Abstract
Background
Cognitive complaints are reported frequently after breast cancer treatments. Their association with neuropsychological (NP) test performance is not well-established.
Methods
Early-stage, posttreatment breast cancer patients were enrolled in a prospective, longitudinal, cohort study prior to starting endocrine therapy. Evaluation included an NP test battery and self-report questionnaires assessing symptoms, including cognitive complaints. Multivariable regression models assessed associations among cognitive complaints, mood, treatment exposures, and NP test performance.
Results
One hundred eighty-nine breast cancer patients, aged 21–65 years, completed the evaluation; 23.3% endorsed higher memory complaints and 19.0% reported higher executive function complaints (>1 SD above the mean for healthy control sample). Regression modeling demonstrated a statistically significant association of higher memory complaints with combined chemotherapy and radiation treatments (P = .01), poorer NP verbal memory performance (P = .02), and higher depressive symptoms (P < .001), controlling for age and IQ. For executive functioning complaints, multivariable modeling controlling for age, IQ, and other confounds demonstrated statistically significant associations with better NP visual memory performance (P = .03) and higher depressive symptoms (P < .001), whereas combined chemotherapy and radiation treatment (P = .05) approached statistical significance.
Conclusions
About one in five post–adjuvant treatment breast cancer patients had elevated memory and/or executive function complaints that were statistically significantly associated with domain-specific NP test performances and depressive symptoms; combined chemotherapy and radiation treatment was also statistically significantly associated with memory complaints. These results and other emerging studies suggest that subjective cognitive complaints in part reflect objective NP performance, although their etiology and biology appear to be multifactorial, motivating further transdisciplinary research.
Cognitive impairment is a feared complication of cancer treatment (1–4) and has been extensively studied in breast cancer patients (5–16). Subjective cognitive complaints have been a driving force behind research in this area since the late 1990s (17). A recent meta-analysis conducted by Jim et al. (18) concluded that in the posttreatment period with standard dose chemotherapy, statistically significant small deficits in neuropsychological (NP) domains of verbal and visuospatial ability could be detected. In most early studies, subjective complaints were more often associated with symptoms of depression and anxiety (5,9,14), calling into question whether patient-reported complaints reflected neurophysiological changes.
Brain imaging studies have begun to provide more information about the biology of cognitive function after cancer treatment exposures. A recent prospective, controlled study (pre- and postchemotherapy) of NP testing and magnetic resonance imaging (MRI) found changes in gray matter associated with chemotherapy treatment, suggesting a physiological basis for cognitive disturbance (19,20). Two additional imaging studies demonstrated that chemotherapy-exposed breast cancer patients reported cognitive complaints (memory and executive function) that aligned with relevant NP test domains, as well as with associated anatomic brain regions (21,22). These emerging data suggest that cognitive complaints may reflect underlying changes in cerebral functioning. The lack of association between NP test performance and subjective cognitive complaints in prior studies with breast cancer patients (23) could reflect the lack of specificity and sensitivity of the self-report tools, or could be due to reliance on global measures rather than domain-specific assessments.
To this end, we examined the relationship between subjective cognitive complaints and NP functioning in the setting of the University of California, Los Angeles (UCLA) Mind Body Study (MBS), a large prospective cohort study of recently diagnosed, early-stage breast cancer patients enrolled after completion of adjuvant therapy and prior to initiation of endocrine therapy (24). A principal goal of the MBS was to assess prospectively the impact of endocrine therapy on subsequent cognitive functioning, while accounting for prior hormone exposures and treatment-associated changes in menstrual status. Cognitive complaints are at their peak in the immediate post–adjuvant treatment period (25), and the MBS baseline assessment provided a rich source of data to examine potential contributing factors. We hypothesized that domain-specific cognitive complaints could be identified in this setting, and that they would be associated with relevant NP domains, as well as with chemotherapy treatment exposure and change in menstrual status.
Patients and Methods
Study Participants, Recruitment, and Design
Eligible women were aged 21–65 years; were newly diagnosed with stage 0, I, II, or IIIA breast cancer; had completed primary breast cancer treatments within the past 3 months; had not yet started endocrine therapy; were available for 12-month follow-up; and were English language proficient. Exclusions/ineligibility included current or past disorder/disease of the central nervous system or medical condition impacting cognitive functioning; head trauma history with prolonged loss of consciousness; epilepsy, dementia, or learning disability; current or past psychotic-spectrum disorder or current major affective disorder; current substance abuse/dependence; daily tobacco and alcohol use; whole brain irradiation or surgery; prior cancer diagnosis or chemotherapy treatment; active autoimmune disorder; insulin-dependent diabetes; uncontrolled allergic condition or asthma; chronic use of oral steroid medication; and hormone therapy (estrogen, progestin compounds) other than vaginal estrogen. Exclusions related to hormones and inflammatory conditions were required due to other MBS aims focused on the biology of cognitive dysfunction (24).
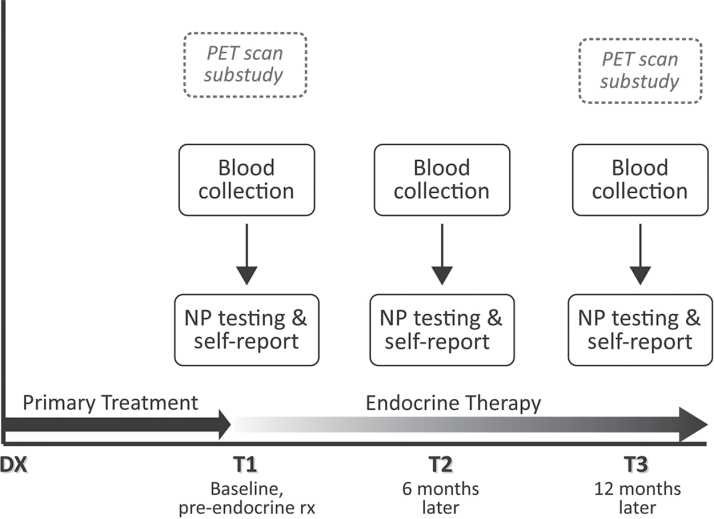
Study recruitment occurred primarily through rapid case ascertainment of stage-eligible patients identified through the Los Angeles County Surveillance Epidemiology and End Results (SEER) registry from selected collaborating physicians and hospitals. Prior publications include details of subject identification, screening, recruitment, and enrollment (24,26). Figure 1 shows the overall study design. Preliminary MBS data describing some baseline symptoms and inflammatory markers are reported elsewhere (26), and preliminary longitudinal data related to inflammatory markers, subjective complaints, and brain imaging are described in another recent report (24). The research was approved by the UCLA Institutional Review Board, and all participants provided written informed consent.
Figure 1.
Overview of the UCLA Mind Body Study, a prospective longitudinal cohort study designed to examine the impact of endocrine therapy for breast cancer on cognitive functioning. DX = breast cancer diagnosis; NP = neuropsychological; PET = positron emission tomography; rx = treatment.
