Abstract
Background
Vascular endothelial growth factor (VEGF) is known to be a potent promoter of angiogenesis under both physiological and pathological conditions. Given its role in regulating tumor vascularization, VEGF has been targeted in various cancer treatments, and anti-VEGF therapy has been used clinically for treatment of several types of cancer. Systems biology approaches, particularly computational models, provide insight into the complexity of tumor angiogenesis. These models complement experimental studies and aid in the development of effective therapies targeting angiogenesis.
Methods
We developed an experiment-based, molecular-detailed compartment model of VEGF kinetics and transport to investigate the distribution of two major VEGF isoforms (VEGF121 and VEGF165) in the body. The model is applied to predict the dynamics of tumor VEGF and, importantly, to gain insight into how tumor VEGF responds to an intravenous injection of an anti-VEGF agent.
Results
The model predicts that free VEGF in the tumor interstitium is seven to 13 times higher than plasma VEGF and is predominantly in the form of VEGF121 (>70%), predictions that are validated by experimental data. The model also predicts that tumor VEGF can increase or decrease with anti-VEGF treatment depending on tumor microenvironment, pointing to the importance of personalized medicine.
Conclusions
This computational study suggests that the rate of VEGF secretion by tumor cells may serve as a biomarker to predict the patient population that is likely to respond to anti-VEGF treatment. Thus, the model predictions have important clinical relevance and may aid clinicians and clinical researchers seeking interpretation of pharmacokinetic and pharmacodynamic observations and optimization of anti-VEGF therapies.
Vascular endothelial growth factor (VEGF) promotes various processes involved in angiogenesis, including endothelial cell proliferation, adhesion, migration, and chemotaxis (1). Angiogenesis is a hallmark of cancer (2) and has been targeted by various cancer therapies, with a focused effort on drugs that inhibit VEGF. Several antiangiogenic agents have been approved by the US Food and Drug Administration (FDA) to treat various cancers and other diseases. Bevacizumab (Genentech, South San Francisco, CA), a recombinant humanized monoclonal antibody to VEGF, is approved for the treatment of metastatic colorectal and kidney cancer, glioblastoma, and non–small cell lung cancer. Ziv-aflibercept (Regeneron, Tarrytown, NY), a soluble decoy receptor for VEGF, is an FDA-approved agent for the treatment of metastatic colorectal cancer and is currently in clinical trials for the treatment of several other cancer types. Other FDA-approved antiangiogenic cancer therapeutics include axitinib, pazopanib, regorafenib, sorafenib, and sunitinib. These agents are small molecule kinase inhibitors with various targets such as VEGF receptors, platelet-derived growth factor receptors, fibroblast growth factor receptors, and Raf kinase.
Systems biology approaches are useful in gaining a broader understanding of the complexity of angiogenesis. Computational models can be applied to generate and test biological hypotheses and can aid in the development of effective therapies that target angiogenesis (3). Additionally, models can provide a framework to predict promising drug targets and identify patient populations that will respond to a particular therapy.
We have developed a molecular-detailed compartment model that is useful in understanding VEGF dynamics in the body. The model is based on detailed biochemical kinetics and molecular transport and has been validated against available experimental data. It is a predictive tool that can provide insight into the distribution of VEGF in the body and the effects of systemic administration of anti-VEGF therapeutics, such as bevacizumab and aflibercept. We have applied the model to understand and explain clinical observations of anti-VEGF agents (4) and predict the effect of the drugs (5,6). Here, we present three important model predictions regarding the pretreatment levels of VEGF121 and VEGF165 and the dynamic response of plasma and tumor VEGF to anti-VEGF treatment. We compare our results with available experimental data and propose clinical applications of the model predictions.
Methods
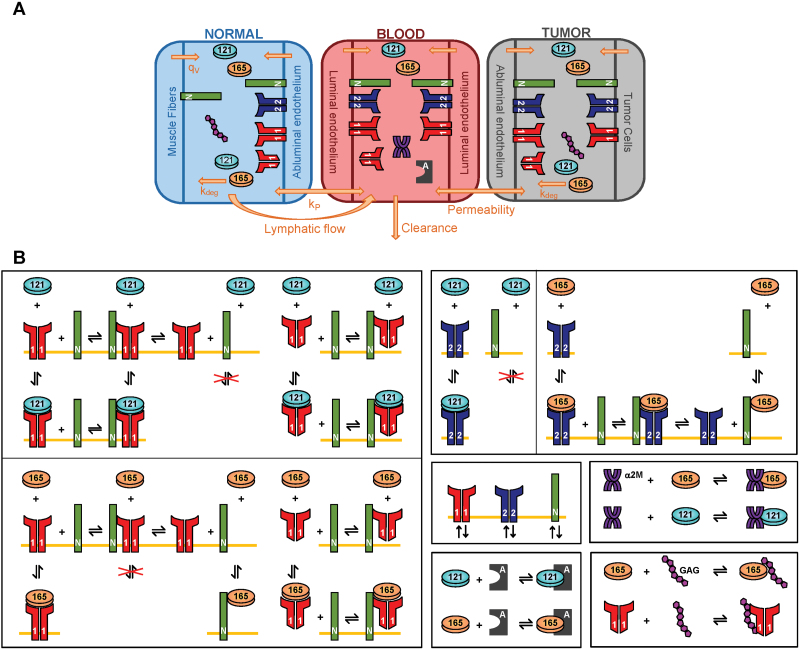
The whole-body model includes normal tissue (“normal compartment,” represented by skeletal muscle), the vasculature (“blood compartment”), and diseased tissue (“tumor compartment”) and has been described in our previous articles (5,6). The normal and tumor compartments consist of parenchymal and endothelial cells and interstitial space (Figure 1A). We include molecular interactions between two major VEGF isoforms (VEGF121 and VEGF165), VEGF receptors (VEGFR1 and VEGFR2), and coreceptor neuropilins (NRP1 and NRP2) (Figure 1B). In this study, we also include VEGF interactions with two soluble factors: soluble VEGFR1 (sVEGFR1) and α-2-macroglobulin (α2M), introduce VEGF secretion by endothelial cells, and modify the permeability between the blood and tumor. The tumor is parameterized as a breast tumor with a volume of 33cm3; however, the model is broadly applicable to any solid tumor. Model elements reflect quantitative experimental characterization of the VEGF system. The model is described in detail in the Supplementary Methods (available online).
Figure 1.
Molecular-detailed compartmental model of vascular endothelial growth factor (VEGF) kinetics and transport in the body. A) The model includes three compartments: normal tissue, blood, and tumor tissue. VEGF is secreted by muscle fibers and tumor cells in the normal tissue and tumor, respectively (qv). VEGF receptors are localized on the luminal and abluminal endothelial surfaces and tumor cells. Only neuropilin 1 (NRP1) is present on muscle fibers. Free and ligand-bound receptors can be internalized (kint). Transport between compartments occurs by transendothelial permeability (kp) and lymph flow (kL). VEGF is also cleared from the blood (cv). B) VEGF165 binds to VEGF receptor 1 (VEGFR1), VEGF receptor 2 (VEGFR2), and coreceptors NRP1 and neuropilin 2 (NRP2), as well as glycosaminoglycan (GAG) chains in the extracellular matrix and basement membranes. VEGF121 binds to VEGFR1 and VEGFR2, but does not bind NRPs. Binding between VEGF and NRP1 or NRP2 follow the same reactions but is governed by different kinetic parameters. Receptors and coreceptors are internalized at the cell surface. The anti-VEGF agent binds to both isoforms.
