Abstract
The classic model of sex determination in mammals states that the sex of the individual is determined by the type of gonad that develops, which in turn determines the gonadal hormonal milieu that creates sex differences outside of the gonads. However, XX and XY cells are intrinsically different because of the cell-autonomous sex-biasing action of X and Y genes. Recent studies of mice, in which sex chromosome complement is independent of gonadal sex, reveal that sex chromosome complement has strong effects contributing to sex differences in phenotypes such as metabolism. Adult mice with two X chromosomes (relative to mice with one X chromosome) show dramatically greater increases in body weight and adiposity after gonadectomy, irrespective of their gonadal sex. When fed a high fat diet, XX mice develop striking hyperinsulinemia and fatty liver, relative to XY mice. The sex chromosome effects are modulated by the presence of gonadal hormones, indicating an interaction of the sex-biasing effects of gonadal hormones and sex chromosome genes. Other cell-autonomous sex chromosome effects are detected in mice in many phenotypes. Birds (relative to eutherian mammals) are expected to show more widespread cell-autonomous sex determination in non-gonadal tissues, because of ineffective sex chromosome dosage compensation mechanisms.
Keywords: sex chromosome, sex determination, X chromosome, Y chromosome, Z chromosome, W chromosome, sexual differentiation, androgens, estrogens
The current dominant model of mammalian sex determination was recently summarized as follows: “In mammals, biological differences between males and females are determined genetically during embryonic development. These differences have a significant impact on the physical, reproductive, psychological, and social life of an individual. Sex development can be divided into two processes, “sex determination”, which is the developmental decision that directs the undifferentiated gonad to develop as a testis or ovary and “sex differentiation”, which occurs once the gonad has developed and is induced by the products of the gonad to establish the phenotypic sex. In mammals, sex determination equates to gonad development” (Eggers and Sinclair 2012).
Based on present evidence, this classical model of sex determination adequately accounts for the forces that cause sex differences in the gonads, external genitalia, internal genitalia (structures derived from Müllerian and Wolffian ducts), and much of sexual differentiation of the mammalian brain that has been investigated. These sexual characteristics have a large impact in determining whether the individual will reproduce as male or female, and are considered by many scientists and lay persons as the characteristics that define the sex of the individual. Moreover, the gender (social implications of sex) of the individual is often affected by and concordant with these characteristics, so that these factors indirectly impact the social environment of the individual.
The problem with the classical model is that it is unable to account for an increasing number of sex differences in important mammalian phenotypes because these sex differences are not downstream of the developmental decision to form testes or ovaries. The sex differences that are thereby defined as “outside” of sex determination are nevertheless quite important. At the top of the list is X inactivation, a process that is ubiquitous in all XX cells outside of the germline. The process profoundly affects the transcriptional landscape of the cell, involves significant epigenetic changes and differential nuclear compartmentalization of chromosomes, and is tightly regulated and requires significant investment of the cell’s resources (Heard and Disteche 2006). X inactivation is fundamentally female, never found in normal XY male cells. As such, it belongs within the framework of a general theory of sex determination, not outside of such a theory. Other sex differences are found prior to sexual differentiation of the gonads, indicating that the sex chromosomes encode factors that act within and outside of the gonads (O et al. 1988; Burgoyne et al. 1995; Bermejo-Alvarez et al. 2011b; Bermejo-Alvarez et al. 2011a; Silversides et al. 2012; (Nef et al. 2005; Dewing et al. 2003). As is discussed below, even after the gonads differentiate, the number of X chromosomes influences sex differences in the phenotype of cells and individuals, independent of the gonadal sex of the individual. The Y chromosome has effects outside of the gonad, and harbors genes that impact sexual phenotype, not just by determining the differentiation of the male’s gonad. Thus, XX and XY individuals with testes are not equivalent, and XX and XY individuals with ovaries are not equivalent.
The thesis of this article is that a more accurate model of sex determination recognizes multiple factors encoded by the sex chromosomes, not just those that determine gonadal sex, that act in parallel to determine sexual bias in tissues throughout the body (Arnold 2011). Accordingly, in this article we define “sex determination” to include any factors that cause sex differences in cells, tissues, and individuals. The discrimination of separate processes of “sex determination”” and “sexual differentiation” is unnecessary and counterproductive. Factors that determine the sex of cells and individuals can be primary (encoded by the sex chromosomes of the zygote), or secondary factors downstream of the primary X and Y factors (Arnold, 2011). In this view, Sry is a primary sex determining factor, and testicular hormones secreted because of the differentiation of testes are secondary sex determinants.
Sex chromosome effects on metabolism and obesity
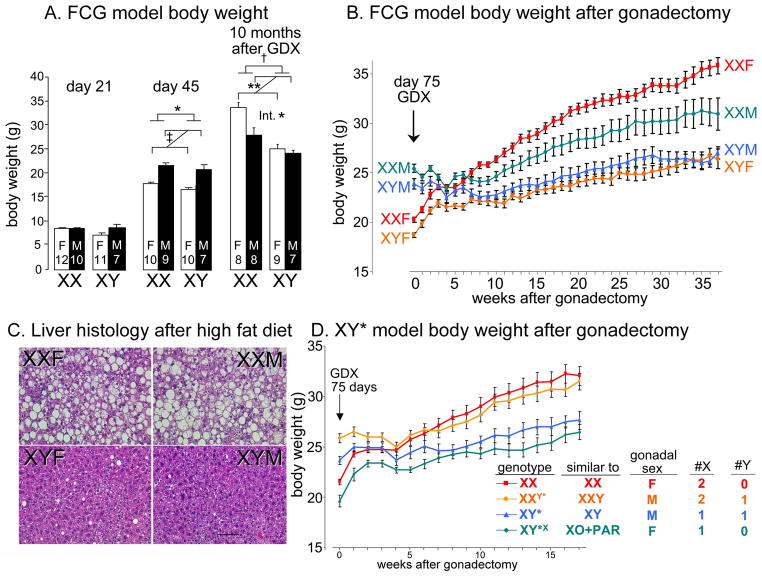
We focus first on recent evidence concerning sex differences in body weight, adiposity, and metabolic disease, because this example is particularly instructive. In mice, adult males weigh more than females. Sex differences in body size have long been known to begin before implantation of the blastocyst, well before the embryo has gonads, and continue into early post-natal life (Burgoyne et al. 1995; Burgoyne et al. 2002) Sex chromosome effects on body weight are also found in adult mice ( Budefeld et al. 2008). These findings establish the existence of sex determinants not acknowledged by the classical dogma. The sex determinants can be further demonstrated using a mouse model—the four core genotypes (FCG)—in which gonadal sex and chromosomal sex are decoupled. FCG mice include XX and XY mice with testes and XY and XX mice with ovaries (De Vries et al. 2002; Arnold and Chen 2009). Analysis of FCG mice revealed that both gonadal secretions and sex chromosome complement influence sex differences in body weight and adiposity (Chen et al. 2012). Thus, at three weeks after birth, no difference in body weight is evident in these mice (day 21, Figure 1A). However, following the onset of puberty, sex differences in gonadal secretions cause sex differences in body size, with gonadal males being larger than females (at day 45, Figure 1A). Adult gonadal males are about 25% heavier than gonadal females, whether they are XX or XY, confirming the importance of gonadal secretions (at 75 days, or week 0 in Figure 1B). However, gonadally intact XX mice are about 7% heavier than XY mice, independent of their gonadal sex, indicating that the sexual phenotype also depends on the sex chromosomes (at 75 days, or week 0 in Figure 1B).