Demographics, Clinical Information, and Symptoms
Information was obtained from self-report and medical record abstraction. The Beck Depression Inventory–II (BDI-II) was used to assess depressive symptoms during the preceding two weeks (27), with higher scores indicating more severe symptoms. We also calculated two subscales of the BDI-II with 14 items representing the cognitive/affective component (eg, sadness, pessimism, guilt, loss of pleasure) and seven items focused on the somatic aspects of depression (eg, loss of energy, changes in sleep or appetite, loss of interest in sex).
Cognitive complaints were assessed with the Patient’s Assessment of Own Functioning Inventory (PAOFI) (28), a self-report instrument with prior evidence for correlation with NP test changes in cancer patient samples (23,29,30). The PAOFI contains 33 questions and is divided into four subscales: memory (10 questions), higher-level cognition (HLC) measuring executive function (nine questions), language and communication (nine questions), and motor-sensory perception (five questions). Each PAOFI complaint item was rated on a 6-point Likert scale from 1 (“almost always”) to 6 (“almost never”). We scored the PAOFI by assigning a score of 1 to each item rated as “almost always,” “very often,” and “fairly often,” whereas items rated “once in awhile,” “very infrequently,” or “almost never” were assigned a score of 0 (28). Thus, the total score for the PAOFI ranges from 0 to 33, and each subscale from 0 to the total number of items in the subscale (ie, the memory subscale ranges from 0 to 10 and the HLC subscale ranges from 0 to 9). As normative data for the PAOFI were lacking, in this report we include comparative data from a healthy control population of women without breast cancer obtained from a concurrent study at the University of California, San Diego (UCSD) (31). In that study, healthy volunteers were recruited to match a prospective, longitudinal study of cognitive function in women receiving breast cancer adjuvant chemotherapy (S. Ancoli-Israel, NCI R01 CA112035). Demographic data, body mass index (BMI), depression and PAOFI scores were available from the 63 healthy women enrolled in the UCSD study.
Fatigue was assessed using the Multidimensional Fatigue Symptom Inventory–Short Form (MFSI-SF), a 30-item questionnaire that includes five subscales: general, physical, emotional, mental fatigue, and vigor (32).
Neuropsychological Assessments
NP testing was conducted by a trained technician, closely supervised by a licensed clinical neuropsychologist using procedures detailed in an earlier report (24). The 120-minute test battery is described in detail in Supplementary Table 1. NP test scores were standardized to z scores, with positive scores indicating outcomes better than age-corrected normative performance scores with a mean of 0 and standard deviation of 1, and negative scores reflecting lower than normative performance (see Supplementary Table 1). These scores were then used to create NP test domains based upon prior factor-analytic studies of larger NP data sets, as well as groupings used in other studies with this population (33). We identified a priori variables most salient for the cognitive domain being studied. An estimate of full-scale IQ, the Wechsler Test of Adult Reading (WTAR), was administered only at baseline.
Statistical Analyses
PAOFI total and subscale scores from the MBS sample were compared to data from the UCSD healthy female controls, using χ2 or t tests as appropriate, permitting definition of a normal range for each PAOFI subscale for comparison with the MBS sample. Only two PAOFI subscales (memory and HLC) showed statistically significant differences; these were examined in subsequent evaluations. Bivariate tables were constructed to identify demographic and treatment characteristics to include as predictors, as well as NP domains, adjusted for age and IQ, to include in multivariable models. MBS patients with PAOFI subscale scores >1 SD above the mean for the healthy controls (high complaints) were compared with patients whose scores were ≤1 SD above the mean (within-normal-limits levels of complaints) using χ2 or t tests. The PAOFI scores were log transformed [log(x+1)] due to nonnormality, in the multivariable linear regression analyses. Multivariable models included age and IQ, as well as statistically significant variables (P < .10) identified in the bivariate analyses. Final models were run with and without BDI-II scores to control for depressive symptoms, excluding the BDI-II item that asked about concentration from the total score. Additional multivariable models were run with the BDI-II somatic and cognitive/affective subscales instead of the BDI-II composite score. Finally, a stepwise linear regression was also performed to assess whether there was any redundancy in predictors selected for other reasons and to summarize the relative contribution of each factor to the overall model variance. For these regressions, the BDI-II somatic and cognitive/affective subscales were entered separately to discern any differential associations. All statistical tests were two-sided and all analyses were conducted in SAS 9.3 (Cary, NC).
Results
Recruitment Results and Patient Characteristics
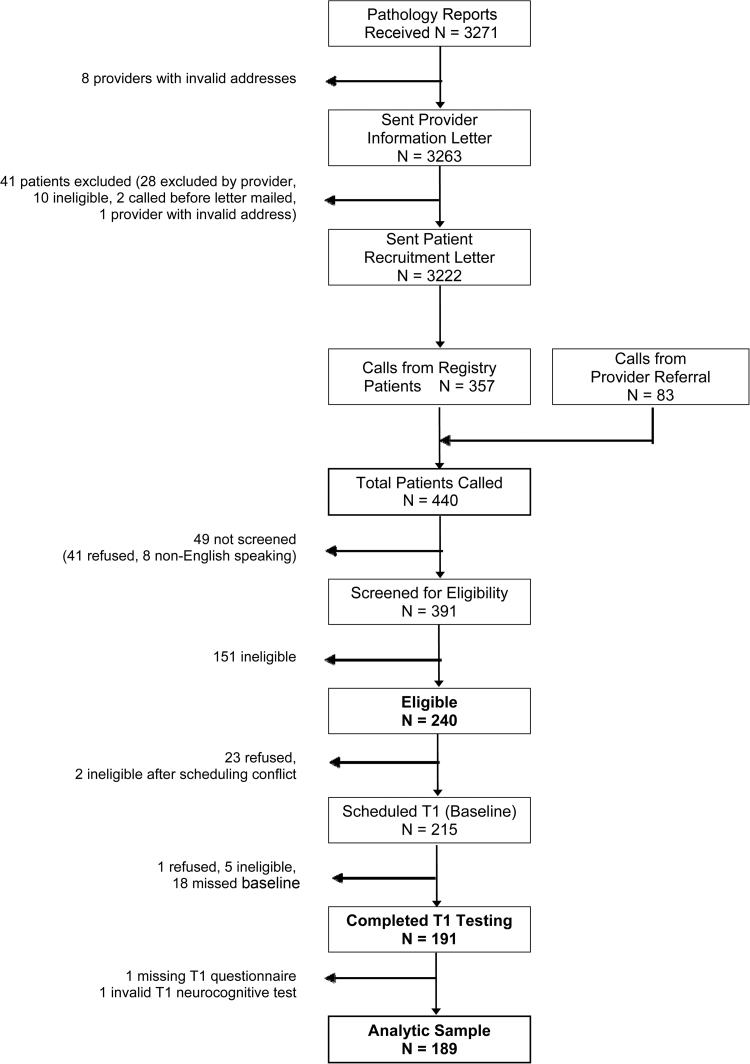
Recruitment began in May 2007 and ended in February 2011. More than 3000 patients identified from the SEER registry were sent invitation letters; we also received referral of 83 patients, independent of the registry recruitment. Details of recruitment results are shown in Figure 2. The most common reasons for ineligibility were initiation of endocrine therapy/beyond enrollment window (53.1%), demographic/medical exclusions (17.9%), and autoimmune and behavioral confounders (eg, alcohol/tobacco use) (15.2%). Two hundred forty patients were eligible after screening and 214 were scheduled for a baseline visit; however, only 189 completed the baseline visit with evaluable data. Nonparticipating eligible patients did not differ from MBS participants in age, marital status, education, type of surgery, chemotherapy status, radiation status, or anticipated endocrine therapy use (all P > .05); however, they were more likely to be nonwhite than enrollees (36.2% vs 19.5%, P = .01).
