Abstract
The N-myc downstream regulated gene 1 (NDRG1) has been identified as a metastasis-suppressor gene in prostate cancer (PCa). Compounds targeting PCa cells deficient in NDRG1 could potentially decrease invasion/metastasis of PCa. A cell based screening strategy was employed to identify small molecules that selectively target NDRG1 deficient PCa cells. DU-145 PCa cells rendered deficient in NDRG1 expression by a lentiviral shRNA-mediated knockdown strategy were used in the primary screen. Compounds filtered from the primary screen were further validated through proliferation and clonogenic survival assays in parental and NDRG1 knockdown PCa cells. Screening of 3360 compounds revealed irinotecan and cetrimonium bromide (CTAB) as compounds that exhibited synthetic lethality against NDRG1 deficient PCa cells. A three-dimensional (3-D) invasion assay was utilized to test the ability of CTAB to inhibit invasion of DU-145 cells. CTAB was found to remarkably decrease invasion of DU-145 cells in collagen matrix. Our results suggest that CTAB and irinotecan could be further explored for their potential clinical benefit in patients with NDRG1 deficient PCa.
Keywords: irinotecan, cetrimonium bromide (CTAB), synthetic lethality, N-myc downstream regulated gene 1 (NDRG1), prostate cancer, invasion, topoisomerase I
Introduction
The majority of morbidity and mortality in prostate cancer (PCa) patients is caused by metastases.1 The ability of cancer cells to metastasize is a multistep process that involves intravasation of cells from their primary site into blood vessels and extravasation into target organs.2 Inhibiting any step of the metastatic process is hypothesized to negatively impact the spread of cancer, thereby providing clinical benefit for cancer patients. Failure of cancer therapies, to a large extent, can be attributed to a failure to halt or contain metastasis, particularly in PCa. To improve the clinical outcome for PCa patients, it is therefore crucial to target key molecular mechanisms/pathways involved in the metastatic process of PCa cells.
In the past two decades, multiple genes have been identified that suppress the formation and growth of cancer metastases without affecting primary growth of the tumor, such as KAI1, CD44, NM23, PEBP1, RECK, MAP2K4 and the N-myc downstream regulated gene 1 (NDRG1).3,4 NDRG1 is downregulated in highly metastatic PCa cells.5 Our group has identified NDRG1 as a Rab4a GTPase effector protein involved in the vesicular recycling of the adhesion molecule E-Cadherin, thereby preventing its degradation and possibly preventing metastasis of cancer cells.6 More recently, Liu et al. reported that NDRG1 negatively modulates Wnt-β-signaling during metastatic progression via interaction with the Wnt receptor LRP6.7 Therefore drugs that selectively target tumor cells deficient in NDRG1 could potentially decrease PCa invasion. However, compounds that selectively targets NDRG1-deficient prostate cancer cells are yet to be identified.
In the current study we aimed to identify compounds that selectively target NDRG1 deficient PCa cells. For this purpose, we raised isogenic DU-145, LNCaP and PC3 cell lines that differed in their NDRG1 expression by stably knocking down (KD) NDRG1 expression using a lentiviral shRNA vector. By performing a drug library screen, we aimed to identify compounds that would be less toxic to cells by themselves, but prove to be synthetically lethal in (PCa) cells that had lower NDRG1 expression. Parental and NDRG1 KD DU-145 cells were utilized for the primary screen; all three PCa cell lines were used for validation of compounds identified from the primary screen. The screen was performed using the Johns Hopkins drug library (JHDL), a small-molecule library with 3,360 compounds consisting mostly of FDA-approved drugs and other bioactive molecules.8
In this study the topoisomerase I inhibitor irinotecan and the cationic surfactant cetrimonium bromide (CTAB) were identified as compounds that are synthetically lethal in vitro in NDRG1 deficient PCa cell lines. These compounds warrant further investigation as potential chemotherapeutic agents that could target NDRG1 deficient invasive PCa cells.
Results
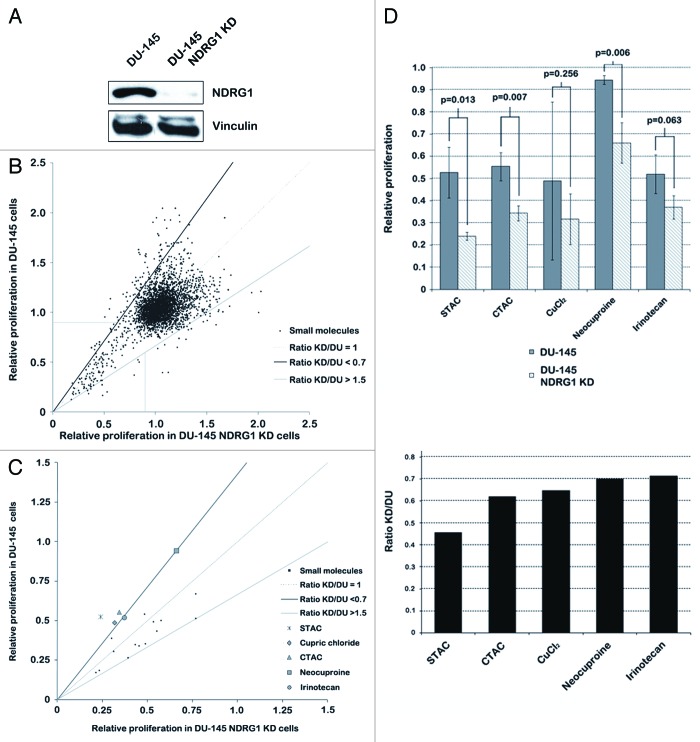
Library screen for compounds targeting DU-145 cells based on NDRG1 expression
To identify novel chemotherapeutic agents that could potentially decrease invasion of PCa cells, a synthetic lethal screen was devised based on NDRG1 expression in DU-145 cells. The goal was to identify PCa targeting compounds that selectively inhibit NDRG1 deficient PCa cells, thereby decreasing invasiveness. For this purpose, we created isogenic PCa cell lines that differed in NDRG1 expression. NDRG1 shRNA lentiviral constructs successfully generated a stable KD of NDRG1 expression in DU-145 cells (DU-145 NDRG1 KD), as judged by the 99% decrease in NDRG1 protein levels in these cells (Fig. 1A). As high-throughput screening assays are prone to generating a high degree of false positives,9 our screen was robustly designed in a three tier fashion to weed out false positives. The primary screen involved treatment of isogenic parental DU-145 and DU-145 NDRG1 KD cells with compounds from the JHDL at a concentration of 10 µM for 48 h. Our objective was to identify compounds that selectively inhibit DU-145 NDRG1 KD cells and parental DU-145 cells. Therefore hits were defined as compounds that selectively inhibited DU-145 NDRG1 KD cells or parental DU-145 cells by changing the “DU-145 NDRG1 KD/DU-145”-proliferation ratio to < 0.7 or > 1.5, respectively. Furthermore, as we were only interested in PCa targeting compounds, only compounds that inhibited cell proliferation of PCa cells by 10% or more were selected. Of the 3,360 compounds in the primary screen, 19 compounds were selected (Fig. 1B). Ten of these compounds selectively inhibited NDRG1 deficient DU-145 cells.
