Abstract
Many studies have provided convincing evidence for hERG as an important diagnostic and prognostic factor in human cancers, as well as a useful target for antineoplastic therapy. Our previous study also revealed that knockdown of herg gene expression by shRNA interference inhibited the growth of neuroblastoma cells in vitro and in vivo. In the experiment, a novel 4-amino piperidine analog, ZC88, was examined for its effect on hERG potassium channels and its antitumor potency was observed in vitro and in vivo. The results showed that ZC88 could block hERG1 and hERG1b channels expressed in Xenopus oocytes in a concentration-dependent manner. ZC88 displayed significant antiproliferative activity in several tumor cell lines and the tumor cells with higher expression of hERG presented higher sensitivity to ZC88. The mitotic progression of tumor cells was markedly suppressed in the presence of ZC88 through arresting cells in G0/G1 phase. ZC88 significantly inhibited the tumor growth in nude mice at a dosage with slight influence on the cardiac QT interval. The antitumor effect of ZC88 was correlated at least partly with its blockage of hERG channels, which implicated a positive role of hERG potassium channel in tumor cell proliferation.
Keywords: 4-aminopiperidine, hERG, potassium channel, SH-SY5Y, neuroblastoma, cancer, cell cycle
Introduction
Targeting ion channels in cancer is a novel frontier in antineoplastic therapy. The expression and activation of different types of channels mark and regulate specific stages of cancer progression, from control of cell proliferation and apoptosis to regulation of invasiveness and metastatic spread.1 Evidence is particularly extensive for the hERG, encoded by human ether-a-go-go-related gene 1(herg1), belong to the family voltage-gated K+ channels. hERG1 channel is typically expressed in excitable cells, such as neurons and cardiac myocytes, where it regulates resting potential and action potential repolarization.2 hERG1 and its truncated N-deleted variant, hERG1b, are preferentially expressed in tumor cell lines of different histogenesis, as well as in primary human cancers, but absent from the healthy cells from which the respective tumor cells are derived.3-6 Both full-length hERG1 and hERG1b proteins were co-expressed and could form heterotetrameric channels on the plasma membrane of tumor cells. Selective pharmacological blockage of the hERG channel dramatically impaired cell growth of hERG-bearing tumor cells.5,6 Our previous study has also revealed that knockdown of herg gene expression by the use of shRNA interference reduced the growth rate, viability and colony formation of neuroblastoma cells. In nude mice, shRNA targeting hERG channels could inhibit the proliferation of tumor cells.7 Both hERG1 and hERG1b produced functional potassium current and play a role in cell proliferation, invasion and vascular endothelial growth factor secretion.8-10 hERG is thus proposed as a novel diagnostic and prognostic factor in human cancers, as well as an attractive oncology target.
ZC88 is a novel compound with 4-amino piperidine scaffold (Fig. 1), designed and synthesized by our institutes. Previous study showed that ZC88 blocked N-type calcium channel currents in a concentration-dependent manner with an IC50 value of 0.45 ± 0.09 μM. Intra-gastric administration of ZC88 (20–80 mg/kg) presented analgesia and antiallodynia effect in acute and neuropathic pain animal models. In addition, ZC88 (10–80 mg/kg) enhanced morphine analgesia and attenuated morphine-induced tolerance and physical dependence.11 Further study showed that ZC88 also inhibited hERG potassium current significantly.
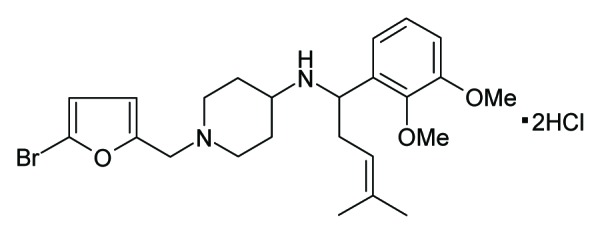
Figure 1. Structure of ZC88
It was reported that approximately 30–50% of all cancer patients experience moderate to severe pain, and 75–95% of patients at advanced-stage or with metastatic cancer, experience substantial life-altering cancer-induced pain.12,13 It suggests that developing antineoplastic agents with the dual efficacy of antineoplastic and analgesia should be an ideal strategy. As the analgesic effect of ZC88 has been confirmed, it is worthwhile to explore the anticancer effect of ZC88 based on its blockage of hERG. In the present study, we further observed the effect of ZC88 on hERG1 and hERG1b potassium channels transiently expressed in Xenopus oocytes and then, determined the antitumor potency of ZC88 in vitro and in vivo. We hope to provide helpful evidence for developing a new kind of antitumor drug with analgesic effect.
Results
ZC88 blocks hERG1 and hERG1b potassium channels transiently expressed in Xenopus oocytes
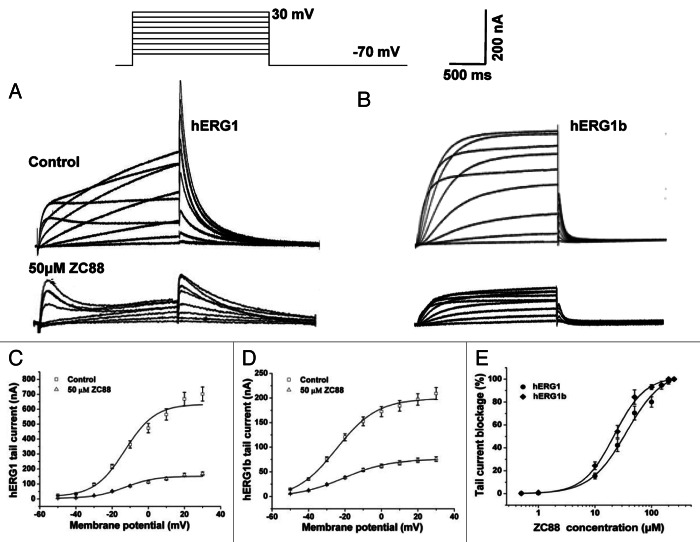
To record the currents from hERG1 or hERG1b channels expressed in oocytes, the cell membrane potential was held at -70 mV and depolarized to voltages between -50 and +30 mV for 2 sec in 10 mV increments. Outward tail current was recorded upon repolarization to -70 mV for 2 sec. Representative hERG1 and hERG1b current traces in the control and in the presence of ZC88 were shown in Figure 2A and B, respectively. hERG1 exhibited a characteristic of high and spiky tail current, whereas its spliced variant hERG1b displayed a small and spiky tail current. When ZC88 (50 μM) was administered, both amplitudes of tail currents of hERG1 and hERG1b were significantly reduced (Fig. 2C and D). The I-V relationship of hERG1 tail currents revealed that the half-point activation voltage values were not affected remarkably (-18.61 ± 1.73 mV and -18.91 ± 1.77 mV for the control and ZC88 group, respectively, Figure 2C). However, the half-point activation voltage values of hERG1b tail currents were significantly shifted from -23.11 ± 1.11 mV to -20.34 ± 0.96 mV (p < 0.05, n = 4, Fig. 2D), suggesting a positive shift in the voltage dependence of activation.
