Abstract
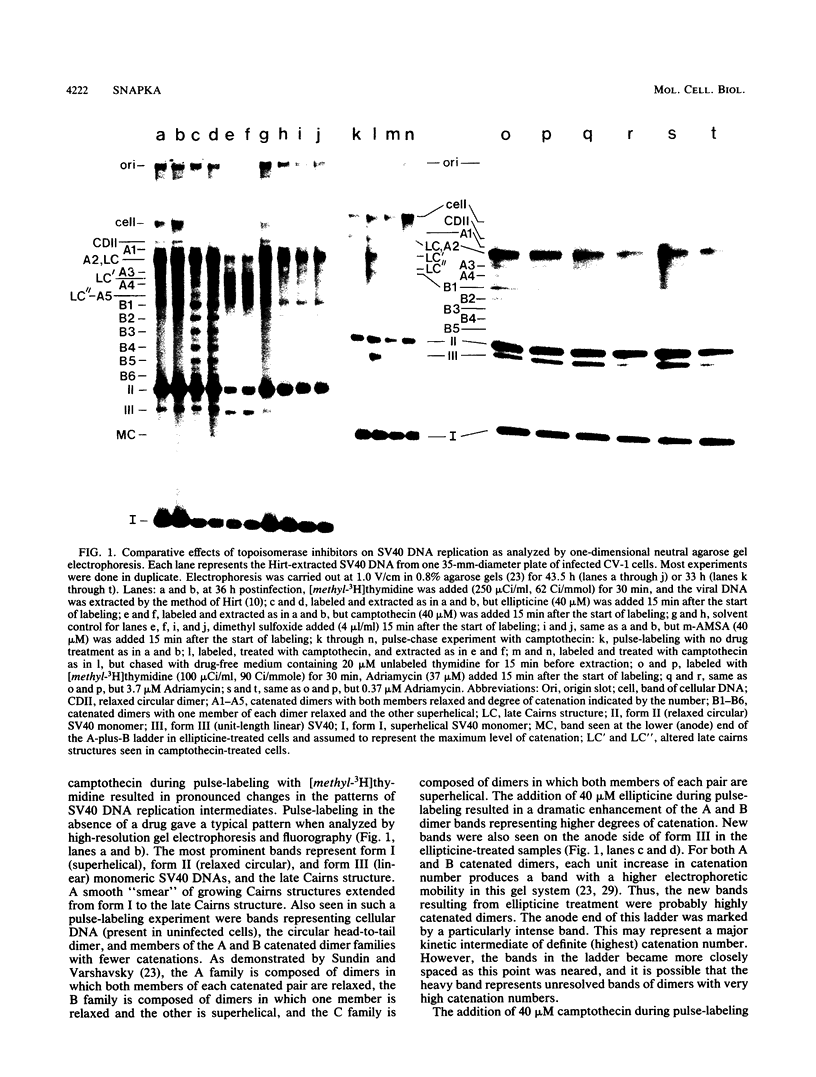
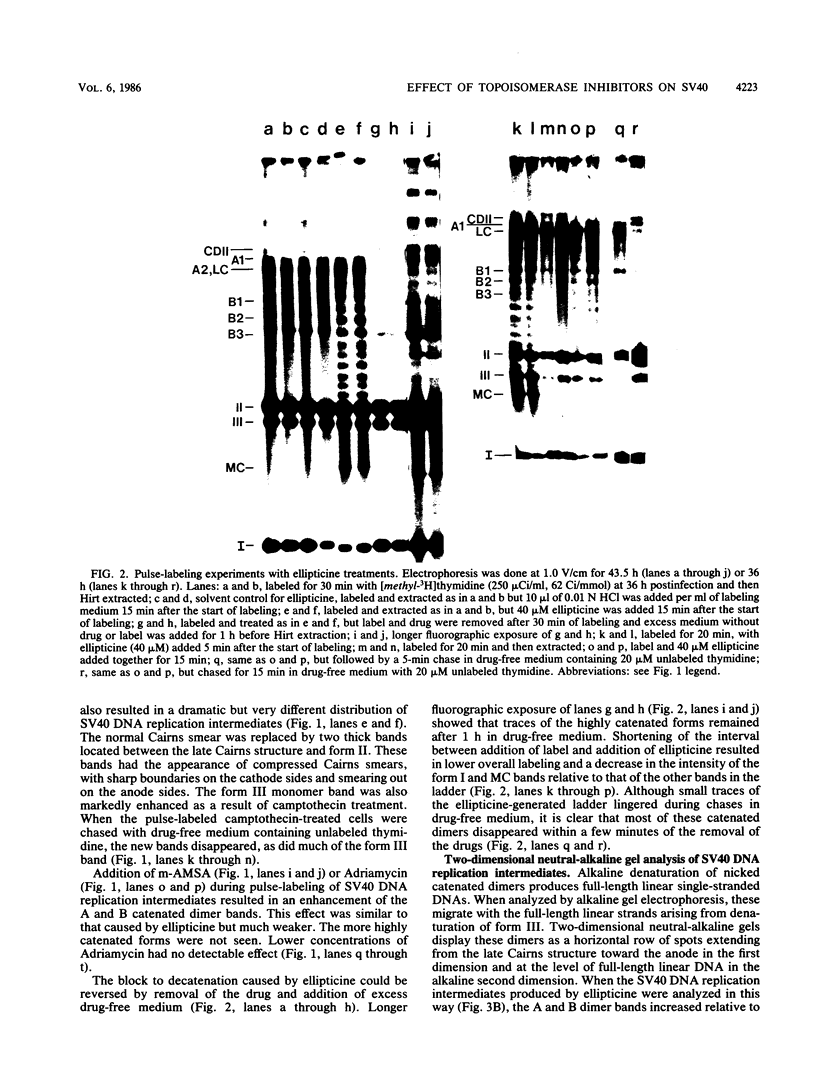
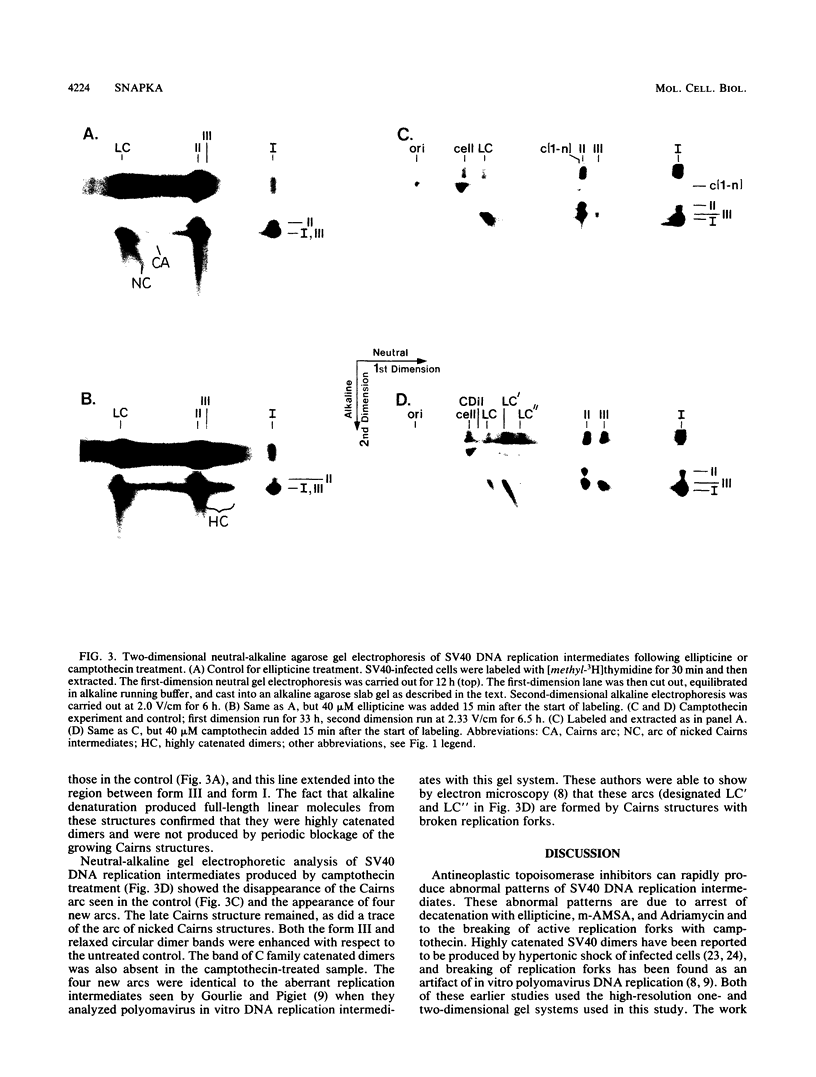
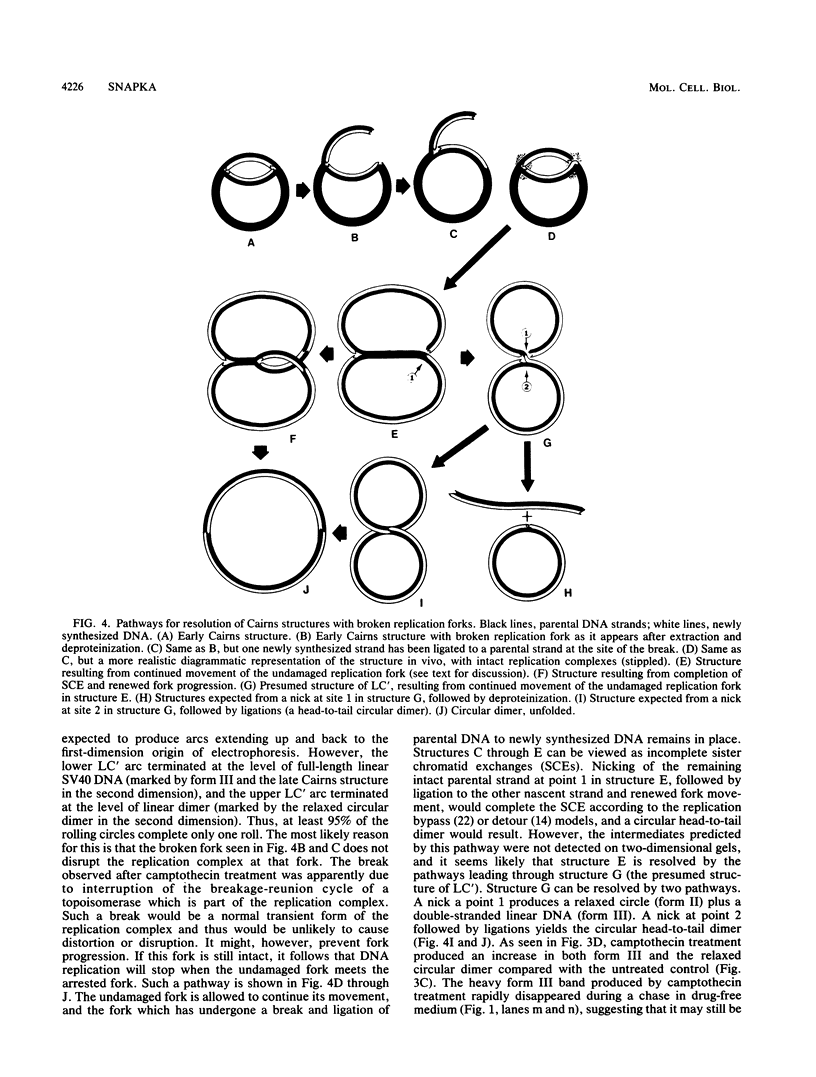
I have found that antineoplastic drugs which are known to be inhibitors of mammalian DNA topoisomerases have pronounced and selective effects on simian virus 40 DNA replication. Ellipticine, 4'-(9-acridinylamino)methanesulfon-m-aniside, and Adriamycin blocked decatenation of newly replicated simian virus 40 daughter chromosomes in vivo. The arrested decatenation intermediates produced by these drugs contained single-strand DNA breaks. Ellipticine in particular produced these catenated dimers rapidly and efficiently. Removal of the drug resulted in rapid reversal of the block and completion of decatenation. The demonstration that these drugs interfere with decatenation suggests that they may exert their cytotoxic and antineoplastic effects by preventing the separation of newly replicated cellular chromosomes. Camptothecin rapidly breaks replication forks in growing Cairns structures. It is likely that the target of camptothecin is the "swivel" topoisomerase required for DNA replication and that it is located at or very near the replication fork in vivo. Evidence is presented that many of the broken Cairns structures are in fact half-completed sister chromatid exchanges. One pathway for the resolution of these structures is completion of the sister chromatid exchange to produce a circular head-to-tail dimer.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bjursell G. Effects of 2'-deoxy-2'-azidocytidine on polyoma virus DNA replication: evidence for rolling circle-type mechanism. J Virol. 1978 Apr;26(1):136–142. doi: 10.1128/jvi.26.1.136-142.