The model predicts the concentration of 154 species using 154 ordinary differential equations. We are able to predict VEGF level in the multiple tissues in the body as well as the distribution of VEGF in the form of unbound ligand or matrix- and receptor-bound complexes. The predicted levels of free VEGF and sVEGFR1 in muscle interstitium (7–13) and plasma (14–19) are within the range of experimental data (Table 1).
Table 1.
Comparison of predicted and experimental steady state vascular endothelial growth factor (VEGF) and soluble VEGF receptor 1 (VEGFR1) levels
| Species | Predicted concentration, pM | Range of experimental measurements | References |
|---|---|---|---|
| Free VEGF | |||
| Normal, muscle | 3.2 | 0.3– 3.0 | (7–12) |
| Plasma | 2.0 | 0.4– 3.0 | (15) |
| Tumor | 26.0 | 8.0– 389.0 | * |
| Soluble VEGFR1 | |||
| Normal, muscle | 0.3 | 0.5 | (13) |
| Plasma | 3.4 | 0.2– 3.0 | (14,16–19) |
| Tumor | 23.0 | † | † |
* See Supplementary Table 1 (available online).
† No data available.
We apply the model to predict the effect of VEGF-neutralizing agents used in cancer therapy. We use the kinetic parameters for bevacizumab in our simulations, although the model is applicable to other anti-VEGF macromolecules. We assume that an indicator of the success of anti-VEGF treatment is a reduction in tumor interstitial VEGF. Therefore, we quantify the response to anti-VEGF therapy by calculating the fold-change, which is the level of free VEGF in the tumor interstitium at 3 weeks after administration of the anti-VEGF agent divided by the pretreatment level. A fold-change of less than one indicates that free VEGF in the tumor decreases after treatment and is termed a therapeutic effect; conversely, a fold-change larger than one indicates a countertherapeutic effect.
Results
Concentration of Free VEGF in the Tumor Interstitium
The model predicts that at steady state the concentration of free VEGF in the tumor interstitium will be approximately 26 pM (1125 pg/mL), based on microvascular permeability between the tumor and blood on the order of 10−7 cm/s, termed low permeability. In the case of high permeability on the order of 10−5 cm/s, based on the data in Chauhan et al. (20), free VEGF in the tumor is predicted to be 13 pM (585 pg/mL). Plasma VEGF is approximately 2 pM in cancer patients (15). Thus, the predicted level of free VEGF in the tumor is an order of magnitude (ie, 7 to 13 times) larger than the level of plasma VEGF.
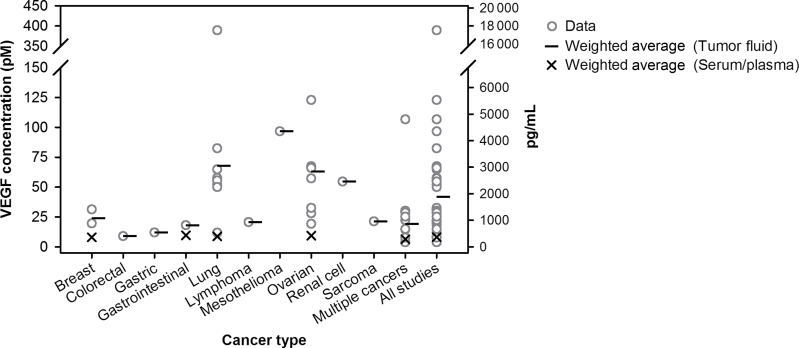
The predicted ratio of tumor interstitial VEGF to plasma VEGF compares well with available experimental data. Although little if any quantitative data are available for the concentration of VEGF in the tumor interstitial fluid, numerous studies have quantified VEGF in tumor ascites fluid and pleural effusions (21−46), which contain proteins derived from the extracellular compartment (47) and should reflect the level of free VEGF. A compilation of these studies is presented in Supplementary Table 1 (available online). Some of the studies also report the concentration of VEGF in the serum or plasma of the patients. These data show that free VEGF in the tumor is two to 10 times larger than the VEGF concentration in serum or plasma across several cancer types (Figure 2). In contrast, microdialysis measurements of tumor interstitial fluid show that tumor extracellular VEGF is approximately 1 pM (48); however, this method may underestimate VEGF because it is unable to completely recover high molecular weight compounds (49). Thus, our model predictions that free VEGF in the tumor can be seven to 13 times higher than plasma VEGF agree with experimental evidence from tumor fluid. Although the experimental data are highly variable and may be subject to artifacts intrinsic to microdialysis and enzyme-linked immunosorbent assay (ELISA) methods, they provide a basis for validating the model predictions.
Figure 2.
Vascular endothelial growth factor (VEGF) levels in cancer patients. VEGF levels in the tumor fluid (ascites or pleural effusions) of cancer patients (open circles). Black lines and “X” denote the weighted average for tumor and blood VEGF, respectively. Additional details of the studies are given in Supplementary Table 1 (available online).
Predominant Isoform in the Tumor Interstitium
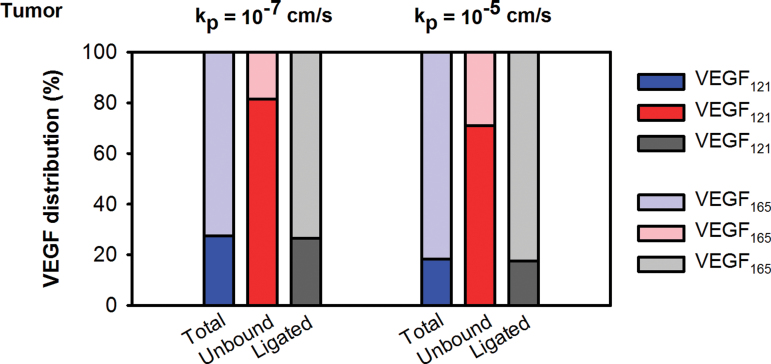
In our model, the VEGF isoforms VEGF121 and VEGF165 are secreted by tumor cells in equal amounts (6) based on experimental data (50–53). Notably, this ratio of VEGF121 to VEGF165 secretion is different from that in skeletal muscle, where it is heavily skewed toward the secretion of the heparin-binding isoform, approximately 8:92 (54). Despite the 50:50 tumor secretion ratio, the model predicts that VEGF121 accounts for 81% or 71% of free VEGF in the tumor interstitium for low and high tumor permeability, respectively (Figure 3). This prediction is a direct result of the different binding profiles of the isoforms. VEGF165 is able to bind to glycosaminoglycan (GAG) chains in the extracellular matrix (ECM) and basement membranes (55) and can directly bind to NRPs (56), which are highly expressed on endothelial and parenchymal cells. In contrast, VEGF121 cannot be sequestered by the ECM or basement membranes (55) and does not directly bind to NRPs. For these reasons, a large percentage of free VEGF is VEGF121.