Figure 2. The blocking effect of ZC88 on hERG1 and hERG1b potassium channel current transiently expressed in Xenopus oocytes. hERG1 and hERG1b currents were activated by applying voltage pulses for 2 sec from -50 to +30 mV (10 mV increments) with a holding potential of -70 mV. Outward tail current was recorded upon repolarization to -70 mV for 2 sec. (A) and (B), representative current traces for control (upper traces) and in the presence of 50 μM ZC88 (lower traces) are respectively shown for hERG1 and hERG1b. (C)I-V relationships for hERG1 tail current before and after application of ZC88 (50 μM). (D)I-V relationships for hERG1b tail current before and after application of ZC88 (50 μM). Concentration dependence of tail current blockage by ZC88. Increasing concentrations of ZC88 (0.5–250 μM) were used to perfuse oocyte cells. The percentage of tail current blockage by ZC88 under 0 mV of the membrane potential was calculated for hERG1 and hERG1b, respectively. The values were plotted against the corresponding ZC88 concentrations and fitted with a Hill equation. Data were expressed as mean ± SEM (n = 3–4 oocytes).
To determine the concentration-effect relationship for ZC88 on hERG1 and hERG1b channels, increasing concentrations of ZC88 (0.5–250 μM) were added to perfuse oocyte cells and current amplitudes were monitored 5 min after drug administration with the same voltage protocol. The percentage of tail current blockage by ZC88 under 0 mV of the membrane potential was calculated for hERG1 and hERG1b, respectively. Then the values were plotted against the corresponding ZC88 concentration as shown in Figure 2E and fitted with a Hill equation. The results showed that the blockage of hERG1 and hERG1b currents by ZC88were dependent on its concentrations, with stronger blockage at higher concentration. The half-maximal IC50 was 35.86 ± 2.48 µM for hERG1 and 21.97 ± 2.13 µM for hERG1b (p < 0.05, n = 3), suggesting that hERG1b was more sensitive than hERG1 to ZC88. The blockage was relieved slowly and incompletely upon removal of ZC88. Control values were not reached even after 10 min wash-out (n = 3 oocytes).
Effects of ZC88 on the viability of several human tumor cell lines with or without hERG K+ channel
Accumulated investigations showed that hERG K+ channel blockage with drug or small RNA interference inhibited the proliferation of tumor cell significantly.7,14-17 To further evaluate the effect of ZC88 on the growth and proliferation of tumor cells, several human tumor cells with or without hERG K+ channels were used and cell viability was assayed. As shown in Figure 3A and B, both hERG1 mRNA and protein were expressed on SH-SY5Y and HT29 cell lines, but not expressed on A549, H157 and H1299 cell lines. The five cell lines were respectively treated with ZC88 (3.125–100 μM) for 24 h and cell viability was determined by the sulforhodamine B (SRB) assay. The results showed that ZC88 suppressed cell growth in a concentration-dependent manner in all five cell lines, but the inhibitory effect was relatively stronger on the two cell lines expressed with hERG1, SH-SY5Y and HT29. As shown in Figure 3C, the 50% growth-inhibitory concentration (GI50) values by ZC88 on SH-SY5Y and HT-29 cells were 12.9 ± 0.5 µM and 13.7 ± 0.62 µM, respectively. In Comparison, A549, H157 and H1299 cells which did not express hERG1 were not much sensitive to ZC88, with GI50 of 22.1 ± 1.4 µM, 26.1 ± 1.5 µM and 31.5 ± 1.5 µM, respectively. One-way ANOVA analysis followed by Dunnett’s t-test showed the statistic difference among cells with or without hERG1 (p < 0.01, n = 3).
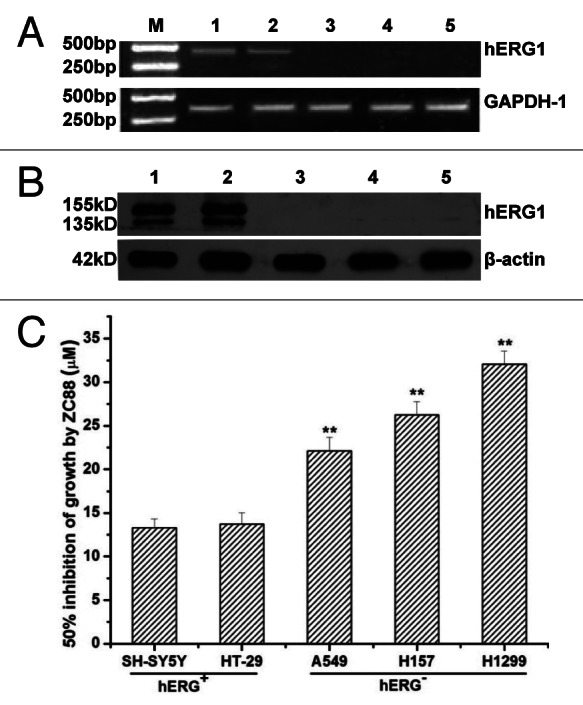
Figure 3. Effects of ZC88 on the viability of several human tumor cell lines with or without hERG K+ channel. (A) and (B), Expression of hERG channel mRNA and protein in several tumor cell lines were identified by RT-PCR (A) and western blot analysis (B), respectively. (C) The cell lines were respectively treated with ZC88 (3.125–100 μM) for 24 h. Cell viability was determined by SRB assay. The GI50 by ZC88 was calculated with Logistic equation. Data were expressed as mean ± SEM **p < 0.01, compared with the GI50 of SH-SY5Y or HT29 cells. (n = 3 individual experiments; M: DNA marker, 1: SH-SY5Y, 2: HT-29, 3: A549, 4: H1299, 5: H157).
