Abstract
Melanoma runs within families, but this may be due to shared genetic or shared environmental influences within those families. The concordance between pairs of non-identical compared to the concordance of identical twins can be used to determine whether familial aggregation is due to genetic or environmental factors. Mandatory reporting of melanoma cases within the state of Queensland yielded approximately 12,000 cases between 1982 and 1990. Twins in this study and from the adjacent state of New South Wales (125 pairs in total) were used to partition variation in liability to melanoma into genetic and environmental factors. Identical twins were more concordant for melanoma (4 of 27 pairs) than non-identical twins (3 of 98 pairs; p-value ≈ 0.04). Identical co-twins of affected individuals were 9.8 times more likely to be affected than by chance. However, non-identical co-twins of affected individuals were only 1.8 times more likely to be affected than by chance. An MZ:DZ recurrence risk ratio of 5.6 suggests that some of the genetic influences on melanoma are due to epistatic (gene-gene) interactions. Using these data and population prevalences, it was estimated that 55% of variation in liability to melanoma is due to genetic influences.
Introduction
A number of investigations have examined the role of familial aggregation for cutaneous melanoma. Between 8% and 12% of melanoma cases are associated with a prior family history of the disease (Goldstein and Tucker, 1995). Duggleby et al. (1981) investigated the rate of melanoma in the relatives of 214 individuals with melanoma compared to the relatives of 194 age- and sex-matched controls. In the relatives of those with melanoma, there were 8 times more cases than expected from the contemporary prevalence rates for that region. Ford et al (1995) showed that there is a 2.24 fold increase in risk of melanoma for those with a family history. However, Cockburn et al (2001), like Nancarrow et al (1993), point out that studies of nuclear families cannot distinguish between familial aggregation due to shared environment (such as similar patterns of sun exposure) or shared genes.
The concordance between identical twins, who share all their genes, compared to non-identical twins, who share approximately half their genes, is an excellent basis on which to partition variation in a trait into genetic and environmental factors. Since identical twins control for genetic effects, it is possible to observe whether differential exposure to environmental factors in these twins concords with discordance for melanoma.
A genetic epidemiologic study of melanoma poses two main problems, regardless of whether we sample cases of twins and determine those with melanoma or vice versa. Firstly, the incidence of melanoma is relatively low. Lichtenstein et al (2000), in a study of 44788 pairs of twins listed in the Swedish, Danish and Finnish twin registries, found that only 290 twin pairs (107 identical, 183 non-identical) were discordant for melanoma and only 3 pairs (1 identical, 2 non-identical) were concordant for melanoma. A study by Milan et al (2002) using all the same sex adult twins living in Finland on the 31st of December, 1975, found that of 12941 twin pairs, there were 60 cases of melanoma, all from discordant pairs. In Queensland, Australia, the incidence was about 50 per 100,000 in 1987 (MacLennan et al, 2002) and about 69 per 100,000 individuals in 2002 (Coory et al, 2006). Therefore, samples from large catchments may not yield sufficient twins with melanoma to perform meaningful analyses. Secondly, a protocol to ascertain twins affected with melanoma or melanoma cases that are twins, may result in bias. For example, Mack et al (2000) advertised in newspapers for twins with cancer or other chronic diseases and found that there were fewer disease discordant non-identical twins and fewer older cases than expected. There may be some difficulty in ascertaining those individuals who have already died from melanoma. Also, it has been noted that ascertainment through newspapers and popular media may be biased toward those who are more educated (Wainwright et al, 2005). This is relevant to this study since education may be associated with behaviour related to sun exposure (Bastuji-Garin et al., 1999).
Fortunately, Australian law has enabled the collection of a data set that mitigates these two problems. In Australia, the law requires that the person in charge of a public or private hospital, or a nursing home, notify the cancer registry when a patient is known to be suffering from cancer. In practice, to capture all incident melanoma cases, this task is usually carried out by a variety of health services, particularly pathology services. The cancer registry for Queensland, the state in which this study was primarily conducted, began operating in 1982.
Using the data from the Queensland Cancer Registry, the Queensland Familial Melanoma Project (QFMP) further investigated families of cases diagnosed between 1982 to 1990. In the family (nontwin) data from this study, Do et al. (2004) investigated the genetics of age of onset of melanoma using the genetic relatedness between affected individuals and their parents, siblings, offspring and other relatives. The results suggested that additive genetic and environmental factors were almost equally important to variation in age of onset. There was also evidence of non-additive genetic effects. Interestingly, environmental effects common to members of a family did not explain the aggregation of age of onset within those families. A hypothesis of Mendelian inheritance of a single major gene was rejected (Do et al., 2004).
With a population of approximately two and half million in the 1980’s and a pair of twins for every one hundred births, one would expect sufficient twin families within Queensland, where at least one twin individual in a pair has melanoma, to provide an adequate sample for genetic analysis. Using twins ascertained through the QFMP and NSW Central Cancer Registry, we perform a classical twin analysis to determine the proportion of genetic and environmental effects of variation in liability to melanoma. We also investigate whether identical twins discordant for melanoma have a differential exposure to environmental factors increasing the risk of melanoma.