Figure 1.
Cell-autonomous effects of sex chromosomes contribute to sex differences in body weight and metabolism in mice. A. Four core genotypes (FCG) mice show little sex difference in body weight at 21 days (weaning). After puberty at day 45, gonadal males weigh more than females. Ten months after gonadectomy (GDX, performed at 75 days of age), XX mice are 24% heavier than XY mice, and an interaction (Int) between sex chromosome complement and gonadal sex is apparent because XX gonadal females are heavier than gonadal males, but XY gonadal males and females are not different. * p<0.05, ** p<0.01 B. Body weight in gonadally intact mice at day 75 and after GDX at day 75. The sex difference caused by gonadal secretions disappears in the first month after GDX, after which XX mice gain more weight than XY mice. XXM, XX gonadal males; XYM, XY gonadal males; XXF, XX gonadal females; XYF, XY gonadal females. C. Liver histology after eating a high fat diet (beginning four weeks after gonadectomy for a total of 16 weeks) shows that XX mice have dramatically greater accumulation of triglycerides in the liver, relative to XY mice, irrespective of gonadal sex. D. Four types of progeny of XY* fathers were compared to assess the effects of one vs. two X chromosomes, or of the Y chromosome. After GDX at 75 days, mice with the equivalent of two X chromosomes gain more weight and fat than mice with one X chromosome, and the presence of the Y chromosome has no apparent effect. From (Chen et al. 2012).
The role of sex chromosome complement is revealed more dramatically when the gonads are removed in adulthood (Figure 1B). At first, the body weights of the four groups of FCG mice converge, as mice that previously had ovaries increase body weight and mice that previously had testes plateau in their weight. Four weeks after gonadectomy, there is no difference in body weight among the four groups. After this point, the body weight of XX mice increases faster than that of XY mice, so that at 10 months after gonadectomy, XX mice are 24% heavier than XY mice. The XX vs. XY difference at this point is as great as the effect of gonadal hormones prior to gonadectomy. The greater weight of XX mice is attributed mostly to 88% greater fat mass in XX than XY (50% greater relative to body weight), although lean mass is also greater in XX than XY. These large differences in adiposity are surprising, considering that the mice are eating a low fat (5% by weight) chow diet. Although XX and XY mice have about the same level of physical activity, XX mice eat more than XY mice during the daytime (their inactive phase), independent of their gonadal sex, which could contribute to their greater adiposity.
The differential role of the sex chromosomes is illustrated further when the mice are fed a high fat diet. Diets such as this are often used by investigators studying metabolic diseases, because they roughly mimic the high caloric diets consumed by people in developed countries. The high caloric content provides a dietary stress that uncovers important pathophysiological effects in mice with varying metabolism. On this diet, XX mice gonadectomized as adults gain weight at an accelerated rate compared to XY mice (Chen et al. 2012), and the livers of gonadectomized XX mice accumulate dramatically higher levels of triglycerides, relative to XY mice, and independent of gonadal sex (Figure 1C). Fatty liver is a major component of metabolic syndrome, and a major risk factor for liver cancer (Sun and Karin 2012). XX mice on a high fat diet, relative to XY, also showed signs of insulin resistance (a two-fold elevation in fasting insulin in plasma, despite similar blood levels of glucose) (Chen et al. 2012). Insulin resistance is commonly associated with obesity in humans, and a key feature of the metabolic syndrome.
The role of sex chromosome complement as a determinant of metabolism and obesity was demonstrated further using the XY* model, which varies the number of X and Y chromosomes independently of each other (see Chen et al., 2008 for description of the XY* model). The analysis of the XY* model shows that mice with two X chromosomes have greater body weight and adiposity than mice with one X chromosome, whereas the presence or absence of a Y chromosome has little effect (Figure 1D). Taken together with data from the FCG model, the results indicate that under hypogonadal conditions, the number of X chromosomes in mice has dramatic effects on critical variables that model human obesity and metabolic disease.
Classic sex determination theory, although seductive in its simplicity, is silent concerning the sexually differentiating role of the number of X chromosomes, and of other possible cell-autonomous effects of sex chromosome complement (e.g., known male-specific non-gonadal effects of Sry or other Y genes; Dewing et al. 2006). A more adequate theory recognizes several classes of factors on the sex chromosomes that make males and females different (Arnold 2009b; Arnold 2011). These include Y genes, such as Sry, that act outside of the gonads, and X genes that escape inactivation or that receive a parental imprint. Candidate X genes include about 3% of X genes that escape inactivation in mice (Yang et al. 2010). Among these are genes that are consistently expressed at higher levels in mice with two X chromosomes compared to mice with one X chromosome (Lopes et al. 2010; Werler et al. 2011; Chen et al. 2012). A second group of X genes that are candidates for the X chromosome effect includes those that receive different parental imprints in XX and XY mice. XY mice experience only a maternal imprint on X genes, but XX mice receive imprints from both parents. These factors all act cell-autonomously, in the gonads and throughout the body, to cause sex differences in cell function. In addition, cells are also sexually differentiated by hormonal factors from the gonads, which are the dominant factors causing sex differences in phenotype throughout the body. Thus, multiple parallel-acting factors encoded by the sex chromosomes have sex-determining effects.
Once several parallel pathways of sex determinants are recognized, it becomes possible to appreciate that the sexually differentiating role of one factor is conditioned by the effects of others, and that sex-biasing factors may counteract or reduce the effects of each other (De Vries 2004). Neither of these important conclusions derives from classic sex-determination theory. For example, the effect of X chromosome number on mouse body weight is present more at some phases of life than at others. In the weanling mouse when gonadal hormones are expected to be low, the effect of X chromosome number on body weight is not apparent, for unknown reasons (day 21, Figure 1A). It becomes obvious by adulthood, when gonads are fully active, when it accounts for a 7% difference in body weight at 75 days of age (week 0, Figure 1B). When the gonads are removed, the effects of X chromosome number slowly emerge over a period of months as a major factor, causing up to 24% difference in body weight and metabolic dysregulation (Fig. 1AB), especially when mice are eating a high fat diet (Chen et al. 2012). Because the sex chromosome effect is larger in the absence of gonads than in their presence, it would appear that gonadal secretions blunt or obscure the effects of sex chromosome complement. Inherent in that idea is that the effects of gonadal hormones differ in XX and XY cells. These ideas need further testing. The data already suggest that the XX vs. XY difference is in the “opposite” direction of the hormone effects, at least for body weight, because female XX chromosome complement increases body weight, but male gonadal hormones produce greater body weight than female gonadal hormones (Figure 1A).
