Abstract
Patients with epilepsy are at high risk for major depression relative to the general population, and both disorders are associated with changes in adult hippocampal neurogenesis, although the mechanisms underlying disease onset remain unknown. The expression of fosB, an immediate early gene encoding FosB and ΔFosB/Δ2ΔFosB by alternative splicing and translation initiation, is known to be induced in neural progenitor cells within the subventricular zone of the lateral ventricles and subgranular zone of the hippocampus, following transient forebrain ischemia in the rat brain. Moreover, adenovirus-mediated expression of fosB gene products can promote neural stem cell proliferation. We recently found that fosB-null mice show increased depressive behavior, suggesting impaired neurogenesis in fosB-null mice. In the current study, we analyzed neurogenesis in the hippocampal dentate gyrus of fosB-null and fosBd/d mice that express ΔFosB/Δ2ΔFosB but not FosB, in comparison with wild-type mice, alongside neuropathology, behaviors, and gene expression profiles. fosB-null but not fosBd/d mice displayed impaired neurogenesis in the adult hippocampus and spontaneous epilepsy. Microarray analysis revealed that genes related to neurogenesis, depression, and epilepsy were altered in the hippocampus of fosB-null mice. Thus, we conclude that the fosB-null mouse is the first animal model to provide a genetic and molecular basis for the comorbidity between depression and epilepsy with abnormal neurogenesis, all of which are caused by loss of a single gene, fosB.
Keywords: immediate early gene, alternative splicing, adult neurogenesis, neural progenitor, proliferation, epilepsy
INTRODUCTION
Neurological and psychiatric disorders are highly prevalent in the general population. It has been shown that patients with epilepsy are at high risk for major depression relative to the general population, with the incidence of depression being 5–20 times higher, but it is very often unrecognized and untreated (Danzer, 2012). Decreased neurogenesis in the adult hippocampus has been implicated in the pathogenesis of anxiety and depression (Snyder et al, 2011). In addition, adult hippocampal neurogenesis is known to be required for mediating the behavioral effects of antidepressants and electroconvulsive therapy (ECT) (Danzer, 2012).
In the adult rodent hippocampus, neurogenesis and expression of fosB (Fosb), an immediate early gene are increased by seizures induced by chemoconvulsants, such as kainic acid (KA), electroconvulsive seizures (ECS), an animal model of ECT, and forebrain ischemia. Among fos family genes, only the fosB gene produces multiple protein products, namely FosB, ΔFosB, and Δ2ΔFosB, by alternative splicing and translation initiation (Chen et al, 1997; Kurushima et al, 2005; Mandelzys et al, 1997; Morris et al, 2000; Nakabeppu and Nathans, 1991; Ohnishi et al, 2008; Ohnishi et al, 2011; Sabatakos et al, 2008). ΔFosB is a translation product of ΔfosB mRNA generated by alternative splicing, and lacks the C-terminal 101 amino acids containing the transactivation domain in the full-length FosB protein (Nakabeppu and Nathans, 1991). Δ2ΔFosB is an N-terminally truncated isoform of ΔFosB, which arises as a consequence of alternative initiation of translation from Met79 of the ΔfosB mRNA and lacks the N-terminal Fos homology domain as well as the C-terminal transactivation domain (Sabatakos et al, 2008). FosB and ΔFosB act in opposition to regulate Jun transactivity, and also regulate cell proliferation, differentiation, and cell death (Kurushima et al, 2005; Nakabeppu et al, 1993; Tahara et al, 2003). ΔFosB, but not Δ2ΔFosB, itself can act as a transcriptional activator or repressor (McClung and Nestler, 2003; Renthal et al, 2008; Sabatakos et al, 2008).
We recently reported that fosB-null mice exhibit depressive-like behaviors, including decreased locomotion, increased immobility during forced swimming, and increased anxiety-like responses (Ohnishi et al, 2011). Interestingly, fosBd/d mice, expressing fosBd alleles coding only the ΔFosB and Δ2ΔFosB proteins, did not show depressive-like behaviors, indicating ΔFosB/Δ2ΔFosB can in part suppress the appearance of depressive-like behaviors. We have also observed that following transient forebrain ischemia in the rat brain, expression of ΔFosB, and to a lesser extent FosB, is induced in neural progenitor cells within the subventricular zone of the lateral ventricles and subgranular zone (SGZ) of the hippocampal dentate gyrus (DG). Furthermore, adenovirus-mediated expression of ΔFosB, and to a lesser extent FosB, promoted neural stem cell proliferation, whereas inhibition of FosB and ΔFosB expression caused neuronal differentiation in rat embryonic cortical cell cultures (Kurushima et al, 2005).
Based on these findings, we hypothesized that the fosB products, FosB, ΔFosB, and/or Δ2ΔFosB, promote basal and injury-induced neurogenesis in the adult hippocampus, and are involved in depression and epilepsy. To test our hypothesis, we analyzed neurogenesis in the hippocampal DG, as well as neuropathology and behaviors, of fosB-null and fosBd/d mice. We show that fosB-null mice exhibit impaired neurogenesis and spontaneous epilepsy, in addition to depressive-like behaviors, while fosBd/d mice do not.
MATERIALS AND METHODS
Animals
fosB+/d mice with a mutant fosBd allele encoding ΔFosB and Δ2ΔFosB but not FosB, and fosB+/G mice with a null-mutant allele, fosBG, were established. Heterozygous (fosB+/d and fosB+/G) mice were backcrossed onto C57BL/6J males (Clea Japan, Tokyo, Japan) and at least one time onto C57BL/6J females for maintenance of the strains (Ohnishi et al, 2011). To yield homozygous mutant mice, fosBd/d and fosB-null (fosBG/G), fosB+/d (N17 generation), or fosB+/G (N12 generation) mice were intercrossed. Homozygous fosBd/d and fosB-null (fosBG/G) mutant mice lines were maintained by inbreeding. Third and fourth generations were used in the present study. C57BL/6J mice were used as wild-type controls for all experiments. Genotyping of the mutant alleles and animal housing conditions are provided in Supplementary Materials and Methods.
KA Treatment
Eight- to Ten-week-old male mice were used in this study. Mice were injected intraperitoneally with either saline (untreated control) or 25 mg/kg KA (Wako, Osaka, Japan). They were then injected twice daily for 3 days with 50 mg/kg BrdU (Sigma-Aldrich Japan, Tokyo, Japan). To determine the intensity of epileptiform seizures, we observed mice 2 h after KA administration and scored the seizure according to previously described criteria (Kajitani et al, 2006; Supplementary Materials and Methods). Animals were killed 1 day after the last BrdU injection, unless otherwise stated.
Antibodies
All antibodies used in the present study are described in Supplementary Materials and Methods.
Tissue Processing and Immunohistochemistry
Brain tissues were prepared and serial coronal sections (40 μm) were cut on a cryostat and collected as free-floating sections. The floating sections were subjected to immunohistochemistry. Detailed methods for tissue processing and immunohistochemistry are in Supplementary Materials and Methods.
Western Blotting
Nuclear extracts were prepared and subjected to western blotting (Supplementary Materials and Methods).
Laser Scanning Confocal Immunofluorescence Microscopy
Free-floating sections were subjected to immunofluorescence microscopy as described in Supplementary Materials and Methods. Confocal images were acquired using an LSM510 META Confocal Microscope System (Carl Zeiss Microimaging Japan, Tokyo, Japan). All sections from each experimental animal, and groups to be compared, were processed in parallel.
Counting BrdU-Positive Cells
Free-floating sections were subjected to BrdU immunodetection, and the density of BrdU-positive cells in the granule cell layer (GCL) or hilus of the DG was determined using the fractionator program of the Stereo Investigator system (MBF Bioscience, Williston, VT) (Supplementary Materials and Methods).
Observation of Spontaneous Seizures
We observed behavioral seizure activity in animals during cage changing for ∼20–30 min per week, and scored them as described previously (Kajitani et al, 2006). We only scored stage 3–5 seizures as seizure episodes, as stage 1–2 seizures were hard to distinguish from normal behavior.