Results
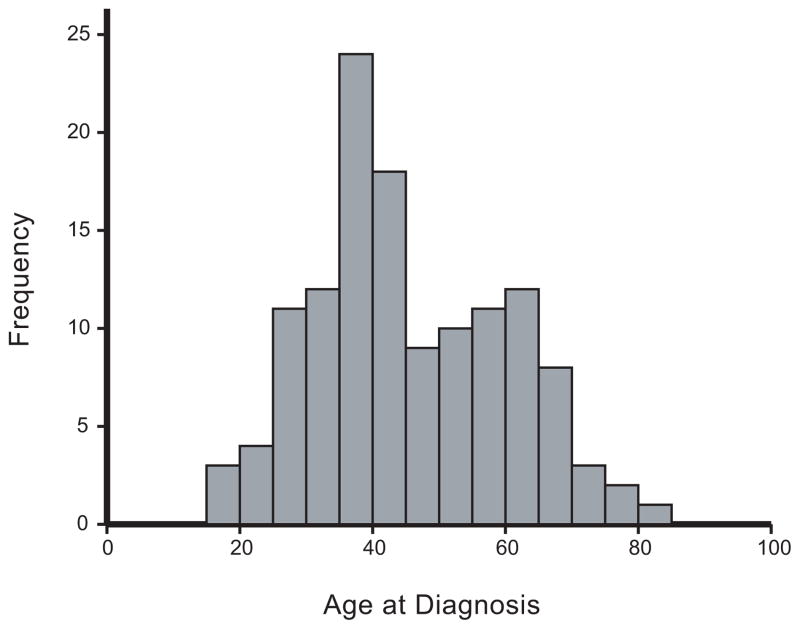
In total, the study identified 181 melanoma cases who were twins from Queensland, between 1982 and 1990, and 144 from New South Wales between 1985 and 1990. A calculation using the estimated population of Queensland, rates of twinning (Doherty and Lancaster, 1989) and rates of melanoma across this period (MacLennan et al., 1992), suggests that 181 twin individuals would be diagnosed with melanoma in Queensland during the period of this study. This provides confidence in the ascertainment procedure. Twin pairs were considered eligible if at least one twin individual was histologically confirmed with melanoma (primarily using pathology reports) and both twins in the pair survived to the age of 20. There were 121 eligible twin individuals from Queensland and 94 eligible twin individuals from New South Wales. Of these 215, there was no significant difference in the proportions of each sex (116 females, 99 males, χ2 1 =0.85). Cumulatively, there were 127 twin pairs for whom both twins were interviewed and for whom there was sufficient information to determine (i) the melanoma status of both twin individuals as well as (ii) their zygosity. However, an interview was not completed by either the proband or the co-twin in the case of 49 pairs and a blood sample could not be obtained for 13 affected probands and 26 co-twins, leaving 127 twin pairs. Two probands (both from discordant pairs) were reviewed and reclassified as unaffected. Of the remaining twin pairs (125), there were 27 identical twin pairs and 98 non-identical twin pairs (18 identical females, 9 identical males, 29 non-identical females, 25 non-identical males, 44 non-identical opposite sex). This ratio of approximately 3 MZ : 10 DZ (after accounting for ascertainment) is significantly different from the 1 MZ : 2 DZ expected in European popluations at the time of ascertainment (χ2 1=8, P ≈ 0.005, Doherty and Lancaster, 1986). If variation in liability to melanoma is due to genetic influences, we would expect a greater proportion of identical twins to concord for melanoma status, with both twins in a pair being either affected or unaffected, than for pairs of non-identical twins. Consequently, we would expect fewer identical twins discordant for melanoma. Here, we ascertain cases where at least one twin in a pair is diagnosed with melanoma. So, the observed ratio of MZ:DZ twins is consistent with the effect of ascertainment on familial cases of a strongly heritable disease. The average age at diagnosis was 45 years (Figure 1). Almost one third of all cases were diagnosed between the ages of 35 and 45.
Figure 1.
Age at diagnosis for affected twin individuals for whom information was available (N=128).
There were 4 identical twin pairs and 3 non-identical twins pairs concordant for melanoma (Table 1). Fisher’s Exact Test (Fisher, 1922) showed that the rate of concordance of melanoma for identical twins is significantly greater than the rate for non-identical twins (p-value ≈ 0.039, one-tailed). A small change in the number of concordant pairs may make this result insignificant at the 0.05 level.
Table 1.
Twin pairs with complete information for melanoma status categorised according to concordance or discordance for melanoma and zygosity. The tetrachoric correlation in liability to melanoma between twin pairs is based on the data presented and estimates of population prevalence.
| Zygosity | Concordant | Discordant | Total | r (95% CI) |
|---|---|---|---|---|
| Identical (MZ) | 4 | 23 | 27 | 0.55 (0.33 – 0.71) |
| Non-identical (DZ) | 3 | 95 | 98 | 0.23 (0.03 – 0.40) |
| Total | 7 | 118 | 125 |
In our sample, the unadjusted probability of developing melanoma if one’s non-identical twin also has melanoma (i.e. recurrence risk) is about 6%. However, the probability of developing melanoma if your identical twin has melanoma is 26%. Adjusting for year of birth, sex and age (or age of being diagnosed if they were affected), we expect 0.4 of the 27 identical co-twins of affected individuals to have melanoma; of the 98 non-identical twins of affected individuals, it is expected that 1.7 individuals will be affected. From the numbers observed in this study, the chance of being affected with melanoma if your identical co-twin is affected is 9.8 (approximate 95% CI: 0.41, 246.90) times higher (Standardised Risk Ratio (SRR)) than for a person in the population with the same sex, age and year of birth; the chance of being affected with melanoma if one’s non-identical co-twin is affected is 1.76 (approximate 95% CI: 0.27, 11.34) higher than predicted by chance. This is approximately a 5.6 fold difference in risk ratio between identical and non-identical twins. Using these data, it is possible to estimate the relative effect of genetic and environmental influences on melanoma. Here, it is assumed that there is complete ascertainment of all twins within the population that have been affected with melanoma. This value is represented by the 325 twins ascertained (322 pairs). Twins for whom zygosity information was not available were allocated a zygosity group in the proportion of 3 MZ : 10 DZ observed. Using this information, we have 3 of the 4 cells in the 2×2 contingency table. The missing cell is the number of twins where both are unaffected for melanoma, for both identical and non-identical twins. Assuming that we have captured all the concordant affected twin pairs and using the expected rate of melanoma (MacLennan et al, 1992) and twinning (Doherty and Lancaster, 1986), we can determine the approximate number of twin pairs for the estimated population catchment. Then, noting that the approximate ratio of identical to non-identical twins is 1:2, we determine that the number of twin pairs for whom both individuals are unaffected is 39,677 (13,258 identical twins and 26,419 nonidentical twins; Table 2). A maximum likelihood estimate of the tetrachoric correlation for the complete contingency table shows that the correlation between identical twins for liability to melanoma is 0.55 (95% CI: 0.33, 0.71) and the correlation between non-identical twins is 0.23 (95% CI: 0.03, 0.40). Although confidence intervals are presented here, it should be noted that they are based largely on expected, but unobserved, data.