Figure 3.
Predictions from compartment model of vascular endothelial growth factor (VEGF) distribution in the body. The VEGF isoforms are present at different relative ratios in total, unbound, and receptor-bound forms (ligated) of VEGF in the tumor.
Experimental measurements of VEGF at the protein level support the model predictions. Poon et al. showed that VEGF165 accounts for 27% of total VEGF (free and bound) in the tumor cytosol for hepatocellular carcinoma patients (57,58). Additionally, a novel ELISA enables quantification of specific VEGF isoforms and was applied to measure the shorter, soluble VEGF isoforms VEGF110 and VEGF121 in tumor lysates from patients with ovarian cancer (59). The data from that study (59) showed that the mean relative amount of VEGF165 in tumor lysates ranged from 24% to 31%. These data suggest that VEGF165 is not the predominant isoform in the tumor and support the model predictions.
Tumor Interstitial Free VEGF After Administration of Bevacizumab
We are interested in predicting the levels of free VEGF in the body after anti-VEGF treatment. Counterintuitively, measurements of VEGF levels in plasma show that circulating VEGF increases after treatment (60–62). We previously applied the model to investigate the mechanism by which plasma VEGF increases after treatment and predicted that the increase is due to the intercompartment transport of VEGF, anti-VEGF, and the VEGF/anti-VEGF complex (4). The tumor model also predicted that free VEGF in the tumor decreased with anti-VEGF treatment (4). Based on those results, we hypothesized that the mechanism of action of anti-VEGF agents is to deplete tumor VEGF rather than circulating intravascular VEGF. It is important to neutralize free VEGF in the tumor because this pool of VEGF can stimulate angiogenesis by binding to and activating abluminal endothelial cell receptors in the tumor. It is also possible for VEGF to promote tumor growth by activating receptors localized on tumor cells, which may or may not be angiogenesis dependent (63,64). Additionally, VEGF can stimulate intracellular pools of VEGFR1 and VEGFR2 by intracrine signaling (65,66).Therefore, we have investigated the response of tumor VEGF to anti-VEGF treatment while varying properties of the tumor microenvironment.
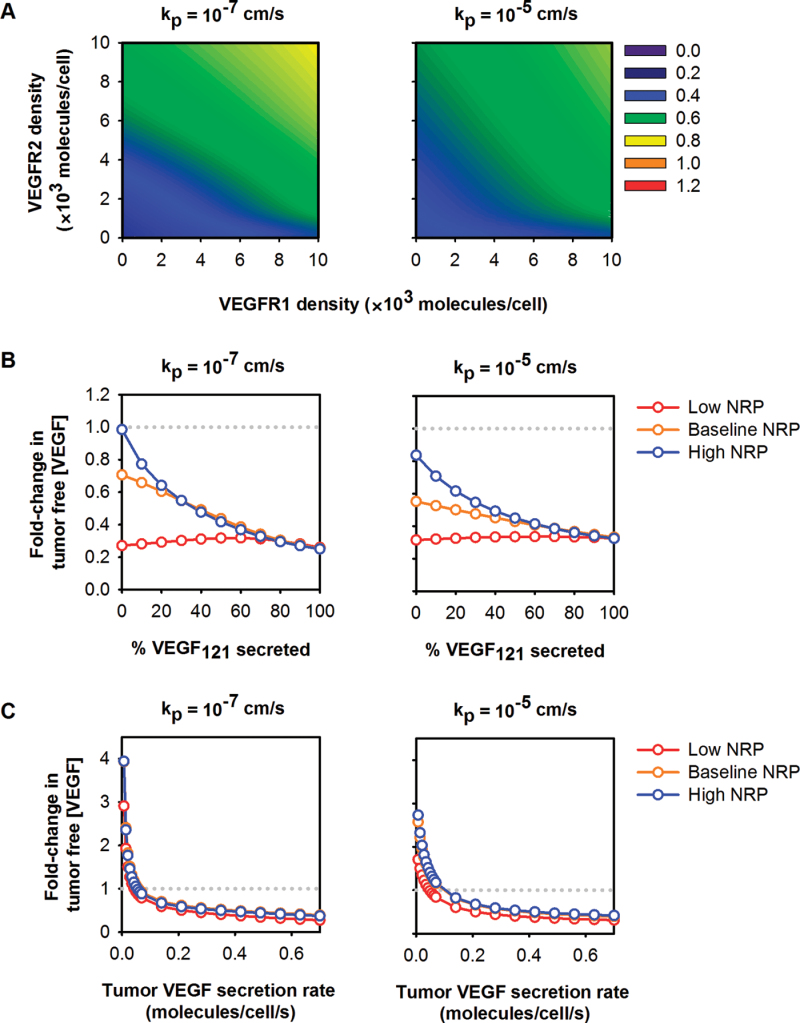
The model predicts that tumor-specific properties influence the behavior of free VEGF in the tumor interstitium after anti-VEGF treatment (6). We have varied the density of VEGF receptors and coreceptors on tumor cells and investigated how receptor density influences the response to anti-VEGF therapy. The model predicts that the response of tumor VEGF to the anti-VEGF treatment is robust across a wide range of receptor expression levels, where free VEGF in the tumor is depleted after treatment (Figure 4A).
Figure 4.
Response of free vascular endothelial growth factor (VEGF) in the tumor to anti-VEGF treatment. A) The fold-change in free VEGF concentration is predicted to be dependent on VEGF receptor 1 (VEGFR1), VEGF receptor 2 (VEGFR2), and neuropilin (NRP) expression on tumor cells for low and high permeability between the blood and tumor. NRP1 = NRP2 = 39 500 molecules/tumor cell. Colors and numbers represent the fold-change in free VEGF in the tumor. A fold-change of less than one indicates a therapeutic response to treatment (ie, tumor interstitial VEGF is reduced). B) The fold-change in free VEGF concentration after anti-VEGF treatment is dependent on the relative amount of VEGF121 secreted by tumor cells and NRP expression levels. C) The absolute rate of VEGF secretion by tumor cells influences the concentration of free VEGF in the tumor after anti-VEGF treatment. For (B) and (C), red: low NRP = 5000 molecules/tumor cell; yellow: baseline NRP = 39 500 molecules/tumor cell; blue: high NRP = 100 000 molecules/tumor cell. The gray dotted line indicates the boundary between therapeutic and countertherapeutic effects.