Recording of Electroencephalogram Discharges for Spontaneous Seizures
To investigate spontaneous electrical seizures using electroencephalogram (EEG) recordings, mice were grouped as follows: clinical seizures observed, fosB-null mice; clinical seizures not observed, fosB-null mice; fosBd/d mice and wild-type mice. Recording electrodes for EEG monitoring were placed on the cerebral cortices and hippocampi of mice. The protocol was identical for all three groups (Supplementary Materials and Methods).
Microarray Hybridization and Data Analysis
Total RNA from the hippocampi of wild-type, fosBd/d, and fosB-null mice was prepared using ISOGEN (Nippon Gene, Tokyo, Japan) and the RNeasy Mini Kit (Qiagen, Tokyo, Japan). For DNA microarray experiments, total RNA (100 ng) was added to poly-A control and treated using the WT Expression Kit (Ambion, Austin, TX, USA), according to manufacturer's instructions. cDNA was labeled using the WT terminal labeling kit (Affymetrix Japan, Tokyo, Japan) and hybridized against the GeneChip Mouse Gene 1.0 ST array (Affymetrix). The resulting list of expressed probes was imported into GeneSpring GX 10.0 (Agilent Technologies, Palo Alto, CA, USA) for data analysis. The microarray data discussed in this publication have been deposited in the NCBI's Gene Expression Omnibus and are accessible through GEO Series accession number, GSE37056.
Real-Time RT–PCR Analysis
Total RNA was prepared from the hippocampus of mice using ISOGEN. First-strand cDNA was synthesized using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems Japan, Tokyo, Japan), according to manufacturer's instructions. For quantitative real-time RT–PCR of Vgf, Smad3, Galanin, Trh, and Sema3e mRNA, reactions were performed in triplicate as described in Supplementary Materials and Methods.
Statistical Analysis
Stereological counts were compared by analysis of variance (ANOVA), by fitting a linear model by least squares, followed by Tukey's honestly significant difference (HSD) test. Microarray data were compared using unpaired t-tests or two-way ANOVA. The p-values were adjusted for false discovery using the Benjamini-Hochberg procedure (Benjamini and Hochberg, 1995). The rest of the data were compared using the Kaplan–Meier method and log-rank tests, or Fisher's exact test as indicated. A value of p<0.05 was considered as statistically significant. Except for microarray analysis data, all statistical analyses were carried out using JMP 9.02 software (SAS Institute, Cary, NC, USA).
RESULTS
Induced Expression of fosB Products in Activated Neural Progenitors, Immature-Newborn and Mature Neurons in the DG After KA Treatment
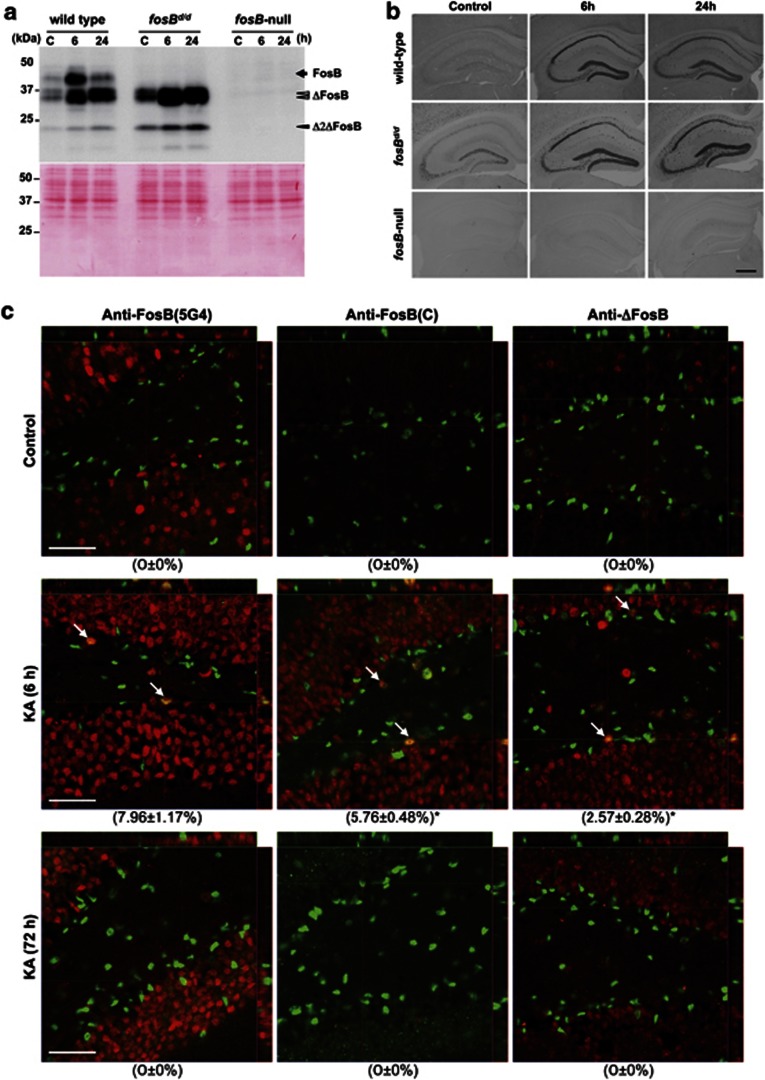
Western blotting analysis of hippocampal nuclear extracts using anti-FosB (5G4), which recognizes all fosB products (FosB, ΔFosB, and Δ2ΔFosB), detected a 43-kDa polypeptide, representing the full-length FosB protein, in wild-type mice only. While 32/36-kDa and 24-kDa polypeptides, representing ΔFosB and Δ2ΔFosB proteins respectively, were detected in wild-type and fosBd/d mice (Figure 1a). No fosB products were detected in fosB-null mice.
Figure 1.
Wild-type, fosBd/d, and fosB-null mice showed differential expression of FosB, ΔFosB, and Δ2ΔFosB in the hippocampus. (a) Western blotting of fosB products in hippocampal nuclear extracts. Nuclear extracts (12.5 μg protein per lane) were prepared from hippocampi at indicated times after saline (C) or KA (6 h, 24 h) administration, and were subjected to western blotting with anti-FosB (5G4) (upper panel). fosB products are highlighted: black arrow (FosB), open arrowheads (ΔFosB), and closed arrowhead (Δ2ΔFosB). Blots were post-stained with Ponceau S (lower panel). Quantified results are shown in Supplementary Figure S1a. (b) Immunohistochemistry of fosB products at 0 h (control), 6 h, or 24 h after KA administration in the adult hippocampus of wild-type, fosBd/d, and fosB-null mice. Anti-FosB (N) was used to detect fosB products (FosB, ΔFosB, and Δ2ΔFosB). Scale bar: 500 μm. (c) Laser scanning confocal immunofluorescence microscopy of fosB products at 0 h (control), 6 h, or 72 h after KA administration in the DG of wild-type mice. Three different antibodies against fosB products, anti-FosB (5G4), anti-FosB (C), and anti-ΔFosB were used to detect fosB products (red). SOX2 protein (green), a marker for neural progenitor cells, was dominantly detected in SGZ of the DG. Arrows indicate cells expressing both fosB products and SOX2. Orthogonal projections throughout the cells are shown. Scale bars: 50 μm. Percentage of anti-FosB IR-positive cells in the SOX2-positive population is shown in parentheses with SEM. More than 100 SOX2-positive cells in each animal (N=3) were observed. *p=0.0421 (anti-FosB (C) IR vs anti-ΔFosB IR-positive population 6 h after KA administration, Fisher's exact test).
Basal levels of ΔFosB and Δ2ΔFosB proteins were marginally higher in fosBd/d compared with wild-type mice. Six hours after KA administration, there was a transient increase in FosB levels in wild-type mice. Levels of ΔFosB and Δ2ΔFosB were stably increased within 24 h after KA administration in both wild-type and fosBd/d mice (Supplementary Figure S1a).
Immunohistochemistry using anti-FosB (N) or anti-FosB(5G4) in untreated wild-type hippocampus detected sparse expression of fosB products in most hippocampal neurons. Levels of fosB products were increased after KA administration (Figure 1b; Supplementary Figure S1b). In wild-type mice, immunoreactivity (IR) in DG granule neurons was highest among all subregions of the hippocampus, and maximal 6 h after KA administration (Figure 1b; Supplementary Figures S1b–d). A similar increase was observed in the hippocampus from fosBd/d mice, 6–24 h after KA administration. No anti-FosB (N) IR was detected in fosB-null hippocampus (Figure 1b).