Figure 2.
CONSORT flow chart of recruitment and screening for the study.
On average, study participants were almost 52 years old and enrolled 6.6 months after their breast cancer diagnosis, with completion of primary treatment about 1 month earlier (Table 1). Most were white, married, college educated, and postmenopausal prior to their breast cancer diagnosis. Two-thirds received breast-conserving surgery; more than half had received chemotherapy; and three-quarters had received radiation therapy. Past hormone therapy exposure and menstrual status are also reported. The BDI-II mean score for the sample was 8.8 (95% CI = 7.9 to 9.8; data not shown), well below the cut point for clinical depression of >19, suggesting that eligibility screening had been effective in excluding clinically depressed individuals. A total of 68.3% were planning to receive endocrine therapy (not started at time of this visit; data not shown).
Table 1.
Patient characteristics by Patient’s Assessment of Own Functioning Inventory (PAOFI) memory and higher-level cognition (HLC) complaint status comparing high and normal groups*
| Memory complaints | HLC complaints | ||||||
|---|---|---|---|---|---|---|---|
| Total N = 189 | High n = 44 | Normal n = 145 | P† | High n = 36 | Normal n = 153 | P† | |
| Age, mean (95% CI), y | 51.8 (50.6 to 53.0) | 50.7 (48.1 to 53.4) | 52.2 (50.8 to 53.5) | .30 | 53.2 (50.6 to 55.8) | 51.5 (50.2 to 52.9) | .28 |
| Months from diagnosis, mean (95% CI) | 6.6 (6.1 to 7.0) | 7.3 (6.1 to 8.4) | 6.3 (5.9 to 6.8) | .09 | 6.7 (5.3 to 8.1) | 6.5 (6.0 to 7.0) | .81 |
| Months from last treatment to T1, mean (95% CI) | 1.2 (1.0 to 1.3) | 1.2 (0.8 to 1.5) | 1.2 (1.0 to 1.4) | .89 | 1.1 (0.8 to 1.5) | 1.2 (1.1 to 1.4) | .56 |
| Months from LMP, mean (95% CI) | 62.9 (50.2 to 75.6) | 60.6 (38.0 to 83.2) | 63.6 (48.4 to 78.8) | .84 | 79.6 (50.5 to 108.8) | 59.1 (44.9 to 73.3) | .22 |
| Race | |||||||
| White, non-Hispanic | 79.9% (151) | 72.7% (32) | 82.1% (119) | .29 | 77.8% (28) | 80.4% (123) | .81 |
| Hispanic | 9.5% (18) | 9.1% (4) | 9.7% (14) | 11.1% (4) | 9.2% (14) | ||
| Black | 2.6% (5) | 4.6% (2) | 2.1% (3) | 2.8% (1) | 2.6% (4) | ||
| Asian | 4.8% (9) | 6.8% (3) | 4.1% (6) | 2.8% (1) | 5.2% (8) | ||
| Other | 2.6% (6) | 6.8% (3) | 2.1% (3) | 5.6% (2) | 2.6% (4) | ||
| Marital status | |||||||
| Married | 65.6% (124) | 70.4% (31) | 64.1% (93) | .44 | 72.2% (26) | 64.0% (98) | .35 |
| Not married | 34.4% (65) | 29.6% (13) | 35.9% (52) | 27.8% (10) | 36.0% (55) | ||
| Education | |||||||
| Postcollege | 52.4% (99) | 59.1% (26) | 50.3% (73) | .15 | 52.8% (19) | 52.3% (80) | .36 |
| College | 29.6% (56) | 18.2% (8) | 33.1% (48) | 22.2% (8) | 31.4% (48) | ||
| No college degree | 18.0% (34) | 22.7% (10) | 16.6% (24) | 25.0% (9) | 16.3% (25) | ||
| IQ, mean (95% CI) | 114.3 (113.0 to 115.6) | 113.7 (110.7 to 116.8) | 114.4 (113.0 to 115.9) | .65 | 111.7 (108.4 to 114.9) | 114.9 (113.5 to 116.3) | .06 |
| Employment status | |||||||
| Full or part-time | 64.6% (122) | 45.4% (20) | 70.3% (102) | .003 | 50.0% (18) | 68.0% (104) | .04 |
| Not employed | 35.4% (67) | 54.6% (24) | 29.7% (43) | 50.0% (18) | 32.0% (49) | ||
| Annual household income | |||||||
| ≥$100,000 | 60.2% (112) | 48.8% (21) | 63.6% (91) | .08 | 55.6% (20) | 61.3% (92) | .53 |
| <$100,000 | 39.8% (74) | 51.1% (22) | 36.4% (52) | 44.4% (16) | 38.7% (58) | ||
| Surgery | |||||||
| Mastectomy | 33.9% (64) | 36.4% (16) | 33.1% (48) | .69 | 30.6% (11) | 34.6% (53) | .64 |
| Lumpectomy | 66.1% (125) | 63.6% (28) | 66.9% (97) | 69.4% (25) | 65.4% (100) | ||
| Treatment | |||||||
| Chemotherapy & radiation | 40.7% (77) | 59.1% (26) | 35.2% (51) | .004 | 58.3% (21) | 36.6% (56) | .02 |
| Chemotherapy only | 10.6% (20) | 15.9% (7) | 9.0% (13) | 16.7% (6) | 9.2% (14) | ||
| Radiation only | 33.9% (64) | 20.4% (9) | 37.9% (55) | 19.4% (7) | 37.2% (57) | ||
| Neither | 14.8% (28) | 4.6% (2) | 17.9% (26) | 5.6% (2) | 17.0% (26) | ||
| Anthracycline use | |||||||
| Yes | 24.7% (24) | 24.2% (8) | 25.0% (16) | .94 | 29.6% (8) | 22.9% (16) | .49 |
| No | 75.3% (73) | 75.8% (25) | 75.0% (48) | 70.4% (19) | 77.1% (55) | ||
| Trastuzumab use | |||||||
| Yes | 14.0% (27) | 25.0% (11) | 11.0% (16) | .02 | 25.0% (9) | 11.8% (18) | .04 |
| No | 85.7% (162) | 75.0% (33) | 89.0% (129) | 75.0% (27) | 88.2% (135) | ||
| Stage at diagnosis | |||||||
| Stage 0 | 13.2% (25) | 2.3% (1) | 16.5% (24) | .04 | 5.6% (2) | 15.0% (23) | .39 |
| Stage I | 46.0% (87) | 43.2% (19) | 46.9% (68) | 44.4% (16) | 46.4% (71) | ||
| Stage II | 31.2% (59) | 43.2% (19) | 27.6% (40) | 38.9% (14) | 29.4% (45) | ||
| Stage III | 9.5% (18) | 11.4% (5) | 9.0% (13) | 11.1% (4) | 9.2% (14) | ||
| Past hormone therapy | |||||||
| Yes | 29.2% (54) | 31.8% (14) | 28.4% (40) | .66 | 51.4% (18) | 24.0% (36) | .001 |
| No | 70.8% (131) | 68.2% (30) | 71.6% (101) | 48.6% (17) | 76.0% (114) | ||
| Change in period | |||||||
| Postmenopausal | 53.2% (100) | 56.8 (25) | 52.1% (75) | .64 | 63.9% (23) | 50.6% (77) | .19 |
| No change | 16.0% (30) | 9.1% (4) | 18.1% (26) | 8.3% (3) | 17.8% (27) | ||
| Became irregular | 5.3% (10) | 4.6% (2) | 5.6% (8) | 0.0% (0) | 6.6% (10) | ||
| Stop but resumed | 4.8% (9) | 6.8% (3) | 4.2% (6) | 8.3% (3) | 4.0% (6) | ||
| Amenorrhea | 20.7% (39) | 22.7% (10) | 20.0% (29) | 19.4% (7) | 21.0% (32) | ||
* High complaints have PAOFI memory or HLC scores >1 SD above the mean for a healthy controls comparison group (2.04 and 0.37, respectively). All P values represent a comparison between the high and normal complaint groups for memory and HLC. CI = confidence interval; LMP = last menstrual period.