Table 2.
Contingency tables used to perform tetrachoric correlations. The numbers represent the total number of identical and non-identical twins pairs expected in the catchment area during the period this study was conducted. These twins are categorised according to their observed and expected melanoma status.
| Identical twins | ||
|---|---|---|
| Affected | Unaffected | |
| Affected | 4 | 36 |
| Unaffected | 35 | 13,258 |
| Non-identical twins | ||
|---|---|---|
| Affected | Unaffected | |
| Affected | 3 | 122 |
| Unaffected | 122 | 26,419 |
Using the differential in correlation between identical and non-identical twin pairs, we can estimate the proportion of genetic and environmental influences (Jinks and Fulker, 1970). Of the total variation, 45% is expected to be due to environmental influences unique to each twin. The remaining 55% is due to genetic influences. Of these genetic influences, two thirds (37% of total variation) is due to additive genetic influences and one third (18% of total variation) is due to non-additive genetic influences. Additive genetic influences are where the effect on the trait is the sum of allelic effects. Non-additive genetic effects include dominance and epistasis (interactions between genes at different loci). Environmental influences common to both twins in a pair were not a significant source of variation. The environmental influences found here have a differential effect between co-twins.
Age at diagnosis and histopathology for the seven pairs concordant for melanoma are shown in Table 3. In one pair of identical male twins, both individuals were diagnosed at the age of 36. One individual was affected with superficial spreading melanoma on the trunk while the other was affected with melanoma, not otherwise specified on the lower limb. A brother of these twins died at the age of 20 as a consequence of cutaneous melanoma on the lower limb (including hip). We have carried out a number of tests to exclude major biases in our study sample. The twin sample was collected within a registry-based study of melanoma which we believe gave complete coverage of all diagnosed cases within the study period. Tumour histological morphology and behaviour did not differ between twin and nontwin cases (chi-square test, P=0.18), or between monozygotic and dizygotic twins. The number of concordantly affected pairs was only small, but comparison of tumour histology did not suggest a large overrepresentation of milder cases (test for an excess of in situ lesions in nonproband twin cases, P=0.53).
Table 3.
Age at diagnosis and melanoma histology for the 4 identical and 3 non-identical twin pairs concordant for melanoma, without any particular order.
| Twin 1 | Twin 2 | |||
|---|---|---|---|---|
| Histology | Age | Histology | Age | |
| Identical | Superficial spreading malignant melanoma | 36 | Malignant melanoma (Not otherwise specified) | 36 |
| Identical | Superficial spreading malignant melanoma | 63 | Malignant melanoma (Not otherwise specified) | 41 |
| Identical | Malignant melanoma (Not otherwise specified) | 43 | Superficial spreading malignant melanoma | 57 |
| Identical | Superficial spreading malignant melanoma | 58 | Melanoma in situ | 62 |
| Non-identical | Nodular melanoma | 84 | Melanoma in situ | 78 |
| Non-identical | Malignant melanoma (Not otherwise specified) | 24 | Superficial spreading malignant melanoma | 41 |
| Non-identical | Superficial spreading malignant melanoma | 72 | Superficial spreading melanoma in situ | 57 |
Twin pairs discordant for melanoma are ideal case-control studies to determine the effect of envi8 ronmental and constitutive factors since they are matched for age and childhood environment and, in the case of identical twins, for genetics. From the results of the 17 comparitive questions, Table 4 shows the number of individuals in the 2×2 table where both twins agreed that one twin had a greater propensity or measure of one risk factor than the other twin. Many of the identical twin individuals indicated that they were concordant for the risk factors or that they could not recall. Nevertheless, four pairs of identical twins suggested that the twin affected with melanoma spent more time at the beach as an adult than their co-twin; no twin pairs recalled that the unaffected individual in a pair of identical twins spent more time at the beach than their affected co-twin. McNemar’s test showed that this difference was not significant at the 0.05 level after accounting for multiple testing (McNemar, 1947). For non-identical twins, some 16 pairs indicated that the unaffected twin tanned more easily while in 6 pairs, the affected twin tanned quicker. This difference was insignificant after accounting for multiple testing (Table 4). To further investigate the effect of sun exposure as an environmental risk factor, we compared reports of sun exposure during adolescence on weekdays in summer for the affected twin compared to their unaffect co-twin (Table 5). A conditional logistic regression found that those unaffected with melanoma reported a greater time spent outdoors during this period than affected twins (χ2 1 ≈ 5.8, P ≈ 0.016). Further, we found that there was no significant difference in deterioration to the reticular patterning on the back of the hand, as measured on the Beagley-Gibson scale, between those affected with melanoma and those unaffected (P ≈ 0.64, N = 32 twin pairs).
Table 4.
Identical and non-identical twin pairs discordant for melanoma categorised according to their comparative responses. Only comparisons on which both twins agree are included in the counts. The chi-square value is for McNemar’s test. After correcting for the 17 tests, no result was significant at the 0.05 level.