To distinguish expression of FosB and ΔFosB/Δ2ΔFosB in wild-type hippocampus, we performed laser scanning confocal immunofluorescence microscopy (LSCIM), using three different antibodies against fosB products, anti-FosB (5G4), anti-FosB (C), and anti-ΔFosB. The latter two antibodies recognize FosB and ΔFosB/Δ2ΔFosB, respectively (Figure 1c). Anti-FosB (C) IR was barely detectable in untreated hippocampus, and was transiently detected in the hippocampus 6 h after KA administration. Seventy-two hours after KA administration, anti-FosB (C) IR had returned to basal levels. Conversely, anti-ΔFosB IR was strongly detected in most neurons from untreated hippocampus, with significantly increased levels observed in hippocampal neurons from 6 to 72 h after KA administration. Moreover, we found that a number of anti-FosB (C) and anti-ΔFosB IR-positive cells, detected in the SGZ of DG 6 h after KA administration, were SOX2 positive, suggesting that expression of both FosB and ΔFosB/Δ2ΔFosB was transiently induced in neural progenitor cells (Lugert et al, 2010).
Strong anti-FosB (5G4) IR was detected in the majority of NeuN-positive neurons throughout the hippocampus, 72 h after KA administration (Supplementary Figure S2), suggesting that increased expression of ΔFosB/Δ2ΔFosB is chronically maintained in most hippocampal neurons after KA administration. After KA administration, anti-FosB (5G4) IR was detected in a few star-shaped glial fibrillary acidic protein (GFAP)-positive astrocytes in the DG hilus (arrowheads in Supplementary Figure S3, left panels), but not in radial glia-like GFAP-positive cells, that have a radial process spanning the entire GCL and ramify in the inner molecular layer of the DG. Anti-FosB (5G4) IR was not observed in Ki67-positive proliferating cells detected in the SGZ, with or without KA administration (Supplementary Figure S3, middle). A proportion of doublecortin (DCX)-positive cells with axon-like processes in the GCL, representing immature newborn neurons (Lugert et al, 2010), exhibited relatively weak anti-FosB (5G4) IR 72 h after KA administration (arrows in Supplementary Figure S3, right panels).
Overall, our results show that excitotoxicity transiently induces FosB expression in SOX2-positive/non-proliferating neural progenitors in the DG, as well as in mature neurons of the hippocampus. Moreover, expression of ΔFosB/Δ2ΔFosB is transiently induced in SOX2-positive neural progenitors, but chronically in mature hippocampal neurons, and to a lesser extent in immature newborn neurons of the DG (Supplementary Figure S4).
fosB-Null Mice Show Decreased Proliferation and Increased Ectopic Migration of Neural Progenitor Cells in the Adult Hippocampus
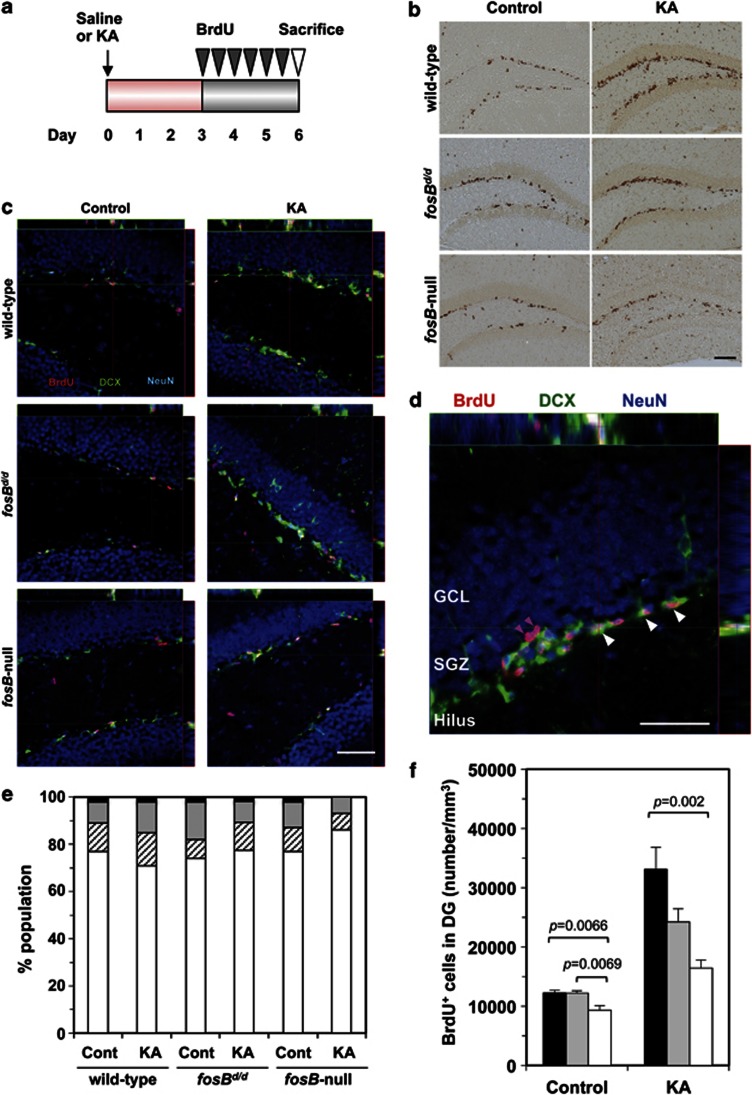
To determine if fosB products affect proliferation of neural progenitor cells in the adult hippocampus, we injected BrdU twice daily for 3 days in wild-type, fosBd/d, and fosB-null mice, following KA or saline injection. Brain sections were prepared 1 day later (Figure 2a). BrdU-positive cells were predominantly distributed in the SGZ (Figure 2b). To confirm that BrdU-positive cells represented newborn neurons, LSCIM was performed using antibodies against BrdU, DCX, and NeuN (Figure 2c; Supplementary Figure S5). Irrespective of KA administration or genotype, >70% of BrdU-positive cells in the DG expressed both DCX and NeuN (Figure 2d and e). The remainder of BrdU-positive cells expressed either DCX or NeuN. Thus, the majority of BrdU-positive cells detected (Figure 2b) represented newborn neurons in the DG.
Figure 2.
Adult fosB-null mice show reduced basal and KA-induced proliferation of neural progenitor cells in the hippocampus. (a) Experimental design. For 3 days following saline (control) or KA injection, mice were injected with BrdU twice daily. Brain sections were prepared on the 6th day, and BrdU-positive cells were detected. (b) Immunohistochemistry for BrdU in adult DG of wild-type, fosBd/d, and fosB-null mice, following saline (control) or KA injection. Scale bar: 200 μm. (c) Laser scanning confocal immunofluorescence microscopy for DCX (green) and NeuN (blue), both neuronal lineage markers, and BrdU (red). Scale bar: 50 μm. (d) Phenotype of BrdU-positive cells in the DG. Orthogonal projections throughout the cells are shown to demonstrate triple (BrdU+/DCX+/NeuN+ shown with white arrowheads) and double-labeled (BrdU+/NeuN+ shown with magenta arrowheads) cells. BrdU+ single-labeled or BrdU+/DCX+ double-labeled cells are not seen in this panel. Scale bar: 50 μm. (e) fosB products do not affect differentiation of newborn neurons. One hundred BrdU-positive cells, within immunofluorescence-stained sections from each animal, and two animals for each group, were examined by confocal microscopy to determine the phenotype of BrdU-positive cells. In all three genotypes, the majority of BrdU-positive cells express DCX and/or NeuN. Bar graphs show percentages of BrdU+/DCX−/NeuN− (black), BrdU+/DCX−/NeuN+ (gray), BrdU+/DCX+/NeuN− (hatched), and BrdU+/DCX+/NeuN+ (open) cells. (f) Density of BrdU-positive cells in the DG was determined. N=5 in each group; error bars, mean±SEM; black bars, wild type; gray bars, fosBd/d; open bars, fosB-null. One-way ANOVA (control, F2,12=9.56, p=0.0033; KA, F2,12=10.08, p=0.0027). The p-values (Tukey–Kramer HSD post hoc comparison) are shown.