Figure 1. Screening of the JHDL identifies stearyltrimethylammonium chloride (STAC), cetrimonium chloride (CTAC), cupric chloride (CuCl2), neocuproine (NCP) and irinotecan as compounds that selectively inhibit NDRG1 deficient DU-145 cells. (A) Western blot for NDRG1 performed with DU-145 cell lysates that had NDRG1 stably knocked down by shRNA. Control cells were transduced with non-mammalian shRNA constructs. (B) MTS assays performed after 48 h treatment of DU-145 cells with 0.05% DMSO (control) or compounds from the JHDL at a final concentration of 10 µM. Compounds were selected for a secondary screen when inhibiting cell proliferation by at least 10% (horizontal and vertical line) and having a “DU-145 NDRG1 KD/DU-145”-ratio of < 0.7 (area above the dark diagonal line) or > 1.5 (area under the light diagonal line). (C) Secondary screen with the selected compounds from the primary screen to discard false positives. MTS assays were performed in triplicate; compounds that changed the “DU-145 NDRG1 KD/DU-145”-ratio to < 0.7 and irinotecan were selected. (D) Bar graphs representing relative proliferation activity (left) and the “DU-145 NDRG1 KD/DU-145”-ratio (right) of selected compounds from the secondary DU-145 screen. Compounds were selected in the secondary screen if the “DU-145 NDRG1 KD/DU-145”-ratio was < 0.7 or > 1.5. STAC, CTAC, CuCl2 and NCP had a “DU-145 NDRG1 KD/DU-145”-ratio < 0.7; irinotecan was selected as it had a “DU-145 NDRG1 KD/DU-145”-ratio of 0.71 and as a relationship between NDRG1 expression and irinotecan resistance is already known in the literature. Ratio KD/DU, “DU-145 NDRG1 KD/DU-145”-ratio.
The 19 compounds identified as hits in the primary screen were put through a secondary screening process wherein MTS assays were performed in triplicates (Fig. 1C). As a result of this approach, four compounds which inhibited NDRG1 deficient cells ≥ 30% more effectively in their proliferation (“DU-145 NDRG1 KD/DU-145”-ratio < 0.7) were identified, namely, stearyltrimethylammonium chloride (STAC), cupric chloride (CuCl2), neocuproine (NCP) and cetyltrimethyl ammonium chloride (CTAC) (Fig. 1D, structure formulas in Fig. S1). Furthermore, irinotecan hydrochloride trihydrate was included in our list of hits as well, as it was just below our threshold with a “DU-145 NDRG1 KD/DU-145”-ratio of 0.71 (Fig. 1C and D) and as previous studies reported a positive correlation between NDRG1 expression and irinotecan resistance in patients with colorectal tumors.10,11 None of the compounds tested resulted in a “DU-145 NDRG1 KD/DU-145”-proliferation ratio > 1.5.
CuCl2 and NCP were excluded from further research. CuCl2 was excluded as the difference in proliferation activity in cells after treatment with this inorganic salt was not significant and could not be repeated in additional experiments (data not shown). NCP was excluded as this compound would most likely not provide any clinical benefit in metastasis-prone PCa patients, the small molecule acting on healthy cells, such as astrocytes, as well.12 The other hits from the secondary screen (STAC, CTAC and irinotecan) were selected for the tertiary screen, in which series of MTS and clonogenic assays were performed at varying concentrations in multiple PCa cell lines to conclusively rule out false positives and to ascertain that the inhibitory effect of the compounds was not merely restricted to one PCa cell line. The results of this tertiary screen will be presented next.
Irinotecan selectively targets NDRG1 deficient PCa cells
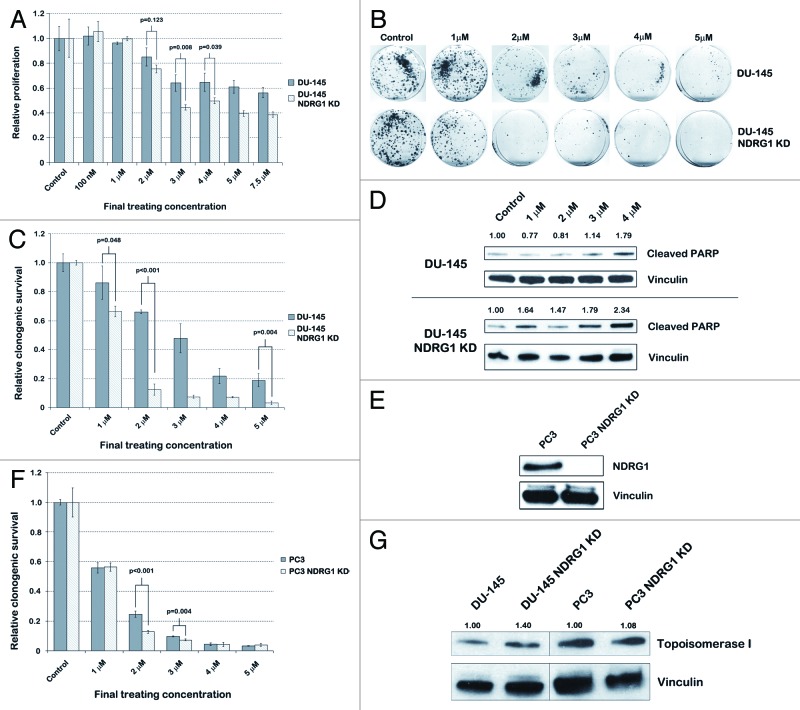
Irinotecan (also known as Camptothecin-11 or CPT-11) is a DNA topoisomerase I inhibitor that is mainly used for the treatment of colorectal carcinomas. Due to the known positive correlation between irinotecan resistance and NDRG1 expression in colon cancer, the compound could be used to validate our screening results.10,11,13 Furthermore, irinotecan was considered an interesting compound to explore for its use in PCa therapy, as the compound is already FDA approved for treatment against other cancer types.
