Abstract
Background
Asthma is a significant disease among children, and its prevalence has increased notably during the last 2 decades. A traditional Korean medicine, So-Cheong-Ryong-Tang (SCRT), has been used for the treatment of asthma in Asia for centuries, but its mechanism for reducing bronchopulmonary inflammation in asthma has yet to be elucidated.
Objective
To investigate whether the herbal extract SCRT inhibits inflammation in a mouse model of cockroach allergen–induced asthma.
Methods
A house dust extract containing endotoxin and cockroach allergens was used for immunization and 2 additional pulmonary challenges in BALB/c mice. Mice were treated with SCRT or vehicle 1 hour before each pulmonary challenge. Respiratory parameters were evaluated by whole-body plethysmography and forced oscillation methods 24 hours after the last challenge. Bronchoalveolar lavage (BAL) fluid was collected, and histologic sections of lung were prepared either 4 or 24 hours after the last house dust extract challenge.
Results
SCRT treatment significantly reduced the hyperreactivity of the airways as measured by whole-body plethysmography and direct measurement of airway resistance. Inflammation was significantly inhibited by SCRT treatment as demonstrated by reduced plasma IgE levels and improved pulmonary histologic characteristics. SCRT significantly reduced the number of neutrophils in the BAL fluid and also significantly reduced the BAL levels of CXC chemokines, providing a potential mechanism for the reduced inflammation. In a similar fashion, SCRT reduced eosinophil recruitment and BAL levels of eotaxin and RANTES.
Conclusion
These data indicate that SCRT treatment alleviates asthma-like pulmonary inflammation via suppression of specific chemokines.
INTRODUCTION
Asthma is a unique form of chronic respiratory disease characterized by reversible airways obstruction and substantial pulmonary inflammation.1 It represents one of the most common chronic inflammatory diseases, affecting an estimated 300 million people worldwide with an expected significant increase to 400 million by 2025.2 The sharply increasing prevalence and incidence of asthma causes global concern in both developing and developed countries.3,4
Conventional remedies, including corticosteroids and β2-agonists, are effective in managing asthma symptoms. However, the concerns regarding the adverse effects of current remedies due to long-term use and the lack of curative therapy lead asthma patients in the Western world to seek complementary and alternative medicine (CAM) treatments.5 In Europe, 30% of allergy patients use CAM.6 In the United States, the number of asthma patients using CAM has increased significantly from 34% in 19907 to 42% in 2001.8 Herbal treatment is the most common form of CAM used by asthma patients and the general public.8 Despite the substantial gain in the popularity of CAM to treat asthma, few studies have reported on either the clinical efficacy or how CAM remedy mechanisms, including herbal treatment, reduce inflammation.5,9
A Korean herbal medicine, So-Cheong-Ryong-Tang (SCRT, also known as Xiao-Qing-Long-Tang in traditional Chinese medicine and as Sho-seiryu-to in Japanese Kampo medicine), has long been prescribed for the treatment of allergic diseases in Korea, China, and Japan. During the past decades, studies have demonstrated the potential efficacy of SCRT in asthma in vivo,10–13 in vitro,12 and in clinical studies.14 In vitro, SCRT corrects the TH2-dominant pathologic disorder by suppressing TH2 cell development via reduction of interleukin (IL)-4 expression and promoting TH1 cell development through increasing interferon-γ expression in mice.12 SCRT treatment reduced airway and pulmonary infiltration of eosinophils induced by allergen challenge in guinea pigs10 and mite-sensitized mice,15 although the mechanisms were not described. In vitro studies showed that SCRT reduces the release of histamine,16 leukotrienes from mast cells,17 and tumor necrosis factor α (TNF-α) from peripheral mononuclear cells.18 These previous reports did not identify the in vivo mechanisms of how SCRT reduces the pulmonary inflammation of an asthmatic response.
To elucidate the mechanism of how SCRT modulates the allergic response, we evaluated the immunomodulatory effects of SCRT in a murine model of asthma induced by a house dust extract (HDE) containing cockroach allergens and endotoxin. In this study, multiple aspects of pulmonary inflammation were examined, including the production of inflammatory mediators and the pulmonary recruitment of inflammatory cells.
METHODS
Mice
Female BALB/c mice (18–20 g) were obtained from Jackson Labs (Bar Harbor, Maine) and maintained under standard laboratory conditions. The mice were housed in a temperature-controlled room (22°C) with a 12-hour dark/light cycle and provided food and water allowed ad libitum. All experiments were approved by the Boston University or the University of Michigan Animal Use Committee.
Experiment Design
The household dust preparation and induction of asthma were performed as reported previously.19 Briefly, collected house dust was resuspended with phosphate-buffered saline (PBS) and assayed for 9 allergens by enzyme-linked immunosorbent assay (ELISA). Our HDE contained high concentrations of cockroach allergens (378 U/mL of Bla g1 and 6,249 ng/mL of Bla g2), low levels of other allergens, and 270 pg/mL of endotoxin. Mice were sensitized with an intraperitoneal injection of a 1:1 mixture of HDE and an adjuvant (TiterMax Gold; CytRx, Norcross, Georgia) on day 0 and received pulmonary challenges on days 14 and 21 via hypopharyngeal administrations. Immunized mice were treated with 2.0 g/kg of SCRT by gastric gavage 1 hour before each pulmonary challenge on days 14 and 21. Control mice received 0.1 mL of PBS.
Preparation of SCRT and Quality Control
SCRT, which contains 8 species of medicinal plants, was prepared and analyzed as described previously.11,20 The types and amounts of standard materials for quantitative analysis of each species of herb in SCRT are listed in Table 1.
Table 1.