| Identical twins (23 pairs) | Non-identical twins (95 pairs) | |||||
|---|---|---|---|---|---|---|
| Affected | Unaffected | χ21 | Affected | Unaffected | χ21 | |
| Who burned more easily? | 0 | 0 | - | 15 | 7 | 2.9 |
| Who tanned more easily (faster)? | 0 | 0 | - | 6 | 16 | 4.5 |
| Who usually had a darker tan? | 0 | 0 | - | 10 | 17 | 1.8 |
| Who spent more hours outdoors in summer | ||||||
| At primary school | ||||||
| on weekdays? | 0 | 0 | - | 1 | 2 | 0.3 |
| on weekends? | 0 | 0 | - | 2 | 2 | 0 |
| on summer holidays? | 0 | 0 | - | 2 | 2 | 0 |
| In your teens (13–19) | ||||||
| on weekdays? | 0 | 1 | 1 | 3 | 3 | 0 |
| on weekends? | 0 | 0 | - | 6 | 9 | 0.6 |
| on summer holidays? | 0 | 0 | - | 5 | 7 | 0.3 |
| Who had more moles as a child? | 0 | 0 | - | 0 | 1 | 1 |
| Who had more moles as an adult? | 1 | 1 | 0 | 5 | 2 | 1.3 |
| Who had more sunburns as a child? | 0 | 0 | - | 8 | 6 | 0.3 |
| Who had more sunburns as an adult? | 1 | 1 | 0 | 9 | 7 | 0.3 |
| Who spent more time on the beach as a child? | 0 | 0 | - | 1 | 2 | 0.3 |
| Who spent more time on the beach as an adult? | 4 | 0 | 4 | 14 | 12 | 0.2 |
| Who tried more to get a tan as a child? | 0 | 0 | - | 5 | 2 | 1.3 |
| Who tried more to get a tan as an adult? | 3 | 0 | 3 | 8 | 8 | 0 |
Table 5.
Number of hours spent outdoors daily during summer between the ages of 13 and 19 comparing twins discordant for melanoma.
| Unaffected | |||||
|---|---|---|---|---|---|
| None | Up to 1 hour | 1–3 hours | More than 3 hrs | ||
| Affected | None | 0 | 0 | 1 | 0 |
| Up to 1 hour | 1 | 6 | 11 | 6 | |
| 1–3 hours | 0 | 5 | 14 | 10 | |
| More than 3 hrs | 0 | 4 | 1 | 8 | |
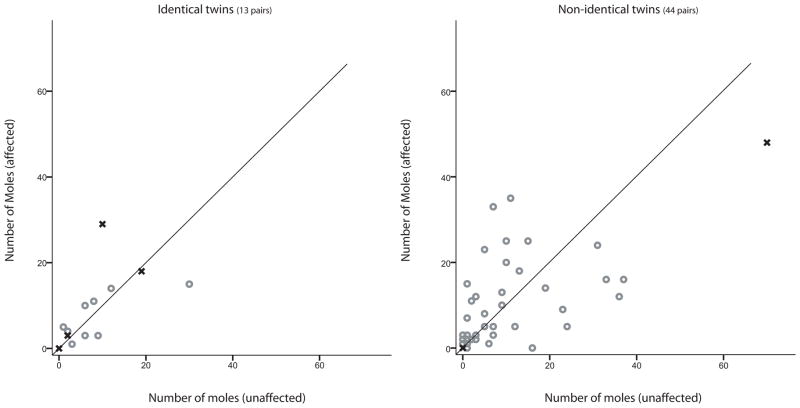
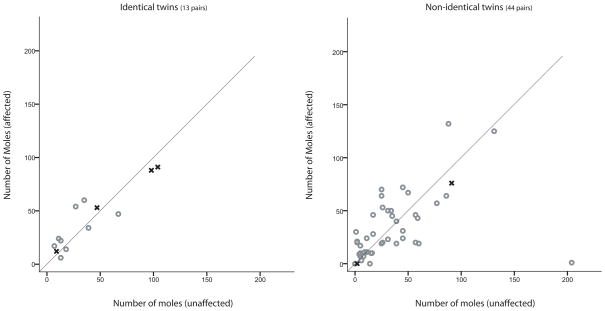
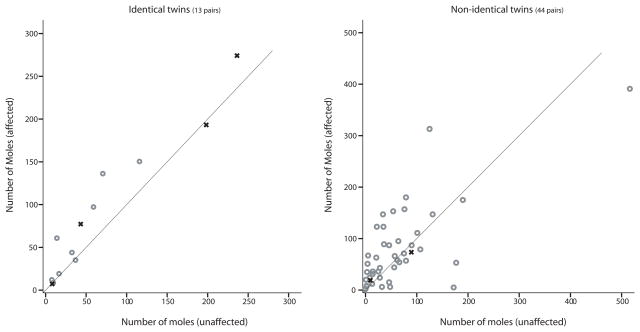
A paired t-test was performed to determine whether there was a significant difference in mole count, freckling or skin reflectance between those individuals affected with melanoma compared to their unaffected co-twins. For pairs of non-identical twins discordant for melanoma, the affected individual had, on average, a greater number of moles on the face, neck and torso, although this difference was not significant (Figures 3 and 4). A paired t-test showed that affected individuals had a greater number of moles on their arms and legs than their unaffected, non-identical, co-twins (p-value ≈ 0.035) and their unaffected, identical, co-twins (p-value ≈ 0.032). A plot of the number of moles on the arms and legs between twin pairs is shown in Figure 2. In pairs of non-identical twins, affected twins had, on average, 80 moles on their arms and legs compared to 65 for their non-identical, unaffected, co-twins. In pairs of identical twins, affected twins had, on average, 71 moles on their arms and legs compared to 46 for their identical, unaffected, co-twins. Inspection of Figure 2 suggests that, in twin pairs where both individuals are affected, they had a similar number of moles on their arms and legs. The total mole count was greater for the affected individual in pairs of non-identical twins discordant for melanoma (p-value ≈ 0.047) but not significantly different between pairs of identical twins discordant for melanoma (p-value ≈ 0.11).
Figure 3.
The number of moles counted on the face and neck comparing individuals affected for melanoma compared to their unaffected co-twin for identical (left) and non-identical (right) twins. Pairs of twins where both individuals are affected are presented (x) without any predefined order. Data on mole count were only collected for two of the three pairs of non-identical twins where both individuals were affected.
Figure 4.
The number of moles counted on the torso comparing individuals affected for melanoma compared to their unaffected co-twin for identical (left) and non-identical (right) twins. Pairs of twins where both individuals are affected are presented (x) without any predefined order. Data on mole count were only collected for two of the three pairs of non-identical twins where both individuals were affected.
Figure 2.
The number of moles counted on arms and legs comparing individuals affected for melanoma compared to their unaffected co-twin for identical (left) and non-identical (right) twins. Pairs of twins where both individuals are affected are presented (x) without any predefined order. Data on mole count were only collected for two of the three pairs of non-identical twins where both individuals were affected.