The data in Figure 1 suggests important other conclusions about the interaction of gonadal hormones and sex chromosome complement. At 10 months after gonadectomy, XX mice that had ovaries have greater body weight than XX mice that had testes (Figure 1B). The phenotypic differences (in body weight and adiposity) between these two groups is caused by the presence or absence of Sry, and is a gonadal hormonal effect that lasts long after the gonads are removed, and/or an effect of Sry outside of the gonads. Interestingly, this effect depends on sex chromosome complement, because XY gonadal male and female mice are not much different in body weight. Because the two groups of factors (hormones and sex chromosome complement) each condition the effects of the other, the various factors (or their downstream effector pathways) must interact directly. The nature of this interaction is completely unexplored at present.
In gonadally intact young adult mice, when all sex-biasing factors are present, gonadal hormones rank first as important determinants of the sex differences in body weight and composition, because the gonadal hormone effect is larger (25%) than the sex chromosome effect (7%). More interesting, perhaps, to aging, post-menopausal, and other hypogonadal humans, is that body weight and adiposity of mice are profoundly influenced by the number of X chromosomes in the absence of gonads. An important goal of research investigating physiological sex differences in disease is to identify sex-biasing factors that protect one sex from disease, as part of a strategy to uncover protective mechanisms that might be targeted by novel therapies. For example, mice with one X chromosome are protected relative to mice with two X chromosomes from obesity and related metabolic dysregulation, including fatty liver and insulin resistance. An improved model of sex determination has the advantage that it focuses attention on multiple new areas for research into such protective mechanisms.
Sex chromosome effects on diverse tissues
The sex chromosome effects on body weight and metabolism, discussed above, illustrate the fascinating interplay of parallel sex-determining mechanisms that operate independently and interdependently. These effects are among a growing list of XX vs. XY differences that are reported to influence a wide variety of phenotypes in numerous tissues. The effects are found in mice in which specific Y genes are manipulated independent of gonadal effects (Dewing et al. 2006), in mice that never developed gonads (Majdic and Tobet 2011), or mice in which the XX vs. XY comparison can be made in both gonadal sexes (Arnold 2004; Arnold 2009b; Arnold 2009a; Arnold and Chen 2009; Arnold 2011; Abel and Rissman 2011). XX vs. XY effects are found in expression of important genes in the brain (De Vries et al. 2002; Gatewood et al. 2006; Chen et al. 2009; Abel et al. 2011; Xu et al. 2002; Xu et al. 2008b; Xu et al. 2008a; Dewing et al. 2006), in brain development (Carruth et al. 2002), and in behaviors such as parental and aggressive behaviors (Gatewood et al. 2006), response to noxious stimuli (Gioiosa et al. 2008a; Gioiosa et al. 2008b), sexual behaviors (Grgurevic et al. 2012; Bonthuis et al. 2012), and social and investigative behaviors (McPhie-Lalmansingh et al. 2008; Cox and Rissman 2011; Grgurevic et al. 2008). An increasing number of mouse models of disease show sex chromosome effects, including studies of autoimmune diseases such as multiple sclerosis and lupus (Palaszynski et al. 2005; Smith-Bouvier et al. 2008; Sasidhar et al. 2012), hypertension (Ji et al. 2010; Caeiro et al. 2011; Ely et al. 2010), neural tube closure defects (Chen et al. 2008), cocksackie viral infection (Robinson et al. 2011), and behavioral tendencies related to addiction and alcohol abuse (Quinn et al. 2007; Barker et al. 2010). Except for direct Sry effects on the brain (Dewing et al. 2006; Czech et al. 2012) and possibly on the adrenal and kidney (Ely et al. 2010), it is not known which X or Y genes cause the sex chromosome effects. However, some studies have implicated the X chromosome as the origin of the sex-biasing factor(s) (Chen et al. 2008; Chen et al. 2009; Chen et al. 2012; Bonthuis et al. 2012).
X inactivation as a defining event of sex determination
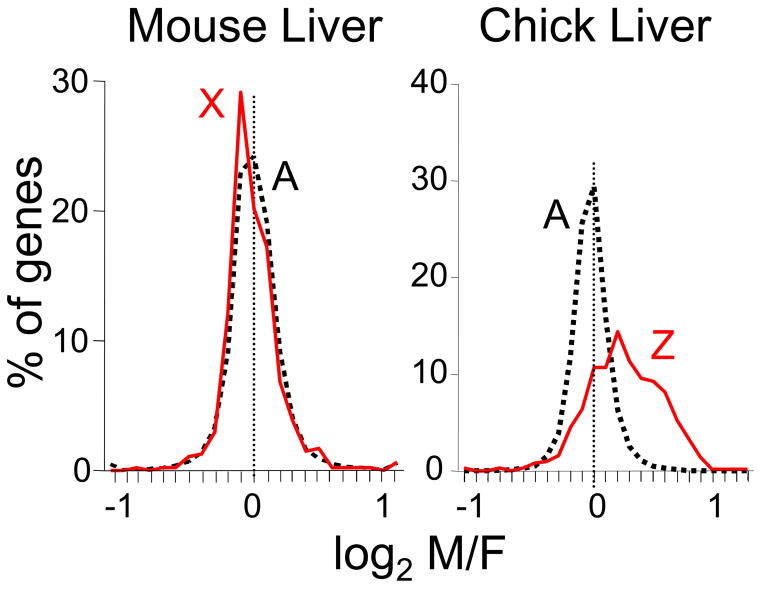
Above we suggested that X inactivation, an event that happens in every XX somatic (i.e., non-germline) cell, but never in XY cells, is properly viewed as a sex-determining event. Certainly it represents a fundamental phenotypic difference between normal male and female cells, but large questions remain concerning the sex-biasing impact of this molecular phenotype. Several points are relevant. Firstly, X inactivation has large effects on gene expression, because it effectively removes what would otherwise be a large female bias in X gene expression (i.e., greater expression of X genes in females than in males, Figure 2). In other animal groups lacking similar chromosome-wide dosage compensation, in birds (see below) and in mammalian blastocysts before X inactivation is ubiquitous, the expected female bias of X gene expression is present (Arnold et al. 2008; Bermejo-Alvarez et al. 2010). Thus, X inactivation is seen as a female-specific mechanism to adjust X gene dose (relative to autosome dose) to a level similar to that of the male. Although calling a factor that reduces sex bias “sex determining” might seem counterintuitive, X inactivation certainly ranks as one of the most profound sex-specific forces in the mammalian genome. Furthermore, its role is predominantly to counteract another female bias, in the number of X chromosomes, which underscores the idea that different sex-determining factors can oppose each other. In the end, the process of shutting down a large chromosome would reasonably be thought to leave XX cells distinctly different from XY cells, but at present there is only a small amount of evidence that the presence of a large heterochromatic chromosome has effects elsewhere in the genome. For example, mice with two X chromosomes, compared to mice with one X chromosome, show greater expression of an autosomal transgene that is a barometer of the status of nearby heterochromatin. The results suggest that the presence of a large heterochromatic inactive X chromosome in XX cells may reduce the availability of heterochromatizing factors generally, affecting gene expression throughout the genome (Wijchers and Festenstein 2011; Wijchers et al. 2010). Moreover, the presence of an inactive X chromosome correlates with reduced methylation of the genome of embryonic stem cells, and XX cells (relative to XY) show reduced expression of the de novo DNA methyltransferases Dnmt3a and Dnmt3b (Zvetkova et al. 2005). The question of genome-wide effects of the inactive X chromosome, not mediated by dosage of the X genes, is ripe for further analysis.