VEGF levels in the tumor after anti-VEGF therapy are sensitive to the relative rate at which the VEGF isoforms are secreted by tumor cells. We varied the relative amount of VEGF121 secreted by tumor cells from zero to 100% to determine how this tumor-specific property affects the level of free VEGF in the tumor after anti-VEGF treatment. Model simulations indicate that the VEGF isoform secretion ratio in the tumor impacts the response to anti-VEGF treatment (Figure 4B). In all cases, free VEGF in the tumor is predicted to decrease after treatment. Interestingly, for tumors that secrete only VEGF165 and have high NRP expression, free VEGF in the tumor is nearly unchanged 3 weeks after the anti-VEGF is administered, as compared with the pretreatment level.
The response of free VEGF in the tumor depends on the absolute rate of VEGF secretion by tumor cells. We varied the rate of VEGF secretion in the tumor from the baseline value, which was set by in vitro measurements, while keeping the isoform secretion ratio at 50:50. Counterintuitively, the model predicted that free VEGF in the tumor can increase after treatment, specifically in tumors that secrete low amounts of VEGF (Figure 4C).
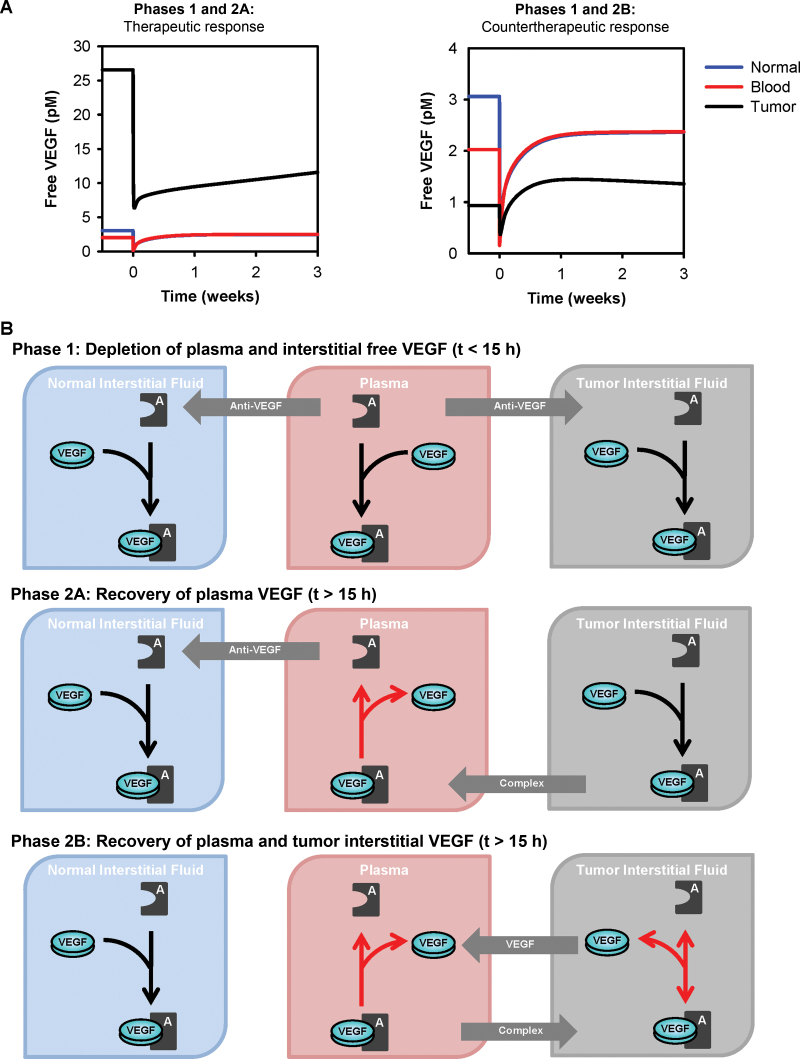
Our simulation results indicate that tumor VEGF can decrease after treatment and identifies conditions under which free VEGF in both plasma and tumor increase with anti-VEGF treatment (Figure 5A). The tumor response to anti-VEGF treatment can be interpreted with a detailed quantitative analysis of the intercompartmental flows and VEGF/anti-VEGF binding reaction, which is divided into two temporal phases (Figure 5B). In the first phase after injection of the anti-VEGF, the drug binds to free VEGF in the plasma, leading to a depletion of plasma VEGF. Unbound anti-VEGF extravasates to the tissue compartments, where it binds to and depletes interstitial VEGF. There are two possibilities for the second phase. In the first, termed phase 2A, the anti-VEGF/VEGF complex in the tissue compartments is transported into the blood. Because of the species concentrations and reversal of the direction of the anti-VEGF binding reaction in the plasma, free VEGF accumulates in the plasma, leading to an increase in plasma VEGF above the pretreatment level. Alternatively, in phase 2B, plasma VEGF increases above the pretreatment level, and tumor VEGF can increase above the pretreatment level. The increase in free VEGF in the tumor is due to extravasation of free VEGF and the anti-VEGF/VEGF complex to the tumor, combined with a net unbinding of the complex in the tumor. These phenomena result from concentration differences among free VEGF, free anti-VEGF, and the anti-VEGF/VEGF complex in the tumor and blood. In summary, the mechanisms by which free VEGF in plasma and tumor increase above the pretreatment level are explained by biochemical and transport processes.
Figure 5.
Response to anti–vascular endothelial growth factor (VEGF) treatment. A) Concentration profiles for free VEGF after intravenous injection of the anti-VEGF agent. Left panel shows therapeutic response to the drug, where the level of free VEGF in the tumor at 3 weeks is decreased, as compared with the pretreatment level. Right panel shows countertherapeutic response, where administration of the drug leads to an increase in free VEGF in the tumor. B) Dynamic levels of free VEGF can be explained by the intercompartment flows and direction of VEGF/anti-VEGF binding reaction. Phase 1: After intravenous administration of the anti-VEGF agent, the agent binds free VEGF in the plasma, leading to a substantial depletion of plasma VEGF. Unbound anti-VEGF partially extravasates to the tissue compartments to bind and deplete interstitial VEGF. In the subsequent phases, both plasma and tumor VEGF begin to increase. Phase 2A: The anti-VEGF/VEGF complex intravasates from the tissue compartments, and the direction of the anti-VEGF binding reaction in the plasma reverses (indicated by red arrow), leading to an increase in plasma VEGF above the pretreatment level. Phase 2B: Extravasation of free VEGF and the anti-VEGF/VEGF complex from blood to tumor, along with a reversal of the direction of the anti-VEGF binding reaction in the tumor leads to an increase in tumor VEGF. Depending on the initial level of interstitial tumor VEGF (determined by the tumor microenvironment), these factors could lead to an increase above the pretreatment level. Plasma VEGF increases above the pretreatment level.
There is a scarcity of data on the response of tumor VEGF to anti-VEGF treatment with which to compare the model results. Studies performed in mice have conflicting results (67–70). To the best of our knowledge, the level of VEGF in tumor tissue, ascites fluid, or pleural effusions in human patients after administration of an anti-VEGF agent has not been measured experimentally. Thus, it is of great interest to quantify human tumor VEGF after anti-VEGF treatment because this would shed light on the mechanism of action of the anti-VEGF agent.