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiNardo S., Voelkel K., Sternglanz R. DNA topoisomerase II mutant of Saccharomyces cerevisiae: topoisomerase II is required for segregation of daughter molecules at the termination of DNA replication. Proc Natl Acad Sci U S A. 1984 May;81(9):2616–2620. doi: 10.1073/pnas.81.9.2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douc-Rasy S., Kayser A., Riou G. F. Inhibition of the reactions catalysed by a type I topoisomerase and catenating enzyme of Trypanosoma cruzi by DNA-intercalating drugs. Preferential inhibition of the catenating reaction. EMBO J. 1984 Jan;3(1):11–16. doi: 10.1002/j.1460-2075.1984.tb01754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fareed G. C., Garon G. F., Salzman N. P. Origin and direction of simian virus 40 deoxyribonucleic acid replication. J Virol. 1972 Sep;10(3):484–491. doi: 10.1128/jvi.10.3.484-491.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert W., Dressler D. DNA replication: the rolling circle model. Cold Spring Harb Symp Quant Biol. 1968;33:473–484. doi: 10.1101/sqb.1968.033.01.055. [DOI] [PubMed] [Google Scholar]
- Glisson B., Gupta R., Hodges P., Ross W. Cross-resistance to intercalating agents in an epipodophyllotoxin-resistant Chinese hamster ovary cell line: evidence for a common intracellular target. Cancer Res. 1986 Apr;46(4 Pt 2):1939–1942. [PubMed] [Google Scholar]
- Glisson B., Gupta R., Smallwood-Kentro S., Ross W. Characterization of acquired epipodophyllotoxin resistance in a Chinese hamster ovary cell line: loss of drug-stimulated DNA cleavage activity. Cancer Res. 1986 Apr;46(4 Pt 2):1934–1938. [PubMed] [Google Scholar]