Figure 2.
Effective sex chromosome dosage compensation in the mouse compared with ineffective dosage compensation in the chick embryo. Microarray mRNA expression profiling was conducted in adult mice and chick embryos of both sexes. The graphs show the distribution of M/F ratios of expression of autosomal genes and X or Z chromosome genes. Autosomal genes have modal M/F ratios near 1 (log of zero), but some genes are expressed higher in males or in females. Log2 M/F ratios are rarely greater that 1 (two-fold higher in males) or less than −1 (two-fold higher in females). In mammals such as the mice, M/F ratios for X genes show a distribution closely matched to autosomal genes, despite the presence of two X chromosomes in females compared to one of males. In birds such as the chick, the distribution of M/F ratios for autosomal genes is similar to that in mammals, but in the absence of chromosome-wide dosage compensation of the Z chromosome, ZZ males have higher expression of Z genes compared to ZW females, for most Z genes. From (Itoh et al. 2007).
The contrast of marsupial (metatherian) and eutherian mammals
Like eutherian mammals, marsupials have an XX-XY sex chromosome system. The Y chromosome contains the testis-determining gene Sry, and many steps of gonadal differentiation are comparable to those in eutherians. However, three major reproductive tissues, pouch, scrotum, and mammary tissue, are sexually differentiated independent of gonadal differentiation. In the tammar wallaby, for example, the anlage of the scrotum, pouch, and mammary tissue begin to form prior to gonadal differentiation, and sex-specific patterns of development are not influenced by changes in gonadal hormone levels. The sex-determining factors appear to be X-linked, because an XXY marsupial (with testes and a penis) also has a pouch and mammary glands, whereas an XO marsupial (with ovaries and uteri) has neither a pouch nor mammary gland (reviewed by Renfree and Short 1988; Renfree et al. 2002; Glickman et al. 2005). Thus, marsupials represent an early and iconic example of important sex-determining factors that are not downstream of gonadal differentiation. To develop a comprehensive understanding of sex determination in marsupials, it is important to discover the X-linked sex determinants that operate in parallel with sex determinants that control sexual differentiation of the gonads.
Birds as models of sex chromosome effects
Birds offer another important contrast to eutherian mammals. Their sex chromosome complement is reversed relative to mammals, with the male being homogametic (ZZ) and the female heterogametic (ZW). More importantly in the present context, birds appear to lack a chromosome-wide mechanism of Z gene dosage compensation comparable to X inactivation (Arnold et al. 2008). In every bird species studied to date, most Z genes are expressed higher in males than in females in numerous tissues before and after gonadal differentiation (Figure 2)(Itoh et al. 2007; Ellegren et al. 2007; Itoh et al. 2010; Zhang et al. 2010; Mank 2009; Chue and Smith 2011; Wolf and Bryk 2011; Naurin et al. 2011). Birds, more than eutherian mammals, may easily evolve diverse cell-autonomous sex-determination mechanisms because of the availability of many more sex-biased (Z-linked) cell-autonomous signals throughout their body, which can evolve a role to control sex differences in diverse phenotypes. In species with effective dosage compensation such as eutherian mammals, most sex chromosome (in this case, X chromosome) genes are expressed at similar levels in the two sexes (Figure 2), and thus are not likely to evolve a primary role in controlling different developmental programs in the two sexes. Perhaps because of the lack of effective Z chromosome dosage compensation that may have favored more cell-autonomous sex determination, the small number of studies of birds has had a major catalytic role in changing attitudes about the gonad-first classic model of sex determination.
In the last half of the 20th Century, many studies of birds showed that sexual phenotype is controlled by gonadal hormones (e.g., Balthazart et al. 2009; Balthazart and Adkins-Regan 2002). One informative model system has been the neural circuit controlling song in Passerine birds (Wade and Arnold 2004). Male zebra finches sing a courtship song that females do not sing, and the brain regions controlling song are 5–6 times larger in males (Nottebohm and Arnold 1976). At first, the search for the sex-determining factors focused on the classical model, and manipulated gonadal hormone levels in females and males. Females treated with estradiol at hatching were permanently masculinized, both in their brain and behavior (Gurney and Konishi 1980). The result was interpreted to support the idea that males normally secrete testosterone, which is converted to estradiol in the brain where it has a permanent masculinizing effect, a finding similar to previous results in mammals. However, several observations were at odds with the hormonal theory. Manipulations of gonadal hormones in males (for example, blocking estradiol action) did not block masculine brain development (Arnold and Schlinger 1993). When genetic females were injected early in development with a drug that caused differentiation of testes in males, those females were not masculinized by the presence of functional testicular tissue (Wade and Arnold 1996). When these studies raised significant doubt about the gonadal hormonal origin of the sex differences, it became reasonable to consider the idea of cell-autonomous factors, encoded by the sex chromosomes, might be the primary cause of the sex difference in brain circuits and the reproductive behaviors that they control (Arnold 1996).