Discussion
Model Limitations
We have not considered the role of additional VEGF isoforms such as VEGF189, which has been shown to be involved in tumor angiogenesis (71) or VEGFxxxb isoforms, which modulate vascularization and can be antiangiogenic (72–75) or weakly proangiogenic (76). However, there is limited quantitative information available on the rate at which these isoforms are secreted by parenchymal cells. Additionally, we have not included blood platelets, which are known to be a rich source of angiogenic factors (77,78) and are able to sequester bevacizumab (79). We assume the normal compartment is skeletal muscle because this composes a large percentage of the body weight in humans. Other tissues and organs have been shown to contain VEGF (80,81); however, quantitative experimental data for the geometric properties, VEGF secretion rate, and density of VEGF receptors in these tissues are largely unavailable. Lastly, although bevacizumab is given with chemotherapy for most cancer types, leading to a reduction in tumor size, we assume a constant tumor size of 33cm3. The model predicts that for a tumor that is 100 times larger (ie, 3300cm3), the concentration of free VEGF in the normal and tumor tissues increases by less than 1%. Plasma VEGF increases 1.5-fold. Therefore, we do not expect that simulating a tumor whose size is dynamic will qualitatively change the model predictions or the conclusions drawn.
The model can be further expanded to include the elements described above as quantitative experimental data become available. It is possible that the absence of one or more of these elements from the model contributes to the counterintuitive prediction that both plasma and tumor VEGF can increase after anti-VEGF treatment. However, we believe that the current model is a minimal, experiment-based model that includes important factors that contribute to VEGF transport and kinetics in the body. The model qualitatively and quantitatively describes the living system and clinical observations. Therefore, we present our results and their clinical implications based on the current state of the model.
Clinical Relevance of Model Predictions
Implication of High Tumor Interstitial VEGF.
The model predicts that unbound VEGF in the tumor interstitium can be an order of magnitude (ie, 7–13 times) higher than plasma VEGF for the range of parameters we considered. This prediction, which is in agreement with experimental data, implies that VEGF signaling in endothelial cells is likely to be dominated by the abluminal rather than luminal VEGF receptors. It diminishes the role of luminal VEGF concentration and emphasizes the role of interstitial tumor concentration. Thus, depleting tumor VEGF may be an important target for antiangiogenic therapies.
Implication of the Predominance of VEGF121 Isoform.
Our model predicts that VEGF121 is a predominant isoform in the tumor interstitium, which may be important to consider when developing treatment strategies that target VEGF. Prior quantitative analysis has shown that targeting this isoform is predicted to result in a therapeutic effect (5). Experimental studies in tumor-bearing mice demonstrate that VEGF121-expressing tumors recruit host vasculature (82) and have increased vascular volume (83) compared with tumors that secrete other isoforms such as VEGF165 or VEGF189. These studies indicate that VEGF121 is a key regulator of tumor angiogenesis, and our model supports this finding.
Implication of Therapeutic or Countertherapeutic Response to Anti-VEGF Treatment.
Our analysis reveals that, depending on the tumor microenvironment, anti-VEGF treatment can have adverse effects of slightly increasing free VEGF in the tumor or not affecting tumor VEGF at all. Incorporating properties of the microenvironment for specific tumor types allows us to investigate why certain tumors respond more favorably to anti-VEGF treatment than others. This prediction would be very instructive in understanding clinical data, including the lack of efficacy of anti-VEGF treatment in specific tumor types or for specific groups of patients, and may aid in the design of future clinical trials. It may lead to stratification of patients into likely responders vs nonresponders based on their microenvironmental parameters and even to personalized medical treatment. An important example is the use of anti-VEGF treatment in breast cancer patients, for which the FDA has revoked approval of bevacizumab for this indication. As shown in Figure 2 and Supplementary Table 1 (available online), the median level of free VEGF in tumor fluids of breast cancer patients is 20 pM; however, the concentration ranges from 1.7 pM to 320 pM (30). The low end of this range corresponds with the low secretion rates shown in Figure 4C, where free VEGF in the tumor is predicted to increase above the pretreatment level after anti-VEGF treatment. This example supports the importance of our model predictions, which indicate that anti-VEGF treatment may lead to countertherapeutic results in certain subpopulations of cancer patients, even those with the same type of cancer. The concentration of VEGF121 is being explored as a biomarker in retrospective studies (84,85). Additionally, VEGFR and NRP expression levels have been investigated as a predictive biomarker in breast (86) and gastric (87) cancers. Thus, our computational studies complement clinical investigations.
In conclusion, our molecular-detailed computational model is useful for understanding the dynamics of VEGF distribution in the body. The model predictions are relevant to the clinical use of VEGF-targeting therapies and testable hypotheses that can aid in elucidating the mechanism of action of anti-VEGF agents. Further experimental and computational studies will need to test whether these predictions are applicable to different cancer types, to primary tumors and metastasis, and to diverse or specific patient populations and whether they can guide personalized medicine. In anticipation of these measurements, we expect that our model predictions will lead to conceptual assessment of the different outcomes of antiangiogenic treatments and will encourage clinical researchers to consider tumor-specific properties that will influence the response to treatment.
Funding
This work was supported by the National Institutes of Health (R01 CA138264 to ASP and F32 CA154213 to SDF) and the UNCF-Merck Science Initiative (to SDF).
Supplementary Material
SDF and ASP designed the study. SDF performed the simulations and wrote the manuscript. All authors analyzed results and prepared the final manuscript.
We thank Drs Feilim Mac Gabhann and Herbert I. Hurwitz for their constructive comments and members of our laboratory for helpful discussions.