† Groups were compared using t tests for continuous variables and χ2 tests (or Fisher exact tests as appropriate) for categorical variables; all tests were two-sided.
Cognitive Complaints at the End of Primary Treatment
PAOFI scores for the MBS sample were compared to the healthy female controls in the UCSD sample. There were no statistically significant differences in age, ethnicity, marital status, BMI, or depressive symptoms between the two groups, but the MBS sample had statistically significantly higher education and income (Supplementary Table 2). For the PAOFI total and subscale scores, the MBS sample had statistically significantly higher total (P = .004), memory (P = .003), and HLC (P = .01) scores, indicating more severe complaints (Supplementary Table 3). There were no statistically significant differences in the remaining PAOFI subscales (language and communication; motor-sensory perception). Thus, subsequent analyses focused on the PAOFI memory and HLC scales.
We classified MBS patients as having higher or normal range/no complaints on the PAOFI memory and HLC subscales to evaluate bivariate relationships between cognitive complaints and potential demographic and clinical variables (see Table 1). Using this classification, 23.3% of patients endorsed higher memory complaints and 19.0% reported higher HLC complaints. Women with higher memory complaints were more likely to have received both chemotherapy and radiation than women with normal complaints (59.1% vs 35.2%, P = .004), as were women with higher HLC complaints (58.3% vs 36.6%, P = .02). There was no statistically significant relationship between higher memory complaints and treatment-induced amenorrhea or menopausal status. Few differences were seen for women with higher HLC complaints with the exception of a history of past hormone therapy (HT) use (51.4% vs 24.0%, P = .001).
The relationship between cognitive complaints and fatigue was examined by performing correlations with the MFSI-SF subscale scores for mental and physical fatigue (data not shown). The mental subscale score was statistically significantly correlated with PAOFI memory (r = 0.605, P < .001), and with the PAOFI HLC (r = 0.473, P < .001); no statistically significant correlations were found for the physical subscale (PAOFI memory r = 0.025, P = .73 and PAOFI HLC r = 0.128, P = .08).
Relationship Between Cognitive Complaints and Neuropsychological Test Performance
Table 2 presents bivariate relationships for the NP test domains adjusted for age and IQ in women with higher and normal PAOFI memory and HLC complaints. Of note, for many of the NP test domains (eg, verbal learning, verbal memory, and psychomotor speed), the MBS sample scores were approximately 0.5 SD above age-based normative reference data regardless of complaint status, likely reflecting the high level of education, socioeconomic status, and estimated IQ for the MBS sample. Women with higher memory complaints had marginally lower NP verbal memory performance than the normal group (P = .08). Women with higher HLC complaints demonstrated worse performance on psychomotor speed (P = .01) and executive functioning (P = .003); however, those with higher complaints tended to perform better on the NP visual memory tests (P = .04).
Table 2.
Mean neuropsychological test scores by Patient’s Assessment of Own Functioning Inventory (PAOFI) memory and higher-level cognition (HLC) complaint status comparing high and normal groups*
| Memory complaints | HLC complaints | ||||||
|---|---|---|---|---|---|---|---|
| Total N = 189 | High n = 44 | Normal n = 145 | P‡ | High n = 36 | Normal n = 153 | P‡ | |
| NP test domain testing, mean (95% CI)† | |||||||
| Verbal learning | 0.514 (0.423 to 0.604) | 0.521 (0.334 to 0.709) | 0.511 (0.407 to 0.614) | .92 | 0.644 (0.427 to 0.862) | 0.486 (0.383 to 0.589) | .20 |
| Verbal memory | 0.712 (0.626 to 0.797) | 0.573 (0.396 to 0.749) | 0.754 (0.657 to 0.852) | .08 | 0.595 (0.392 to 0.798) | 0.738 (0.642 to 0.834) | .22 |
| Visual learning | –0.008 (–0.105 to 0.088) | 0.047 (–0.152 to 0.245) | –0.024 (–0.134 to 0.087) | .54 | 0.121 (–0.102 to 0.344) | –0.016 (–0.122 to 0.090) | .28 |
| Visual memory | –0.050 (–0.166 to 0.066) | 0.093 (–0.146 to 0.333) | –0.092 (–0.225 to 0.041) | .18 | 0.221 (–0.044 to 0.485) | –0.099 (–0.224 to 0.027) | .04 |
| Visuospatial function | –0.357 (–0.458 to –0.257) | –0.324 (–0.534 to –0.115) | –0.368 (–0.483 to -0.252) | .72 | –0.345 (–0.586 to –0.105) | –0.360 (–0.473 to -0.246) | .92 |
| Psychomotor speed | 0.506 (0.415 to 0.597) | 0.470 (0.281 to 0.658) | 0.518 (0.414 to 0.622) | .66 | 0.275 (0.063 to 0.488) | 0.580 (0.479 to 0.680) | .01 |
| Executive function | 0.323 (0.231 to 0.427) | 0.302 (0.099 to 0.506) | 0.337 (0.225 to 0.449) | .77 | 0.025 (–0.202 to 0.253) | 0.418 (0.310 to 0.525) | .003 |
| Motor speed | –0.256 (–0.396 to –0.116) | –0.449 (–0.738 to –0.160) | –0.195 (–0.355 to –0.036) | .13 | –0.486 (–0.818 to –0.154) | –0.188 (–0.345 to –0.031) | .12 |
* High complaints have a PAOFI memory or HLC score >1 SD above the mean for a healthy controls comparison group (2.04 and 0.37, respectively). CI = confidence interval; HLC = higher-level cognition.