Next, we estimated the density of BrdU-positive cells in the DG using stereological counting methods (Figure 2f). The density of BrdU-positive cells in controls, without KA administration, was significantly decreased in fosB-null compared with wild-type mice (p=0.0066, Tukey–Kramer HSD post hoc comparison), while there was a slight, albeit non-significant, increase in fosBd/d mice (Figure 2f, left). In all three genotypes, KA administration significantly increased the density of BrdU-positive cells in comparison with each untreated control (wild type, p=0.0006; fosBd/d, p=0.0006; fosB-null, p=0.0015, t-test). The density of BrdU-positive cells in fosB-null mice, after KA administration, was significantly lower compared with wild-type mice (p=0.002, Tukey–Kramer HSD post hoc comparison) (Figure 2f, right).
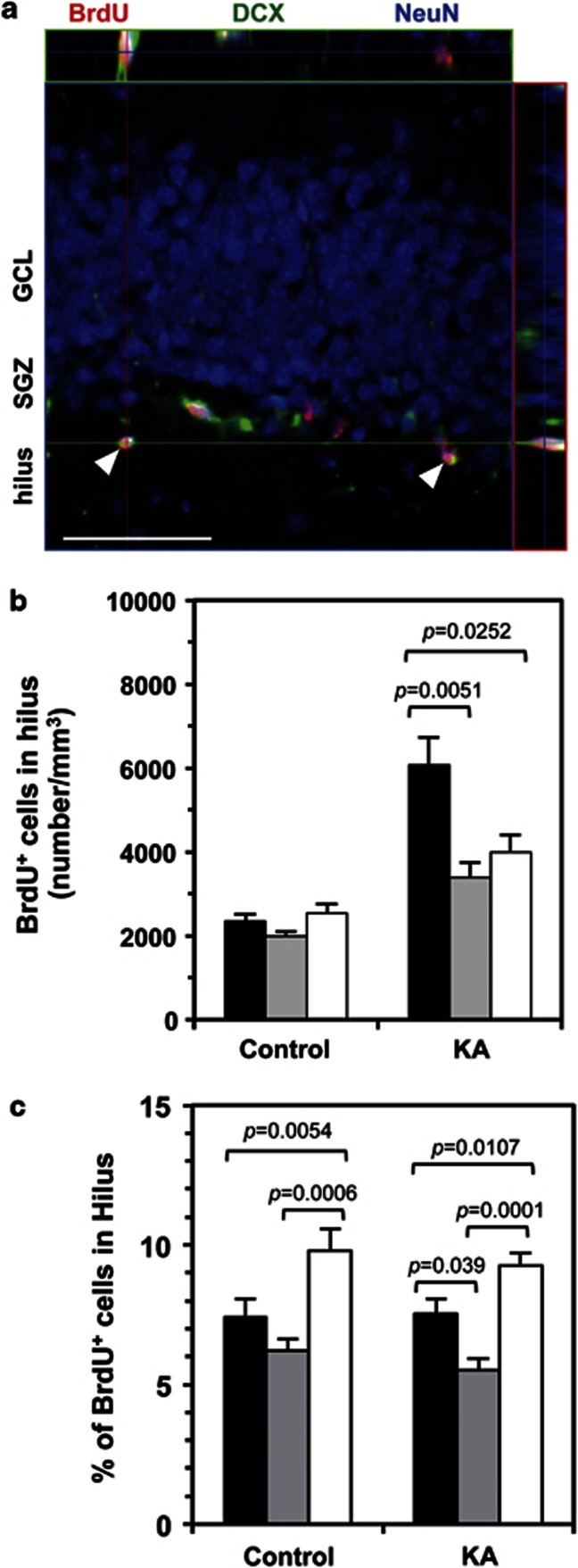
LSCIM identified the majority of BrdU-positive cells in the hilus as newborn neurons, expressing DCX and NeuN, either individually or in combination (Figure 3a). We compared the density of BrdU-positive cells in the hilus, and found that without KA administration, fosB-null mice had a slightly higher density of BrdU-positive cells compared with wild-type and fosBd/d mice. Following KA administration, the density of BrdU-positive cells in both fosBd/d and fosB-null mice was increased slightly, but to a lesser extent compared with wild-type mice (Figure 3b). The percentage of BrdU-positive cells in the hilus (calculated from the total number of BrdU-positive cells in the whole DG) was highest in fosB-null and lowest in fosBd/d mice, regardless of KA administration, suggesting that newborn neurons in fosB-null, but not fosBd/d mice, show increased migration into the hilus in comparison with wild type (Figure 3c).
Figure 3.
Adult fosB-null mice show increased ectopic migration of neural progenitor cells in the hippocampus. As shown in Figure 2a, for 3 days following saline (control) or KA injection, mice were injected with BrdU twice daily. Brain sections were prepared on the sixth day, and BrdU-positive cells were detected. (a) Laser scanning confocal immunofluorescence microscopy for BrdU (red), DCX (green), and NeuN (blue) in the hilus of fosB-null mice. The arrowheads indicate ectopic neuroblasts or immature neurons (BrdU+/DCX+/NeuN+). Orthogonal projections throughout the cells are shown. Scale bar: 50 μm. (b) The density of BrdU-positive cells in the hilus. N=5 in each group; error bars, mean±SEM; black bars, wild type; gray bars, fosBd/d; open bars, fosB-null. One-way ANOVA (control: F2,12=2.53, p=0.121; KA: F2,12=8.58, p=0.0049). The p-values (Tukey–Kramer HSD post hoc comparison) are shown. (c) The percentage of BrdU-positive cells in the hilus, calculated from the total number of BrdU-positive cells in the entire DG. N=5 in each group; error bars, mean±SEM; black bars, wild-type; gray bars, fosBd/d; open bars, fosB-null. One-way ANOVA (control: F2,12=14.53, p=0.0006; KA: F2,12=20.17, p=0.0001). The p-values (Tukey–Kramer HSD post hoc comparison) are shown.
We next examined survival of newborn neurons in the DG. BrdU was injected into wild-type and fosB-null mice twice daily for 3 days, following saline administration (Supplementary Figure S6a). The density of BrdU-positive cells in the DG was determined 1 day and 70 days after the last BrdU injection. There was no significant difference in the survival of BrdU-positive cells in the DG after 70 days (wild type: 19.47±3.50% fosB-null: 14.85±0.66%) (Supplementary Figure S6b). In both mouse lines, all BrdU-positive cells were NeuN positive and were detected in the GCL/SGZ and hilus (Supplementary Figure S6c), suggesting that fosB products do not affect survival and maturation of newborn neurons.
We compared seizure scores and hippocampal damage in wild-type, fosBd/d, and fosB-null mice after KA administration. We found no significant difference in both seizure response 2 h after KA administration and hippocampal damage 6 days after KA administration, among the three mouse lines (Supplementary Figure S7).