Effect of ZC88 on the viability of human embryonic kidney (HEK) 293 cells stably transfected with hERG
To further evaluate the role of hERG1 expression in ZC88-mediated cytostatic effect, we determined cell growth suppression induced by ZC88 in cells of HEK293 overexpressed with hERG1. HEK293 cells were transfected with a plasmid of pcDNA3.1 encoding hERG1or vector only. After geneticin selection, HEK293 cell clones expressing hERG1 were identified by western blot analysis with hERG1 antibody (Fig. 4A). Vector-transfected and hERG1-transfected HEK293 cells and parental cells were then analyzed for their susceptibility to ZC88 by SRB assay. The results showed that ZC88 induced marked growth suppression on the hERG1 expressed HEK293 clone, when compared with vector-transfected HEK293 cells. The GI50 values of ZC88 on vector- and hERG1-transfected HEK293 cells were 31.65 ± 1.34 µM and 15.93 ± 1.00 µM, respectively (Fig. 4B, p < 0.01). The result further proved the role of hERG1 in ZC88-mediated antiproliferative effect, coinciding with the data from tumor cells with hERG1 expression.
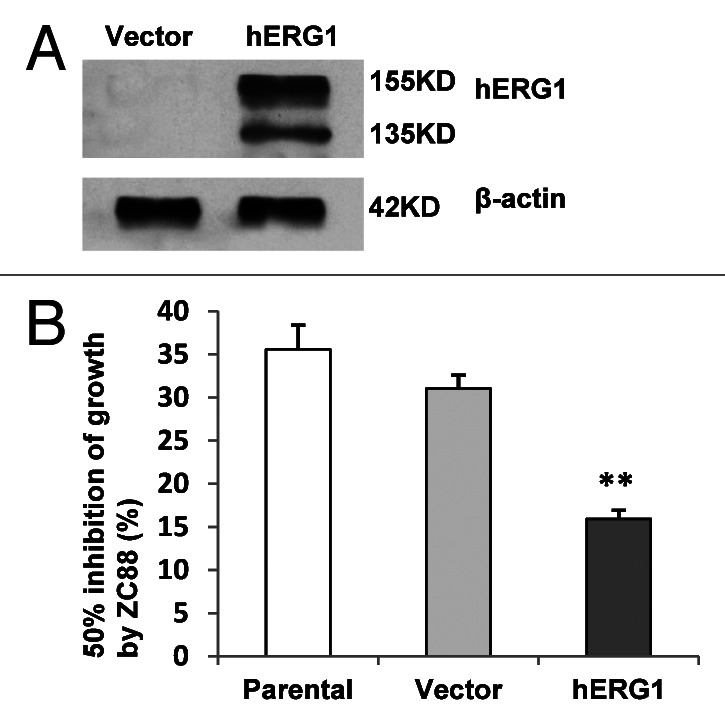
Figure 4. Effect of ZC88 on the viability of HEK293 cells stably transfected with hERG1. (A) HEK293 cells were transfected with a plasmid of pcDNA3.1 encoding hERG1 or vector only. HEK293 clone expressing hERG1 was identified by western blot analysis with hERG1 antibody. (B) The cell lines stably transfected with hERG1or pcDNA3.1 vector and parental HEK293 cells were respectively treated with ZC88 (3.125–100 μM) for 24 h. Cell viability was determined by SRB assay. The GI50 by ZC88 was calculated with Logistic equation. Data were expressed as mean ± SEM ** p < 0.01, compared with the GI50 of vector-transfected HEK293 cells. (n = 3 individual experiments).
ZC88 inhibits cell cycle progression in SH-SY5Y cells
It was known that hERG K+ channel was involved in the regulation of cell mitotic progression and cell proliferation in tumor cells. To confirm the inhibitory effect of ZC88 on cell growth, we further evaluated the effect of ZC88 on cell growth cycle in SH-SY5Y cells by flow cytometric analysis. The results showed that the percentage of G0/G1 phase increased from 40.8% to 62.04%, and that of S phase decreased from 52.92% to 30.31%, 24 h after ZC88 treatment (3.125–25 μM). This assay was repeated and similar result was obtained. The data indicated that ZC88 markedly suppressed the cell mitotic progression in a concentration-dependent manner through arresting cells in G0/G1 phase and decreasing DNA synthesis (Fig. 5).
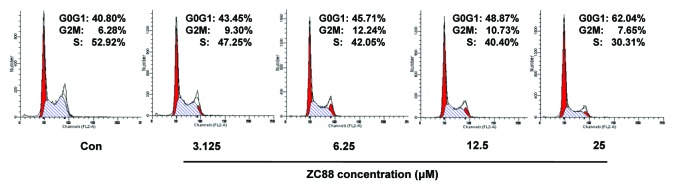
Figure 5. Effect of ZC88 on the cell cycle progression of SH-SY5Y cells. Cells were treated by ZC88 (3.125–25 μM) for 24 h. The cells were harvested and labeled with PI. Cell cycle distribution was assayed by flow cytometric analysis. The histograms displayed here were representative of two experiments.
ZC88-mediated antiproliferative effect is not correlated with its N-type voltage-dependent calcium channel blockage
Considering the blockage of N-type calcium channels by ZC88 in the previous study,11 we further determined the potential relationship between ZC88-mediated cell grown inhibition and calcium channel blockage. Ziconotide, a synthetic neuroactive peptide equivalent of an omega conotoxin derived from the venom of a marine snail, is a neuron-specific N-type calcium channel blocker. After SH-SY5Y cells were treated with Ziconotide (5–50 μM) or ZC88 (5–50 μM) for 24 h, cell viability was assayed (Fig. 6A). The result indicated that Ziconotide didn’t have any effect on the growth and proliferation of SH-SY5Y cell. Similar result was obtained from HT29 cells (data not shown). Further, the mRNA expression of functional α1B subunit of the N-type calcium channel was detected in several tumor cells including SH-SY5Y and HT29. RT-PCR data showed that both SH-SY5Y and HT-29 cells with hERG channel didn’t express N-type calcium channel mRNA (Fig. 6B). Therefore, ZC88-mediated antiproliferative effect may not be related with its calcium channel blockage.