† Scores were calculated by comparing subjects’ scores against age-corrected normative scores with a mean of 0 and standard deviation of 1, with positive (ie, >0) scores representing higher/better performance.
‡ Groups were compared by t tests of the least squares means from general linearized models, adjusted for age and IQ for memory complaints, and age, IQ, and hormone therapy use for HLC; all tests were two-sided.
Multivariate models were then conducted to examine associations between PAOFI memory and HLC complaints and these four NP domains (NP verbal memory performance, psychomotor speed, executive functioning, and visual memory), controlling for known factors associated with NP performance (age, IQ, and depressive symptoms), as well as statistically significant treatment associated variables identified in the bivariate comparisons (see Table 1). The PAOFI memory subscale results are shown in Table 3. The base model without depressive symptoms found that poorer NP verbal memory (P = .01) and combined chemotherapy and radiation (P = .002) were statistically significantly associated with the PAOFI memory subscale score with a model adjusted R 2 = 0.15. With the BDI-II in the model, these statistically significant results were retained (higher memory complaints associated with combined chemotherapy and radiation treatments, P = .01; poorer NP verbal memory performance, P = .02), with only modest reduction in parameter estimates; the BDI-II score also was statistically significant (P < .001), increasing the adjusted R 2 to 0.30.
Table 3.
Linear regression models predicting Patient’s Assessment of Own Functioning Inventory (PAOFI) memory (n = 188)*
| Base model adjusted R 2 = 0.15 | Base model with BDI-II adjusted R 2 = 0.30 | |||
|---|---|---|---|---|
| Variable | Estimate (95% CI) | P† | Estimate (95% CI) | P † |
| Age | –0.005 (–0.017 to 0.007) | .445 | –0.002 (–0.013 to 0.009) | .739 |
| IQ | 0.00004 (–0.011 to 0.011) | .994 | 0.001 (–0.009 to 0.011) | .833 |
| Treatment | ||||
| Radiation | –0.003 (–0.304 to 0.297) | .983 | 0.017 (–0.257 to 0.290) | .903 |
| Chemotherapy | 0.279 (–0.113 to 0.671) | .162 | 0.090 (–0.272 to 0.452) | .624 |
| Chemotherapy & radiation | 0.466 (0.174 to 0.758) | .002 | 0.374 (0.107 to 0.641) | .006 |
| Verbal memory§ | –0.213 (–0.376 to -0.051) | .010 | –0.174 (–0.322 to –0.025) | .022 |
| BDI-II|| | ----- | ----- | 0.045 (0.030 to 0.059) | <.001 |
* PAOFI memory score transformed with log(x+1). BDI-II = Beck Depression Inventory–II; CI = confidence interval.
† Estimates were tested for H0: estimate = 0 with a t statistic, controlling for all other variables in the regression model; all tests were two-sided.
§ Scores were calculated by comparing participants’ scores against age-corrected normative scores with a mean of 0 and SD of 1, with positive (ie, > 0) scores representing higher/better performance.
|| BDI-II is calculated without the item pertaining to concentration.
Table 4 shows the model results for the PAOFI HLC subscale, with and without the BDI-II. In both models, poorer NP executive function performance was associated, though not significantly, with higher HLC complaints (P = .09 without and P = .07 with depressive symptoms in the regression model). In the base model, better NP visual memory (P = .01), combined chemotherapy and radiation (P = .03), and HT use (P = .01) were all statistically significantly associated with higher HLC complaints, with an adjusted R 2 of 0.21. When the BDI-II was added to the model, past HT use was no longer statistically significant (P = .09); however, better NP visual performance remained statistically significant (P = .03), whereas combined chemotherapy and radiation treatment (P = .05) only approached statistical significance, and the BDI-II was statistically significantly associated (P < .001), increasing the model adjusted R 2 to 0.34.
Table 4.
Linear regression models Predicting Patient’s Assessment of Own Functioning Inventory (PAOFI) higher-level cognition (HLC) (n = 182)*
| Base model adjusted R 2 = 0.21 | Base model plus BDI-II adjusted R 2 = 0.34 | |||
|---|---|---|---|---|
| Variable | Estimate (95% CI) | P† | Estimate (95% CI) | P† |
| Age | 0.002 (–0.008 to 0.013) | .691 | 0.007 (–0.003 to 0.017) | .145 |
| IQ | –0.003 (–0.012 to 0.007) | .582 | –0.002 (–0.010 to 0.006) | .659 |
| Treatment | ||||
| Radiation | 0.030 (–0.204 to 0.265) | .799 | 0.064 (–0.151 to 0.278) | .558 |
| Chemotherapy | 0.282 (–0.019 to 0.584) | .066 | 0.151 (–0.128 to 0.429) | .286 |
| Chemotherapy & radiation | 0.258 (0.029 to 0.486) | .027 | 0.208 (–0.001 to 0.417) | .051 |
| Hormone therapy | 0.234 (0.047 to 0.421) | .015 | 0.150 (–0.023 to 0.323) | .089 |
| Visual memory§ | 0.135 (0.037 to 0.233) | .007 | 0.101 (0.011 to 0.191) | .029 |
| Psychomotor speed§ | –0.080 (–0.236 to 0.077) | .315 | –0.037 (–0.181 to 0.106) | .611 |
| Executive function§ | –0.126 (–0.271 to 0.019) | .088 | –0.120 (–0.252 to 0.012) | .074 |
| BDI-II|| | ----- | ----- | 0.035 (0.023 to 0.046) | <.001 |
* PAOFI HLC score transformed with log(x+1). BDI-II = Beck Depression Inventory–II; CI = confidence interval.
† Estimates were tested for H0: estimate=0 with a t statistic, controlling for all other variables in the regression model; all tests were two-sided.
§ Scores were calculated by comparing participants’ scores against age-corrected normative scores with a mean of 0 and SD of 1, with positive (ie, > 0) scores representing higher/better performance.
|| BDI-II is calculated without the item pertaining to concentration.
A final regression was conducted that included the two BDI-II subscales instead of the BDI-II composite score (data not shown). For both memory and HLC models, the parameter estimates for the NP tests were statistically similar to the estimates in the regressions with the BDI-II composite score. The BDI-II cognitive/affective subscale was statistically significant for both PAOFI memory and HLC (P = .01 and P < .001, respectively), and the BDI-II somatic subscale was statistically significant for memory (P = .01) but not for HLC (P = 0.17).