Rare avian gynandromorphs are dramatic intersex animals, sometimes with a sharp line at the animal’s midline separating male plumage on one side from female plumage on the other side. Other sexual characters are also lateralized, such as type of gonad and body size. As early as 1939, based on analysis of gynandromorphs, Witschi concluded that “many of the secondary sex characters of … birds are not under hormonal but direct intracellular control” (Witschi 1939). The use of modern molecular reagents to analyze gynandromorphs has confirmed this conclusion. In a gynandromorphic zebra finch, the right side had male plumage, masculine brain characteristics, and a testis, whereas the left side had female plumage, less masculine brain, and an ovary (Agate et al. 2003). The W chromosome, normally only found in ZW females, was enriched in the genomic DNA of the animal’s left side (ruling out a ZO genotype). Expression of W-linked genes was very high on the entire left side of the brain, but nearly absent (except for a small number of cells) on the right. Expression of Z genes was also uniformly but modestly higher on the right side compared to the left, compatible with ZZ genotype on the right and a single Z on the left. Because gonadal hormones would be expected to affect both sides of the brain and body, the greater masculinization of the brain on the right side suggested that it was controlled in a cell-autonomous fashion by sex chromosome complement of brain cells
Analysis of several gynandromorphic chickens, using BAC probes to determine the number of Z and W chromosomes in specific cells, proved that the birds had a mixture of ZZ and ZW cells on the two sides of the body, with ZZ predominating on one side and ZW on the other (Zhao et al. 2010). On the ZZ side, the phenotypes of numerous tissues were more masculine than on the ZW side, including muscle, bone, leg, plumage, and wattles. Moreover, when ZZ cells were implanted into ZW embryos or vice versa, the cells retained sexual phenotypes consistent with their own sex chromosome complement after the cells took up residence within the somatic component of the recipient gonads (Zhao et al. 2010), confirming that the genetic sex of somatic gonadal cells plays a significant role in determining their sexual phenotype.
Cell-autonomous sex determination probably explains the development of a larger number of sexually dimorphic phenotypes in birds than in eutherian mammals, because of the lack of Z chromosome-wide dosage compensation that results in male bias in expression of most Z gene expression throughout the body. Nevertheless, most of the phenotypes that show a sex chromosome effect in birds (different phenotype in ZZ vs. ZW) are often also influenced by gonadal hormone levels. A salient example is the chicken’s comb and wattle, large in males and small in females. These structures have long been known to be sensitive to androgens, so that the sex difference was thought to be downstream of gonadal determination. Yet, the chicken gynandromorph has a larger comb and wattle on the male side, indicating that sex chromosome complement also contributes to the sex difference (Zhao et al. 2010). Similar roles for both factors (hormonal and genetic) are proposed in the zebra finch song system (Agate et al. 2003; Kim et al. 2004; Chen et al. 2005). Hormonal manipulations show hormonal effects, and genetic manipulations show genetic effects, and often both are important. That conclusion would seem to apply to both birds and mammals, although the difficulty of performing the genetic manipulations means that there is much less information about that type of sex-biasing factor.
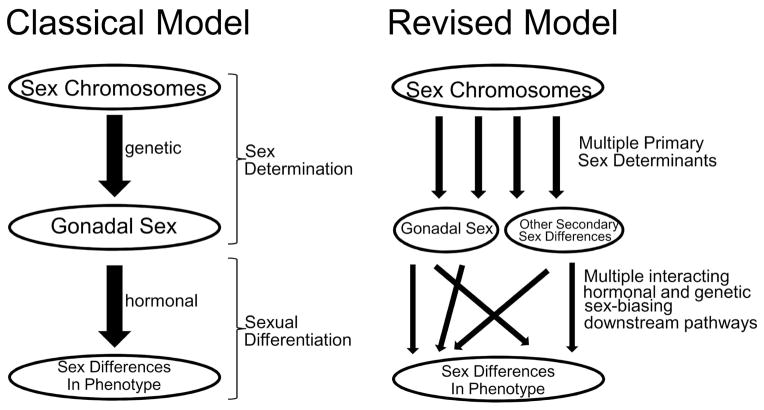
Above we proposed a revised definition and framework for thinking about sex determination. This framework undermines the classic distinction between two distinct processes, sex determination and sexual differentiation, which was originally rationalized based on the order of events (sex determination preceding and causing sexual differentiation), and on different mechanisms (genetic sex determination vs. hormonal sexual differentiation). In a revision of the classic model (Figure 3), it is appropriate to recognize multiple sex chromosome mechanisms, acting along parallel mechanistic pathways, that are inherently different in various types of male vs. female cells, which lead to downstream sex differences. In many phenotypes, we can expect an interaction of the parallel pathways, so that both hormonal and sex chromosome factors modify the effect of the other. A new concept of sex determination frees us from inaccurate and misleading over-simplifications such as “in mammals, sex is determined genetically”, or “in birds, sex is cell-autonomous”. Instead, we can recognize that even eutherian mammals have significant cell-autonomous sex determinants, and in birds hormonal and genetic factors interact. We propose that taxonomic differences in the importance of cell-autonomous factors may be related to the effectiveness of sex-chromosome dosage compensation, which affects the number of inherently sexually dimorphic signals within each male and female cell that can evolve a sex-determining function.
Figure 3.
Schematic contrast of sex determination models. In the classical model of sex determination of vertebrates with heteromorphic sex chromosomes (left), sex determination is defined as the processes leading to differentiation of the gonads, which then secrete gonadal hormones that cause sexual differentiation of non-gonadal tissues. The revised model recognizes multiple primary parallel-acting factors encoded by the sex chromosomes, which activate numerous secondary downstream pathways, hormonal and genetic, that interact with each other (summing with each other or reducing the effects of each other) to cause or reduce sex differences in phenotype. In the revised model, gonadal secretions dominate as the most important group of secondary factors causing sex differences in phenotype of eutherian mammals.
Future Directions
Numerous mouse models are now widely available for the routine measurement of the importance of sex chromosome complement on sexual phenotypes (Arnold 2009a; Chen et al. 2012). These models will be used in increasing numbers of experiments to uncover differences in the phenotype of cells and tissues throughout the body. Three major goals are to identify the phenotypic effects of sex chromosome complement, to identify the X and Y genes that cause these differences and their downstream effectors, and to study how those genes interact with the effects of gonadal hormones. These studies have a strong rationale within the context of biomedical research, because one sex is often protected from important diseases such as cardiovascular disease, metabolic syndrome and obesity, cancer, etc. Thus, discovering the sex-biased mechanisms that protect from disease should increase understanding of the disease in both sexes, and could uncover novel protective factors and therapies. Because of the similarity of gene content of X and Y chromosomes of the mouse and human, discoveries in mice could be relevant to understanding and treating human disease.
Although birds are not as easily rationalized as a model system for human disease, they offer huge advantages for studying the operation of the same gene networks in two states, when the male and female transcriptomes are fundamentally different. These studies will bear strongly on the evolution of dosage compensation mechanisms and sex differences in development.
Bullet points.
Sex differences are caused by cell-autonomous effects of XX vs. XY sex chromosomes interacting with gonadal hormones.
Sex chromosome complement produces sex differences in many tissues and phenotypes, including those relevant to diseases such as autoimmune disease, neural tube closure defects, addiction, and hypertension.
Two X chromosomes in gonadectomized adult mice increases body weight, fat mass, and produces metabolic dysregulation in response to a high fat diet.
Birds show more dramatic cell-autonomous effects of sex chromosome complement perhaps because of the lack of chromosome-wide sex chromosome dosage compensation.
Acknowledgments
Supported by NIH DK083561, NS043196, DC000217.