- Gourlie B. B., Krauss M. R., Buckler-White A. J., Benbow R. M., Pigiet V. Polyoma virus minichromosomes: a soluble in vitro replication system. J Virol. 1981 Jun;38(3):805–814. doi: 10.1128/jvi.38.3.805-814.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourlie B. B., Pigiet V. P. Polyoma virus minichromosomes: characterization of the products of in vitro DNA synthesis. J Virol. 1983 Feb;45(2):585–593. doi: 10.1128/jvi.45.2.585-593.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Holm C., Goto T., Wang J. C., Botstein D. DNA topoisomerase II is required at the time of mitosis in yeast. Cell. 1985 Jun;41(2):553–563. doi: 10.1016/s0092-8674(85)80028-3. [DOI] [PubMed] [Google Scholar]
- Hsiang Y. H., Hertzberg R., Hecht S., Liu L. F. Camptothecin induces protein-linked DNA breaks via mammalian DNA topoisomerase I. J Biol Chem. 1985 Nov 25;260(27):14873–14878. [PubMed] [Google Scholar]
- Hsu T. C., Pathak S., Kusyk C. J. Continuous induction of chromatid lesions by DNA-intercalating compounds. Mutat Res. 1975 Dec;33(2-3):417–420. doi: 10.1016/0027-5107(75)90219-5. [DOI] [PubMed] [Google Scholar]
- Ishii Y., Bender M. A. Effects of inhibitors of DNA synthesis on spontaneous and ultraviolet light-induced sister-chromatid exchanges in Chinese hamster cells. Mutat Res. 1980 Sep;79(1):19–32. doi: 10.1016/0165-1218(80)90144-5. [DOI] [PubMed] [Google Scholar]
- Nelson E. M., Tewey K. M., Liu L. F. Mechanism of antitumor drug action: poisoning of mammalian DNA topoisomerase II on DNA by 4'-(9-acridinylamino)-methanesulfon-m-anisidide. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1361–1365. doi: 10.1073/pnas.81.5.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi H., Prem veer Reddy G., Pardee A. B. Rapid incorporation of label from ribonucleoside disphosphates into DNA by a cell-free high molecular weight fraction from animal cell nuclei. Cell. 1983 Feb;32(2):443–451. doi: 10.1016/0092-8674(83)90464-6. [DOI] [PubMed] [Google Scholar]