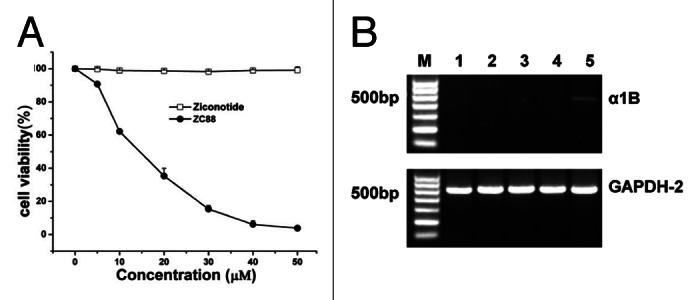
Figure 6. Effect of N-type calcium channel blocker ziconotide on SH-SY5Y cell viability. (A) Cell viability assay. The cells were treated by ziconotide or ZC88 (5–50 μM) for 24 h and cell viability was determined by SRB assay. Cells without treatment were used as control, with viability set at 100%. Each data point represented the mean ± SEM of three independent experiments. (B) α1B subunit of N-type calcium channel mRNA expression on cancer cells by RT-PCR. M: DNA marker, 1: SH-SY5Y, 2: HT-29, 3: A549, 4: H157, 5: H1299.
In vivo antitumor activity of ZC88 in SH-SY5Y xenografted nude mice
To assess the potential effect of ZC88 on tumor growth in vivo, SH-SY5Y cells were inoculated into the dorsal flank of each mouse and grown as xenografts in Balb/c nude mice. Intraperitoneal injection with ZC88 or saline was initiated after the tumors grew to 5 mm in diameter. The results showed that mean tumor size was smaller in ZC88 treated animals over the treated days, as compared with the saline group. And the tumor suppression of ZC88 was better at 50 mg/kg dose than lower doses (Fig. 7A). As shown in Figure 7B, mean tumor weight in ZC88 (50 mg/kg) treated group was markedly less than that of saline group at the endpoint of the study (1.52 ± 0.35 g and 3.08 ± 0.53 g, respectively, p < 0.05, n = 7–8). During the investigation, body weights were monitored every two days to assess toxicity. Slight body weight loss was seen in 50 mg/kg group, which led to the alteration of injection times in this group, from every two days designed originally to every four days. At the end of the experiment, mean body weight loss was about 10% in ZC88 (50 mg/kg) treated mice and other signs of toxicity were not seen.
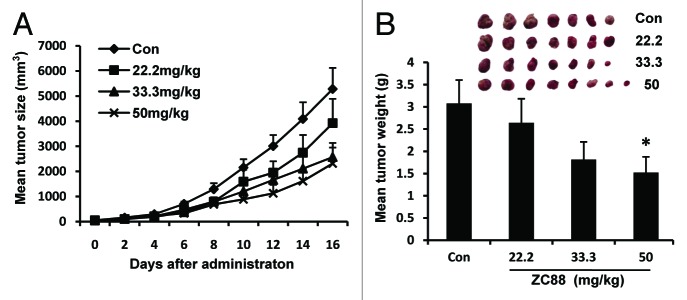
Figure 7. Antitumor activity of ZC88 in xenografted nude mice. (A) Changes of tumor size over days after administration. (B) Mean tumor weight at the end point of experiment. Treatment was initiated at D0 when the tumor nodules reached 5 × 5 mm. For the control and the groups of 22.2 and 33.3 mg/kg ZC88, saline or ZC88 was given intraperitoneally every two days. For the group of 50 mg/kg, ZC88 was given intraperitoneally every four days. Tumor volumes (mm3) were determined every two days by caliper measurements and were calculated by the formula: ab2/2. At the end of the experiment, the tumor nodules were excised and weighed. Data were expressed as mean ± SEM *p < 0.05, compared with saline control, n = 7–8.
Effect of ZC88 on the corrected QT (QTc) interval in anaesthetized guinea pigs
To evaluate the effects of ZC88 on the cardiac action potentials, guinea pigs were used as an animal model. The results showed that ZC88 at the dose of 20 and 30 mg/kg did not significantly influence the QTc values of guinea pigs (Fig. 8, n = 5, p > 0.05). However, ZC88 (50 mg/kg) in guinea pigs could prolong the QTc values of electrocardiograms (ECGs) as early as 15 min after administration. The QTc values were prolonged by 18.74 ± 5.15% at 45 min after administration (Fig. 8, n = 5, p < 0.01).
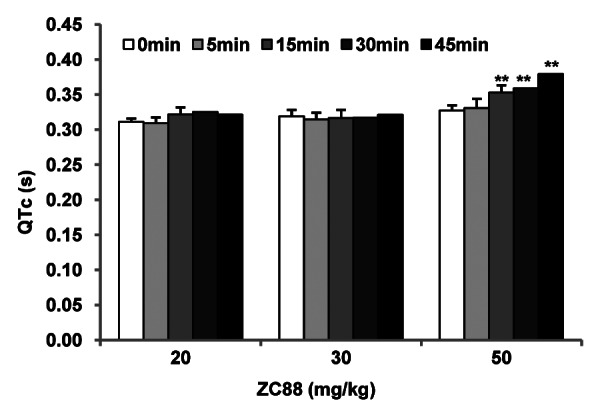
Figure 8. Effect of ZC88 on the QTc interval in anaesthetized guinea pigs. Guinea pigs were anesthetized with sodium pentobarbital. Needle electrodes, inserted subcutaneously, were used to record the ECG of standard limb lead II. After 10 min of equilibration for a stable baseline measurement (0 min), 20, 30 or 50 mg/kg of ZC88 was given by intraperitoneal injection and the ECGs were recorded at 5, 15, 30 and 45 min thereafter. The QTc was calculated with the Bazett formula (QT c = QT × RR-1/2). Data were expressed as mean ± SEM **p < 0.01, compared with the QTc of 50 mg/kg group at 0 min (n = 5).
