDA receptor antagonist, CPP, into the mPFC impaired accuracy and caused a loss of inhibitory response control as shown by increased premature and perseverative responding. In addition, it increased GLU, DA, and GABA release in the dm-STR. The D1-like antagonist SCH23390 reversed the CPP-induced accuracy deficit and increase in premature responses without having any effect on the CPP-induced perseverative responding. In contrast, the D2-like antagonist haloperidol reduced the CPP-induced perseverative and premature responding but had no effects on the accuracy deficit (see Figure 7 for schematic representations of the proposed sites of action and the behavioral and neurochemical changes).
Figure 7.
The cortico-striatal circuitry, neurotransmitters, and their receptors involved in the 5-CSRT task. (a) The behavioral outcome and changes in GLU, DA, and GABA levels in the mPFC and dm-STR caused by CPP injected into the mPFC. (b, c) Schematic representations of the proposed sites of action and the behavioral outcomes resulting from blockade of D1-like or D2-like receptors by antagonists SCH23390 (b) and haloperidol (c) in the dm-STR of rats injected in the mPFC with CPP. The symbols represent: (↑) increase; (↓) decrease; (=) no effect; or (+) reversal of the CPP-induced effects.
The selective role of dorsal striatal D1-like and D2-like receptors in these functions is further supported by data showing that (in the absence of CPP injection in the mPFC) activation of the D1-like receptors by SKF38393 impaired accuracy and increased premature responses but had no effect on perseverative responding whereas the D2-like agonist quinpirole increased premature and perseverative responding but had no effects on accuracy.
Role for D1-like but not D2-like Receptors in Attentional Functioning
Despite the lack of effects under baseline conditions, SCH23390 prevented the CPP-induced impairment in accuracy. The effect was not dose dependent and already reached its maximum at the lowest dose tested (10 ng/side). Increasing the dose did not further increase accuracy but other effects emerged such as the reduction of CPP-induced impulsivity. Similar improvements in accuracy after SCH23390 have been reported in rats with excitotoxic lesion to the mPFC (Passetti et al, 2003). SCH23390 on its own had no effect on accuracy. This may be because the doses we used were too low as much higher doses (100 and 300 ng/side, injected respectively in the NAC or prelimbic region of the PFC (PrL)) have been reported to reduce accuracy in the 5-CSRT task (Granon et al, 2000; Pezze et al, 2007) though no effect has been reported too (Pattij et al, 2007). However, in our previous study, 100 ng/side SCH23390 in the dm-STR had no effects on accuracy, suggesting that under basal conditions D1-like receptors in this brain area do not exert tonic control over attention (Agnoli and Carli, 2011).
Activation of dm-STR D1 receptors by SKF38393 impaired accuracy, suggesting that too much activity at D1-like receptors in the dm-STR will result in less than optimal attention leading to less accuracy in the 5-CSRT task. In previous studies, similar doses of SKF38393 injected into the dorsolateral striatum had no effect but when injected into the NAC or PrL they increased accuracy (Granon et al, 2000; Pezze et al, 2007). However, the improvements were apparent only when accuracy was low (Granon et al, 2000), not when it was high as in the present experiments (almost 90%). These differences in the effects of D1-like receptor activation on accuracy are not surprising, as it has been repeatedly shown that the effects of D1-like receptor manipulation on task performance depend on the optimal levels of DA function in the PFC (Arnsten, 1997; Granon et al, 2000; Sawaguchi and Goldman-Rakic, 1991; Zahrt et al, 1997). This is reminiscent of the Yerkes-Dodson principle based on the inverted U-shaped function relating levels of arousal/activation with efficiency of behavioral performance (Robbins, 2005). Although there is no direct evidence for the U-shaped relationship between D1-like receptor function in the dm-STR and attention we suggest that like for PFC, the inverted U-shaped function could best explain our findings. Thus, in rats performing the 5-CSRT task at a very high level of efficiency, the activity of D1-like receptors may already be at a maximum and any further activation could have detrimental effects. However, while in the PrL frontal and NAC nodes of the corticostriatal circuitry D1-like receptor activity exerts a tonic control on attention functioning (Granon et al, 2000; Pezze et al, 2007), the D1-like receptor in the dm-STR may come into play only under special conditions such as increased (present study) or decreased corticostriatal neurotransmission (Agnoli and Carli, 2011).
In contrast to SCH23390 and SKF38393, haloperidol and quinpirole had no effects on accuracy. It could be argued that higher doses of haloperidol or quinpirole may have resulted in some effects. However, with higher doses of haloperidol rats stopped responding or made mostly omissions (particularly when injected with both haloperidol and CPP) (unpublished results). Similarly, a higher dose of quinpirole (1000 ng/side) had no effect on accuracy but increased the omissions (unpublished results). These data contrast with the ability of systemic or intra-NAC l-sulpiride, a D2/3 receptor antagonist, to prevent the accuracy deficit induced by mPFC lesion (Passetti et al, 2003; Pezze et al, 2009). However, l-sulpiride does not distinguish D2 and D3 receptors whereas haloperidol is more selective for D2 than D3 receptors (Missale et al, 1998). D3 receptors are particularly abundant in the NAC but relatively scarce in the dorsal striatum (Sokoloff et al, 1990) and this may help explain the lack of effect of haloperidol on accuracy deficit.