fosB-Null Mice Display Spontaneous Epilepsy
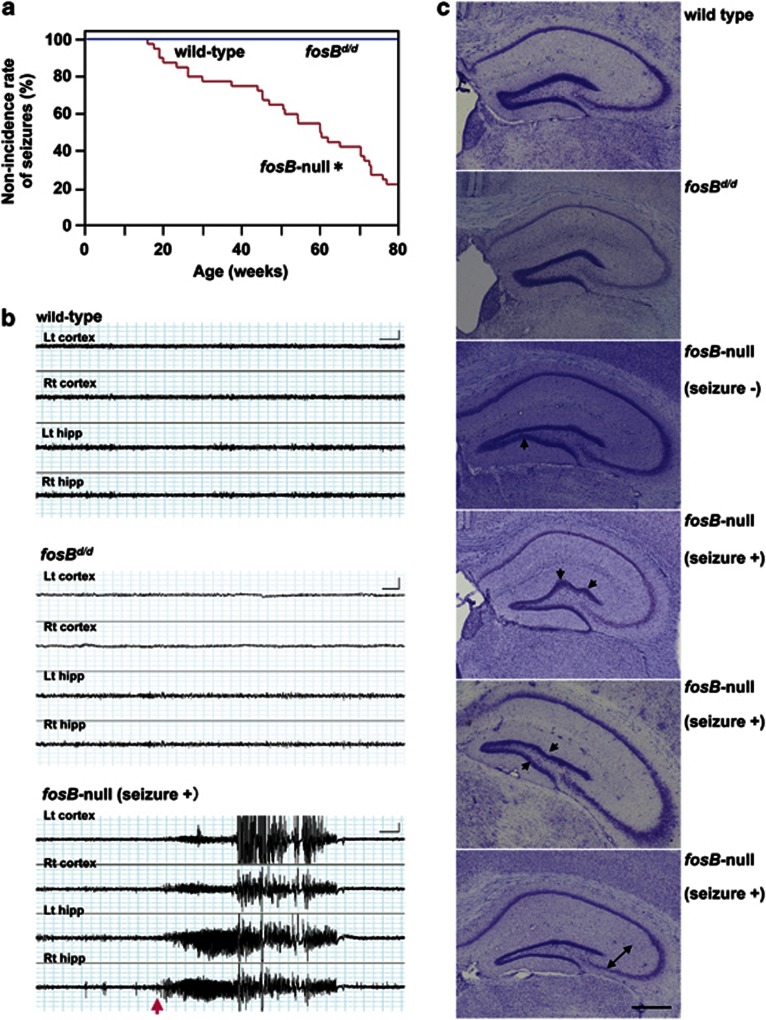
fosB-null mice occasionally exhibited spontaneous seizures, often associated with behavioral arrest and forelimb clonus (Supplementary video S1). Seizures were observed from 13 weeks of age and the non-incidence rate of seizures decreased as age increased. Approximately, 80% of fosB-null mice exhibited spontaneous seizures by 80 weeks (Figure 4a). The seizures were similar to KA-induced seizures that are widely accepted as a model of temporal lobe epilepsy (Ben-Ari and Cossart, 2000). Neither wild-type nor fosBd/d mice displayed seizures.
Figure 4.
Aged fosB-null mice exhibit spontaneous seizures with epileptiform electroencephalograph discharges that originate in the hippocampus, and an abnormal DG structure. (a) The non-incidence rate of spontaneous seizures. Wild-type and fosBd/d mice, N=10; fosB-null mice, N=40. *p<0.0001, Kaplan–Meier method and log-rank test (χ2=40.94, df=2). (b) Concurrent hippocampal and cortical EEGs in 50- to 70-week wild-type (upper panel), fosBd/d (middle panel), and fosB-null mice (lower panel). Lt cortex, left cortex; Rt cortex, right cortex; Lt hipp, left hippocampus; Rt hipp, right hippocampus. N=5 in each group. Scale bars: X axis, 4 s; Y axis, 0.5 mV. (c) Nissl stained coronal hippocampal sections from wild-type, fosBd/d, and fosB-null mice. Characteristic abnormal arrangements (arrows) and thinning (bidirectional arrow) of the GCL are observed in fosB-null mice regardless of seizures. Seizure−, without any seizures; Seizure+, with seizures. Scale bar: 500 μm.
To confirm electrophysiological changes of epilepsy in fosB-null mice, EEG recordings were performed using 50- to 70-week-old wild-type, fosBd/d, and fosB-null mice (Supplementary Figure S8; Figure 4b). Wild-type and fosBd/d mice displayed low baseline amplitude throughout the observation period (upper and middle panels in Figure 4b). However, in fosB-null mice, typical spontaneous electrographic seizures were observed, with the onset of epileptiform discharge from the hippocampus and secondary generalization to the cortex (bottom panel in Figure 4b). Spontaneous epileptiform EEG activity was verified in 27.3% of fosB-null mice examined. The spontaneous seizures had durations of up to 47 s. The majority of recorded seizures were strong, with generalized tonic-clonic episodes that occurred in the hippocampus and spread to the cortex. We concluded that fosB-null mice display hippocampal-onset epileptic seizures with secondary generalization to tonic-clonic convulsions.
An abnormal hippocampal cell arrangement including thinning of the GCL of the DG was characteristic of fosB-null mice regardless of behavioral seizures (Figure 4c). Neither wild-type nor fosBd/d mice showed any histological abnormalities. We also examined the density of parvalbumin-positive interneurons in the DG, and found that the density was slightly decreased in both fosBd/d and fosB-null mice compared with wild-type mice, albeit non-significantly (Supplementary Figure S9). Thus, our results indicate that expression of ΔFosB and/or Δ2ΔFosB is sufficient to suppress spontaneous epilepsy, as determined by an absence of spontaneous seizures, epileptic EEGs, and abnormal DG structures in the adult brain of wild-type and fosBd/d mice.
fosB Products Regulate Expression of Various Genes Involved in Neurogenesis, Depression, Migration, and Epilepsy
To examine fosB regulation of neurogenesis, depression, and epilepsy, we compared the gene expression profiles of wild-type, fosBd/d, and fosB-null mice. Total RNA was isolated from hippocampi of wild-type, fosBd/d, and fosB-null mice 24 h after administration of saline or KA, and subjected to microarray analysis.
Expression levels of 63 genes were significantly altered in fosB-null mice compared with wild-type and/or fosBd/d mice, with or without KA administration (Supplementary Table S1). In young saline controls, 23 genes were significantly downregulated and one gene significantly upregulated in fosB-null mice compared with fosBd/d or wild-type mice. Twenty-four hours after KA administration in young mice, 29 genes were significantly downregulated and 12 genes significantly upregulated in fosB-null mice compared with fosBd/d or wild-type mice. In aged untreated mice, expression of two genes was significantly higher in fosB-null mice compared with wild-type mice. Expression levels of Vgf, Smad3, Gal, Trh, and Sema3e were confirmed by quantitative RT–PCR analysis (Supplementary Table S2; Supplementary Figure S10).
Our results indicate that fosB differentially regulates expression of a number of genes, particularly following KA administration. Among them, 10 genes (Vgf, Dlk1, Smad3, Gal, Trh, Islr2, Penk, Srxn1, Serinc2, and Car12; Table 1) are likely to be involved in neurogenesis, depression, migration, or epilepsy, as discussed later. Among the 10 genes, Vgf, Gal, Trh, Penk, Srxn1, and Smad3 are known to be regulated by the activator protein-1 (AP-1) complex. Vgf, Gal, Trh, Penk, and Srxn1 have AP-1 sites in their promoter regions, whereas SMAD3 interacts with AP-1 complexes (FOS, JUN, and JUNB), to regulate TGF-β signaling (Zhang et al, 1998; Table 1; Supplementary Figure S11).
Table 1. Candidate Genes for Neurogenesis, Depression, and Epilepsy Regulated by fosB Products.
|
Fold change |
Literaturea |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Untreated |
24 h after KA |
|||||||||||
| Symbol | Gene name | null/wt | dd/wt | null/dd | null/wt | dd/wt | null/dd | AP-1 | ECS | Neurogenesis | Depression | Epilepsy or seizures |
| Young | ||||||||||||
| Vgf | VGF nerve growth factor inducible | −1.29 | −1.42 | −1.23 | Canu et al (1997) | ↑ Wang et al (2010) | Thakker-Varia et al (2007) | Thakker-Varia et al (2007) | Newton et al (2003) | |||
| Dlk1 | Delta-like 1 homolog (Drosophila) | −1.22 | Surmacz et al (2011) | |||||||||
| Smad3 | MAD homolog 3 (Drosophila) | −1.67 | Verrecchia et al (2001) | Wang and Symes (2010) | ||||||||
| Gal | Galanin prepropeptide | −2.30* | −1.86 | −1.24 | Anouar et al (1999) | ↑ Wang et al (2010) | Abbosh et al (2011) | Abbosh et al (2011) | Mazarati et al (2000) | |||
| Trh | Thyrotropin-releasing hormone | −3.26* | −2.50* | −1.30 | Cote-Velez et al (2005) | ↑ Wang et al (2010) | Sattin et al (1994) | Deng et al (2006) | ||||
| Islr2 | Immunoglobulin superfamily containing leucine-rich repeat 2 | −1.33 | Pramparo et al (2011) | |||||||||
| Penk | Preproenkephalin 1 | −2.17* | −1.51 | −1.44 | Hyman et al (1988) | Ogden et al (2004) | Lason et al (1994) | |||||
| Srxn1 | Sulfiredoxin 1 homolog (S. cerevisiae) | −1.21 | Soriano et al (2008) | Soriano et al (2008) | ||||||||
| Serinc2 | Serine incorporator 2 | −1.38 | Inuzuka et al (2005) | |||||||||
| Aged | ||||||||||||
| Ca12 | Carbonic anhydrase XII | 2.21 | ND | ND | ND | Halmi et al (2006); Winum et al (2009) | ||||||
Among 63 genes altered in the hippocampus of fosB-null mice, 10 genes are related to neurogenesis, depression, or epilepsy. Fold changes (null/wt, fosB-null vs wt; dd/wt, fosBd/d vs wt; null/dd, fosB-null vs fosBd/d). Numbers in bold indicate fold change >1.2 or<−1.2, and p<0.05 by unpaired t-test. Numbers with an asterisk indicate fold change >2.0 or<−2.0, and p<0.05 by ANOVA. p-value computation; Asymptotic Multiple Testing Correction; Benjamini Hochberg FDR (Benjamini and Hochberg, 1995).