A conditional logistic regression of pairs discordant for melanoma found that the odds ratio for each additional mole (on the arm or leg) against no moles, to the onset of melanoma, is 1.010 (χ21 ≈ 4, 95% CI: 0.99, 1.021). There was no significant difference between identical and non-identical twins (χ2 1 ≈ 3.10). Since melanoma is rare, this odds ratio is equivalent to the relative risk (Zhang and Yu, 1998). So, the risk of developing melanoma is 1.01n, where n is the number moles on the arms and legs. This risk is relative to someone with no moles. So, those with 100 moles on their arms and legs are 2.7 times more likely to develop melanoma than those with no moles.
Discussion
Lichtenstein et al (2000), from 44,788 twin pairs registered in Sweden, Denmark and Finland, found 3 concordant pairs from 293 twin pairs where at least one individual had melanoma. Perhaps because of the generally higher incidence of melanoma in Australian in general and Queensland specifically, and the mandatory reporting of melanoma cases to cancer registries, we found 7 twin pairs concordant for melanoma from a total of 125 twin pairs. Although this number is small, it was sufficient to indicate that genetic influences were a significant source of variation in liability to melanoma. Although there are wide confidence intervals on the recurrence risks, identical twins were more than four times more likely to be affected with melanoma if they had an affected co-twin than non-identical twins, suggesting that non-additive genetic influences (dominance, epistasis) are a significant source of variation (Risch, 1990). This was echoed in genetic modelling of variance in liability, where the point estimate is that 18% of variation in liability to melanoma is due to non-additive genetic influences. Do et al (2004), who performed a genetic analysis of the non-twin data (from the larger study of which this is a part) for age of onset to melanoma, also found a point estimate suggesting non-additive genetic influence. We found, like Do et al (2004), that variation in liability to melanoma was almost equally divided between genetic and environmental influences. Similarly, we also found that environmental influences common between twins in a pair were not a significant source of variation. This suggests that, for the most part, the aggregation of melanoma within families is due to genetic influences.
The second part of this investigation sought to observe whether there was a difference in environmental factors between those affected with melanoma and their unaffected co-twins. Since identical twins control for genetic factors, discordance in melanoma status can only arise from the different environmental conditions experienced by the affected twin compared to the unaffected twin including stochastic events. Using data collected from questionnaires, there was a trend to suggest that those affected with melanoma had less routine time outdoors during adolesence and subsequently greater intermittent exposure to the sun compared to their unaffected co-twins. This accords with the meta-analysis performed by Gandini et al (2005b) on the relationship between sun exposure and melanoma. However, it should be noted that public health campaigns outlining appropriate behaviour in relation to the sun have been running in these catchment areas for some time (Coory et al, 2006). It is possible that respondents’ recall of events, after knowing that one twin individual has melanoma and understanding various risk factors, may also explain these results.
It is known that moles are a risk factor for melanoma (Gandini et al, 2005a). However, it is important to know the extent to which this risk is due to genetic and environmental influences shared between mole count and melanoma. In this study, those affected with melanoma had a greater number of moles on their arms and legs than their unaffected co-twins. This was the case for both identical and non-identical twins. Since identical twins control for genetic influences, this suggests the importance of environmental factors which affect both moliness and risk of melanoma. Exposure to sunlight is the most obvious candidate (Gandini et al, 2005b). There was no significant difference in the number of moles on the torso or the head/neck between those affected with melanoma and their unaffected co-twins. However, there are few moles on the head/neck and the torso is, by convention, generally covered.
Many studies have investigated the increase in risk of developing melanoma with increased number of nevi. Green and Swerdlow (1989) compared the relative risk of developing melanoma for nine case-control studies from various geographical locations. Although there are differences in the method and site of nevus count, the results suggest quite an appreciable increase in risk for each nevus. For example, Green et al (1985) show that the relative risk of developing melanoma with 2–4 nevi greater than 2mm is 15.7 compared to those with no nevi. Holman and Armstrong (1984) found that those with 1–4 raised pigmented nevi had twice the risk of developing melanoma compared to those with no nevi. More recently, studies have shown that the relative risk for developing melanoma for those with greater than 100 nevi compared to those less than 10 nevi is about 10 (Garbe et al., 1994; Grulich et al., 1996). We looked at the effect of each additional mole on the arms and legs using twins discordant for melanoma as cases and controls. Naturally, they are matched for age and childhood environment. The increased risk of 1% for each additional mole (Recurrence risk of 1.01) found here is smaller than that suggested by these previous studies. However, this risk appears broadly similar to that found using the 5135 non-twin individuals in the QFMP with information on nevi. Using these individuals, Aitken et al (1994) found that the adjusted relative risk of developing melanoma for those with few nevi (compared to no nevi) is 1.9 and the relative risk for those with many nevi is 2.94.
Overall, these results confirm the heritability of risk towards developing cutaneous melanoma. They also show that the correlation between mole count and melanoma is not purely due to genetic effects common between them. Others have found that mutations in the BRAF gene are more common in melanomas occurring on intermittently sun exposed skin (Pollock et al., 2003; Rivers, 2004). So, some of the variation in mole count may be a proxy for sun exposure and may directly increase the risk of melanoma.
Methods
The data used in this study were collected as part of a larger study of individuals diagnosed with cutaneous melanoma between 1st January 1982 (coinciding with the beginning of the Queensland Cancer Registry) and 3rd December 1990. The design and general characteristics of participants of the Queensland Familial Melanoma Project (QFMP) have been outlined by Aitken et al (1996) and Baxter et al (2008). Briefly, of the 12,016 eligible cases, 8,412 returned a family history questionnaire and provided their consent and 7,784 of these agreed to answering further questionnaires. This family history questionnaire included a question that asked Are you a twin? Those that answered ‘yes’ were recruited for the study. To obtain additional twin pairs for this study, we also recruited melanoma cases in the adjacent state of New South Wales between January 1985 and December 1990. As far as possible, the protocol and questionnaires used in New South Wales were identical to those used in Queensland. For this study, twins were eligible if both individuals in a pair reached 20 years of age, when the cumulative risk of developing melanoma was thought to be appreciable. Further questionnaires were administered to affected twins and their co-twins regardless of whether those co-twins were affected. This investigation conforms to the principles set forth in the Declaration of Helsinki and approval for the study was provided by the Human Research Ethics Committee at the Queensland Institute of Medical Research.