We assessed the sensitivity of parental DU-145 and DU-145 NDRG1 KD cells to irinotecan by performing MTS assays with varying concentrations of the compound. After 48 h of treatment, the IC50 of parental DU-145 cells was > 7.5 µM, while the IC50 of NDRG1 KD cells was 2.5 µM (Fig. 2A). Furthermore, at concentrations between 3 and 7.5 µM, DU-145 cells demonstrated a statistically significant difference in sensitivity to irinotecan based on the NDRG1 status of the DU-145 cells, DU-145 NDRG1 KD cells having up to 30% decreased cell viability compared with parental DU-145 cells (p < 0.04). These results were in line with the results from the secondary screen (compare Fig. 2A to Fig. 1D). Long-term clonogenic survival demonstrated that DU-145 NDRG1 KD cells have about 50% less clonogenic survival after irinotecan treatment as compared with parental DU-145 cells (p < 0.05) (Figs. 2B–C). Furthermore, western blots for cleaved PARP and FACS analysis for annexin V confirmed that apoptosis was increased after 48 h of irinotecan treatment in NDRG1 deficient DU-145 cells (Fig. 2D and Fig. S4). To exclude that the differential effect caused by irinotecan was not a cell-line dependent response, we performed clonogenic assays in another PCa cell line as well, namely PC3 cells. Stable KD of NDRG1 in PC3 cells significantly reduced NDRG1 protein expression levels (Fig. 2E). PC3 NDRG1 KD cells were significantly more sensitive to irinotecan at final treating concentrations between 2 and 3 µM (p < 0.01) (Fig. 2F). Furthermore, PC3 cells were more sensitive to irinotecan compared with DU-145 cells in general, as irinotecan at a concentration of 4 µM effectively inhibited PC3 cells regardless of their NDRG1 expression, decreasing clonogenic survival to < 5% compared with untreated controls. Since increase in topoisomerase I expression is reported to be one of six general mechanisms for cellular resistance against irinotecan,14 we analyzed topoisomerase I expression through western blotting (Fig. 2G). We did find a slight increase in topoisomerase I expression in NDRG1KD DU-145 and PC3 cells. To rule out that sensitivity to irinotecan in NDRG1 deficient cells is due to off target effects, we performed a transient knockdown of topoisomerase I in DU-145 and DU-145 NDRG1KD cells and evaluated proliferation through MTS assay. In concordance with the irinotecan data NDRG1 deficient cells showed a decrease in proliferation after topoisomerase I knockdown (Fig. S3)
Figure 2. Irinotecan selectively targets NDRG1 deficient DU-145 and PC3 cells by induction of apoptosis. (A) MTS assays performed after 48 h treatment of parental DU-145 and DU-145 NDRG1 KD cells with irinotecan to assess differences in sensitivity based on NDRG1 expression. Student’s t-tests were performed to assess p-values. (B) Images displaying clonogenic survival of parental DU-145 and DU-145 NDRG1 KD cells after 48 h treatment with irinotecan. (C) Bar graph quantifying the percentage of clonogenic survival in DU-145 cells. This percentage was determined by dividing the average number of colonies in treated dishes by the average number of colonies in control dishes. Student’s t-tests were performed to assess p-values. (D) Protein expression levels of cleaved PARP, an apoptotic marker, were assessed after treatment of DU-145 cells with irinotecan for 48 h. Densitometry was performed with ImageJ, in which density of the cleaved PARP bands was normalized to the density of the housekeeper vinculin. (E) Western blots confirmed successful knockdown (KD) of NDRG1 protein expression in PC3 cells after transduction of the cells with shRNA that stably knocks down NDRG1 expression. (F) Bar graph quantifying the percentage of clonogenic survival in PC3 cells after 48 h of irinotecan treatment. Student’s t-tests were performed to assess p-values. G, western blots for topoisomerase I were performed with cell lysates from untreated DU-145 and PC3 cells and their respective KD. Protein expression levels of topoisomerase I matched with the sensitivity of the cells to irinotecan. Densitometry was performed with ImageJ, in which density of the topoisomerase I bands was corrected for density of the housekeeper vinculin.
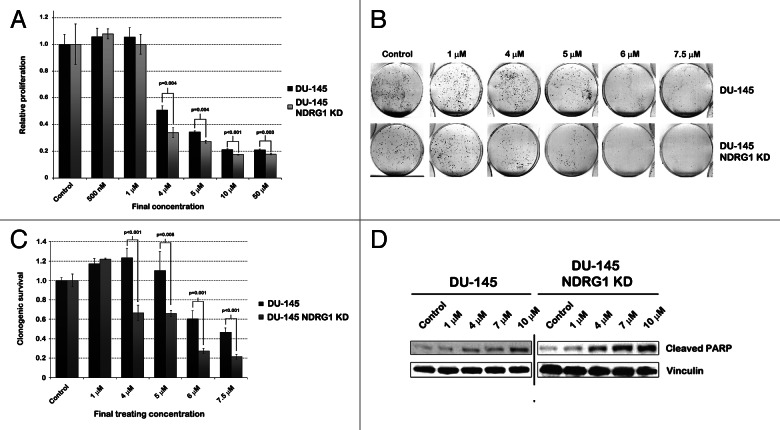
CTAB selectively targets NDRG1 deficient PCa cells
Two other hits in our secondary screen of the JHDL were STAC and CTAC, two cationic surfactants with similar characteristics. Of these two compounds, CTAC was more selective for NDRG1 deficient DU-145 cells than STAC (Fig. 1C). CTAC was more selective for NDRG1 deficient LNCaP cells compared with parental LNCaP cells as well (Fig. S2). The two compounds drew our interest, as similar cationic surfactants such as cetriumonium bromide (CTAB) have shown to have an anti-tumor effect.15-17 As CTAC was more selective for NDRG1 deficient PCa cells than STAC and, as CTAC and CTAB, which only differ in the halide groups, have a similar efficacy as anti-tumor agents, we continued our study with CTAB.15 This compound was also preferred as efficacy and safety in mammals have been studied more extensively with CTAB.
We performed MTS assays with varying concentrations of CTAB in parental DU-145 and DU-145 NDRG1 KD cells (Fig. 3A). In general CTAB appeared more potent in inhibiting DU-145 and DU-145 NDRG1 KD cells than CTAC, which might be caused by the difference in the halide group (compare Fig. 3A with Fig. 1D). Although the IC50 in parental DU-145 and DU-145 NDRG1 KD cells differed by 0.5 µM only (4 and 3.5 µM, respectively, data not shown), multiple independently performed MTS assays indicated a significant decrease in cell viability of DU-145 NDRG1 KD cells compared with parental DU-145 cells at concentrations of 4 µM and above, DU-145 NDRG1 KD cells being up to 15% more effectively inhibited (p ≤ 0.004). Next we assessed differences in long-term survival by performing clonogenic assays. At final concentrations between 4 and 7.5 µM, NDRG1 deficient DU-145 cells clearly exhibited less clonogenic survival than parental DU-145 cells (Figs. 3B–C). Due to a lack of viable cells after 48 h treatment with 10 µM CTAB, no clonogenics could be performed at this concentration. As the difference between parental DU-145 and DU-145 NDRG1 KD cells was more pronounced in the long-term clonogenic survival assay than in the short term MTS assay, we hypothesized that CTAB causes a differential induction of apoptosis in the cell lines. To test this hypothesis, we performed a western blot for cleaved PARP, a surrogate marker for apoptosis. Cells were treated with CTAB for 48 h and lysates were probed for cleaved PARP. As expected, NDRG1 deficient DU-145 cells had increased levels of cleaved PARP and Annexin V staining as compared with parental DU-145 cells (Fig. 3D and Fig. S4). In line with previous results, this difference in cleaved PARP expression was most pronounced at concentrations of 4 and 7 µM.