- Pommier Y., Zwelling L. A., Kao-Shan C. S., Whang-Peng J., Bradley M. O. Correlations between intercalator-induced DNA strand breaks and sister chromatid exchanges, mutations, and cytotoxicity in Chinese hamster cells. Cancer Res. 1985 Jul;45(7):3143–3149. [PubMed] [Google Scholar]
- Pulleyblank D. E., Booth G. M. Improved methods for the fluorographic detection of weak beta-emitting radioisotopes in Agarose and acrylamide gel electrophoresis media. J Biochem Biophys Methods. 1981 Jun;4(5-6):339–346. doi: 10.1016/0165-022x(81)90074-9. [DOI] [PubMed] [Google Scholar]
- Ross W. E. DNA topoisomerases as targets for cancer therapy. Biochem Pharmacol. 1985 Dec 15;34(24):4191–4195. doi: 10.1016/0006-2952(85)90273-4. [DOI] [PubMed] [Google Scholar]
- Ross W. E., Smith M. C. Repair of deoxyribonucleic acid lesions caused by adriamycin and ellipticine. Biochem Pharmacol. 1982 May 15;31(10):1931–1935. doi: 10.1016/0006-2952(82)90500-7. [DOI] [PubMed] [Google Scholar]
- Rowe T. C., Chen G. L., Hsiang Y. H., Liu L. F. DNA damage by antitumor acridines mediated by mammalian DNA topoisomerase II. Cancer Res. 1986 Apr;46(4 Pt 2):2021–2026. [PubMed] [Google Scholar]
- Sundin O., Varshavsky A. Arrest of segregation leads to accumulation of highly intertwined catenated dimers: dissection of the final stages of SV40 DNA replication. Cell. 1981 Sep;25(3):659–669. doi: 10.1016/0092-8674(81)90173-2. [DOI] [PubMed] [Google Scholar]
- Sundin O., Varshavsky A. Terminal stages of SV40 DNA replication proceed via multiply intertwined catenated dimers. Cell. 1980 Aug;21(1):103–114. doi: 10.1016/0092-8674(80)90118-x. [DOI] [PubMed] [Google Scholar]
- Tewey K. M., Chen G. L., Nelson E. M., Liu L. F. Intercalative antitumor drugs interfere with the breakage-reunion reaction of mammalian DNA topoisomerase II. J Biol Chem. 1984 Jul 25;259(14):9182–9187. [PubMed] [Google Scholar]