Role for D2-like but not D1-like Receptors in Perseverative Responding
Haloperidol but not SCH23390 reduced CPP-induced perseverative over-responding. The pattern of effects reported after haloperidol injections in the dm-STR is remarkably similar to that after systemic haloperidol, which reduced CPP-induced perseverative over-responding and impulsivity but had no effect on accuracy (Baviera et al, 2008) and suggests that striatal D2 receptors are the likely site for the effects of systemic haloperidol. Accordingly, quinpirole, injected in the dm-STR dose dependently increased perseverative responding with no effects on accuracy, similar to the effects of these doses injected in the NAC core (Pezze et al, 2007).
This ‘compulsive' behavior is exemplified by the effects of frontal and also striatal lesions in man and monkeys (Clarke et al, 2008; Divac et al, 1967; Fuster, 1989) and may be alleviated by haloperidol (Ridley et al, 1993). That the DA nigrostriatal system plays a role in these repetitive responses in the 5-CSRT task is further suggested by a paradoxical increase in perseverative responding after striatal DA depletion (Baunez and Robbins, 1999), most likely reflecting the supersensitivity of D2 receptors after 6-hydroxydopamine lesion (Ungerstedt, 1971).
Our findings also agree with other studies that have linked changes in D2-like receptor function to flexible modification of behavior; PFC-dependent perseveration in a task requiring an egocentric response strategy depends on tonic DA release and D2-like receptor stimulation in the striatum (Goto and Grace, 2005) and mice over-expressing D2 receptors in the striatum make more perseverative errors in a PFC-dependent working memory task (Kellendonk et al, 2006). The D2-like receptor stimulation by quinpirole increases perseverative but not learning errors in a spatial reversal task (Boulougouris et al, 2009) and D2-like receptor availability in the dorsal striatum was related to the probability of a perseverative response in a three-choice reversal task (Groman et al, 2011).
The lack of effect of D1-like agents on perseverative responding in the 5-CSRT task is consistent with other reports of no effect on this form of behavioral flexibility (Barnes et al, 2012; Granon et al, 2000; Pezze et al, 2007). These findings contrast with evidence that D1-like receptors in the mPFC and NAC contribute to other forms of flexibility such as that involving shifts between strategies, rules or working memory Haluk and Floresco, 2009); Ragozzino, 2002; Zahrt et al, 1997). The perseverative over-responding in the 5-CSRT task may have resulted from a deficit related to the selection and integration of an adequate response in a long sequence, leading to reinforcement rather than inability to flexibly adapt to the shifts between rules, strategies and sets. It is interesting that the PFC and dorsal striatum with its DA afferents have been implicated in the organization of complex sequences of actions and ordering of movements within a behavioral sequence (Bailey and Mair, 2006; Graybiel, 1998; Hikosaka et al, 1998) and that neurons in the striatum and substantia nigra can signal the start and end of self-paced action sequences (Jin and Costa, 2010).
D1-like and D2-like Receptors in Impulsivity
Activation of D1-like and D2-like receptors by SKF38393 and quinpirole increased impulsivity (ie, premature responses) while SCH23390 and haloperidol reduced those induced by CPP. The fact that on their own D1-like and D2-like receptor antagonists did not reduce premature responses may reflect the very small number of premature responses under baseline conditions. However, a higher dose of SCH23390 in the dm-STR reduced premature responses (Agnoli and Carli, 2011). These data are consistent with a general performance-scaling function of tonic DA activity (Cagniard et al, 2006; Robbins, 2002). The D1-like receptor agonist's ability to reduce the number of omissions, latency to make a correct response and to collect the food pellet is also in line with the response-rate invigorating effects, which are also seen, for example after d-amphetamine (Cole and Robbins, 1987). This effect has been mostly attributed to the mesolimbic not the nigrostriatal DA system as enhanced impulsivity induced by d-amphetamine in the 5-CSRT task was not blocked by dorsal striatal DA depletion (Baunez and Robbins, 1999) but was abolished by ventral striatal DA depletion (Cole and Robbins, 1989). However, recent evidence suggests that D2 receptor availability in the dorsal striatum is also associated with impulsiveness (Lee et al, 2009). Interestingly, DA depletion in the dorsal striatum reversed the impulsivity induced by lesion to the subthalamic nucleus (Baunez and Robbins, 1999), suggesting that dorsal striatal DA mediation of impulsivity may be detected in particular conditions.
The CPP-injected rats made more omissions and had longer correct response latencies. Although this may indicate a motivational deficit (Carli and Samanin, 1992), it could not be excluded that rats were unable to maintain voluntary attentional control over sustained performance (Robbins, 2002) or to schedule behavior on account of motor hyperactivity (Mirjana et al, 2004). Haloperidol and SCH23390 did not reduce the CPP-induced increases in omissions and latency to make a correct response or motor hyperactivity (L Agnoli and M Carli, unpublished results), suggesting that control over performance or motivation may not depend on DA mechanisms in the dm-STR.
Blockade of NMDA Receptors in the mPFC Causes Hyperactivity in Corticostriatal Circuitry
As in previous reports, CPP infused in the mPFC raised extracellular levels of cortical GLU and DA (Calcagno et al, 2009; Ceglia et al, 2004; Yonezawa et al, 1998) but lowered GABA. It is likely that CPP facilitates GLU and DA by inhibiting GABA release through blockade of NMDA receptors on GABA interneurons in the PFC (DeBiasi et al, 1996; Homayoun and Moghaddam, 2007). This mechanism is likely to account for the cortical activation in rodents (Gozzi et al, 2008; Homayoun and Moghaddam, 2007; Jackson et al, 2004) and man after NMDA receptor antagonists (Breier et al, 1997; Vollenweider et al, 1997).