Figure 3. CTAB selectively targets DU-145 NDRG1 KD cells by induction of apoptosis. (A) MTS assays performed after 48 h treatment of parental DU-145 and DU-145 NDRG1 KD cells with CTAB. Student’s t-tests were performed to assess p-values. (B) Representative images of clonogenic survival assay results. Parental DU-145 and DU-145 NDRG1 KD cells had been treated for 48 h with CTAB prior to the survival assay. (C) Bar graph quantifying the percentage of clonogenic survival in DU-145 cells after CTAB treatment. This percentage was determined by dividing the average number of colonies in treated dishes by the average number of colonies in control dishes. Student’s t-tests were performed to assess p-values. (D) Both parental DU-145 and DU-145 NDRG1 KD cells were treated with CTAB for 48 h, after which proteins were extracted and western blots for cleaved PARP were performed, to assess levels of apoptosis after CTAB treatment in these cells.
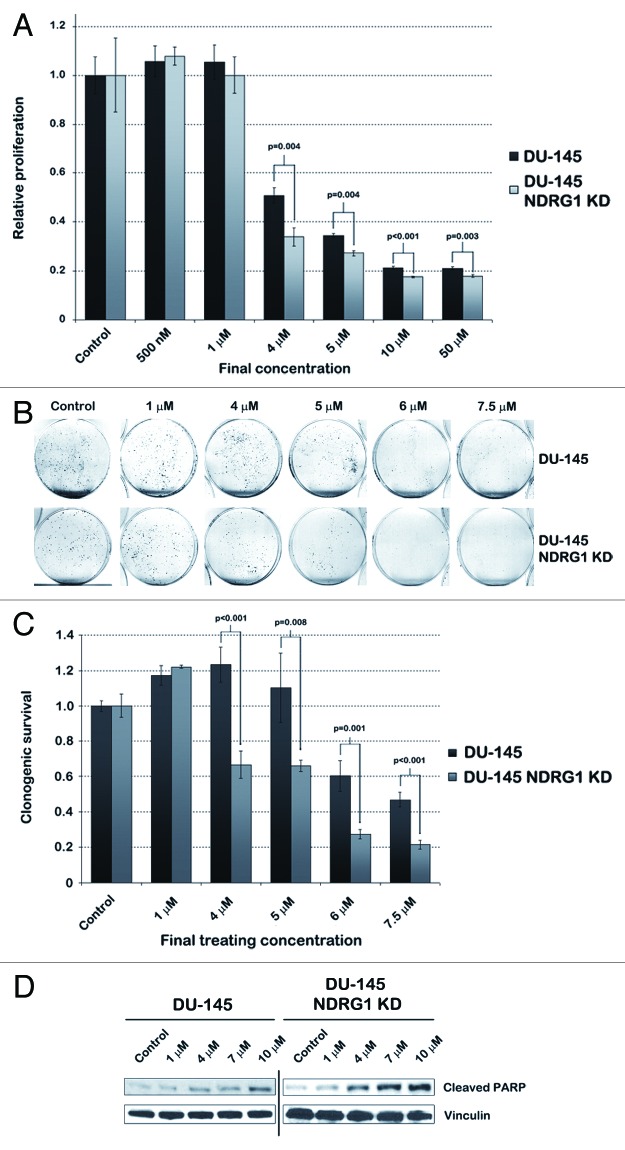
To rule out that the inhibitory effect of CTAB observed in cancer cells is not caused by general toxicity, we treated human prostate fibroblast cells with CTAB at concentrations at which it is effective in inhibiting PCa cells and performed MTS assays (Fig. 4A). In human prostate fibroblasts, the IC50 for CTAB was about 30 µM, while in parental DU-145 cells the IC50 was about 4 µM (p < 0.05 at concentrations of ≥ 3µM). This result encouraged us to determine the efficacy of CTAB in other PCa cell lines as well. In concordance with earlier observations, cell proliferation in NDRG1 deficient LNCaP cells was more effectively inhibited compared with parental LNCaP cells at concentrations above 5 µM, as determined by MTS assays (p < 0.02) (Fig. 4B). Again in general CTAB was more potent than CTAC (compare Figure 4B to Fig. S2B). Since LNCaP cells have weak adhesive properties compared with other PCa cell lines, technical limitations prevented confirming this result by clonogenic assays. Therefore PC3 cells were used to confirm and validate the effect of CTAB in clonogenic assays. This assay indicated that CTAB selectively inhibited NDRG1 deficient PC3 cells at final concentrations between 2.5 and 6 µM (p < 0.001) (Figs. 4C–D).
Figure 4. While non-cancerous human prostate fibroblasts are relatively unaffected by CTAB, NDRG1 KD cells are selectively targeted in multiple PCa cell lines. (A) MTS assays were performed with human prostate fibroblasts and DU-145 cells after 48 h of CTAB treatment, to assess the difference in IC50 between both cell lines. (B) MTS assays performed after 48 h treatment of parental LNCaP and LNCaP NDRG1 KD cells with CTAB. Student’s t-tests were performed to assess p-values. (C) Images displaying clonogenic assay results performed with parental PC3 and PC3 NDRG1 KD cells after 48 h treatment with CTAB prior to the survival assay. (D) Bar graph quantifying the percentage of clonogenic survival in PC3 cells after CTAB treatment. This percentage was determined by dividing the average number of colonies in treated dishes by the average number of colonies in control dishes. Student’s t-tests were performed to assess p-values.