Reference List
- Abel JL, Rissman EF. Location, location, location: Genetic regulation of neural sex differences. Rev Endocr Metab Disord. 2011;13:151–161. doi: 10.1007/s11154-011-9186-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abel JM, Witt DM, Rissman EF. Sex Differences in the Cerebellum and Frontal Cortex: Roles of Estrogen Receptor Alpha and Sex Chromosome Genes. Neuroendocrinol. 2011;93:230–240. doi: 10.1159/000324402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agate RJ, Grisham W, Wade J, Mann S, Wingfield J, Schanen C, Palotie A, Arnold AP. Neural not gonadal origin of brain sex differences in a gynandromorphic finch. Proc Natl Acad Sci USA. 2003;100:4873–4878. doi: 10.1073/pnas.0636925100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold AP. Genetically triggered sexual differentiation of brain and behavior. Horm Behav. 1996;30:495–505. doi: 10.1006/hbeh.1996.0053. [DOI] [PubMed] [Google Scholar]
- Arnold AP. Sex chromosomes and brain gender. Nat Rev Neurosci. 2004;5:701–708. doi: 10.1038/nrn1494. [DOI] [PubMed] [Google Scholar]
- Arnold AP. Mouse models for evaluating sex chromosome effects that cause sex differences in non-gonadal tissues. J Neuroendocrinol. 2009a;21:377–386. doi: 10.1111/j.1365-2826.2009.01831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold AP. The organizational-activational hypothesis as the foundation for a unified theory of sexual differentiation of all mammalian tissues. Horm Behav. 2009b;55:570–578. doi: 10.1016/j.yhbeh.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold AP. The end of gonad-centric sex determination in mammals. Trends Genet. 2011;28:55–61. doi: 10.1016/j.tig.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold AP, Chen X. What does the "four core genotypes" mouse model tell us about sex differences in the brain and other tissues? Front Neuroendocrinol. 2009;30:1–9. doi: 10.1016/j.yfrne.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold AP, Itoh Y, Melamed E. A bird’s-eye view of sex chromosome dosage compensation. Annu Rev Genomics Hum Genet. 2008;9:109–127. doi: 10.1146/annurev.genom.9.081307.164220. [DOI] [PubMed] [Google Scholar]
- Arnold AP, Schlinger BA. Sexual differentiation of brain and behavior: the zebra finch is not Just a flying rat. Brain Behav Evol. 1993;42:231–241. doi: 10.1159/000114157. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Adkins-Regan E. Sexual differentiation of brain and behavior in birds. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, Brain, and Behavior. San Diego: Academic Press; 2002. pp. 223–302. [Google Scholar]
- Balthazart J, Arnold AP, Adkins-Regan E. Sexual differentiation of brain and behavior in birds. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, Brain, and Behavior. San Diego: Elsevier; 2009. pp. 1745–1788. [Google Scholar]
- Barker JM, Torregrossa MM, Arnold AP, Taylor JR. Dissociation of genetic and hormonal influences on sex differences in alcoholism-related behaviors. J Neurosci. 2010;30:9140–9144. doi: 10.1523/JNEUROSCI.0548-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermejo-Alvarez P, Rizos D, Lonergan P, Gutierrez-Adan A. Transcriptional sexual dimorphism during preimplantation embryo development and its consequences for developmental competence and adult health and disease. Reproduction. 2011a;141:563–570. doi: 10.1530/REP-10-0482. [DOI] [PubMed] [Google Scholar]
- Bermejo-Alvarez P, Rizos D, Lonergan P, Gutierrez-Adan A. Transcriptional sexual dimorphism in elongating bovine embryos: implications for XCI and sex determination genes. Reproduction. 2011b;141:801–808. doi: 10.1530/REP-11-0006. [DOI] [PubMed] [Google Scholar]
- Bermejo-Alvarez P, Rizos D, Rath D, Lonergan P, Gutierrez-Adan A. Sex determines the expression level of one third of the actively expressed genes in bovine blastocysts. Proc Natl Acad Sci U S A. 2010;107:3394–3399. doi: 10.1073/pnas.0913843107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonthuis PJ, Cox KH, Rissman EF. X-chromosome dosage affects male sexual behavior. Horm Behav. 2012;64:565–572. doi: 10.1016/j.yhbeh.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budefeld T, Grgurevic N, Tobet SA, Majdic G. Sex differences in brain developing in the presence or absence of gonads. Dev Neurobiol. 2008;68:981–995. doi: 10.1002/dneu.20638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgoyne PS, Ojarikre OA, Turner JM. Evidence that postnatal growth retardation in XO mice is due to haploinsufficiency for a non-PAR X gene. Cytogenet Genome Res. 2002;99:252–256. doi: 10.1159/000071601. [DOI] [PubMed] [Google Scholar]
- Burgoyne PS, Thornhill AR, Boudrean SK, Darling SM, Bishop CE, Evans EP. The genetic basis of XX-XY differences present before gonadal sex differentiation in the mouse. Philos Trans R Soc Lond B Biol Sci. 1995;350:253–260. doi: 10.1098/rstb.1995.0159. [DOI] [PubMed] [Google Scholar]
- Caeiro XE, Mir FR, Vivas LM, Carrer HF, Cambiasso MJ. Sex Chromosome Complement Contributes to Sex Differences in Bradycardic Baroreflex Response. Hypertension. 2011;58:505–511. doi: 10.1161/HYPERTENSIONAHA.111.175661. [DOI] [PubMed] [Google Scholar]
- Carruth LL, Reisert I, Arnold AP. Sex chromosome genes directly affect brain sexual differentiation. Nat Neurosci. 2002;5:933–934. doi: 10.1038/nn922. [DOI] [PubMed] [Google Scholar]
- Chen X, Agate RJ, Itoh Y, Arnold AP. Sexually dimorphic expression of trkB, a Z-linked gene, in early posthatch zebra finch brain. Proc Natl Acad Sci USA. 2005;102:7730–7735. doi: 10.1073/pnas.0408350102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Grisham W, Arnold AP. X chromosome number causes sex differences in gene expression in adult mouse striatum. Eur J Neurosci. 2009;29:768–776. doi: 10.1111/j.1460-9568.2009.06610.x. [DOI] [PubMed] [Google Scholar]
- Chen X, McClusky R, Chen J, Beaven SW, Tontonoz P, Arnold AP, Reue K. The number of X chromosomes causes sex differences in adiposity in mice. PLoS Genet. 2012;8:e1002709. doi: 10.1371/journal.pgen.1002709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Watkins R, Delot E, Reliene R, Schiestl RH, Burgoyne PS, Arnold AP. Sex difference in neural tube defects in p53-null mice is caused by differences in the complement of X not Y genes. Dev Neurobiol. 2008;68:265–273. doi: 10.1002/dneu.20581. [DOI] [PubMed] [Google Scholar]