Discussion
Many expression and channel activity studies have indicated a tight association between hERG and cancers. Either blocking hERG channels or reducing hERG expression inhibited cell proliferation, invasion and therefore disease progression in many tissues of cancer. These results provide important clues for the potential pharmacological use of hERG modulators in cancer treatment.18-20
ZC88 is a novel compound designed and synthesized by our institute based on a 4-aminopiperidine scaffold. Previous study showed that ZC88 exhibited a marked N-type calcium channel blockage. N-type calcium channels has been validated as a potential target for pain relief, which are located mainly on pre-synaptic terminal in central and peripheral neurons, directly mediated spinal transmission of pain signals from the peripheral to the central nervous system.21 Ziconotide, a selective N-type calcium channel inhibitor, has been clinically used for the amelioration of severe and chronic pain. Further study showed that ZC88 could inhibit hERG potassium current. So it is intriguing to know whether ZC88 has antitumor activity based on its blockage of hERG channels.
In the present study, it was found that ZC88 significantly inhibited hERG1 and hERG1b potassium channel currents transiently expressed in Xenopus oocytes in a concentration-dependent manner. ZC88 had evident antiproliferative activity in several cancer cell lines. The cells with hERG channels (SH-SY5Y and HT29) presented higher sensitivity to ZC88 than the cells without hERG channels (H157, H1299 and A549), which implied the participation of hERG channel blockage. To further confirm the relationship between the antitumor effect of ZC88 and hERG channel blockage, a HEK293 cell clone stably transfected with hERG1 cDNA was established and the antiproliferative effect of ZC88 on the hERG1-transfected and vector-transfected control cells was compared. Similar correlation between hERG expression and chemosensitivity to ZC88 was observed in hERG-transfected HEK293 cells. Consistently, another report from Zhen’s group observed the effect of sparfloxacin, a hERG blocker, on both hERG-transfected HEK293 cells and hERG siRNA knockdown HCT116 cells. They demonstrated that the cells with hERG channel were more sensitive than the counterparts, with a merely 2–2.5 fold difference (IC50) in chemosensitivity to sparfloxacin.14 Meanwhile, we found Ziconotide, the N-type calcium channel blocker, did not have similar cell growth inhibition on both SH-SY5Y and HT-29 cells, which suggested that N-type calcium channel might not be involved in the effect of ZC88. These data further implied the participation of hERG channel in the antiproliferative effect of ZC88, although other mechanisms could not be completely excluded. Further mechanisms investigation showed that ZC88 induced significant cell cycle arresting in G0/G1 phase in SH-SY5Y cells, which was one of the characteristics of hERG channel blockage.7,22,23 Thus, all the data supported that the effect of ZC88 on tumor cell growth inhibition was correlated at least partly with its hERG channel blockage, which implied a positive role of hERG potassium channel in tumor cell proliferation.
The in vivo antitumor activity of ZC88 was studied in SH-SY5Y xenografted nude mice, which showed that ZC88 at the dose of 33.3 and 50 mg/kg inhibited the tumor growth by 41% and 50%, respectively. This result again emphasized the role of hERG channel in tumor progression and suggested that blockage of hERG channel induced the tumor growth suppression in vivo. However, hERG blocker alone could not evoke the actual regression of measurable tumors completely. This result was consistent with our previous study with shRNA interference and Zhen’s investigation with sparfloxacin.7,14 The study with shRNA showed that intra-tumor injection of shRNA-herg1/1b remarkably inhibited the growth of SH-SY5Y tumors xenografted in nude mice by about 50%.7 Similarly, Zhen et al. found sparfloxacin at the dose of 800 and 1200 mg/kg inhibited the growth of human colon cancer HCT116 xenografts in nude mice, with a moderate inhibition (44.2 and 58.0%, respectively). However, a combination of 800 mg/kg sparfloxacin and 5-FU at a lower dose (20 mg/kg) inhibited the tumor growth more effectively (62.4%) than sparfloxacin or 5-FU alone (44.2 and 24.9%, respectively).14 In addition, there have been some other reports on hERG channel blocking drugs as an adjuvant cancer therapy.15-17 For example, cisapride, as a specific blocker of hERG K+ channel, prevented gastric cancer cell proliferation.15 and erythromycin, a macrolide antibiotic with hERG-blocking properties, behaved as a chemosensitivity enhancer.16
Recently, Glassmeier G et al. reported that hERG1 protein was involved in the growth of human small cell lung cancer cells, whereas the ion-conducting function of hERG1 was not important for cell growth. Because they found that hERG1 knockdown by siRNA technique inhibited cell proliferation, while blockage of hERG channels by E-4031 did not influence cell proliferation.24 In contrast, both our study and many other groups’ work showed that pharmacological blockage of hERG channels with selective blockers significantly reduced cell proliferation in several tumor cells, including primary leukemia cells,5 colon cancer cells,10 melanoma cells19 and gastric cancer cells.15 This divergence might be derived from the difference among the tumor types. The roles of hERG channels and protein expression for cell growth in different tumor types still need further investigation.
It is well known that blockage of hERG channel will induce prolonged QT interval, leading to potential fatal ventricular tachyarrhythmia known as torsade de pointes.25,26 Therefore, it is inevitable for concerning about the safety of any new drug targeting hERG. In this study, the potential effect of ZC88 on the QTc interval in anaesthetized guinea pigs was evaluated. The results showed that ZC88 at the dose of 30 mg/kg did not significantly influence the QTc values of ECGs in guinea pigs. The QTc values were prolonged by 18.74 ± 5.15% at the dose of 50 mg/kg. Based on the body surface area conversion in different species, ZC88 at the dose of 30 mg/kg in guinea pigs was approximately equivalent to the dose of 50 mg/kg in mice, which meant that 50 mg/kg ZC88 used in vivo antitumor activity in mice might have minor cardiac adverse effect.