A follow-up questionnaire administered to the twins asked their primary place of residence for each decade of life, their outdoor behaviour in relation to the sun, their hair, eye and skin colour and the sensitivity of their skin to acute and chronic sun exposure. Each twin in a pair was asked whether they, or their co-twin, had a greater propensity to burn from sun exposure and tan from sun exposure, which twin had more moles and who was exposed to more sunlight. Trained research nurses visited both twins and their co-twins, regardless of their melanoma status, and measured their age, skin reflectance on the back of the left hand and the inner upper left arm, and number of moles greater than 2 mm in diameter on the head/neck, torso and arms/legs. In addition, an impression of the back of the left hand, while it was held in a loose-grip position, was taken using a quick-setting silicon solution to assess photoageing (Fritschi et al., 1995). Venous blood samples were also collected. A final questionnaire requested information about their birth and asked questions to elucidate whether the twins were identical or non-identical and whether they were separated from their co-twin for longer than a year. Zygosity was determined from the results of questions regarding physical similarity and confusion by others as to identity between twins. Bonnelykke et al (1989) show that the rate of misclassification using this method is approximately 4% (entirely composed of non-identical twins misclassified as identical). Martin and Martin (1975) found complete agreement between zygosity tested using blood samples and zygosity assessed from a questionnaire for 47 twin pairs.
Some individuals in the QFMP were contacted again between 2002 and 2005 and a computer assisted telephone interview (CATI) was conducted. Further detail of this follow-up can be found in Baxter et al (2008). Of interest to this investigation is a CATI question that asked the amount of time spent outdoors daily in summer between the hours of 9am to 5pm from the age of 13 to the age of 19 on weekdays. The possible responses were none, less than 1 hour, between 1 and 3 hours and greater than 3 hours. For this question, information was collected for 67 complete twin pairs.
Analysis
Using these data, we perform analyses to determine whether identical twins are more concordant for melanoma status than non-identical twins. If identical twins are more concordant, it would suggest that there are genes influencing liability to melanoma. Using the known basis for similarity of monozygotic and dizygotic twins, correlations between twin pairs can be used to decompose the variance of a trait into genetic and environmental influences. Genetic variation can be partitioned into additive (A) and non-additive (D) influences. If the effect of an allele on a trait is independent of the other allele at that locus or any other allele at any other loci, then additive genetic influences are the sum of the effects of all these independent alleles as they affect the trait (A). Identical twin pairs are perfectly correlated for additive genetic effects while dizygotic twin pairs, who share roughly half their genes, are expected to correlate about 0.5. Non-additive influences can be allelic interactions (dominance) or non-allelic interactions (epistasis). Allelic interactions are where the effect of an allele at a particular locus is dependent upon the other allele at that locus (Mendelian inheritance). Epistasis, also known as gene-gene interaction, is where the effect of an allele at a particular locus is dependent upon an allele at another locus or alleles at multiple loci. There is no possibility of distinguishing between dominance and epistasis in a classical twin design so the non-additive term is usually called “dominance”, with dizygotic twins expected to correlate 0.25 (Jinks and Fulker, 1970).
Variation due to the environment can be partitioned into either that influencing both twins (C) or that influencing each twin disproportionately (E). Twins are expected to correlate for common environmental factors (c) independent of whether they are identical or non-identical. By definition, twins are not correlated for unique environmental effects (Jinks and Fulker, 1970).
The estimates of common environmental influences (C) and non-additive genetic influences (D) are both derived from the relationship between monozygotic and dizygotic twin pair correlations, and are negatively confounded. When the correlation between dizygotic twin pairs is less than half the correlation between monozygotic twin pairs, non additive genetic effects have greater influence than common environmental effects. Based on inspection of the monozygotic and dizygotic tetrachoric correlations, an ADE model was fitted to the data. Maximum likelihood estimation of tetrachoric correlations and proportions of genetic and environmental influence on liability to melanoma were performed using the computer program Mx v1.54a (Neale et al., 2002). The analysis assumes a bivariate normal distribution underlying susceptibility to melanoma with a threshold distinguishing those affected from those unaffected (Neale and Cardon, 1992).
Information on the cumulative risk of melanoma for each age, year of birth and sex was obtained from the Australian Institute of Health and Welfare (http://www.aihw.gov.au/). These data were used to estimate the number of people we would expect to be diagnosed with melanoma if we had selected individuals from the population with the same year of birth, sex and age (or age of diagnosis if they were affected) as the co-twins of affected twins. This is calculated by summing the proportion of people affected for the cohort representing the year of birth, sex and age at diagnosis (or current age if unaffected) for each co-twin of an affected individual. Considering that the difference in incidence of melanoma within Queensland compared to that across Australia (Lens and Dawes, 2004) was relatively small considering the purpose of this study, the latter was used for greater accuracy.
In the second part of this study, we investigate whether exposure to different environmental factors is associated with discordance in melanoma status within a twin pair. In particular, we focus on identical twins since they control for genetic influences. The 2×2 contingency tables from questions requesting twins to compare their risk factors against their co-twins were analysed using McNemar’s test (McNemar, 1947). The p-value resulting from the chi-square was adjusted for multiple testing using the Bonferroni correction.
Statistical tests were performed in SPSS v15 and R (R Core Development Team, 2008). In analyses where co-twins were used as controls, the number of moles was transformed toward normality using log10(x+1) where x is the number of moles, before performing paired t-tests. An analysis was performed to determine the increased risk of melanoma for each additional mole on an arm or leg. This conditional logistic regression matched those with melanoma with their co-twins.