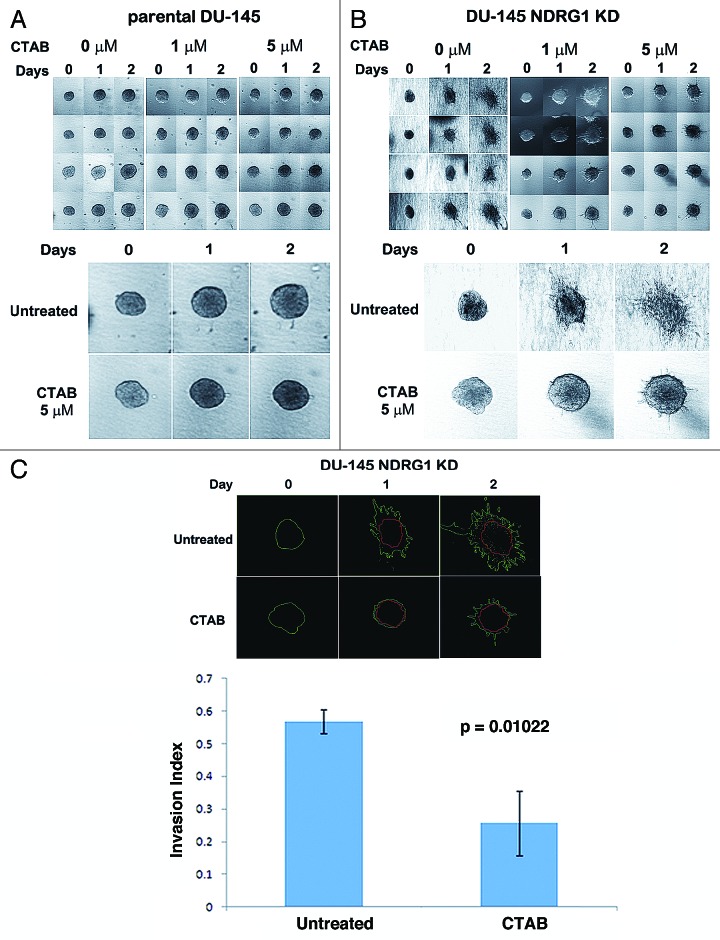
Our results indicate that NDRG1 deficient PCa cells are more sensitive to CTAB than NDRG1 expressing cells at concentrations which were well tolerated in xenografts.15 To test whether CTAB also decreases invasion of PCa at these concentrations, we conducted a 3-D invasion assay in collagen type I matrix (Fig. 4). A 3-D invasion assay is superior to the Boyden chamber invasion assay, as the cells exhibit properties and behavior which are physiologically closer to in vivo settings. Untreated spheroids, consisting of parental DU-145 cells, increased in size over the course of 48 h. However, barely any invasive structures were observed (Fig. 5A). On the other hand, untreated spheroids consisting of DU-145 NDRG1 KD cells exhibited highly invasive behavior (Fig. 5B).18 Treatment of spheroids with 5 µM CTAB resulted in a decrease in growth of spheroids in both cell lines, indicating that CTAB was effectively targeting both DU-145 cell lines. Invasion of the DU-145 NDRG1 KD spheroids into the collagen matrix was markedly reduced after CTAB treatment, the invasion index of untreated controls being 0.57, while the invasion index of CTAB treated cells was 0.26 (Fig. 5C). Thus, apart from selectively targeting NDRG1 deficient DU-145 cells, CTAB significantly reduced invasion in NDRG1 deficient DU-145 cells as well (p = 0.01).
Figure 5. CTAB treatment results in decreased invasion of DU-145 NDRG1 KD cells. (A–B) 3-D invasion assays were performed with parental DU-145 (A) and DU-145 NDRG1 KD (B) cells. Cells were grown as spheroids in a Collagen type I matrix and treated with CTAB (except for the untreated control). Pictures of four spheroids per treatment group were taken on day 0, 1 and 2. Both changes in size of the spheroids and collective migratory behavior were assessed. (C) The invasion index of CTAB-treated and untreated DU-145 NDRG1 KD cells after 48 h of treatment was determined with ImageJ. For days 1 and 2 the inner (red) area contains non-invading cells and the outer (green) area contains both invading and non-invading cells. The invasion index was calculated as mentioned in the methods section. The graph depicts the invasion index on day 2 of CTAB treatment for DU-145 NDRG1 KD cells. Student’s t-tests were performed to assess the p-value.
Discussion
In the constant search for improved therapy options for cancer patients, there is a growing need for novel chemotherapeutic agents that target metastatic tumors. Chemotherapeutics that target PCa invasion is limited, a major limitation for the development of such chemotherapeutics being the lack of a good model for invasive PCa. We reasoned that engineering PCa cell lines through overexpression of metastasis related oncogenes or KD/knockout of metastasis suppressor genes could be useful in identifying compounds that selectively inhibit PCa invasion. For this purpose, the PCa metastasis suppressor gene NDRG1 was knocked down to generate a cell based screen in this study. By performing a synthetically lethal screen, comparing sensitivity for compounds between wildtype PCa cells and the engineered cells, compounds that selectively target metastasis-prone PCa cells were identified. This type of screens has gained prominence due to its success in identifying cytotoxic agents that target cells with mutations in a particular gene.19,20 The JHDL used in our study has previously been used to identify new anti-tumor agents, such as mycophenolic acid, nitroxoline, itraconazole and ketoconazole as potential inhibitors of angiogenesis;8,21,22 ebselen oxide as an inhibitor of α -methylacyl coenzyme A racemase (AMACR) in PCa, a metabolic enzyme that stimulates PCa cell growth;23 glafenine as an inhibitor of the ATP-binding cassette transporter ABCG2, an enzyme associated with multidrug resistance;24 and digoxin and other cardiac glycosides as inhibitors of HIF-1α synthesis, thereby blocking tumor growth.19,25 As most small molecules in the JHDL are FDA-approved, pharmacokinetic and safety profiles are already known, and the identified compounds can progress rapidly from preclinical development to phase II clinical trials.26 Such a repurposing strategy saves time between preclinical research and implementation of the drug in clinic and reduces costs.26,27
Although these chemical libraries have demonstrated their success in identifying novel compounds for cancer treatment,19 they do have certain limitations which have to be taken into account during screening. Some limitations are inherent to the nature of semi/high-throughput screens, while others include practical limitations related to the nature of the compounds present in the library. A few limitations worth mentioning include: (1) screening libraries often go through many freezing/thawing cycles, therefore the structure and activity of drugs may alter over time; (2) screening libraries are incomplete and often not up-to-date with the most recent drug developments; (3) concentrations chosen for the screen may be outside the effective range of the particular compound, creating false-negative results; (4) screening results contain false-positive results due to experimental limitations (i.e., to limit the use of resources the primary screen is performed only once); and (5) cutoff values to select for hits are chosen arbitrarily. However, robustness of the primary screen in this study was underscored by the identification of known antitumor agents such as cardiac glycosides and the type II topoisomerase inhibitor mitoxantrone as inhibitors of DU-145 proliferation (data not shown).19 The strength of our screen was further enhanced as compounds were validated rigorously through a three tier approach, weeding out false positives.
Due to aforementioned limitations of high-throughput screens, it is important to interpret the data in the light of known information about the drugs. This is well illustrated in our screening results with irinotecan. Although compounds were selected in our secondary screen when having a “DU-145 NDRG1 KD/DU-145”-ratio of ≤ 0.70, hence we selected compounds that selectively target NDRG1 KD cells 30% more effectively than parental DU-145 cells, irinotecan was taken ahead for further investigation as well, despite its “DU-145 NDRG1 KD/DU-145”-ratio of 0.71. The rationale for selection of this compound is based on reports which indicate a negative correlation between NDRG1 expression and sensitivity of cells to irinotecan;10,11,13 our data confirms this inverse relationship. Therefore irinotecan may prove to be of clinical use in patients with advanced NDRG1 deficient PCa.28 Irinotecan is FDA approved for its use as a chemotherapeutic agent in metastatic colorectal cancer. The compound has also been studied in PCa: its effect in PCa was demonstrated for the first time in 1996, when it was being tested successfully in PC3 cells in vitro and in the Dunning R3327 AT6.3 rat model in vivo.29 Subsequently, a phase II clinical trial was performed in hormone-refractory PCa patients.30 In this study, the best response established was stable disease in eight out of 15 patients (53%), which was defined as a PSA decrease of < 50% or a PSA increase of < 25% for at least four weeks. As NDRG1 expression of the tumors was not assessed in the clinical trial, it is tempting to speculate that patients with stable disease might have had PCa with low NDRG1 protein expression.31 However, it is also possible that most patients selected for this study had higher NDRG1 expression levels, as the ECOG performance status of all PCa patients but one was 0/1, irinotecan being given as a first line therapy to patients with hormone-refractory PCa, while patients with NDRG1 deficient PCa generally have a more aggressive disease.