Contents of SCRT and the Amounts of Standard Materials in SCRT Extract Mixture
| Herbal medicines | Raw material amount, g (%) | Standard materials | Standard material amount, mg/1 g of extract |
|---|---|---|---|
| Ephedrae Herba | 6.0 (15) | NA | NA |
| Paeoniae Radix Alba | 6.0 (15) | Paeoniflorin | 34.42 ± 1.367 |
| Schizandrae Fructus | 6.0 (15) | Schizandrin | 2.39 ± 0.010 |
| Pinelliae Rhizoma | 6.0 (15) | Homogentistic acid | 17.07 ± 0.318 |
| Asari Herba Cum Radice | 4.0 (10) | α-Asarone | 0.18 ± 0.029 |
| Zingiberis Rhizoma | 4.0 (10) | 6-Gingerol | 2.11 ± 0.251 |
| Cinnamomi Ramulus | 4.0 (10) | Cinnamaldehyde | 1.84 ± 0.438 |
| Glycyrrhizae Radix | 4.0 (10) | Glycyrrhizic acid | 15.39 ± 0.283 |
Abbreviations: NA, not assayed; SCRT, So-Cheong-Ryong-Tang.
Measurement of Respiratory Functions
Airway hyperresponsiveness (AHR) was measured 24 hours after the final allergen challenge via whole-body plethysmography (Buxco, Troy, New York) in response to increasing doses of aerosolized acetyl β-methacholine (Sigma, St. Louis, Missouri) in unrestrained and conscious mice as previously reported.21
In a separate group of mice, airway resistance was measured by a forced oscillation technique22 with a FlexiVent instrument (Scireq Scientific Respiratory Equipment, Montreal, Quebec, Canada). Mice were anesthetized with an intraperitoneal injection of 1:5 diluted pentobarbital (Nembutal, 0.016 mL per gram of body weight Ovation Pharmaceutical, Deerfield, Illinois) combined with the paralytic pancuronium (Sigma) at 0.5 mg per gram of body weight. Once adequate surgical sedation was established, determined by a firm squeeze of the foot pad, a tracheotomy was performed with insertion of an 18-g polyethylene cannula into the distal trachea. The mouse was then placed on the FlexiVent mechanical ventilator (Scireq) and ventilated at breaths per minute with positive-end expiratory pressure set at 3 cm H2O. Measurement of airway resistance in response to increasing concentrations of aerosolized methacholine was obtained through periodic, computer-generated, “snapshot 150,” forced-maneuver interruptions in ventilation. Data are then presented as resistance change from baseline (in centimeters of water per milliliter per second).
Sample Collection
Blood was collected from the retro-orbital venous plexus into tubes containing 169mM tripotassium EDTA (Sigma) before the mice were sacrificed. Bronchoalveolar lavage (BAL) was collected through the trachea after intubation as previously reported.19 Differential counting was performed by counting 300 Diff-Quick (Baxter, Detroit, Michigan) stained cells. After the BAL fluid was collected, the right lung was removed and processed for the myeloperoxidase assay as described in our previous publication.23
Histopathologic Analysis
Immediately after collecting the BAL, the left lung was removed and processed for routine histologic analysis. Hematoxylin-eosin–stained tissue sections were examined by a board-certified pathologist (D.G.R.). The severity of the inflammation in the perivascular and peribronchial area in the lung was scored on a scale of 0 (no inflammation) to 4 (severe inflammation). All slides were scored in a masked manner.
Cytokine and Chemokine Analysis
All chemokine, cytokine, and IgE measurements were performed simultaneously to reduce errors due to interassay variation. The concentrations of all chemokines and cytokines were measured by ELISA using matched antibody pairs (R&D Systems Inc, Minneapolis, Minnesota) as previously described.24 IgE in blood and BAL were also measured by ELISA23 using antibody pairs purchased from Bethyl Laboratories Inc (Montgomery, Texas).
Statistical Analyses
The mean (SEM) was used for summary statistics in all figures. Differences between all treatments groups were compared by unpaired t test or 1-way analysis of variance with the Turkey posttest using GraphPad Prism, version 5.0 (GraphPad Software, San Diego, California). Statistical significance was achieved when P ≤ .05 at the 95% confidence interval.
RESULTS
SCRT Reduces AHR
AHR was significantly reduced by SCRT treatment as measured by either whole-body plethysmography or forced oscillation. AHR was evaluated by measuring enhanced pause (Penh) in response to nebulized methacholine. Bronchopulmonary hyperresponsiveness was significantly reduced in SCRT-treated mice when compared with vehicle-treated (PBS) mice (Fig 1A). To verify the unrestrained whole-body plethysmography data, which are not considered authoritative,22,25–27 the result was verified by the forced oscillation technique to directly assess airways resistance. SCRT treatment significantly reduced the airway resistance when compared with PBS treatment (Fig 1B).
Figure 1.
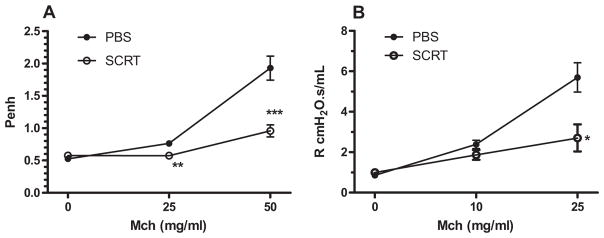
Herbal extract (So-Cheong-Ryong-Tang [SCRT]) treatment significantly improves pulmonary respiratory parameters. Penh (A) and resistance (R) (B) were measured in SCRT- and phosphate-buffered saline (PBS)–treated mice 24 hours after final house dust extract challenge via whole-body plethysmography or forced oscillation technique, respectively. Groups of mice received 2 treatments of SCRT or PBS on days 14 and 21, 1 hour before house dust extract challenge. Data are represented as mean ± SEM (n = 12 mice per group for Fig 1A and n = 3–4 per group for Fig 1B). Statistically significant differences were analyzed by using 1-way analysis of variance. *P ≤ .05, **P ≤ .01 and ***P ≤ .001 when compared with the PBS-treated group. MCh, methacholine.