In the dm-STR extracellular levels of GLU, DA and GABA increased after CPP infusion in the mPFC. The activation of GLU neurotransmission in the mPFC and increased firing activity of pyramidal-projecting neurons (Homayoun and Moghaddam, 2007; Jackson et al, 2004) might explain the increase in endogenous GLU release in the dm-STR, which in turn may increase GABA and DA release. The NMDA/GABA interaction is important in the regulation of DA levels in the PFC and striatum (Balla et al, 2009). Reducing GABA transmission in the mPFC with the GABAA receptor antagonist bicuculline or infusion of GLU increased DA release in the dorsal-STR and these effects were abolished by intracortical infusion of MK801 or the GABAA agonist muscimol (Matsumoto et al, 2003). Activation of NMDA receptors on striatal GABA neurons also facilitated GABA release (Morari et al, 1996; Morari et al, 1993, 1994; Young and Bradford, 1993). Thus, it is likely that GABA release is enhanced by a direct action of GLU on its NMDA receptors on GABA MS neurons or interneurons.
One possible limit to the relationship between changes in neurotransmission in the mPFC and dm-STR and attention performance in the 5-CSRT task is that in microdialysis experiments CPP was infused in behaviorally naive rats. Although it cannot be excluded that performance on the task affects neurotramsmitter levels in these areas, there is evidence that performance in the 5-CSRT task had no effect on dopamine, serotonin, or noradrenaline levels in the PFC (Dalley et al, 2001, 2002; Passetti et al, 2000).
There is also, evidence that DA governs GLU- and GABA-evoked responses in the striatum. In a condition of increased GLU, GABA, and DA release, the simultaneous activation of NMDA receptors coupled to D1-induced attenuation of GABA-evoked responses and lateral inhibition (Cepeda et al, 1993, 1998; Flores-Hernandez et al, 2002; Flores-Hernandez et al, 2000; Levine et al, 1996; Taverna et al, 2005) would predispose certain ensembles of MS neurons to become or remain dominant over other competing ensembles. The D1 receptor's action on this inhibition may represent a functional substrate for the filtering and selecting mechanism of attention in the striatum (Pennartz et al, 1994; Redgrave and Gurney, 2006). Further support for this hypothesis comes from data showing that a critical level of co-activation of NMDA and D1-like receptors in the dm-STR may be necessary for focusing attention (Agnoli and Carli, 2011).
The D2 receptors act by reducing the activity of L-type Ca2+ channels and also the excitability of MS neurons of the indirect pathway and their response to glutamatergic AMPA synaptic input (Hernandez-Lopez et al, 2000; Onn et al, 2000) whereas blockade of D2 receptors in the striatum increases the firing and responsiveness of striatal neurons to electrical stimulation of the PFC (West and Grace, 2002). Thus, overstimulation of D2-like receptors may shut down mPFC inputs, which might explain perseverative responding observed after quinpirole. D2 receptors in the striatum negatively control GABA release (Chapman and See, 1996; Robertson et al, 1992). In conditions of increased corticostriatal glutamate neurotransmission and increased GABA release, it is likely that the activity of MS striatopallidal output neurons is suppressed. Over-stimulation of D2-like receptors due to increased DA release may further attenuate the activity of MS neurons of the indirect pathway, leading to less inhibition of unwanted responses. Possibly, haloperidol, by reducing this GABA inhibition on the MS neurons of the indirect pathway, may prevent the rat from engaging in compulsive repetition of previously reinforced responses.
Conclusions
The findings suggest that under a condition of increased activity of corticostriatal inputs the dopamine D1-like receptor in the dorsal striatum is a key mechanism for the control of the input selection process of attention (Lustig et al, 2012), while dopamine D2-like receptors control the ability to switch from one act/response to the next in a complex motor sequence, permitting rapid adjustment of behavior. The data provide further evidence that NMDA receptor blockade activates corticostriatal projections, most likely by lowering inhibitory GABA tone on PFC pyramidal neurons. Thus, activation of DA neurotransmission in the dm-STR probably contributes, at least in part, to the impairment in attentional performance induced by blockade of NMDA receptors in the mPFC. These findings cast further light on the role of glutamate, GABA, and DA neurotransmission in corticostriatal circuitry that regulates attention and different aspects of response control, and possibly also on the neural pathology that underlies executive dysfunctions in some neuropsychiatric disorders where impairments in attention and behavioral flexibility are prominent.