Acknowledgments
The authors would like to thank the research nurses who spent a great deal of time travelling around the vast expanse of rural Queensland and New South Wales to collect the information from the twins. These include Jane Palmer and Kate. The authors would also like to thank Lea Jackman, Judy Symmons, John Pearson, Ros Patterson and David Symth for their help in data management. Finally, we would like to thank the twins and their families for their time in participating in this study. This work was supported by NHMRC project grants 870774, 900536 and 930223 and NHMRC Research Fellowships to D.W., D.D., and N.H., the US National Cancer Institute (CA88363), the Cooperative Research Centre for Discovery of Genes for Common Diseases (project support). This research is also supported by The Cancer Council Queensland and The Cancer Council NSW.
References
- Aitken JF, Duffy DL, Green A, Youl P, MacLennan R, Martin NG. Heterogeneity of melanoma risk in families of melanoma patients. Am J Epidemiol. 1994;140:961–973. doi: 10.1093/oxfordjournals.aje.a117203. [DOI] [PubMed] [Google Scholar]
- Aitken JF, Green AC, MacLennan R, Youl P, Martin NG. The Queensland Familial Melanoma Project: study design and characteristics of participants. Melanoma Res. 1996;6:155–165. doi: 10.1097/00008390-199604000-00011. [DOI] [PubMed] [Google Scholar]
- Bastuji-Garin S, Grob JJ, Grognard C, Grosjean F, Guillaume JC. Melanoma prevention: evaluation of a health education campaign for primary schools. Arch Dermatol. 1999;135:936–940. doi: 10.1001/archderm.135.8.936. [DOI] [PubMed] [Google Scholar]
- Baxter AJ, Hughes MC, Kvaskoff M, Siskind V, Shekar S, Aitken JF, et al. The Queensland Study of Melanoma: Environmental and Genetic Associations (Q-MEGA); Study Design, Baseline Characteristics, and Repeatability of Phenotype and Sun Exposure Measures. Twin Res Hum Genet. 2008;11:183–196. doi: 10.1375/twin.11.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnelykke B, Hauge M, Holm N, Kristoffersen K, Gurtler H. Evaluation of zygosity diagnosis in twin pairs below age seven by means of a mailed questionnaire. Acta Genet Med Gemellol (Roma) 1989;38:305–313. doi: 10.1017/s0001566000002713. [DOI] [PubMed] [Google Scholar]
- Brown KM, Macgregor S, Montgomery GW, Craig DW, Zhao ZZ, Iyadurai K, et al. Common sequence variants on 20q11.22 confer melanoma susceptibility. Nat Genet. 2008 doi: 10.1038/ng.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockburn M, Black W, McKelvey W, Mack T. Determinants of melanoma in a case-control study of twins (United States) Cancer Causes Control. 2001;12:615–625. doi: 10.1023/a:1011271117496. [DOI] [PubMed] [Google Scholar]
- Coory M, Baade P, Aitken J, Smithers M, McLeod GR, Ring I. Trends for in situ and invasive melanoma in Queensland, Australia, 1982–2002. Cancer Causes Control. 2006;17:21–27. doi: 10.1007/s10552-005-3637-4. [DOI] [PubMed] [Google Scholar]
- Do KA, Aitken JF, Green AC, Martin NG. Analysis of melanoma onset: assessing familial aggregation by using estimating equations and fitting variance components via Bayesian random effects models. Twin Res. 2004;7:98–113. doi: 10.1375/13690520460741480. [DOI] [PubMed] [Google Scholar]
- Doherty JD, Lancaster PA. The secular trend of twinning in Australia, 1853–1982. Acta Genet Med Gemellol (Roma) 1986;35:61–76. doi: 10.1017/s0001566000006279. [DOI] [PubMed] [Google Scholar]
- Duffy DL. Screening a 2 cM genetic map for allelic association: a simulated oligogenic trait. Genet Epidemiol. 1995;12:595–600. doi: 10.1002/gepi.1370120612. [DOI] [PubMed] [Google Scholar]
- Duggleby WF, Stoll H, Priore RL, Greenwald P, Graham S. A genetic analysis of melanoma--polygenic inheritance as a threshold trait. Am J Epidemiol. 1981;114:63–72. doi: 10.1093/oxfordjournals.aje.a113175. [DOI] [PubMed] [Google Scholar]
- Fisher RA. On the interpretation of χ2 from contingency tables, and the calculation of P. Journal of the Royal Statistical Society. 1922;85:87–94. [Google Scholar]
- Ford D, Bliss JM, Swerdlow AJ, Armstrong BK, Franceschi S, Green A, et al. Risk of cutaneous melanoma associated with a family history of the disease. The International Melanoma Analysis Group (IMAGE) Int J Cancer. 1995;62:377–381. doi: 10.1002/ijc.2910620403. [DOI] [PubMed] [Google Scholar]
- Fritschi L, Battistutta D, Strutton GM, Green A. A non-invasive measure of photoageing. Int J Epidemiol. 1995;24:150–154. doi: 10.1093/ije/24.1.150. [DOI] [PubMed] [Google Scholar]
- Gandini S, Sera F, Cattaruzza MS, Pasquini P, Abeni D, Boyle P, et al. Meta-analysis of risk factors for cutaneous melanoma: I. Common and atypical naevi. Eur J Cancer. 2005a;41:28–44. doi: 10.1016/j.ejca.2004.10.015. [DOI] [PubMed] [Google Scholar]
- Gandini S, Sera F, Cattaruzza MS, Pasquini P, Picconi O, Boyle P, et al. Meta-analysis of risk factors for cutaneous melanoma: II. Sun exposure. Eur J Cancer. 2005b;41:45–60. doi: 10.1016/j.ejca.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Garbe C, Buttner P, Weiss J, Soyer HP, Stocker U, Kruger S, et al. Risk factors for developing cutaneous melanoma and criteria for identifying persons at risk: multicenter case-control study of the Central Malignant Melanoma Registry of the German Dermatological Society. J Invest Dermatol. 1994;102:695–699. doi: 10.1111/1523-1747.ep12374280. [DOI] [PubMed] [Google Scholar]