In summary, the present study revealed that ZC88, as a hERG channel blocker, inhibited the growth of neuroblastoma SH-SY5Y cells in vitro and in vivo and arrested SH-SY5Y cells in G0/G1 phase. However, ZC88 alone could not evoke the complete regression of the tumor. At the dosage with remarkable antineoplastic effect, ZC88 might have slight influence on the QT interval. The results suggested that ZC88 may be applied in combination with other chemotherapeutic drugs as a chemosensitivity enhancer, which obviously deserve further investigation.
Materials and Methods
Preparation of hERG cRNA
pGh19/hERG1 and pGh19/hERG1b plasmid were kindly presented by Dr Gail A. Robertson (University of Wisconsin-Madison, WI). The plasmid cDNAs were linearized by XbaI restriction enzyme. cRNAs were synthesized in vitro from linearized template cDNA using a RiboMAXTM large scale RNA production system (Promega) and stored at -70°C.
Xenopus oocyte expression and electrophysiology
Oocytes (stages V–VI) were surgically removed from mature Xenopus laevis frogs anesthetized under iced bath. The follicular cell layer was removed by incubating oocytes in Ca2+-free solution containing NaCl 100, KCl 2.0, MgCl2 2.8, HEPES 5.0 (mM, pH 7.6) and penicillin 1 × 105 U/L, streptomycin 100 mg/L plus 1.5 mg/ml collagenase type I (Sigma) for 1.5 h at room temperature. Oocytes were rinsed several times, sorted and maintained at 18°C in ND-96 storage solution containing NaCl 96, KCl 2.0, CaCl2 1.8, MgCl2 1.0, HEPES 5.0 and sodium pyruvate 5.0 (mM, pH 7.6) and penicillin 1 × 105 U/L, streptomycin 100 mg/L. The oocyte was injected with 75 ng of hERG1 or hERG1b channel cRNA in a total of 50 nl RNase-free H2O using a Nanoliter 2000 microinjector (WPI, USA). Injected cells were maintained in ND-96 solution at 18°C for 2–6 d prior to electrophysiological recording.
hERG1 and hERG1b channel proteins were expressed in Xenopus oocytes 48 h after injection of in vitro transcribed mRNAs. K+ channel currents were recorded with the two-microelectrode voltage-clamp technique (Axoclamp2B amplifier, Axon Instruments, USA). The microelectrodes were filled with 3 mol/L KCl. Currents were recorded in ND 96 bath solution at the temperature of 20–23°C. Oocytes showing < 15% change in current amplitude over a 10 min incubation period were used in the experiment.
Cell culture and transfection
The human neuroblastoma SH-SY5Y cell line and colon cancer HT29 cell line were cultured in DMEM/F12 medium (Hyclone) supplemented with 10% and 5% fetal bovine serum, respectively. The human non-small cell lung cancer A549, H157 and H1299 cell lines, human embryonic kidney 293 cell lines were cultured in RPMI 1640 medium (Sigma) supplemented with 10% fetal bovine serum (Hyclone). All cell lines were bought from the Cell Resource Center, IBMS, CAMS/PUMC (Beijing, China) and incubated at 37°C in a humidified atmosphere with 5% CO2.
2.5 × 105 HEK 293 cells were seeded in a 6-well plate to reach 50–70% confluency. Twenty-four h after incubation, the cells were transfected with pcDNA3.1/hERG1 plasmid or pcDNA3.1 vector only via Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. The cell line stably transfected with hERG1 or vector only was obtained by selection under the pressure of geneticin (800 μg/mL) and the expression of hERG1 was identified by western blot.
Cell viability assay
Cells were seeded at a density of 7.5 × 103 cells/well in 96-well plates. After overnight incubation, the cells were treated with ZC88 (3.125–100 μM) for 24 h. Stocks of ZC88 were prepared in distilled water. The inhibitory effects of ZC88 on cell growth were determined by SRB assay, as described previously.27 Each experiment was performed in quadruplicate and repeated for a total of at least three times. Cells treated with medium were used as a control, with viability set at 100%. The GI50 values were calculated with Logistic equation after curve fitting by ORIGIN 7.5 software.
RT-PCR
Total RNA was extracted from cells using TRIzol reagent (Invitrogen). A 2 μg aliquot of each RNA sample was reverse-transcribed in a 25 μl reaction volume using a two-step RT-PCR kit (Takara). PCR was performed in 25 μl of a reaction mixture with the following conditions: 95°C for 5 min, 35 cycles at 94°C for 30 sec, 55°C for 1 min and 72°C for 1 min. The following primer sequences for the genes were used: hERG1 (467 bp), sense 5′-CCTAAGATAAAGGAGCGAACCC-3′, antisense 5′-CCAGAGCCGAAGATGAGCAG-3′; GAPDH-1 (339 bp), sense 5′-GATTTGGTCGTATTGGGGCGC-3′, antisense 5′-CAGAGATGACCCTTTTGGCTCC-3′; α1B subunit of N-type calcium channel (468 bp), sense 5′-TCCCTTCTTCTTCGTCA-3′, antisense 5′-TTCGTTTCCGCAATCT-3′; GAPDH-2 (508 bp), sense 5′-TCAACGGATTTGGTCGTATT-3′, antisense 5′-CTGTGGTCATGAGTCCTTCC-3′. The PCR products were separated on 1% agarose gel.
Western blot
Cells were harvested and subjected to lysis in RIPA buffer. Equal amounts of lysates (50 μg) were separated by 7% SDS-PAGE and then transferred to nitrocellulose membranes (Amersham Biosciences). Membranes were then blocked with PBS buffer containing 5% low fat milk and 0.05% Tween (PBST) for 1 h and then incubated with anti-hERG primary antibodies (1:10000, PA3860, Thermo Scientific) overnight at 4°C. After being washed three times with PBST, membranes were incubated with peroxidase-conjugated secondary antibodies for 1 h at room temperature. The membranes were washed with PBST again and developed with the enhanced chemiluminescence detection kit (ECL kit; Amersham Biosciences). β-actin (Beijing Zhongshan Jinqiao Biotechnology) was used as a loading control.