SCRT Decreases Plasma and BAL IgE Concentrations
Cross-linking of allergen specific IgE antibodies on the surface of mast cells causes degranulation and subsequent AHR.28 To investigate the systemic effects of oral administration of SCRT, IgE levels were measured in blood and BAL because elevated expression of IgE is a key characteristic of the systemic asthmatic response. Plasma levels of IgE (Fig 2A) and IgE levels in the BAL (Fig 2B) were significantly decreased in SCRT-treated mice in both the early (4 hours) and late (24 hours) responses. As expected, the plasma levels of IgE were much greater than those in the BAL.
Figure 2.
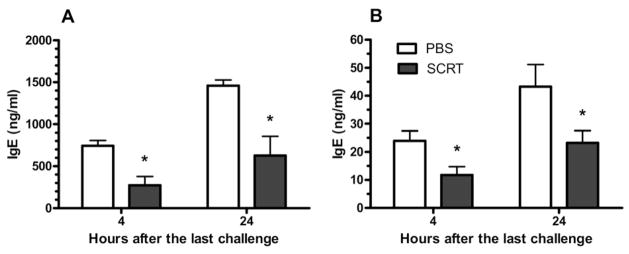
Reduced IgE antibody levels in bronchoalveolar lavage (BAL) and blood by So-Cheong-Ryong-Tang (SCRT) treatment. A, IgE concentrations in plasma of SCRT- or phosphate-buffered saline (PBS)–treated mice; B, IgE levels in BAL fluid. Plasma and BAL fluid were collected at 4 or 24 hours after final allergen challenge and IgE levels measured by enzyme-linked immunosorbent assay. Data are represented as mean ± SEM (n = 4–8 mice per group). Differences between the 2 groups were analyzed by the unpaired t test. *P ≤ .05 compared with PBS-treated mice.
SCRT Modulation of Pulmonary Infiltration of Inflammatory Cells and Expression of Mediators
The effects of oral administration of SCRT on the pulmonary infiltration of inflammatory cells and inflammatory mediators were examined. SCRT treatment resulted in a significant reduction of the pulmonary inflammation as determined by histologic examination of lung tissue (Fig 3). PBS-treated mice (Fig 3A) showed substantial infiltration of inflammatory cells that included lymphocytes and eosinophils. These cells were located in both the peribronchial and perivascular spaces. The pulmonary infiltration of inflammatory cells was reduced by SCRT treatment (Fig 3B). The pulmonary findings were scored in a masked manner, and SCRT significantly reduced the pulmonary inflammation induced by the cockroach allergens (Fig 3C).
Figure 3.
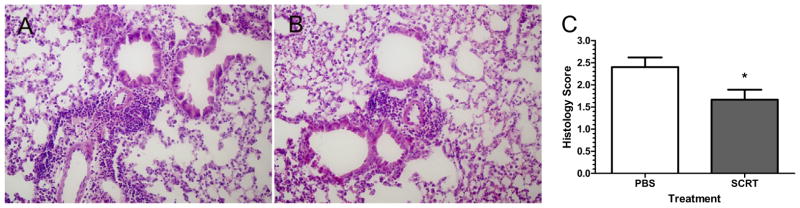
Pulmonary histologic analysis from mice treated with So-Cheong-Ryong-Tang (SCRT) or phosphate-buffered saline (PBS). Lungs were harvested 24 hours after the last pulmonary challenge and after bronchoalveolar lavage fluid was collected. (A) Representative hematoxylineosin (HE)–stained image from a PBS-treated mouse with a marked peribronchial and perivascular infiltration of eosinophils and lymphocytes. (B) Representative HE histologic image from SCRT-treated mouse with reduced inflammation. Both pictures are in the same magnification (×100). (C) HE-stained tissue sections were scored on a scale of 0 (no inflammation) to 4 (severe inflammation). All slides were scored in a masked manner. Values represent mean ± SEM with 10 to 12 mice in each group. Differences between the 2 groups were analyzed by the unpaired t test. *P ≤ .05 when compared with PBS-treated mice.
The effects of SCRT treatment on the pulmonary inflammatory response was further investigated by measuring the expression of inflammatory mediators in the BAL fluid. Because both IL-13 and TNF have been implicated as critical components of the asthmatic inflammatory response, we examined whether the improved pulmonary histologic findings could be due to reduced levels of these cytokines. The pulmonary expression of proinflammatory cytokines, TNF-α, and IL-13 were measured by ELISA (Fig 4). IL-13 expression was immediately increased after the last pulmonary challenge and remained elevated for 24 hours. The TNF-α concentration in the BAL fluid significantly increased with HDE challenge and sharply declined by 24 hours. Although both these cytokines were elevated, the expression of these 2 cytokines was not decreased by SCRT treatment (Fig 4).
Figure 4.
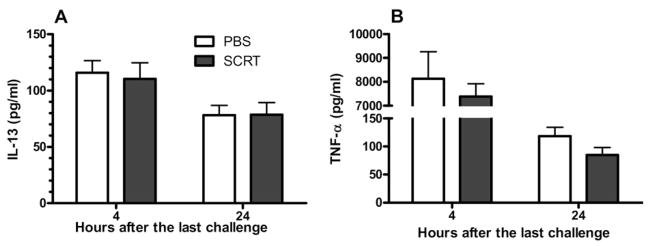
Bronchoalveolar lavage (BAL) levels of cytokines, interleukin 13 (IL-13) (A), and tumor necrosis factor α (TNF-α) (B). Mice were treated with So-Cheong-Ryong-Tang (SCRT) (2.0 g/kg) or phosphate-buffered saline (PBS) by gastric lavage 1 hour before each pulmonary challenge. BAL levels were determined 4 or 24 hours after the last intratracheal challenge. Differences between the 2 groups were analyzed by the unpaired t test. Values represent mean ± SEM with 10 to 12 mice in each group.