Literature columns indicate previous evidence for the following categories. AP-1: existence of AP-1 sites in the promoter regions or reported interactions with AP-1 transcription factors (+); ECS: evidence of upregulation by ECS (↑); NG: involvement in neurogenesis; DP: involvement in depression; E/S: seizure- or epilepsy-associated regulation. ND, not done.
DISCUSSION
fosB-Null Mice Display Impaired Neurogenesis in the Adult Hippocampus, Increased Depressive-like Behaviors and Spontaneous Epilepsy
In the current study, we have shown that fosB-null mice exhibit decreased proliferation of neuronal progenitor cells, increased ectopic migration of newborn neurons during basal and KA-induced neurogenesis in the adult hippocampus, and adult-onset spontaneous epileptic seizures, epileptic EEGs, and abnormal hippocampal structures. Conversely, compared with wild-type mice, fosBd/d mice exhibited a similar level of basal proliferation of neuronal progenitor cells, with decreased KA-induced proliferation and decreased ectopic migration of newborn neurons in the hippocampus, but importantly, fosBd/d mice did not exhibit spontaneous seizures, epileptic EEGs, or abnormal hippocampal structures.
In the present study, we compared various phenotypes of fosBd/d and fosB-null mutant mice with those of C57BL/6J mice as a wild-type strain, onto which the mutant mouse lines were backcrossed >12 generations. However, a considerable amount of allelic differences are still expected near the mutated fosB locus on chromosome 7 between C57BL/6J and mutant mice (Flaherty and Bolivar, 2007). fosB-null mice exhibited largely different phenotypes in comparison with C57BL/6J and fosBd/d mice, the latter two exhibited rather similar phenotypes regarding spontaneous epilepsy, depressive-like behaviors, and extent of adult neurogenesis in the hippocampus, and these phenotypes were maintained through the generations of backcross (N=4 to N=12 or 17) (unpublished observation). Although it remains possible that allelic differences in the chromosome 7 modify the phenotypes, our results indicate that the phenotypes reported here are determined by the fosB locus, at least in part.
We previously found that fosB-null mice exhibit increased depressive-like behaviors, whereas fosB+/d mice, with reduced expression of FosB and enhanced expression of ΔFosB and Δ2ΔFosB, exhibit antidepressive-like behaviors (Ohnishi et al, 2011). These data indicate that fosB products, especially ΔFosB and/or Δ2ΔFosB, promote and maintain intact neurogenesis in the adult hippocampus, and ensure suppression of depression and spontaneous epilepsy. A previous study demonstrated that repeated ECS induces expression of fosB products in the prefrontal cortex and tolerance to repeated ECS is significantly attenuated in fosB-null mice (Hiroi et al, 1998). Taken together, the results suggest that fosB products have multiple effects on various aspects of seizures in multiple regions of the brain.
fosB Regulates Specific Genes to Maintain Intact Neurogenesis and Suppress Spontaneous Epilepsy
Gene expression profiling suggests that expression of Vgf, Gal, Trh, Penk, and Srxn1 is likely to be regulated by FosB and to a lesser extent ΔFosB/Δ2ΔFosB. Among them, Vgf and Gal are reported to be involved in both neurogenesis and seizures or epilepsy (Abbosh et al, 2011; Mazarati et al, 2000; Newton et al, 2003; Thakker-Varia et al, 2007), and whose expression is likely to be upregulated by both FosB and ΔFosB/Δ2ΔFosB (Supplementary Figure S10). These results may explain why only fosB-null, but not fosBd/d, mice exhibit spontaneous epilepsy together with a severe decline in neurogenesis (Figures 2f and 4).
It is well known that GABAergic interneurons play an important role in suppressing epilepsy (Perucca and Mula, 2012). In this study, we found a slight decrease in the density of parvalbumin-positive interneurons in both fosBd/d and fosB-null DG, the latter only exhibiting spontaneous epilepsy (Supplementary Figure S9). However, fosB-null mice exhibited the lowest expression of mRNA for TRH, which promotes GABA release (Deng et al, 2006), after KA administration, while fosBd/d mice exhibited the highest TRH mRNA level in untreated conditions (Supplementary Figure S10). This may also contribute to the spontaneous epilepsy seen in fosB-null mice. It is noteworthy that the level of Car12 mRNA encoding carbonic anhydrase 12 was increased >2-fold only in aged fosB-null mice (Table 1). Because topiramate 1, an inhibitor of carbonic anhydrases, is a broad-spectrum anti-epileptic drug (Winum et al, 2009), it is likely that ΔFosB and/or Δ2ΔFosB as well as FosB repress Car12 expression, thereby resulting in epilepsy suppression.
ΔFosB and/or Δ2ΔFosB Maintain the Intact Structure of Adult Hippocampus
fosB-null mice exhibited abnormalities in hippocampal structures, which were similar to those observed in brains of rodent models and human patients with epilepsy (Fahrner et al, 2007). Undulation or thinning of the GCL of the DG was observed in fosB-null mice, and may be due to either a neuronal migration defect or decreased hippocampal neurogenesis. Tyrosine kinase fyn-deficient mice have previously been reported to have a similar undulation of the DG (Grant et al, 1992), and also show increased susceptibility to audiogenic seizures (Miyakawa et al, 1995). fosBd/d mice did not show these structural abnormalities, indicating that the presence of ΔFosB and/or Δ2ΔFosB was sufficient to suppress them.
Among the 10 fosB-regulated genes, Islr2, which encodes the immunoglobulin superfamily containing leucine-rich repeat 2, is downregulated in mouse models of human neuronal migration defects, and is also mutated in seizure patients (Pramparo et al, 2011). SMAD3 and DLK1 are also reported to be involved in cell migration (Wang and Symes, 2010; Yin et al, 2006). We previously reported that ΔFosB/Δ2ΔFosB positively, whereas FosB negatively, regulates cell–matrix adhesion and the TGF-β1 signaling pathway in mouse embryonic stem cells (Ohnishi et al, 2008). Moreover, E-cadherin, an important cell–cell adhesion molecule, is an indirect target of fosB gene products in the adult mouse striatum and hippocampus (Ohnishi et al, 2011), suggesting that fosB products indirectly regulate cell migration and structural maintenance of the hippocampus.
fosB May Suppress Depressive-Like Behaviors Through Regulation of Downstream Genes That Enhance Neurogenesis
Recently, it was reported that adult-born hippocampal granule neurons dynamically regulate stress reactivity at both the endocrine and behavioral levels (Snyder et al, 2011). VGF and GAL exert antidepressant action by enhancing neurogenesis in the hippocampus and/or through other effects. Vgf is downregulated in animal models of depression and human bipolar postmortem brain (Thakker-Varia et al, 2010, 2007). GAL exhibits antidepressant-like effects in human patients with depression (Murck et al, 2004), which may be partly mediated by a direct effect of GAL on hippocampal neurogenesis (Abbosh et al, 2011). Moreover, hippocampal TRH induction has also been proposed to have antidepressant effects (Sattin et al, 1994). Taken together, our present findings suggest that fosB suppresses depressive-like behavior through upregulation of these genes (Supplementary Figure S11).
fosB Targets May Act in an Autocrine/Paracrine Manner
Expression of fosB products was significantly increased in mature hippocampal neurons, SOX2-positive/GFAP-negative neural progenitor cells, and immature newborn neurons in the DG after exposure to KA. Ki67-positive proliferating cells did not express fosB products. Since exogenous expression of FosB or ΔFosB in quiescent fibroblasts and embryonic cortical cells induces their proliferation (Kurushima et al, 2005; Nakabeppu et al, 1993), it is likely that brain stimuli such as KA treatment or ischemic insults immediately and transiently induce fosB expression in neural stem-like cells, thus stimulating their own proliferation.