- Chue J, Smith CA. Sex determination and sexual differentiation in the avian model. FEBS J. 2011;278:1027–1034. doi: 10.1111/j.1742-4658.2011.08032.x. [DOI] [PubMed] [Google Scholar]
- Cox KH, Rissman EF. Sex differences in juvenile mouse social behavior are influenced by sex chromosomes and social context. Genes Brain Behav. 2011;10:465–472. doi: 10.1111/j.1601-183X.2011.00688.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czech DP, Lee J, Sim H, Parish CL, Vilain E, Harley VR. The human testis-determining factor SRY localizes in midbrain dopamine neurons and regulates multiple components of catecholamine synthesis and metabolism. J Neurochem. 2012 doi: 10.1111/j.1471-4159.2012.07782.x. Epub May 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vries GJ. Minireview: Sex differences in adult and developing brains: compensation, compensation, compensation. Endocrinol. 2004;145:1063–1068. doi: 10.1210/en.2003-1504. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Rissman EF, Simerly RB, Yang LY, Scordalakes EM, Auger CJ, Swain A, Lovell-Badge R, Burgoyne PS, Arnold AP. A model system for study of sex chromosome effects on sexually dimorphic neural and behavioral traits. J Neurosci. 2002;22:9005–9014. doi: 10.1523/JNEUROSCI.22-20-09005.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewing P, Shi T, Horvath S, Vilain E. Sexually dimorphic gene expression in mouse brain precedes gonadal differentiation. Brain Res Mol Brain Res. 2003;118:82–90. doi: 10.1016/s0169-328x(03)00339-5. [DOI] [PubMed] [Google Scholar]
- Dewing P, Chiang CWK, Sinchak K, Sim H, Fernagut PO, Kelly S, Chesselet MF, Micevych PE, Albrecht KH, Harley VR, Vilain E. Direct regulation of adult brain function by the male-specific factor SRY. Curr Biol. 2006;16:415–420. doi: 10.1016/j.cub.2006.01.017. [DOI] [PubMed] [Google Scholar]
- Eggers S, Sinclair A. Mammalian sex determination-insights from humans and mice. Chromosome Res. 2012;20:215–238. doi: 10.1007/s10577-012-9274-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellegren H, Hultin-Rosenberg L, Brunstrom B, Denker L, Kultimaa K, Scholz B. Faced with inequality: Chicken does not have a general dosage compensation of sex-linked genes. BMC Biol. 2007;5:40. doi: 10.1186/1741-7007-5-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ely D, Underwood A, Dunphy G, Boehme S, Turner M, Milsted A. Review of the Y chromosome, Sry and hypertension. Steroids. 2010;75:747–753. doi: 10.1016/j.steroids.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatewood JD, Wills A, Shetty S, Xu J, Arnold AP, Burgoyne PS, Rissman EF. Sex chromosome complement and gonadal sex influence aggressive and parental behaviors in mice. J Neurosci. 2006;26:2335–2342. doi: 10.1523/JNEUROSCI.3743-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gioiosa L, Chen X, Watkins R, Klanfer N, Bryant CD, Evans CJ, Arnold AP. Sex chromosome complement affects nociception in tests of acute and chronic exposure to morphine in mice. Horm Behav. 2008a;53:124–130. doi: 10.1016/j.yhbeh.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gioiosa L, Chen X, Watkins R, Umeda EA, Arnold AP. Sex chromosome complement affects nociception and analgesia in newborn mice. J Pain. 2008b;9:962–969. doi: 10.1016/j.jpain.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glickman SE, Short RV, Renfree MB. Sexual differentiation in three unconventional mammals: spotted hyenas, elephants and tammar wallabies. Horm Behav. 2005;48:403–417. doi: 10.1016/j.yhbeh.2005.07.013. [DOI] [PubMed] [Google Scholar]
- Grgurevic N, Budefeld T, Rissman EF, Tobet SA, Majdic G. Aggressive behaviors in adult SF-1 knockout mice that are not exposed to gonadal steroids during development. Behav Neurosci. 2008;122:876–884. doi: 10.1037/0735-7044.122.4.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grgurevic N, Budefeld T, Spanic T, Tobet SA, Majdic G. Evidence that sex chromosome genes affect sexual differentiation of female sexual behavior. Horm Behav. 2012;61:719–724. doi: 10.1016/j.yhbeh.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurney ME, Konishi M. Hormone-induced sexual differentiation of brain and behavior in zebra finches. Science. 1980;208:1380–1382. doi: 10.1126/science.208.4450.1380. [DOI] [PubMed] [Google Scholar]
- Heard E, Disteche CM. Dosage compensation in mammals: fine-tuning the expression of the X chromosome. Genes & Development. 2006;20:1848–1867. doi: 10.1101/gad.1422906. [DOI] [PubMed] [Google Scholar]
- Itoh Y, Melamed E, Yang X, Kampf K, Wang S, Yehya N, Van Nas A, Replogle K, Band MR, Clayton DF, Schadt EE, Lusis AJ, Arnold AP. Dosage compensation is less effective in birds than in mammals. J BIol. 2007;6:2. doi: 10.1186/jbiol53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh Y, Replogle K, Kim YH, Wade J, Clayton DF, Arnold AP. Sex bias and dosage compensation in the zebra finch versus chicken genomes: general and specialized patterns among birds. Genome Res. 2010;20:512–518. doi: 10.1101/gr.102343.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji H, Zheng W, Wu X, Liu J, Ecelbarger CM, Watkins R, Arnold AP, Sandberg K. Sex Chromosome Effects Unmasked in Angiotensin II-Induced Hypertension. Hypertension. 2010;55:1275–1282. doi: 10.1161/HYPERTENSIONAHA.109.144949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YH, Perlman WR, Arnold AP. Expression of androgen receptor mRNA in zebra finch song system: Developmental regulation by estrogen. J Comp Neurol. 2004;469:535–547. doi: 10.1002/cne.11033. [DOI] [PubMed] [Google Scholar]