We then examined the infiltration of inflammatory cells in BAL fluid by measuring the total number of cells within the BAL fluid, as well as the types of cells present. As previous work in this model showed,19 the number of total cells infiltrated into airway sharply increases within 4 hours after the HDE challenge and remained substantially elevated 24 hours after the last HDE challenge (Fig 5A). The total number of cells recovered in the BAL was significantly decreased by SCRT treatment 4 and 24 hours after the last allergen challenge (Fig 5A). As anticipated, the number of lymphocytes recruited to the airspaces increased between 4 and 24 hours after the HDE challenge (Fig 5B). The SCRT treatment significantly reduced the number of lymphocytes in the BAL fluid 24 hours after the last HDE challenge (Fig 5B). The number of macrophages remained similar between the early and the late responses and was not changed with SCRT treatment (Fig 5C).
Figure 5.
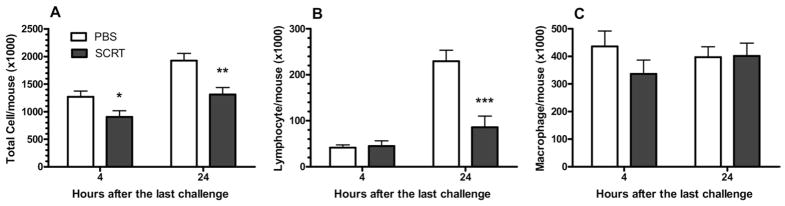
So-Cheong-Ryong-Tang (SCRT) treatment decreases bronchoalveolar lavage (BAL) total cell (A) and lymphocyte (B) infiltrated into airway without altering macrophages (C). Mice were treated with SCRT (2.0 g/kg) or phosphate-buffered saline (PBS) by gastric gavage 1 hour before each pulmonary challenge and BAL cells determined 4 or 24 hours after the last intratracheal challenge. Values represent mean ± SEM with 10 to 12 mice for each group. Differences between the 2 groups were analyzed by the unpaired t test. *P ≤ .05, **P ≤ .01, or ***P ≤ .001 when compared with PBS-treated mice.
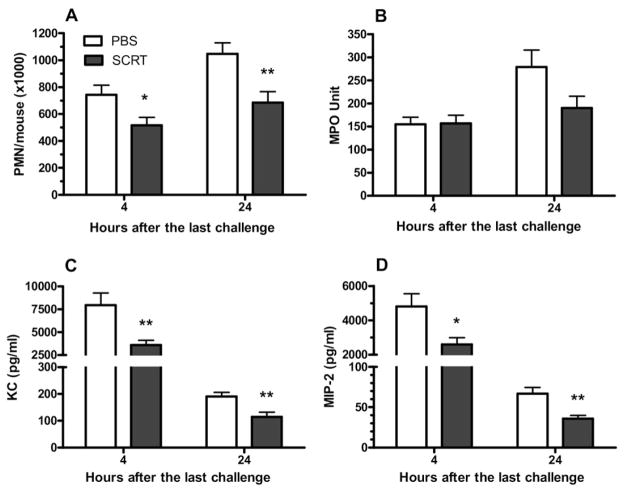
SCRT-Treatment Reduced Pulmonary Polymorphonuclear Leukocytes and CXC Chemokines
Pulmonary recruitment of neutrophils occurs as an early event in the asthmatic response, which may persist to later time points.19 Four hours after the last pulmonary challenge, the numbers of neutrophils in the BAL was markedly decreased in SCRT-treated mice, and this substantial reduction of inflammatory cells in the lung lavage was still present when mice were killed 24 hours after the last pulmonary challenge (Fig 6A). The effect of SCRT treatment on the pulmonary recruitment of neutrophils was further evaluated by measurement of pulmonary myeloperoxidase activity in the lung (Fig 6B). Myeloperoxidase activities were measured on the lungs after lavage, and the data represent the neutrophils sequestered within the interstitial space. Despite a reduction in the number of neutrophils recruited into the alveolar space (Fig 6A), SCRT did not decrease the number of polymorphonuclear leukocytes (PMNs) sequestered within the lung (Fig 6B).
Figure 6.
So-Cheong-Ryong-Tang (SCRT) treatment decreases neutrophil recruitment and neutrophil chemotactic factors. BALB/c mice were treated with SCRT (2.0 g/kg) or phosphate-buffered saline (PBS) by gastric gavage 1 hour before each pulmonary challenge and bronchoalveolar lavage (BAL) cells determined 4 or 24 hours after the last intratracheal challenge. The recruitment of polymorphonuclear leukocytes (PMNs) into the bronchoalveolar space was reduced by SCRT (A) without a significant reduction in the number of sequestered neutrophils measured by myeloperoxidase (MPO) (B). The CXC chemokine KC (C) and macrophage inflammatory protein 2 (MIP-2) (D) were significantly reduced. Values represent mean ± SEM with 10 to 12 mice in each group. Differences between the 2 groups were analyzed by the unpaired t test. *P ≤ .05, **P ≤ .01 when compared with PBS-treated mice.