The other compounds we identified that selectively target NDRG1 deficient cells and could potentially be used in the clinic are CTAB/CTAC and STAC. CTAB is a cationic micellar surfactant, part of the group of quaternary ammonium compounds. It is used as a topical antiseptic and part of a group of molecules that (potentially) plays a role in cancer treatment in diverse ways. A cell-based phenotype-driven high-throughput screen recently identified CTAB as a potential compound in the treatment of head and neck cancer (HNC), inducing caspase activated apoptosis by inhibition of H+-ATP synthase activity and depolarization of the mitochondrial membrane potential, thereby decreasing ATP levels in the cell.15 In in vivo experiments CTAB inhibited tumor formation and delayed tumor growth. At the concentrations used in this study, mice had no evidence of toxicity or lethality, similar to our in vitro findings in human prostate fibroblasts. Furthermore, cetrimide (of which CTAB is one component) is used as an effective cytotoxic lavage solution for use during surgery of breast carcinomas,32 and other quaternary ammonium compounds, such as benzethonium chloride, have shown to exhibit anti-tumor activity as well.33
CTAB was identified in our screen as a compound that selectively inhibits proliferation of PCa cells in general and NDRG1 deficient PCa cells in particular. While screenings involving simple endpoint proliferation assays often yield the development of highly selective and potent compounds, such assays provide limited information on how potential therapeutics influence complex multifaceted biological events such as tumor invasion. To investigate whether CTAB affects the invasive capacity of NDRG1 deficient cells, we performed a 3-D spheroid invasion assay. Our data demonstrate that NDRG1 deficient PCa cells exhibit increased invasion on a 3-D collagen matrix, which gets significantly inhibited after CTAB treatment. These results, combined with the anti-tumor activity of CTAB in HNC as indicated by Ito et al.15 urge further exploration of CTAB for its use in cancer therapy.
In summary, this study identifies cetrimonium bromide (CTAB) and irinotecan as clinically established compounds that selectively inhibit NDRG1 deficient PCa cells.7 Future in vivo and clinical studies need to be performed to assess whether administration of CTAB or irinotecan to patients with NDRG1 deficient PCa is clinically beneficial. If these studies confirm the preclinical data presented here, CTAB or irinotecan could potentially be used in personalized medicine in combination with conventional or new PCa treatment methods, to prevent invasion of tumor cells in patients with NDRG1 deficient advanced PCa.
Materials and Methods
Cell lines and treatment
PC3, DU-145 and LNCaP PCa cell lines were obtained from ATCC. Human prostate fibroblasts were obtained from a prostate biopsy on a 62-y old PCa patient with a Gleason score of 4 and kindly provided by Dr J. Isaacs. All cell lines were grown and maintained in RPMI-1640 media (Invitrogen) supplemented with 10% fetal bovine serum (complete RPMI media). Cells were maintained in a humidified incubator at 37°C in a 5% CO2 atmosphere.
JHDL compounds were stored at -20°C in 200 μM stock solutions in DMSO; compounds were dissolved in H2O when they were not soluble in DMSO. Irinotecan (I1406, Sigma-Aldrich) and CTAB (H9151, Sigma-Aldrich) were stored as 10 mM stock solutions in DMSO at -20°C. For experiments the compounds were diluted in complete RPMI media to obtain the desired final concentration.
Knockdown experiment
Stable shRNA KD for NDRG1 in PCa cell lines were generated using MISSION lentiviral systems (Sigma) according to the manufacturer’s recommendations. Control cells were generated using non-mammalian shRNA constructs. Briefly, 1.6 × 104 cells were plated in a 96-well plate and incubated overnight. Cells were transduced with lentiviral particles at a Multiplicity of Infection (MOI) of 1, 2 and 3 with hexadimethrine bromide at a final concentration of 8 µg/ml. Transduced clones were selected with puromycin. KDs were assessed by western blotting. Clones that demonstrated maximum KD with the lowest MOI were selected. For topoisomerase I knockdown siRNA for topoisomerase I (Santacruz Biotech.) was transfected in DU-145 cells using Lipofectamine 2000 reagent (Invitrogen) and knockdown was assessed 48h after transfection by western blotting.
Drug library screen
Parental DU-145 cells and DU-145 NDRG1 KD (DU-145 NDRG1 KD) cells were seeded in 100 μl complete RPMI media in 96-well plates (1.5 × 103 cells/well). The cells were incubated overnight to allow for attachment and subsequently treated with compounds from the JHDL at a final concentration of 10 μM. Cells treated with 0.05% DMSO were used as negative controls. Plates were incubated for 48 h, after which cell viability was measured using CellTiter 96™ AQueous Non-Radioactive Cell Proliferation Assay (Promega) according to the manufacturer’s specifications. Absorption at 490 nm was determined using a SoftMax Pro plate reader (Molecular Devices). Viability of treated cells was compared with viability of DMSO-treated control cells (relative cell viability). Then relative cell viability of DU-145 NDRG1 KD cells was compared with relative cell viability of DU-145 cells (“DU-145 NDRG1 KD/DU-145”-ratio).
IC50 assessment
To assess IC50 values of selected compounds from the library screen in various PCa cell lines, MTS assays were performed in a similar way as in the aforementioned screen. In brief, cells were plated (DU-145 cells at 1.5 × 103 cells/well, LNCaP cells at 2 x 103 cells/well and PC3 cells at 1 × 103 cells/well; all cells were dissolved in 100 µl complete RPMI media), allowed to adhere overnight and treated with the small molecules for a duration of 48 h. MTS reagent (Promega) was added for 2–3 h, after which a colorimetric reading was performed using the SoftMax Pro plate reader (Molecular Devices). Viability of treated cells was compared with viability of DMSO-treated control cells (relative cell viability). Independent two-sample t-tests were performed to compare the relative cell viability in parental cells to the relative cell viability in NDRG1 KD cells. Variances were assumed to be equal in parental and NDRG1 KD cells of the same cell line. When comparing DU-145 cells to human prostate fibroblasts, the variance was assumed to be unequal.
Clonogenic assay
Clonogenic assays were performed to assess long-term survival after treatment with small molecules. Briefly, cells were plated in 60 mm dishes and treated for 48 h. Following treatment, 1 × 103 cells from each dish were plated in triplicate in 60 mm dishes and incubated for 10–12 d. Colonies were fixed and stained in a crystal violet solution (Sigma); dishes were scanned with a computer scanner (Microtek) and counted manually. Student’s t-tests were performed to assess p-values.