Acknowledgments
We thank Professor Trevor W Robbins for his helpful discussion of these data. This work was supported by intramural funds. Laura Agnoli was a recipient of the fellowship of the Monzino Foundation, Milan, Italy.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Agnoli L, Carli M. Synergistic interaction of dopamine D(1) and glutamate N-methyl-d-aspartate receptors in the rat dorsal striatum controls attention. Neuroscience. 2011;185:39–49. doi: 10.1016/j.neuroscience.2011.04.044. [DOI] [PubMed] [Google Scholar]
- Amalric M, Ouagazzal A, Baunez C, Nieoullon A. Functional interactions between glutamate and dopamine in the rat striatum. Neurochem Int. 1994;25:123–131. doi: 10.1016/0197-0186(94)90031-0. [DOI] [PubMed] [Google Scholar]
- Anden NE, Hfuxe K, Hamberger B, Hokfelt T. A quantitative study on the nigro-neostriatal dopamine neuron system in the rat. Acta Physiol Scand. 1966;67:306–312. doi: 10.1111/j.1748-1716.1966.tb03317.x. [DOI] [PubMed] [Google Scholar]
- Arnsten AF. Catecholamine regulation of the prefrontal cortex. J Psychopharmacol. 1997;11:151–162. doi: 10.1177/026988119701100208. [DOI] [PubMed] [Google Scholar]
- Bailey KR, Mair RG. The role of striatum in initiation and execution of learned action sequences in rats. J Neurosci. 2006;26:1016–1025. doi: 10.1523/JNEUROSCI.3883-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balla A, Nattini ME, Sershen H, Lajtha A, Dunlop DS, Javitt DC. GABAB/NMDA receptor interaction in the regulation of extracellular dopamine levels in rodent prefrontal cortex and striatum. Neuropharmacology. 2009;56:915–921. doi: 10.1016/j.neuropharm.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balleine BW, O'Doherty JP. Human and rodent homologies in action control: corticostriatal determinants of goal-directed and habitual action. Neuropsychopharmacology. 2010;35:48–69. doi: 10.1038/npp.2009.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes SA, Young JW, Neill JC. D receptor activation improves vigilance in rats as measured by the 5-choice continuous performance test. Psychopharmacology (Berl) 2012;220:129–141. doi: 10.1007/s00213-011-2460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baunez C, Robbins TW. Effects of dopamine depletion of the dorsal striatum and further interaction with subthalamic nucleus lesions in an attentional task in the rat. Neuroscience. 1999;92:1343–1356. doi: 10.1016/s0306-4522(99)00065-2. [DOI] [PubMed] [Google Scholar]
- Baviera M, Invernizzi RW, Carli M. Haloperidol and clozapine have dissociable effects in a model of attentional performance deficits induced by blockade of NMDA receptors in the mPFC. Psychopharmacology (Berl) 2008;196:269–280. doi: 10.1007/s00213-007-0959-9. [DOI] [PubMed] [Google Scholar]
- Boulougouris V, Castane A, Robbins TW. Dopamine D2/D3 receptor agonist quinpirole impairs spatial reversal learning in rats: investigation of D3 receptor involvement in persistent behavior. Psychopharmacology (Berl) 2009;202:611–620. doi: 10.1007/s00213-008-1341-2. [DOI] [PubMed] [Google Scholar]
- Breier A, Malhotra AK, Pinals DA, Weisenfeld NI, Pickar D. Association of ketamine-induced psychosis with focal activation of the prefrontal cortex in healthy volunteers. Am J Psychiatry. 1997;154:805–811. doi: 10.1176/ajp.154.6.805. [DOI] [PubMed] [Google Scholar]
- Cagniard B, Beeler JA, Britt JP, McGehee DS, Marinelli M, Zhuang X. Dopamine scales performance in the absence of new learning. Neuron. 2006;51:541–547. doi: 10.1016/j.neuron.2006.07.026. [DOI] [PubMed] [Google Scholar]
- Calcagno E, Carli M, Baviera M, Invernizzi RW. Endogenous serotonin and serotonin2C receptors are involved in the ability of M100907 to suppress cortical glutamate release induced by NMDA receptor blockade. J Neurochem. 2009;108:521–532. doi: 10.1111/j.1471-4159.2008.05789.x. [DOI] [PubMed] [Google Scholar]
- Calcagno E, Invernizzi RW. Strain-dependent serotonin neuron feedback control: role of serotonin 2C receptors. J Neurochem. 2010;114:1701–1710. doi: 10.1111/j.1471-4159.2010.06880.x. [DOI] [PubMed] [Google Scholar]
- Carli M, Baviera M, Invernizzi RW, Balducci C. Dissociable contribution of 5-HT1A and 5-HT2A receptors in the medial prefrontal cortex to different aspects of executive control such as impulsivity and compulsive perseveration in rats. Neuropsychopharmacology. 2006;31:757–767. doi: 10.1038/sj.npp.1300893. [DOI] [PubMed] [Google Scholar]
- Carli M, Robbins TW, Evenden JL, Everitt BJ. Effects of lesions to ascending noradrenergic neurones on performance of a 5-choice serial reaction task in rats; implications for theories of dorsal noradrenergic bundle function based on selective attention and arousal. Behav Brain Res. 1983;9:361–380. doi: 10.1016/0166-4328(83)90138-9. [DOI] [PubMed] [Google Scholar]
- Carli M, Samanin R. Serotonin2 receptor agonists and serotonergic anorectic drugs affect rats' performance differently in a five-choice serial reaction time task. Psychopharmacology (Berl) 1992;106:228–234. doi: 10.1007/BF02801977. [DOI] [PubMed] [Google Scholar]