We sought to determine the mechanism of reduced PMN recruitment into the lavage fluid even when the cells were still sequestered within the lung. Because CXC chemokines, such as keratinocyte-derived chemokine and macrophage inflammatory protein 2 (MIP-2), recruit neutrophils to sites of inflammation, we examined whether SCRT would decrease BAL levels of these mediators. Compared with the PBS-treated mice, SCRT-treated mice had significantly reduced concentrations of the chemokines at both 4 and 24 hours after the last challenge (Fig 6C and D). The total combined reduction of the BAL chemokines was thousands of picograms per milliliter, which would substantially reduce the chemotactic gradient into the lung.29
Reduced Airway Eosinophils Infiltration and CC Chemokines
Although neutrophils are important in the asthmatic response, eosinophils represent the signature cell of asthma30 and typically arrive at a later time point.19 Eosinophil recruitment and the expression of eosinophil-recruiting chemokines within the lung tissue were investigated (Fig 7A). As previously reported, the number of eosinophils in BAL fluid was slightly increased within 4 hours after the HDE challenge, with substantially greater numbers of eosinophils recruited into the lung 24 hours after the last HDE challenge. Even at the early time point (4 hours after the last pulmonary challenge), the numbers of eosinophils in the BAL was significantly decreased with SCRT treatment. More substantial reduction of inflammatory cells in the lung lavage was demonstrated when mice were killed 24 hours after the last pulmonary challenge (Fig 7A).
Figure 7.
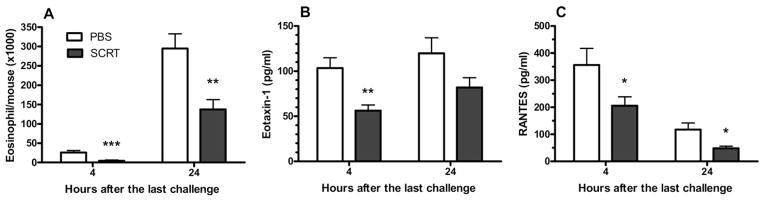
So-Cheong-Ryong-Tang (SCRT) treatment decreases bronchoalveolar lavage (BAL) eosinophil infiltration (A) and airway expression of the eosinophil-attracting CC chemokines eotaxin (B) and RANTES (C). Mice were treated with SCRT (2.0 g/kg) or phosphate-buffered saline (PBS) by gastric gavage 1 hour before each pulmonary challenge and BAL cells determined 4 or 24 hours after the last intratracheal challenge. Values represent mean (SEM) with 10 to 12 mice in each group. Differences between 2 groups were analyzed by the unpaired t test. *P ≤ .05, **P ≤ .01, or ***P ≤ .001 when compared with PBS-treated mice.
Similar to the mechanisms of neutrophil recruitment, there are chemotactic mediators responsible for the recruitment of eosinophils. Eotaxin and RANTES are considered to be the most important eosinophil-selective chemoattractant chemokines.30,31 Eotaxin in BAL fluid was clearly present 4 hours after the last challenge and remained elevated 24 hours after the last pulmonary challenge (Fig 7B). At 4 hours, the eotaxin level in the BAL fluid was significantly reduced by SCRT treatment. Pulmonary expression of RANTES also present after the last HDE challenge (Fig 7C). Significantly lower levels of RANTES were expressed in the BAL of SCRT-treated mice in both the early (4 hours) and late (24 hours) responses. As we indicated with the CXC chemokines and neutrophil infiltration, our data demonstrate that pulmonary infiltration of eosinophils is preceded by the expression of the CC chemokines eotaxin and RANTES in the lung. SCRT treatment reduced the expression of chemokines in the airways, providing a plausible mechanism for the significant reduction in eosinophil recruitment.
DISCUSSION
In the present study, we hypothesized that SCRT treatment would reduce pulmonary inflammation and that the mechanism of the suppressed inflammatory cell recruitment would be due to decreased production of chemotactic factors. We demonstrated that oral feeding of SCRT 1 hour before each intratracheal allergen challenge substantially decreased AHR, pulmonary infiltration of neutrophils and eosinophils, and airway expression of CXC chemokines, including KC and MIP-2, and CC chemokines, including eotaxin and RANTES, when compared with PBS-treated mice. Our data demonstrated that the mechanism of suppressed asthma-like pulmonary inflammation by SCRT probably occurred through the decreased pulmonary expression of CXC and CC chemokines. Our results suggest that the suppression of pulmonary inflammation by SCRT treatment is restricted to discrete aspects of the inflammatory response. The number of eosinophils and neutrophils were reduced significantly in both early and late asthmatic responses, whereas the number of macrophages remained unaffected. This selected suppression of inflammation may be compared with our previous study32 and other studies using dexamethasone.33 This previous study using the same mouse model of asthma demonstrated that dexamethasone treatment induced a nonspecific immunosuppression, including a substantial reduction of the number of macrophages and eosinophils and neutrophils in the airway after allergen challenge.32 Recent studies showed that dexamethasone treatment decreased the pulmonary inflammation via the suppression of a broad spectrum of proinflammatory mediators at the messenger RNA33 and protein level34 in a mouse model of asthma induced by ovalbumin. In addition to selected suppression of inflammatory mediators and cells, SCRT treatment decreased the concentration of allergen specific IgE, in agreement with previous reports that demonstrated a significant reduction of ovalbumin specific IgE in BAL13 and plasma12 by SCRT treatment in an ovalbumin-sensitized allergic inflammation model.