Immunoblotting and densitometry
Immunoblotting and densitometry were performed as described previously.34 Cleaved PARP primary antibody (9541, Cell Signaling Technology) and topoisomerase I primary antibody (TG2012–2, TopoGEN) were diluted 1:1,000, NDRG1 primary antibody35 1:4,000, Vinculin primary antibody [05–286, Millipore (Upstate)] 1:10,000; all antibodies were dissolved in blocking buffer [5% milk in TBST (100 mM TRIS-HCl pH 7.4, 0.1% Tween 20, 150mM NaCl in H2O)]. Secondary antibodies were dissolved in blocking buffer (5% milk in TBST) at 1:4,000 dilution.
Flow cytometry
Cells were plated in 60 mm dishes and allowed to adhere overnight before treatment with the compounds. Forty eight hours after treatment, cells were processed for annexin V (BD Bioscience) staining as recommended by the manufacturer. Annexin V and PI positive cells were scored using FACS (BD Biosciences FACSCalibur) to detect early apoptotic and late apoptosis respectively.
3-D invasion assay
Trypsinized DU-145 and DU-145 NDRG1 KD cells were suspended in complete RPMI media containing 0.5% methylcellulose. Ten microliters of suspended cells were placed on the inner side of the lid of a sterile bacterial Petri plate and cultured as a hanging drop over a humidified plate in a CO2 incubator for 16 h. The generated spheroids were used for 3-D invasion assays by embedding them in collagen matrix (BD Biosciences), which was prepared according to the manufacturer’s instructions. CTAB was administered and the spheroids were imaged daily using a Nikon Eclipse Ti microscope (Nikon) under phase contrast. For calculation of the invasion index the total area over which the spheroid had dispersed (including invading and non-invading cells) and the area of non-invading cells (at the center of the spheroid) were measured using ImageJ. Values were expressed as an average of at least 3 invasion index calculations using the formula invasion index = 1–(non-invading area/total area). Comparisons were done using the student’s t-test.
Supplementary Material
Acknowledgments
The authors wish to thank Profs. E. Van der Wall, H. Gelderblom and J.W.R. Nortier for their support and discussion and Dr J.T. Isaacs and S. Chen for their provision of the human prostate fibroblast cell line. The study was supported by the Flight Attendant Medical Research Institute (FAMRI), Koch Award, NCI SPORE Grant P50CA58236 and NIH CA122814. MDW was financially supported by fellowships from the Dr Saal van Zwanenbergstichting, the HSP Talentenprogramma and Leiden University. JM is supported by an AEGON International Fellowship.
Glossary
Abbreviations:
- PCa
prostate cancer
- HNC
head and neck cancer
- NDRG1
N-myc downstream regulated gene 1
- KD
knockdown
- AMACR
α -methylacyl coenzyme A racemase
- CTAB
cetrimonium bromide
- CTAC
cetrimonium bromide
- STAC
stearyltrimethylammonium chloride
- NCP
neocuproine
- CuCl2
cupric chloride
- NaCl
sodium chloride
- JHDL
Johns Hopkins Drug Library
- FDA
Food and Drug Administration
- shRNA
short hairpin RNA
- MOI
Multiplicity of Infection
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/cbt/article/23759
References
- 1.seer.cancer.gov. [Web site]. Table 23.8 Cancer of the Prostate (Invasive): 5-Year Relative and Period Survival (Percent) by Race, Diagnosis Year, Stage and Age. Available at: http://seer.cancer.gov/csr/1975_2007/results_merged/sect_23_prostate.pdf Accessed: August 20, 2012.
- 2.Suva LJ, Washam C, Nicholas RW, Griffin RJ. Bone metastasis: mechanisms and therapeutic opportunities. Nat Rev Endocrinol. 2011;7:208–18. doi: 10.1038/nrendo.2010.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karayi MK, Markham AF. Molecular biology of prostate cancer. Prostate Cancer Prostatic Dis. 2004;7:6–20. doi: 10.1038/sj.pcan.4500697. [DOI] [PubMed] [Google Scholar]
- 4.Shoushtari AN, Szmulewitz RZ, Rinker-Schaeffer CW. Metastasis-suppressor genes in clinical practice: lost in translation? Nat Rev Clin Oncol. 2011;8:333–42. doi: 10.1038/nrclinonc.2011.65. [DOI] [PubMed] [Google Scholar]
- 5.Bandyopadhyay S, Pai SK, Gross SC, Hirota S, Hosobe S, Miura K, et al. The Drg-1 gene suppresses tumor metastasis in prostate cancer. Cancer Res. 2003;63:1731–6. [PubMed] [Google Scholar]
- 6.Kachhap SK, Faith D, Qian DZ, Shabbeer S, Galloway NL, Pili R, et al. The N-Myc down regulated Gene1 (NDRG1) Is a Rab4a effector involved in vesicular recycling of E-cadherin. PLoS One. 2007;2:e844. doi: 10.1371/journal.pone.0000844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu W, Xing F, Iiizumi-Gairani M, Okuda H, Watabe M, Pai SK, et al. N-myc downstream regulated gene 1 modulates Wnt-β-catenin signalling and pleiotropically suppresses metastasis. EMBO Mol Med. 2012;4:93–108. doi: 10.1002/emmm.201100190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chong CR, Qian DZ, Pan F, Wei Y, Pili R, Sullivan DJ, Jr., et al. Identification of type 1 inosine monophosphate dehydrogenase as an antiangiogenic drug target. J Med Chem. 2006;49:2677–80. doi: 10.1021/jm051225t. [DOI] [PubMed] [Google Scholar]
- 9.Sink R, Gobec S, Pečar S, Zega A. False positives in the early stages of drug discovery. Curr Med Chem. 2010;17:4231–55. doi: 10.2174/092986710793348545. [DOI] [PubMed] [Google Scholar]
- 10.Motwani M, Sirotnak FM, She Y, Commes T, Schwartz GK. Drg1, a novel target for modulating sensitivity to CPT-11 in colon cancer cells. Cancer Res. 2002;62:3950–5. [PubMed] [Google Scholar]
- 11.Shah MA, Kemeny N, Hummer A, Drobnjak M, Motwani M, Cordon-Cardo C, et al. Drg1 expression in 131 colorectal liver metastases: correlation with clinical variables and patient outcomes. Clin Cancer Res. 2005;11:3296–302. doi: 10.1158/1078-0432.CCR-04-2417. [DOI] [PubMed] [Google Scholar]
- 12.Chen SH, Lin JK, Liu SH, Liang YC, Lin-Shiau SY. Apoptosis of cultured astrocytes induced by the copper and neocuproine complex through oxidative stress and JNK activation. Toxicol Sci. 2008;102:138–49. doi: 10.1093/toxsci/kfm292. [DOI] [PubMed] [Google Scholar]