References
- 1. Jayson GC, Hicklin DJ, Ellis LM. Antiangiogenic therapy—evolving view based on clinical trial results. Nat Rev Clin Oncol. 2012;9(5):297–303 [DOI] [PubMed] [Google Scholar]
- 2. Hanahan D, Wienberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674 [DOI] [PubMed] [Google Scholar]
- 3. Arrell DK, Terzic A. Network systems biology for drug discovery. Clin Pharmacol Ther. 2010;8(1):120–125 [DOI] [PubMed] [Google Scholar]
- 4. Stefanini MO, Wu FTH, Mac Gabhann F, Popel AS. Increase of plasma VEGF after intravenous administration of bevacizumab is predicted by a pharmacokinetic model. Cancer Res. 2010;70(23):9886–9894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Finley SD, Popel AS. Predicting the effects of anti-angiogenic agents targeting specific VEGF isoforms. AAPS J. 2012;14(3):500–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Finley SD, Engel-Stefanini MO, Imoukhuede PI, Popel AS. Pharmacokinetics and pharmacodynamics of VEGF-neutralizing antibodies. BMC Syst Biol. 2011;5:193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gavin TP, Ruster RS, Carrithers JA, et al. No difference in the skeletal muscle angiogenic response to aerobic exercise training between young and aged men. J Physiol. 2007;585(1):231–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rullman E, Rundqvist H, Wagsater D, et al. A single bout of exercise activates matrix metalloproteinase in human skeletal muscle. J Appl Physiol. 2007;102(6):2346–2351 [DOI] [PubMed] [Google Scholar]
- 9. Hellsten Y, Rufener N, Nielsen JJ, Hoier B, Krustrup P, Bangsbo J. Passive leg movement enhances interstitial VEGF protein, endothelial cell proliferation, and eNOS mRNA content in human skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2008;294(3):R975–R982 [DOI] [PubMed] [Google Scholar]
- 10. Hansen-Algenstaedt N, Nielsen JJ, Saltin B, Hellsten Y. Exercise training normalizes skeletal muscle vascular endothelial growth factor levels in patients with essential hypertension. J Hypertens. 2010;28(6): 1176–1185 [DOI] [PubMed] [Google Scholar]
- 11. Hoier B, Rufener N, Bojsen-Moller J, Bangsbo J, Hellsten Y. The effect of passive movement training on angiogenic factors and capillary growth in human skeletal muscle. J Physiol. 2010;588(19):3833–3845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hoier B, Nordsborg N, Andersen S, et al. Pro- and anti-angiogenic factors in human skeletal muscle in response to acute exercise and training. J Physiol. 2012;590(3):595–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hoier B, Passos M, Bangsbo J, Hellsten Y. Intense intermittent exercise provides weak stimulus for VEGF secretion and capillary growth in skeletal muscle. Exp Physiol. 2013;98(2):585–597 [DOI] [PubMed] [Google Scholar]
- 14. Hoar FJ, Lip GYH, Belgore F, Stonelake PS. Circulating levels of VEGF-A, VEGF-D and soluble VEGF-A receptor (sFlt-1) in human breast cancer. Int J Biol Markers. 2004;19(3):229–235 [DOI] [PubMed] [Google Scholar]
- 15. Kut C, Mac Gabhann F, Popel AS. Where is VEGF in the body? A meta-analysis of VEGF distribution in cancer. Br J Cancer. 2007;97(7):978–985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Aref S, El Sherbiny M, Goda T, Fouda M, Al Askalany H, Abdalla D. Soluble VEGF/sFLt1 ratio is an independent predictor of AML patient out come. Hematology. 2005;10(2):131–134 [DOI] [PubMed] [Google Scholar]
- 17. Caine GJ, Blann AD, Stonelake PS, Ryan P, Lip GYH. Plasma angiopoietin-2 ant tie-2 in breast and prostate cancer: a comparison with VEGF and flt-1. Eur J Clin Invest. 2003;33(10):883–890 [DOI] [PubMed] [Google Scholar]
- 18. Ustuner A, Saip P, Yasasever V, et al. Prognostic and predictive value of vascular endothelial growth factor and its soluble receptors, VEGFR-1 and VEGFR-2 levels in the sera of small cell lung cancer patients. Med Oncol. 2008;25(4):394–399 [DOI] [PubMed] [Google Scholar]
- 19. Chang YT, Chang MC, Wei SC, et al. Serum vascular endothelial growth factor/soluble vascular endothelial growth factor receptor 1 ratio is an independent prognostic marker in pancreatic cancer. Pancreas. 2008;37(2):145–150 [DOI] [PubMed] [Google Scholar]
- 20. Chauhan VP, Stylianopoulos T, Martin JD, et al. Normalization of tumour blood vessels improves the delivery of nanomedicines in a size-dependent manner. Nat Nanotechnol. 2012;7(6):383–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Duysinx BC, Corhay J-L, Hubin L, Nguyen D, Henket M, Louis R. Diagnostic value of interleukin-6, transforming growth factor-beta 1 and vascular endothelial growth factor in malignant pleural effusions. Respir Med. 2008;102(12):1708–1714 [DOI] [PubMed] [Google Scholar]
- 22. Economidou F, Antoniou KM, Tzanakis N, Sfiridaki K, Siafakas NM, Schiza SE. Angiogenic molecule Tie-2 and VEGF in the pathogenesis of pleural effusions. Respir Med. 2008;102(5):774–779 [DOI] [PubMed] [Google Scholar]
- 23. Gieseler F, Luhr I, Kunze T, et al. Activated coagulation factors in human malignant effusions and their contribution to cancer cell metastasis and therapy. Thromb Haemost. 2007;97(6):1023–1030 [PubMed] [Google Scholar]
- 24. Hamed EA, El-Noweihi AM, Mohamed AZ, Mahmoud A. Vasoactive mediators (VEGF and TNF-a) in patients with malignant and tuberculous pleural effusions. Respirology. 2004;9(1):81–86 [DOI] [PubMed] [Google Scholar]
- 25. Harlozinska A, Sedlaczek P, Kulpa J, et al. Vascular endothelial growth factor (VEGF) concentration in sera and tumor effusions from patients with ovarian carcinoma. Anticancer Res. 2004;24(2C):1149–1158 [PubMed] [Google Scholar]
- 26. Ishimoto O, Saijo Y, Narumi K, et al. High level of vascular endothelial growth factor in hemorrhagic pleural effusion of cancer. Oncology. 2002;63(1):70–75 [DOI] [PubMed] [Google Scholar]
- 27. Jin HY, Lee KS, Jin SM, Lee Y-C. Vascular endothelial growth factor correlates with matrix metalloproteinase-9 in the pleural effusion. Respir Med. 2004;98(2):115–122 [DOI] [PubMed] [Google Scholar]
- 28. Kishiro I, Kato S, Fuse D, Yoshida T, Machida S, Kaneko N. Clinical significance of vascular endothelial growth factor in patients with primary lung cancer. Respirology. 2002;7(2):93–98 [DOI] [PubMed] [Google Scholar]
- 29. Kotyza J, Havel D, Vrzalova J, Kulda V, Pesek M. Diagnostic and prognostic significance of inflammatory markers in lung cancer-associated pleural effusions. Int J Biol Markers. 2010;25(1):12–20 [DOI] [PubMed] [Google Scholar]
- 30. Kraft A, Weindel K, Ochs A, et al. Vascular endothelial growth factor in the sera and effusions of patients with malignant and nonmalignant disease. Cancer Metastasis Rev. 1999;85(1):178–187 [PubMed] [Google Scholar]