To further refine our understanding of the contribution of each variable to these self-reported cognitive complaints, and in particular to assess whether any predictors were potentially redundant, stepwise linear regression models were examined (Supplementary Tables 4 and 5). For PAOFI memory complaints, we found that the NP verbal memory domain contributed 0.03 to the model’s variance (10% of the total variance) and the BDI-II somatic subscale contributed 0.13, explaining about 43% of the total variance with the cognitive/affective subscale contributing 0.03 or 7% of the total variance. For the PAOFI HLC model, the three NP domains together contributed 0.07 to the model (about 21% of the total variance) and the BDI–II somatic subscale contributed 0.08 to the model, (about 24% of the total variance), whereas the BDI-II cognitive/affective subscale contributed 0.06 to the model (16% of the variance).
Exploration of the Relationship Between Higher-Level Cognition Complaints and NP Visual Memory
Women who have used long-term postmenopausal HT have been reported to have better visual memory on NP testing and doing a visual memory task during brain imaging (34,35). In the MBS sample, past HT was associated with higher HLC complaints. Thus, we hypothesized that the finding of better visual memory in these women could be a result of the prior HT exposure, with higher executive function complaints at the end of treatment representing a perceived decline in NP visual memory as a result of discontinuation of HT at the time of breast cancer diagnosis, or an interaction with chemotherapy and radiation exposure. To test this hypothesis, we examined the trajectory of subsequent NP visual memory performance in the following year among the women with higher and normal HLC complaints. There were 34 high-HLC-complaint women and 140 normal-HLC-complaint women who were tested 12 months later, with about 70% of each group receiving adjuvant endocrine therapy in the following year (no statistically significant difference in use). For the high-HLC-complaint group, the unadjusted baseline mean NP visual memory domain score was 0.185 (95% CI = 0.059 to 0.428) and 12 months later it was –0.324 (95% CI = –0.600 to –0.048), for a mean within-subjects change of –0.509 (95% CI = –0.796 to –0.222, P = .001). For the normal-HLC-complaint group, the unadjusted mean NP visual memory domain score at baseline was –0.077 (95% CI = –0.217 to 0.063) and 12 months later was –0.063 (95% CI = –0.214 to 0.089), for a mean within-subjects change of –0.006 (95% CI = –0.177 to 0.164, P = .94).
Discussion
Cognitive complaints are common after breast cancer treatments, but prior research had not identified consistent association with NP test abnormalities (23). In this study, we investigated clinical and treatment factors likely to be associated with post–adjuvant treatment cognitive complaints. We identified two specific domains of cognitive complaints (memory and executive functioning) for examination based on comparison with data from a normative reference sample, and hypothesized that more precise evaluation of subjective complaints would facilitate identification of an association with domain-specific NP test abnormalities. Contrary to prediction, recent onset of amenorrhea was not associated with higher cognitive complaints in either domain, but past HT exposure was statistically significantly associated with executive function complaints. In multivariable models, poorer NP verbal memory performance was statistically significantly associated with higher memory complaints, whereas NP executive function was not statistically significantly associated with higher executive function complaints. Higher memory complaints were statistically significantly associated with the combination of chemotherapy and radiation treatment (not chemotherapy alone). Depressive symptoms as measured by the BDI-II were statistically significant in both the memory and executive complaint models; however, it should be noted that this was not a clinically depressed sample, and that the somatic subscale of the BDI-II (eg, sleep disturbance, loss of interest in sex, fatigue)—constitutional symptoms that are common after adjuvant therapy (36)—was only statistically significant in the memory complaint model.
Interestingly, higher executive function complaints were associated with past postmenopausal HT in the base model, but not when controlling for depressive symptoms. In the absence of precancer treatment NP evaluation of these women, we cannot fully evaluate the temporal relationship between these past exposures and the NP evaluation that was done after cancer treatment was concluded. However, a post hoc examination of the trajectory of subsequent NP visual memory scores over the course of the follow-up year comparing the high HLC complaint group to the normal HLC complaint group showed a statistically significant decline in NP visual memory scores among high complainers. Thus, it is possible that women with higher executive function complaints post–adjuvant treatment may be perceiving a changing performance with regard to visual memory, which plays a key role in learning, memory, and executive function. There is an executive function component to visual memory tasks such as the ones used in this study, and it may be that those who are particularly strong on such tasks are more sensitive to disruption or change in efficiency.
Our finding of statistically significantly better NP visual memory performance among women with executive function complaints is a novel finding, although a recent functional MRI study in women who had received long-term HT found enhanced activation during a visual working memory task (34,37). In addition, recent studies of women transitioning through natural menopause suggest that there may be a window of opportunity in which early initiation of HT may protect against elements of cognitive decline (38–41). One large cohort study of older French women examined lifetime estrogen exposure, reproductive factors, and cognitive function longitudinally and found that women on current HT performed statistically significantly better on a task of visual memory (35). Given that visual memory activity is localized to the nondominant hippocampus, a region of the brain known to be rich in hormone receptors, our finding of this association may have some biological significance; however, our cross-sectional findings of an association with executive function complaints may also be a chance finding without clinical significance.
Recent studies have now found that subjective cognitive complaints in midlife women going through the menopausal transition are associated with objective NP test abnormalities, as well as menopausal and depressive symptoms (42,43). Of note, both of these studies used self-report scales that focused on everyday function and behaviors that require attention and memory. A brain imaging study of healthy postmenopausal midlife women used functional MRI and a working-memory task to examine brain activity among women with increased cognitive complaints compared to women who did not endorse cognitive complaints on a screening questionnaire (44). Although no statistically significant differences were found between the two groups on an extensive NP test battery or other variables, those with increased cognitive complaints showed greater activation in a broad network involved in working memory during an increasingly difficult memory task, compared to noncomplainers. The authors concluded that subjective cognitive complaints in postmenopausal women might be associated with increased cortical activity during the effort of demanding cognitive tasks (44).
There has been much interest in developing a better understanding of the biological mechanisms associated with objective and subjective changes in cognitive function after a cancer diagnosis and treatment (45–47). We have previously reported an association between memory complaints, an increase in the soluble tumor necrosis alpha (TNF-α) receptor II, and changes in brain metabolism on positron emission tomographic imaging, in an earlier longitudinal report from the MBS study that was focused on chemotherapy exposure (24). Kesler et al. (48) have found a statistically significant association between increased cognitive complaints, increased plasma cytokines (including TNF-α), decreased hippocampal volume, and verbal memory performance in long-term breast cancer survivors compared to controls. These two studies (24,48) provide support for an underlying proinflammatory biological mechanism for the relationship between breast cancer treatments and subjective cognitive complaints, as proposed by Miller et al. (49), and would be consistent with the biology of persistent fatigue in breast cancer patients (50). Our finding that cognitive complaints were most strongly associated with the combination of chemotherapy and radiation suggests their potential synergy in increasing posttreatment inflammation that may be an etiology for subjective cognitive complaints.
The strengths of this study include its large sample size, thorough evaluation of cognitive complaints with use of a domain-specific approach to examining associations with a comprehensive NP test battery, and careful evaluation of potential treatment exposures, including changes in menstrual status and past HT exposure. Limitations include the exclusion of older women (>65 years), women with chronic inflammatory conditions, and those with active clinical depression, which were ineligibility criteria required to reduce confounding for other study endpoints focused on inflammatory biology and posttreatment symptoms (24,26). These analyses are also limited by their cross-sectional nature, with the exception of our exploration of subsequent changes in visual memory NP testing among women with and without higher executive function complaints.