- 13.Zheng Y, Wang LS, Xia L, Han YH, Liao SH, Wang XL, et al. NDRG1 is down-regulated in the early apoptotic event induced by camptothecin analogs: the potential role in proteolytic activation of PKC delta and apoptosis. Proteomics. 2009;9:2064–75. doi: 10.1002/pmic.200800031. [DOI] [PubMed] [Google Scholar]
- 14.Xu Y, Villalona-Calero MA. Irinotecan: mechanisms of tumor resistance and novel strategies for modulating its activity. Ann Oncol. 2002;13:1841–51. doi: 10.1093/annonc/mdf337. [DOI] [PubMed] [Google Scholar]
- 15.Ito E, Yip KW, Katz D, Fonseca SB, Hedley DW, Chow S, et al. Potential use of cetrimonium bromide as an apoptosis-promoting anticancer agent for head and neck cancer. Mol Pharmacol. 2009;76:969–83. doi: 10.1124/mol.109.055277. [DOI] [PubMed] [Google Scholar]
- 16.Boca SC, Astilean S. Detoxification of gold nanorods by conjugation with thiolated poly(ethylene glycol) and their assessment as SERS-active carriers of Raman tags. Nanotechnology. 2010;21:235601. doi: 10.1088/0957-4484/21/23/235601. [DOI] [PubMed] [Google Scholar]
- 17.Hauck TS, Ghazani AA, Chan WC. Assessing the effect of surface chemistry on gold nanorod uptake, toxicity, and gene expression in mammalian cells. Small. 2008;4:153–9. doi: 10.1002/smll.200700217. [DOI] [PubMed] [Google Scholar]
- 18.Friedl P, Hegerfeldt Y, Tusch M. Collective cell migration in morphogenesis and cancer. Int J Dev Biol. 2004;48:441–9. doi: 10.1387/ijdb.041821pf. [DOI] [PubMed] [Google Scholar]
- 19.Zhang H, Qian DZ, Tan YS, Lee K, Gao P, Ren YR, et al. Digoxin and other cardiac glycosides inhibit HIF-1alpha synthesis and block tumor growth. Proc Natl Acad Sci U S A. 2008;105:19579–86. doi: 10.1073/pnas.0809763105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guo W, Wu S, Liu J, Fang B. Identification of a small molecule with synthetic lethality for K-ras and protein kinase C iota. Cancer Res. 2008;68:7403–8. doi: 10.1158/0008-5472.CAN-08-1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chong CR, Xu J, Lu J, Bhat S, Sullivan DJ, Jr., Liu JO. Inhibition of angiogenesis by the antifungal drug itraconazole. ACS Chem Biol. 2007;2:263–70. doi: 10.1021/cb600362d. [DOI] [PubMed] [Google Scholar]
- 22.Shim JS, Matsui Y, Bhat S, Nacev BA, Xu J, Bhang HE, et al. Effect of nitroxoline on angiogenesis and growth of human bladder cancer. J Natl Cancer Inst. 2010;102:1855–73. doi: 10.1093/jnci/djq457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilson BA, Wang H, Nacev BA, Mease RC, Liu JO, Pomper MG, et al. High-throughput screen identifies novel inhibitors of cancer biomarker α-methylacyl coenzyme A racemase (AMACR/P504S) Mol Cancer Ther. 2011;10:825–38. doi: 10.1158/1535-7163.MCT-10-0902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Y, Byun Y, Ren YR, Liu JO, Laterra J, Pomper MG. Identification of inhibitors of ABCG2 by a bioluminescence imaging-based high-throughput assay. Cancer Res. 2009;69:5867–75. doi: 10.1158/0008-5472.CAN-08-4866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.clinicaltrials.gov. [Internet]. Bethesda (MD): US National Institutes of Health (NIH); 2010 [updated 2011 Nov 29; cited 2012 Dec 6]. Available from: http://clinicaltrials.gov/ct2/show/NCT01162135?
- 26.Chong CR, Sullivan DJ., Jr. New uses for old drugs. Nature. 2007;448:645–6. doi: 10.1038/448645a. [DOI] [PubMed] [Google Scholar]
- 27.DiMasi JA, Hansen RW, Grabowski HG. The price of innovation: new estimates of drug development costs. J Health Econ. 2003;22:151–85. doi: 10.1016/S0167-6296(02)00126-1. [DOI] [PubMed] [Google Scholar]
- 28.Song Y, Oda Y, Hori M, Kuroiwa K, Ono M, Hosoi F, et al. N-myc downstream regulated gene-1/Cap43 may play an important role in malignant progression of prostate cancer, in its close association with E-cadherin. Hum Pathol. 2010;41:214–22. doi: 10.1016/j.humpath.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 29.Lievano G, Mirochnik Y, Rubenstein M, Shaw M, Guinan P. Antitumor effect of CPT-11, a new derivative of camptothecin, against human prostate cancer (PC-3) in vitro and prostate rat tumor (AT-3) in vivo. Methods Find Exp Clin Pharmacol. 1996;18:659–62. [PubMed] [Google Scholar]
- 30.Reese DM, Tchekmedyian S, Chapman Y, Prager D, Rosen PJ. A phase II trial of irinotecan in hormone-refractory prostate cancer. Invest New Drugs. 1998;16:353–9. doi: 10.1023/A:1006120910380. [DOI] [PubMed] [Google Scholar]
- 31.Caruso RP, Levinson B, Melamed J, Wieczorek R, Taneja S, Polsky D, et al. Altered N-myc downstream-regulated gene 1 protein expression in African-American compared with caucasian prostate cancer patients. Clin Cancer Res. 2004;10:222–7. doi: 10.1158/1078-0432.CCR-0604-3. [DOI] [PubMed] [Google Scholar]
- 32.Park KG, Chetty U, Scott W, Miller W. The activity of locally applied cytotoxics to breast cancer cells in vitro. Ann R Coll Surg Engl. 1991;73:96–9. [PMC free article] [PubMed] [Google Scholar]
- 33.Yip KW, Mao X, Au PY, Hedley DW, Chow S, Dalili S, et al. Benzethonium chloride: a novel anticancer agent identified by using a cell-based small-molecule screen. Clin Cancer Res. 2006;12:5557–69. doi: 10.1158/1078-0432.CCR-06-0536. [DOI] [PubMed] [Google Scholar]
- 34.Kachhap SK, Rosmus N, Collis SJ, Kortenhorst MS, Wissing MD, Hedayati M, et al. Downregulation of homologous recombination DNA repair genes by HDAC inhibition in prostate cancer is mediated through the E2F1 transcription factor. PLoS One. 2010;5:e11208. doi: 10.1371/journal.pone.0011208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Piquemal D, Joulia D, Balaguer P, Basset A, Marti J, Commes T. Differential expression of the RTP/Drg1/Ndr1 gene product in proliferating and growth arrested cells. Biochim Biophys Acta. 1999;1450:364–73. doi: 10.1016/S0167-4889(99)00056-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.