- Ceglia I, Carli M, Baviera M, Renoldi G, Calcagno E, Invernizzi RW. The 5-HT receptor antagonist M100,907 prevents extracellular glutamate rising in response to NMDA receptor blockade in the mPFC. J Neurochem. 2004;91:189–199. doi: 10.1111/j.1471-4159.2004.02704.x. [DOI] [PubMed] [Google Scholar]
- Cepeda C, Buchwald NA, Levine MS. Neuromodulatory actions of dopamine in the neostriatum are dependent upon the excitatory amino acid receptor subtypes activated. Proc Natl Acad Sci USA. 1993;90:9576–9580. doi: 10.1073/pnas.90.20.9576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepeda C, Colwell CS, Itri JN, Chandler SH, Levine MS. Dopaminergic modulation of NMDA-induced whole cell currents in neostriatal neurons in slices: contribution of calcium conductances. J Neurophysiol. 1998;79:82–94. doi: 10.1152/jn.1998.79.1.82. [DOI] [PubMed] [Google Scholar]
- Cepeda C, Hurst RS, Altemus KL, Flores-Hernandez J, Calvert CR, Jokel ES, et al. Facilitated glutamatergic transmission in the striatum of D2 dopamine receptor-deficient mice. J Neurophysiol. 2001;85:659–670. doi: 10.1152/jn.2001.85.2.659. [DOI] [PubMed] [Google Scholar]
- Chapman MA, See RE. Differential effects of unique profile antipsychotic drugs on extracellular amino acids in the ventral pallidum and globus pallidus of rats. J Pharmacol Exp Ther. 1996;277:1586–1594. [PubMed] [Google Scholar]
- Chase TN, Oh JD. Striatal dopamine- and glutamate-mediated dysregulation in experimental parkinsonism. Trends Neurosci. 2000;23 (10 Suppl:S86–91. doi: 10.1016/s1471-1931(00)00018-5. [DOI] [PubMed] [Google Scholar]
- Christakou A, Robbins TW, Everitt BJ. Functional disconnection of a prefrontal cortical-dorsal striatal system disrupts choice reaction time performance: implications for attentional function. Behav Neurosci. 2001;115:812–825. doi: 10.1037//0735-7044.115.4.812. [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Baunez C, Robbins TW. Functional disconnection of the medial prefrontal cortex and subthalamic nucleus in attentional performance: evidence for corticosubthalamic interaction. J Neurosci. 2003;23:5477–5485. doi: 10.1523/JNEUROSCI.23-13-05477.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chudasama Y, Muir JL. Visual attention in the rat: a role for the prelimbic cortex and thalamic nuclei. Behav Neurosci. 2001;115:417–428. [PubMed] [Google Scholar]
- Clarke HF, Robbins TW, Roberts AC. Lesions of the medial striatum in monkeys produce perseverative impairments during reversal learning similar to those produced by lesions of the orbitofrontal cortex. J Neurosci. 2008;28:10972–10982. doi: 10.1523/JNEUROSCI.1521-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole BJ, Robbins TW. Amphetamine impairs the discriminative performance of rats with dorsal noradrenergic bundle lesions on a 5-choice serial reaction time task: new evidence for central dopaminergic-noradrenergic interactions. Psychopharmacology (Berl) 1987;91:458–466. doi: 10.1007/BF00216011. [DOI] [PubMed] [Google Scholar]
- Cole BJ, Robbins TW. Effects of 6-hydroxydopamine lesions of the nucleus accumbens septi on performance of a 5-choice serial reaction time task in rats: implications for theories of selective attention and arousal. Behav Brain Res. 1989;33:165–179. doi: 10.1016/s0166-4328(89)80048-8. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Miezin FM, Dobmeyer S, Shulman GL, Petersen SE. Selective and divided attention during visual discriminations of shape, color, and speed: functional anatomy by positron emission tomography. J Neurosci. 1991;11:2383–2402. doi: 10.1523/JNEUROSCI.11-08-02383.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, McGaughy J, O'Connell MT, Cardinal RN, Levita L, Robbins TW. Distinct changes in cortical acetylcholine and noradrenaline efflux during contingent and noncontingent performance of a visual attentional task. J Neurosci. 2001;21:4908–4914. doi: 10.1523/JNEUROSCI.21-13-04908.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Theobald DE, Eagle DM, Passetti F, Robbins TW. Deficits in impulse control associated with tonically-elevated serotonergic function in rat prefrontal cortex. Neuropsychopharmacology. 2002;26:716–728. doi: 10.1016/S0893-133X(01)00412-2. [DOI] [PubMed] [Google Scholar]
- DeBiasi S, Minelli A, Melone M, Conti F. Presynaptic NMDA receptors in the neocortex are both auto- and heteroreceptors. Neuroreport. 1996;7:2773–2776. doi: 10.1097/00001756-199611040-00073. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Morelli M, Consolo S. Modulatory functions of neurotransmitters in the striatum: ACh/dopamine/NMDA interactions. Trends Neurosci. 1994;17:228–233. doi: 10.1016/0166-2236(94)90005-1. [DOI] [PubMed] [Google Scholar]
- Divac I, Rosvold HE, Szwarcbart MK. Behavioral effects of selective ablation of the caudate nucleus. J Comp Physiol Psychol. 1967;63:184–190. doi: 10.1037/h0024348. [DOI] [PubMed] [Google Scholar]
- Donzanti BA, Yamamoto BK. A rapid and simple HPLC microassay for biogenic amines in discrete brain regions. Pharmacol Biochem Behav. 1988;30:795–799. doi: 10.1016/0091-3057(88)90102-5. [DOI] [PubMed] [Google Scholar]