- 31. Lee HK, Chae HS, Kim JS, et al. Vascular endothelial growth factor levels in ascites between chemonaive and chemotreated patients. Yonsei Med J. 2008;49(3):429–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Matsuyama W, Hashiguchi T, Mizoguchi A, et al. Serum levels of vascular endothelial growth factor dependent on the stage progression of lung cancer. Chest. 2000;118(4):948–951 [DOI] [PubMed] [Google Scholar]
- 33. Nascimento I, Schaer R, Lemaire D, et al. Vascular endothelial growth factor (VEGF) levels as a tool to discriminate between malignant and nonmalignant ascites. Acta Pathol Microbiol Scand. 2004;112(9):585–587 [DOI] [PubMed] [Google Scholar]
- 34. Richardson M, Gunawan J, Hatton MWC, Seidlitz E, Hirte HW, Signh G. Malignant ascites fluid (MAF), including ovarian-cancer-associated MAF, contains angiogstatin and other factor(s) which inhibit angiogenesis. Gynecol Oncol. 2002;86(3):279–287 [DOI] [PubMed] [Google Scholar]
- 35. Rudlowski C, Pickarts A-K, Fuhljahns C, et al. Prognostic significance of vascular endothelial growth factor expression in ovarian cancer patients: a long-term follow-up. Int J Gynecol Cancer. 2006;16(S1):183–189 [DOI] [PubMed] [Google Scholar]
- 36. Sack U, Hoffmann M, Zhao XJ, et al. Vascular endothelial growth factor in pleural effusions of different origin. Eur Respir J. 2005;25(4):600–604 [DOI] [PubMed] [Google Scholar]
- 37. Shu J, Sun G, Liu H, Liu J. Clinical utility of vascular endothelial growth factor in diagnosing malignant pleural effusions. Acta Oncol. 2007;46(7): 1004–1011 [DOI] [PubMed] [Google Scholar]
- 38. Sun X-M, Dont W-G, Gao L-C. Detection of VEGF levels in ascites and peritoneal fluid. Chin J Cancer Res. 2003;15(4):310–314 [Google Scholar]
- 39. Thickett DR, Armstrong L, Millar AB. Vascular endothelial growth factor (VEGF) in inflammatory and malignant pleural effusions. Thorax. 1999;54(8):707–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Verheul HM, Hoekman K, Jorna AS, Smit EF, Pinedo HM. Targeting vascular endothelial growth factor blockade: ascites and pleural effusion formation. Oncologist. 2000;5(Suppl 1):45–50 [DOI] [PubMed] [Google Scholar]
- 41. Xue K, Siong S, Xiong W. Clincial value of vascular endothelial growth factor combined with interferon-gamma in diagnosing malignant pleural effusion and tuberculous pleural effusion. J Huazhong Univ Sci Technol (Med Sci). 2007;27(5):495–497 [DOI] [PubMed] [Google Scholar]
- 42. Yabushita H, Shimazu M, Noguchi M, et al. Vascular endothelial growth factor activating matrix metalloproteinase in ascitic fluid during peritoneal dissemination of ovarian cancer. Oncol Rep. 2003;10(1):89–95 [PubMed] [Google Scholar]
- 43. Yamamoto S, Konishi I, Mandai M, et al. Expression of vascular endothelial growth factor (VEGF) in epithelial ovarian neoplasms: correlation with clinicopathology and patient survival, and analysis of serum VEGF levels. Br J Cancer. 1997;76(9):1221–1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yanagawa H, Takeuchi E, Suzuki Y, Ohmoto Y, Bando H, Sone S. Vascular endothelial growth factor in malignant pleural effusion associated with lung cancer. Cancer Immunol Immunother. 1999;48(7):396–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zebrowski BK, Yano S, Liu W, et al. Vascular endothelial growth factor levels and induction of permeability in malignant pleural effusions. Clin Cancer Res. 1999;5(11):3364–3368 [PubMed] [Google Scholar]
- 46. Zhou W-B, Bai M, Jin Y. Diagnostic value of vascular endothelial growth factor and endostatin in malignant pleural effusions. Int J Tuberc Lung Dis. 2009;13(3):381–386 [PubMed] [Google Scholar]
- 47. Hoskins ER, Hood BL, Sun M, Krivak TC, Edwards RP, Conrads TP. Proteomic analysis of ovarian cancer proximal fluids: validation of elevated peroxiredoxin 1 in patient perifpheral circulation. PLoS One. 2011;6(9):e25056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Garvin S, Dabrosin C. In vivo measurement of tumor estradiol and vascular endothelial growth factor in breast cancer patients. BMC Cancer. 2008;8:73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wiig H, Tenstad O, Iversen PO, Kalluri R, Bjerkvig R. Interstitial fluid: the overlooked component of the tumor microenvironment? Fibrogenesis and Tissue Repair. 2010;3:12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ljungberg B, Jacobsen J, Haggstrom-Rudolfssson S, Rasmuson T, Lindh G, Grankvist K. Tumor vascular endothelial growth factor (VEGF) mRNA in relation to serum VEGF protein levels and tumour progression in human renal cell carcinoma. Urol Res. 2003;31(5):335–340 [DOI] [PubMed] [Google Scholar]
- 51. Stimpfl M, Tong D, Fasching B, et al. Vascular endothelial growth factor splice variants and their prognostic value in breast and ovarian cancer. Clin Cancer Res. 2002;8(7):2253–2259 [PubMed] [Google Scholar]
- 52. Yuan A, Yu CJ, Luh KT, Lin FY, Kuo SH, Yang PC. Quantification of VEGF mRNA expression in non-small cell lung cancer using a real-time quantitative reverse transcription-PCR assay and a comparison with quantitative competitive reverse transcription-PCR. Lab Invest. 2000;2000(80):11 [DOI] [PubMed] [Google Scholar]
- 53. Zygalaki E, Tsaroucha EG, Kaklamanis L, Lianidou ES. Quantitative real-time reverse transcription-PCR study of the expression of vascular endothelial growth factor (VEGF) splice variants and VEGF receptors (VEGFR-1 and VEGFR-2) in non-small cell lung cancer. Clin Chem. 2007;53(8):1433–1439 [DOI] [PubMed] [Google Scholar]
- 54. Ng YS, Rohan R, Sunday ME, Demello DE, D’Amore PA. Differential expression of VEGF isoforms in mouse during development and in the adult. Dev Dyn. 2001;220(2):112–121 [DOI] [PubMed] [Google Scholar]
- 55. Houck K, Leung DW, Rowland AM, Winer J, Ferrara N. Dual regulation of vascular endothelial growth factor bioavailability by genetic and proteolytic mechanisms. J Biol Chem. 1992;268(36):26031–26037 [PubMed] [Google Scholar]
- 56. Soker S, Miao H-Q, Nomi M, Takashima S, Klagsbrun M. VEGF165 mediates formation of complexes containing VEGFR-2 and neuropilin-1 that enhance VEGF165-receptor binding. J Cell Biochem. 2002;85(2):357–368 [DOI] [PubMed] [Google Scholar]
- 57. Poon RT-P, Lau CP-Y, Cheung S-T, Yu W-C, Fan S-T. Quantitative correlation of serum levels and tumor expression of vascular endothelial growth factor in patients with hepatocellular carcinoma. Cancer Res. 2003;63(12):3121–3216 [PubMed] [Google Scholar]
- 58. Poon RT-P, Lau CP-Y, Ho JW-Y, Yu W-C, Fan S-T, Wong J. Tissue factor expression correlates with tumor angiogenesis and invasiveness in human hepatocellular carcinoma. Clin Cancer Res. 2003;9(14):5339–5345 [PubMed] [Google Scholar]