SCRT-induced downregulation of pulmonary eosinophilia via reduced CC chemokines has been previously reported and also agrees with our findings. Kao at al reported significant reduction of airway expression of eotaxin followed by decreased eosinophil recruitment in SCRT-treated mice15 and guinea pigs.10 As one of the most efficient eosinophil recruiting chemokines, eotaxin plays a critical role in the development of allergic pulmonary inflammation35. The number of pulmonary infiltrating eosinophils correlates with the concentration of eotaxin expressed in airway.30 In our previous study19 the number of pulmonary eosinophils in response to cockroach allergen challenge was substantially reduced by antibody neutralization of eotaxin. This result was verified by other investigators,36 suggesting that this chemokine plays an important role in pulmonary recruitment of eosinophils. In addition, the coexpression of RANTES with other chemokines, including eotaxin 1 and eotaxin 2, has been reported to regulate the pulmonary recruitment of eosinophils in the airways of asthmatic children.37 These CC chemokines regulate pulmonary recruitment of eosinophils through the CCR3 receptor, which is upregulated on eosinophils.38
In addition to pulmonary eosinophilia, neutrophils also infiltrate into airway in murine models of asthma.19,39,40 The CXC chemokines KC and MIP-2 are considered the hallmark neutrophil chemoattractants released in the lung in many animal models of airway inflammation.23,41 Neutralization of KC and MIP-2 by antibody administration in a mouse model significantly reduced the number of airway neutrophils when compared with the mice treated with control antibody.23
Although the active compound(s) exerting the anti-inflammatory effects of the SCRT have not been identified, several substances found in the herbal components of SCRT have shown various immunomodulatory effects. Schizandrae Fructus extract treatment of lung epithelial cells reduced eotaxin expression and eosinophil recruitment.42 Paeoniflorin, one of the major bioactive components of the peony, has demonstrated various pharmacologic activities, including the suppression of inflammatory mediators, such as TNF-α.43,44 It exhibits an antiallergic response in rats via inhibition of mast cell degranulation.45 Pinellic acid from another herbal component of SCRT, Pinellia ternata, also showed antiallergic activities, including reduced allergen specific IgE concentrations in the BAL from sensitized mice.13 Glycyrrhizin is one of the major bioactive components of licorice root (Glycyrrhiza glabra) and has multiple pharmacologic activities.46 Glycyrrhizin treatment alleviates the asthmatic features in mice, including inhibition of AHR, suppression of pulmonary eosinophilia, and reduction of allergen specific IgE expression in an allergic asthma model of mouse.47
Taken together, our data indicate that the SCRT treatment alleviates the asthma-like pulmonary inflammation via suppression of the cell-specific chemoattractants, with subsequent reduction of the number of BAL neutrophils and eosinophils in our mouse model of asthma. Our data provide mechanistic insights to further our understanding of the basic biology of how herbal medicines work and permit the use of CAM in combination with conventional therapies to achieve the best clinical outcome.
Acknowledgments
Funding Sources: This study was supported by a grant from the National Institutes of Health R01 ES013528 and a grant of the Oriental Medicine R & D Project, Ministry of Health & Welfare, Republic of Korea (HMP-00-CO-02-0002).
Footnotes
Disclosures: Authors have nothing to disclose.
References
- 1.Busse WW, Lemanske RF., Jr Asthma. N Engl J Med. 2001;344:350–362. doi: 10.1056/NEJM200102013440507. [DOI] [PubMed] [Google Scholar]
- 2.Masoli M, Fabian D, Holt S, Beasley R Global Initiative for Asthma. The global burden of asthma: executive summary of the GINA dissemination committee report. Allergy. 2004;59:469–478. doi: 10.1111/j.1398-9995.2004.00526.x. [DOI] [PubMed] [Google Scholar]
- 3.Eder W, Ege MJ, von Mutius E. The asthma epidemic. N Engl J Med. 2006;355:2226–2235. doi: 10.1056/NEJMra054308. [DOI] [PubMed] [Google Scholar]
- 4.Watts J. Doctors blame air pollution for china’s asthma increases. Lancet. 2006;368:719–720. doi: 10.1016/S0140-6736(06)69267-2. [DOI] [PubMed] [Google Scholar]
- 5.Li XM, Brown L. Efficacy and mechanisms of action of traditional Chinese medicines for treating asthma and allergy. J Allergy Clin Immunol. 2009;123:297–307. doi: 10.1016/j.jaci.2008.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schafer T. Epidemiology of complementary alternative medicine for asthma and allergy in Europe and Germany. Ann Allergy Asthma Immunol. 2004;93(2 suppl 1):S5–S10. doi: 10.1016/s1081-1206(10)61481-0. [DOI] [PubMed] [Google Scholar]
- 7.Eisenberg DM, Davis RB, Ettner SL, et al. Trends in alternative medicine use in the United States, 1990–1997: results of a follow-up national survey [see comment] JAMA. 1998;280:1569–1575. doi: 10.1001/jama.280.18.1569. [DOI] [PubMed] [Google Scholar]