- Goldstein AM, Tucker MA. Genetic epidemiology of familial melanoma. Dermatol Clin. 1995;13:605–612. [PubMed] [Google Scholar]
- Green A, MacLennan R, Siskind V. Common acquired naevi and the risk of malignant melanoma. Int J Cancer. 1985;35:297–300. doi: 10.1002/ijc.2910350303. [DOI] [PubMed] [Google Scholar]
- Green A, Swerdlow AJ. Epidemiology of melanocytic nevi. Epidemiol Rev. 1989;11:204–221. doi: 10.1093/oxfordjournals.epirev.a036037. [DOI] [PubMed] [Google Scholar]
- Grulich AE, Bataille V, Swerdlow AJ, Newton-Bishop JA, Cuzick J, Hersey P, et al. Naevi and pigmentary characteristics as risk factors for melanoma in a high-risk population: a case-control study in New South Wales, Australia. Int J Cancer. 1996;67:485–491. doi: 10.1002/(SICI)1097-0215(19960807)67:4<485::AID-IJC4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Holman CD, Armstrong BK. Pigmentary traits, ethnic origin, benign nevi, and family history as risk factors for cutaneous malignant melanoma. J Natl Cancer Inst. 1984;72:257–266. [PubMed] [Google Scholar]
- Jinks JL, Fulker DW. Comparison of the biometrical genetical, MAVA, and classical approaches to the analysis of human behavior. Psychol Bull. 1970;73:311–349. doi: 10.1037/h0029135. [DOI] [PubMed] [Google Scholar]
- Lens MB, Dawes M. Global perspectives of contemporary epidemiological trends of cutaneous malignant melanoma. Br J Dermatol. 2004;150:179–185. doi: 10.1111/j.1365-2133.2004.05708.x. [DOI] [PubMed] [Google Scholar]
- Lichtenstein P, Holm NV, Verkasalo PK, Iliadou A, Kaprio J, Koskenvuo M, et al. Environmental and heritable factors in the causation of cancer--analyses of cohorts of twins from Sweden, Denmark, and Finland. N Engl J Med. 2000;343:78–85. doi: 10.1056/NEJM200007133430201. [DOI] [PubMed] [Google Scholar]
- Mack TM, Deapen D, Hamilton AS. Representativeness of a roster of volunteer North American twins with chronic disease. Twin Res. 2000;3:33–42. doi: 10.1375/136905200320565670. [DOI] [PubMed] [Google Scholar]
- MacLennan R, Green AC, McLeod GR, Martin NG. Increasing incidence of cutaneous melanoma in Queensland, Australia. J Natl Cancer Inst. 1992;84:1427–1432. doi: 10.1093/jnci/84.18.1427. [DOI] [PubMed] [Google Scholar]
- Martin NG. The inheritance of scholastic abilities in a sample of twins. II. Genetical analysis of examinations results. Ann Hum Genet. 1975;39:219–229. doi: 10.1111/j.1469-1809.1975.tb00125.x. [DOI] [PubMed] [Google Scholar]
- McNemar Q. Note on the sampling error of the difference between correlated proportions or percentages. Psychometrika. 1947;12:153–157. doi: 10.1007/BF02295996. [DOI] [PubMed] [Google Scholar]
- Milan T, Verkasalo PK, Kaprio J, Koskenvuo M, Pukkala E. Malignant skin cancers in the Finnish Twin Cohort: a population-based study, 1976–97. Br J Dermatol. 2002;147:509–512. doi: 10.1046/j.1365-2133.2002.04870.x. [DOI] [PubMed] [Google Scholar]
- Nancarrow DJ, Mann GJ, Holland EA, Walker GJ, Beaton SC, Walters MK, et al. Confirmation of chromosome 9p linkage in familial melanoma. Am J Hum Genet. 1993;53:936–942. [PMC free article] [PubMed] [Google Scholar]
- Neale MC, Boker SM, Xie G, Maes HH. Mx: Statistical Modelling. 6. Department of Psychiatry; VCU Box 900126, Richmond, VA 23298: 2002. [Google Scholar]
- Neale MC, Cardon LR. Methodology for Genetic Studies of Twins and Families (NATO ASI Series D) Dordrecht: Kluwer Academic; 1992. [Google Scholar]
- Pollock PM, Harper UL, Hansen KS, Yudt LM, Stark M, Robbins CM, et al. High frequency of BRAF mutations in nevi. Nat Genet. 2003;33:19–20. doi: 10.1038/ng1054. [DOI] [PubMed] [Google Scholar]
- R Core Development Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2008. [Google Scholar]
- Risch N. Linkage strategies for genetically complex traits. I. Multilocus models. Am J Hum Genet. 1990;46:222–228. [PMC free article] [PubMed] [Google Scholar]
- Rivers JK. Is there more than one road to melanoma? Lancet. 2004;363:728–730. doi: 10.1016/S0140-6736(04)15649-3. [DOI] [PubMed] [Google Scholar]
- SPSS for Windows, Rel 15.0.0. Chicago: SPSS Inc; 2006. [Google Scholar]
- Wainwright MA, Wright MJ, Geffen GM, Luciano M, Martin NG. The genetic basis of academic achievement on the Queensland Core Skills Test and its shared genetic variance with IQ. Behav Genet. 2005;35:133–145. doi: 10.1007/s10519-004-1014-9. [DOI] [PubMed] [Google Scholar]
- Zhang J, Yu KF. What’s the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. Jama. 1998;280:1690–1691. doi: 10.1001/jama.280.19.1690. [DOI] [PubMed] [Google Scholar]
- Zhu G, Duffy DL, Eldridge A, Grace M, Mayne C, O’Gorman L, et al. A major quantitative-trait locus for mole density is linked to the familial melanoma gene CDKN2A: a maximum likelihood combined linkage and association analysis in twins and their sibs. Am J Hum Genet. 1999;65:483–492. doi: 10.1086/302494. [DOI] [PMC free article] [PubMed] [Google Scholar]