Of the genes listed in Table 1, 10 are expressed in hippocampal neurons, as determined by in situ hybridization (ISH) images (http://mouse.brain-map.org/) provided by the Allen Institute for Brain Science. As VGF, TRH, GAL, and PENK are secretary molecules, it is feasible that these proteins are expressed and function within fosB-expressing hippocampal neurons, but also act in an autocrine/paracrine manner to regulate neighboring progenitor cells.
Conclusion
The present study demonstrates that the fosB-null mouse is the first animal model to provide a genetic and molecular basis for the comorbidity of epilepsy and depression with abnormal neurogenesis, all of which are caused by loss of a single gene, fosB. Since depression and epilepsy are known to share a genetic basis (Hesdorffer et al, 2012), it will be important in the future to determine if alterations in the fosB gene, or its expression, are associated with these disorders in humans. Identifying alterations will provide insight into the diagnosis and treatment of these complicated disorders.
Acknowledgments
We thank Dr Z Sheng (Kyushu University, Fukuoka, Japan), Dr N Osumi (Tohoku University, Sendai, Japan) and Dr M Matsumata (Brain Science of Institute, RIKEN, Wako, Japan) for technical advice, and Ms S Kitamura, Ms A Matsuyama, and Ms K Nakabeppu for technical help. This work was supported by grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan, the Japan Society for the Promotion of Science (18300124, 22221004, and 23657116 to Y Nakabeppu), and the Kyushu University Global COE Program (Cell-rate Decision Function and Dysfunction in Homeostasis).
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Abbosh C, Lawkowski A, Zaben M, Gray W. GalR2/3 mediates proliferative and trophic effects of galanin on postnatal hippocampal precursors. J Neurochem. 2011;117:425–436. doi: 10.1111/j.1471-4159.2011.07204.x. [DOI] [PubMed] [Google Scholar]
- Anouar Y, Lee HW, Eiden LE. Both inducible and constitutive activator protein-1-like transcription factors are used for transcriptional activation of the galanin gene by different first and second messenger pathways. Mol Pharmacol. 1999;56:162–169. doi: 10.1124/mol.56.1.162. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Cossart R. Kainate, a double agent that generates seizures: two decades of progress. Trends Neurosci. 2000;23:580–587. doi: 10.1016/s0166-2236(00)01659-3. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol. 1995;57:289–300. [Google Scholar]
- Canu N, Possenti R, Rinaldi AM, Trani E, Levi A. Molecular cloning and characterization of the human VGF promoter region. J Neurochem. 1997;68:1390–1399. doi: 10.1046/j.1471-4159.1997.68041390.x. [DOI] [PubMed] [Google Scholar]
- Chen J, Kelz MB, Hope BT, Nakabeppu Y, Nestler EJ. Chronic Fos-related antigens: stable variants of ΔFosB induced in brain by chronic treatments. J Neurosci. 1997;17:4933–4941. doi: 10.1523/JNEUROSCI.17-13-04933.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cote-Velez A, Perez-Martinez L, Diaz-Gallardo MY, Perez-Monter C, Carreon-Rodriguez A, Charli JL, et al. Dexamethasone represses cAMP rapid upregulation of TRH gene transcription: identification of a composite glucocorticoid response element and a cAMP response element in TRH promoter. J Mol Endocrinol. 2005;34:177–197. doi: 10.1677/jme.1.01634. [DOI] [PubMed] [Google Scholar]
- Danzer SC. Depression, stress, epilepsy and adult neurogenesis. Exp Neurol. 2012;233:22–32. doi: 10.1016/j.expneurol.2011.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng PY, Porter JE, Shin HS, Lei S. Thyrotropin-releasing hormone increases GABA release in rat hippocampus. J Physiol. 2006;577:497–511. doi: 10.1113/jphysiol.2006.118141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahrner A, Kann G, Flubacher A, Heinrich C, Freiman TM, Zentner J, et al. Granule cell dispersion is not accompanied by enhanced neurogenesis in temporal lobe epilepsy patients. Exp Neurol. 2007;203:320–332. doi: 10.1016/j.expneurol.2006.08.023. [DOI] [PubMed] [Google Scholar]
- Flaherty L, Bolivar V.2007Congenic and consomic strainsIn: Jones BC, Mormede P (eds).Neurobehavioral Genetics CRC Press, Taylor & Francis: Boca Raton, FL; 115–127. [Google Scholar]
- Grant SG, O'Dell TJ, Karl KA, Stein PL, Soriano P, Kandel ER. Impaired long-term potentiation, spatial learning, and hippocampal development in fyn mutant mice. Science. 1992;258:1903–1910. doi: 10.1126/science.1361685. [DOI] [PubMed] [Google Scholar]
- Halmi P, Parkkila S, Honkaniemi J. Expression of carbonic anhydrases II, IV, VII, VIII and XII in rat brain after kainic acid induced status epilepticus. Neurochem Int. 2006;48:24–30. doi: 10.1016/j.neuint.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Hesdorffer DC, Caplan R, Berg AT. Familial clustering of epilepsy and behavioral disorders: evidence for a shared genetic basis. Epilepsia. 2012;53:301–307. doi: 10.1111/j.1528-1167.2011.03351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiroi N, Marek GJ, Brown JR, Ye H, Saudou F, Vaidya VA, et al. Essential role of the fosB gene in molecular, cellular, and behavioral actions of chronic electroconvulsive seizures. J Neurosci. 1998;18:6952–6962. doi: 10.1523/JNEUROSCI.18-17-06952.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman SE, Comb M, Lin YS, Pearlberg J, Green MR, Goodman HM. A common trans-acting factor is involved in transcriptional regulation of neurotransmitter genes by cyclic AMP. Mol Cell Biol. 1988;8:4225–4233. doi: 10.1128/mcb.8.10.4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inuzuka M, Hayakawa M, Ingi T. Serinc, an activity-regulated protein family, incorporates serine into membrane lipid synthesis. J Biol Chem. 2005;280:35776–35783. doi: 10.1074/jbc.M505712200. [DOI] [PubMed] [Google Scholar]
- Kajitani K, Yamaguchi H, Dan Y, Furuichi M, Kang D, Nakabeppu Y. MTH1, an oxidized purine nucleoside triphosphatase, suppresses the accumulation of oxidative damage of nucleic acids in the hippocampal microglia during kainate-induced excitotoxicity. J Neurosci. 2006;26:1688–1698. doi: 10.1523/JNEUROSCI.4948-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurushima H, Ohno M, Miura T, Nakamura TY, Horie H, Kadoya T, et al. Selective induction of ΔFosB in the brain after transient forebrain ischemia accompanied by an increased expression of galectin-1, and the implication of ΔFosB and galectin-1 in neuroprotection and neurogenesis. Cell Death Differ. 2005;12:1078–1096. doi: 10.1038/sj.cdd.4401648. [DOI] [PubMed] [Google Scholar]
- Lason W, Przewlocka B, Van Luijtelaar G, Coenen A. Proenkephalin and prodynorphin mRNA level in brain of rats with absence epilepsy. Neuropeptides. 1994;27:343–347. doi: 10.1016/0143-4179(94)90060-4. [DOI] [PubMed] [Google Scholar]
- Lugert S, Basak O, Knuckles P, Haussler U, Fabel K, Gotz M, et al. Quiescent and active hippocampal neural stem cells with distinct morphologies respond selectively to physiological and pathological stimuli and aging. Cell Stem Cell. 2010;6:445–456. doi: 10.1016/j.stem.2010.03.017. [DOI] [PubMed] [Google Scholar]