- 8.Blanc PD, Trupin L, Earnest G, Katz PP, Yelin EH, Eisner MD. Alternative therapies among adults with a reported diagnosis of asthma or rhinosinusitis: data from a population-based survey. Chest. 2001;120:1461–1467. doi: 10.1378/chest.120.5.1461. [DOI] [PubMed] [Google Scholar]
- 9.Brinkhaus B, Hummelsberger J, Kohnen R, et al. Acupuncture and Chinese herbal medicine in the treatment of patients with seasonal allergic rhinitis: a randomized-controlled clinical trial. Allergy. 2004;59:953–960. doi: 10.1111/j.1398-9995.2004.00540.x. [DOI] [PubMed] [Google Scholar]
- 10.Kao ST, Lin CS, Hsieh CC, Hsieh WT, Lin JG. Effects of Xiao-Qing-Long-Tang (XQLT) on bronchoconstriction and airway eosinophil infiltration in ovalbumin-sensitized guinea pigs: in vivo and in vitro studies. Allergy. 2001;56:1164–1171. doi: 10.1034/j.1398-9995.2001.00982.x. [DOI] [PubMed] [Google Scholar]
- 11.Ko E, Rho S, Lee EJ, et al. Traditional Korean medicine (SCRT) modulate th1/th2 specific cytokine production in mice cd4+ t cell. J Ethnopharmacol. 2004;92:121–128. doi: 10.1016/j.jep.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 12.Ikeda Y, Kaneko A, Yamamoto M, Ishige A, Sasaki H. Possible involvement of suppression of Th2 differentiation in the anti-allergic effect of Sho-seiryu-to in mice. Jpn J Pharmacol. 2002;90:328–336. doi: 10.1254/jjp.90.328. [DOI] [PubMed] [Google Scholar]
- 13.Nagai T, Arai Y, Emori M, et al. Anti-allergic activity of a kampo (Japanese herbal) medicine “Sho-seiryu-to (Xiao-Qing-Long-Tang)” On airway inflammation in a mouse model. Int Immunopharmacol. 2004;4:1353–1365. doi: 10.1016/j.intimp.2004.05.021. [DOI] [PubMed] [Google Scholar]
- 14.Miyamoto T, Inoue H, Kitaura S, et al. Effect of Tsumura Sho-seiryu-to (tj-19) on bronchitis in a double-blind placebo-controlled study. J Clin Ther Med. 2001;17:1189–1214. [Google Scholar]
- 15.Kao ST, Wang SD, Wang JY, Yu CK, Lei HY. The effect of Chinese herbal medicine, Xiao-Qing-Long Tang (XQLT), on allergen-induced bronchial inflammation in mite-sensitized mice. Allergy. 2000;55:1127–1133. doi: 10.1034/j.1398-9995.2000.00728.x. [DOI] [PubMed] [Google Scholar]
- 16.Sakaguchi M, Mase A, Iizuka A, et al. Further pharmacological study on Sho-seiryu-to as an antiallergic. Methods Findings Exp Clin Pharmacol. 1997;19:707–713. [PubMed] [Google Scholar]
- 17.Nyunt AK, Takeuchi Y, Yokomuro K, Miyanaga Y. Comparative studies on the antiallergic effects of kampo medicines used for the therapy of respiratory diseases. Arerugi. 1995;44:503–512. [PubMed] [Google Scholar]
- 18.Tanaka A, Ohashi Y, Kakinoki Y, et al. The herbal medicine Sho-seiryu-to inhibits allergen-induced synthesis of tumour necrosis factor alpha by peripheral blood mononuclear cells in patients with perennial allergic rhinitis. Acta Otolaryngol Suppl. 1998;538:118–125. [PubMed] [Google Scholar]
- 19.Kim J, Merry AC, Nemzek JA, Bolgos GL, Siddiqui J, Remick DG. Eotaxin represents the principal eosinophil chemoattractant in a novel murine asthma model induced by house dust containing cockroach allergens. J Immunol. 2001;167:2808–2815. doi: 10.4049/jimmunol.167.5.2808. [DOI] [PubMed] [Google Scholar]
- 20.Ko E, Rho S, Cho C, et al. So-Cheong-Ryong-Tang, traditional Korean medicine, suppresses Th2 lineage development. Biol Pharm Bull. 2004;27:739–743. doi: 10.1248/bpb.27.739. [DOI] [PubMed] [Google Scholar]
- 21.Vaickus LJ, Bouchard J, Kim J, Natarajan S, Remick DG. Assessing pulmonary pathology by detailed examination of respiratory function. Am J Pathol. 2010;177:1861–1869. doi: 10.2353/ajpath.2010.100053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vanoirbeek JAJ, Rinaldi M, De Vooght V, et al. Noninvasive and invasive pulmonary function in mouse models of obstructive and restrictive respiratory diseases. Am J Respir Cell Mol Biol. 2010;42:96–104. doi: 10.1165/rcmb.2008-0487OC. [DOI] [PubMed] [Google Scholar]
- 23.McKinley L, Kim J, Bolgos GL, Siddiqui J, Remick DG. CXC chemokines modulate IgE secretion and pulmonary inflammation in a model of allergic asthma. Cytokine. 2005;32:178–185. doi: 10.1016/j.cyto.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 24.Nemzek JA, Siddiqui J, Remick DG. Development and optimization of cytokine ELISAs using commercial antibody pairs. J Immunol Methods. 2001;255:149–157. doi: 10.1016/s0022-1759(01)00419-7. [DOI] [PubMed] [Google Scholar]
- 25.Finkelman FD. Use of unrestrained, single-chamber barometric plethysmography to evaluate sensitivity to cholinergic stimulation in mouse models of allergic airway disease. J Allergy Clin Immunol. 2008;121:334–335. doi: 10.1016/j.jaci.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 26.Inman MD. Trends and recommendations in studies of mouse airway function. Clin Exp Allergy. 2010;40:524–527. doi: 10.1111/j.1365-2222.2010.03485.x. [DOI] [PubMed] [Google Scholar]