Despite skepticism regarding the validity of cognitive complaints and their relationship to NP test performance in cancer patients (23), this study suggests that patient self-report is associated with relevant domains of NP function, which is strongest for memory complaints. There is an emerging literature supporting the ability of individuals to subjectively detect changes in cognitive function that precedes statistically significant changes in NP performance or structural imaging abnormalities (51). Our findings add further support for the value of patient-reported outcomes as a central measurement in evaluation of cancer treatment-related morbidities (52). We encourage continued research on the biopsychosocial factors associated with subjective cognitive complaints in cancer survivors.
Funding
This research was supported by funding from the National Cancer Institute R01 CA 109650 and the Breast Cancer Research Foundation (to PAG); the National Institutes of Health and National Cancer Institute R01-AG034588; R01-AG026364; R01-CA119159; R01-HL079955; R01 HL095799; P30-AG028748; UL RR 033176 (to MRI), and the Cousins Center for Psychoneuroimmunology (to MRI); the National Cancer Institute R01CA112035 (to SA-I).
Supplementary Material
The study sponsors had no role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication. SA-I is a consultant for the following companies: AstraZeneca, Ferring Pharmaceuticals Inc, GlaxoSmithKline, Hypnocore, Johnson & Johnson, Merck, NeuroVigil, Inc, Orphagen Pharmaceuticals, Pfizer, Philips, Purdue Pharma LP, and Sanofi-Aventis. We express our appreciation to the women who volunteered for this study, as well as the Los Angeles County SEER registry and the many Los Angeles–area physicians for assisting us in recruitment.
References
- 1. Meyers CA. Neurocognitive dysfunction in cancer patients. Oncology (Wiliston Park). 2000; 14(1):75–79 [PubMed] [Google Scholar]
- 2. Butler RW, Hill JM, Steinherz PG, Meyers PA, Finlay JL. Neuropsychologic effects of cranial irradiation, intrathecal methotrexate, and systemic methotrexate in childhood cancer. J Clin Oncol. 1994; 12(12):2621–2629 [DOI] [PubMed] [Google Scholar]
- 3. Meyers CA. How chemotherapy damages the central nervous system. J Biol. 2008; 7(4):11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wefel JS, Witgert ME, Meyers CA. Neuropsychological sequelae of non-central nervous system cancer and cancer therapy. Neuropsychol Rev. 2008; 18(2):121–131 [DOI] [PubMed] [Google Scholar]
- 5. Ahles TA, Saykin AJ, Furstenberg CT, et al. Neuropsychologic impact of standard-dose systemic chemotherapy in long-term survivors of breast cancer and lymphoma. J Clin Oncol. 2002; 20(2):485–493 [DOI] [PubMed] [Google Scholar]
- 6. Schagen SB, van Dam FS, Muller MJ, Boogerd W, Lindeboom J, Bruning PF. Cognitive deficits after postoperative adjuvant chemotherapy for breast carcinoma. Cancer. 1999; 85(3):640–650 [DOI] [PubMed] [Google Scholar]
- 7. Schagen SB, Hamburger HL, Muller MJ, Boogerd W, van Dam FS. Neurophysiological evaluation of late effects of adjuvant high-dose chemotherapy on cognitive function. J Neurooncol. 2001; 51(2):159–165 [DOI] [PubMed] [Google Scholar]
- 8. Schagen SB, van Dam FS, Muller MJ, Boogerd W, Lindeboom J, Bruning PF. Cognitive deficits after postoperative chemotherapy for breast carcinoma. Cancer. 1999; 85(3):640–650 [DOI] [PubMed] [Google Scholar]
- 9. van Dam FS, Schagen SB, Muller MJ, et al. Impairment of cognitive function in women receiving adjuvant treatment for high-risk breast cancer: high-dose versus standard-dose chemotherapy. J Natl Cancer Inst. 1998; 90(3):210–218 [DOI] [PubMed] [Google Scholar]
- 10. Schagen SB, Muller MJ, Boogerd W, et al. Late effects of adjuvant chemotherapy on cognitive function: a follow-up study in breast cancer patients. Ann Oncol. 2002; 13(9):1387–1397 [DOI] [PubMed] [Google Scholar]
- 11. Kreukels BP, Schagen SB, Ridderinkhof KR, Boogerd W, Hamburger HL, van Dam FS. Electrophysiological correlates of information processing in breast-cancer patients treated with adjuvant chemotherapy. Breast Cancer Res Treat. 2005; 94:53–61 [DOI] [PubMed] [Google Scholar]
- 12. Wefel JS, Lenzi R, Therlault RL, Davis RN, Meyers CA. The cognitive sequelae of standard-dose adjuvant chemotherapy in women with breast carcinoma. Cancer. 2004; 100(11):2292–2299 [DOI] [PubMed] [Google Scholar]
- 13. Brezden CB, Phillips KA, Abdolell M, Bunston T, Tannock IF. Cognitive function in breast cancer patients receiving adjuvant chemotherapy. J Clin Oncol. 2000; 18(14):2695–2701 [DOI] [PubMed] [Google Scholar]
- 14. Castellon SA, Ganz PA, Bower JE, Petersen LA, Abraham L, Greendale GA. Neurocognitive performance in breast cancer survivors exposed to adjuvant chemotherapy and tamoxifen. J Clin Exp Neuropsychol. 2004; 26(7):955–969 [DOI] [PubMed] [Google Scholar]
- 15. Jenkins V, Shilling V, Fallowfield L, Howell A, Hutton S. Does hormone therapy for the treatment of breast cancer have a detrimental effect on memory and cognition? A pilot study. Psychooncology. 2004; 13(1):61–66 [DOI] [PubMed] [Google Scholar]
- 16. Jenkins V, Shilling V, Deutsch G, et al. A 3-year prospective study of the effects of adjuvant treatments on cognition in women with early stage breast cancer. Br J Cancer. 2006; 94:828–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ganz PA. Cognitive dysfunction following adjuvant treatment of breast cancer: a new dose-limiting toxic effect? J Natl Cancer Inst. 1998; 90(3):182–183 [DOI] [PubMed] [Google Scholar]
- 18. Jim HSL, Phillips KM, Chait S, et al. Meta-analysis of cognitive functioning in breast cancer survivors previously treated with standard-dose chemotherapy. J Clin Oncol. 2012; 30(29):3578–3587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McDonald B, Conroy S, Ahles T, West J, Saykin A. Gray matter reduction associated with systemic chemotherapy for breast cancer: a prospective MRI study. Breast Cancer Res Treat. 2010; 123(3):819–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McDonald BC, Conroy SK, Ahles TA, West JD, Saykin AJ. Alterations in brain activation during working memory processing associated with breast cancer and treatment: a prospective functional magnetic resonance imaging study. J Clin Oncol. 2012; 30(20):2500–2508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Deprez S, Amant F, Smeets A, et al. Longitudinal assessment of chemotherapy-induced structural changes in cerebral white matter and its correlation with impaired cognitive functioning. J Clin Oncol. 2012; 30(3):274–281 [DOI] [PubMed] [Google Scholar]