- Lopes AM, Burgoyne PS, Ojarikre A, Bauer J, Sargent CA, Amorim A, Affara NA. Transcriptional changes in response to X chromosome dosage in the mouse: implications for X inactivation and the molecular basis of Turner Syndrome. BMC Genomics. 2010;11:82. doi: 10.1186/1471-2164-11-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majdic G, Tobet S. Cooperation of sex chromosomal genes and endocrine influences for hypothalamic sexual differentiation. Front Neuroendocrinol. 2011;32:137–145. doi: 10.1016/j.yfrne.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mank JE. The W, X, Y and Z of sex-chromosome dosage compensation. Trends Genet. 2009;25:226–233. doi: 10.1016/j.tig.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPhie-Lalmansingh AA, Tejada LD, Weaver JL, Rissman EF. Sex chromosome complement affects social interactions in mice. Horm Behav. 2008;54:565–570. doi: 10.1016/j.yhbeh.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naurin S, Hansson B, Hasselquist D, Kim YH, Bensch S. The sex-biased brain: sexual dimorphism in gene expression in two species of songbirds. BMC Genomics. 2011;12:37. doi: 10.1186/1471-2164-12-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nef S, Schaad O, Stallings NR, Cederroth CR, Pitetti JL, Schaer G, Malki S, Dubois-Dauphin M, Boizet-Bonhoure B, Descombes P, Parker KL, Vassalli JD. Gene expression during sex determination reveals a robust female genetic program at the onset of ovarian development. Dev Biol. 2005;287:361–377. doi: 10.1016/j.ydbio.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Nottebohm F, Arnold AP. Sexual dimorphism in vocal control areas of the song bird brain. Science. 1976;194:211–213. doi: 10.1126/science.959852. [DOI] [PubMed] [Google Scholar]
- OW-S, Short R, Renfree MB, Shaw G. Primary genetic control of somatic sexual differentiation in a mammal. Nature. 1988;331:716–717. doi: 10.1038/331716a0. [DOI] [PubMed] [Google Scholar]
- Palaszynski KM, Smith DL, Kamrava S, Burgoyne PS, Arnold AP, Voskuhl RR. A Yin-Yang effect between sex chromosome complement and sex hormones on the immune response. Endocrinol. 2005;146:3280–3285. doi: 10.1210/en.2005-0284. [DOI] [PubMed] [Google Scholar]
- Quinn JJ, Hitchcott PK, Umeda EA, Arnold AP, Taylor JR. Sex chromosome complement regulates habit formation. Nat Neurosci. 2007;10:1398–1400. doi: 10.1038/nn1994. [DOI] [PubMed] [Google Scholar]
- Renfree MB, Short RV. Sex determination in marsupials: evidence for a marsupial-eutherian dichotomy. Philosophical Transactions of the Royal Society of London B:Biological Sciences. 1988;322:41–53. doi: 10.1098/rstb.1988.0112. [DOI] [PubMed] [Google Scholar]
- Renfree MB, Wilson JD, Shaw G. The hormonal control of sexual development. Novartis Found Symp. 2002;244:136–152. [PubMed] [Google Scholar]
- Robinson DP, Huber SA, Moussawi M, Roberts B, Teuscher C, Watkins R, Arnold AP, Klein SL. Sex chromosome complement contributes to sex differences in Coxsackievirus B3 but not Influenza A virus pathogenesis. Biol Sex Differ. 2011;2:8. doi: 10.1186/2042-6410-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasidhar MV, Itoh N, Gold SM, Lawson GW, Voskuhl RR. The XX sex chromosome complement in mice is associated with increased spontaneous lupus compared with XY. Ann Rheum Dis. 2012 doi: 10.1136/annrheumdis-2011-201246. epub May 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silversides DW, Raiwet DL, Souchkova O, Viger RS, Pilon N. Transgenic mouse analysis of Sry expression during the pre- and peri-implantation stage. Dev Dyn. 2012;241:1192–1204. doi: 10.1002/dvdy.23798. [DOI] [PubMed] [Google Scholar]
- Smith-Bouvier DL, Divekar AA, Sasidhar M, Du S, Tiwari-Woodruff SK, King JK, Arnold AP, Singh RR, Voskuhl RR. A role for sex chromosome complement in the female bias in autoimmune disease. J Exp Med. 2008;205:1099–1108. doi: 10.1084/jem.20070850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade J, Arnold AP. Functional testicular tissue does not masculinize development of the zebra finch song system. Proc Natl Acad Sci U S A. 1996;93:5264–5268. doi: 10.1073/pnas.93.11.5264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade J, Arnold AP. Sexual differentiation of the zebra finch song system. Ann N Y Acad Sci. 2004;1016:540–559. doi: 10.1196/annals.1298.015. [DOI] [PubMed] [Google Scholar]
- Werler S, Poplinski A, Gromoll J, Wistuba J. Expression of selected genes escaping from X inactivation in the 41, XX(Y) * mouse model for Klinefelter’s syndrome. Acta Paediatr. 2011;100:885–891. doi: 10.1111/j.1651-2227.2010.02112.x. [DOI] [PubMed] [Google Scholar]
- Wijchers PJ, Festenstein RJ. Epigenetic regulation of autosomal gene expression by sex chromosomes. Trends Genet. 2011;27:132–140. doi: 10.1016/j.tig.2011.01.004. [DOI] [PubMed] [Google Scholar]
- Wijchers PJ, Yandim C, Panousopoulou E, Ahmad M, Harker N, Saveliev A, Burgoyne PS, Festenstein R. Sexual dimorphism in mammalian autosomal gene regulation is determined not only by Sry but by sex chromosome complement as well. Dev Cell. 2010;19:477–484. doi: 10.1016/j.devcel.2010.08.005. [DOI] [PubMed] [Google Scholar]
- Witschi E. Modification of the development of sex in lower vertebrates and in mammals. In: Allen E, Danforth CH, Doisy EA, editors. Sex and Internal Secretions. Baltimore: Williams & Wilkins; 1939. pp. 145–226. [Google Scholar]
- Wolf JB, Bryk J. General lack of global dosage compensation in ZZ/ZW systems? Broadening the perspective with RNA-seq. BMC Genomics. 2011;12:91. doi: 10.1186/1471-2164-12-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Burgoyne PS, Arnold AP. Sex differences in sex chromosome gene expression in mouse brain. Hum Mol Genet. 2002;11:1409–1419. doi: 10.1093/hmg/11.12.1409. [DOI] [PubMed] [Google Scholar]
- Xu J, Deng X, Disteche CM. Sex-specific expression of the X-linked histone demethylase gene Jarid1c in brain. PLoS One. 2008a;3:e2553. doi: 10.1371/journal.pone.0002553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Deng X, Watkins R, Disteche CM. Sex-specific differences in expression of histone demethylases Utx and Uty in mouse brain and neurons. J Neurosci. 2008b;28:4521–4527. doi: 10.1523/JNEUROSCI.5382-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F, Babak T, Shendure J, Disteche CM. Global survey of escape from X inactivation by RNA-sequencing in mouse. Genome Res. 2010;20:614–622. doi: 10.1101/gr.103200.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SO, Mathur S, Hattem G, Tassy O, Pourquie O. Sex-dimorphic gene expression and ineffective dosage compensation of Z-linked genes in gastrulating chicken embryos. BMC Genomics. 2010;11:13. doi: 10.1186/1471-2164-11-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao D, McBride D, Nandi S, McQueen HA, McGrew MJ, Hocking PM, Lewis PD, Sang HM, Clinton M. Somatic sex identity is cell autonomous in the chicken. Nature. 2010;464:237–242. doi: 10.1038/nature08852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvetkova I, Apedaile A, Ramsahoye B, Mermoud JE, Crompton LA, John R, Feil R, Brockdorff N. Global hypomethylation of the genome in XX embryonic stem cells. Nat Genet. 2005;37:1274–1279. doi: 10.1038/ng1663. [DOI] [PubMed] [Google Scholar]