- 59. Gutierrez J, Konecny GE, Hong K, et al. A new ELISA for use in a 3-ELISA system to assess concentrations of VEGF splice variants and VEGF110 in ovarian cancer tumors. Clin Chem. 2008;54(3):597–601 [DOI] [PubMed] [Google Scholar]
- 60. Segerstrom L, Fuchs D, Backman U, Holmquist K, Christofferson R, Azarbayjani F. The anti-VEGF antibody bevacizumab potently reduces the growth rate of high-risk neuroblastoma xenografts. Pediatr Res. 2006; 60(5):576–581 [DOI] [PubMed] [Google Scholar]
- 61. Willett CG, Boucher Y, Duda DG, et al. Surrogate markers for antiangiogenic therapy and dose-limiting toxicities for bevacizumab with radiation and chemotherapy: continued experience of a phase I trial in rectal cancer patients. J Clin Oncol. 2005;23(31):8136–8139 [DOI] [PubMed] [Google Scholar]
- 62. Yang JC, Haworth L, Sherry RM, et al. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. New Engl J Med. 2003;349(5):427–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lee J, Ku T, Yu H, et al. Blockade of VEGF-A suppresses tumor growth via inhibition of autocrine signaling through FAK and AKT. Cancer Lett. 2012;318(2):221–225 [DOI] [PubMed] [Google Scholar]
- 64. Cao Y, E G, Wang E, et al. VEGF exerts an angiogenesis-independent function in cancer cells to promote their malignant progression. Cancer Res. 2012;72(16):3912–3918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Adamcic U, Skowronski K, Peters C, Morrison J, Coomber BL. The effect of bevacizumab on human malignant melanoma cells with function VEGF/VEGFR2 autocrine and intracrine signaling loops. Neoplasia. 2012;14(7):612–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lee TH, Seng S, Sekine M, Hinton C, Avraham HK, Avraham S. Vascular endothelial growth factor mediates intracrine survival in human breast carcinoma cells through internally expressed VEGFR1/FLT1. PLoS Med. 2007;4(6):e186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Zhang J, Jia Z, Li Q, et al. Elevated expression of vascular endothelial growth factor correlates with increased angiogenesis and decreased progression-free survival among patients with low-grade neuroendocrine tumors. Cancer. 2007;109(8):1478–1486 [DOI] [PubMed] [Google Scholar]
- 68. Ricci AG, Olivares CN, Biolotas MA, Meresman GF, Baranao RI. Effect of vascular endothelial growth factor inhibition on endometrial implant development in a murine model of endometriosis. Reprod Sci. 2011;18(7):614–622 [DOI] [PubMed] [Google Scholar]
- 69. Lan K-L, Ou-Yang F, Yen S-H, Shih H-L, Lan K-H. Cationic liposome coupled endostatin gene for treatment of peritoneal colon cancer. Clin Exp Metastasis. 2010;27(5):307–318 [DOI] [PubMed] [Google Scholar]
- 70. Okada Y, Akisue T, Hara H, et al. The effect of bevacizumab on tumour growth of malignant fibrous histiocytoma in an animal model. Anticancer Res. 2010;30(9):3391–3395 [PubMed] [Google Scholar]
- 71. Yuan A, Lin C-Y, Chou C-H, et al. Functional and structural characteristics of tumor angiogenesis in lung cancers overexpressing different VEGF isoforms assessed by DCE- and SSCE-MRI. PLoS One. 2011;6(1):e16062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Rennel ES, Harper SJ, Bates DO. Therapeutic potential of manipulating VEGF splice isoforms in oncology. Future Oncol. 2009;5(5):703–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Dokun AO, Annex BH. The VEGF165b “ICE-o-form” puts a chill on the VEGF story. Circ Res. 2011;109(3):246–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Manetti M, Guiducci S, Romano E, et al. Overexpression of VEGF165b, an inhibitory splice variant of vascular endothelial growth factor, leads to insufficient angiogenesis in patients with systemic sclerosis. Circ Res. 2011;109(3):e14–e26 [DOI] [PubMed] [Google Scholar]
- 75. Nowak DG, Woolard J, Amin EM, et al. Expression of pro- and anti-angiogenic isoforms of VEGF is differentially regulated by splicing and growth factors. J Cell Sci. 2008;121(20):3487–3495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Catena R, Larzabal L, Larrayoz M, et al. VEGF121b and VEGF165b are weakly angiogenic isoforms of VEGF-A. Mol Cancer. 2010;9:320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Italiano JE, Richardson JL, Patel-Hett S, et al. Angiogenesis is regulated by a novel mechanism: pro- and antiangiogenic proteins are organized into separate platelet a-granules and differentially released. Blood. 2008;111(3): 1227–1233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Battinelli EM, Markens BA, Italiano JE., Jr Release of angiogenesis regulatory proteins from platelet alpha granules: modulation of physiologic and pathologic angiogenesis. Blood. 2011;118(5):1359–1369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Verheul HMW, Lolkema MPJ, Quian DZ, et al. Platelets take up the monoclonal antibody bevacizumab. Clin Cancer Res. 2007;13(18):5341–5347 [DOI] [PubMed] [Google Scholar]
- 80. Zhang Q-X, Magover CJ, Mack CA, Budenbender KT, Ko W, Rosengart T. Vascular endothelial growth factor is the major angiogenic factor in omentum: mechanism of the omentum-mediated angiogenesis. J Surg Res. 1997;67(2):147–154 [DOI] [PubMed] [Google Scholar]
- 81. Maharaj ASR, D’Amore PA. Roles for VEGF in adult. Microvasc Res. 2007;74:100–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Grunstein J, Masbad JJ, Hickey R, Giordano F, Johnson RS. Isoforms of vascular endothelial growth factor act in a coordinate fashion to recruit and expand tumor vasculature. Mol Cell Biol. 2000;20(19):7292–7291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Tozer GM, Akerman S, Cross NA, et al. Blood vessel maturation and response to vascular-disrupting therapy in single vascular endothelial growth factor-A isoform-producing tumors. Cancer Res. 2008;68(7):2301–2311 [DOI] [PubMed] [Google Scholar]
- 84. Sanmartin E, Jantus E, Blasco A, et al. Plasma levels of VEGF-A and VEGFR-2 in advanced NSCLC. J Clin Oncol. 2010;28(15s):10623 [Google Scholar]
- 85. Bernaards C, Hegde P, Chen D, et al. Circulating vascular endothelial growth factor (VEGF) as a biomarker for bevacizumab-based therapy in metastatic colorectal, non-small cell lung, and renal cell cancers: analysis of phase III studies. J Clin Oncol. 2010;28(15s):10519 [Google Scholar]
- 86. Jubb AM, Miller KD, Rugo HS, et al. Impact of exploratory biomarkers on the treatment effect of bevacizumab in metastatic breast cancer. Clin Cancer Res. 2011;17(2):372–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Van Cutsem E, de Haas S, Kang Y-K, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a biomarker evaluation from the AVAGAST randomized phase III trial. J Clin Oncol. 2012;30(17):2119–2127 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.