Cell cycle analysis
The flow cytometric assay was performed to determine the effect of ZC88 on cell cycle progression in SH-SY5Y cells. In brief, cells were seeded at a density of 2.0 × 105 cells/well in six-well plates and allowed to grow overnight. The cells were treated with ZC88 (3.125–25 μM) for 24 h. After treatment, the cells were harvested with trypsin, washed once with PBS and then fixed by incubation with 70% ethanol overnight at 4°C. Before flow cytometric analysis, cells were stained with propidium iodide buffer (PI, containing 200 μg/mL DNase-free RNase A and 50 μg/mL PI) for 30 min. One × 104 cells per sample were analyzed using a FACSCalibur flow cytometer (BD Biosciences). Data were evaluated using Modfit software.
Tumor xenografts in nude mice
Male Balb/c nude mice of 6–8 weeks old were purchased from Beijing Animal Center, China. All animals were kept under specific pathogen-free conditions. The experiment was performed in accordance with the NIH guidelines for the care and use of laboratory animals (1996) and the institutional guidelines. The experimental protocol was approved by the Animal Research Advisory Committee of Beijing institute of pharmacology and toxicology. Tumor was raised by subcutaneous injection of 1 × 106 SH-SY5Y cells/mouse in 100 μl PBS buffer into the flanks of the nude mice. When the tumor nodules reached 5 × 5 mm, the tumor-bearing mice were randomly divided into saline control and three treated groups (22.2, 33.3 and 50 mg/kg ZC88, dissolved in saline). Mice were treated intraperitoneally every two days (totally seven injections) with either normal saline (for control group) or ZC88 (for 22.2 and 33.3 mg/kg group). The mice of 50 mg/kg group were treated only at day 0, 4, 8 (totally three injections). Body weights were monitored to assess toxicity. Tumor volumes (mm3) were determined every two days by caliper measurements and were calculated by the formula: ab2/2, where a means the length and b means the width of the tumor. At the end of the experiment, the mice were sacrificed by dislocation and the tumor nodules were excised and weighed.
ECG recording in guinea pigs
Guinea pigs (weighing 400–500 g) of either sex were used in this study, which were also bought from Beijing Animal Center and cared and used in accordance with the NIH and institutional guidelines. The animals were anesthetized with sodium pentobarbital (40mg/kg, intraperitoneal injection). Needle electrodes, inserted subcutaneously, were used to record the ECG of standard limb lead II (BIOPAC systems Mp150 electrophysiolograph, USA). The RR interval and QT interval were measured respectively. The QTc was calculated with the Bazett formula (QTc = QT × RR-1/2) to reduce the influence of the fluctuation of the heart rate on the measurement of the QT interval. After 10 min of equilibration for a stable baseline measurement, different doses of ZC88 were given by intraperitoneal injection and the ECGs were recorded at 5, 15, 30 and 45 min thereafter.
Statistics and curve fitting
The currents evoked from hERG channels were recorded and analyzed with pCLAMP 9.0 software (Axon Instruments, USA) and ORIGIN 7.5 software (Origin Lab Corporation, Northampton, MA, USA). The concentration-response curves were fitted with Hill equation to obtain the IC50 values. To determine the voltage dependence of hERG current activation, ORIGIN software was used to fit tail current amplitudes to Boltzmann equation. All values were presented as mean ± SEM. For difference between two groups, student’s t-test was performed. For differences among three groups or more, analysis of variance (ANOVA) followed by Dunnett’s t-test was performed. A P value of less than 0.05 was considered significant.
Acknowledgments
This work was supported by “Integrated drug discovery technology platform” (2012ZX09301003–001) of National Science and Technology Major Projects.
We would also like to thank National Center of Biomedical Analysis (Beijing, China) for technical contributions to flow cytometric analysis.
Glossary
Abbreviations:
- HERG
human ether-a-go-go-related gene
- HEK293
human embryonic kidney 293
- SRB
sulforhodamine B
- GI50
50% growth-inhibitory concentration
- ECG
electrocardiogram
- QTc
corrected QT interval
Disclosure of Potential Conflicts of Interest
No potential conflict of interest was declared.
Footnotes
Previously published online: www.landesbioscience.com/journals/cbt/article/24423
References
- 1.Arcangeli A, Crociani O, Lastraioli E, Masi A, Pillozzi S, Becchetti A. Targeting ion channels in cancer: a novel frontier in antineoplastic therapy. Curr Med Chem. 2009;16:66–93. doi: 10.2174/092986709787002835. [DOI] [PubMed] [Google Scholar]
- 2.Sanguinetti MC, Tristani-Firouzi M. hERG potassium channels and cardiac arrhythmia. Nature. 2006;440:463–9. doi: 10.1038/nature04710. [DOI] [PubMed] [Google Scholar]
- 3.Bianchi L, Wible B, Arcangeli A, Taglialatela M, Morra F, Castaldo P, et al. herg encodes a K+ current highly conserved in tumors of different histogenesis: a selective advantage for cancer cells? Cancer Res. 1998;58:815–22. [PubMed] [Google Scholar]
- 4.Cherubini A, Taddei GL, Crociani O, Paglierani M, Buccoliero AM, Fontana L, et al. HERG potassium channels are more frequently expressed in human endometrial cancer as compared to non-cancerous endometrium. Br J Cancer. 2000;83:1722–9. doi: 10.1054/bjoc.2000.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pillozzi S, Brizzi MF, Balzi M, Crociani O, Cherubini A, Guasti L, et al. HERG potassium channels are constitutively expressed in primary human acute myeloid leukemias and regulate cell proliferation of normal and leukemic hemopoietic progenitors. Leukemia. 2002;16:1791–8. doi: 10.1038/sj.leu.2402572. [DOI] [PubMed] [Google Scholar]