- 27.Lomask M. Further exploration of the Penh parameter. Exp Toxicol Pathol. 2006;57(suppl 2):13–20. doi: 10.1016/j.etp.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 28.Bradding P, Walls AF, Holgate ST. The role of the mast cell in the pathophysiology of asthma. J Allergy Clin Immunol. 2006;117:1277–1284. doi: 10.1016/j.jaci.2006.02.039. [DOI] [PubMed] [Google Scholar]
- 29.Call DR, Nemzek JA, Ebong SJ, Bolgos GL, Newcomb DE, Remick DG. Ratio of local to systemic chemokine concentrations regulates neutrophil recruitment. Am J Pathol. 2001;158:715–721. doi: 10.1016/S0002-9440(10)64014-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rankin SM, Conroy DM, Williams TJ. Eotaxin and eosinophil recruitment: Implications for human disease. Mol Med Today. 2000;6:20–27. doi: 10.1016/s1357-4310(99)01635-4. [DOI] [PubMed] [Google Scholar]
- 31.Bandeira-Melo C, Herbst A, Weller PF. Eotaxins: contributing to the diversity of eosinophil recruitment and activation [comment] Am J Respir Cell Mol Biol. 2001;24:653–657. doi: 10.1165/ajrcmb.24.6.f209. [DOI] [PubMed] [Google Scholar]
- 32.Kim J, McKinley L, Siddiqui J, Bolgos GL, Remick DG. Prevention and reversal of pulmonary inflammation and airway hyperresponsiveness by dexamethasone treatment in a murine model of asthma induced by house dust. Am J Physiol Lung Cell Mol Physiol. 2004;287:L503–509. doi: 10.1152/ajplung.00433.2003. [DOI] [PubMed] [Google Scholar]
- 33.Herbert C, Hettiaratchi A, Webb DC, Thomas PS, Foster PS, Kumar RK. Suppression of cytokine expression by roflumilast and dexamethasone in a model of chronic asthma. Clin Exp Allergy. 2008;38:847– 856. doi: 10.1111/j.1365-2222.2008.02950.x. [DOI] [PubMed] [Google Scholar]
- 34.Zhao J, Yeong LH, Wong WS. Dexamethasone alters bronchoalveolar lavage fluid proteome in a mouse asthma model. Int Arch Allergy Immunol. 2007;142:219–229. doi: 10.1159/000097024. [DOI] [PubMed] [Google Scholar]
- 35.Conroy DM, Williams TJ. Eotaxin and the attraction of eosinophils to the asthmatic lung. Respir Res. 2001;2:150–156. doi: 10.1186/rr52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gonzalo JA, Lloyd CM, Wen D, et al. The coordinated action of cc chemokines in the lung orchestrates allergic inflammation and airway hyperresponsiveness. J Exp Med. 1998;188:157–167. doi: 10.1084/jem.188.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rojas-Ramos E, Avalos AF, Perez-Fernandez L, Cuevas-Schacht F, Valencia-Maqueda E, Teran LM. Role of the chemokines RANTES, monocyte chemotactic proteins-3 and -4, and eotaxins-1 and -2 in childhood asthma. Eur Respir J. 2003;22:310–316. doi: 10.1183/09031936.03.00084802. [DOI] [PubMed] [Google Scholar]
- 38.Fujisawa T, Kato Y, Nagase H, et al. Chemokines induce eosinophil degranulation through ccr-3. J Allergy Clin Immunol. 2000;106:507–513. doi: 10.1067/mai.2000.108311. [DOI] [PubMed] [Google Scholar]
- 39.Bogaert P, Naessens T, De Koker S, et al. Inflammatory signatures for eosinophilic versus neutrophilic allergic pulmonary inflammation reveal critical regulatory checkpoints. Am J Physiol Lung Cell Mol Physiol. 2011;300:L679L90. doi: 10.1152/ajplung.00202.2010. [DOI] [PubMed] [Google Scholar]
- 40.Trifilieff A, El-Hashim A, Bertrand C. Time course of inflammatory and remodeling events in a murine model of asthma: effect of steroid treatment. Am J Physiol Lung Cell Mol Physiol. 2000;279:L1120–L1128. doi: 10.1152/ajplung.2000.279.6.L1120. [DOI] [PubMed] [Google Scholar]
- 41.De Filippo K, Henderson RB, Laschinger M, Hogg N. Neutrophil chemokines kc and macrophage-inflammatory protein-2 are newly synthesized by tissue macrophages using distinct TLR signaling pathways. J Immunol. 2008;180:4308–4315. doi: 10.4049/jimmunol.180.6.4308. [DOI] [PubMed] [Google Scholar]
- 42.Oh BG, Lee H, Kim Y, et al. Inhibitory effects of Schizandrae Fructus on eotaxin secretion in a549 human epithelial cells and eosinophil migration. Phytomedicine. 2009;16:814–822. doi: 10.1016/j.phymed.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 43.Zhang LL, Wei W, Wang NP, et al. Paeoniflorin suppresses inflammatory mediator production and regulates G protein-coupled signaling in fibroblast-like synoviocytes of collagen induced arthritic rats. Inflamm Res. 2008;57:388–395. doi: 10.1007/s00011-007-7240-x. [DOI] [PubMed] [Google Scholar]
- 44.Jiang WL, Chen XG, Zhu HB, Gao YB, Tian JW, Fu FH. Paeoniflorin inhibits systemic inflammation and improves survival in experimental sepsis. Basic Clin Pharmacol Toxicol. 2009;105:64–71. doi: 10.1111/j.1742-7843.2009.00415.x. [DOI] [PubMed] [Google Scholar]
- 45.Lee B, Shin YW, Bae EA, et al. Antiallergic effect of the root of Paeonia lactiflora and its constituents paeoniflorin and paeonol. Arch Pharm Res. 2008;31:445–450. doi: 10.1007/s12272-001-1177-6. [DOI] [PubMed] [Google Scholar]
- 46.Marjan Nassiri A, Hossein H. Review of pharmacological effects of glycyrrhiza sp. and its bioactive compounds. Phytother Res. 2008;22:709–724. doi: 10.1002/ptr.2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ram A, Mabalirajan U, Das M, et al. Glycyrrhizin alleviates experimental allergic asthma in mice. Int Immunopharmacol. 2006;6:1468. doi: 10.1016/j.intimp.2006.04.020. [DOI] [PubMed] [Google Scholar]