- Mandelzys A, Gruda MA, Bravo R, Morgan JI. Absence of a persistently elevated 37 kDa fos-related antigen and AP-1-like DNA-binding activity in the brains of kainic acid-treated fosB null mice. J Neurosci. 1997;17:5407–5415. doi: 10.1523/JNEUROSCI.17-14-05407.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazarati AM, Hohmann JG, Bacon A, Liu H, Sankar R, Steiner RA, et al. Modulation of hippocampal excitability and seizures by galanin. J Neurosci. 2000;20:6276–6281. doi: 10.1523/JNEUROSCI.20-16-06276.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung CA, Nestler EJ. Regulation of gene expression and cocaine reward by CREB and ΔFosB. Nat Neurosci. 2003;6:1208–1215. doi: 10.1038/nn1143. [DOI] [PubMed] [Google Scholar]
- Miyakawa T, Yagi T, Taniguchi M, Matsuura H, Tateishi K, Niki H. Enhanced susceptibility of audiogenic seizures in Fyn-kinase deficient mice. Brain Res Mol Brain Res. 1995;28:349–352. doi: 10.1016/0169-328x(94)00251-9. [DOI] [PubMed] [Google Scholar]
- Morris TA, Jafari N, DeLorenzo RJ. Chronic ΔFosB expression and increased AP-1 transcription factor binding are associated with the long term plasticity changes in epilepsy. Brain Res Mol Brain Res. 2000;79:138–149. doi: 10.1016/s0169-328x(00)00112-1. [DOI] [PubMed] [Google Scholar]
- Murck H, Held K, Ziegenbein M, Kunzel H, Holsboer F, Steiger A. Intravenous administration of the neuropeptide galanin has fast antidepressant efficacy and affects the sleep EEG. Psychoneuroendocrinology. 2004;29:1205–1211. doi: 10.1016/j.psyneuen.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Nakabeppu Y, Nathans D. A naturally occurring truncated form of FosB that inhibits Fos/Jun transcriptional activity. Cell. 1991;64:751–759. doi: 10.1016/0092-8674(91)90504-r. [DOI] [PubMed] [Google Scholar]
- Nakabeppu Y, Oda S, Sekiguchi M. Proliferative activation of quiescent Rat-1A cells by ΔFosB. Mol Cell Biol. 1993;13:4157–4166. doi: 10.1128/mcb.13.7.4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton SS, Collier EF, Hunsberger J, Adams D, Terwilliger R, Selvanayagam E, et al. Gene profile of electroconvulsive seizures: induction of neurotrophic and angiogenic factors. J Neurosci. 2003;23:10841–10851. doi: 10.1523/JNEUROSCI.23-34-10841.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden CA, Rich ME, Schork NJ, Paulus MP, Geyer MA, Lohr JB, et al. Candidate genes, pathways and mechanisms for bipolar (manic-depressive) and related disorders: an expanded convergent functional genomics approach. Mol Psychiatry. 2004;9:1007–1029. doi: 10.1038/sj.mp.4001547. [DOI] [PubMed] [Google Scholar]
- Ohnishi YN, Ohnishi YH, Hokama M, Nomaru H, Yamazaki K, Tominaga Y, et al. FosB is essential for the enhancement of stress tolerance and antagonizes locomotor sensitization by ΔFosB. Biol Psychiatry. 2011;70:487–495. doi: 10.1016/j.biopsych.2011.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnishi YN, Sakumi K, Yamazaki K, Ohnishi YH, Miura T, Tominaga Y, et al. Antagonistic regulation of cell-matrix adhesion by FosB and ΔFosB/Δ2ΔFosB encoded by alternatively spliced forms of fosB transcripts. Mol Biol Cell. 2008;19:4717–4729. doi: 10.1091/mbc.E07-08-0768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perucca P, Mula M.2012Antiepileptic drug effects on mood and behavior: Molecular targets Epilepsy Behav(in press), doi: 10.1016/j.yebeh.2012.09.018 [DOI] [PubMed]
- Pramparo T, Libiger O, Jain S, Li H, Youn YH, Hirotsune S, et al. Global developmental gene expression and pathway analysis of normal brain development and mouse models of human neuronal migration defects. PLoS Genet. 2011;7:e1001331. doi: 10.1371/journal.pgen.1001331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renthal W, Carle TL, Maze I, Covington HE, Truong HT, Alibhai I, et al. ΔFosB mediates epigenetic desensitization of the c-fos gene after chronic amphetamine exposure. J Neurosci. 2008;28:7344–7349. doi: 10.1523/JNEUROSCI.1043-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatakos G, Rowe GC, Kveiborg M, Wu M, Neff L, Chiusaroli R, et al. Doubly truncated FosB isoform (Δ2ΔFosB) induces osteosclerosis in transgenic mice and modulates expression and phosphorylation of Smads in osteoblasts independent of intrinsic AP-1 activity. J Bone Miner Res. 2008;23:584–595. doi: 10.1359/JBMR.080110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattin A, Pekary AE, Lloyd RL. TRH gene products are implicated in the antidepressant mechanisms of seizures. Ann NY Acad Sci. 1994;739:135–153. doi: 10.1111/j.1749-6632.1994.tb19815.x. [DOI] [PubMed] [Google Scholar]
- Snyder JS, Soumier A, Brewer M, Pickel J, Cameron HA. Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature. 2011;476:458–461. doi: 10.1038/nature10287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano FX, Leveille F, Papadia S, Higgins LG, Varley J, Baxter P, et al. Induction of sulfiredoxin expression and reduction of peroxiredoxin hyperoxidation by the neuroprotective Nrf2 activator 3H-1,2-dithiole-3-thione. J Neurochem. 2008;107:533–543. doi: 10.1111/j.1471-4159.2008.05648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmacz B, Noisa P, Risner-Janiczek JR, Hui K, Ungless M, Cui W, et al. DLK1 promotes neurogenesis of human and mouse pluripotent stem cell-derived neural progenitors via modulating Notch and BMP signalling. Stem Cell Rev. 2011;8:459–471. doi: 10.1007/s12015-011-9298-7. [DOI] [PubMed] [Google Scholar]
- Tahara K, Tsuchimoto D, Tominaga Y, Asoh S, Ohta S, Kitagawa M, et al. ΔFosB, but not FosB, induces delayed apoptosis independent of cell proliferation in the Rat1a embryo cell line. Cell Death Differ. 2003;10:496–507. doi: 10.1038/sj.cdd.4401173. [DOI] [PubMed] [Google Scholar]
- Thakker-Varia S, Jean YY, Parikh P, Sizer CF, Jernstedt Ayer J, Parikh A, et al. The neuropeptide VGF is reduced in human bipolar postmortem brain and contributes to some of the behavioral and molecular effects of lithium. J Neurosci. 2010;30:9368–9380. doi: 10.1523/JNEUROSCI.5987-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakker-Varia S, Krol JJ, Nettleton J, Bilimoria PM, Bangasser DA, Shors TJ, et al. The neuropeptide VGF produces antidepressant-like behavioral effects and enhances proliferation in the hippocampus. J Neurosci. 2007;27:12156–12167. doi: 10.1523/JNEUROSCI.1898-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verrecchia F, Vindevoghel L, Lechleider RJ, Uitto J, Roberts AB, Mauviel A. Smad3/AP-1 interactions control transcriptional responses to TGF-β in a promoter-specific manner. Oncogene. 2001;20:3332–3340. doi: 10.1038/sj.onc.1204448. [DOI] [PubMed] [Google Scholar]
- Wang Y, Symes AJ. Smad3 deficiency reduces neurogenesis in adult mice. J Mol Neurosci. 2010;41:383–396. doi: 10.1007/s12031-010-9329-x. [DOI] [PubMed] [Google Scholar]
- Wang YY, Smith P, Murphy M, Cook M. Global expression profiling in epileptogenesis: does it add to the confusion. Brain Pathol. 2010;20:1–16. doi: 10.1111/j.1750-3639.2008.00254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winum JY, Poulsen SA, Supuran CT. Therapeutic applications of glycosidic carbonic anhydrase inhibitors. Med Res Rev. 2009;29:419–435. doi: 10.1002/med.20141. [DOI] [PubMed] [Google Scholar]
- Yin D, Xie D, Sakajiri S, Miller CW, Zhu H, Popoviciu ML, et al. DLK1: increased expression in gliomas and associated with oncogenic activities. Oncogene. 2006;25:1852–1861. doi: 10.1038/sj.onc.1209219. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Feng XH, Derynck R. Smad3 and Smad4 cooperate with c-Jun/c-Fos to mediate TGF-β-induced transcription. Nature. 1998;394:909–913. doi: 10.1038/29814. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.