- Flores-Hernandez J, Cepeda C, Hernandez-Echeagaray E, Calvert CR, Jokel ES, Fienberg AA, et al. Dopamine enhancement of NMDA currents in dissociated medium-sized striatal neurons: role of D1 receptors and DARPP-32. J Neurophysiol. 2002;88:3010–3020. doi: 10.1152/jn.00361.2002. [DOI] [PubMed] [Google Scholar]
- Flores-Hernandez J, Hernandez S, Snyder GL, Yan Z, Fienberg AA, Moss SJ, et al. D(1) dopamine receptor activation reduces GABA(A) receptor currents in neostriatal neurons through a PKA/DARPP-32/PP1 signaling cascade. J Neurophysiol. 2000;83:2996–3004. doi: 10.1152/jn.2000.83.5.2996. [DOI] [PubMed] [Google Scholar]
- Fuster JM.1989The Prefrontal CortexSecond editionedn, Raven Press: New York [Google Scholar]
- Goto Y, Grace AA. Dopaminergic modulation of limbic and cortical drive of nucleus accumbens in goal-directed behavior. Nat Neurosci. 2005;8:805–812. doi: 10.1038/nn1471. [DOI] [PubMed] [Google Scholar]
- Gozzi A, Large CH, Schwarz A, Bertani S, Crestan V, Bifone A. Differential effects of antipsychotic and glutamatergic agents on the phMRI response to phencyclidine. Neuropsychopharmacology. 2008;33:1690–1703. doi: 10.1038/sj.npp.1301547. [DOI] [PubMed] [Google Scholar]
- Granon S, Passetti F, Thomas KL, Dalley JW, Everitt BJ, Robbins TW. Enhanced and impaired attentional performance after infusion of D1 dopaminergic receptor agents into rat prefrontal cortex. J Neurosci. 2000;20:1208–1215. doi: 10.1523/JNEUROSCI.20-03-01208.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graybiel AM. The basal ganglia and chunking of action repertoires. Neurobiol Learn Mem. 1998;70:119–136. doi: 10.1006/nlme.1998.3843. [DOI] [PubMed] [Google Scholar]
- Groman SM, Lee B, London ED, Mandelkern MA, James AS, Feiler K, et al. Dorsal striatal D2-like receptor availability covaries with sensitivity to positive reinforcement during discrimination learning. J Neurosci. 2011;31:7291–7299. doi: 10.1523/JNEUROSCI.0363-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haluk DM, Floresco SB. Ventral striatal dopamine modulation of different forms of behavioral flexibility. Neuropsychopharmacology. 2009;34:2041–2052. doi: 10.1038/npp.2009.21. [DOI] [PubMed] [Google Scholar]
- Hernandez-Lopez S, Tkatch T, Perez-Garci E, Galarraga E, Bargas J, Hamm H, et al. D2 dopamine receptors in striatal medium spiny neurons reduce L-type Ca2+ currents and excitability via a novel PLC[beta]1-IP3-calcineurin-signaling cascade. J Neurosci. 2000;20:8987–8995. doi: 10.1523/JNEUROSCI.20-24-08987.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersch SM, Ciliax BJ, Gutekunst CA, Rees HD, Heilman CJ, Yung KK, et al. Electron microscopic analysis of D1 and D2 dopamine receptor proteins in the dorsal striatum and their synaptic relationships with motor corticostriatal afferents. J Neurosci. 1995;15 (7 Pt 2:5222–5237. doi: 10.1523/JNEUROSCI.15-07-05222.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins GA, Enderlin M, Haman M, Fletcher PJ. The 5-HT2A receptor antagonist M100,907 attenuates motor and 'impulsive-type' behaviours produced by NMDA receptor antagonism. Psychopharmacology (Berl) 2003;170:309–319. doi: 10.1007/s00213-003-1549-0. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Miyashita K, Miyachi S, Sakai K, Lu X. Differential roles of the frontal cortex, basal ganglia, and cerebellum in visuomotor sequence learning. Neurobiol Learn Mem. 1998;70:137–149. doi: 10.1006/nlme.1998.3844. [DOI] [PubMed] [Google Scholar]
- Homayoun H, Moghaddam B. NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. J Neurosci. 2007;27:11496–11500. doi: 10.1523/JNEUROSCI.2213-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Invernizzi R, Pozzi L, Vallebuona F, Bonini I, Sacchetti G, Samanin R. Effect of amineptine on regional extracellular concentrations of dopamine and noradrenaline in the rat brain. J Pharmacol Exp Ther. 1992;262:769–774. [PubMed] [Google Scholar]
- Jackson ME, Homayoun H, Moghaddam B. NMDA receptor hypofunction produces concomitant firing rate potentiation and burst activity reduction in the prefrontal cortex. Proc Natl Acad Sci USA. 2004;101:8467–8472. doi: 10.1073/pnas.0308455101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry. 1991;148:1301–1308. doi: 10.1176/ajp.148.10.1301. [DOI] [PubMed] [Google Scholar]
- Jin X, Costa RM. Start/stop signals emerge in nigrostriatal circuits during sequence learning. Nature. 2010;466:457–462. doi: 10.1038/nature09263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellendonk C, Simpson EH, Polan HJ, Malleret G, Vronskaya S, Winiger V, et al. Transient and selective overexpression of dopamine D2 receptors in the striatum causes persistent abnormalities in prefrontal cortex functioning. Neuron. 2006;49:603–615. doi: 10.1016/j.neuron.2006.01.023. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, et al. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry. 1994;51:199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- Lee B, London ED, Poldrack RA, Farahi J, Nacca A, Monterosso JR, et al. Striatal dopamine d2/d3 receptor availability is reduced in methamphetamine dependence and is linked to impulsivity. J Neurosci. 2009;29:14734–14740. doi: 10.1523/JNEUROSCI.3765-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine MS, Altemus KL, Cepeda C, Cromwell HC, Crawford C, Ariano MA, et al. Modulatory actions of dopamine on NMDA receptor-mediated responses are reduced in D1A-deficient mutant mice. J Neurosci. 1996;16:5870–5882. doi: 10.1523/JNEUROSCI.16-18-05870.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustig C, Kozak R, Sarter M, Young JW, Robbins TW.2012CNTRICS final animal model task selection: control of attention Neurosci Biobehav Reve-pub ahead of print 5 June 2012. [DOI] [PMC free article] [PubMed]