- Tewey K. M., Rowe T. C., Yang L., Halligan B. D., Liu L. F. Adriamycin-induced DNA damage mediated by mammalian DNA topoisomerase II. Science. 1984 Oct 26;226(4673):466–468. doi: 10.1126/science.6093249. [DOI] [PubMed] [Google Scholar]
- Uemura T., Tanagida M. Mitotic spindle pulls but fails to separate chromosomes in type II DNA topoisomerase mutants: uncoordinated mitosis. EMBO J. 1986 May;5(5):1003–1010. doi: 10.1002/j.1460-2075.1986.tb04315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura T., Yanagida M. Isolation of type I and II DNA topoisomerase mutants from fission yeast: single and double mutants show different phenotypes in cell growth and chromatin organization. EMBO J. 1984 Aug;3(8):1737–1744. doi: 10.1002/j.1460-2075.1984.tb02040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver D. T., Fields-Berry S. C., DePamphilis M. L. The termination region for SV40 DNA replication directs the mode of separation for the two sibling molecules. Cell. 1985 Jun;41(2):565–575. doi: 10.1016/s0092-8674(85)80029-5. [DOI] [PubMed] [Google Scholar]
- Yang L., Rowe T. C., Liu L. F. Identification of DNA topoisomerase II as an intracellular target of antitumor epipodophyllotoxins in simian virus 40-infected monkey cells. Cancer Res. 1985 Nov;45(11 Pt 2):5872–5876. [PubMed] [Google Scholar]
- Zwelling L. A. DNA topoisomerase II as a target of antineoplastic drug therapy. Cancer Metastasis Rev. 1985;4(4):263–276. doi: 10.1007/BF00048092. [DOI] [PubMed] [Google Scholar]
- Zwelling L. A., Michaels S., Erickson L. C., Ungerleider R. S., Nichols M., Kohn K. W. Protein-associated deoxyribonucleic acid strand breaks in L1210 cells treated with the deoxyribonucleic acid intercalating agents 4'-(9-acridinylamino) methanesulfon-m-anisidide and adriamycin. Biochemistry. 1981 Nov 10;20(23):6553–6563. doi: 10.1021/bi00526a006. [DOI] [PubMed] [Google Scholar]