- 6.Crociani O, Guasti L, Balzi M, Becchetti A, Wanke E, Olivotto M, et al. Cell cycle-dependent expression of HERG1 and HERG1B isoforms in tumor cells. J Biol Chem. 2003;278:2947–55. doi: 10.1074/jbc.M210789200. [DOI] [PubMed] [Google Scholar]
- 7.Zhao J, Wei XL, Jia YS, Zheng JQ. Silencing of herg gene by shRNA inhibits SH-SY5Y cell growth in vitro and in vivo. Eur J Pharmacol. 2008;579:50–7. doi: 10.1016/j.ejphar.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 8.Smith GA, Tsui HW, Newell EW, Jiang X, Zhu XP, Tsui FWL, et al. Functional up-regulation of HERG K+ channels in neoplastic hematopoietic cells. J Biol Chem. 2002;277:18528–34. doi: 10.1074/jbc.M200592200. [DOI] [PubMed] [Google Scholar]
- 9.Masi A, Becchetti A, Restano-Cassulini R, Polvani S, Hofmann G, Buccoliero AM, et al. hERG1 channels are overexpressed in glioblastoma multiforme and modulate VEGF secretion in glioblastoma cell lines. Br J Cancer. 2005;93:781–92. doi: 10.1038/sj.bjc.6602775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lastraioli E, Guasti L, Crociani O, Polvani S, Hofmann G, Witchel H, et al. herg1 gene and HERG1 protein are overexpressed in colorectal cancers and regulate cell invasion of tumor cells. Cancer Res. 2004;64:606–11. doi: 10.1158/0008-5472.CAN-03-2360. [DOI] [PubMed] [Google Scholar]
- 11.Meng G, Wu N, Zhang C, Su RB, Lu XQ, Liu Y, et al. Analgesic activity of ZC88, a novel N-type voltage-dependent calcium channel blocker, and its modulation of morphine analgesia, tolerance and dependence. Eur J Pharmacol. 2008;586:130–8. doi: 10.1016/j.ejphar.2008.02.066. [DOI] [PubMed] [Google Scholar]
- 12.Mercadante S. Recent progress in the pharmacotherapy of cancer pain. Expert Rev Anticancer Ther. 2001;1:487–94. doi: 10.1586/14737140.1.3.487. [DOI] [PubMed] [Google Scholar]
- 13.Mantyh PW, Clohisy DR, Koltzenburg M, Hunt SP. Molecular mechanisms of cancer pain. Nat Rev Cancer. 2002;2:201–9. doi: 10.1038/nrc747. [DOI] [PubMed] [Google Scholar]
- 14.Gong JH, Liu XJ, Shang BY, Chen SZ, Zhen YS. HERG K+ channel related chemosensitivity to sparfloxacin in colon cancer cells. Oncol Rep. 2010;23:1747–56. doi: 10.3892/or_00000820. [DOI] [PubMed] [Google Scholar]
- 15.Shao XD, Wu KC, Hao ZM, Hong L, Zhang J, Fan DM. The potent inhibitory effects of cisapride, a specific blocker for human ether-a-go-go-related gene (HERG) channel, on gastric cancer cells. Cancer Biol Ther. 2005;4:295–301. doi: 10.4161/cbt.4.3.1500. [DOI] [PubMed] [Google Scholar]
- 16.Chen SZ, Jiang M, Zhen YS. HERG K+ channel expression-related chemosensitivity in cancer cells and its modulation by erythromycin. Cancer Chemother Pharmacol. 2005;56:212–20. doi: 10.1007/s00280-004-0960-5. [DOI] [PubMed] [Google Scholar]
- 17.Ganapathi SB, Kester M, Elmslie KS. State-dependent block of HERG potassium channels by R-roscovitine: implications for cancer therapy. Am J Physiol Cell Physiol. 2009;296:C701–10. doi: 10.1152/ajpcell.00633.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arcangeli A. Expression and role of hERG channels in cancer cells. Novartis Found Symp. 2005;266:225–32, discussion 232-4. doi: 10.1002/047002142X.ch17. [DOI] [PubMed] [Google Scholar]
- 19.Afrasiabi E, Hietamäki M, Viitanen T, Sukumaran P, Bergelin N, Törnquist K. Expression and significance of HERG (KCNH2) potassium channels in the regulation of MDA-MB-435S melanoma cell proliferation and migration. Cell Signal. 2010;22:57–64. doi: 10.1016/j.cellsig.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 20.Dolderer JH, Schuldes H, Bockhorn H, Altmannsberger M, Lambers C, von Zabern D, et al. HERG1 gene expression as a specific tumor marker in colorectal tissues. Eur J Surg Oncol. 2010;36:72–7. doi: 10.1016/j.ejso.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 21.Miljanich GP, Ramachandran J. Antagonists of neuronal calcium channels: structure, function, and therapeutic implications. Annu Rev Pharmacol Toxicol. 1995;35:707–34. doi: 10.1146/annurev.pa.35.040195.003423. [DOI] [PubMed] [Google Scholar]
- 22.Lang F, Föller M, Lang KS, Lang PA, Ritter M, Gulbins E, et al. Ion channels in cell proliferation and apoptotic cell death. J Membr Biol. 2005;205:147–57. doi: 10.1007/s00232-005-0780-5. [DOI] [PubMed] [Google Scholar]
- 23.Jehle J, Schweizer PA, Katus HA, Thomas D. Novel roles for hERG K(+) channels in cell proliferation and apoptosis. Cell Death Dis. 2011;2:e193. doi: 10.1038/cddis.2011.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Glassmeier G, Hempel K, Wulfsen I, Bauer CK, Schumacher U, Schwarz JR. Inhibition of HERG1 K+ channel protein expression decreases cell proliferation of human small cell lung cancer cells. Pflugers Arch. 2012;463:365–76. doi: 10.1007/s00424-011-1045-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Curran ME, Splawski I, Timothy KW, Vincent GM, Green ED, Keating MT. A molecular basis for cardiac arrhythmia: HERG mutations cause long QT syndrome. Cell. 1995;80:795–803. doi: 10.1016/0092-8674(95)90358-5. [DOI] [PubMed] [Google Scholar]
- 26.Sanguinetti MC, Jiang C, Curran ME, Keating MT. A mechanistic link between an inherited and an acquired cardiac arrhythmia: HERG encodes the IKr potassium channel. Cell. 1995;81:299–307. doi: 10.1016/0092-8674(95)90340-2. [DOI] [PubMed] [Google Scholar]
- 27.Rubinstein LV, Shoemaker RH, Paull KD, Simon RM, Tosini S, Skehan P, et al. Comparison of in vitro anticancer-drug-screening data generated with a tetrazolium assay versus a protein assay against a diverse panel of human tumor cell lines. J Natl Cancer Inst. 1990;82:1113–8. doi: 10.1093/jnci/82.13.1113. [DOI] [PubMed] [Google Scholar]