- Matsumoto M, Kanno M, Togashi H, Ueno K, Otani H, Mano Y, et al. Involvement of GABAA receptors in the regulation of the prefrontal cortex on dopamine release in the rat dorsolateral striatum. Eur J Pharmacol. 2003;482:177–184. doi: 10.1016/j.ejphar.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Mirjana C, Baviera M, Invernizzi RW, Balducci C. The serotonin 5-HT2A receptors antagonist M100907 prevents impairment in attentional performance by NMDA receptor blockade in the rat prefrontal cortex. Neuropsychopharmacology. 2004;29:1637–1647. doi: 10.1038/sj.npp.1300479. [DOI] [PubMed] [Google Scholar]
- Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: from structure to function. Physiol Rev. 1998;78:189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- Moghaddam B, Adams B, Verma A, Daly D. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci. 1997;17:2921–2927. doi: 10.1523/JNEUROSCI.17-08-02921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam B, Adams BW. Reversal of phencyclidine effects by a group II metabotropic glutamate receptor agonist in rats. Science. 1998;281:1349–1352. doi: 10.1126/science.281.5381.1349. [DOI] [PubMed] [Google Scholar]
- Morari M, O'Connor WT, Ungerstedt U, Fuxe K. Dopamine D1 and D2 receptor antagonism differentially modulates stimulation of striatal neurotransmitter levels by N-methyl-D-aspartic acid. Eur J Pharmacol. 1994;256:23–30. doi: 10.1016/0014-2999(94)90611-4. [DOI] [PubMed] [Google Scholar]
- Morari M, O'Connor WT, Ungerstedt U, Bianchi C, Fuxe K. Functional neuroanatomy of the nigrostriatal and striatonigral pathways as studied with dual probe microdialysis in the awake rat--II. Evidence for striatal N-methyl-D-aspartate receptor regulation of striatonigral GABAergic transmission and motor function. Neuroscience. 1996;72:89–97. doi: 10.1016/0306-4522(95)00556-0. [DOI] [PubMed] [Google Scholar]
- Morari M, O'Connor WT, Ungerstedt U, Fuxe K. N-methyl-D-aspartic acid differentially regulates extracellular dopamine, GABA, and glutamate levels in the dorsolateral neostriatum of the halothane-anesthetized rat: an in vivo microdialysis study. J Neurochem. 1993;60:1884–1893. doi: 10.1111/j.1471-4159.1993.tb13416.x. [DOI] [PubMed] [Google Scholar]
- Onn SP, West AR, Grace AA. Dopamine-mediated regulation of striatal neuronal and network interactions. Trends Neurosci. 2000;23 (10 Suppl:S48–56. doi: 10.1016/s1471-1931(00)00020-3. [DOI] [PubMed] [Google Scholar]
- Owen AM, James M, Leigh PN, Summers BA, Marsden CD, Quinn NP, et al. Fronto-striatal cognitive deficits at different stages of Parkinson's disease. Brain. 1992;115 (Pt 6:1727–1751. doi: 10.1093/brain/115.6.1727. [DOI] [PubMed] [Google Scholar]
- Passetti F, Dalley JW, O'Connell MT, Everitt BJ, Robbins TW. Increased acetylcholine release in the rat medial prefrontal cortex during performance of a visual attentional task. Eur J Neurosci. 2000;12:3051–3058. doi: 10.1046/j.1460-9568.2000.00183.x. [DOI] [PubMed] [Google Scholar]
- Passetti F, Levita L, Robbins TW. Sulpiride alleviates the attentional impairments of rats with medial prefrontal cortex lesions. Behav Brain Res. 2003;138:59–69. doi: 10.1016/s0166-4328(02)00229-2. [DOI] [PubMed] [Google Scholar]
- Pattij T, Janssen MC, Vanderschuren LJ, Schoffelmeer AN, van Gaalen MM. Involvement of dopamine D1 and D2 receptors in the nucleus accumbens core and shell in inhibitory response control. Psychopharmacology (Berl) 2007;191:587–598. doi: 10.1007/s00213-006-0533-x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press: Sidney; 1998. [Google Scholar]
- Pennartz CM, Groenewegen HJ, Lopes da Silva FH. The nucleus accumbens as a complex of functionally distinct neuronal ensembles: an integration of behavioural, electrophysiological and anatomical data. Prog Neurobiol. 1994;42:719–761. doi: 10.1016/0301-0082(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Pezze MA, Dalley JW, Robbins TW. Differential roles of dopamine D1 and D2 receptors in the nucleus accumbens in attentional performance on the five-choice serial reaction time task. Neuropsychopharmacology. 2007;32:273–283. doi: 10.1038/sj.npp.1301073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezze MA, Dalley JW, Robbins TW. Remediation of attentional dysfunction in rats with lesions of the medial prefrontal cortex by intra-accumbens administration of the dopamine D(2/3) receptor antagonist sulpiride. Psychopharmacology (Berl) 2009;202:307–313. doi: 10.1007/s00213-008-1384-4. [DOI] [PubMed] [Google Scholar]