- 22. Kesler SR, Kent JS, O’Hara R. Prefrontal cortex and executive function impairments in primary breast cancer. Arch Neurol. 2011; 68(11):1447–1453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pullens MJJ, De Vries J, Roukema JA. Subjective cognitive dysfunction in breast cancer patients: a systematic review. Psycho-Oncology. 2010; 19(11):1127–1138 [DOI] [PubMed] [Google Scholar]
- 24. Ganz PA, Bower JE, Kwan L, et al. Does tumor necrosis factor-alpha (TNF-α) play a role in post-chemotherapy cerebral dysfunction? Brain Behav Immun. 2013; 30(suppl):S99–S108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ganz PA, Kwan L, Stanton AL, Bower JE, Belin TR. Physical and psychosocial recovery in the year after primary treatment of breast cancer. J Clin Oncol. 2011; 29(9):1101–1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bower JE, Ganz PA, Irwin MR, Kwan L, Breen EC, Cole SW. Inflammation and behavioral symptoms after breast cancer treatment: do fatigue, depression, and sleep disturbance share a common underlying mechanism? J Clin Oncol. 2011; 29(26):3517–3522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Beck AT, Steer RA, Brown GK. BDI-II; Beck Depression Inventory. 2nd ed Boston, MA: Harcourt Brace Inc, 1996. [Google Scholar]
- 28. Chelune CJ, Heaton RK, Lehman RAW. Neuropsychological and personality correlates of patients’ complaints of disability. In: Goldstein G, Tarter RE, eds. Advances in Clinical Neuropsychology., Volume 3 New York, NY: Plenum Press; 1986:95–126 [Google Scholar]
- 29. Bender CM, Sereika SM, Berga SL, et al. Cognitive impairment associated with adjuvant therapy in breast cancer. Psychooncology. 2006; 15:422–430 [DOI] [PubMed] [Google Scholar]
- 30. Bender CM, Pacella ML, Sereika SM, et al. What do perceived cognitive problems reflect? J Support Oncol. 2008; 6:238–242 [PMC free article] [PubMed] [Google Scholar]
- 31. Liu L, Rissling M, Neikrug A, et al. Fatigue and circadian activity rhythms in breast cancer patients before and after chemotherapy: a controlled study. Fatigue. 2012; 1(1–2): 12–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stein KD, Jacobsen PB, Blanchard CM, Thors C. Further validation of the multidimensional fatigue symptom inventory-short form. J Pain Symptom Manage. 2004; 27(1):14–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tulsky DS, Price LR. The joint WAIS-III and WMS-III factor structure: development and cross-validation of a six-factor model of cognitive functioning. Psychol Assess. 2003; 15(2):149–162 [DOI] [PubMed] [Google Scholar]
- 34. Berent-Spillson A, Persad CC, Love T, et al. Early menopausal hormone use influences brain regions used for visual working memory. Menopause. 2010; 17(4):692–699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ryan J, Carrière I, Scali J, Ritchie K, Ancelin ML. Life-time estrogen exposure and cognitive functioning in later life. Psychoneuroendocrinology. 2009; 34(2):287–298 [DOI] [PubMed] [Google Scholar]
- 36. Ganz PA, Kwan L, Stanton AL, et al. Quality of life at the end of primary treatment of breast cancer: first results from the moving beyond cancer randomized trial. J Natl Cancer Inst. 2004; 96(5):376–387 [DOI] [PubMed] [Google Scholar]
- 37. Henderson VW, Greicius MD. Functional magnetic resonance imaging and estrogen effects on the brain: cautious interpretation of a BOLD finding. Menopause. 2010; 17(4):669–671 [DOI] [PubMed] [Google Scholar]
- 38. Greendale GA, Wight RG, Huang MH, et al. Menopause-associated symptoms and cognitive performance: results from the study of women’s health across the nation. Am J Epidemiol. 2010; 171(11):1214–1224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Greendale GA, Derby CA, Maki PM. Perimenopause and cognition. Obstet Gynecol Clin N Am. 2011; 38(3):519–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Boulware M, Kent B, Frick K. The impact of age-related ovarian hormone loss on cognitive and neural function. In: Pardon MC, Bondi MW, eds. Behavioral Neurobiology of Aging. 10th ed Berlin, Germany: Springer; 2012:165–184 [DOI] [PubMed] [Google Scholar]
- 41. Maki PM, Dennerstein L, Clark M, et al. Perimenopausal use of hormone therapy is associated with enhanced memory and hippocampal function later in life. Brain Res. 2011; 1379(0):232–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Schaafsma M, Homewood J, Taylor A. Subjective cognitive complaints at menopause associated with declines in performance of verbal memory and attentional processes. Climacteric. 2010; 13(1):84–98 [DOI] [PubMed] [Google Scholar]
- 43. Weber MT, Mapstone M, Staskiewicz J, Maki PM. Reconciling subjective memory complaints with objective memory performance in the menopausal transition. Menopause. 2012; 19(7):735–741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dumas JA, Kutz AM, McDonald BC, et al. Increased working memory-related brain activity in middle-aged women with-cognitive complaints. Neurobiol Aging. 2013; 34(4):1145–1147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ahles TA, Saykin AJ. Candidate mechanisms for chemotherapy-induced cognitive changes. Nat Rev Cancer. 2007; 7(3):192–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Seruga B, Zhang H, Bernstein LJ, Tannock IF. Cytokines and their relationship to the symptoms and outcome of cancer. Nat Rev Cancer. 2008; 8(11):887–899 [DOI] [PubMed] [Google Scholar]
- 47. Ahles TA. Brain vulnerability to chemotherapy toxicities. Psychooncology. 2012; 21(11):1141–1148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kesler S, Janelsins M, Koovakkattu D, et al. Reduced hippocampal volume and verbal memory performance associated with interleukin-6 and tumor necrosis factor-alpha levels in chemotherapy-treated breast cancer survivors [published online ahead of print]. Brain Behav Immun. 2013; 30(suppl):S109–S116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Miller AH, Ancoli-Israel S, Bower JE, Capuron L, Irwin MR. Neuroendocrine-immune mechanisms of behavioral comorbidities in patients with cancer. J Clin Oncol. 2008; 26(6):971–982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bower JE, Lamkin DM. Inflammation and cancer-related fatigue: mechanisms, contributing factors, and treatment implications. Brain Behav Immun. 2013; 30(suppl):S48–S57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Saykin AJ, Wishart HA, Rabin LA, et al. Older adults with cognitive complaints show brain atrophy similar to that of amnestic MCI. Neurology. 2006; 67(5):834–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ganz PA. Doctor, Will the treatment you are recommending cause chemobrain? J Clin Oncol. 2012; 30(3):229–231 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.