- Ragozzino ME. The effects of dopamine D(1) receptor blockade in the prelimbic-infralimbic areas on behavioral flexibility. Learn Mem. 2002;9:18–28. doi: 10.1101/lm.45802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redgrave P, Gurney K. The short-latency dopamine signal: a role in discovering novel actions. Nat Rev Neurosci. 2006;7:967–975. doi: 10.1038/nrn2022. [DOI] [PubMed] [Google Scholar]
- Ridley RM, Clark BA, Durnford LJ, Baker HF. Stimulus-bound perseveration after frontal ablations in marmosets. Neuroscience. 1993;52:595–604. doi: 10.1016/0306-4522(93)90409-9. [DOI] [PubMed] [Google Scholar]
- Robbins TW. The case of frontostriatal dysfunction in schizophrenia. Schizophr Bull. 1990;16:391–402. doi: 10.1093/schbul/16.3.391. [DOI] [PubMed] [Google Scholar]
- Robbins TW. The 5-choice serial reaction time task: behavioural pharmacology and functional neurochemistry. Psychopharmacology (Berl) 2002;163:362–380. doi: 10.1007/s00213-002-1154-7. [DOI] [PubMed] [Google Scholar]
- Robbins TW.2005Role of cortical and striatal dopamine in cognitive functionIn: Dunnett SB, Bentivoglio M, Bjorklund A (eds)Dopamine Vol 21Elsevier B. V.: Amsterdam; 395–434. [Google Scholar]
- Robertson GS, Vincent SR, Fibiger HC. D1 and D2 dopamine receptors differentially regulate c-fos expression in striatonigral and striatopallidal neurons. Neuroscience. 1992;49:285–296. doi: 10.1016/0306-4522(92)90096-k. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Whishaw IQ. Normalization of extracellular dopamine in striatum following recovery from a partial unilateral 6-OHDA lesion of the substantia nigra: a microdialysis study in freely moving rats. Brain Res. 1988;450:209–224. doi: 10.1016/0006-8993(88)91560-0. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Baunez C, Everitt BJ, Robbins TW. Lesions of the medial and lateral striatum in the rat produce differential deficits in attentional performance. Behav Neurosci. 2001;115:799–811. doi: 10.1037//0735-7044.115.4.799. [DOI] [PubMed] [Google Scholar]
- Sawaguchi T, Goldman-Rakic PS. D1 dopamine receptors in prefrontal cortex: involvement in working memory. Science. 1991;251:947–950. doi: 10.1126/science.1825731. [DOI] [PubMed] [Google Scholar]
- Smith Y, Bevan MD, Shink E, Bolam JP. Microcircuitry of the direct and indirect pathways of the basal ganglia. Neuroscience. 1998;86:353–387. doi: 10.1016/s0306-4522(98)00004-9. [DOI] [PubMed] [Google Scholar]
- Sokoloff P, Giros B, Martres MP, Bouthenet ML, Schwartz JC. Molecular cloning and characterization of a novel dopamine receptor (D3) as a target for neuroleptics. Nature. 1990;347:146–151. doi: 10.1038/347146a0. [DOI] [PubMed] [Google Scholar]
- Taverna S, Canciani B, Pennartz CM. Dopamine D1-receptors modulate lateral inhibition between principal cells of the nucleus accumbens. J Neurophysiol. 2005;93:1816–1819. doi: 10.1152/jn.00672.2004. [DOI] [PubMed] [Google Scholar]
- Ungerstedt U. Postsynaptic supersensitivity after 6-hydroxy-dopamine induced degeneration of the nigro-striatal dopamine system. Acta Physiol Scand Suppl. 1971;367:69–93. doi: 10.1111/j.1365-201x.1971.tb11000.x. [DOI] [PubMed] [Google Scholar]
- van Schouwenburg MR, den Ouden HE, Cools R. The human basal ganglia modulate frontal-posterior connectivity during attention shifting. J Neurosci. 2010;30:9910–9918. doi: 10.1523/JNEUROSCI.1111-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollenweider FX, Leenders KL, Oye I, Hell D, Angst J. Differential psychopathology and patterns of cerebral glucose utilisation produced by (S)- and (R)-ketamine in healthy volunteers using positron emission tomography (PET) Eur Neuropsychopharmacol. 1997;7:25–38. doi: 10.1016/s0924-977x(96)00042-9. [DOI] [PubMed] [Google Scholar]
- Wang H, Pickel VM. Dopamine D2 receptors are present in prefrontal cortical afferents and their targets in patches of the rat caudate-putamen nucleus. J Comp Neurol. 2002;442:392–404. doi: 10.1002/cne.10086. [DOI] [PubMed] [Google Scholar]
- West AR, Grace AA. Opposite influences of endogenous dopamine D1 and D2 receptor activation on activity states and electrophysiological properties of striatal neurons: studies combining in vivo intracellular recordings and reverse microdialysis. J Neurosci. 2002;22:294–304. doi: 10.1523/JNEUROSCI.22-01-00294.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonezawa Y, Kuroki T, Kawahara T, Tashiro N, Uchimura H. Involvement of gamma-aminobutyric acid neurotransmission in phencyclidine-induced dopamine release in the medial prefrontal cortex. Eur J Pharmacol. 1998;341:45–56. doi: 10.1016/s0014-2999(97)01435-0. [DOI] [PubMed] [Google Scholar]
- Young AM, Bradford HF. N-methyl-D-aspartate releases gamma-aminobutyric acid from rat striatum in vivo: a microdialysis study using a novel preloading method. J Neurochem. 1993;60:487–492. doi: 10.1111/j.1471-4159.1993.tb03176.x. [DOI] [PubMed] [Google Scholar]
- Zahrt J, Taylor JR, Mathew RG, Arnsten AF. Supranormal stimulation of D1 dopamine receptors in the rodent prefrontal cortex impairs spatial working memory performance. J Neurosci. 1997;17:8528–8535. doi: 10.1523/JNEUROSCI.17-21-08528.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.